Abstract
The transcription factor NF-κB is critical for the induction of cancer, including adult T-cell leukemia, which is linked to infection by human T-cell leukemia virus type 1 and the expression of its regulatory protein Tax. Although activation of the NF-κB pathway by Tax involves its interaction with the regulatory subunit of the IκB kinase (IKK) complex, NEMO/IKKγ, the mechanism by which Tax activates specific cellular genes in the nucleus remains unknown. Here, we demonstrate that the attachment of SUMO-1 to Tax regulates its localization in nuclear bodies and the recruitment of both the RelA subunit of NF-κB and free IKKγ in these nuclear structures. However, this sumoylation step is not sufficient for the activation of the NF-κB pathway by Tax. This activity requires the prior ubiquitination and colocalization of ubiquitinated Tax with IKK complexes in the cytoplasm and the subsequent migration of the RelA subunit of NF-κB to the nucleus. Thus, the ubiquitination and sumoylation of Tax function in concert to result in the migration of RelA to the nucleus and its accumulation with IKKγ in nuclear bodies for activation of gene expression. These modifications may result in targets for the treatment of adult T-cell leukemia.
The human T-cell leukemia virus type 1 (HTLV-1) is the etiological agent of adult T-cell leukemia and a virus-associated neurodegenerative disease, HTLV-1-associated myelopathy/tropical spastic paraparesis (31, 36, 47). These two diseases have been linked to the expression of the HTLV-1 regulatory protein Tax (10, 13, 29, 41). Tax is a potent transcriptional activator of viral genes as well as specific cellular genes and has a clear oncogenic potential since Tax is able to transform T lymphocytes and fibroblasts and induces tumors in transgenic mice (19). A substantial part of the oncogenic properties of Tax is associated with its ability to activate the expression of cellular genes that control T-cell proliferation and differentiation by inducing constitutive activation of the NF-κB pathway (2, 38).
In nonstimulated cells, inactive NF-κB complexes composed of p50 and RelA heterodimers are retained in the cytoplasm by NF-κB inhibitors of the IκB family (15, 23, 40). Tax activation of the NF-κB pathway involves its interaction with the regulatory subunit of the IκB kinase (IKK) complex, NEMO/IKKγ, leading to the activation of the two catalytic subunits, IKKα and IKKβ (8, 14). Activation of the IκB kinase complex by Tax determines the phosphorylation of the IκB proteins, leading to their ubiquitination and degradation by the proteasome and to the migration of the NF-κB complexes to the nucleus (18, 45, 46). It also determines the phosphorylation of the RelA subunit of NF-κB, a prerequisite for activation of RelA and p50 heterodimers in the nucleus (30). However, Tax also colocalizes in discrete nuclear bodies (NB) with the two subunits of NF-κB, p50 and RelA, in addition to RNA polymerase II, and the assembly of these nuclear structures correlates with Tax transcriptional activity (4-6, 37). Thus, Tax-mediated activation of gene expression may require the presence of Tax both in the cytoplasm and in the nucleus (3).
Ubiquitin (Ub) and the small ubiquitin-like modifier (SUMO) are polypeptides which are attached to the lysine residues of a number of proteins via isopeptidic bonds, leading to the subsequent formation of poly-ubiquitinated or poly-sumoylated branched molecules. Ubiquitination and sumoylation often compete for the same lysine residues and have opposite effects in regulating the function of a variety of transcription factors by altering their intracellular targeting, their interaction with specific partners, and/or their stability (9, 22, 24, 27). Although poly-ubiquitinated proteins are often targeted to the proteasome, SUMO modification results in the targeting to subnuclear domains called nuclear bodies, as was first observed for the promyelocytic leukemia protein (PML) (27, 48). Activation of the NF-κB pathway following genotoxic stress requires the sequential sumoylation and nuclear targeting of IKKγ, followed by its ubiquitination, permitting IKK activation in the cytoplasm (16).
Tax is ubiquitinated on carboxy-terminal lysine residues, and this modification is critical for Tax interaction with the proteasome (7, 34). In this work, we demonstrate that Tax is sumoylated and ubiquitinated on overlapping lysine residues. These two modifications determine the partitioning of Tax in the nuclear and cytoplasmic compartments. Colocalized Tax and ubiquitin molecules were detected in the cytoplasm only, in association with IKK complexes, and this modification plays a critical role in the translocation of the RelA subunit of NF-κB to the nucleus. However, the sumoylation of Tax and the subsequent formation of the Tax nuclear bodies that contained both RelA and IKKγ must also occur for Tax-mediated activation of gene expression via the NF-κB pathway. These results demonstrate that both the sumoylation and the ubiquitination of Tax are critical for Tax-mediated transcriptional activity.
MATERIALS AND METHODS
Plasmid constructs.
The pSG5-Tax plasmid was described previously (7), and the arginine-for-lysine substitution mutants were kindly provided by C. Pique. The sequences coding for these Tax mutants were subcloned in pSG5-Tax, followed by sequence coding for the six-histidine tag (Tax-6His). The constructs of vectors for the expression of wild-type Tax (WT) or mutant Tax-6His fusions with ubiquitin or SUMO-1 were obtained by PCR amplification of the ubiquitin or SUMO-1 coding sequences and the cloning of the resulting fragments between the Tax coding sequence and the 6His tag. In these constructs, the di-glycine motif involved in the conjugation reaction at the end of the ubiquitin and SUMO-1 sequences were replaced by Gly-Ala during the course of PCR amplification to prevent conjugation of the fusions to other substrates. The vectors for the expression of ubiquitin (7) or SUMO-1, SUMO-2, and SUMO-3 (42) fused at their amino termini to the influenza virus hemagglutinin epitope (HA-Ub, HA-SUMO-1, HA-SUMO-2, and HA-SUMO-3, respectively) and the vectors for the expression of IKKα or IKKβ fused at their amino termini to the Flag epitope, Flag-IKKα and Flag-IKKβ (25), have previously been described and were kindly provided by R. Hay and S. Ghosh. Human immunodeficiency virus type 1 (HIV-1) or HTLV1 long terminal repeat (LTR)-luciferase reporter plasmids were constructed by the cloning of a KpnI-HindIII fragment containing the HIV-1 promoter or a SmaI-KpnI fragment containing the HTLV-1 promoter upstream of the luciferase gene in the pGL2 vector (Promega).
Antibodies.
Antibodies directed against HA (Y-11), ubiquitin (P4D1), SUMO-1 (FL-101), RelA (C-20), IKKγ (FL-419), and PML (PG-M3) were purchased from Santa Cruz Biotechnology. Anti-Flag (M2) was from Sigma, the anti-HA monoclonal antibody (MAb) was from Cell Signaling Technology, and the anti-GM130 MAb was from BD Transduction Laboratories. Tax proteins were revealed with the anti-Tax MAb from hybridoma 168-A51 cells (AIDS Research and Reagent Program, National Institutes of Health) or a polyclonal serum obtained by immunizing rabbits with a purified maltose binding protein-Tax fusion produced in bacteria.
Cell culture and transient-expression experiments.
293T cells were maintained in Dulbecco's modified Eagle's medium supplemented with 2 mM l-glutamine, 10% fetal calf serum, 1% penicillin-streptomycin, and 1 mM sodium pyruvate (Gibco). HTLV-1-infected (HUT102) or noninfected (Jurkat) T lymphocytes were maintained in RPMI 1640 medium with Glutamax-I (Gibco) supplemented with 10% fetal calf serum. 293T cells were transfected using the calcium phosphate precipitation procedure or using FuGENE 6 reagent (Roche) according to the manufacturer's instructions. The CEM T-lymphocyte cell line (2 × 107 cells) was transfected by electroporation with a Gene Pulser electroporator (Bio-Rad Laboratories).
Ni-NTA pulldown assays.
293T and CEM T cells were lysed 30 h and 48 h, respectively, after transfection, and Ni-nitrilotriacetic acid (NTA) pulldown assays were done as described before (7). Briefly, cell pellets were lysed under reducing and highly denaturing conditions using buffer A (6 M guanidinium-HCl, 0.1 M Na2HPO4-NaH2PO4, 0.01 M Tris-Cl, pH 8.0, 5 mM imidazole, 10 mM β-mercaptoethanol) and incubated with Ni2+-NTA beads for 4 h at 4°C. The beads were washed with buffers A, B (8 M urea, 0.1 M Na2HPO4-NaH2PO4, 0.01 M Tris-Cl, pH 8.0, 10 mM imidazole, 10 mM β-mercaptoethanol), and C (8 M urea, 0.1 M Na2HPO4-NaH2PO4, 0.01 M Tris-Cl, pH 6.3, 10 mM imidazole, 10 mM β-mercaptoethanol), and the bound proteins were eluted with buffer D (300 mM imidazole, 0.15 M Tris-Cl, pH 6.7, 30% glycerol, 0.72 M β-mercaptoethanol, 5% sodium dodecyl sulfate). All solutions contained a cocktail of inhibitors, including 50 mM NaF, 20 mM β-glycerophosphate, 1 mM orthovanadate, 50 mM N-ethylmaleimide, and the protease inhibitor cocktail (Roche). To detect modified forms of endogenous Tax in HTLV-1-infected T lymphocytes, cells were lysed for 20 min in buffer A. The lysates were precipitated for 10 min on ice in 20% trichloroacetic acid. The precipitates were washed with acetone, dried, and solved in phosphate-buffered saline (PBS). The purified proteins were submitted to electrophoresis on 4 to 12% bis-Tris NuPAGE gel (Invitrogen), transferred to a Hybond enhanced-chemiluminescence nitrocellulose membrane (Amersham Pharmacia Biotech), and immunoblotted with primary antibodies and corresponding secondary antibodies. The detection was performed using Lumi-Light Western blotting substrate (Roche). Detection and quantitation of chemiluminescent signals were performed with the Chemi-Smart 5000 apparatus and Bio-1D software (Vilber Lourmat, France).
Cell fractionation.
