Abstract
Protein kinase Cδ (PKCδ) is involved in the apoptosis of various cells in response to diverse stimuli. In this study, we characterized the role of PKCδ in the apoptosis of C6 glioma cells in response to etoposide. We found that etoposide induced apoptosis in the C6 cells within 24 to 48 h and arrested the cells in the G1/S phase of the cell cycle. Overexpression of PKCδ increased the apoptotic effect induced by etoposide, whereas the PKCδ selective inhibitor rottlerin and the PKCδ dominant-negative mutant K376R reduced this effect compared to control cells. Etoposide-induced tyrosine phosphorylation of PKCδ and its translocation to the nucleus within 3 h was followed by caspase-dependent cleavage of the enzyme. Using PKC chimeras, we found that both the regulatory and catalytic domains of PKCδ were necessary for its apoptotic effect. The role of tyrosine phosphorylation of PKCδ in the effects of etoposide was examined using cells overexpressing a PKCδ mutant in which five tyrosine residues were mutated to phenylalanine (PKCδ5). These cells exhibited decreased apoptosis in response to etoposide compared to cells overexpressing PKCδ. Likewise, activation of caspase 3 and the cleavage of the PKCδ5 mutant were significantly lower in cells overexpressing PKCδ5. Using mutants of PKCδ altered at individual tyrosine residues, we identified tyrosine 64 and tyrosine 187 as important phosphorylation sites in the apoptotic effect induced by etoposide. Our results suggest a role of PKCδ in the apoptosis induced by etoposide and implicate tyrosine phosphorylation of PKCδ as an important regulator of this effect.
Protein kinase C (PKC) comprises a family of phospholipid-dependent serine-threonine kinases which play important roles in various cellular functions (45, 46, 56). PKC consists of 12 isoforms showing diversity in their structures, cellular distributions, and biological functions (27). Based on their structures and cofactor regulation, the PKC isoforms are divided into the classical PKCs (α, β1, β2, and γ), the novel PKCs (δ, ɛ, η, and θ), and the atypical PKCs (PKCζ and PKCι/λ). In addition, two other members, PKCμ and PKCν, exhibit unique characteristics (28). All PKC isoforms can be divided into an N-terminal regulatory domain and a C-terminal catalytic domain with serine-threonine kinase activity (26, 44). PKC chimeras have been used to study the role of the regulatory and catalytic domains of different PKC isoforms (2, 57).
Various PKC isoforms have been reported to play important roles in cell apoptosis (10, 17). Thus, PKCα and PKCɛ inhibit apoptosis by phosphorylating or increasing the expression of Bcl2, respectively (23, 52). In contrast, PKCδ, -θ, and -μ have been implicated as proapoptotic kinases, mostly by being targets of caspase 3 (14, 34). Thus, apoptotic stimuli, such as ionizing radiation and etoposide, induced the cleavage of PKCδ and the accumulation of the PKCδ catalytic fragment, which is constitutively active (21, 34). Overexpression of the catalytic domain of PKCδ in HeLa cells induced nuclear fragmentation and cell apoptosis (21). PKCδ has also been reported to be essential for the spontaneous apoptosis of neutrophils (48), for the apoptosis of parotid cells in response to etoposide (49), and for the apoptosis of keratinocytes and LaNCap cells in response to phorbol myristate acetate (PMA) (19, 36). Likewise, PKCθ has been shown to be cleaved by caspase 3 in vitro and in vivo and the catalytic domain of PKCθ-induced apoptosis in U937 cells (11). Similarly, PKCζ can be cleaved at the hinge region by caspases in response to apoptotic stimuli, such as tumor necrosis factor alpha or etoposide (18).
Various studies suggest that PKCδ associates with different tyrosine kinases and undergoes tyrosine phosphorylation in response to various stimuli. PKCδ has been shown to be tyrosine phosphorylated in response to PMA, epidermal growth factor (EGF), platelet-derived growth factor (PDGF) (5, 13, 37), and ligands for the immunoglobulin E (IgE) receptor (25, 54). In addition, apoptotic stimuli, such as H2O2 (32) and γ-irradiation (58), induce tyrosine phosphorylation of PKCδ, and c-Abl is implicated in these latter responses. Depending on the stimulus, tyrosine residues in both the regulatory and catalytic domains may undergo phosphorylation.
In a recent study (5), we reported that phosphorylation of PKCδ on distinct tyrosine residues plays important roles in C6 cell proliferation and in the expression of the astrocytic marker glutamine synthetase. In the present study, we find that tyrosine phosphorylation of PKCδ is essential for the cleavage of caspase 3 and PKCδ and for the apoptotic effect of PKCδ in response to etoposide.
MATERIALS AND METHODS
Materials.
An affinity-purified polyclonal anti-PKCɛ antibody against a polypeptide corresponding to amino acids 726 to 737 of PKCɛ was purchased from GIBCO-BRL Life Technologies (Gaithersburg, Md.). Monoclonal anti-PKCδ antibody directed against the regulatory domain was obtained from Transduction Laboratories (Lexington, Ky.), and polyclonal anti-PKC antibodies were from Santa Cruz (Santa Cruz, Calif.). Etoposide was from Alexis Co. (San Diego, Calif.), and an anti-active caspase 3 antibody was obtained from New England Biolabs (Beverly, Mass.). The caspase inhibitors DEVD-FMK, Z-VAD-FMK, and YVAD were obtained from Calbiochem (La Jolla, Calif.). Leupeptin, aprotinin, phenylmethylsulfonyl fluoride (PMSF), and sodium vanadate were obtained from Sigma Chemical Co. (St. Louis, Mo.). The Caspase 3 Cellular Activity Assay Kit PLUS was obtained from BIOMOL (Plymouth Meeting, Pa.), and the Cell Death Detection enzyme-linked immunosorbent assay (ELISA) Kit was from Roche Molecular Biochemicals.
Generation of PKC chimeras.
The PKC chimeras were generated by exchanging the regulatory and catalytic domains of PKCα and PKCδ as previously described (1). PKCα/δ refers to the chimera with the PKCα regulatory domain and the PKCδ catalytic domain, and PKCδ/α refers to the reciprocal chimera. The PKC cDNAs were subcloned into the metallothionein promoter-driven eukaryotic expression vector (MTH). The vector sequence encodes a C-terminal PKCɛ-derived 12-amino-acid tag (ɛMTH) that is added to the expressed proteins (47). The expression of these chimeras and their activities in C6 cells were recently described (5).
Site-directed mutagenesis of PKCδ.
Mouse PKCδ was cloned into the pGEM-T vector (Promega, Madison, Wis.) as described previously (5). This plasmid served as our “master” vector for the site-directed mutagenesis, using the Transformer Site-Directed Mutagenesis Kit from Clontech (Palo Alto, Calif.). Conversion of tyrosine residues at sites 52, 64, 155, 187, and 565 into phenylalanine was performed as previously described (5). PKCδ and the PKCδ mutants were subcloned into the metallothionein promoter-driven eukaryotic expression vector (ɛMTH). A PKCδ K376R dominant mutant was generated as previously described (36).
Construction of PKCδ-GFP fusion protein.
cDNAs encoding the murine PKCδ and the PKCδ5 mutant were fused into the N-terminal enhanced green fluorescent protein (GFP) vector pEGFP-N1 (Clontech Laboratories). The original pEGFP-N1 vector was modified by the insertion of an MluI site into the plasmid polylinker. The restriction site was created by ligating a phosphorylated linker containing the MluI site into pEGFP-N1 digested with SmaI. The construct was verified by sequencing. The clones containing GFP-PKCδ or GFP-PKCδ5 were constructed by the excision of PKCδ or PKCδ5 from MTH-PKC plasmids by digestion with XhoI and MluI. The inserts were then ligated into the modified GFP vector by using the same restriction sites. DNA sequencing of the GFP-PKC constructs confirmed the intended reading frame.
C6 glial cultures and cell transfection.
C6 cells (105 cells/ml) were seeded on tissue culture dishes (10-cm diameter) and were grown in medium consisting of Dulbecco’s modified Eagle’s medium containing 10% heat-inactivated fetal calf serum, 2 mM glutamine, penicillin (50 U/ml), and streptomycin (0.05 mg/ml). The cells were transfected either with the empty vectors or with the PKCδ and PKCδ5 expression vectors by using Lipofectamine (Gibco-BRL Life Technologies) as previously described (6). Experiments were routinely carried out on a clone of the transfected cells, but all the results were confirmed on one pool and two additional individual clones.
For overexpression of the GFP-PKCδ fusion proteins, C6 cells were seeded onto 40-mm round glass coverslips at a density of 5 × 104 cells/coverslip. Twenty-four hours later, cells were transfected with the different GFP-PKCδ constructs using Lipofectamine Plus reagent according to the manufacturer’s instructions. All experiments were performed 48 h posttransfection.
Measurements of cell apoptosis.
Cell apoptosis was measured using propidium iodide (PI) staining and analysis by flow cytometry and by ELISA (Cell Death Detection ELISA Kit) using anti-histone antibodies. Cells (1 × 106/ml) were plated in six-well plates and treated with the indicated treatments for 24 h. Detached cells and trypsinized adherent cells were pooled, fixed in 70% ethanol for 1 h on ice, washed with phosphate-buffered saline (PBS), and treated for 15 min with RNase (50 μM) at room temperature. Cells were then stained with PI (5 μg/ml) and analyzed on a Becton-Dickinson cell sorter.
For anti-histone ELISA (Cell Death Detection ELISA kit), extracts of cells containing histone-associated DNA fragments were incubated in 96-well plates coated with anti-histone antibodies for 2 h. Plates were then washed and incubated with anti-DNA antibodies conjugated to peroxidase for an additional 2 h. Substrate solution was added and absorbance was measured at 405 nm.
Cell viability was also quantitatively assessed by the measurement of lactate dehydrogenase (LDH) in the medium.
Measurement of caspase 3 activity.
