Abstract
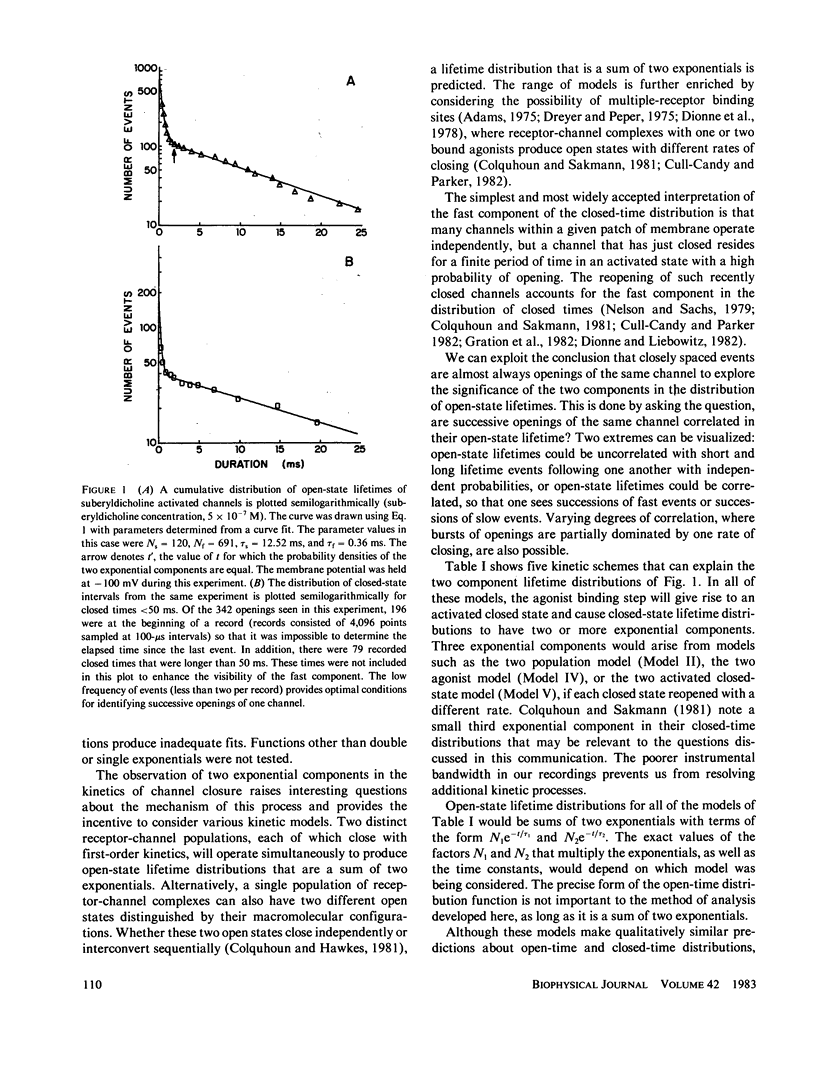
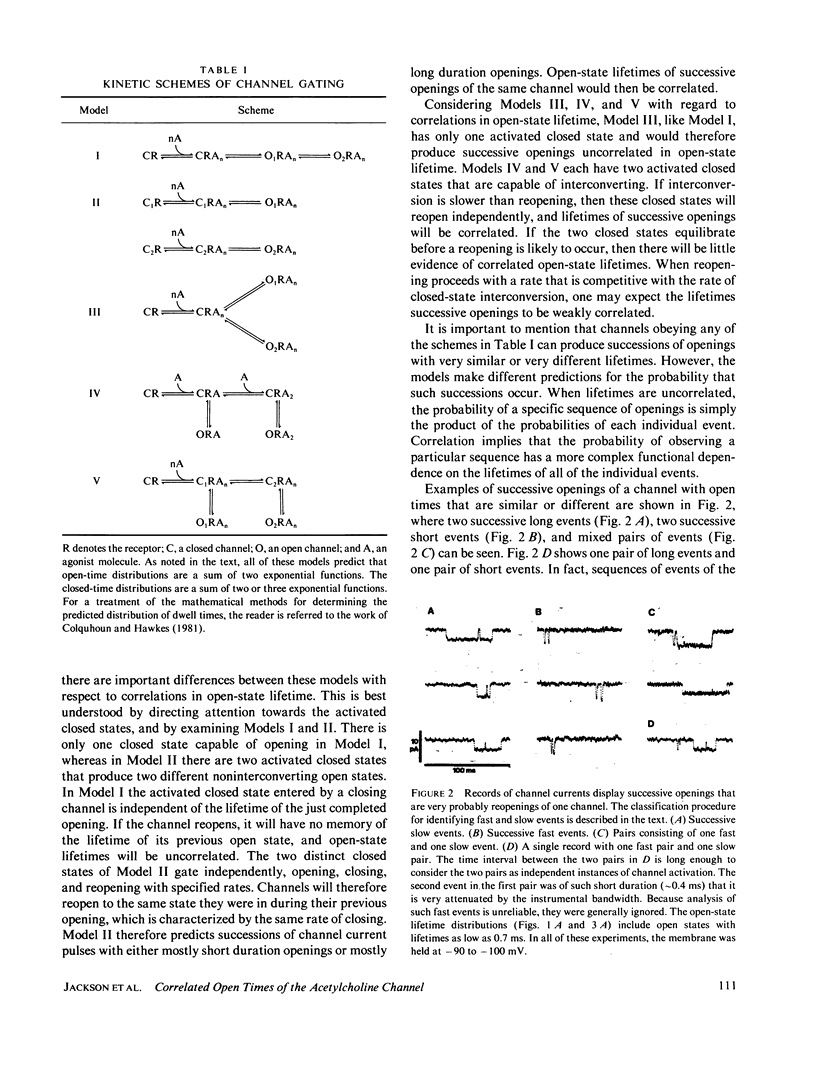
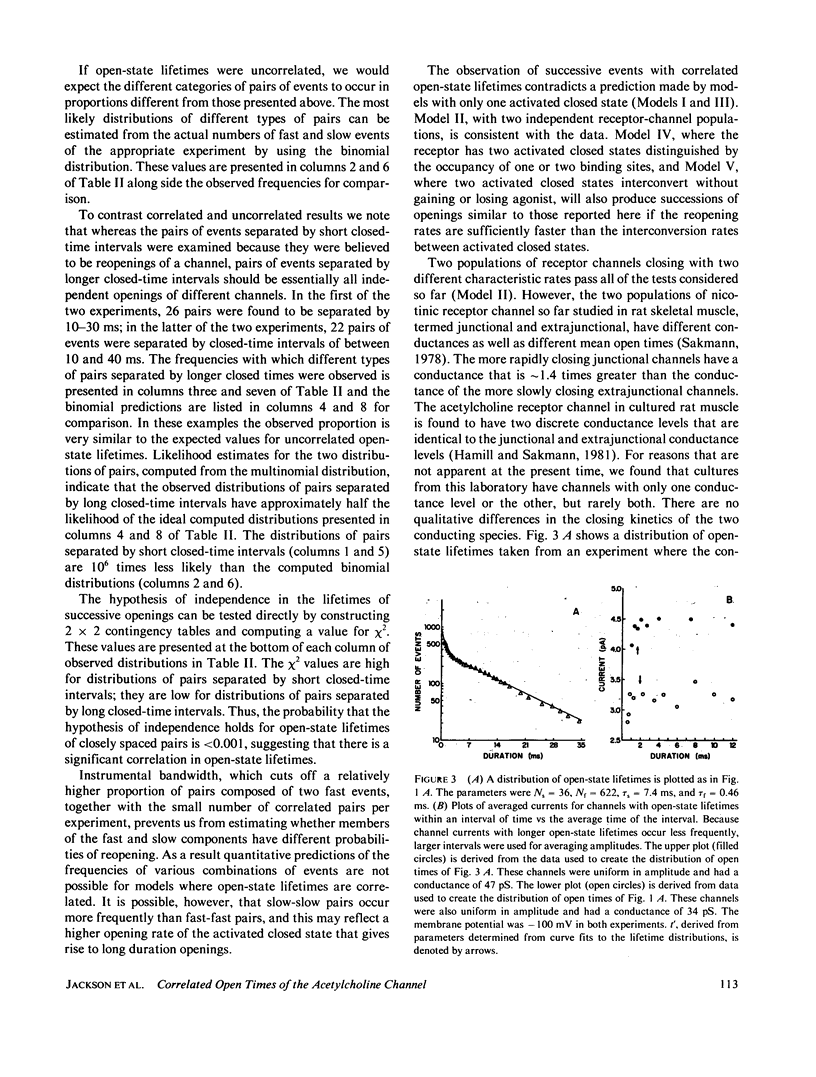
Previous analysis of single-channel current records has shown that both the opening and closing transitions of chemically activated ion channels are operated by fast and slow kinetic processes. The fast component in the kinetics of channel opening has been interpreted as the reopening of a channel that has just closed. The fast component in the kinetics of channel closure has many possible explanations and is therefore more difficult to interpret. We can gain insight into the closing process by asking whether the lifetimes of successive openings of an acetylcholine receptor channel are correlated in open-state lifetime. Five kinetic models of channel closure are considered. Two of these models predict uncorrelated open-state lifetimes, one predicts correlated open-state lifetimes, and for two others a range of behavior is possible. Acetylcholine receptor channel data from cultured rat muscle are analyzed to show that open-state lifetimes are correlated, eliminating two models of channel gating.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams P. R. An analysis of the dose-response curve at voltage-clamped frog-endplates. Pflugers Arch. 1975 Oct 28;360(2):145–153. doi: 10.1007/BF00580537. [DOI] [PubMed] [Google Scholar]
- Colquhoun D., Hawkes A. G. On the stochastic properties of single ion channels. Proc R Soc Lond B Biol Sci. 1981 Mar 6;211(1183):205–235. doi: 10.1098/rspb.1981.0003. [DOI] [PubMed] [Google Scholar]
