Abstract
The regulated release of hormones and neurotransmitters is a fundamental process throughout the animal kingdom. The short time scale for the calcium triggering of vesicle fusion in regulated secretion suggests that the calcium sensor synaptotagmin and the soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) membrane fusion machinery are well ordered before the calcium signal. To gain insight into the organization of the prefusion protein assembly in regulated exocytosis, we undertook a structural/functional study of the vesicular synaptotagmin1 and the plasma membrane SNARE proteins, which copurify from the brain in the absence of calcium. Based on an evolutionary analysis, mutagenesis screens, and a computational protein docking approach, we now provide the first testable description of the supramolecular prefusion assembly. Perturbing the determined synaptotagmin/SNARE-interacting interface in several models of regulated exocytosis altered the secretion of hormones and neurotransmitters. These mutations also disrupted the constitutive synaptotagmin/SNARE link in full agreement with our model. We conclude that the interaction of synaptotagmin with preassembled plasma membrane SNARE proteins, before the action of calcium, can provide a precisely organized “tethering” scaffold that underlies regulated secretion throughout evolution.
INTRODUCTION
In regulated secretion, fusion of docked secretory vesicles with the plasma membrane is triggered by the rapid elevation of the intracellular calcium concentration (Katz and Miledi, 1967). The membrane fusion itself is brought about by the action of the three soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) proteins, whereas the vesicular protein synaptotagmin (SYT) acts as the calcium sensor (Sollner et al., 1993; Sudhof and Scheller, 2001; Bonifacino and Glick, 2004). In neuroendocrine cells, the principal SNAREs are syntaxin1 and synaptosome-associated protein of 25 kDa (SNAP-25) on the plasma membrane (so-called target SNAREs or t-SNAREs), and vesicular synaptobrevin, also known as VAMP. Interaction between synaptobrevin and the two t-SNAREs leads to the formation of the ternary SNARE complex, a twisted parallel four-helical bundle that probably drives membrane fusion (Sutton et al., 1998). With all the major players involved in vesicle fusion identified, it is becoming possible to tackle a central problem of this cellular process— how does calcium trigger vesicle fusion? Arguably, to understand the action of calcium it is essential to know 1) the extent of the assembly of the SNARE fusion proteins and 2) their organization in relation to the calcium sensor, SYT, before calcium-triggered events.
It would be reasonable to assume that the SNAREs are at least partially preassembled, and the plasma membrane syntaxin and SNAP-25 are the first obvious candidates for being in such a ready state (An and Almers, 2004; Rickman et al., 2004b). Importantly, synaptobrevin binds syntaxin and SNAP-25 with high-affinity only when the two plasma membrane proteins are together and not apart (Hayashi et al., 1994). Likewise, SYT can interact, in the absence of calcium (constitutively), with the preassembled syntaxin and SNAP-25, but not with the individual proteins (Rickman and Davletov, 2003; Rickman et al., 2004a). SYT possesses two C2 domains, C2A and C2B, both being capable of calcium-dependent phospholipid binding in in vitro reactions (Davletov and Sudhof, 1993; Fernandez et al., 2001). The original observed property of native SYT, however, was its ability to copurify with assembled SNAREs, in the absence of calcium, from brain extracts (Sollner et al., 1993; Mehta et al., 1996; Leveque et al., 2000). The ability of the assembled, rather than individual, t-SNARE proteins to bind SYT and synaptobrevin may be a reflection of large structural changes in syntaxin and SNAP-25 upon their interaction (Fasshauer et al., 1997).
Theoretical models, based mostly on genetic data, have recently been put forward to indicate the spatial arrangement of SYT with the SNAREs (Yoshihara and Littleton, 2002; Koh and Bellen, 2003; Nishiki and Augustine, 2004). In view of the absence of experimental data for the organization of this supramolecular assembly before the action of calcium, we decided to investigate molecular and structural determinants for the constitutive SYT/SNARE association in detail. Through evolutionary analysis of SNAP-25 and mutagenesis screens, we identified a highly conserved region in the N-terminal helix of SNAP-25 as being essential for both constitutive SYT coupling and regulated exocytosis. Using this information and the previously identified SNARE-binding epitope on SYT (Rickman et al., 2004a), we used a computational protein docking approach to generate a structure-based model for the constitutive SYT link to the preassembled t-SNAREs. Our model provides a unifying molecular explanation for the phenotypes of secretion-deficient mutations previously identified in genetic studies.
MATERIALS AND METHODS
Plasmids and Protein Preparation
Plasmids encoding glutathione S-transferase (GST) fusion proteins with syntaxin1A (aa 1–261), synaptobrevin2 (aa 1–96), SYT1 C2AB (aa 95–421), C2B (aa 248–421), and complexin2 were described previously (Hu et al., 2002b; Rickman et al., 2004a). The full-length SNAP-25A and SNAP-25B were kind gifts of Drs. R. Jahn (Max-Planck-Institute for Biochemical Chemistry, Gottingen, Germany) and Y.-K. Shin (Iowa State University, Ames, Iowa) (Fasshauer et al., 1999; Kweon et al., 2003). GST-SNAP-25, carrying cysteine-to-alanine mutations, exhibits improved expression in bacteria with no changes detected in biochemical or biophysical properties (Fasshauer et al., 1999). His-tagged versions of syntaxins 1A and 1B were a kind gift of Dr. J. Blasi (University of Barcelona, Barcelona, Spain) (Perez-Branguli et al., 2002). Mammalian plasmids encoding C2AB of SYT1 (aa 96–421) and SNAP-25R carrying a BoNT/E-resistant mutation [R179W,I180E,E182I] in the C-terminal end of SNAP-25 were described previously (Washbourne et al., 1999; Rickman et al., 2004a). Mutagenesis was performed using the QuikChange system (Stratagene, La Jolla, CA) with mutants identified by DNA sequencing by Cytomyx (Cambridge, UK). Native SYT was purified as a by-product of the SNARE isolation from bovine brains (Rickman and Davletov, 2003). To isolate the brain syntaxin, the native SNARE complex was disrupted by heating for 3 min at 95°C, and the SNARE proteins were subjected to electroelution (Hu et al., 2002a). The eluted syntaxin was refolded by replacing SDS with 0.8% β-octylglucoside on a gel-filtration column. The isolated syntaxin was active in SNARE, synaptotagmin, and complexin binding reactions and also in reconstituted fusion assays (Hu et al., 2002b; Rickman et al., 2004a, 2005). The recombinant syntaxin1A (1-261) fails to substitute for the brain-purified syntaxin in the constitutive SYT binding even when subjected to the same electroelution procedure.
Protein Binding Assays and Circular Dichroism (CD) Spectroscopy
All binding reactions were performed for 30 min in the absence of calcium (2 mM EDTA) at 22°C. GST-tagged proteins (3 μg each) were immobilized on glutathione-Sepharose beads and incubated in 100 μl of buffer A (100 mM NaCl, 0.1% Triton X-100, 2 mM EDTA, and 20 mM HEPES, pH 7.2) in the presence of 3 μg of partner proteins. Beads were washed with buffer A, and bound protein was analyzed by SDS-PAGE followed by Coomassie staining (50% of reaction) or Western immunoblotting (1% of reaction). For immunoblotting, protein bands were detected using syntaxin (clone HPC-1; Sigma-Aldrich, St. Louis, MO) and SNAP-25 (clone 71.2; Synaptic Systems, Goettingen, Germany) antibodies and chemiluminescence (West Dura; Pierce Chemical, Rockford, IL). The chemiluminescent signal was imaged using a ChemiDoc XRS (Bio-Rad, Hercules, CA) and quantified using Quantity One software (Bio-Rad). Real-time binding of C2B, without the GST tag, to the SNAP-25/syntaxin complex was performed using a Biacore 2000 system (Biacore, Uppsala, Sweden). A single channel of CM5 chip (Biacore) was used to immobilize GST-SNAP-25 protein that was then loaded with brain-derived syntaxin before C2B binding. Syntaxin and C2B binding to immobilized GST-SNAP-25 was highly reproducible between cycles (SD <2%). C2B (2 μM) was applied to the chip at a rate of 2 μl/min in buffer A. In circular dichroism experiments, far UV-CD spectra were obtained by averaging five scans with a step size of 0.2 nm on a Jasco J-810 CD spectrometer (Jasco, Tokyo, Japan) at 25°C. Measurements were performed in a quartz cuvette (0.2 cm; Hellma, Plainview, NY) using normalized protein amounts. The CD spectra measurements of the t-SNARE assembly were performed at equilibrium. In thermal denaturation experiments, the ellipticity was measured at 222 nm. Formation of the SDS-resistant SNARE complex was analyzed in 30-min reactions at 22°C as described previously (Hu et al., 2002a).