Caspase 3 activity was measured using the caspase 3 colorimetric assay kit obtained from BIOMOL by using Ac-DEVD-pNA as a substrate according to the manufacturer’s recommendations.
Nuclear and cytosolic fractionation.
Nuclear proteins were prepared according to the method described by Haglund and Rothblum (24). Cells (1 × 106 to 5 × 106/ml) were washed once with 1 ml of PBS and once with 1 ml of lysis buffer (10 mM HEPES, 10 mM KCl, 1.5 mM MgCl2, 0.5 mM PMSF, 10 μg of leupeptin/ml, pH 7.9). Cells were lysed by suspending the cell pellet in 20 μl of lysis buffer containing 0.1% Nonidet P-40 (NP-40) for 10 min on ice. To isolate nuclei, the lysates were microcentrifuged for 5 min at 12,000 × g, and the nuclear pellet was washed with lysis buffer without NP-40. Nuclear proteins were obtained by resuspending the nuclear pellet in 20 μl of extraction buffer (420 mM NaCl, 20 mM HEPES, 1.5 mM MgCl2, 0.2 mM EDTA, and 25% glycerol, pH 7.9) for 10 min at 4°C. The nuclear suspension was microcentrifuged, the pellet was discarded, and the supernatant was diluted in dilution buffer (50 mM KCl, 20 mM HEPES, 0.2 mM EDTA, and 20% glycerol, pH 7.9). Lack of contamination of the nuclear fraction by the plasma membrane was confirmed using the plasma membrane marker Na-K ATPase.
Preparation of cell homogenates.
Cells were washed and resuspended in serum-free medium. The plates were placed on ice, scraped with a rubber policeman, and centrifuged at 1,400 rpm for 10 min. The supernatants were aspirated, and the cell pellets were resuspended in 100 μl of lysis buffer (25 mM Tris-HCl, pH 7.4, 50 mM NaCl, 0.5% Na deoxycholate, 2% NP-40, 0.2% sodium dodecyl sulfate [SDS], 1 mM PMSF, 50 μg of aprotinin/ml, 50 μM leupeptin, 0.5 mM Na3VO4) on ice for 15 min. The cell lysates were centrifuged for 15 min at 14,000 rpm in an Eppendorf microcentrifuge, supernatants were removed, and 2× sample buffer was added.
Immunoblot analysis.
Lysates (40 μg of protein) were resolved by SDS-polyacrylamide gel electrophoresis (PAGE) (10% polyacrylamide) and were transferred to nitrocellulose membranes. The membranes were blocked with 5% dry milk in PBS and subsequently stained with the primary antibody. Specific reactive bands were detected using a goat anti-rabbit or goat anti-mouse IgG conjugated to horseradish peroxidase (Bio-Rad, Hercules, Calif.), and the immunoreactive bands were visualized by the ECL Western blotting detection kit (Amersham, Arlington Heights, Ill.).
Immunoprecipitation.
Immunoprecipitation was performed as previously described (5). Briefly, C6 cells overexpressing PKCδ or PKCδ5 were serum starved overnight and treated for different periods of time with PMA (10 nM) or PDGF (100 ng/ml). The samples were preabsorbed with 25 μl of protein A/G-Sepharose (50%) for 10 min, and immunoprecipitation was performed using 4 μg of antibody/ml for 1 h at 4°C and then incubated with 30 μl of protein A/G-Sepharose for an additional hour. Following washes, the pellets were resuspended in 25 μl of SDS sample buffer and boiled for 5 min. The entire supernatants were subjected to Western blotting. Membranes were incubated with horseradish peroxidase-conjugated secondary antibodies for 1 h at room temperature. The membranes were washed and visualized by the enhanced chemiluminescence (ECL) system.
Immunofluorescence staining.
Cells were grown on glass coverslips. Following etoposide treatment (3 to 24 h), cells were washed with PBS and fixed in 4% paraformaldehyde (PFA) for 10 min. Subsequently, cells were washed in PBS, and after blocking with staining buffer (2% bovine serum albumin [BSA] and 0.1% Triton X-100 in PBS) for 30 min at room temperature, cells were incubated with a mouse (regulatory domain) or a rabbit (catalytic domain) anti-PKC antibody. Following washes in PBS, cells were incubated with an anti-rabbit antibody or with Alexa FluorTM 546 goat anti-mouse IgG for an additional 60 min and were mounted in FluoroGuard antifade reagent. Cells were viewed and photographed using confocal microscopy with ×63 magnification at excitation wavelengths of 543 and 488 nm.
For the translocation of GFP-PKCδ and GFP-PKCδ5, cells were treated for 6 and 24 h with etoposide, were fixed in 4% PFA for 10 min, and were mounted in FluoroGuard antifade reagent.
Statistical analysis.
The results are presented as the mean values ± standard error (SE). Data were analyzed using analysis of variance (ANOVA) and a paired Student’s t test to determine the level of significance between the different groups.
RESULTS
Etoposide induces apoptosis and cell cycle arrest in C6 glioma cells.
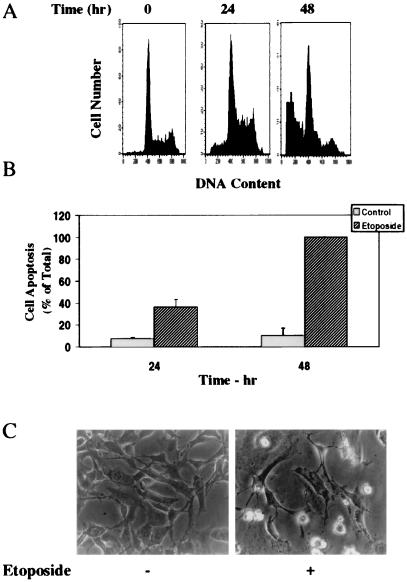
Treatment of the cells with etoposide arrested the cells in the G1/S phase of the cell cycle and induced cell apoptosis (Fig. 1A). Using PI staining and fluorescence-activated cell sorter (FACS) analysis, we found that approximately 22% ± 3.9% of the cells underwent apoptosis in response to etoposide after 24 h of treatment, whereas approximately 63% ± 7.1% of the cells were apoptotic after 48 h. Similar results were obtained using anti-histone ELISA (Fig. 1B) and using measurements of LDH levels in the cell supernatants (data not shown).
FIG. 1.
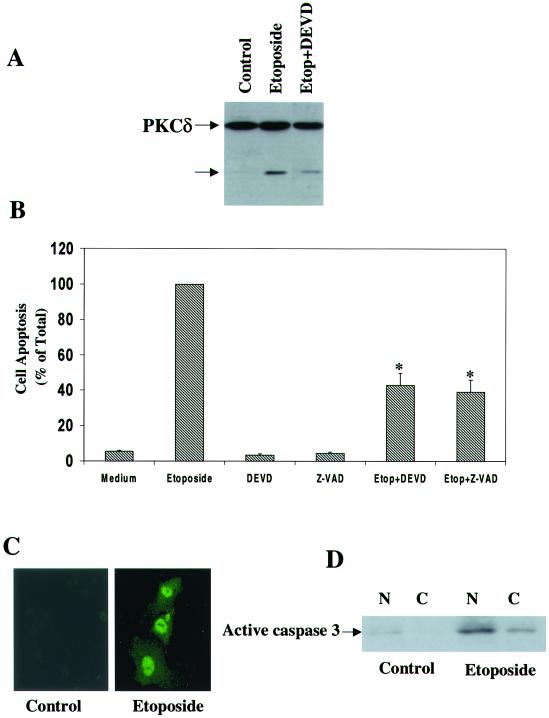
Etoposide induces apoptosis in C6 glioma cells. C6 cells were treated with etoposide (50 μM) for 24 or 48 h. Cell apoptosis was determined using PI staining and FACS analysis (A) or anti-histone ELISA (B). The results are from one representative experiment out of six similar experiments. (A) The optical densities of etoposide-treated cells (48 h) were designated 100% (total apoptosis), and all other values are presented as percent of this total. (B) The results represent the means ± SE of triplicate measurements in each of three experiments. (C) The morphology of the cells (48 h) was monitored under a phase contrast light microscope. The results are representative of four similar experiments.
Treatment of the cells with etoposide also resulted in lower cell number and in the appearance of rounded and detached cells, which are characteristic of apoptotic cells (Fig. 1C).
Role of PKCδ in apoptotic effect of etoposide.
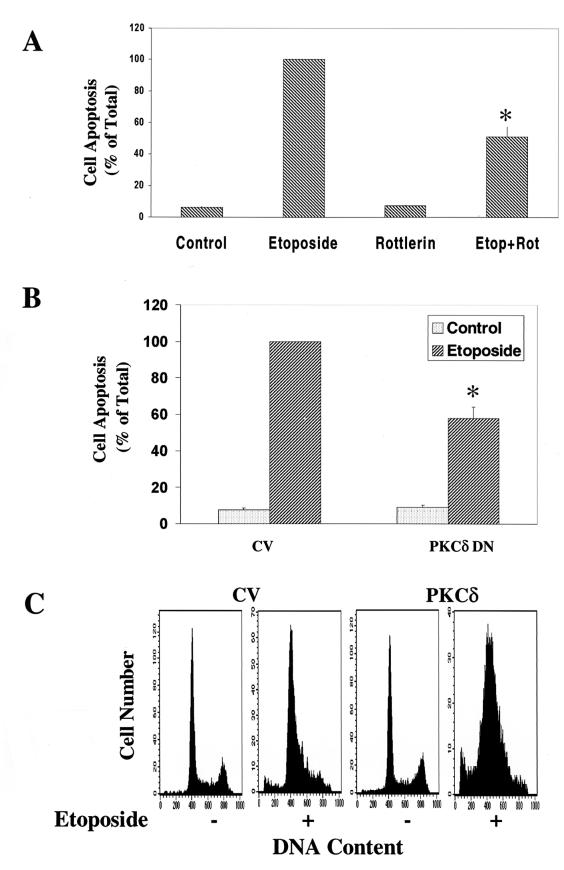
Recent studies suggested that PKCδ plays a role in the apoptotic effect of etoposide in salivary gland acinar cells (49). To explore the role of PKCδ in the effect of etoposide on C6 cells, we utilized rottlerin, which has been reported to be a PKCδ selective inhibitor (22). Treatment of C6 cells with rottlerin (5 μM) did not affect the basal level of cell apoptosis; however, rottlerin inhibited the apoptotic effect of etoposide (Fig. 2A), reducing cell apoptosis by approximately 50%. Higher concentrations of rottlerin itself induced morphologic changes in the cells and some cell apoptosis and therefore could not be used.