293T cells expressing Tax were washed in Tp I (20 mM HEPES buffer, pH 7.9, 2 mM MgCl2, 1 mM dithiothreitol) containing inhibitors as described above and then lysed on ice for 10 min in the same buffer containing 0.2% NP-40. The samples were centrifuged to collect the cytoplasmic fraction. The whole process was repeated a second time on the pellet to complete the extraction of cytoplasmic components from the nuclear pellet. The final pellet containing the nuclear fraction was lysed in buffer Tp II (Tp I supplemented with 420 mM KCl, 25% glycerol, and 1 mM EDTA) on ice for 20 min and centrifuged for 10 min to collect the nuclear fraction. Finally, each cytoplasmic or nuclear fraction was submitted to a Ni-NTA pulldown assay as described above.
Luciferase assays.
Tax-mediated transactivation of the HTLV-1 and HIV-1 promoters via the activating transcription factor (ATF)/CREB and NF-κB pathways, respectively, was assayed by dual-luciferase assays. 293T cells (1.25 × 105 cells) were transfected into 12-well plates with 50 ng of phRG-TK (Promega), which was used for monitoring transfection efficiency, 500 ng of HTLV-LTR or HIV-1-LTR-luciferase reporter plasmids, and either 50 ng or increasing amounts (100, 200, and 400 ng) of the constructs coding for wild-type or mutant Tax as indicated in the figure legends. Total amounts of DNA were equalized by adding the empty vector into the transfection mixture. Cells were lysed and subjected to luciferase assay 30 h after transfection with a TD-20/20 luminometer (Turner Designs) using the dual-luciferase reporter assay system (Promega). The results represent averages and standard deviations for at least six independent experiments.
Immunocytochemistry and confocal microscopy.
Cells cultured on glass coverslips were transfected with 1 μg of expression plasmids for 28 h and fixed with Immunohistofix (A Phase Inc., Belgium), a zinc fixative free of aldehyde, to stabilize cellular structures for 10 min at room temperature (33), followed by incubation in 100% methanol at −20°C for 6 min. The cells were washed with PBS, blocked in PBS containing 0.5% gelatin (Bio-Rad) and 0.25% bovine serum albumin (Gibco), and incubated with the primary antibodies diluted in the blocking solution. The preparations were then washed with PBS containing 0.2% gelatin and incubated with the secondary antibodies, goat anti-mouse immunoglobulin G (IgG) conjugated to Alexa Fluor 488, goat anti-rabbit IgG conjugated to Alexa Fluor 546 (Molecular Probes), goat anti-mouse IgG2a conjugated to fluorescein isothiocyanate, or goat anti-mouse IgG1 coupled to biotin (Southern Biotechnology Associates, Ala.) for dual- and triple-immunofluorescence staining. Samples were washed and, when biotinylated goat anti-mouse IgG1 was used as the secondary antibody, cells were incubated for 20 min with Cy5-conjugated streptavidin (Jackson ImmunoResearch). Controls to test for secondary-antibody cross-reaction demonstrated the desired specificity of the fluorescent reagents. Samples were then mounted in DABCO-based medium (ICN Biomedicals) and analyzed with a laser scanning confocal microscope (LSM 510; Zeiss) using a 63× objective and light source wavelengths of 488, 543, and 633 nm.
RESULTS
Differential intracellular partitioning of colocalized Tax and SUMO or Tax and ubiquitin molecules.
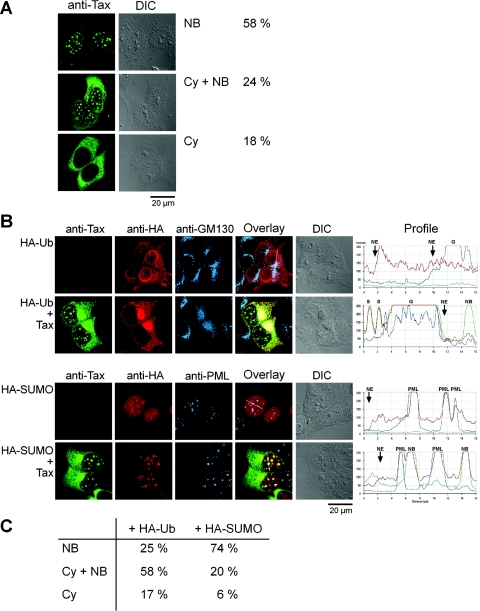
We previously demonstrated that Tax localizes in prominent nuclear bodies that include transcription factors, among which are the two subunits of NF-κB, p50 and RelA (4), and that Tax was modified by ubiquitination (7). In this work, we analyzed by dual-immunofluorescence staining what compartment of the cells contained colocalized Tax and ubiquitin molecules. In addition, since SUMO modification of proteins correlates with their partitioning in subnuclear domains, we also studied the relative intracellular localizations of Tax and SUMO. First, 293T cells expressing Tax were analyzed by immunofluorescence staining with a monoclonal antibody directed against Tax and confocal microscopy (Fig. 1A). Examination of 200 Tax-expressing cells indicated that 82% of these cells exhibited Tax-containing nuclear bodies, including 58% of the Tax-expressing cells containing NB only and 24% of the cells with an additional cytoplasmic staining. The remaining 18% displayed a predominant cytoplasmic distribution of Tax without the formation of Tax-containing nuclear bodies. The boundaries of the nuclei are clearly pictured on the differential interference contrast image in Fig. 1A.
FIG. 1.
Differential intracellular partitioning of colocalized Tax and SUMO or Tax and ubiquitin molecules. 293T cells were transfected with vectors expressing (A) Tax alone or (B) Tax in combination with HA-Ub or HA-SUMO-1 and stained by immunofluorescence with (A) an anti-Tax MAb or (B) the anti-Tax MAb (IgG2a), an anti-HA rabbit polyclonal antibody, and a MAb (IgG1) recognizing either the Golgi matrix marker GM130 (upper panels) or the promyelocytic leukemia protein (lower panels). The cells were analyzed by laser scanning confocal microscopy, with one confocal section being depicted in panel A and the projection of Z-series confocal images depicted in panel B. Diagrams depict the intensity of the fluorescence for each staining along lines drawn on the overlay images with the boundaries of the nuclei (NE, nuclear envelope), the Tax NB, the Golgi apparatus (G), and the PML bodies (PML) indicated. The percentages of cells having Tax in nuclear bodies only (NB), in the cytoplasm and in the nuclear bodies (Cy + NB), or in the cytoplasm only (Cy) with or without overexpression of HA-ubiquitin or HA-SUMO-1 are depicted in panels A and C. DIC, differential inference contrast.
To study the relative intracellular localizations of Tax and ubiquitin or SUMO, 293T cells were cotransfected with vectors expressing HA-tagged ubiquitin or SUMO-1 (HA-Ub and HA-SUMO) in combination with the Tax expression vector. The cells were analyzed by immunofluorescence staining by using anti-Tax and anti-HA antibodies (Fig. 1B). Since cytoplasmic Tax was recently shown to be associated with the Golgi apparatus in HTLV-1-infected T lymphocytes (28) and since SUMO is a component of the PML bodies, a monoclonal antibody directed against GM130, a marker of the Golgi matrix, or an anti-PML monoclonal antibody was included in the triple-immunofluorescence staining depicted in Fig. 1B. In cells transfected with the HA-Ub expression vector alone, ubiquitin was localized both in the nucleus and in the cytoplasm, with an intense staining of the nuclear periphery. No increased concentration of ubiquitin in the Golgi apparatus was observed in these cells, as indicated by the intensity of the immunofluorescence staining for both ubiquitin (red) and GM130 (blue) along a line drawn across this structure. Coexpression of Tax with ubiquitin led to the colocalization of both proteins only in cells displaying cytoplasmic Tax, and colocalized Tax and exogenous ubiquitin were concentrated in two distinct cytoplasmic structures, a cytoplasmic speckle and a prominent cytoplasmic inclusion that appeared as a result of ubiquitin overexpression in 40% of the Tax-expressing cells. These cytoplasmic inclusions were closely connected to the nuclear envelope and included GM130, which indicated that this Tax- and ubiquitin-containing structure was associated with the Golgi apparatus. Vimentin did not colocalize with the Tax-containing cytoplasmic inclusions, indicating that these structures were not aggresomes assembled as a result of Tax overexpression (data not shown). By contrast, no ubiquitin staining was detected in the whole volume of the nuclei, including the nuclear bodies. Thus, colocalized Tax and ubiquitin molecules were detected only in the cytoplasm. Expression of Tax resulted in the redistribution of ubiquitin molecules in the cells, emptying the nuclear pool and concentrating ubiquitin in two distinguishable Tax-containing cytoplasmic structures.
Overexpressed SUMO-1 was detected exclusively in the nucleus as a diffuse nucleoplasmic staining with superimposed discrete bodies, which were PML bodies as demonstrated by the colocalized HA (SUMO) and PML staining. Coexpression of Tax and SUMO-1 led to the colocalization of Tax with exogenously expressed SUMO-1 in nuclear bodies but not in the cytoplasm, even in cells displaying intense cytoplasmic Tax staining. The formation of the Tax- and SUMO-1-containing nuclear bodies did not alter the localization of SUMO-1 in the PML bodies. There was no colocalization of the Tax and PML staining, but the majority of PML bodies were in close spatial proximity to Tax bodies, forming complex nuclear structures in which the Tax bodies were surrounded by one or more PML bodies. We concluded that colocalized Tax and ubiquitin molecules were detected only in the cytoplasm and that Tax colocalized with SUMO-1 only in the nuclear bodies.
We then tested whether overexpression of ubiquitin or SUMO altered the fraction of cells containing Tax in the cytoplasm, in the nuclear bodies, or both. The percentages of cells in each population were estimated by counting at least 200 Tax-expressing cells in the experiment depicted in Fig. 1B. The results presented in Fig. 1C are compared with those obtained in cells expressing only Tax in Fig. 1A. In cells overexpressing Tax and ubiquitin, the fraction of cells that had Tax both in the cytoplasm and in the nuclear bodies increased by a factor of 2. Inversely, the fraction of cells that had Tax in nuclear bodies decreased only by a factor of 2. In addition, the fraction of cells that displayed a concentration of Tax in cytoplasmic inclusions associated with the Golgi apparatus increased from 5% to 40%. In contrast, overexpression of SUMO-1 increased the fraction of cells with Tax in nuclear bodies only, whereas the fraction of cells having Tax in the cytoplasm was reduced. These results indicated that overexpression of ubiquitin favored the localization of Tax in the cytoplasm and the formation of the Tax-containing cytoplasmic inclusions associated with the Golgi apparatus and, inversely, that overexpression of SUMO-1 favored the localization of Tax in the nuclear bodies.