Computational Protein Docking
A set of in silico complexes was generated, on the basis of shape complementarity, by a systematic search for possible, rigid body, docking orientations of the atomic coordinates of the SNARE ternary complex (Protein Data Bank [PDB] code: 1sfc) with the C2A or C2B domains of SYT1 (PDB codes: 1byn and 1k5w, respectively) using the fast Fourier transform-based FTDOCK program (Gabb et al., 1997). Further refinement was performed using a MULTIDOCK program to minimize atom clashes and to improve bonding of the side chains of each interacting component (Jackson et al., 1998). In the resulting complexes, the electrostatic and shape complementarities of the individual molecules upon complexation were specifically assessed as 1) the difference of electrostatic potentials (Eelect) between the bound-unbound states using a finite difference Poisson–Boltzmann solver (Honig and Nicholls, 1995; Grant et al., 2001); and 2) the amount of buried area (BA) upon complexation calculated using the NACCES program (Hubbard et al., 1991). For the electrostatic calculations, the relative dielectric constants for protein and solvent were taken to be 2.0 and 78.54, respectively, considering an electrolyte concentration of 100 mM. The energetic contributions were combined into a total binding energy (Etotal) defined as Etotal = Eelect + γ× BA, where γ is equal to –0.005 kcal × mol–1 × Å–2. Favorable complexes, i.e., those with total binding energies less than –10 kcal/mol (Erickson, 1989), were subsequently filtered using the mutagenesis data. The presented model was chosen based on its very low energy (–27 kcal/mol) and the interfacial bonding of the C2B K326,K327 and SNAP-25 D51,E52,E55 residues. Small rotations and translations of the individual interacting components are allowed and will be valid within the accuracy of our calculations.
Analysis of Protein Mutants in Release Assays
The effect of the SNAP-25 [D51K,E52K,E55K] mutation on the release of growth hormone (GH) was investigated in PC12 cells. LipofectAMINE 2000 (Invitrogen, Carlsbad, CA) was used to transiently transfect cells with plasmids expressing human growth hormone (pXGH5) and the botulinum neurotoxin E light chain (BoNT/E) in the presence of either control (pcDNA3) or BoNT/E-resistant SNAP-25R-encoding vectors (Graham et al., 2002). Seventy-two hours later, transfected cells were challenged with 0 or 10 μM calcium in the presence of 20 μM digitonin-containing Krebs-Ringer buffer (145 mM NaCl, 5 mM KCl, 1.3 mM MgCl2, 1.2 mM NaH2PO4, 10 mM glucose, and 20 mM HEPES, pH 7.4). GH release over a 15-min period was assayed using a GH assay kit (Roche Diagnostics, Lewes, United Kingdom).
The ability of rat SYT1 C2AB, mutated or not, to compete with the native vesicular form was probed in bovine chromaffin cells by amperometry as described previously (Rickman et al., 2004a). Briefly, 3 d after electroporation, cells transfected with C2AB were identified through coexpression of enhanced green fluorescent protein. A carbon fiber electrode was positioned in contact with fluorescent, or control, cells, and exocytosis was triggered by pressure ejection of a buffer containing 20 μM digitonin and 10 μM calcium. Amperometric responses were collected at 4 kHz and digitized with a Digidata 1322A acquisition system (Molecular Devices, Sunnyvale, CA). Similar levels of expression of control and mutant proteins in mammalian cells were confirmed by immunoblotting using either PC12 cells (SNAP-25R) or HeLa cells (C2AB).
RESULTS
Complementary Binding of Two Vesicular Proteins to t-SNAREs
We have recently identified the residues on the C2B domain of SYT1 involved in the constitutive association with syntaxin and SNAP-25, the latter two being purified from a native source, the bovine brain (Rickman et al., 2004a). To experimentally map the binding interface on the SNAREs, recombinant proteins amenable to mutagenesis were required. We found that bacterially expressed SNAP-25 can be used for the constitutive SYT binding. As shown in Figure 1A, the cytoplasmic part of SYT (C2AB) constitutively binds brain-derived syntaxin1 and the recombinant SNAP-25B when they are together in a binary complex, being unable to bind to individual proteins. Both forms of SNAP-25, A and B, were equally effective, whereas neither recombinant syntaxin1A nor syntaxin1B, both lacking their transmembrane regions, could efficiently support calcium-independent C2AB binding (our unpublished data). The deficiency of bacterially expressed syntaxin1 may be because of either the lack of the transmembrane region or the absence of yet uncharacterized posttranslational modifications. Therefore, we used the brain-purified syntaxin in all subsequent reactions and focused on SNAP-25 mutagenesis.
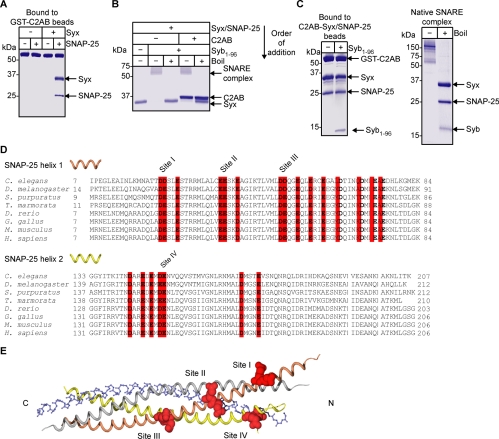
Figure 1.
Constitutive binding of two vesicular proteins, SYT and synaptobrevin, to the plasma membrane proteins syntaxin and SNAP-25. (A) Immobilized GST-C2AB of SYT1 binds constitutively to the t-SNARE assembly, containing recombinant SNAP-25 and brain-derived syntaxin (syx), but not the individual t-SNAREs. Coomassie-stained gel. (B) C2AB does not inhibit formation of the SDS-resistant SNARE complex. Syntaxin was incubated with SNAP-25 and C2AB for 30 min before addition of synaptobrevin (syb, aa 1–96). SNARE complex assembly was detected by the appearance of a 60-kDa band in a Coomassie-stained gel. Boiling disrupts the SDS-resistant SNARE complex. (C) t-SNAREs, when prebound to C2AB, can engage synaptobrevin (left) with a stoichiometry similar to that found in the native SNARE complex (right). Coomassie-stained gels. (D) Sequence alignment of SNAP-25 from evolutionarily distant organisms. Conserved acidic amino acids are highlighted in red. Double acidic residue sites are numbered. (E) The four conserved sites are shown in spacefill (red) with SNARE helices as colored ribbons (gray, syntaxin; orange, SNAP-25 helix1; yellow, SNAP-25 helix2; blue backbone designates synaptobrevin).
Before analyzing SNAP-25 residues, we investigated the relationship between SYT and synaptobrevin binding to the two t-SNAREs. Because SYT can bind the t-SNARE assembly in the absence of calcium, C2AB may act as a molecular clamp, physically interfering with synaptobrevin engagement of t-SNAREs. We tested whether C2AB could block formation of the ternary SNARE complex in defined reactions. Figure 1B shows that preincubation of syntaxin and SNAP-25 with C2AB had no effect on the ability of the two t-SNAREs to form, upon the addition of synaptobrevin, the tight SDS-resistant ternary SNARE complex implicated in membrane fusion (Hayashi et al., 1994; Sutton et al., 1998). Next, the two t-SNAREs were first prebound to the immobilized GST-C2AB, and then the triple protein assembly— C2AB/syntaxin/SNAP-25—was incubated with synaptobrevin. Synaptobrevin bound the C2AB/t-SNARE triple assembly with a stoichiometry similar to that found in the native SDS-resistant SNARE complex purified from bovine brain (Figure 1C). Therefore, SYT by itself does not prevent the soluble synaptobrevin engagement with the two t-SNAREs providing an explanation for copurification of all four proteins from the brain detergent extracts (Sollner et al., 1993). Because the SYT/t-SNARE interaction is not diminished after synaptobrevin binding (Figure 1C), the two vesicular proteins do not compete with each other and may have independent binding sites. Indeed, whereas the SYT interaction with t-SNAREs is primarily electrostatic (Rickman et al., 2004a), synaptobrevin binds to a hydrophobic groove formed by syntaxin and SNAP-25 (Sutton et al., 1998).