FIG. 2.
Effects of rottlerin, PKCδ DN, and PKCδ overexpression on the apoptosis of C6 cells induced by etoposide. C6 cells were treated with etoposide (50 μM) in the absence and presence of rottlerin (5 μM) for 48 h (A) or cells overexpressing control vector (CV), PKCδ DN (B), or PKCδ (C) were treated with etoposide. Cell apoptosis was determined using anti-histone ELISA (A and B) or PI staining and FACS analysis (C). The optical densities of etoposide-treated cells (A) or of etoposide-treated CV cells (B) were designated 100% (total apoptosis), and all other values are presented as percent of this total. (A and B) The results represent the means ± SE of triplicate measurements in each of three experiments. (C) Distributions are from a representative experiment. ∗, P < 0.001, compared to control cells.
Since the in vitro inhibitory effect of rottlerin on PKCδ activity has recently been subject to controversy (12, 22, 30), we further explored the role of PKCδ in the apoptotic effect of etoposide by employing cells overexpressing a PKCδ dominant-negative mutant (K376R) (36). Cells stably expressing the PKCδ DN mutant were treated with ZnCl2 for 24 h (to induce overexpression through its MTH promoter), followed by a 48-h treatment with etoposide (50 μM). Overexpression of PKCδ DN did not affect the basal level of C6 cell apoptosis; however, it reduced the apoptosis induced by etoposide by 40% (Fig. 2B).
The role of PKCδ in the apoptotic effect of etoposide was also demonstrated using cells stably overexpressing PKCδ. The expression of PKCδ in these cells was seven- to eightfold higher than control vector cells (35). These cells exhibited an increased rate of apoptosis in response to etoposide (45%) compared to cells expressing the control vector (20%; Fig. 2C).
Etoposide induces nuclear translocation of PKCδ.
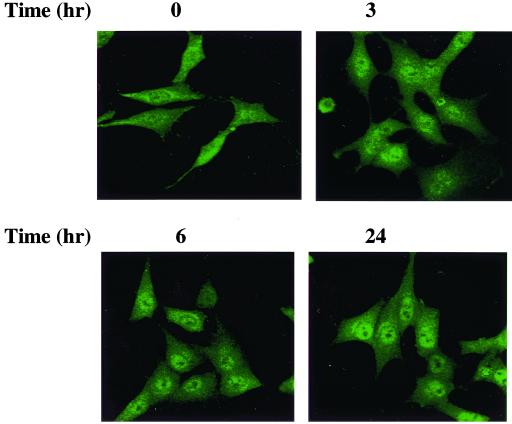
Activation of PKC by different stimuli results in distinct patterns of translocation, which are cell and stimulus dependent. To examine the effect of etoposide on the translocation of PKCδ, we treated C6 cells with etoposide for various periods of time and followed the expression of PKCδ using immunofluorescence and confocal microscopy. In control cells, PKCδ was found largely in the cytosol, but with some expression in the nucleus (Fig. 3). Treatment with etoposide induced further translocation of PKCδ to the nucleus. Translocation was observed already after 3 h, and the expression of PKCδ in the nucleus was observed up to 24 h following treatment. We confirmed that etoposide did not induce translocation of PKCδ to the Golgi, endoplasmic reticulum, or the mitochondria, as determined by costaining with these organelle-specific markers (data not shown).
FIG. 3.
Translocation of PKCδ in C6 cells treated with etoposide. C6 cells were treated with etoposide (50 μM) for 0, 3, 6, and 24 h, and the translocation of PKCδ was assessed using immunofluorescence staining. Following fixation with 4% PFA, cells were incubated with a rabbit anti-PKCδ antibody for 1 h and with an anti-rabbit antibody conjugated to fluorescein isothiocyanate. Cells were visualized by confocal microscopy. The results are from one representative experiment out of five similar experiments.
No translocation of PKCα, -β, -ζ, -μ, and -ι was observed in etoposide-treated cells, whereas some translocation of PKCɛ to the perinuclear membrane was observed (data not shown).
Cleavage of PKCδ by etoposide.
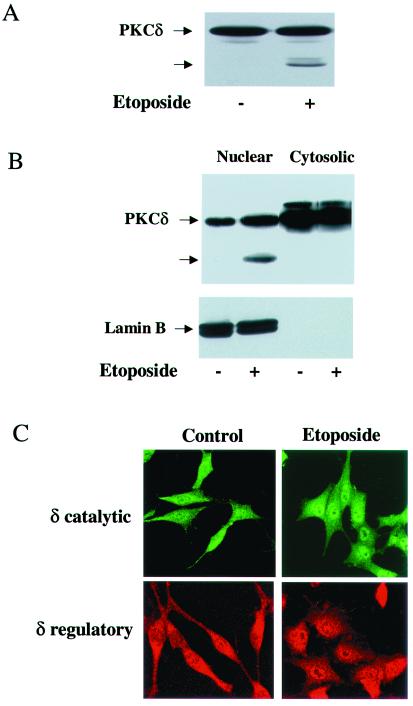
PKCδ undergoes cleavage in response to various apoptotic stimuli (21, 49). To examine the effect of etoposide on the cleavage of PKCδ in our system, we treated C6 cells with etoposide (50 μM) for various periods of time and analyzed cell lysates by using Western blotting. The level of PKCδ decreased following 24 h of etoposide treatment, and a 40-kDa cleavage product of PKCδ appeared and started to accumulate (Fig. 4A). No significant cleavage of PKCα, -β, -ɛ, -ζ, and -μ was observed in response to etoposide, and no cleavage products were detected (data not shown).
FIG. 4.
Nuclear cleavage and translocation of PKCδ in etoposide-treated cells. C6 cells were treated with etoposide (50 μM) for 24 h, and the cleavage of PKCδ was determined by Western blotting using an anti-PKCδ antibody (rabbit; Santa Cruz). (A) A 40-kDa cleaved form is marked by an arrow. For the detection of PKCδ in the nucleus, extracts from control and etoposide-treated C6 cells (24 h) were fractionated and the nuclear and cytoplasmic fractions (50 μg/ml) were examined for the presence of PKCδ and for its cleaved form by using Western blot analysis. (B) The extracts were also examined for the expression of the nuclear marker lamin B. (C) The nuclear translocation of PKCδ in C6 cells treated with etoposide for 6 h was monitored by immunofluorescence using anti-PKCδ antibody directed against the catalytic domain (fluorescein isothiocyanate) or against the regulatory domain (Alexa FluorTM 546 goat anti-mouse IgG). The results shown represent one of three separate experiments, which yielded similar results.
Since the nuclear translocation of PKCδ preceded its caspase-dependent cleavage, we examined if PKCδ cleavage occurred in the nucleus. We first performed cell fractionation and separated the nuclear and cytosolic fractions. We found that in untreated cells, PKCδ was mainly expressed in the cytosol with some expression in the nucleus. Following etoposide treatment, PKCδ translocated to the nucleus and a cleaved form of PKCδ accumulated only in the nuclear fraction (Fig. 4B). The nuclear marker lamin B was also detected only in the nuclear fraction. Using anti-PKCδ antibodies directed against the regulatory and catalytic domains, we found that the immunostaining of PKCδ using these two antibodies resulted in a similar pattern of nuclear translocation (Fig. 4C). The results of these two experiments suggest that PKCδ translocated to the nucleus, where it underwent cleavage. Since some PKCδ was present in the nucleus of the untreated cells without being cleaved, the cleavage must depend on the etoposide treatment itself as well as on its location.
Caspase 3 is involved in cleavage of PKCδ and apoptosis is induced by etoposide.
PKCδ can be cleaved by a caspase-dependent process (21, 34). We therefore examined the effects of the cell-permeable caspase 3 inhibitor DEVD.FMK (20 μM) on the cleavage of PKCδ in the C6 cells. As seen in Fig. 5A, pretreatment of the cells for 1 h with DEVD.FMK significantly reduced the accumulation of the PKCδ cleavage product in response to etoposide. Similar results were obtained with another caspase inhibitor, Z-VAD.FMK (data not shown).
FIG. 5.
Role of caspase 3 in cleavage of PKCδ and C6 cell apoptosis induced by etoposide. C6 cells were treated with etoposide for 24 h in the absence and presence of DEVD (20 μM) and Z-VAD (25 μM). The cleavage of PKCδ was determined using Western blot analysis (A), and cell apoptosis was determined using anti-histone ELISA (B). The results are representative of four similar experiments (A) or represent the means ± SE of three separate experiments (B). The expression of active caspase 3 in the nucleus of etoposide-treated cells (24 h) was determined using immunofluorescence staining (C) and Western blot analysis of cytosolic (C) and nuclear (N) fractions (D). ∗, P < 0.001
We also examined the ability of the caspase inhibitors to block the apoptosis induced by etoposide in C6 cells. Pretreatment of the cells with either DEVD.FMK or Z-VAD.FMK reduced the apoptotic effect of etoposide by approximately 60% (Fig. 5B), suggesting a role for caspase 3 in the apoptotic response. In contrast, the caspase 1 inhibitor YVAD did not affect etoposide effects in these systems (data not shown).
Since the cleavage of PKCδ occurred in the nucleus, we examined whether active caspase 3 was also expressed in the nucleus in etoposide-treated cells. Using immunofluorescence staining, we found that the active form of caspase 3 was expressed in the nucleus of etoposide-treated cells, whereas a very low expression of active caspase 3 was detected in control cells (Fig. 5C). Similar results were obtained using Western blot analysis of nuclear and cytosolic fractions (Fig. 5D).