Tax is modified by conjugation of overlapping lysine residues to SUMO and ubiquitin.
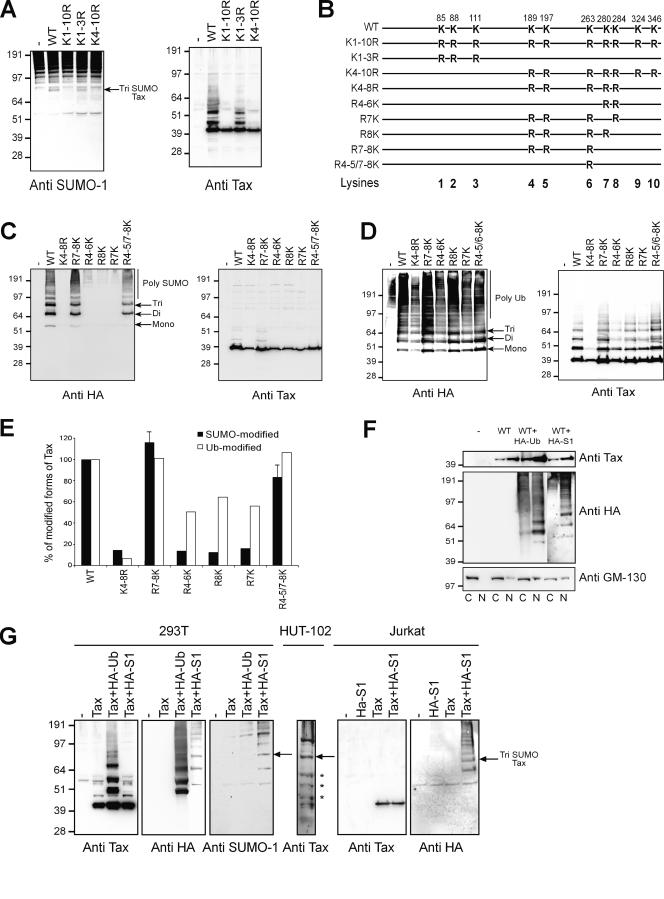
Since Tax colocalized with SUMO in nuclear bodies, we wondered whether Tax was conjugated to SUMO. To answer this question, 293T cells were transfected with a vector expressing Tax-6His. Following transfection, cells were lysed under highly denaturing conditions to inhibit the actions of desumoylating enzymes. The proteins were subjected to Ni-NTA pulldown assay, and the purified proteins were analyzed by immunoblotting with antibodies directed against SUMO-1 or Tax (Fig. 2A). Expression of Tax caused the appearance of an 80-kDa species, which was recognized by the anti-SUMO-1 antibody. This species was not detected in cells that did not express Tax or in cells expressing various Tax mutants (see below). It was faintly detected on the anti-Tax immunoblot among a number of other retarded Tax species previously attributed to ubiquitinated Tax molecules (7).
FIG. 2.
Tax is modified by conjugation to SUMO and ubiquitin. (A) 293T cells were transfected with vectors expressing wild-type or mutant Tax-6His. The cells extracts were purified by Ni-NTA pulldown assay followed by Western blotting with anti-SUMO-1 or anti-Tax rabbit polyclonal antibodies. (B) Schematic representation of the Tax proteins with the positions of the lysines replaced in the different Tax mutants used in this work. (C and D) 293T cells were cotransfected with vectors expressing wild-type or mutant Tax-6His and either (C) HA-SUMO-1 or (D) HA-Ub. Proteins were revealed with anti-HA or anti-Tax rabbit polyclonal antibodies. (E) Quantitation was performed on the anti-HA immunoblots in panels C and D after subtraction of the background in lanes 1 and normalization to an equal amount of the unmodified 40-kDa Tax species on the anti-Tax immunoblots. The values representing the percentages of modified forms of Tax mutants relative to wild-type Tax are reported in the diagram for both SUMO-modified (black bars) and ubiquitin-modified (white bars) Tax species. Error bars for quantitation of the SUMO-modified forms of the Tax mutants R7-8K and R4-5/7-8K represent the standard deviation in three independent experiments. (F) 293T cells either not transfected or transfected with a vector expressing Tax in combination with vectors expressing either HA-Ub or HA-SUMO-1 (HA-S1) were fractionated, and the cytoplasmic (C) and nuclear (N) fractions were immunoblotted with anti-Tax, anti-HA, or anti-GM130 antibodies. (G) HUT102 HTLV-1-transformed T lymphocytes were lysed under highly denaturing conditions and immunoblotted with anti-Tax rabbit polyclonal serum in parallel with extracts from 293T cells or Jurkat T lymphocytes transfected with a vector expressing wild-type Tax-6His with or without vectors for the expression of HA-Ub or HA-SUMO-1 that were revealed with anti-Tax, anti-HA, or anti-SUMO-1 antibodies. The position of the tri-sumoylated Tax species is indicated as well as the mono-, di-, and tri-ubiquitinated forms (*). Molecular mass markers (in kilodaltons) are noted at the left of the blots.
To determine which lysine residue was involved in the conjugation of Tax to SUMO, leading to the appearance of the 80-kDa modified species, vectors expressing Tax-6His mutant proteins that had one or several of the 10 lysine residues replaced by arginines (7) (Fig. 2B) were examined by a Ni-NTA pulldown assay as described above (Fig. 2A). The Tax-6His mutant that had the three amino-terminal lysines replaced by arginines (K1-3R) still produced the 80-kDa species, whereas this species was absent in cells expressing mutants with substitutions of all the 10 lysines (K1-10R) or the 7 carboxy-terminal lysines (K4-10R). These Tax mutants were expressed at rather similar levels, as indicated by the intensity of the 40-kDa unmodified form of Tax present on the anti-Tax immunoblot. Thus, expression of Tax led to the detection of an 80-kDa SUMO-modified form of Tax which may correspond to endogenously tri-sumoylated Tax, according to the molecular weight increase attributed to one conjugated SUMO molecule. In addition, substitution of the seven carboxy-terminal lysines resulted in the disappearance of this sumoylated species.
To confirm and extend these observations, 293T cells were cotransfected with vectors expressing wild-type Tax-6His or a mutant that had all five central lysines from K4 to K8 (K4-8R Tax-6His) replaced by arginines (Fig. 2B) in combination with the vector for the expression of HA-SUMO-1. The proteins purified by Ni-NTA pulldown assay were analyzed by immunoblotting with anti-HA or anti-Tax antibodies (Fig. 2C). No reacting products were found in the absence of Tax, showing the high specificity of the Ni-NTA pulldown assay. Coexpression of wild-type Tax and HA-SUMO-1 led to the appearance of three prominent HA-containing species of 53, 66, and 80 kDa, with molecular masses compatible for mono-, di-, and tri-sumoylated products, as well as a ladder of poly-sumoylated slower-migrating forms. Interestingly, these sumoylated forms of Tax were not observed in mutant K4-8R-expressing cells. Band quantitation obtained by normalizing the sumoylated Tax products on the anti-HA immunoblot to an equal amount of unmodified 40-kDa Tax on the anti-Tax immunoblot (Fig. 2E) indicated that this mutant maintained only 14% of wild-type Tax sumoylation. These results indicated that the SUMO-modified species detected with the anti-HA antibody must be attributed to sumoylated Tax forms even though our rabbit polyclonal serum was unable to reveal these forms, presumably due to epitope masking.
Then, a new series of Tax mutants was constructed by reintroducing individual or multiple lysine residues in the context of mutant K4-8R (Fig. 2B). Reintroduction of single lysine K7 (R7K) or K8 (R8K) or all three lysines from K4 to K6 (R4-6K) did not increase the amount of sumoylated Tax products (Fig. 2C and E). By contrast, reintroduction of the two lysine residues K7 and K8 (R7-8K) increased the amount of sumoylated Tax products to 117% of the amount found in wild-type Tax-expressing cells. Reintroduction of lysines K4 and K5 in addition to K7 and K8 (R4-5/7-8K) restored sumoylation to 82%, suggesting that lysine residues K4 and K5 might exert an inhibitory effect on Tax sumoylation. Similar results were obtained when wild-type or mutant Tax proteins were coexpressed with either HA-SUMO-2 or HA-SUMO-3, the two other members of the SUMO family (data not shown). We concluded that the integrity of both lysine residues K7 and K8 was critical for Tax sumoylation and that restoration of individual lysine K7 or K8 resulted in defective Tax conjugation to SUMO.
Since the seven carboxy-terminal lysines of Tax are also implicated in the conjugation of Tax to ubiquitin (7), we analyzed the ability of the K4-8R mutant and the lysine restoration mutants for their ability to be ubiquitinated. Vectors for expression of these mutants were cotransfected into 293T cells with the HA-Ub expression vector, and proteins recovered by Ni-NTA pulldown assay were immunoblotted for the detection of HA or Tax (Fig. 2D). High-molecular-weight Tax products recognized by the anti-HA antibody were detected in wild-type Tax-expressing cells as previously demonstrated (7). These ubiquitin-conjugated Tax species were highly reduced in cells expressing mutant K4-8R (6% of wild-type Tax ubiquitination) (Fig. 2E). The wild-type level of ubiquitination was recovered by reintroduction of both lysines K7 and K8 (R7-8K) with or without the additional lysines K4 and K5 (R4-5/7-8K). Restoration of individual lysine K7 (R7K) or K8 (R8K) increased the levels of Tax ubiquitination to 56% and 65%, respectively, whereas the lysine restoration mutant R4-6K displayed 50% of wild-type Tax ubiquitination. These results indicated that lysine residues K7 and K8 were involved in Tax conjugation to both SUMO and ubiquitin. However, contrary to what was observed for SUMO conjugation, each of the K7 or K8 lysines could be individually ubiquitinated. In addition, lysine residues K4, K5, and/or K6 contributed to a significant extent to Tax ubiquitination but not to Tax sumoylation.
