Abstract
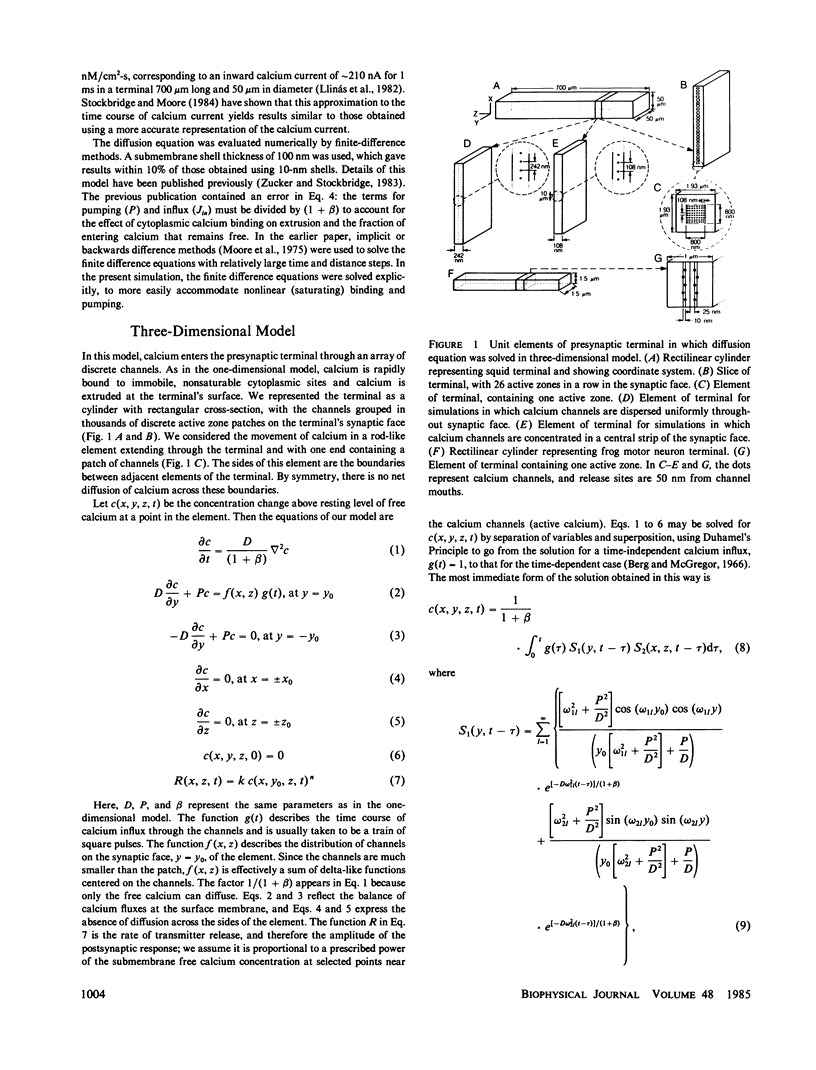
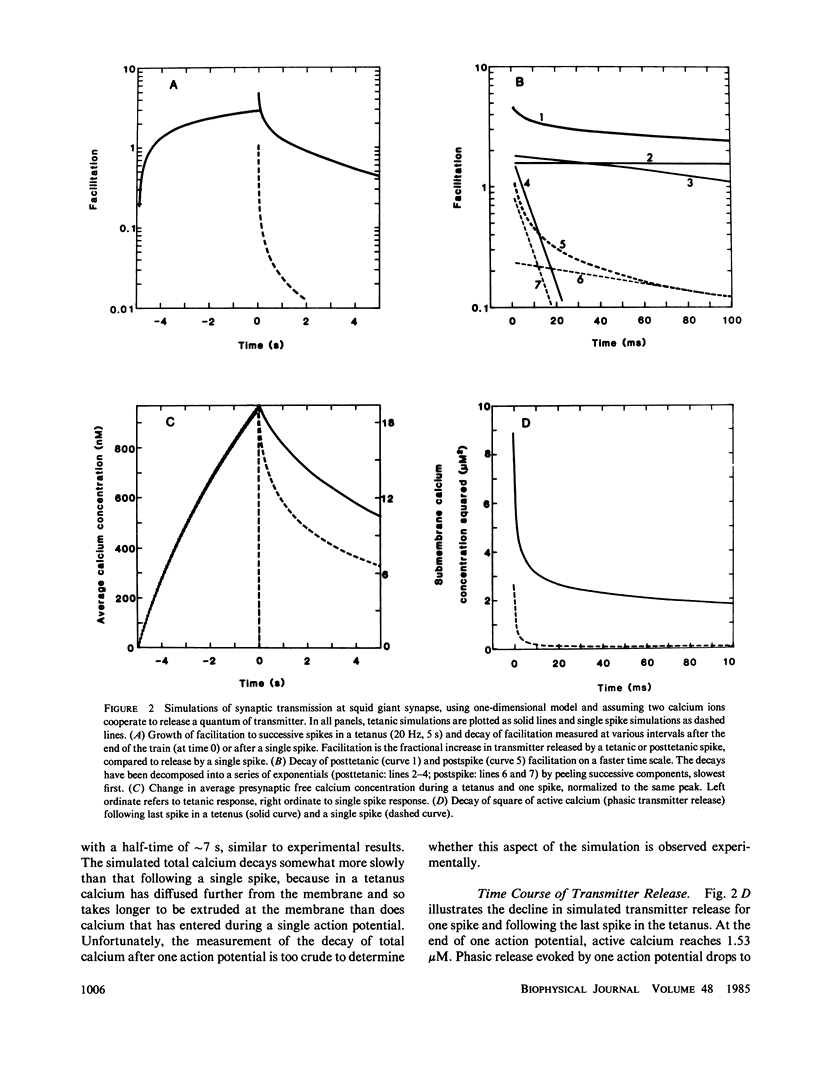
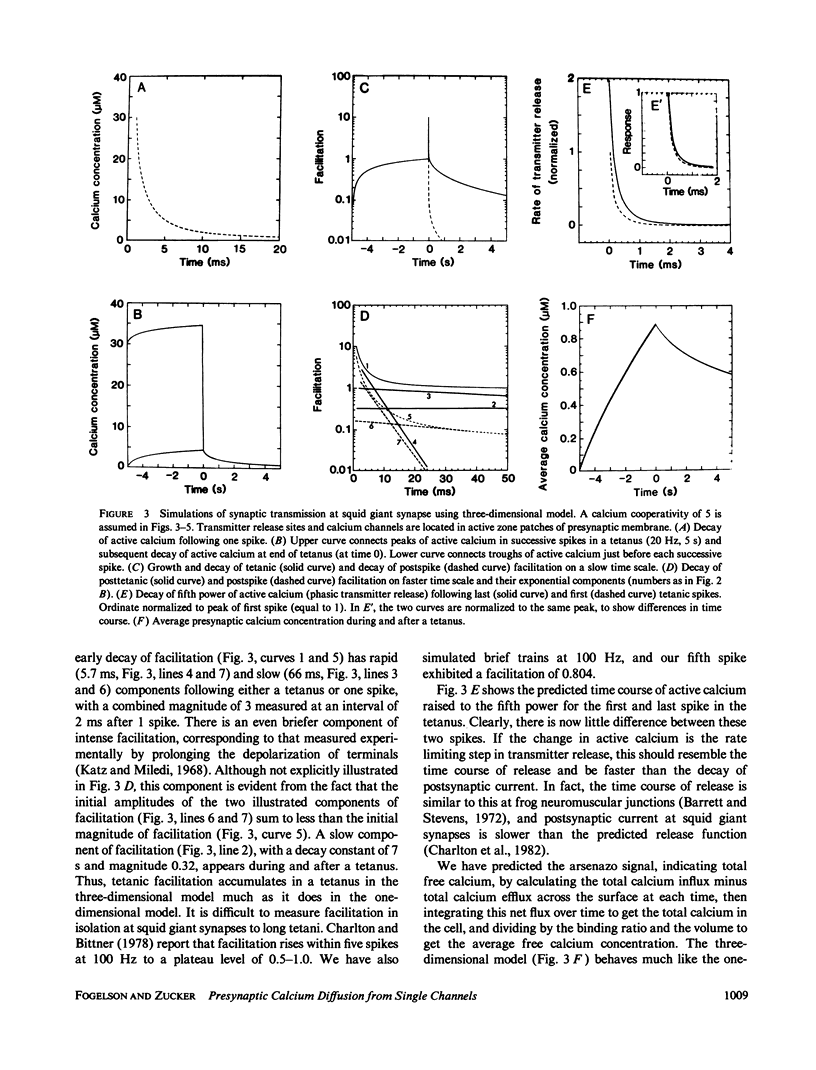
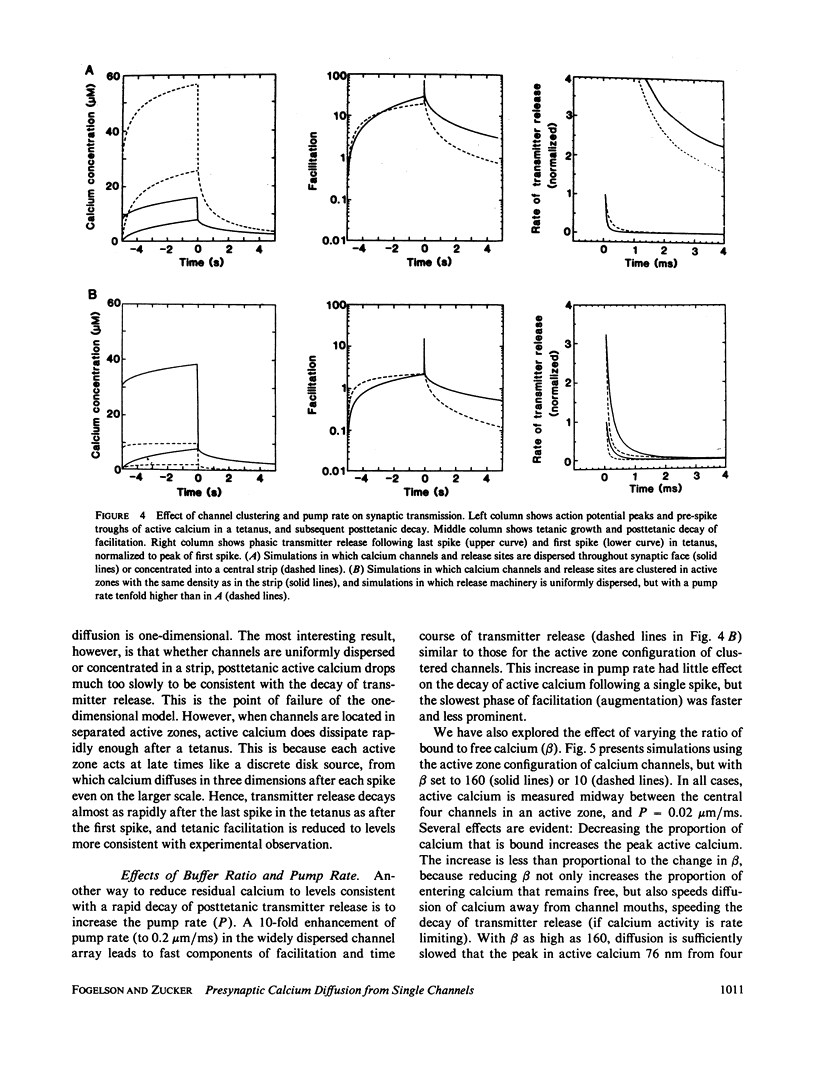
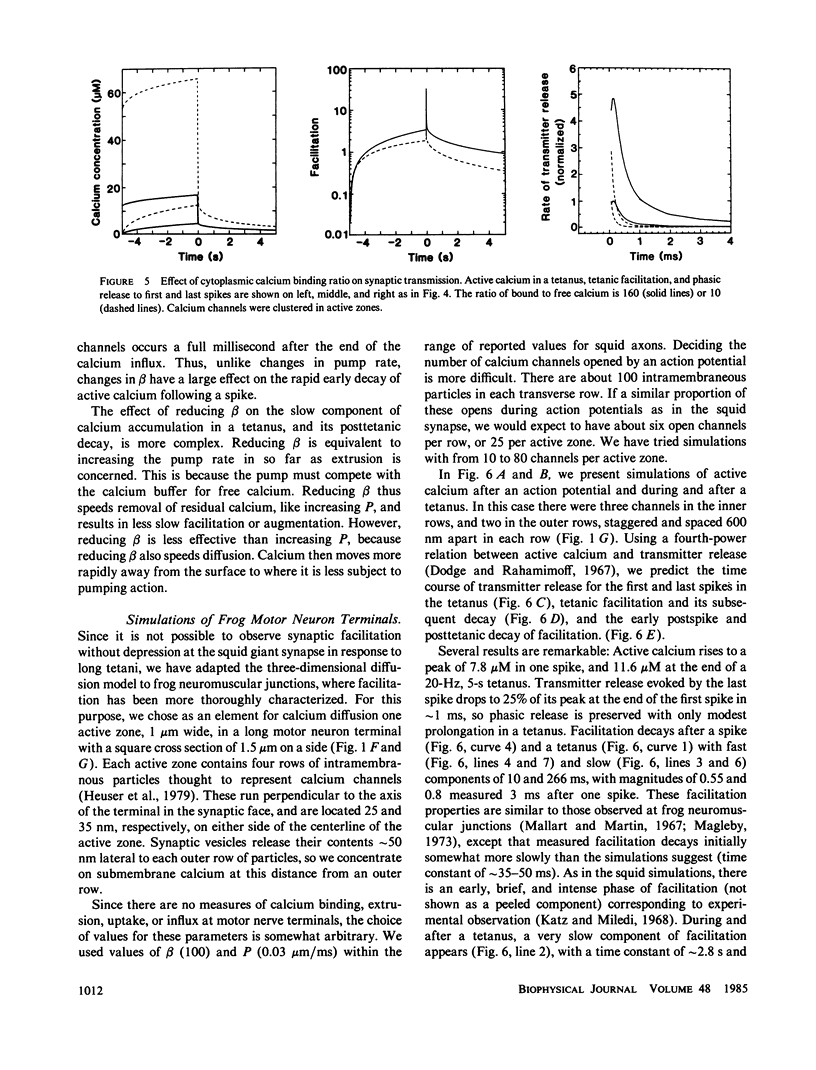
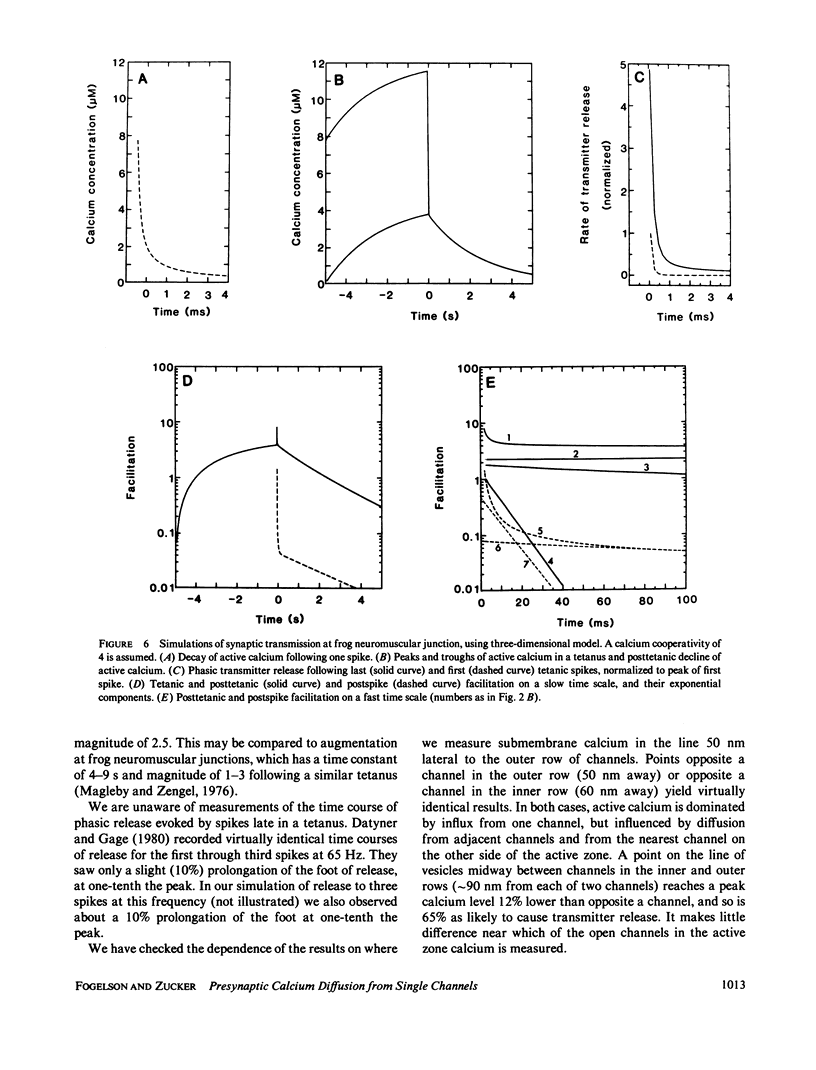
A one-dimensional model of presynaptic calcium diffusion away from the membrane, with cytoplasmic binding, extrusion by a surface pump, and influx during action potentials, can account for the rapid decay of phasic transmitter release and the slower decay of synaptic facilitation following one spike, as well as the very slow decline in total free calcium observed experimentally. However, simulations using this model, and alternative versions in which calcium uptake into organelles and saturable binding are included, fail to preserve phasic transmitter release to spikes in a long tetanus. A three-dimensional diffusion model was developed, in which calcium enters through discrete membrane channels and acts to release transmitter within 50 nm of entry points. Analytic solutions of the equations of this model, in which calcium channels were distributed in active zone patches based on ultrastructural observations, were successful in predicting synaptic facilitation, phasic release to tetanic spikes, and the accumulation of total free calcium. The effects of varying calcium buffering, pump rate, and channel number and distribution were explored. Versions appropriate to squid giant synapses and frog neuromuscular junctions were simulated. Limitations of key assumptions, particularly rapid nonsaturable binding, are discussed.