Inhibition of PKCδ blocks cleavage of caspase 3 and PKCδ.
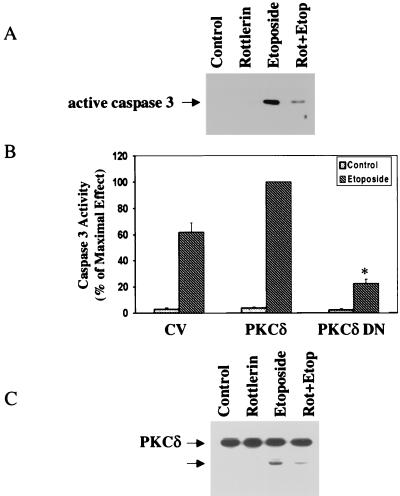
To examine the importance of PKCδ activity in its ability to undergo cleavage, we employed the selective PKCδ inhibitor rottlerin. Pretreatment of the cells with rottlerin (5 μM) reduced the cleavage of caspase 3 in response to etoposide (Fig. 6A). Similarly, cells overexpressing PKCδ DN exhibited a significantly lower caspase 3 activity than control vector cells (Fig. 6B). Finally, the cleavage of PKCδ and the accumulation of the PKCδ catalytic domain were reduced in rottlerin-treated cells (Fig. 6C), suggesting that the cleavage of both caspase 3 and PKCδ is dependent on an active PKCδ.
FIG. 6.
The PKCδ inhibitor rottlerin and PKCδ DN decrease the activation of caspase 3 and the cleavage of PKCδ in etoposide-treated cells. C6 cells were treated with etoposide (50 μM) in the absence or presence of rottlerin (5 μM) for 24 h. The cleavage of caspase 3 (A) and of PKCδ (C) was detected using Western blot analysis. The membranes were probed with antibodies against active caspase 3 (17 kDa) (A) or PKCδ (C). The results represent one of three separate experiments which yielded similar results. (B) Cells overexpressing PKCδ DN were treated with etoposide for 24 h, and the activity of caspase 3 was measured using the Caspase 3 Cellular Activity Assay Kit as described in Materials and Methods. Caspase 3 activity was calculated and expressed as picomoles/minute/microgram of protein, and the percent of maximal effect was determined. The results represent the means ± SE of four experiments. ∗, P < 0.001
Both regulatory and catalytic domains of PKCδ are required for apoptotic effect induced by etoposide.
In a recent study, we demonstrated that the regulatory domain of PKCδ mediated its inhibitory effect on C6 cell proliferation and on the expression of the astrocytic marker GS (5). The regulatory domain of PKCδ was phosphorylated on tyrosines 155 and 187 in response to PMA and PDGF, respectively (5, 35), and the translocation of the PKCδ/α chimera resembled that of PKCδ (40). To examine the relative contributions of the regulatory and catalytic domains of PKCδ to its apoptotic effects, we used chimeras between the regulatory and catalytic domains of PKCα and -δ combined at the highly conserved hinge region. The expression and activity of C6 cells overexpressing the different chimeras were already described (5).
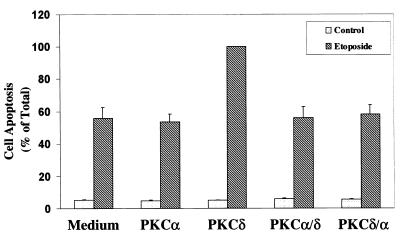
Cells were pretreated for 24 h with ZnCl2, followed by etoposide treatment (50 μM) for an additional 48 h. Cells overexpressing PKCδ/δ exhibited levels of apoptosis similar to those of control vector-transfected cells. Treatment of the control vector cells with etoposide induced about 60% apoptosis, similar to the results obtained with the parental C6 cells, whereas cells overexpressing PKCδ exhibited increased levels of apoptosis. Cells overexpressing PKCα/α exhibited a rate of cell apoptosis similar to that of the control vector cells. Interestingly, cells overexpressing the chimera containing the regulatory domain of PKCδ (δ/α) or the chimera containing the catalytic domain of PKCδ (α/δ) exhibited a similar level of apoptosis to that of control vector cells and did not resemble cells overexpressing PKCδ (Fig. 7). These results suggest that both domains are required for the apoptotic effect of PKCδ.
FIG. 7.
Apoptosis of C6 cells overexpressing different PKC chimeras in response to etoposide. C6 cells overexpressing PKCα, PKCδ, and the chimeras PKCα/δ and PKCδ/α were treated with etoposide for 48 h. Cell apoptosis was determined using anti-histone ELISA. The optical densities of etoposide-treated PKCδ-overexpressing cells were designated 100% (total apoptosis), and all other values are presented as a percent of this total. The results represent the means ± SE of triplicate measurements in each of three experiments.
Etoposide induces tyrosine phosphorylation of PKCδ.
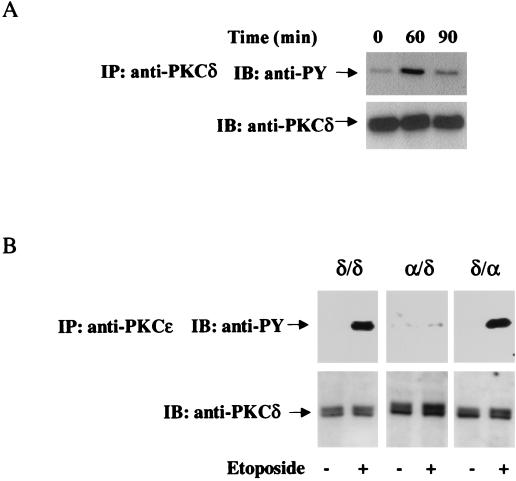
PKCδ has been shown to undergo tyrosine phosphorylation in response to PMA, growth factors, and apoptotic stimuli, such as H2O2 and γ-irradiation (5, 35, 58). To examine the effect of etoposide on tyrosine phosphorylation of PKCδ, we treated C6 cells with etoposide for 15 to 180 min. PKCδ was immunoprecipitated, and the membrane was blotted with anti-phosphotyrosine antibody. As illustrated in Fig. 8A, a low basal level of tyrosine phosphorylation was observed in untreated cells. Etoposide induced tyrosine phosphorylation of PKCδ in a time-dependent manner. Initial phosphorylation was observed after 45 min (data not shown), maximal phosphorylation was obtained after 60 min and was decreased thereafter (Fig. 8A). Tyrosine phosphorylation of PKCδ in response to etoposide was lower than that induced by PMA and exhibited slower kinetics (5, 35). The tyrosine phosphorylation induced by etoposide was specific for PKCδ. We did not observe tyrosine phosphorylation of any of the other isoforms (data not shown).
FIG. 8.
Etoposide induces tyrosine phosphorylation of PKCδ in the regulatory domain. Parental C6 cells or cells stably transfected with PKCδ or the chimeras PKCδ/α and PKCα/δ were treated with etoposide (50 μM) for various periods of time. Cells were then harvested, and immunoblotting (IB) and immunoprecipitation (IP) of PKCδ were performed using anti-PKCδ (A) or anti-PKCɛ (B) antibodies as described in Materials and Methods. Following SDS-PAGE, membranes were stained with anti-phosphotyrosine antibody (anti-PY) or with anti-PKCδ antibodies. The results are from one representative experiment out of four separate experiments.
We next examined whether the tyrosine phosphorylation in response to etoposide occurred in the regulatory or catalytic domains. For these experiments, we used the PKC chimeras α/δ and δ/α. Treatment of the chimeras with etoposide for 60 min resulted in the phosphorylation of PKCδ/δ and of the chimera containing the regulatory domain of PKCδ (PKCδ/α). The chimera containing the catalytic domain of PKCδ did not exhibit an increase in tyrosine phosphorylation in response to etoposide (Fig. 8B), suggesting that the tyrosine phosphorylation occurred in the regulatory domain.
Tyrosine phosphorylation of PKCδ is essential for its apoptotic effect.
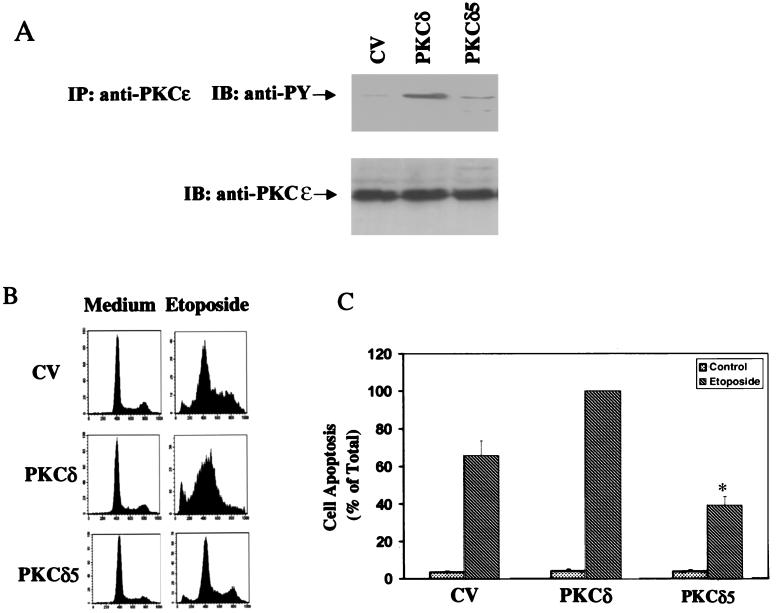
In previous studies, we identified five putative tyrosine phosphorylation sites in PKCδ and generated a PKCδ5 mutant, in which these five tyrosine phosphorylation sites were mutated to phenylalanine (5). The expression and activity of C6 cells overexpressing this mutant were already described (5). Overexpression of the PKCδ5 mutant in C6 cells abolished the decrease in the expression of the astrocytic marker GS induced by PMA or PDGF and the decrease in cell proliferation induced by PMA. Expression of the PKCδ5 mutant also resulted in a lower level of tyrosine phosphorylation of PKCδ in response to these treatments (5). Using cells expressing PKCδ5, we found that etoposide did not induce a significant increase in tyrosine phosphorylation of PKCδ5 (Fig. 9A). Similarly, treatment of these cells with etoposide resulted in low levels of apoptosis similar to the response observed in the control vector cells when measured after 24 h (Fig. 9B) and in lower levels of apoptosis than control vector cells after 48 h of treatment (Fig. 9C). Thus, the tyrosine phosphorylation of PKCδ in the regulatory domain induced by etoposide was essential for the apoptotic effect of PKCδ in response to this drug.