We then used cell fractionation to analyze the intracellular localization of ubiquitinated and sumoylated Tax molecules (Fig. 2F). Cytoplasmic and nuclear fractions from 293T cells expressing Tax alone or in combination with HA-Ub or HA-SUMO-1 were first analyzed by immunoblotting for the detection of Tax. The result indicated that Tax was predominantly localized in the nuclear fractions even in cells that overexpressed either ubiquitin or SUMO-1, although a significant amount of Tax was also present in the cytoplasmic fractions. The same fractions were then immunoblotted with an antibody directed against HA to detect the ubiquitinated or sumoylated Tax species. As expected from the immunofluorescence results (see above), the sumoylated Tax species were detected only in the nuclear fraction. Surprisingly, in contrast to what was observed by using immunofluorescence staining, ubiquitinated Tax species were present both in the cytoplasmic and in the nuclear fractions. The discrepancy between the results obtained from both techniques and the fact that both ubiquitin and Tax were highly concentrated in cytoplasmic inclusions connected to the nuclear envelope led us to analyze the purity of the nuclear fractions. We thus immunoblotted the fractionated cell extracts with the antibody directed against the Golgi marker GM130. This result indicated that GM130 was present both in the cytoplasmic and in the nuclear fractions. Any attempt to prevent copurification of GM130 with the nuclear fraction failed. Thus, ubiquitinated Tax molecules present in the nuclear fraction likely originated from Tax-containing cytoplasmic inclusions that copurified with the nuclei. It is interesting to note that the suspected nucleus-associated cytoplasmic inclusions predominantly contained poorly branched ubiquitinated Tax species, unlike the soluble cytoplasmic fraction.
At this point it was critical to demonstrate that Tax was modified by sumoylation in HTLV-1-transformed T lymphocytes. Cell extracts from HTLV-1-transformed HUT102 T lymphocytes lysed under highly denaturing conditions to preserve modifications were immunoblotted with an anti-Tax antibody, which revealed the 40-kDa unmodified Tax form and clearly distinguishable higher-molecular-weight Tax species (Fig. 2G). Alignment of this blot with control blots from Ni-NTA-purified Tax-6His species produced in 293T cells or Jurkat T cells with or without the expression of HA-Ub or HA-SUMO-1 enabled the identification of the tri-sumoylated 80-kDa Tax species along with mono-, di-, and tri-ubiquitinated Tax. We concluded that sumoylation and ubiquitination occurred not only on Tax molecules overproduced in fibroblasts and T cells but also on endogenous Tax molecules expressed by HTLV-1-transformed T lymphocytes.
Lysines K7 and K8 are critical for the ability of Tax to assemble nuclear bodies.
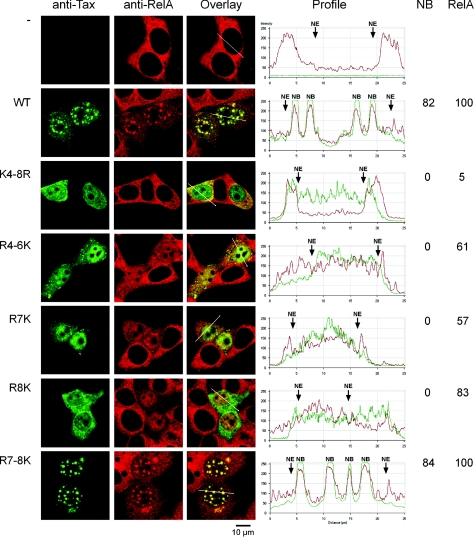
We then asked about the incidence of modifications occurring on lysine residues K4 to K8 on Tax intracellular localization. 293T cells were transfected with vectors for the expression of wild-type Tax, the Tax mutant K4-8R, or each of the lysine restoration mutants R4-6K, R7K, R8K, and R7-8K and analyzed by immunofluorescence staining with an anti-Tax monoclonal antibody. Since colocalization of Tax and the RelA subunit of NF-κB in nuclear bodies is an important characteristic of these bodies (4), an antibody directed against RelA was included in the dual-immunofluorescence staining procedure (Fig. 3). The fraction of cells that displayed Tax-containing nuclear bodies was estimated by counting at least 100 cells that expressed each of these Tax mutants. The measurement of the intensity of the fluorescence along lines crossing the nuclei also enabled us to estimate the fraction of cells in which the translocation of RelA to the nucleus had occurred.
FIG. 3.
Lysines K7 and K8 are critical for the ability of Tax to localize in nuclear bodies. 293T cells were transfected with vectors expressing wild-type Tax or the Tax mutants as indicated. The cells were stained by dual immunofluorescence with an anti-Tax MAb and an anti-RelA rabbit polyclonal antibody. Diagrams depict the intensity of the fluorescence for each staining along lines drawn on the overlay images with the boundaries of the nuclei (NE) and the nuclear bodies (NB) indicated. Cells were considered positive for the translocation of RelA to the nucleus when the intensity of the fluorescence in the nuclei was at least twice that found in cells that did not express Tax. The percentages of cells displaying Tax-containing nuclear bodies or the translocation of RelA to the nucleus are indicated.
In cells that did not express Tax, RelA had a diffuse cytoplasmic staining and was not detected in the nucleus. Expression of wild-type Tax led to the formation of nuclear bodies in the majority of the cells, and these nuclear bodies included RelA, confirming our previous observations (4). Translocation of RelA to the nucleus was evident in all the cells expressing wild-type Tax, including those that had Tax localized only in the cytoplasm. Mutant K4-8R, which was minimally sumoylated and ubiquitinated, was distributed either in the cytoplasm or in the nucleus or both and no Tax-containing nuclear bodies could be detected in cells expressing this mutant. The lysine restoration mutants R4-6K, R7K, and R8K, which were minimally sumoylated but maintained at least 50% of wild-type Tax ubiquitination, displayed a distribution similar to that of mutant K4-8R, with no formation of nuclear bodies. By contrast, mutant R7-8K, which was fully ubiquitinated and sumoylated, had a distribution very similar to that of wild-type Tax. Colocalization of Tax with RelA in nuclear bodies was also observed in HTLV-1-transformed T lymphocytes (HUT102) and in Jurkat cells expressing wild-type Tax but not in Jurkat cells expressing the K4-8R Tax mutant (see the supplemental material). These results indicated that lysine residues K7 and K8 at amino acid positions 280 and 284 are major determinants for the assembly of the Tax-containing nuclear bodies and suggested that formation of these nuclear structures involved conjugation of Tax to SUMO.
Interestingly, among the mutants that were not able to assemble nuclear bodies (K4-8R, R4-6K, R7K, and R8K), mutant K4-8R was the sole mutant which was defective for the translocation of RelA to the nucleus. Only 5% of the cells expressing this mutant were able to translocate RelA to the nucleus. By contrast, mutants R4-6K, R7K, and R8K, which were ubiquitinated to at least 50% of wild-type Tax, displayed a diffuse nuclear distribution of RelA in 61%, 57%, and 83% of the cells, respectively. Thus, a correlation was observed between the level of ubiquitination and the ability of Tax mutants to translocate the RelA subunit of NF-κB to the nucleus.
Lysines K7 and K8 are critical for Tax activation of gene expression via the NF-κB pathway.
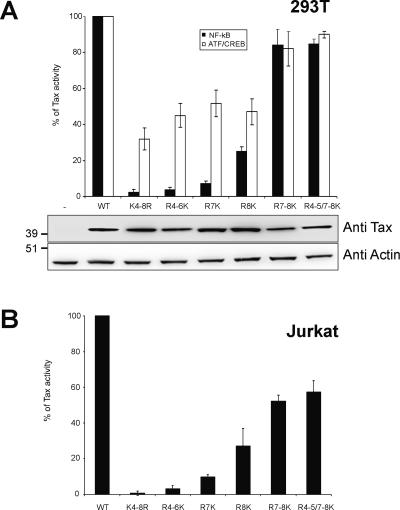
Tax activates viral and cellular gene expression via modulation of the activity of either the ATF/CREB or the NF-κB pathway. We thus asked about the ability of the Tax mutants to activate gene expression via these pathways. The vectors expressing wild-type or mutant Tax were cotransfected in 293T cells with either an HIV-1 LTR or an HTLV-1 LTR-luciferase reporter to assay for the effects of Tax on the NF-κB or ATF/CREB pathway (Fig. 4A). Wild-type Tax activated gene expression from both the HIV-1 LTR (7.4-fold ± 1-fold) and the HTLV-1 LTR (288-fold ± 45-fold). Mutant K4-8R and the lysine restoration mutants R4-6K and R7K were highly affected in their ability to activate gene expression via the NF-κB pathway, whereas mutant R8K displayed an intermediary phenotype. By contrast, mutants R7-8K and R4-5/7-8K were almost not affected. Immunoblot analysis performed on the extracts from the luciferase assays demonstrated that the wild-type and mutant Tax proteins were expressed at similar levels. Of note, the four mutants that were highly affected in their ability to activate the NF-κB pathway were also affected in their ability to activate the ATF/CREB pathway although the effects were less stringent on the latter pathway. At this point, it was important to test the ability of these mutants to activate gene expression in a T-lymphocyte cell line. Jurkat cells were cotransfected with wild-type or mutant Tax expression vectors and the HIV-1 LTR-luciferase reporter plasmid and analyzed as described above (Fig. 4B). Results obtained with Jurkat cells were similar to results obtained with 293T cells.
FIG. 4.
Lysines K7 and K8 are critical for Tax activation of gene expression via the NF-κB pathway. (A) 293T cells or (B) Jurkat T lymphocytes were cotransfected with either the HIV-1 LTR or the HTLV-1 LTR-luciferase reporter plasmids and vectors expressing wild-type or mutant Tax. Luciferase activities are represented as percentages of the activity seen with wild-type Tax. The values are averages from at least six independent experiments, with the standard deviations indicated. The 293T cell extracts from the luciferase assays were immunoblotted with the anti-Tax and antiactin antibodies (A), which indicates similar levels of wild-type and mutant Tax proteins in each extract.
These results indicated that the integrity of lysine K8 and, to a lesser extent, lysine K7 were critical for the Tax-mediated activation of gene expression via the NF-κB pathway in fibroblasts and in T lymphocytes. These lysine residues also played a role in the activation of gene expression via the ATF/CREB pathway. Remarkably, mutant R4-6K, which was able to translocate the RelA subunit of NF-κB to the nucleus in 61% of the cells (Fig. 3), was unable to activate gene expression via the NF-κB pathway. This result indicated that the migration of RelA to the nucleus was not sufficient for the Tax-mediated activation of gene expression and suggested that other events, including the sumoylation of Tax and the subsequent formation of the Tax-containing nuclear bodies, might also be required.