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alemà S., Calissano P., Rusca G., Giuditta A. Identification of a calcium-binding, brain specific protein in the axoplasm of squid giant axons. J Neurochem. 1973 Mar;20(3):681–689. doi: 10.1111/j.1471-4159.1973.tb00028.x. [DOI] [PubMed] [Google Scholar]
- Baker P. F., Schlaepfer W. W. Uptake and binding of calcium by axoplasm isolated from giant axons of Loligo and Myxicola. J Physiol. 1978 Mar;276:103–125. doi: 10.1113/jphysiol.1978.sp012222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barish M. E., Thompson S. H. Calcium buffering and slow recovery kinetics of calcium-dependent outward current in molluscan neurones. J Physiol. 1983 Apr;337:201–219. doi: 10.1113/jphysiol.1983.sp014620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett E. F., Stevens C. F. The kinetics of transmitter release at the frog neuromuscular junction. J Physiol. 1972 Dec;227(3):691–708. doi: 10.1113/jphysiol.1972.sp010054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton S. B., Cohen I. S., van der Kloot W. The calcium dependence of spontaneous and evoked quantal release at the frog neuromuscular junction. J Physiol. 1983 Apr;337:735–751. doi: 10.1113/jphysiol.1983.sp014652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaustein M. P., Ratzlaff R. W., Schweitzer E. S. Calcium buffering in presynaptic nerve terminals. II. Kinetic properties of the nonmitochondrial Ca sequestration mechanism. J Gen Physiol. 1978 Jul;72(1):43–66. doi: 10.1085/jgp.72.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinley F. J., Jr Calcium buffering in squid axons. Annu Rev Biophys Bioeng. 1978;7:363–392. doi: 10.1146/annurev.bb.07.060178.002051. [DOI] [PubMed] [Google Scholar]