The SYT Binding Epitope on SNAP-25
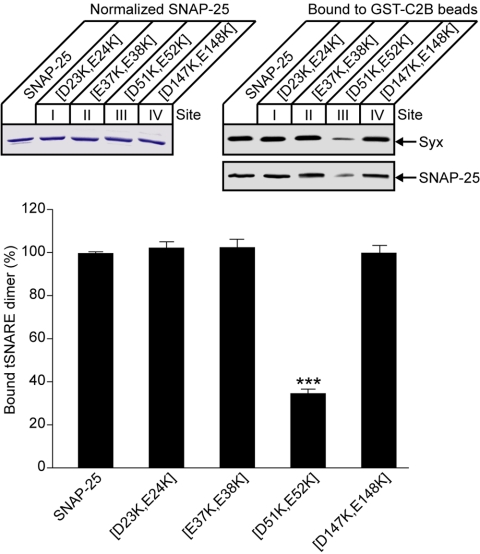
The constitutive interaction of SYT with the preassembled t-SNAREs is mediated by a conserved polybasic motif on the C2B domain and principally involves a pair of lysines, 326 and 327 (Rickman et al., 2004a). The ability of the recombinant SNAP-25 to replicate this function allowed us to map the binding site on SNAP-25 through site-directed mutagenesis. First, we analyzed the two helices of SNAP-25 for the presence of conserved acidic amino acids in evolutionarily distant organisms. Such an analysis is now used, in the postgenomic era, to uncover potential areas for protein interactions (Lichtarge and Sowa, 2002). In the two helices, there are four conserved double acidic residue sites, labeled I to IV and highlighted on the ribbon representation of the helical crystal structure of the ternary SNARE complex (Figure 1, D and E). To test for involvement of these four sites in SYT binding, the relevant aspartate and glutamate residues were mutated to lysines. After protein normalization, SNAP-25 mutants were incubated, in the presence of syntaxin, with immobilized GST-C2B. For initial screens, bound material was analyzed by Western immunoblotting. Only mutation of site III [D51K,E52K] resulted in a reduction in bound t-SNAREs (Figure 2). Notably, this mutation reduced binding of not only SNAP-25 but also syntaxin, supporting the fact that t-SNAREs bind to SYT as a singular molecular entity.
Figure 2.
Identification of the constitutive SYT binding site on the SNAP-25 molecule. Mutational screen of double acidic amino acid sites on SNAP-25. GST-C2B, immobilized on Sepharose beads, was incubated with normalized SNAP-25 (top left, Coomassie-stained gel) in the presence of brain-purified syntaxin. Bound material (1%) was analyzed by Western immunoblotting using t-SNARE antibodies (top right). The histogram represents a summary of the mutational analysis of SNAP-25 assessed by quantitation of the chemiluminescent signals. Error bars are SEM (n = 3); ***p < 0.0001.
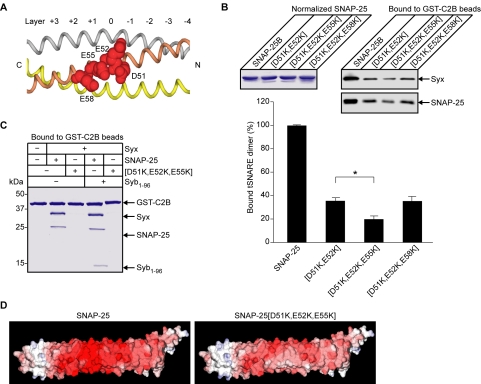
Closer inspection of site III reveals two additional glutamate residues next to D51 and E52 (Figure 3A). This site is at the ionic layer (layer 0) observed in the crystal structure of the ternary SNARE complex (Sutton et al., 1998). To analyze whether these additional residues, E55 and E58, also play a role in the SYT binding, triple mutants were generated. Quantification of bound t-SNAREs showed that the further mutation of [E55K] but not [E58K] had an additive effect on the SNAP-25[D51K,E52K] mutant (Figure 3B), highlighting the specificity of the SYT interaction. Finally, we tested the ability of SYT to interact with either the binary or ternary SNARE complexes containing SNAP-25[D51K,E52K,E55K]. The triple mutation severely disrupted C2B binding of both t-SNAREs and of the ternary SNARE complex as judged by Coomassie staining (Figure 3C). Figure 3D illustrates the effect of the [D51K,E52K,E55K] mutation on the electrostatic potential of SNAP-25 in the context of the SNARE complex.
Figure 3.
The synaptotagmin binding epitope on SNAP-25 consists of D51, E52, and E55. (A) Representation of the SYT binding site on the SNAP-25 helix1. Acidic residues D51, E52, E55, and E58 are shown in spacefill with SNARE helices colored as in Figure 1E. The layers involved in ternary complex formation are numbered with the ionic layer marked 0. (B) Further mutational analysis of the synaptotagmin binding site on SNAP-25. GST-C2B, on Sepharose beads, was incubated with syntaxin and normalized SNAP-25 (Coomassie-stained gel), carrying mutations or not. Bound material (1%) was analyzed by Western immunoblotting (top right) with quantitation of the chemiluminescent signal (bottom). Error bars are SEM (n = 3), *p <0.05. (C) The triple mutation of SNAP-25 abrogates association of both the t-SNARE assembly and the ternary SNARE complex with immobilized GST-C2B as judged by Coomassie staining. (D) Electrostatic potential of SNAP-25 before and after the [D51K,E52K,E55K] mutation in the context of the ternary SNARE complex (–10 to +10 kT/e). Blue, positive charge; red, negative charge.
The SYT Binding Epitope on SNAP-25 Is Essential for Regulated Exocytosis
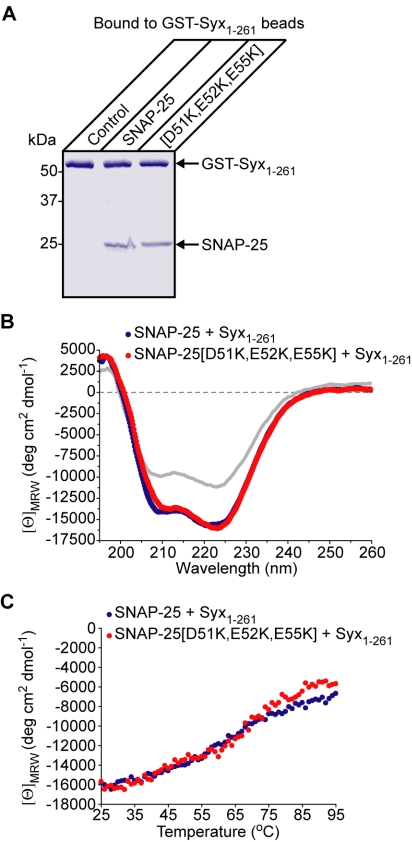
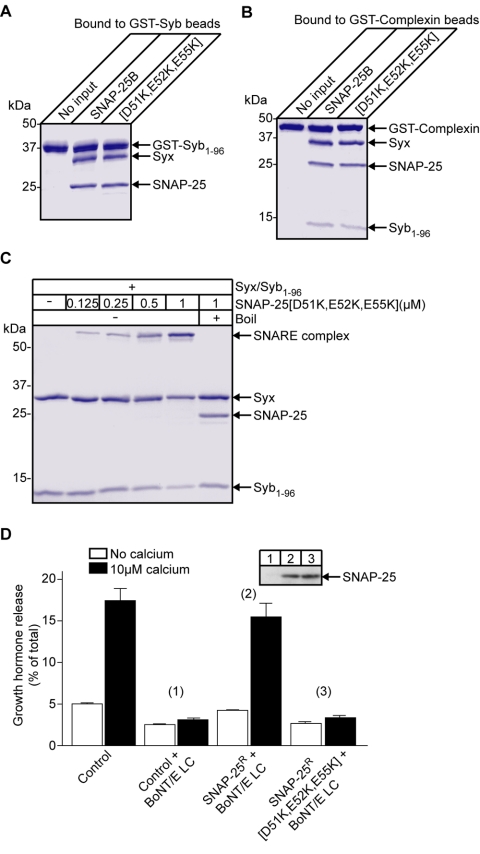
Before testing the impact of the SNAP-25 [D51K,E52K,E55K] mutation on regulated exocytosis, it was important to determine whether these changes were specifically disrupting the SYT/t-SNARE link or were simply compromising the ability of SNAP-25 to associate with syntaxin. In a direct binding reaction, SNAP-25[D51K,E52K,E55K] readily bound to GST-syntaxin in a similar manner to the nonmutated molecule (Figure 4A). Next, CD spectroscopy demonstrated that syntaxin and SNAP-25, when mixed, exhibit a significant increase in α-helicity compared with the calculated spectra for noninteracting molecules, in agreement with a previous study (Fasshauer et al., 1997). Comparison of CD spectra for syntaxin with either the nonmutated or SNAP-25[D51K,E52K,E55K] demonstrated a similar increase in α-helical spectra (Figure 4B), indicating that the two forms of SNAP-25 generate comparable helical structures. To assess the relative stability of the t-SNARE assembly, thermal denaturation was used with α-helical content monitored by CD (Figure 4C). There was no significant difference between the t-SNARE assemblies containing SNAP-25 with or without the triple mutation. Furthermore, we tested the ability of the t-SNAREs to bind synaptobrevin. Syntaxin, preincubated with either the wild-type SNAP-25 or SNAP-25[D51K,E52K,E55K], was added to synaptobrevin immobilized on Sepharose beads (Figure 5A). The triple mutation had no detectable effect on the synaptobrevin/t-SNARE interaction. Complexin, a soluble protein that specifically interacts with the fully folded ternary SNARE complex (Chen et al., 2002), exhibited comparable binding of the SNARE complexes made with SNAP-25, carrying the triple mutation or not (Figure 5B). Although this experiment provided strong evidence that folding of the SNARE helices is not affected by the mutation, it was essential to test the ability of the mutated SNAP-25 to form the SDS-resistant SNARE complex implicated in the fusion process (Sutton et al., 1998). Titration of SNAP-25 demonstrated that the mutated SNAP-25 was fully capable of entering into the “tight” SNARE complex (Figure 5C). We conclude therefore that the triple [D51K,E52K,E55K] mutation of SNAP-25 disrupts specifically the constitutive binding of SYT with the assembled t-SNAREs but not interactions between the SNAREs themselves.