FIG. 9.
Tyrosine phosphorylation and cell apoptosis in response to etoposide in cells overexpressing PKCδ and PKCδ5. C6 cells overexpressing control vector (CV), PKCδ, or PKCδ5 were treated with etoposide (50 μM) for 60 min (A), 24 h (B), or 48 h (C). (A) Cells were then harvested, and immunoprecipitation of PKCδ was performed using anti-PKCɛ antibody as described in Materials and Methods. Following SDS-PAGE, membranes were stained with anti-phosphotyrosine antibody (anti-PY) or with an anti-PKCδ antibody (rabbit; Santa Cruz). The results represent one of three separate experiments, which yielded similar results. For the measurement of apoptosis, cells were harvested after 24 h (B) or 48 h (C) of treatment and were analyzed using PI staining and FACS analysis (B) or by anti-histone ELISA (C). The optical densities of etoposide-treated PKCδ-overexpressing cells were designated 100% (total apoptosis), and all other values are presented as a percent of this total. The results represent the means ± SE of triplicate measurements in each of three experiments. ∗, P < 0.001.
Cleavage of caspase 3 and PKCδ in cells overexpressing PKCδ5 mutant.
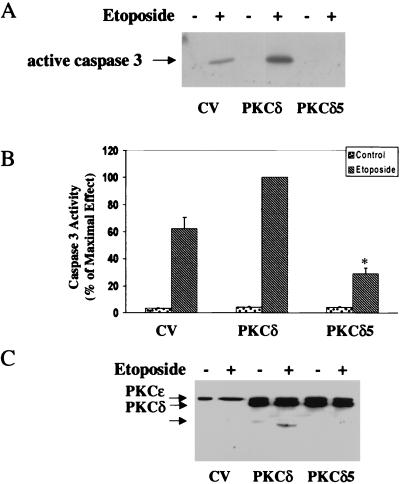
To further explore the role of tyrosine phosphorylation of PKCδ in the apoptosis induced by etoposide, we compared the activation of caspase 3 in cells overexpressing control vector, PKCδ, and the PKCδ5 mutant. Using a specific antibody recognizing the cleaved product (17 kDa) of caspase 3, we found that etoposide induced caspase 3 cleavage in the control vector cells. Cells overexpressing PKCδ showed a larger amount of the cleaved product, whereas cells overexpressing the PKCδ5 mutant exhibited very low levels of the 17-kDa fragment (Fig. 10A). Similar results were obtained for caspase 3 activity as measured by a caspase 3 colorimetric assay (Fig. 10B). These results suggest that the activation of caspase 3 required a tyrosine phosphorylated form of PKCδ.
FIG. 10.
Activation of caspase 3 and cleavage of PKCδ in cells overexpressing PKCδ and PKCδ5. C6 cells overexpressing control vector (CV), PKCδ, or PKCδ5 were treated with etoposide (50 μM) for 24 h, and the activation of caspase 3 (A and B) and cleavage of PKCδ (C) were determined. Cells were harvested and subjected to SDS-PAGE and Western blot analysis. The membranes were probed with active caspase 3 antibody (A) or with anti-PKCɛ that recognizes the ɛ tag which is located in the catalytic domain of the PKCδ constructs (C). The results represent one of three separate experiments which yielded similar results. The activity of caspase 3 was measured using the Caspase 3 Cellular Activity Assay Kit as described in Materials and Methods (B). Caspase 3 activity was calculated and expressed as picomoles/minute/microgram of protein, and the percent of maximal effect was determined. The results represent the means ± SE of three experiments.
We then examined the cleavage of PKCδ and PKCδ5 in response to etoposide. Using the ɛ tag, we were able to detect the cleaved catalytic domain of the exogenous PKCδ and PKCδ5. Using the anti-PKCɛ antibody, we found no detectable cleaved product in the etoposide-treated control vector cells. Accumulation of the catalytic fragment was observed in cells overexpressing PKCδ, whereas cells overexpressing the PKCδ5 mutant displayed no detectable cleavage (Fig. 10C). Since the kinetics of PKCδ phosphorylation is faster than the cleavage and is transient, the phosphorylation presumably plays a role upstream from the actual cleavage.
Translocation of PKCδ and the PKCδ 5 mutant in response to etoposide.
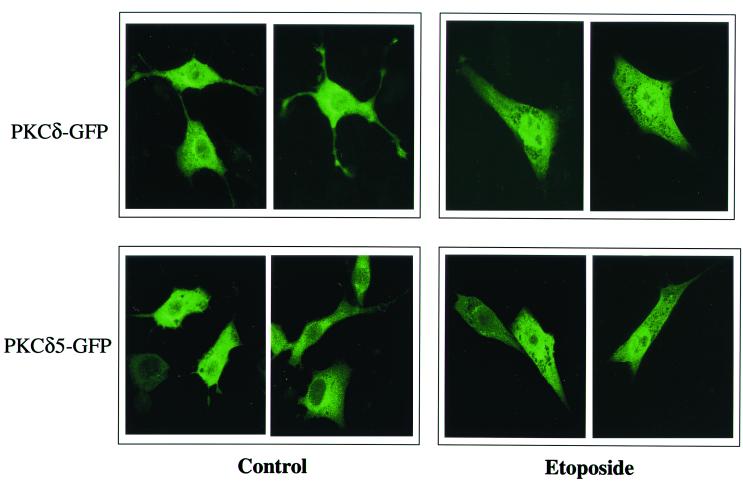
One possible explanation for the differential effects of PKCδ and the PKCδ5 mutant on cell apoptosis is their differential translocation following etoposide treatment. We therefore examined the translocation of PKCδ and the PKCδ5 mutant in response to etoposide. For these experiments, we used GFP-tagged PKCδ wild type or the PKCδ5 mutant. Cells were transiently transfected with the specific construct, and the response of the cells to etoposide was monitored after 6 and 24 h.
We found that etoposide induced a similar pattern of translocation for both PKCδ and PKCδ5 (Fig. 11). Thus, stimulation of the cells with etoposide for 6 h induced translocation of PKCδ-GFP to the nucleus in about 90 to 95% of the cells, similar to the results obtained using immunofluorescent staining of the endogenous PKCδ. Likewise, PKCδ5-GFP translocated to the nucleus in response to etoposide in about 90% of the cells. A similar pattern of nuclear translocation of PKCδ and PKCδ5 was also obtained after 24 h of etoposide treatment (data not shown).
FIG. 11.
Translocation of PKCδ and PKCδ5 in etoposide-treated C6 cells. Cells were transiently transfected with GFP-PKCδ or GFP-PKCδ5. After 48 h, cells were treated with etoposide (50 μM) for 6 h and cells were viewed using confocal microscopy. Cells shown are representative of four independent experiments
Role of single tyrosine mutants in apoptotic response of etoposide.
To identify the specific tyrosines that are involved in the inhibitory effect of PKCδ on cell apoptosis, we examined the role of the single tyrosines 52, 64, 155, 187, and 565. C6 cells were stably transfected with the different PKCδ mutants in which each one of these tyrosines was individually mutated to phenylalanine. The expression and activity of C6 cells overexpressing the different PKC mutants were previously described (35).
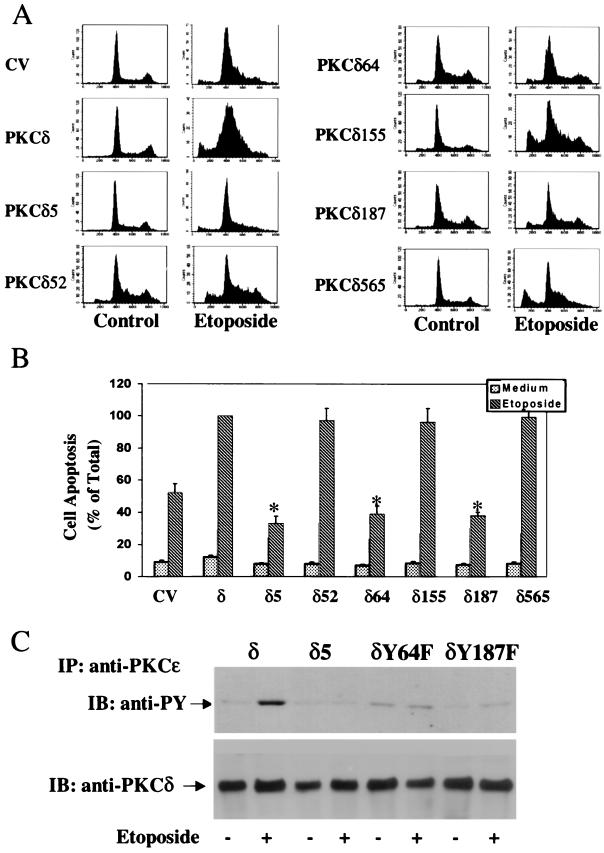
The apoptotic effect of etoposide was examined in cells overexpressing the different mutants using PI staining and FACS analysis. We found that cells overexpressing PKCδY52F, PKCδY155F, and PKCδY565F exhibited an enhanced apoptotic response to etoposide similar to that of cells overexpressing PKCδ. In contrast, treatment with etoposide of cells overexpressing PKCδY64F or PKCδY187F resulted in a lower apoptotic response, similar to the response observed with cells overexpressing the PKCδ5 mutant (Fig. 12A). Similar results were obtained with anti-histone ELISA (Fig. 12B).