- Brinley F. J., Jr, Tiffert T., Scarpa A. Mitochondria and other calcium buffers of squid axon studied in situ. J Gen Physiol. 1978 Jul;72(1):101–127. doi: 10.1085/jgp.72.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chad J. E., Eckert R. Calcium domains associated with individual channels can account for anomalous voltage relations of CA-dependent responses. Biophys J. 1984 May;45(5):993–999. doi: 10.1016/S0006-3495(84)84244-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlton M. P., Atwood H. L. Synaptic transmission: temperature-sensitivity of calcium entry in presynaptic terminals. Brain Res. 1979 Jul 20;170(3):543–546. doi: 10.1016/0006-8993(79)90972-7. [DOI] [PubMed] [Google Scholar]
- Charlton M. P., Bittner G. D. Facilitation of transmitter release at squid synapses. J Gen Physiol. 1978 Oct;72(4):471–486. doi: 10.1085/jgp.72.4.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlton M. P., Smith S. J., Zucker R. S. Role of presynaptic calcium ions and channels in synaptic facilitation and depression at the squid giant synapse. J Physiol. 1982 Feb;323:173–193. doi: 10.1113/jphysiol.1982.sp014067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datyner N. B., Gage P. W. Phasic secretion of acetylcholine at a mammalian neuromuscular junction. J Physiol. 1980 Jun;303:299–314. doi: 10.1113/jphysiol.1980.sp013286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dipolo R., Requena J., Brinley F. J., Jr, Mullins L. J., Scarpa A., Tiffert T. Ionized calcium concentrations in squid axons. J Gen Physiol. 1976 Apr;67(4):433–467. doi: 10.1085/jgp.67.4.