- Colquhoun D., Sakmann B. Fluctuations in the microsecond time range of the current through single acetylcholine receptor ion channels. Nature. 1981 Dec 3;294(5840):464–466. doi: 10.1038/294464a0. [DOI] [PubMed] [Google Scholar]
- Cull-Candy S. G., Parker I. Rapid kinetics of single glutamate-receptor channels. Nature. 1982 Feb 4;295(5848):410–412. doi: 10.1038/295410a0. [DOI] [PubMed] [Google Scholar]
- Dionne V. E., Leibowitz M. D. Acetylcholine receptor kinetics. A description from single-channel currents at snake neuromuscular junctions. Biophys J. 1982 Sep;39(3):253–261. doi: 10.1016/S0006-3495(82)84515-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dionne V. E., Steinbach J. H., Stevens C. F. An analysis of the dose-response relationship at voltage-clamped frog neuromuscular junctions. J Physiol. 1978 Aug;281:421–444. doi: 10.1113/jphysiol.1978.sp012431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyer F., Peper K. Density and dose-response curve of acetylcholine receptors in frog neuromuscular junction. Nature. 1975 Feb 20;253(5493):641–643. doi: 10.1038/253641a0. [DOI] [PubMed] [Google Scholar]
- Gration K. A., Lambert J. J., Ramsey R. L., Rand R. P., Usherwood P. N. Closure of membrane channels gated by glutamate receptors may be a two-step process. Nature. 1982 Feb 18;295(5850):599–603. doi: 10.1038/295599a0. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Sakmann B. Multiple conductance states of single acetylcholine receptor channels in embryonic muscle cells. Nature. 1981 Dec 3;294(5840):462–464. doi: 10.1038/294462a0. [DOI] [PubMed] [Google Scholar]