Figure 4.
The triple mutation of SNAP-25 does not prevent the syntaxin/SNAP-25 interaction. (A) Immobilized syntaxin (GST-Syx1-261) was incubated with SNAP-25, mutated or not, and bound material was analyzed by SDS-PAGE. Coomassie-stained gel. (B) CD spectra of the t-SNARE assembly formed with SNAP-25 mutated (red) or not (blue). The calculated noninteracting mean residue ellipticities (gray) are shown for comparison. (C) Thermal denaturation of the t-SNARE assemblies monitored by CD spectroscopy at 222 nm.
Figure 5.
Mutation of the SYT-binding epitope on SNAP-25 does not disrupt formation of the ternary SNARE complex and yet abrogates regulated exocytosis. (A) Binding of the t-SNARE assembly by synaptobrevin is not affected by the triple mutation of SNAP-25. Immobilized synaptobrevin (GST-Syb) was incubated with syntaxin and SNAP-25, with or without the triple mutation. Bound material was analyzed by SDS-PAGE and Coomassie staining. (B) Complexin binding to the ternary SNARE complex. GST-complexin, immobilized on Sepharose beads, was incubated with the ternary SNARE complexes containing SNAP-25 with or without the triple mutation. Coomassie-stained gel. (C) SNAP-25[D51K,E52K,E55K] forms the SDS-resistant SNARE complex at the indicated concentrations in a 30-min reaction. Coomassie-stained gel. (D) The triple mutation in SNAP-25 potently blocks exocytosis in BoNT/E-resistant PC12 cells. On expression of the light chain of BoNT/E, the release of GH is blocked in control cells but not in cells expressing BoNT/E-insensitive SNAP-25, SNAP-25R. Introduction of the D51K,E52K,E55K mutation into SNAP-25R abrogates regulated exocytosis. The amount of GH collected for 15 min is expressed as a percentage of total cellular GH. Expression levels of the constructs (numbered) were verified by Western immunoblotting using a SNAP-25 antibody (inset).
To investigate the impact of the triple SNAP-25 mutation on regulated exocytosis, we sought to express the mutant SNAP-25 in the absence of endogenous SNAP-25. This was accomplished by 1) using botulinum neurotoxin E light chain (BoNT/E LC) that cleaves endogenous SNAP-25, leading to its removal from the plasma membrane syntaxin (Bajohrs et al., 2004); and 2) expressing a BoNT/E-resistant form of SNAP-25, SNAP-25R, to restore exocytosis (Graham et al., 2002). Mutagenesis of this SNAP-25R molecule thereby permits to assess the contribution of specific SNAP-25 residues in regulated exocytosis. We used neuroendocrine PC12 cells that serve as a popular model for studies of regulated exocytosis (Chen et al., 1999). Transfection of PC12 cells with the plasmid expressing BoNT/E LC alone resulted in an efficient blockade of exocytosis, as measured by the growth hormone release assay, whereas its cotransfection with SNAP-25R resulted in restoration of exocytosis (Figure 5D). The triple mutation [D51K,E52K,E55K] was then introduced in SNAP-25R. The extent of exocytosis upon expression of SNAP-25R[D51K,E52K,E55K] was greatly reduced, despite the same level of protein expression as for SNAP-25R (Figure 5D). Evidently, the SYT-binding epitope on SNAP-25 helix1 does play a critical role in regulated exocytosis. Because this epitope does not disrupt the interaction between the SNAREs themselves, it is reasonable to conclude that it affects the SYT/SNARE link, which is vital for efficient exocytosis (Sudhof and Scheller, 2001; Koh and Bellen, 2003).
In Silico Modeling of the SYT/SNARE Complex
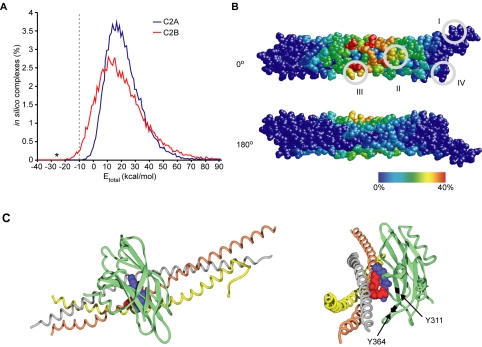
The requirement for the full-length brain-purified syntaxin, mentioned above, to form the stoichiometric SYT/SNARE supramolecular assembly precludes crystallization trials. However, the available crystal structures of the SYT1 C2 domains and of the ternary SNARE complex offered an alternative way to gain further insight into the constitutive SYT/SNARE interaction, by using an in silico docking approach. This approach has recently been designed to derive protein complexes from known atomic coordinates of the individual components with no prior knowledge of their interacting surfaces (Smith and Sternberg, 2002; van Dijk et al., 2005). We used the FTDOCK program, which was developed for docking native structures and was shown to successfully predict protein complexes in test conditions (Gabb et al., 1997). An initial set of ∼27,000 binding geometries was generated by systematically docking different orientations of the atomic coordinates of the SNARE complex (Sutton et al., 1998) to individual C2 domains (Sutton et al., 1995; Fernandez et al., 2001). Total binding energies for each possible complex were then derived from their buried area and the electrostatic complementarity. Considering that ∼10 kcal/mol is required to overcome the entropy loss upon complexation (Erickson, 1989), the number of favorable complexes between the C2B domain and the SNARE complex was still relatively large, 296 (Figure 6A). By contrast, none of the C2A-involving complexes could fulfill such a criterion consistent with the inability of C2A to bind t-SNAREs in the absence of calcium (Rickman et al., 2004a).
Figure 6.