FIG. 12.
Apoptosis and tyrosine phosphorylation in C6 cells overexpressing different PKCδ tyrosine mutants. C6 cells overexpressing PKCδ or the PKCδ mutants were plated in the absence and presence of etoposide (50 μM). (A) Cell apoptosis was measured by PI staining and FACS analysis after 24 h. The results are from one representative experiment out of four separate experiments. (B) Cell apoptosis was also determined after 48 h using anti-histone ELISA. The optical densities of etoposide-treated PKCδ-overexpressing cells were designated 100% (total apoptosis), and all other values are presented as percent of this total. The results represent the means ± SE of triplicate measurements in each of five experiments. Tyrosine phosphorylation of PKCδ, PKCδ5, PKCδY64F, and PKCδY187F was determined following 1 h of etoposide treatment. (C) Cells were harvested, and immunoprecipitation of PKCδ was performed using anti-PKCɛ antibody as described in Materials and Methods. Following SDS-PAGE, membranes were stained with anti-phosphotyrosine antibody (anti-PY) or with an anti-PKCδ antibody. The results represent one of three separate experiments which yielded similar results.
We also found that etoposide did not induce a significant increase in the tyrosine phosphorylation of PKCδY64F and PKCδY187F, similar to the results obtained with PKCδ5 (Fig. 12C). Finally, we found no detectable cleaved product of PKCδ in the etoposide-treated PKCδY64F and PKCδY187F overexpressors (data not shown).
Apoptosis induced by etoposide is not inhibited by Src-kinase inhibitors PP1 and PP2.
In a previous study, we showed that Fyn and Lyn can associate with PKCδ and that Fyn associates with PKCδ via tyrosine 187 (35). To examine the role of Src-related kinases in the apoptotic response of etoposide, we employed the Src-kinase inhibitors PP1 and PP2. Pretreatment of the cells with either PP1 (10 μM) or PP2 (10 μM) did not affect the apoptotic response of etoposide (data not shown), suggesting that Src-related kinases are probably not involved in the tyrosine phosphorylation of PKCδ and in the apoptotic response of etoposide.
DISCUSSION
In this study, we explored the importance of PKCδ and its tyrosine phosphorylation for the induction of apoptosis in C6 glioma cells upon treatment with etoposide. We found that etoposide induced apoptosis of C6 cells, that this response was partially inhibited by the PKCδ inhibitor rottlerin and PKCδ DN, and that overexpression of PKCδ enhanced the apoptotic effect of etoposide. Etoposide, an inhibitor of topoisomerase II, has been reported to induce apoptosis in a broad range of different cells (29), and PKCδ has previously been implicated in the induction of apoptosis (34, 49). Thus, our results demonstrate that C6 glioma cells exhibit a response to etoposide similar to those of other tumor cells.
Etoposide induced translocation of PKCδ to the nucleus within 3 h of treatment. Various PKC isoforms can be found in the nucleus and in subnuclear compartments (7), and PKC isoforms can undergo nuclear translocation in response to various stimuli, including phorbol esters (55), growth factors (39, 43), and apoptotic stimuli, such as γ-irradiation (58). In addition, increases in the levels of nuclear PKC isoforms were reported in the context of cell proliferation and differentiation (16, 31), suggesting a role of nuclear PKC in these processes. It is presently unclear how PKCδ is transported to the nucleus since PKC isoforms do not contain any known nuclear localization signal, but PKC-binding proteins probably play a role in this process (7). Indeed, in a recent study, it was reported that PKCδ associates with c-Abl via its SH3 domain and undergoes tyrosine phosphorylation and nuclear translocation in complex with c-Abl in response to γ-irradiation (58).
In some systems, the apoptotic effect of PKCδ has been associated with a cleavage of the catalytic domain from the regulatory domain (34, 49). Cleavage of the catalytic domain of PKCδ by caspases has been reported for cells treated with ionizing radiation, tumor necrosis factor alpha, and etoposide (34, 38, 49). We found that etoposide induced cleavage of PKCδ in C6 cells and that this cleavage occurred in the nucleus. The absolute extent of cleavage was somewhat lower than that reported for parotid cells (49), presumably reflecting differences between these cell types, including a lower sensitivity of the C6 cells to etoposide. Both the cleavage of PKCδ by etoposide and its apoptotic effect were partially inhibited by the caspase inhibitor DEVD.FMK, suggesting a role for caspase 3 in these effects, as was reported for other systems (21, 49). Similarly, we found that the inhibition of PKCδ blocked the cleavage of caspase 3 and the cleavage of PKCδ was induced by etoposide. These results suggest the presence of a positive loop between PKCδ and caspase 3, which is dependent on PKCδ activity. Consistent with these results, recent studies demonstrated that caspases, including caspase 3, translocate and act in the nucleus following apoptotic stimuli (15). The partial inhibition of the caspase inhibitors on the apoptotic effect of etoposide was reported for other systems as well (44), and it is probably due to the presence of additional, non-caspase-dependent mechanisms involved in the effects of etoposide (50).
Both the regulatory and catalytic domains of PKCδ were important for the apoptotic effects of PKCδ in response to etoposide. These results are different from those of our previous studies, in which the regulatory domain of PKCδ was responsible for the effects of this isoform on C6 cell proliferation and on GS expression (5, 35). In various studies, it was suggested that, once cleaved, the catalytic domain of PKCδ mediates the apoptotic effect of this isoform since overexpression of the catalytic fragment alone was able to induce apoptosis (21). Taken together, our results suggest that the regulatory domain contains signals that are important for the cleavage and activation of the catalytic domain.
Etoposide induced tyrosine phosphorylation of PKCδ in the regulatory domain. Tyrosine phosphorylation of PKCδ has been reported in the response to PMA and PDGF in C6 cells (5), in EGF-stimulated keratinocytes (13), in response to activation of the IgE receptor in RBL-2H3 cells (25, 54), and in response to apoptotic stimuli, such as γ-irradiation (58) and H2O2 (32). Phosphorylation of tyrosine residues occurs in either the regulatory or catalytic domains of PKCδ. Thus, PDGF and PMA induced tyrosine phosphorylation on tyrosines 187 and 155, respectively (35). Similarly, activation of the IgE receptor induced tyrosine phosphorylation on tyrosine 52 (54). In contrast, stimulation of the cells with H2O2 and γ-irradiation induced phosphorylation on tyrosines in the catalytic domain and in the hinge region (32, 33).
The tyrosine phosphorylation of PKCδ by etoposide appeared to be essential for the apoptosis induced by PKCδ, since the increase in cell apoptosis obtained in cells overexpressing PKCδ was absent in cells overexpressing the PKCδ5 mutant. These results are similar to our recent findings, which showed a role for tyrosine phosphorylation of PKCδ in the inhibitory effect of this isoform on the expression of the astrocytic marker GS (5) and on C6 cell proliferation (35). Thus, the PKCδ5 mutant also appears to act in an opposite way to PKCδ in its effect on cell apoptosis.
We found that caspase 3 was cleaved in response to etoposide and that this cleavage was enhanced in cells overexpressing PKCδ but inhibited in cells overexpressing the PKCδ5 mutant. The mechanisms involved in the enhanced cleavage of caspase 3 in response to overexpression of PKCδ are not known. PKCδ could act directly on caspase 3 to induce its phosphorylation or act indirectly via the phosphorylation of upstream caspases, such as caspase 8 or caspase 9 or other unknown upstream proteins. Indeed, a study by Martins et al. (41) reported that etoposide induced phosphorylation of caspases in HL-60 cells. Similarly, caspase 9, which is upstream of caspase 3, has been shown to be phosphorylated and directly regulated by phosphorylation (8, 20). Whatever the mechanism is for the effects of PKCδ, our data suggest that tyrosine phosphorylation of PKCδ is essential for the ability of this isoform to cause activation of caspase 3. In accordance with our data and with other studies suggesting that the cleavage of PKCδ is mediated by caspase 3 (21, 49), we found that the cleavage of the PKCδ5 mutant in response to etoposide was also inhibited. The difference in the ability of the PKCδ5 mutant to markedly inhibit etoposide-induced caspase-dependent cleavage and caspase 3 activity compared to its partial inhibition of etoposide-induced cell apoptosis suggests that tyrosine phosphorylation of PKCδ mediates the caspase-dependent component of the apoptosis induced by etoposide, whereas it is not involved in the caspase-independent pathways.
The effects of tyrosine phosphorylation on the activity of PKCδ or on its function are complex and dependent on the specific system and stimulus. Tyrosine phosphorylation of PKCδ in various systems has been reported to reduce or increase PKCδ activity and may do so in a substrate-specific manner (13, 25). Thus, the phosphorylation of PKCδ in response to etoposide may act by changing the affinity of PKCδ towards specific caspases or upstream proteins.
One of the factors that could provide the basis for the different effects of PKCδ and PKCδ5 is a distinct pattern of translocation. Translocation of PKC to specific cellular compartments could lead to different effects due to the phosphorylation of different substrates and to the association of PKCδ with specific proteins present in these locations. One determinant of the localization of PKC following its activation is association with receptors for activated C kinases (RACKs) (42). It is presently not clear to what extent tyrosine kinases can act as RACKs and affect the translocation of PKC isoforms. In a recent study, we found that mutations in tyrosine residues did not alter the translocation of PKCδ in response to PMA or PDGF (35). In contrast, Ron et al. suggested that Fyn might act as a RACK of PKCθ (51). We found that etoposide induced nuclear translocation of both PKCδ and PKCδ5. Thus, the nuclear translocation of PKCδ does not depend on its phosphorylation, and the differential effects of PKCδ and PKCδ5 on cell apoptosis in response to etoposide do not appear to reflect their different translocation following activation.