- Jackson M. B., Lecar H., Askanas V., Engel W. K. Single cholinergic receptor channel currents in cultured human muscle. J Neurosci. 1982 Oct;2(10):1465–1473. doi: 10.1523/JNEUROSCI.02-10-01465.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson M. B., Lecar H., Mathers D. A., Barker J. L. Single channel currents activated by gamma-aminobutyric acid, muscimol, and (-)-pentobarbital in cultured mouse spinal neurons. J Neurosci. 1982 Jul;2(7):889–894. doi: 10.1523/JNEUROSCI.02-07-00889.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson M. B., Lecar H. Single postsynaptic channel currents in tissue cultured muscle. Nature. 1979 Dec 20;282(5741):863–864. doi: 10.1038/282863a0. [DOI] [PubMed] [Google Scholar]
- Neher E., Sakmann B. Single-channel currents recorded from membrane of denervated frog muscle fibres. Nature. 1976 Apr 29;260(5554):799–802. doi: 10.1038/260799a0. [DOI] [PubMed] [Google Scholar]
- Nelson D. J., Sachs F. Single ionic channels observed in tissue-cultured muscle. Nature. 1979 Dec 20;282(5741):861–863. doi: 10.1038/282861a0. [DOI] [PubMed] [Google Scholar]
- Nelson P., Christian C., Nirenberg M. Synapse formation between clonal neuroblastoma X glioma hybrid cells and striated muscle cells. Proc Natl Acad Sci U S A. 1976 Jan;73(1):123–127. doi: 10.1073/pnas.73.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakmann B. Acetylcholine-induced ionic channels in rat skeletal muscle. Fed Proc. 1978 Oct;37(12):2654–2659. [PubMed] [Google Scholar]