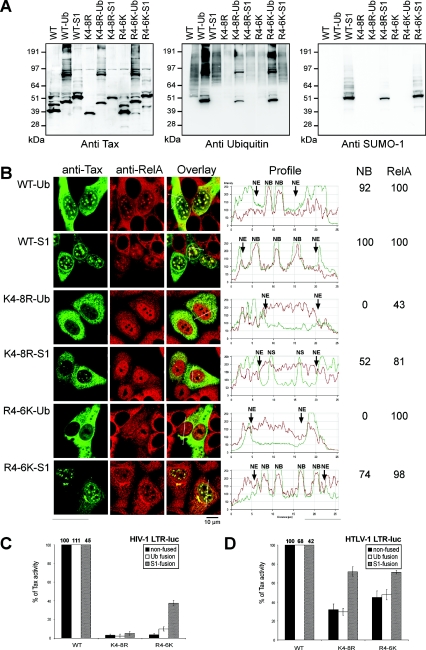
Fusion of ubiquitin or SUMO to the modification-deficient K4-8R mutant restores the translocation of RelA to the nucleus but does not restore transcriptional activity.
To study the specific role of Tax ubiquitination and sumoylation in Tax transcriptional activities, we next produced vectors for the expression of constitutively ubiquitinated or sumoylated Tax mutants that were intrinsically defective for ubiquitin and/or SUMO conjugation (mutants K4-8R and R4-6K). This was achieved by in-frame fusion of the ubiquitin or the SUMO-1 coding sequence to the 3′ end of the sequence coding for these Tax mutants. This resulted in the production of vectors for the expression of K4-8R-Ub, K4-8R-SUMO-1, R4-6K-Ub, and R4-6K-SUMO-1. In addition, to check whether the fusion of ubiquitin or SUMO-1 to the carboxy terminus of wild-type Tax had no major inhibitory effects on Tax transcriptional activities, we also constructed vectors for the expression of the WT-Ub and WT-SUMO-1 fusions.
First, all these fusions were purified from transfected 293T cells by Ni-NTA pulldown assay and analyzed by immunoblotting for the detection of Tax, ubiquitin, or SUMO-1 (Fig. 5A). The fusions to SUMO-1 or ubiquitin were recognized by anti-SUMO-1 or antiubiquitin antibodies, respectively, and they migrated at molecular masses expected for constitutively mono-ubiquitinated (47-kDa) or mono-sumoylated (50-kDa) Tax proteins. All the fusions to ubiquitin displayed additional species with molecular masses expected for dimers and trimers (80 kDa and 120 kDa), indicating that the mutation of the di-glycine motif at the 3′ end of the Tax-Ub fusions was unable to completely prevent the formation of branched Tax-Ub fusions. As expected from the results reported in Fig. 2C, the antiubiquitin immunoblot indicated that both the WT-SUMO-1 and R4-6K-SUMO-1 fusions could still be intrinsically ubiquitinated, unlike the K4-8R-SUMO-1 fusion.
FIG.5.
Phenotypes of Tax mutants fused to ubiquitin or SUMO. (A) 293T cells were transfected with a vector expressing either wild-type or mutant K4-8R or mutant R4-6K Tax-6His not fused or fused to either ubiquitin or SUMO-1. The proteins were purified by Ni-NTA pulldown assay and immunoblotted with anti-Tax or anti-SUMO-1 rabbit polyclonal antibodies or an anti-Ub MAb. WT-S1, K4-8R-S1, and R4-6K-S1, WT-SUMO-1, K4-8R-SUMO-1, and R4-6K-SUMO-1, respectively. (B) 293T cells were transfected with the vector expressing wild-type or mutant Tax fusions to ubiquitin or SUMO-1 and stained by dual-immunofluorescence staining with an anti-Tax MAb and an anti-RelA rabbit polyclonal antibody. Diagrams depict the intensity of the fluorescence for each staining along lines drawn on the overlay images with the boundaries of the nuclei (NE, nuclear envelope) and nuclear speckles (NS) or nuclear bodies indicated. The percentages of cells displaying Tax-containing nuclear bodies or the translocation of RelA to the nucleus is indicated. (C and D) 293T cells were cotransfected with either (C) the HIV-1 LTR luciferase or (D) the HTLV-1 LTR-luciferase reporter plasmids and vectors expressing wild-type or mutant ubiquitin or SUMO-1 Tax fusions. Luciferase activities are represented as percentages of the activities obtained with the homologous wild-type Tax or the Tax-Ub or Tax-SUMO-1 fusions. The luciferase activities of the WT-Ub and WT-SUMO-1 fusions relative to that of nonfused wild-type Tax are presented on top of the bars. The values shown represent averages from at least six independent experiments, with the standard deviations indicated.
We next analyzed the phenotypes of the WT-Ub and WT-SUMO-1 fusions. Dual immunofluorescence staining of 293T cells transfected with the WT-Ub or WT-SUMO-1 expression vectors was performed with anti-Tax and anti-RelA antibodies (Fig. 5B). The WT-Ub and WT-SUMO-1 fusions were able to form nuclear bodies that included the RelA subunit of NF-κB. However, the majority of the cells expressing the WT-Ub fusion had Tax distributed both in the cytoplasm and in nuclear bodies, with an intense concentration of WT-Ub in cytoplasmic inclusions in 78% of the Tax-Ub-expressing cells. In contrast, most of the cells expressing WT-SUMO-1 displayed only nuclear bodies. Thus, constitutive ubiquitination or sumoylation of Tax favored the retention of Tax either in the cytoplasm or in the nucleus, respectively, in a way similar to the effects observed when Tax was coexpressed with HA-Ub or HA-SUMO (Fig. 1). We next tested the transcriptional activity of these wild-type Tax fusions. Luciferase reporter assays performed as described above indicated that levels of activation of HIV and HTLV gene expression by the WT-Ub fusion were 111% ± 9% and 68% ± 8%, respectively, and that the levels of activation by the WT-SUMO-1 fusion were 45% ± 6% and 42% ± 8% of the activities seen with nonfused Tax, respectively (Fig. 5C and D). We concluded that the fusion of ubiquitin or SUMO to the carboxy terminus of Tax had no major detrimental effects on the ability of Tax to assemble nuclear bodies that included the RelA subunit of NF-κB and on Tax transcriptional activities.
We then tested the phenotypes of the constitutively ubiquitinated or sumoylated Tax mutant K4-8R, which was intrinsically deficient for both modifications. As reported above, the nonfused Tax K4-8R mutant was distributed in the cytoplasm and/or in the nucleus, with no formation of nuclear bodies, and this mutant was unable to induce the migration of RelA to the nucleus (Fig. 3). Remarkably, both the K4-8R-Ub and K4-8R-SUMO-1 fusions translocated RelA to the nucleus in 43% and 81% of the cells, respectively (Fig. 5B). These fusions were predominantly localized in the cytoplasm. In addition, the K4-8R-SUMO-1 fusion was able to assemble punctate nuclear structures in 52% of the cells. These structures were, however, smaller than the NB assembled by wild-type Tax, and the RelA subunit of NF-κB was not recruited in these structures, as indicated by the intensity of the fluorescence on the diagrams in Fig. 5B. The intracellular localization of the R4-6K fusions will be analyzed below. We then tested the transcriptional activity of the nonfused K4-8R mutant or the fusions K4-8R-Ub and K4-8R-S1 by luciferase reporter assays after cotransfection with the HIV-LTR-luciferase reporter in 293T cells. The luciferase activities were normalized to the activity of each of the homologous WT, WT-Ub, and WT-SUMO-1 proteins (Fig. 5C). The results indicated that both K4-8R fusions were as defective for the activation of gene expression via the NF-κB pathway as the nonfused K4-8R mutant. Thus, as was observed for mutant R4-6K (Fig. 3 and 4A), the K4-8R-Ub and K4-8R-SUMO-1 fusions were able to induce the migration of the RelA subunit of NF-κB to the nucleus in a significant fraction of the cells, but they were unable to activate gene expression via the NF-κB pathway.
Fusion of SUMO to the carboxy terminus of Tax R4-6K partly restores the formation of Tax- and RelA-containing nuclear bodies and transcriptional activity.
We finally analyzed the phenotypes of the ubiquitin or SUMO-1 fusions to the R4-6K mutant, which was partly intrinsically ubiquitinated but not sumoylated. Dual-immunofluorescence staining of cells expressing R4-6K-Ub or R4-6K-SUMO-1 (Fig. 5B) indicated that the R4-6K-Ub fusion was distributed in the cytoplasm of the majority of the cells and was able to translocate the RelA subunit of NF-κB to the nucleus in 100% of the cells. In contrast, the R4-6K-SUMO-1 fusion had a punctate distribution in the nucleus in 74% of the cells, with at least partial recruitment of the RelA subunit of NF-κB in the nuclear structures in 32% of the cells that displayed nuclear bodies. Remarkably, fusion of SUMO-1 to the R4-6K mutant resulted in a significant increase in the ability of this mutant to activate gene expression via the NF-κB pathway (10.5-fold), whereas fusion of ubiquitin resulted in only a 2.7-fold increase compared to the level of activation of the nonfused R4-6K mutant (Fig. 5C). The same results were obtained when luciferase activities were measured in cells transfected with increasing amounts of the different Tax expression vectors (see the supplemental material). We similarly tested the ability of the K4-8R and R4-6K fusions to activate gene expression via the ATF/CREB pathway (Fig. 5D). Interestingly, although the K4-8R-Ub and the R4-6K-Ub fusions displayed ATF/CREB activities similar to those of the homologous nonfused mutants, fusion of SUMO-1 to both of these mutants significantly increased their ability to activate gene expression via the ATF/CREB pathway.