PKCδ has been reported to associate with different tyrosine kinases. Thus, p60Src (4, 53, 54, 59), Lyn (54), Fyn (35), and c-Abl (58) can associate with PKCδ in either a phosphorylation-dependent or -independent manner. In addition, various reports indicate that PKCδ can undergo tyrosine phosphorylation on specific tyrosine residues by Src, Fyn, and c-Abl. The ability of PKCδ to be tyrosine phosphorylated on more than one tyrosine suggests that PKCδ can associate with different tyrosine kinases. In a recent study, we found that PKCδ underwent tyrosine phosphorylation on tyrosines 155 and 187 which was associated with the inhibitory effects of PKCδ on cell proliferation and the expression of GS, respectively (35). Our present results indicate that phosphorylation of PKCδ on tyrosines 64 and 187 is essential for its apoptotic effect. Our results suggest that Src-related kinases which are involved in the tyrosine phosphorylation of PKCδ in response to PDGF are not involved in the apoptosis induced by etoposide. The tyrosine kinase which phosphorylates PKCδ in etoposide-treated cells remains to be identified.
In summary, we demonstrated that the apoptosis induced by etoposide involves activation of an as-yet-unidentified tyrosine kinase(s) which phosphorylates PKCδ in the regulatory domain. PKCδ is then translocated to the nucleus where it directly or indirectly activates caspase 3. Caspase 3 in turn cleaves PKCδ and releases the δ catalytic domain, which can phosphorylate known apoptosis-related proteins, such as lamin B (9) or DNA-PK (3). It is presently not clear whether the effect of the tyrosine-phosphorylated PKCδ on the apoptosis in C6 cells is mediated via altered affinity of PKCδ towards downstream PKC substrates or via activation of a tyrosine kinase(s) that is associated with PKCδ. However, the results of this study further extend our previous findings that tyrosine phosphorylation plays an important role in the regulation of PKCδ activity and in determining the nature of its effects.
Acknowledgments
This work was supported by the James S. McDonnell Foundation 21st Century Scientist Award/Brain Cancer Research (C.B.).
REFERENCES
- 1.Acs, P., K. Bogi, P. S. Lorenzo, A. M. Marquez, T. Biro, Z. Szallasi, and P. M. Blumberg. 1997. The catalytic domain of protein kinase C chimeras modulates the affinity and targeting of phorbol ester-induced translocation. J. Biol. Chem. 272:22148–22153. [DOI] [PubMed] [Google Scholar]
- 2.Acs, P., Q. J. Wang, K. Bogi, A. M. Marquez, P. S. Lorenzo, T. Biro, Z. Szallasi, J. F. Mushinski, and P. M. Blumberg. 1997. Both the catalytic and regulatory domains of protein kinase C chimeras modulate the proliferative properties of NIH 3T3 cells. J. Biol. Chem. 272:28793–28799. [DOI] [PubMed] [Google Scholar]
- 3.Bharti, A., S. K. Kraeft, M. Gounder, P. Pandey, S. Jin, Z. M. Yuan, S. P. Lees-Miller, R. Weichselbaum, D. Weaver, L. B. Chen, D. Kufe, and S. Kharbanda. 1998. Inactivation of DNA-dependent protein kinase by protein kinase Cδ: implications for apoptosis. Mol. Cell. Biol. 18:6719–6728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blake, R. A., P. Garcia-Paramio, P. J. Parker, and S. A. Courtneidge. 1999. Src promotes PKCδ degradation. Cell Growth Differ. 10:231–241. [PubMed] [Google Scholar]
- 5.Brodie, C., K. Bogi, P. Acs, P. S. Lorenzo, L. Baskin, and P. M. Blumberg. 1998. Protein kinase Cδ (PKCδ) inhibits the expression of glutamine synthetase in glial cells via the PKCδ regulatory domain and its tyrosine phosphorylation. J. Biol. Chem. 273:30713–30718. [DOI] [PubMed] [Google Scholar]
- 6.Brodie, C., I. Kuperstein, P. Acs, and P. M. Blumberg. 1998. Differential role of specific PKC isoforms in the proliferation of glial cells and the expression of the astrocytic markers GFAP and glutamine synthetase. Brain Res. Mol. Brain Res. 56:108–117. [DOI] [PubMed] [Google Scholar]
- 7.Buchner, K. 2000. The role of protein kinase C in the regulation of cell growth and in signalling to the cell nucleus. J. Cancer Res. Clin. Oncol. 126:1–11. [DOI] [PubMed] [Google Scholar]
- 8.Cardone, M. H., N. Roy, H. R. Stennicke, G. S. Salvesen, T. F. Franke, E. Stanbridge, S. Frisch, and J. C. Reed. 1998. Regulation of cell death protease caspase-9 by phosphorylation. Science 282:1318–1321. [DOI] [PubMed] [Google Scholar]
- 9.Cross, T., G. Griffiths, E. Deacon, R. Sallis, M. Gough, D. Watters, and J. M. Lord. 2000. PKC-δ is an apoptotic lamin kinase. Oncogene 19:2331–2337. [DOI] [PubMed] [Google Scholar]
- 10.Cross, T. G., D. Scheel-Toellner, N. V. Henriquez, E. Deacon, M. Salmon, and J. M. Lord. 2000. Serine/threonine protein kinases and apoptosis. Exp. Cell Res. 256:34–41. [DOI] [PubMed] [Google Scholar]
- 11.Datta, R., H. Kojima, K. Yoshida, and D. Kufe. 1997. Caspase-3-mediated cleavage of protein kinase Cθ in induction of apoptosis. J. Biol. Chem. 272:20317–20320. [DOI] [PubMed] [Google Scholar]
- 12.Davies, S. P., H. Reddy, M. Caivano, and P. Cohen. 2000. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem. J. 351:95–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Denning, M. F., A. A. Dlugosz, D. W. Threadgill, T. Magnuson, and S. H. Yuspa. 1996. Activation of the epidermal growth factor receptor signal transduction pathway stimulates tyrosine phosphorylation of protein kinase Cδ. J. Biol. Chem. 271:5325–5331. [DOI] [PubMed] [Google Scholar]
- 14.Endo, K., E. Oki, V. Biedermann, H. Kojima, K. Yoshida, F. J. Johannes, D. Kufe, and R. Datta. 2000. Proteolytic cleavage and activation of protein kinase Cμ by caspase-3 in the apoptotic response of cells to 1-β-d-arabinofuranosylcytosine and other genotoxic agents. J. Biol. Chem. 275:18476–18481. [DOI] [PubMed] [Google Scholar]
- 15.Faleiro, L., and Y. Lazebnik. 2000. Caspases disrupt the nuclear-cytoplasmic barrier. J. Cell Biol. 151:951–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fields, A. P., G. Tyler, A. S. Kraft, and W. S. May. 1990. Role of nuclear protein kinase C in the mitogenic response to platelet-derived growth factor. J. Cell Sci. 96:107–114. [DOI] [PubMed] [Google Scholar]
- 17.Franklin, R. A., and J. A. McCubrey. 2000. Kinases: positive and negative regulators of apoptosis. Leukemia 14:2019–2034. [DOI] [PubMed] [Google Scholar]
- 18.Frutos, S., J. Moscat, and M. T. Diaz-Meco. 1999. Cleavage of ζPKC but not λ/ιPKC by caspase-3 during UV-induced apoptosis. J. Biol. Chem. 274:10765–10770. [DOI] [PubMed] [Google Scholar]
- 19.Fujii, T., M. L. Garcia-Bermejo, J. L. Bernabo, J. Caamano, M. Ohba, T. Kuroki, L. Li, S. H. Yuspa, and M. G. Kazanietz. 2000. Involvement of protein kinase Cδ (PKCδ) in phorbol ester-induced apoptosis in LNCaP prostate cancer cells. Lack of proteolytic cleavage of PKCδ. J. Biol. Chem. 275:7574–7582. [DOI] [PubMed] [Google Scholar]
- 20.Fujita, E., A. Jinbo, H. Matuzaki, H. Konishi, U. Kikkawa, and T. Momoi. 1999. Akt phosphorylation site found in human caspase-9 is absent in mouse caspase-9. Biochem. Biophys. Res. Commun. 264:550–555. [DOI] [PubMed] [Google Scholar]
- 21.Ghayur, T., M. Hugunin, R. V. Talanian, S. Ratnofsky, C. Quinlan, Y. Emoto, P. Pandey, R. Datta, Y. Huang, S. Kharbanda, H. Allen, R. Kamen, W. Wong, and D. Kufe. 1996. Proteolytic activation of protein kinase Cδ by an ICE/CED 3-like protease induces characteristics of apoptosis. J. Exp. Med. 184:2399–2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gschwendt, M., H. J. Muller, K. Kielbassa, R. Zang, W. Kittstein, G. Rincke, and F. Marks. 1994. Rottlerin, a novel protein kinase inhibitor. Biochem. Biophys. Res. Commun. 199:93–98. [DOI] [PubMed] [Google Scholar]
- 23.Gubina, E., M. S. Rinaudo, Z. Szallasi, P. M. Blumberg, and R. A. Mufson. 1998. Overexpression of protein kinase C isoform ɛ but not δ in human interleukin-3-dependent cells suppresses apoptosis and induces bcl-2 expression. Blood 91:823–829. [PubMed] [Google Scholar]
- 24.Haglund, R. E., and L. I. Rothblum. 1987. Isolation, fractionation and reconstitution of a nuclear extract capable of transcribing ribosomal DNA. Mol. Cell Biochem. 73:11–20. [DOI] [PubMed] [Google Scholar]