Computational analysis of the SYT/SNARE binding interface. (A) The distribution of total binding energies for all complexes generated by the FTDOCK program expressed as a percentage of the total number (∼27,000 for each C2 domain). C2B, and not C2A, can produce complexes with the SNARE helices with low values of total binding energy (–10 kcal/mol indicated by a dashed line). The asterisk indicates the complex displayed in Figure 6C. (B) Calculated frequency of individual SNARE amino acids to be a part of the interface in favorable interactions with the C2B domain. The SNARE helices are shown in spacefill in the orientation as in Figure 1E (0°) with a 180° rotation about the longitudinal axis (180°). Amino acids are colored according to their frequency of involvement in C2B interactions from 0% (blue) to 40% (red). The four conserved sites identified in Figure 1 are enclosed in gray circles. (C) The low-energy docking orientation (Etotal = –27 kcal/mol) for the constitutive C2B/SNARE association where the K326 and K327 SYT residues (blue) interact with the SNAP-25 amino acids D51, E52, and E55 (red). SNARE helices are shown as colored ribbons (gray, syntaxin; orange, SNAP-25 helix1; and yellow, SNAP-25 helix2) with the interacting residues depicted in spacefill. The carboxy-terminus is to the left in the left panel and foremost in the right panel. Calcium-binding loops of C2B (green) are oriented downward. The model suggests a contribution of the Y311 residue, changed in the AD3 mutation to asparagine, to the binding interface. In the rotated view about the y-axis (right), Y311 and the neighboring Y364, used in a control reaction, are highlighted in black.
The systematic docking of C2B to the SNARE complex surface residues, with the set electrostatic and buried area constraints, indicated amino acids that are likely to participate in energetically favorable complexes (Figure 6B). In the SNAP-25 helices, the residues centered on E52 exhibited the highest calculated frequency to take part in favorable complexes, thereby lending a structure-based support for our experimental data. The closely located syntaxin residues E224 and E228 also showed a strong tendency for interaction with the C2B domain; however, as evident from our experimental data (Figures 3C and 5D), the triple mutation of SNAP-25 is sufficient to disrupt the SYT/SNARE association in vitro and regulated exocytosis in vivo. Rotation of the SNARE complex by 180° highlights the overall selectivity of the in silico docking approach (Figures 6B). The face of the SNARE complex with the lowest frequency for the C2B interaction (Figure 6B, blue color) corresponds to the synaptobrevin helix (Sutton et al., 1998).
Taking into account the limitations of computational docking (van Dijk et al., 2005), we used our mutagenesis data of SNAP-25 (Figures 2 and 3) and of C2B (Rickman et al., 2004a) to determine a complex with interfacial bonding for all experimentally identified residues. The selected C2B/t-SNARE complex, shown in Figure 6C, represents the highest ranked prediction based on 1) the lowest total binding energy, –27 kcal/mol; and 2) participation of the C2B lysines 326 and 327 and the SNAP-25 three acidic residues D51, E52, E55 in the interaction. It presents both a very favorable electrostatic energy (–17 kcal/mol) and fulfills the essential requirement for shape complementarity (Lo Conte et al., 1999). In this near perpendicular arrangement of the two structures, the concave face of C2B partially envelops the SNARE cylinder, resulting in a buried area of almost 2000 Å2. In this configuration, the bound C2B domain is on the opposite face to the synaptobrevin-binding groove, consistent with the complementary interaction of the two vesicular proteins with syntaxin and SNAP-25 (Figure 1).
Examination of the AD3 Mutation in the SYT C2B Domain
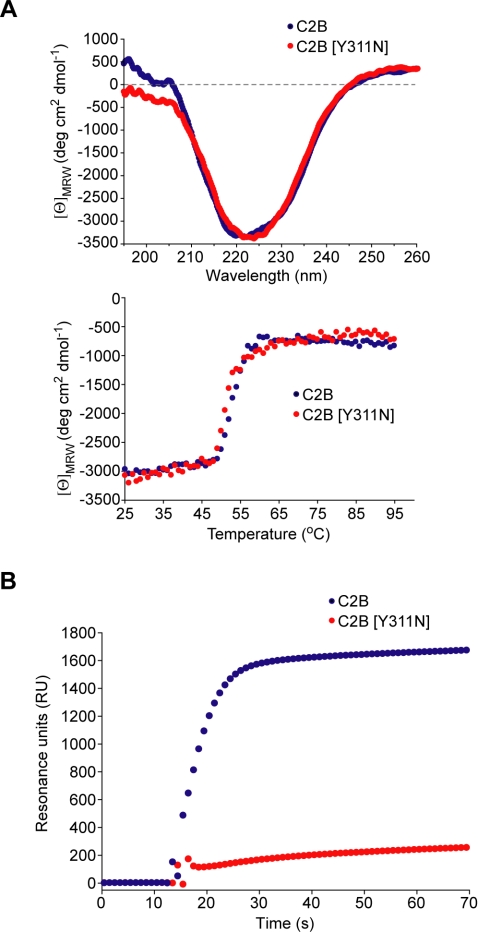
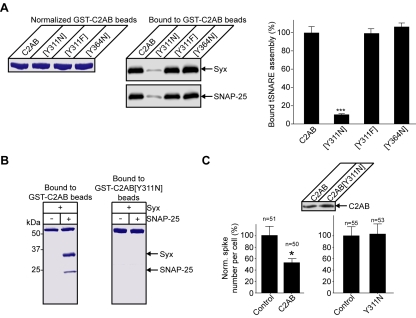
Analysis of the literature for mutations residing at the SYT/SNARE binding interface revealed that a well characterized mutation, named AD3 in the original Drosophila study (DiAntonio et al., 1993), is in proximity to the interacting residues (Figure 6C, right). This mutant carries only a single amino acid substitution of tyrosine to asparagine but exhibits a pronounced decrease in evoked release (DiAntonio et al., 1993; Yoshihara and Littleton, 2002). Intriguingly, this mutation, corresponding to Y311 in the rat protein, affects neither the calcium-triggered phospholipid binding nor the reported calcium-dependent interactions with recombinant SNAREs (Littleton et al., 2001). We hypothesized that the Y311N mutation may impact upon the constitutive SYT/SNARE interaction providing an immediate test for the relevance of our model. Comparative CD spectroscopy of C2B demonstrated that the overall β-sheet content and its stability are not affected by this single mutation (Figure 7A). We then immobilized GST-SNAP-25 with brain-derived syntaxin on a surface plasmon resonance chip and tested constitutive binding of the C2B domain, mutated or not. The Y311N mutation strongly decreased the ability of the untagged C2B domain to associate with the t-SNAREs (Figure 7B). It was essential to determine whether the Y311N mutation, even in the context of the tandem C2A-C2B structure, would be sufficient to disrupt the constitutive SYT/SNARE interaction. Normalized amounts of the wild-type and mutated GST-C2AB domain were immobilized on Sepharose beads and incubated with the t-SNAREs. The Y311N mutation severely disrupted C2AB binding to the t-SNARE assembly in the quantitative pull-down assay (Figure 8A). In a control experiment, mutation of a neighboring tyrosine (Y364, highlighted in Figure 6C) to asparagine had no impact on the t-SNARE binding, indicating the specific importance of Y311. Mutation of tyrosine 311 to a different hydrophobic amino acid—phenylalanine [Y311F]— did not affect the constitutive t-SNARE binding (Figure 8A), arguing that the deleterious effect of the AD3 mutation is not due to disrupting a specific amino acid interaction but is likely a result of a local structural change in the C2B domain. Indeed, this amino acid is packed between neighboring side chains and is largely buried, indicating a structural role (Fernandez et al., 2001). The direct staining of pull-down reactions with Coomassie stain confirms the disruptive effect of the AD3 mutation on the constitutive SYT/t-SNARE interaction (Figure 8B).
Figure 7.
Effect of the Y311N mutation on the ability of C2B to bind the t-SNARE assembly. (A) CD measurements (top) and the melting spectra (bottom) of the wild-type or mutant C2B show no major disruption of the secondary structure. (B) Real-time binding analysis of the constitutive association of the wild-type, or mutant, C2B with immobilized GST-SNAP-25/syntaxin by surface plasmon resonance. Only wild-type C2B can efficiently associate with the assembled t-SNAREs.
Figure 8.
Specific disruption of the constitutive SYT/SNARE link by the AD3 mutation. (A) Y311N, but not control mutations Y364N and Y311F, in the C2AB domain affect the constitutive t-SNARE binding. GST-C2ABs were normalized (left, Coomassie-stained gel), immobilized on Sepharose beads, and incubated with syntaxin and SNAP-25. Bound t-SNAREs were analyzed by immunoblotting (middle) with quantitation of the chemiluminescent signals (right). Error bars are SEM (n = 3), ***p < 0.0001. (B) Immobilized C2AB carrying the Y311N mutation fails to support constitutive t-SNARE binding. Coomassie-stained gel. (C) Expression of C2AB in bovine chromaffin cells impedes regulated catecholamine release (left). The Y311N mutation abrogates the ability of C2AB to suppress regulated exocytosis (right). Bar charts show normalized number of amperometric spikes per transfected cell, after stimulation, as a percentage of nontransfected cells (control). *p < 0.02. Expression levels of the constructs were verified, in HeLa cells, by Western immunoblotting using a SYT1 antibody (inset).