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodge F. A., Jr, Rahamimoff R. Co-operative action a calcium ions in transmitter release at the neuromuscular junction. J Physiol. 1967 Nov;193(2):419–432. doi: 10.1113/jphysiol.1967.sp008367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorogi P. L., Rabl C. R., Neumann E. Kinetic scheme for Ca2+-arsenazo III interactions. Biochem Biophys Res Commun. 1983 Mar 29;111(3):1027–1033. doi: 10.1016/0006-291x(83)91403-1. [DOI] [PubMed] [Google Scholar]
- Dudel J., Parnas I., Parnas H. Neurotransmitter release and its facilitation in crayfish muscle. VI. Release determined by both, intracellular calcium concentration and depolarization of the nerve terminal. Pflugers Arch. 1983 Sep;399(1):1–10. doi: 10.1007/BF00652515. [DOI] [PubMed] [Google Scholar]
- Heuser J. E., Reese T. S., Dennis M. J., Jan Y., Jan L., Evans L. Synaptic vesicle exocytosis captured by quick freezing and correlated with quantal transmitter release. J Cell Biol. 1979 May;81(2):275–300. doi: 10.1083/jcb.81.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. Further study of the role of calcium in synaptic transmission. J Physiol. 1970 May;207(3):789–801. doi: 10.1113/jphysiol.1970.sp009095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. The role of calcium in neuromuscular facilitation. J Physiol. 1968 Mar;195(2):481–492. doi: 10.1113/jphysiol.1968.sp008469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusano K., Landau E. M. Depression and recovery of transmission at the squid giant synapse. J Physiol. 1975 Feb;245(1):13–32. doi: 10.1113/jphysiol.1975.sp010832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lester H. A. Transmitter release by presynaptic impulses in the squid stellate ganglion. Nature. 1970 Aug 1;227(5257):493–496. doi: 10.1038/227493a0. [DOI] [PubMed] [Google Scholar]