- 25.Haleem-Smith, H., E. Y. Chang, Z. Szallasi, P. M. Blumberg, and J. Rivera. 1995. Tyrosine phosphorylation of protein kinase C-δ in response to the activation of the high-affinity receptor for immunoglobulin E modifies its substrate recognition. Proc. Natl. Acad. Sci. USA 92:9112–9116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hug, H., and T. F. Sarre. 1993. Protein kinase C isoenzymes: divergence in signal transduction? Biochem. J. 291:329–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jaken, S. 1996. Protein kinase C isozymes and substrates. Curr. Opin. Cell Biol. 8:168–173. [DOI] [PubMed] [Google Scholar]
- 28.Johannes, F. J., J. Prestle, S. Eis, P. Oberhagemann, and K. Pfizenmaier. 1994. PKCu is a novel, atypical member of the protein kinase C family. J. Biol. Chem. 269:6140–6148. [PubMed] [Google Scholar]
- 29.Kaufmann, S. H. 1998. Cell death induced by topoisomerase-targeted drugs: more questions than answers. Biochim. Biophys. Acta 1400:195–211. [DOI] [PubMed] [Google Scholar]
- 30.Keenan, C., N. Goode, and C. Pears. 1997. Isoform specificity of activators and inhibitors of protein kinase C γ and δ. FEBS Lett. 415:101–108. [DOI] [PubMed] [Google Scholar]
- 31.Kiley, S. C., and P. J. Parker. 1995. Differential localization of protein kinase C isozymes in U937 cells: evidence for distinct isozyme functions during monocyte differentiation. J. Cell Sci 108:1003–1016. [DOI] [PubMed] [Google Scholar]
- 32.Konishi, H., M. Tanaka, Y. Takemura, H. Matsuzaki, Y. Ono, U. Kikkawa, and Y. Nishizuka. 1997. Activation of protein kinase C by tyrosine phosphorylation in response to H2O2. Proc. Natl. Acad. Sci. USA 94:11233–11237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Konishi, H., E. Yamauchi, H. Taniguchi, T. Yamamoto, H. Matsuzaki, Y. Takemura, K. Ohmae, U. Kikkawa, and Y. Nishizuka. 2001. Phosphorylation sites of protein kinase C δ in H2O2-treated cells and its activation by tyrosine kinase in vitro. Proc. Natl. Acad. Sci. USA 98:6587–6592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koriyama, H., Z. Kouchi, T. Umeda, T. C. Saido, T. Momoi, S. Ishiura, and K. Suzuki. 1999. Proteolytic activation of protein kinase Cδ and ɛ by caspase-3 in U937 cells during chemotherapeutic agent-induced apoptosis. Cell. Signal. 11:831–838. [DOI] [PubMed] [Google Scholar]
- 35.Kronfeld, I., G. Kazimirsky, P. S. Lorenzo, S. H. Garfield, P. M. Blumberg, and C. Brodie. 2000. Phosphorylation of PKCδ on distinct tyrosine residues regulates specific cellular functions. J. Biol. Chem. 275:35491–35498. [DOI] [PubMed] [Google Scholar]
- 36.Li, L., P. S. Lorenzo, K. Bogi, P. M. Blumberg, and S. H. Yuspa. 1999. Protein kinase Cδ targets mitochondria, alters mitochondrial membrane potential, and induces apoptosis in normal and neoplastic keratinocytes when overexpressed by an adenoviral vector. Mol. Cell. Biol. 19:8547–8558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li, W., X. H. Chen, C. A. Kelley, M. Alimandi, J. Zhang, Q. Chen, D. P. Bottaro, and J. H. Pierce. 1996. Identification of tyrosine 187 as a protein kinase C-δ phosphorylation site. J. Biol. Chem. 271:26404–26409. [DOI] [PubMed] [Google Scholar]
- 38.Majumder, P. K., P. Pandey, X. Sun, K. Cheng, R. Datta, S. Saxena, S. Kharbanda, and D. Kufe. 2000. Mitochondrial translocation of protein kinase C δ in phorbol ester-induced cytochrome c release and apoptosis. J. Biol. Chem. 275:21793–21796. [DOI] [PubMed] [Google Scholar]
- 39.Maloney, J. A., O. Tsygankova, A. Szot, L. Yang, Q. Li, and J. R. Williamson. 1998. Differential translocation of protein kinase C isozymes by phorbol esters, EGF, and ANG II in rat liver WB cells. Am. J. Physiol. 274:C974–C982. [DOI] [PubMed] [Google Scholar]
- 40.Mandil, R., E. Ashkenazi, M. Blass, I. Kronfeld, G. Kazimirsky, G. Rosenthal, F. Umansky, P. S. Lorenzo, P. M. Blumberg, and C. Brodie. 2001. Protein kinase Cα and protein kinase Cδ play opposite roles in the proliferation and apoptosis of glioma cells. Cancer Res. 61:4612–4619. [PubMed] [Google Scholar]
- 41.Martins, L. M., T. J. Kottke, S. H. Kaufmann, and W. C. Earnshaw. 1998. Phosphorylated forms of activated caspases are present in cytosol from HL-60 cells during etoposide-induced apoptosis. Blood 92:3042–3049. [PubMed] [Google Scholar]
- 42.Mochly-Rosen, D., and A. S. Gordon. 1998. Anchoring proteins for protein kinase C: a means for isozyme selectivity. FASEB J. 12:35–42. [PubMed] [Google Scholar]
- 43.Neri, L. M., A. M. Billi, L. Manzoli, S. Rubbini, R. S. Gilmour, L. Cocco, and A. M. Martelli. 1994. Selective nuclear translocation of protein kinase C α in Swiss 3T3 cells treated with IGF-I, PDGF and EGF. FEBS Lett. 347:63–68. [DOI] [PubMed] [Google Scholar]
- 44.Newton, A. C. 1995. Protein kinase C. Seeing two domains. Curr. Biol. 5:973–976. [DOI] [PubMed] [Google Scholar]
- 45.Nishizuka, Y. 1984. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature 308:693–698. [DOI] [PubMed] [Google Scholar]
- 46.Nishizuka, Y. 1988. The molecular heterogeneity of protein kinase C and its implications for cellular regulation. Nature 334:661–665. [DOI] [PubMed] [Google Scholar]
- 47.Olah, Z., C. Lehel, G. Jakab, and W. B. Anderson. 1994. A cloning and epsilon-epitope-tagging insert for the expression of polymerase chain reaction-generated cDNA fragments in Escherichia coli and mammalian cells. Anal. Biochem. 221:94–102. [DOI] [PubMed] [Google Scholar]
- 48.Pongracz, J., P. Webb, K. Wang, E. Deacon, O. J. Lunn, and J. M. Lord. 1999. Spontaneous neutrophil apoptosis involves caspase 3-mediated activation of protein kinase C-δ. J. Biol. Chem. 274:37329–37334. [DOI] [PubMed] [Google Scholar]
- 49.Reyland, M. E., S. M. Anderson, A. A. Matassa, K. A. Barzen, and D. O. Quissell. 1999. Protein kinase Cδ is essential for etoposide-induced apoptosis in salivary gland acinar cells. J. Biol. Chem. 274:19115–19123. [DOI] [PubMed] [Google Scholar]
- 50.Robertson, J. D., V. Gogvadze, B. Zhivotovsky, and S. Orrenius. 2000. Distinct pathways for stimulation of cytochrome c release by etoposide. J. Biol. Chem. 275:32438–32443. [DOI] [PubMed] [Google Scholar]
- 51.Ron, D., E. W. Napolitano, A. Voronova, N. J. Vasquez, D. N. Roberts, B. L. Calio, R. H. Caothien, S. M. Pettiford, S. Wellik, J. B. Mandac, and L. M. Kauvar. 1999. Direct interaction in T-cells between θPKC and the tyrosine kinase p59fyn. J. Biol. Chem. 274:19003–19010. [DOI] [PubMed] [Google Scholar]
- 52.Ruvolo, P. P., X. Deng, B. K. Carr, and W. S. May. 1998. A functional role for mitochondrial protein kinase Cα in Bcl2 phosphorylation and suppression of apoptosis. J. Biol. Chem. 273:25436–25442. [DOI] [PubMed] [Google Scholar]
- 53.Shanmugam, M., N. L. Krett, C. A. Peters, E. T. Maizels, F. M. Murad, H. Kawakatsu, S. T. Rosen, and M. Hunzicker-Dunn. 1998. Association of PKC δ and active Src in PMA-treated MCF-7 human breast cancer cells. Oncogene 16:1649–1654. [DOI] [PubMed] [Google Scholar]
- 54.Song, J. S., P. G. Swann, Z. Szallasi, U. Blank, P. M. Blumberg, and J. Rivera. 1998. Tyrosine phosphorylation-dependent and -independent associations of protein kinase C-δ with Src family kinases in the RBL-2H3 mast cell line: regulation of Src family kinase activity by protein kinase C-δ. Oncogene 16:3357–3368. [DOI] [PubMed] [Google Scholar]
- 55.Thomas, T. P., H. S. Talwar, and W. B. Anderson. 1988. Phorbol ester-mediated association of protein kinase C to the nuclear fraction in NIH 3T3 cells. Cancer Res. 48:1910–1919. [PubMed] [Google Scholar]
- 56.Toker, A. 1998. Signaling through protein kinase C. Front. Biosci. 3:D1134–D1147. [DOI] [PubMed] [Google Scholar]
- 57.Wang, Q. J., P. Acs, J. Goodnight, T. Giese, P. M. Blumberg, H. Mischak, and J. F. Mushinski. 1997. The catalytic domain of protein kinase C-δ in reciprocal δ and ɛ chimeras mediates phorbol ester-induced macrophage differentiation of mouse promyelocytes. J. Biol. Chem. 272:76–82. [DOI] [PubMed] [Google Scholar]
- 58.Yuan, Z. M., T. Utsugisawa, T. Ishiko, S. Nakada, Y. Huang, S. Kharbanda, R. Weichselbaum, and D. Kufe. 1998. Activation of protein kinase C δ by the c-Abl tyrosine kinase in response to ionizing radiation. Oncogene 16:1643–1648. [DOI] [PubMed] [Google Scholar]
- 59.Zang, Q., Z. Lu, M. Curto, N. Barile, D. Shalloway, and D. A. Foster. 1997. Association between v-Src and protein kinase C δ in v-Src-transformed fibroblasts. J. Biol. Chem. 272:13275–13280. [DOI] [PubMed] [Google Scholar]