The fact that the fusion of SUMO to the two intrinsically nonsumoylated Tax mutants K4-8R and R4-6K restored their ability to assemble punctate nuclear structures demonstrated that the sumoylation of Tax is critical for the assembly of the Tax nuclear bodies. Since the fusion of ubiquitin or SUMO to the unmodifiable Tax mutant K4-8R resulted in the restoration of the ability to translocate RelA to the nucleus, we concluded that SUMO and ubiquitin have the common property of conferring to Tax the ability to translocate RelA to the nucleus. However, the fact that these fusions were not able to activate gene expression via the NF-κB pathway indicated that the translocation of RelA to the nucleus is insufficient for Tax-mediated NF-κB activity. Since fusion of SUMO to the R4-6K mutant partly restored the ability of Tax to activate the NF-κB pathway, we concluded that transcriptional activity requires in addition the formation of nuclear bodies in which the RelA subunit of NF-κB is recruited. In contrast to the intrinsically ubiquitinated R4-6K-SUMO-1 fusion, the nonubiquitinated K4-8R-SUMO-1 fusion was not able to recruit RelA in the nuclear bodies and had no transcriptional activity. From this observation, we conclude that the formation of nuclear bodies that include RelA requires not only the sumoylation of Tax but also its ubiquitination in the cytoplasm. Finally, our results also suggested that the ubiquitination of Tax was dispensable for the activation of gene expression via the ATF/CREB pathway. However, sumoylation and the subsequent assembly of Tax-containing nuclear bodies may be also important for activation of gene expression via this pathway.
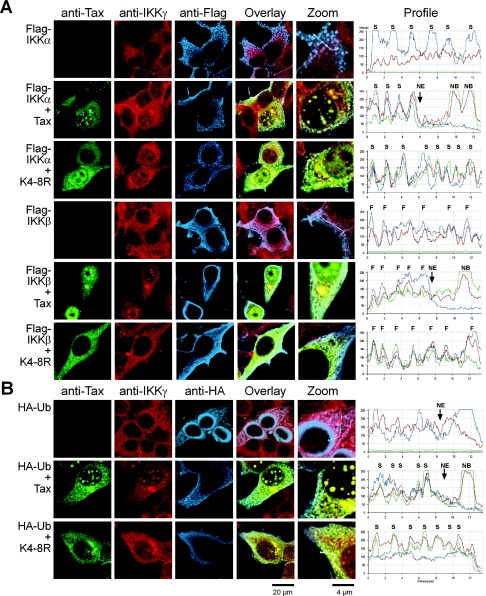
Tax colocalizes with free IKKγ in nuclear bodies and with IKK complexes in the cytoplasm.
Since Tax activation of the NF-κB pathway involves its interaction with the regulatory subunit of the IKK complex, NEMO/IKKγ (8, 14, 21), we finally asked about the relative intracellular localizations of Tax and components of the IKK complex, IΚΚα, IKKβ, and IKKγ. 293T cells were cotransfected with the vectors for the expression of wild-type Tax or the K4-8R mutant in combination with either a Flag-IKKα or a Flag-IKKβ expression vector, and the cells were analyzed by triple-immunofluorescence staining with monoclonal antibodies directed against Tax and Flag and a rabbit polyclonal antibody directed against IKKγ. Z-series confocal images were collected, and the projections are depicted in Fig. 6A.
FIG. 6.
Tax colocalizes with IKK complexes in the cytoplasm and with free IKKγ in the nucleus. (A) 293T cells were cotransfected with the vector for expression of either Flag-IKKα or Flag-IKKβ, alone or in combination with wild-type Tax or the Tax mutant K4-8R. The cells were submitted to triple-immunofluorescence staining with the anti-Tax MAb (IgG2a), an anti-Flag MAb (IgG1), and an anti-IKKγ rabbit polyclonal antibody. (B) 293T cells were transfected as described for panel A, except that HA-Ub was expressed instead of the IKK subunits and an anti-HA MAb (IgG1) was used during the triple-immunofluorescence staining. The images represent projections of Z-series confocal images. A fivefold enlargement of a field in the overlay images (Zoom) and diagrams of the intensity of the fluorescence for each of the staining along lines crossing cellular structures (S, cytoplasmic speckles; F, cytoplasmic fibers; NE, nuclear envelope) are shown.
In the cytoplasm of 293T cells that did not express Tax, each component of the IKK complexes had differential localizations, with a diffuse distribution of endogenous IKKγ and a granular distribution of exogenously expressed IKKα, whereas IKKβ was distributed along cytoplasmic fibers. IKKγ did not colocalize with IKKα in cytoplasmic speckles, and a limited colocalization of IKKγ and overexpressed IKKβ was observed in cytoplasmic fibers. The nuclei of these cells did not contain detectable IKKα or IKKβ, but they displayed a diffuse IKKγ staining. In wild-type-Tax-expressing cells, the Tax-containing NB included IKKγ but no detectable IKKα or IKKβ. In addition, Tax colocalized with both IKKγ and IKKα in the cytoplasmic speckles and with both IKKγ and IKKβ in cytoplasmic fibers. Tax also colocalized with IKKγ in the cytoplasmic inclusion closely connected to the nuclear envelope, as shown by the yellow color of these structures on the overlay images in Fig. 6A. However, these Tax-containing Golgi apparatus-associated cytoplasmic inclusions were devoid of both IKKα and IKKβ staining.
Surprisingly, the nonubiquitinated and nonsumoylated K4-8R mutant also colocalized with IKK complexes in the cytoplasmic speckles and fibers, although this mutant, which was unable to assemble nuclear bodies, did not colocalize with IKKγ in the nucleus (Fig. 6A). Similar to the nonfused K4-8R mutant, the K4-8R-Ub and K4-8R-SUMO-1 fusions colocalized with IKKα and IKKβ in discrete cytoplasmic structures (see the supplemental material). To confirm this result, 293T cells were cotransfected with the HA-Ub expression vector and vectors expressing either wild-type Tax or the K4-8R mutant, and the cells were stained with anti-Tax, anti-IKKγ, and anti-HA antibodies (Fig. 6B). As expected from the results reported above, the cytoplasmic speckles containing wild-type Tax included both IKKγ and ubiquitin, whereas the cytoplasmic speckles containing the K4-8R mutant included IKKγ but no ubiquitin. These results indicated that, in the cytoplasm, Tax altered the intracellular localization of components of the IKK complexes in an ubiquitin-independent manner, leading to their colocalization with Tax in cytoplasmic speckled and fibrous structures. However, as demonstrated before, translocation of the RelA subunit of NF-κB to the nucleus was strictly dependent on Tax modification. Thus, conjugation of Tax to ubiquitin was dispensable for the assembly of the IKK complexes but necessary for the activation of these complexes. In addition, sumoylated Tax molecules determined the nuclear localization of free IKKγ and its concentration with Tax in the nuclear bodies.
DISCUSSION
In this work, we present evidence that the Tax regulatory protein of HTLV-1 is ubiquitinated and sumoylated in HTLV-1-transformed T lymphocytes and in cells transduced with Tax expression vectors and that both modifications are required for Tax-mediated activation of the NF-κB pathway. Tax colocalized with ubiquitin and IKK complexes in the cytoplasm, leading to the translocation of the RelA subunit of NF-κB to the nucleus. However, this function was insufficient for Tax transcriptional activity. In addition, sumoylated Tax molecules had to assemble nuclear bodies in which Tax colocalized with RelA and free IKKγ. A schematic diagram depicting a model for concerted ubiquitin and SUMO conjugation of the HTLV-1 Tax protein in Tax-mediated activation of the NF-κB pathway is presented in Fig. 7. By using reintroduction of single or multiple lysine residues in the context of a Tax mutant deficient for both ubiquitination and sumoylation (mutant K4-8R), we also demonstrate that ubiquitination and sumoylation of Tax occur on overlapping lysine residues. Each of the lysine residues K4, K5, and K6 as well as K7 and K8 contributed individually to Tax ubiquitination, whereas sumoylation required the simultaneous integrity of both lysine residues K7 and K8 at amino acid positions 280 and 284.
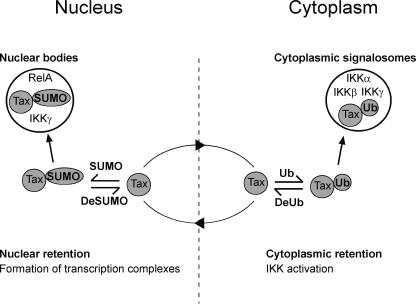
FIG. 7.
Schematic diagram depicting a model for concerted ubiquitin and SUMO conjugation of the HTLV-1 Tax protein in Tax-mediated activation of the NF-κB pathway. In the cytoplasm, ubiquitin-conjugated Tax activates the IKK signalosomes, resulting in the translocation of the RelA subunit of NF-κB to the nucleus. Deubiquitinated Tax migrates to the nucleus, in which it is sumoylated. Sumoylated Tax assembles nuclear bodies that include the RelA subunit of NF-κB and free IKKγ along with other transcription and splicing factors, leading to the activation of specific Tax-responsive target genes. Tax can further be desumoylated, which reveals its nuclear export signal, leading to the exit of Tax from the nucleus.
Characterization of the specific roles of Tax conjugation to ubiquitin or SUMO.
Our observations that the colocalization of Tax and ubiquitin was detectable only in the cytoplasm and that Tax colocalized with SUMO only in the nucleus helped us to solve the respective roles of Tax ubiquitin and SUMO conjugation. Since either overexpression of ubiquitin or fusion of ubiquitin to Tax increased the fraction of cells displaying Tax in the cytoplasm, we concluded that ubiquitin conjugation is involved in the cytoplasmic retention of Tax. Inversely, overexpression of SUMO or the fusion of SUMO to Tax increased the fraction of cells with Tax localized in nuclear bodies, indicating that SUMO conjugation favored the retention of Tax in the nucleus. Thus, the two modifications are exclusive and occur in different cellular compartments. This observation is in line with the idea that SUMO modification and demodification are coupled to the shuttling of proteins into and out of the nucleus (12, 35). Ubiquitinated Tax molecules were associated with two distinct cytoplasmic structures, a cytoplasmic speckle, which contained IKK complexes, and the Golgi apparatus, which included the Golgi matrix marker GM130 and free IKKγ. In addition, the latter structure also included the RelA subunit of NF-κB (data not shown). The functional role of the association of ubiquitinated Tax with the Golgi apparatus is presently unknown. However, the specific concentration of poorly branched ubiquitinated Tax, free IKKγ, and RelA in the cytoplasmic inclusions and their close vicinity to the nuclear envelope suggest that these structures might be involved in Tax ubiquitination and/or desubiquitination and the transport of Tax along with free IKKγ and RelA into the nucleus.