- Llinás R., Steinberg I. Z., Walton K. Relationship between presynaptic calcium current and postsynaptic potential in squid giant synapse. Biophys J. 1981 Mar;33(3):323–351. doi: 10.1016/S0006-3495(81)84899-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R., Sugimori M., Simon S. M. Transmission by presynaptic spike-like depolarization in the squid giant synapse. Proc Natl Acad Sci U S A. 1982 Apr;79(7):2415–2419. doi: 10.1073/pnas.79.7.2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lux H. D., Brown A. M. Patch and whole cell calcium currents recorded simultaneously in snail neurons. J Gen Physiol. 1984 May;83(5):727–750. doi: 10.1085/jgp.83.5.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magleby K. L. The effect of repetitive stimulation on facilitation of transmitter release at the frog neuromuscular junction. J Physiol. 1973 Oct;234(2):327–352. doi: 10.1113/jphysiol.1973.sp010348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magleby K. L., Zengel J. E. A quantitative description of stimulation-induced changes in transmitter release at the frog neuromuscular junction. J Gen Physiol. 1982 Oct;80(4):613–638. doi: 10.1085/jgp.80.4.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magleby K. L., Zengel J. E. Augmentation: A process that acts to increase transmitter release at the frog neuromuscular junction. J Physiol. 