To test the impact of the Y311N mutation in a mammalian system, we used mature bovine chromaffin cells, which strictly rely on the SYT1 isoform in regulated exocytosis (Burgoyne and Morgan, 2003). Expression of the soluble C2AB fragment in the cytoplasm is known to impede catecholamine release because this soluble C2AB competes with the native vesicular SYT for its molecular target. Mutation of this inhibitory C2AB allows analysis of the residues that contribute to this competition. By inference, this provides insight into functionally important amino acids in the native vesicular form of SYT as has previously been shown for the C2B lysines 326 and 327 (Rickman et al., 2004a). Amperometric recordings demonstrated that introduction of the Y311N mutation in the soluble C2AB is sufficient to relieve the blockade of exocytosis (Figure 8C). Therefore, the soluble C2AB carrying an equivalent of the Drosophila AD3 mutation cannot compete with the vesicular SYT function in exocytosis. This highlights the functional importance of the Y311 tyrosine residue at the concave face of the C2B domain, further indicating an essential role of the constitutive SYT/SNARE link in regulated exocytosis.
DISCUSSION
The identification of the SNARE fusion machinery and the calcium sensor SYT led to a number of hypothetical models of their mutual organization between the vesicular and plasma membranes in regulated exocytosis (Yoshihara and Littleton, 2002; Koh and Bellen, 2003; Nishiki and Augustine, 2004). Here, we have provided a structure-based model for the constitutive SYT/SNARE assembly that was derived and tested through complementary experimental and theoretical approaches. Availability of sequence information in evolutionarily distant organisms allowed us to narrow down our search to conserved sites with a potential role in SYT/SNARE binding. Our reliance on the evolutionary conservation of amino acids is valid because the basic properties of neuroendocrine exocytosis are known to be conserved throughout the animal kingdom (Bellen, 1999). Through mutagenesis analysis, we have determined the SYT binding site on the SNAREs, showing that it resides on helix1 of SNAP-25 and is adjacent to the amino acids involved in the ionic layer of the ternary SNARE complex (Figures 2 and 3). The conservation of this binding motif mirrors the strong conservation of the SNARE-binding epitope on the C2B domain of SYT (Rickman et al., 2004a). The recently published atomic structures of the C2 domains of SYT1 and of the ternary SNARE complex allowed us to use an in silico docking approach and computational power to further confirm the likely interface for the SYT/SNARE interaction. Our computational analysis relied on the assumption that the t-SNARE helices are folded similarly whether in the presence or absence of synaptobrevin. This is valid because 1) the assembly of syntaxin and SNAP-25 is highly α-helical (Fasshauer et al., 1997) and 2) the SNAP-25 [D51K,E52K,E55K] mutation similarly affects constitutive SYT binding to both the t-SNARE assembly and the ternary SNARE complex (Figure 3C).
The ability of SYT to interact equally with either the two assembled t-SNAREs or the ternary complex is consistent with a notion that SYT and synaptobrevin bind the plasma membrane SNAREs through different epitopes and likely in a sequential manner. Here, however, an important question arises: does SYT bind the t-SNARE assembly before or after synaptobrevin? The initial preassembly of all three SNAREs, as observed in brain detergent extracts, was proposed a decade ago (Sollner et al., 1993), but a number of observations contradict such a detergent-based hypothesis. First, studies in intact cells using botulinum toxins, which cannot cleave complexed SNAREs but still potently block exocytosis, demonstrated that no stable ternary complex exists before the action of calcium (Chen et al., 1999; Schiavo et al., 2000). Second, injections of synaptobrevin peptides into resting nerve terminals block exocytosis, indicating that vesicular synaptobrevin and the plasma membrane SNAREs are not tightly associated (Hunt et al., 1994). Third, the reported synaptobrevin interaction with vesicular membranes can potently restrict it from premature engagement of the t-SNAREs (Hu et al., 2002a; Kweon et al., 2003). Importantly, this vesicular restriction of synaptobrevin is abolished when phospholipid membranes are dissolved by detergents allowing instant, unregulated, ternary complex formation in the absence of calcium (Hu et al., 2002a). This spontaneous synaptobrevin engagement with the t-SNAREs upon preparation of detergent cell extracts can explain why the SYT/ternary SNARE complex predominates over the SYT/binary t-SNARE assembly upon purification. Together, it is possible that, in the native membrane environment, the relatively large SYT molecule, through its distant C2B domain, can associate with the t-SNAREs well before synaptobrevin and that this constitutive association may serve, as discussed below, an important function in regulated exocytosis. Our conclusions are consistent with a view that the accurate targeting and docking of transport vesicles throughout the cell, including calcium-triggered secretion, requires factors additional to SNAREs (Pfeffer, 1999; Whyte and Munro, 2002).
The proposed model is detailed in Figure 9. The two t-SNAREs, forming a single molecular entity, are bound by the C2B domain of SYT through the SNARE epitope identified here. This interaction may serve several important roles. First, it would restrict the diffusion of the t-SNAREs in the plane of the plasma membrane and position them at the shortest possible distance to the vesicular membrane, increasing the probability of eventual synaptobrevin engagement. Second, the SNAP-25 link would allow the C2B domain to be positioned close to the membrane phospholipids—its likely calcium-triggered target (Fernandez et al., 2001; Mackler et al., 2002). Third, the short linker between the two C2 domains would limit the orientations that the C2A domain can adopt, enhancing the rapid response to the calcium signal. In this orientation, C2A may respond to calcium entry either through binding electrostatically to the phospholipid membrane (Fernandez-Chacon et al., 2001) or, as has also been suggested, through the inducible, calcium-dependent, binding to SNAREs (Zhang et al., 2002; Bai et al., 2004). This inducible SYT/SNARE interaction exhibits properties very different from those characteristic of the constitutive association. The calcium-triggered interaction is less specific, with SYT binding individual SNAREs, regardless of whether they are assembled or not (Bai et al., 2004), and also non-SNARE proteins, e.g., tubulin (Honda et al., 2002). Importantly, in the context of native synaptic membranes, a calcium-dependent dissociation of SYT from the SNAREs has been observed, stressing an important role for the constitutive SYT/SNARE association (Mehta et al., 1996; Leveque et al., 2000).
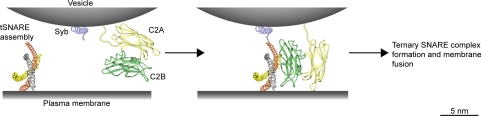
Figure 9.
Model for the constitutive interaction of vesicular SYT with the SNARE fusion proteins, before the action of calcium, in the context of two opposing membranes. The preassembled t-SNAREs (gray helix, syntaxin; orange and yellow, SNAP-25) are bound by the C2B domain (green ribbons), positioning the calcium binding loops of both C2A and C2B toward the plasma membrane. This interaction also positions the t-SNARE assembly at the point of shortest distance between the vesicular and plasma membranes enhancing the probability of full synaptobrevin (Syb, blue helix) engagement with the t-SNAREs, upon calcium action (not shown for brevity). The vesicular membrane was drawn taking into account an average diameter of synaptic vesicles of 40 nm and in scale with the size of the prefusion protein assembly. Bar, 5 nm.
The constitutive SYT coupling to the syntaxin/SNAP-25 assembly may be sufficient to provide an organized scaffold for a calcium-enhanced collision of nascent SNARE α-helices, resulting in rapid formation (Myers and Oas, 2001) of the fusogenic ternary SNARE complex. The proposed model highlights the pivotal role for C2B in linking the vesicular and the plasma membrane fusion machineries before calcium-triggered events and is in agreement with available in vivo findings. Genetic knockouts of SYT1 result in dramatic suppression of evoked release in several model organisms (Bellen, 1999; Sudhof and Scheller, 2001). Of the two C2 domains, mutagenesis of C2B has a more severe effect on the SYT-controlled fast synchronous release (Fernandez-Chacon et al., 2001; Mackler et al., 2002; Robinson et al., 2002). In Drosophila melanogaster, a single mutation of tyrosine to asparagine in the C2B domain severely disrupts calcium-triggered exocytosis (Yoshihara and Littleton, 2002). This mutation, called AD3, impacts on the distribution of synaptic vesicles at the plasma membrane, suggesting that a molecular link between the vesicular and plasma membrane has been severed (Reist et al., 1998). We herein demonstrated that this tyrosine residue lies at the interface of the C2B domain with the SNARE helices. The AD3 mutation efficiently disrupts the constitutive SYT interaction with the t-SNARE assembly even in the context of the tandem C2 domains (Figure 8, A and B), providing clear evidence that the C2B domain alone is necessary and sufficient for the constitutive link to the t-SNARE assembly.