Both a nuclear localization signal (39) and a nuclear export signal (NES) (1) have been identified in the Tax protein. Remarkably, Alefantis et al. mentioned that the Tax NES likely exists as a conditionally masked signal because the deletion of the carboxy-terminal sequence from amino acid 214 revealed this export signal. Thus, conjugation of Tax to SUMO-1 on lysine residues at position 280 and/or position 284 might prevent NES effects and Tax nuclear export. A number of proteins that shuttle between the cytoplasm and the nucleus might undergo differential intracellular retention by exclusive ubiquitination and sumoylation on identical or closely spaced lysine residues. The available list includes IκBα (9), IKKγ/NEMO (16), and the herein described HTLV-1 Tax regulatory protein.
Activation of IKK complexes by ubiquitinated Tax.
Our results indicate that ubiquitinated Tax colocalized with components of the IKK complexes, IKKα, IKKβ, and IKKγ, in cytoplasmic speckled and fibrous structures. The redistribution of IKK components by Tax is in complete concordance with the known physical binding of Tax to IKKγ, with subsequent recruitment of Tax to the IKK catalytic subunits IKKα and IKKβ, leading to sustained phosphorylation and the turnover of IκBα as well as the phosphorylation of RelA and subsequent migration of the RelA subunit of NF-κB to the nucleus (8, 14, 17, 21, 30). Interestingly, the ubiquitination-deficient Tax mutant K4-8R also colocalized with IKK complexes in the cytoplasm, but it was unable to induce the translocation of RelA to the nucleus, and the fusion of ubiquitin to this mutant restored the latter property. This result indicates that the activation of IKK complexes but not the assembly of these complexes requires Tax ubiquitination. In support of this conclusion, it is interesting to note that the coimmunoprecipitation experiments demonstrating the interaction between Tax and IKKγ did not likely preserve Tax conjugation to ubiquitin and that the GST-Tax fusion produced in bacteria readily interacted with IKKγ in vitro (8, 17, 21). Tax interaction with IKKγ might simply involve a domain that was mapped to the leucine-rich region between amino acids 106 and 141 (44). Surprisingly, the fusion of SUMO-1 to the carboxy terminus of the K4-8R mutant also restored the migration of RelA to the nucleus. This result suggests that both ubiquitin and SUMO-1 conjugation might confer to Tax the ability to activate IKK complexes. However, activation of cytoplasmic IKK complexes by sumoylated Tax molecules is a rather artificial situation created by the fusion to SUMO since intrinsically sumoylated Tax molecules were not detected in the cytoplasm.
Sumoylation-dependent assembly of Tax-containing nuclear bodies.
Here, we demonstrate that Tax conjugation to SUMO-1 is a prerequisite for Tax to form nuclear bodies. All the Tax mutants that were not sumoylated were unable to assemble nuclear bodies, and the fusion of SUMO-1, but not ubiquitin, to their carboxy termini significantly restored the ability to form these nuclear structures. Interestingly, the fusion of SUMO-1 to the intrinsically unmodified K4-8R mutant restored both the translocation of RelA to the nucleus and the formation of punctate nuclear structures that did not include RelA, and this fusion was defective for activation of the NF-κB pathway. In contrast, the fusion of SUMO-1 to the R4-6K mutant, which was intrinsically ubiquitinated but not sumoylated, restored the formation of RelA-containing nuclear bodies and significantly increased transcriptional activity. We conclude that the presence of ubiquitinated Tax in the cytoplasm is required not only for the migration of RelA to the nucleus but also for the subsequent concentration of RelA in the nuclear bodies. Thus, both ubiquitination and sumoylation are required for the Tax-mediated activation of gene expression via the NF-κB pathway.
We previously demonstrated that the nuclear bodies assembled by Tax included components of the transcription and splicing complexes, including the two subunits of NF-κB, p50 and RelA, and the transcriptional coactivator CBP/p300 (4, 6). In this paper, we demonstrate that IKKγ was recruited in the Tax-containing nuclear bodies but that neither IKKα nor IKKβ was detected in these nuclear structures. Sumoylated IKKγ was detected in the nucleus following genotoxic stress and, in concert with ubiquitinated IKKγ, permitted IKK activation in the cytoplasm (16). Thus, Tax and IKKγ interact with each other and share similar routes in the cell. However, it is not clear presently whether Tax alters SUMO conjugation to IKKγ or whether IKKγ is brought to the nucleus as a consequence of its interaction which Tax. In addition to its cytoplasmic role in assembling IKK complexes, Verma et al. identified a nuclear function of IKKγ that involves its binding to an amino-terminal domain of the transcriptional coactivator CBP, counteracting the positive action of CBP/RelA/IKKα complexes on the transcription initiated at NF-κB-responsive promoters (43). It is worth mentioning that Tax also interacts with the amino terminus of CBP and that this interaction is critical for Tax transcriptional activities (20). Since Tax colocalizes in nuclear bodies with CBP, RelA, and IKKγ, it will be important to determine whether IKKγ affects Tax and RelA binding to CBP. The mechanistic role of the presence of IKKγ in the nucleus in NF-κB activation needs further investigations.
A role for Tax nuclear bodies as “transcription factories.”
Recent studies have demonstrated that active genes migrate to preassembled shared nuclear subcompartments called transcription factories and that movement in and out of these factories results in the activation or abatement of transcription (26, 32). In addition, specific gene loci have been found to preferentially localize in close spatial proximity to each of a variety of proteinaceous nuclear structures, including PML bodies, Cajal bodies, and the nucleoli (11). Our observations that the Tax-containing nuclear bodies include not only components of transcription and splicing complexes but also the mRNA from a gene specifically activated by Tax via the NF-κB pathway (4), that they are in close spatial contact with decondensed chromatin (our unpublished data), and that the defect of assembly of these structures leads to the loss of transcriptional activation (this paper) strongly suggest that the unique nuclear structures assembled by Tax are directly involved in Tax activation of gene expression. The fact that nonsumoylated Tax mutants were still able to partly activate gene expression via the ATF/CREB pathway seems to be in contradiction with this hypothesis. However, it is important to mention that our assays for transcriptional activity utilize transiently transfected reporter genes that do not have the complexity of chromatin templates. A role for the nuclear bodies in the activation of gene expression via the latter pathway was indicated by the significant increase of gene expression by SUMO fusion to intrinsically sumoylation-defective Tax mutants. A more stringent dependency on nuclear body structure might be observed for activation of gene expression on in vivo templates.
Since ubiquitination and sumoylation play a critical role in Tax transcriptional activation, these modifications are potential targets for the treatment of adult T-cell leukemia.
Supplementary Material
Acknowledgments
We thank C. Pique (Hôpital Saint-Louis, France) for providing vectors for expression of the Tax lysine mutants. We are most grateful to R. Hay (University of St. Andrews, United Kingdom) for HA-SUMO and to S. Ghosh (Yale University School of Medicine) for the Flag-IKKα and Flag-IKKβ expression vectors.
I.L. and S.L. are recipients of grants from the Belgian National Fund for Scientific Research and FNRS-Télévie, and this work was supported by grants from the Belgian National Fund for Scientific Research and from the Internationale Brachet Stiftung.
Footnotes
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Alefantis, T., K. Barmak, E. W. Harhaj, C. Grant, and B. Wigdahl. 2003. Characterization of a nuclear export signal within the human T cell leukemia virus type I transactivator protein Tax. J. Biol. Chem. 278:21814-21822. [DOI] [PubMed] [Google Scholar]
- 2.Baeuerle, P. A., and T. Henkel. 1994. Function and activation of NF-kappa B in the immune system. Annu. Rev. Immunol. 12:141-179. [DOI] [PubMed] [Google Scholar]
- 3.Bex, F., and R. B. Gaynor. 1998. Regulation of gene expression by HTLV-I Tax protein. Methods 16:83-94. [DOI] [PubMed] [Google Scholar]
- 4.Bex, F., A. McDowall, A. Burny, and R. Gaynor. 1997. The human T-cell leukemia virus type 1 transactivator protein Tax colocalizes in unique nuclear structures with NF-κB proteins. J. Virol. 71:3484-3497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bex, F., K. Murphy, R. Wattiez, A. Burny, and R. B. Gaynor. 1999. Phosphorylation of the human T-cell leukemia virus type 1 transactivator tax on adjacent serine residues is critical for tax activation. J. Virol. 73:738-745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bex, F., M. J. Yin, A. Burny, and R. B. Gaynor. 1998. Differential transcriptional activation by human T-cell leukemia virus type 1 Tax mutants is mediated by distinct interactions with CREB binding protein and p300. Mol. Cell. Biol. 18:2392-2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chiari, E., I. Lamsoul, J. Lodewick, C. Chopin, F. Bex, and C. Pique. 2004. Stable ubiquitination of human T-cell leukemia virus type 1 tax is required for proteasome binding. J. Virol. 78:11823-11832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chu, Z. L., Y. A. Shin, J. M. Yang, J. A. DiDonato, and D. W. Ballard. 1999. IKKgamma mediates the interaction of cellular IkappaB kinases with the tax transforming protein of human T cell leukemia virus type 1. J. Biol. Chem. 274:15297-15300. [DOI] [PubMed] [Google Scholar]