1976 May;257(2):449–470. doi: 10.1113/jphysiol.1976.sp011378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallart A., Martin A. R. An analysis of facilitation of transmitter release at the neuromuscular junction of the frog. J Physiol. 1967 Dec;193(3):679–694. doi: 10.1113/jphysiol.1967.sp008388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore J. W., Ramón F., Joyner R. W. Axon voltage-clamp simulations. I. Methods and tests. Biophys J. 1975 Jan;15(1):11–24. doi: 10.1016/S0006-3495(75)85788-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa Y., Harafuji H., Kurebayashi N. Comparison of the characteristics of four metallochromic dyes as potential calcium indicators for biological experiments. J Biochem. 1980 May;87(5):1293–1303. doi: 10.1093/oxfordjournals.jbchem.a132867. [DOI] [PubMed] [Google Scholar]
- Parnas H., Dudel J., Parnas I. Neurotransmitter release and its facilitation in crayfish. I. Saturation kinetics of release, and of entry and removal of calcium. Pflugers Arch. 1982 Mar;393(1):1–14. doi: 10.1007/BF00582384. [DOI] [PubMed] [Google Scholar]
- Pumplin D. W., Reese T. S., Llinás R. Are the presynaptic membrane particles the calcium channels? Proc Natl Acad Sci U S A. 1981 Nov;78(11):7210–7213. doi: 10.1073/pnas.78.11.7210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pumplin D. W., Reese T. S. Membrane ultrastructure of the giant synapse of the squid Loligo pealei. Neuroscience. 1978;3(8):685–696. doi: 10.1016/0306-4522(78)90065-9. [DOI] [PubMed] [Google Scholar]
- Rahamimoff R., Lev-Tov A., Meiri H. Primary and secondary regulation of quantal transmitter release: calcium and sodium. J Exp Biol. 1980 Dec;89:5–18. doi: 10.1242/jeb.89.1.5. [DOI] [PubMed] [Google Scholar]