Mutations of the calcium-binding residues in the C2B domain block evoked release (Mackler et al., 2002), indicating that a further calcium-triggered SYT action is required for efficient exocytosis. A detailed analysis of these mutants revealed a close correlation between the decrease in neurotransmitter release and a reduction in calcium/phospholipid interaction rather than the reported calcium-triggered oligomerization of the C2 domains (Mackler et al., 2002). The severity of the AD3 mutation was initially explained through the inability of SYT to oligomerize (Littleton et al., 2001), but this has been subsequently challenged (Borden et al., 2005). In addition, calcium-induced oligomerization of a vesicular protein cannot readily account for the change in the distribution of the vesicles at the plasma membrane in resting nerve terminals (Reist et al., 1998). The down-regulation of vesicle fusion in the case of the AD3 mutation, and the changes in vesicle distribution at the presynaptic membrane, can now be rationally explained through the disruption of the prefusion tethering assembly that underlies the regulated exocytosis (Figure 9).
We have demonstrated that the cytoplasmic domain of SYT does not physically prevent the engagement of soluble synaptobrevin with the t-SNAREs (Figure 1). In the context of synaptic membranes, however, synaptotagmin may fulfill the role of a clamp that keeps the fusion partners at a distance (Figure 9) to allow calcium-triggered high-probability synchronous release. Understanding the exact sequence of events triggered by calcium will require mechanistic studies of fast protein rearrangements in the context of phospholipid membranes. Our testable model provides a conceptual basis for uncovering these, calcium-driven, molecular events.
Acknowledgments
We thank Drs. Yeon-Kyun Shin, Reinhard Jahn, and Juan Blasi for recombinant SNARE plasmids. We are grateful to Graham Smith for the latest version of FTDOCK.
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E05–07–0620) on November 2, 2005.
References
- An, S. J., and Almers, W. (2004). Tracking SNARE complex formation in live endocrine cells. Science 306, 1042–1046. [DOI] [PubMed] [Google Scholar]
- Bai, J., Wang, C. T., Richards, D. A., Jackson, M. B., and Chapman, E. R. (2004). Fusion pore dynamics are regulated by synaptotagmin*t-SNARE interactions. Neuron 41, 929–942. [DOI] [PubMed] [Google Scholar]
- Bajohrs, M., Rickman, C., Binz, T., and Davletov, B. (2004). A molecular basis underlying differences in the toxicity of botulinum serotypes A and E. EMBO Rep. 5, 1090–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellen, H. J. (ed.) (1999). Neurotransmitter Release, Oxford, UK: Oxford University Press.
- Bonifacino, J. S., and Glick, B. S. (2004). The mechanisms of vesicle budding and fusion. Cell 116, 153–166. [DOI] [PubMed] [Google Scholar]
- Borden, C. R., Stevens, C. F., Sullivan, J. M., and Zhu, Y. (2005). Synaptotagmin mutants Y311N and K326/327A alter the calcium dependence of neurotransmission. Mol. Cell. Neurosci. 29, 462–470. [DOI] [PubMed] [Google Scholar]
- Burgoyne, R. D., and Morgan, A. (2003). Secretory granule exocytosis. Physiol. Rev. 83, 581–632. [DOI] [PubMed] [Google Scholar]
- Chen, X., Tomchick, D. R., Kovrigin, E., Arac, D., Machius, M., Sudhof, T. C., and Rizo, J. (2002). Three-dimensional structure of the complexin/SNARE complex. Neuron 33, 397–409. [DOI] [PubMed] [Google Scholar]
- Chen, Y. A., Scales, S. J., Patel, S. M., Doung, Y. C., and Scheller, R. H. (1999). SNARE complex formation is triggered by Ca2+ and drives membrane fusion. Cell 97, 165–174. [DOI] [PubMed] [Google Scholar]
- Davletov, B. A., and Sudhof, T. C. (1993). A single C2 domain from synaptotagmin I is sufficient for high affinity Ca2+/phospholipid binding. J. Biol. Chem. 268, 26386–26390. [PubMed] [Google Scholar]
- DiAntonio, A., Parfitt, K. D., and Schwarz, T. L. (1993). Synaptic transmission persists in synaptotagmin mutants of Drosophila. Cell 73, 1281–1290. [DOI] [PubMed] [Google Scholar]
- Erickson, H. P. (1989). Co-operativity in protein-protein association. The structure and stability of the actin filament. J. Mol. Biol. 206, 465–474. [DOI] [PubMed] [Google Scholar]
- Fasshauer, D., Antonin, W., Margittai, M., Pabst, S., and Jahn, R. (1999). Mixed and non-cognate SNARE complexes. Characterization of assembly and biophysical properties. J. Biol. Chem. 274, 15440–15446. [DOI] [PubMed] [Google Scholar]
- Fasshauer, D., Bruns, D., Shen, B., Jahn, R., and Brunger, A. T. (1997). A structural change occurs upon binding of syntaxin to SNAP-25. J. Biol. Chem. 272, 4582–4590. [DOI] [PubMed] [Google Scholar]
- Fernandez-Chacon, R., Konigstorfer, A., Gerber, S. H., Garcia, J., Matos, M. F., Stevens, C. F., Brose, N., Rizo, J., Rosenmund, C., and Sudhof, T. C. (2001). Synaptotagmin I functions as a calcium regulator of release probability. Nature 410, 41–49. [DOI] [PubMed] [Google Scholar]
- Fernandez, I., Arac, D., Ubach, J., Gerber, S. H., Shin, O., Gao, Y., Anderson, R. G., Sudhof, T. C., and Rizo, J. (2001). Three-dimensional structure of the synaptotagmin 1 c(2)b-domain. Synaptotagmin 1 as a phospholipid binding machine. Neuron 32, 1057–1069. [DOI] [PubMed] [Google Scholar]
- Gabb, H. A., Jackson, R. M., and Sternberg, M. J. (1997). Modelling protein docking using shape complementarity, electrostatics and biochemical information. J. Mol. Biol. 272, 106–120. [DOI] [PubMed] [Google Scholar]
- Graham, M. E., Washbourne, P., Wilson, M. C., and Burgoyne, R. D. (2002). Molecular analysis of SNAP-25 function in exocytosis. Ann. N.Y. Acad. Sci. 971, 210–221. [DOI] [PubMed] [Google Scholar]
- Grant, J. A., Pickup, B. T., and Nicholls, A. (2001). A smooth permittivity function for Poisson-Boltzmann solvation methods. J. Comput. Chem. 22, 608–640. [Google Scholar]
- Hayashi, T., McMahon, H., Yamasaki, S., Binz, T., Hata, Y., Sudhof, T. C., and Niemann, H. (1994). Synaptic vesicle membrane fusion complex: action of clostridial neurotoxins on assembly. EMBO J. 13, 5051–5061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda, A., Yamada, M., Saisu, H., Takahashi, H., Mori, K. J., and Abe, T. (2002). Direct, Ca2+-dependent interaction between tubulin and synaptotagmin I: a possible mechanism for attaching synaptic vesicles to microtubules. J. Biol. Chem. 277, 20234–20242. [DOI] [PubMed] [Google Scholar]
- Honig, B., and Nicholls, A. (1995). Classical electrostatics in biology and chemistry. Science 268, 1144–1149. [DOI] [PubMed] [Google Scholar]
- Hu, K., Carroll, J., Fedorovich, S., Rickman, C., Sukhodub, A., and Davletov, B. (2002a). Vesicular restriction of synaptobrevin suggests a role for calcium in membrane fusion. Nature 415, 646–650. [DOI] [PubMed] [Google Scholar]
- Hu, K., Carroll, J., Rickman, C., and Davletov, B. (2002b). Action of complexin on SNARE complex. J. Biol. Chem. 277, 41652–41656. [DOI] [PubMed] [Google Scholar]