- 9.Desterro, J. M., M. S. Rodriguez, and R. T. Hay. 1998. SUMO-1 modification of IkappaBalpha inhibits NF-kappaB activation. Mol. Cell 2:233-239. [DOI] [PubMed] [Google Scholar]
- 10.Franchini, G. 1995. Molecular mechanisms of human T-cell leukemia/lymphotropic virus type I infection. Blood 86:3619-3639. [PubMed] [Google Scholar]
- 11.Freiman, R. N., and R. Tjian. 2003. Regulating the regulators: lysine modifications make their mark. Cell 112:11-17. [DOI] [PubMed] [Google Scholar]
- 12.Gill, G. 2004. SUMO and ubiquitin in the nucleus: different functions, similar mechanisms? Genes Dev. 18:2046-2059. [DOI] [PubMed] [Google Scholar]
- 13.Grassmann, R., S. Berchtold, I. Radant, M. Alt, B. Fleckenstein, J. G. Sodroski, W. A. Haseltine, and U. Ramstedt. 1992. Role of human T-cell leukemia virus type 1 X region proteins in immortalization of primary human lymphocytes in culture. J. Virol. 66:4570-4575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harhaj, E. W., and S. C. Sun. 1999. IKKgamma serves as a docking subunit of the IkappaB kinase (IKK) and mediates interaction of IKK with the human T-cell leukemia virus Tax protein. J. Biol. Chem. 274:22911-22914. [DOI] [PubMed] [Google Scholar]
- 15.Huang, T. T., N. Kudo, M. Yoshida, and S. Miyamoto. 2000. A nuclear export signal in the N-terminal regulatory domain of IkappaBalpha controls cytoplasmic localization of inactive NF-kappaB/IkappaBalpha complexes. Proc. Natl. Acad. Sci. USA 97:1014-1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang, T. T., S. M. Wuerzberger-Davis, Z. H. Wu, and S. Miyamoto. 2003. Sequential modification of NEMO/IKKgamma by SUMO-1 and ubiquitin mediates NF-kappaB activation by genotoxic stress. Cell 115:565-576. [DOI] [PubMed] [Google Scholar]
- 17.Jin, D. Y., V. Giordano, K. V. Kibler, H. Nakano, and K. T. Jeang. 1999. Role of adapter function in oncoprotein-mediated activation of NF-kappaB. Human T-cell leukemia virus type I Tax interacts directly with IkappaB kinase gamma. J. Biol. Chem. 274:17402-17405. [DOI] [PubMed] [Google Scholar]
- 18.Karin, M., and M. Delhase. 2000. The I kappa B kinase (IKK) and NF-kappa B: key elements of proinflammatory signalling. Semin. Immunol. 12:85-98. [DOI] [PubMed] [Google Scholar]
- 19.Kehn, K., R. Berro, F. C. de La, K. Strouss, E. Ghedin, S. Dadgar, M. E. Bottazzi, A. Pumfery, and F. Kashanchi. 2004. Mechanisms of HTLV-1 transformation. Front. Biosci. 9:2347-2372. [DOI] [PubMed] [Google Scholar]
- 20.Kwok, R. P., M. E. Laurance, J. R. Lundblad, P. S. Goldman, H. Shih, L. M. Connor, S. J. Marriott, and R. H. Goodman. 1996. Control of cAMP-regulated enhancers by the viral transactivator Tax through CREB and the co-activator CBP. Nature 380:642-646. [DOI] [PubMed] [Google Scholar]
- 21.Li, X. H., K. M. Murphy, K. T. Palka, R. M. Surabhi, and R. B. Gaynor. 1999. The human T-cell leukemia virus type-1 Tax protein regulates the activity of the IkappaB kinase complex. J. Biol. Chem. 274:34417-34424. [DOI] [PubMed] [Google Scholar]
- 22.Mahajan, R., C. Delphin, T. Guan, L. Gerace, and F. Melchior. 1997. A small ubiquitin-related polypeptide involved in targeting RanGAP1 to nuclear pore complex protein RanBP2. Cell 88:97-107. [DOI] [PubMed] [Google Scholar]
- 23.Malek, S., Y. Chen, T. Huxford, and G. Ghosh. 2001. IkappaBbeta, but not IkappaBalpha, functions as a classical cytoplasmic inhibitor of NF-kappaB dimers by masking both NF-kappaB nuclear localization sequences in resting cells. J. Biol. Chem. 276:45225-45235. [DOI] [PubMed] [Google Scholar]
- 24.Matunis, M. J., E. Coutavas, and G. Blobel. 1996. A novel ubiquitin-like modification modulates the partitioning of the Ran-GTPase-activating protein RanGAP1 between the cytosol and the nuclear pore complex. J. Cell Biol. 135:1457-1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.May, M. J., R. B. Marienfeld, and S. Ghosh. 2002. Characterization of the Ikappa B-kinase NEMO binding domain. J. Biol. Chem. 277:45992-46000. [DOI] [PubMed] [Google Scholar]
- 26.Misteli, T. 2004. Spatial positioning; a new dimension in genome function. Cell 119:153-156. [DOI] [PubMed] [Google Scholar]
- 27.Muller, S., M. J. Matunis, and A. Dejean. 1998. Conjugation with the ubiquitin-related modifier SUMO-1 regulates the partitioning of PML within the nucleus. EMBO J. 17:61-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nejmeddine, M., A. L. Barnard, Y. Tanaka, G. P. Taylor, and C. R. Bangham. 2005. Human T-lymphotrophic virus, type 1, tax protein triggers microtubule reorientation in the virological synapse. J. Biol. Chem. 280:29653-29660. [DOI] [PubMed] [Google Scholar]
- 29.Nerenberg, M., S. H. Hinrichs, R. K. Reynolds, G. Khoury, and G. Jay. 1987. The tat gene of human T-lymphotropic virus type 1 induces mesenchymal tumors in transgenic mice. Science 237:1324-1329. [DOI] [PubMed] [Google Scholar]
- 30.O'Mahony, A. M., M. Montano, K. Van Beneden, L. F. Chen, and W. C. Greene. 2004. Human T-cell lymphotropic virus type 1 tax induction of biologically active NF-kappaB requires IkappaB kinase-1-mediated phosphorylation of RelA/p65. J. Biol. Chem. 279:18137-18145. [DOI] [PubMed] [Google Scholar]
- 31.Osame, M., K. Usuku, S. Izumo, N. Ijichi, H. Amitani, A. Igata, M. Matsumoto, and M. Tara. 1986. HTLV-I associated myelopathy, a new clinical entity. Lancet i:1031-1032. [DOI] [PubMed] [Google Scholar]
- 32.Osborne, C. S., L. Chakalova, K. E. Brown, D. Carter, A. Horton, E. Debrand, B. Goyenechea, J. A. Mitchell, S. Lopes, W. Reik, and P. Fraser. 2004. Active genes dynamically colocalize to shared sites of ongoing transcription. Nat. Genet. 36:1065-1071. [DOI] [PubMed] [Google Scholar]
- 33.Pajak, B., T. De Smedt, V. Moulin, C. De Trez, R. Maldonado-Lopez, G. Vansanten, E. Briend, J. Urbain, O. Leo, and M. Moser. 2000. Immunohistowax processing, a new fixation and embedding method for light microscopy, which preserves antigen immunoreactivity and morphological structures: visualisation of dendritic cells in peripheral organs. J. Clin. Pathol. 53:518-524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peloponese, J. M., Jr., H. Iha, V. R. Yedavalli, A. Miyazato, Y. Li, K. Haller, M. Benkirane, and K. T. Jeang. 2004. Ubiquitination of human T-cell leukemia virus type 1 tax modulates its activity. J. Virol. 78:11686-11695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pichler, A., and F. Melchior. 2002. Ubiquitin-related modifier SUMO1 and nucleocytoplasmic transport. Traffic 3:381-387. [DOI] [PubMed] [Google Scholar]
- 36.Poiesz, B. J., F. W. Ruscetti, A. F. Gazdar, P. A. Bunn, J. D. Minna, and R. C. Gallo. 1980. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc. Natl. Acad. Sci. USA 77:7415-7419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Semmes, O. J., and K. T. Jeang. 1996. Localization of human T-cell leukemia virus type 1 tax to subnuclear compartments that overlap with interchromatin speckles. J. Virol. 70:6347-6357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smith, M. R., and W. C. Greene. 1991. Molecular biology of the type I human T-cell leukemia virus (HTLV-I) and adult T-cell leukemia. J. Clin. Investig. 87:761-766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smith, M. R., and W. C. Greene. 1992. Characterization of a novel nuclear localization signal in the HTLV-I tax transactivator protein. Virology 187:316-320. [DOI] [PubMed] [Google Scholar]
- 40.Tam, W. F., and R. Sen. 2001. IkappaB family members function by different mechanisms. J. Biol. Chem. 276:7701-7704. [DOI] [PubMed] [Google Scholar]
- 41.Tanaka, A., C. Takahashi, S. Yamaoka, T. Nosaka, M. Maki, and M. Hatanaka. 1990. Oncogenic transformation by the tax gene of human T-cell leukemia virus type I in vitro. Proc. Natl. Acad. Sci. USA 87:1071-1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tatham, M. H., E. Jaffray, O. A. Vaughan, J. M. Desterro, C. H. Botting, J. H. Naismith, and R. T. Hay. 2001. Polymeric chains of SUMO-2 and SUMO-3 are conjugated to protein substrates by SAE1/SAE2 and Ubc9. J. Biol. Chem. 276:35368-35374. [DOI] [PubMed] [Google Scholar]
- 43.Verma, U. N., Y. Yamamoto, S. Prajapati, and R. B. Gaynor. 2004. Nuclear role of I kappa B kinase-gamma/NF-kappa B essential modulator (IKK gamma/NEMO) in NF-kappa B-dependent gene expression. J. Biol. Chem. 279:3509-3515. [DOI] [PubMed] [Google Scholar]
- 44.Xiao, G., E. W. Harhaj, and S. C. Sun. 2000. Domain-specific interaction with the I kappa B kinase (IKK) regulatory subunit IKK gamma is an essential step in tax-mediated activation of IKK. J. Biol. Chem. 275:34060-34067. [DOI] [PubMed] [Google Scholar]
- 45.Yamamoto, Y., and R. B. Gaynor. 2004. IkappaB kinases: key regulators of the NF-kappaB pathway. Trends Biochem. Sci. 29:72-79. [DOI] [PubMed] [Google Scholar]
- 46.Yin, M. J., L. B. Christerson, Y. Yamamoto, Y. T. Kwak, S. Xu, F. Mercurio, M. Barbosa, M. H. Cobb, and R. B. Gaynor. 1998. HTLV-I Tax protein binds to MEKK1 to stimulate IkappaB kinase activity and NF-kappaB activation. Cell 93:875-884. [DOI] [PubMed] [Google Scholar]
- 47.Yoshida, M., M. Seiki, K. Yamaguchi, and K. Takatsuki. 1984. Monoclonal integration of human T-cell leukemia provirus in all primary tumors of adult T-cell leukemia suggests causative role of human T-cell leukemia virus in the disease. Proc. Natl. Acad. Sci. USA 81:2534-2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhong, S., S. Muller, S. Ronchetti, P. S. Freemont, A. Dejean, and P. P. Pandolfi. 2000. Role of SUMO-1-modified PML in nuclear body formation. Blood 95:2748-2752. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.