- Scarpa A., Brinley F. J., Jr, Dubyak G. Antipyrylazo III, a "middle range" Ca2+ metallochromic indicator. Biochemistry. 1978 Apr 18;17(8):1378–1386. doi: 10.1021/bi00601a004. [DOI] [PubMed] [Google Scholar]
- Smith S. J., Augustine G. J., Charlton M. P. Transmission at voltage-clamped giant synapse of the squid: evidence for cooperativity of presynaptic calcium action. Proc Natl Acad Sci U S A. 1985 Jan;82(2):622–625. doi: 10.1073/pnas.82.2.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockbridge N., Moore J. W. Dynamics of intracellular calcium and its possible relationship to phasic transmitter release and facilitation at the frog neuromuscular junction. J Neurosci. 1984 Mar;4(3):803–811. doi: 10.1523/JNEUROSCI.04-03-00803.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zengel J. E., Magleby K. L. Changes in miniature endplate potential frequency during repetitive nerve stimulation in the presence of Ca2+, Ba2+, and Sr2+ at the frog neuromuscular junction. J Gen Physiol. 1981 May;77(5):503–529. doi: 10.1085/jgp.77.5.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker R. S., Lara-Estrella L. O. Post-tetanic decay of evoked and spontaneous transmitter release and a residual-calcium model of synaptic facilitation at crayfish neuromuscular junctions. J Gen Physiol. 1983 Mar;81(3):355–372. doi: 10.1085/jgp.81.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker R. S., Stockbridge N. Presynaptic calcium diffusion and the time courses of transmitter release and synaptic facilitation at the squid giant synapse. J Neurosci. 1983 Jun;3(6):1263–1269. doi: 10.1523/JNEUROSCI.03-06-01263.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]