- Hubbard, S. J., Campbell, S. F., and Thornton, J. M. (1991). Molecular recognition. Conformational analysis of limited proteolytic sites and serine proteinase protein inhibitors. J. Mol. Biol. 220, 507–530. [DOI] [PubMed] [Google Scholar]
- Hunt, J. M., Bommert, K., Charlton, M. P., Kistner, A., Habermann, E., Augustine, G. J., and Betz, H. (1994). A post-docking role for synaptobrevin in synaptic vesicle fusion. Neuron 12, 1269–1279. [DOI] [PubMed] [Google Scholar]
- Jackson, R. M., Gabb, H. A., and Sternberg, M. J. (1998). Rapid refinement of protein interfaces incorporating solvation: application to the docking problem. J. Mol. Biol. 276, 265–285. [DOI] [PubMed] [Google Scholar]
- Katz, B., and Miledi, R. (1967). The timing of calcium action during neuromuscular transmission. J. Physiol. 189, 535–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh, T. W., and Bellen, H. J. (2003). Synaptotagmin I, a Ca2+ sensor for neurotransmitter release. Trends Neurosci. 26, 413–422. [DOI] [PubMed] [Google Scholar]
- Kweon, D. H., Kim, C. S., and Shin, Y. K. (2003). Regulation of neuronal SNARE assembly by the membrane. Nat. Struct. Biol. 10, 440–447. [DOI] [PubMed] [Google Scholar]
- Leveque, C., Boudier, J. A., Takahashi, M., and Seagar, M. (2000). Calcium-dependent dissociation of synaptotagmin from synaptic SNARE complexes. J. Neurochem. 74, 367–374. [DOI] [PubMed] [Google Scholar]
- Lichtarge, O., and Sowa, M. E. (2002). Evolutionary predictions of binding surfaces and interactions. Curr. Opin. Struct. Biol. 12, 21–27. [DOI] [PubMed] [Google Scholar]
- Littleton, J. T., Bai, J., Vyas, B., Desai, R., Baltus, A. E., Garment, M. B., Carlson, S. D., Ganetzky, B., and Chapman, E. R. (2001). synaptotagmin mutants reveal essential functions for the C2B domain in Ca2+-triggered fusion and recycling of synaptic vesicles in vivo. J. Neurosci. 21, 1421–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo Conte, L., Chothia, C., and Janin, J. (1999). The atomic structure of protein-protein recognition sites. J. Mol. Biol. 285, 2177–2198. [DOI] [PubMed] [Google Scholar]
- Mackler, J. M., Drummond, J. A., Loewen, C. A., Robinson, I. M., and Reist, N. E. (2002). The C(2)B Ca(2+)-binding motif of synaptotagmin is required for synaptic transmission in vivo. Nature 418, 340–344. [DOI] [PubMed] [Google Scholar]
- Mehta, P. P., Battenberg, E., and Wilson, M. C. (1996). SNAP-25 and synaptotagmin involvement in the final Ca(2+)-dependent triggering of neurotransmitter exocytosis. Proc. Natl. Acad. Sci. USA 93, 10471–10476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers, J. K., and Oas, T. G. (2001). Preorganized secondary structure as an important determinant of fast protein folding. Nat. Struct. Biol. 8, 552–558. [DOI] [PubMed] [Google Scholar]
- Nishiki, T., and Augustine, G. J. (2004). Dual roles of the C2B domain of synaptotagmin I in synchronizing Ca2+-dependent neurotransmitter release. J. Neurosci. 24, 8542–8550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Branguli, F., Muhaisen, A., and Blasi, J. (2002). Munc 18a binding to syntaxin 1A and 1B isoforms defines its localization at the plasma membrane and blocks SNARE assembly in a three-hybrid system assay. Mol. Cell Neurosci. 20, 169–180. [DOI] [PubMed] [Google Scholar]
- Pfeffer, S. R. (1999). Transport-vesicle targeting: tethers before SNAREs. Nat. Cell Biol. 1, E17–E22. [DOI] [PubMed] [Google Scholar]
- Reist, N. E., Buchanan, J., Li, J., DiAntonio, A., Buxton, E. M., and Schwarz, T. L. (1998). Morphologically docked synaptic vesicles are reduced in synaptotagmin mutants of Drosophila. J. Neurosci. 18, 7662–7673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickman, C., Archer, D. A., Meunier, F. A., Craxton, M., Fukuda, M., Burgoyne, R. D., and Davletov, B. (2004a). Synaptotagmin interaction with the syntaxin/SNAP-25 dimer is mediated by an evolutionarily conserved motif and is sensitive to inositol hexakisphosphate. J. Biol. Chem. 279, 12574–12579. [DOI] [PubMed] [Google Scholar]
- Rickman, C., and Davletov, B. (2003). Mechanism of calcium-independent synaptotagmin binding to target SNAREs. J. Biol. Chem. 278, 5501–5504. [DOI] [PubMed] [Google Scholar]
- Rickman, C., Hu, K., Carroll, J., and Davletov, B. (2005). Self-assembly of SNARE fusion proteins into star-shaped oligomers. Biochem. J. 388, 75–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickman, C., Meunier, F. A., Binz, T., and Davletov, B. (2004b). High affinity interaction of syntaxin and SNAP-25 on the plasma membrane is abolished by botulinum toxin E. J. Biol. Chem. 279, 644–651. [DOI] [PubMed] [Google Scholar]
- Robinson, I. M., Ranjan, R., and Schwarz, T. L. (2002). Synaptotagmins I and IV promote transmitter release independently of Ca(2+) binding in the C(2)A domain. Nature 418, 336–340. [DOI] [PubMed] [Google Scholar]
- Schiavo, G., Matteoli, M., and Montecucco, C. (2000). Neurotoxins affecting neuroexocytosis. Physiol. Rev. 80, 717–766. [DOI] [PubMed] [Google Scholar]
- Smith, G. R., and Sternberg, M. J. (2002). Prediction of protein-protein interactions by docking methods. Curr. Opin. Struct. Biol. 12, 28–35. [DOI] [PubMed] [Google Scholar]
- Sollner, T., Whiteheart, S. W., Brunner, M., Erdjument-Bromage, H., Geromanos, S., Tempst, P., and Rothman, J. E. (1993). SNAP receptors implicated in vesicle targeting and fusion. Nature 362, 318–324. [DOI] [PubMed] [Google Scholar]
- Sudhof, T. C., and Scheller, R. H. (2001). Mechanism and regulation of neurotransmitter release. In: Synapses, ed. W. M. Cowan, T. C. Sudhof, and C. F. Stevens, Baltimore: The Johns Hopkins University Press.
- Sutton, R. B., Davletov, B. A., Berghuis, A. M., Sudhof, T. C., and Sprang, S. R. (1995). Structure of the first C2 domain of synaptotagmin I: a novel Ca2+/phospholipid-binding fold. Cell 80, 929–938. [DOI] [PubMed] [Google Scholar]
- Sutton, R. B., Fasshauer, D., Jahn, R., and Brunger, A. T. (1998). Crystal structure of a SNARE complex involved in synaptic exocytosis at 2.4 A resolution. Nature 395, 347–353. [DOI] [PubMed] [Google Scholar]
- van Dijk, A. D., Boelens, R., and Bonvin, A. M. (2005). Data-driven docking for the study of biomolecular complexes. FEBS J. 272, 293–312. [DOI] [PubMed] [Google Scholar]
- Washbourne, P., Bortoletto, N., Graham, M. E., Wilson, M. C., Burgoyne, R. D., and Montecucco, C. (1999). Botulinum neurotoxin E-insensitive mutants of SNAP-25 fail to bind VAMP but support exocytosis. J. Neurochem. 73, 2424–2433. [DOI] [PubMed] [Google Scholar]
- Whyte, J. R., and Munro, S. (2002). Vesicle tethering complexes in membrane traffic. J. Cell Sci. 115, 2627–2637. [DOI] [PubMed] [Google Scholar]
- Yoshihara, M., and Littleton, J. T. (2002). Synaptotagmin I functions as a calcium sensor to synchronize neurotransmitter release. Neuron 36, 897–908. [DOI] [PubMed] [Google Scholar]
- Zhang, X., Kim-Miller, M. J., Fukuda, M., Kowalchyk, J. A., and Martin, T. F. (2002). Ca2+-dependent synaptotagmin binding to SNAP-25 is essential for Ca2+-triggered exocytosis. Neuron 34, 599–611. [DOI] [PubMed] [Google Scholar]