Abstract
Autosomal recessive Charcot-Marie-Tooth disease (CMT) type 4 (CMT4) is a complex group of demyelinating hereditary motor and sensory neuropathies presenting genetic heterogeneity. Five different subtypes that correspond to six different chromosomal locations have been described. We hereby report a large inbred Lebanese family affected with autosomal recessive CMT4, in whom we have excluded linkage to the already-known loci. The results of a genomewide search demonstrated linkage to a locus on chromosome 19q13.1-13.3, over an 8.5-cM interval between markers D19S220 and D19S412. A maximum pairwise LOD score of 5.37 for marker D19S420, at recombination fraction [θ] .00, and a multipoint LOD score of 10.3 for marker D19S881, at θ=.00, strongly supported linkage to this locus. Clinical features and the results of histopathologic studies confirm that the disease affecting this family constitutes a previously unknown demyelinating autosomal recessive CMT subtype known as “CMT4F.” The myelin-associated glycoprotein (MAG) gene, located on 19q13.1 and specifically expressed in the CNS and the peripheral nervous system, was ruled out as being the gene responsible for this form of CMT.
Hereditary motor and sensory neuropathy (HMSN), also known as “Charcot-Marie-Tooth disease” (CMT [MIM 118300]), is a heterogeneous group of disorders characterized by chronic distal weakness with progressive muscular atrophy and sensory loss in the distal extremities (Dyck et al. 1993). With a frequency of ∼1/2,500, CMT is the most common inherited neurological disorder (Skre et al. 1974). Clinically, two major groups are defined; they are differentiated on the basis of peripheral nerve pathology and the results of nerve conduction velocity (NCV) studies. CMT type 1 (CMT1) is characterized by low NCVs resulting from demyelination in the motor and sensory nerves, whereas CMT type 2 (CMT2) refers to neuropathies with normal NCVs and no evidence of demyelination (Bell 1997). Modes of inheritance could be either autosomal dominant, autosomal recessive, or X-linked. Autosomal recessive demyelinating CMT type 4 (CMT4) is less frequent and more severe than the two other modes of inheritance. Five different CMT4 subtypes, each of which presents particular ethnic, pathological, or clinical characteristics, are known. They are CMT4A (MIM 214400) on 8q13-21.1 (Ben Othmane et al. 1993); CMT4B, which includes CMT4B1 (MIM 601382) on 11q22 (Bolino et al. 1996), caused by mutations in the MTMR2 gene (MIM 603557) encoding myotubularin-related protein-2 (Bolino et al. 2000), and CMT4B2 (MIM 604563) on 11p15 (Ben Othmane et al. 1999); CMT4C (MIM 601596) on 5q23-33 (Leguern et al. 1996); CMT4D (MIM 601455) on 8q24 (Kalaydjieva et al. 1996); and CMT4E on 10q21-22. The fifth subtype, CMT4E, corresponds to a congenital hypomyelinating neuropathy in which a homozygous missense mutation has been identified in an early-growth-response gene (EGR2; Warner et al. 1998).
We now report the assignment of a new locus for an autosomal recessive CMT4 subtype on chromosome 19q13.1-13.3 in a large inbred Lebanese family, using homozygosity mapping (Lander and Botstein 1987) and DNA pooling. The family presented with clinical features that may define a new CMT4F subtype. Involvement of the myelin-associated glycoprotein (MAG) gene, which is located on 19q13.1 and is specifically expressed in Schwann cells in the peripheral nervous system or in oligodendrocytes in the CNS, was ruled out in this study.
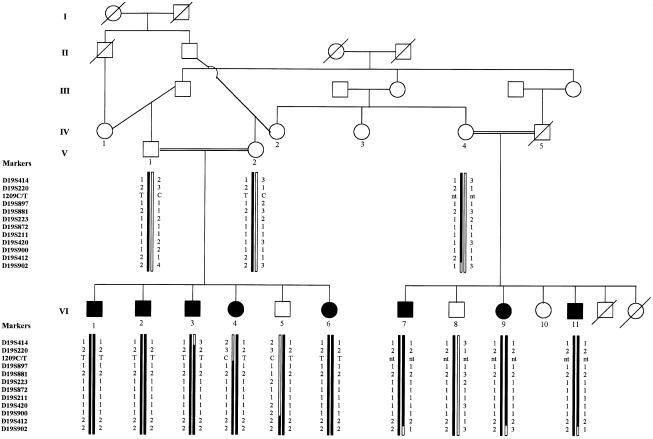
Thirteen members of a Shiite Muslim family originating from a village in the south of Lebanon were investigated (fig. 1). All affected members, with the exception of patient VI-6, who was too young to present all the clinical features, had nearly identical clinical, radiological, and laboratory findings and were therefore described together.
Figure 1.
Haplotype reconstruction in a Lebanese family affected with autosomal recessive demyelinating CMT at 19q13.1-13.3. Markers are reported from centromere (top) to telomere (bottom). 1209C/T denotes a polymorphism in MAG. Blackened symbols represent affected individuals. The disease-bearing chromosome is blackened. nt = not tested.
Gestation and delivery were unremarkable, with no history of exposure to pre- or perinatal environmental toxins in the family. All patients had a delay in motor development. They started sitting by age 12–18 mo and began walking and talking at age ∼2 years. From the beginning, gait was described as wide-based and mildly ataxic. At age 9–10 years, lower-limb distal muscle weakness and atrophy with pes cavus deformity were evident. By age 14–15 years, weakness of the upper limbs—in particular, of the hands—and finger retraction appeared. Upon examination, head circumferences and statures were normal. The neurological examination showed important sensory ataxia, bilateral steppage gait, mild kyphoscoliosis, symmetrical atrophy of intrinsic hand muscles and of muscles below the knees, pes cavus deformity, slightly diminished distal muscle strength of the upper and lower limbs, with paresthesia on the distal portions of the lower limbs, absent osteotendinous reflexes in four limbs, and distal sensory abnormalities—including deficits of position, vibration, pain, and temperature—in both the upper and lower extremities. No seizures, cranial nerve abnormalities, or facial weakness were noted. Intelligence and speech were normal. The progression of the disease had been very slow. Only patient VI-7 could no longer walk, essentially because of a severe left pes equinovarus. Total body X-rays revealed the presence of scoliosis in all patients. Magnetic-resonance imaging (MRI), which was performed for patients VI-1 and VI-2, showed a large cisterna magna. The results of abdominal ultrasound, echocardiography, and electroencephalography; evoked potentials; auditory brain-stem responses; and the results of ophthalmologic evaluation performed in some of the patients were all unremarkable. The results of routine laboratory tests as well as levels of plasma very-long-chain fatty acids and results of white-blood-cell enzyme assays were within the normal range. The karyotype was normal (46,XY; patient VI-1). The results of NCV studies performed on five affected individuals revealed the total absence of any sensory or motor evoked response in the upper and lower limbs. Electromyograms (EMGs) showed normal insertional activity, absence of spontaneous activity, and moderately reduced recruitment of motor-unit potentials in the distal muscles of four limbs. The results of EMG and NCV studies performed on parents were completely normal.
Histopathologic examination of the whole sural nerve–biopsy specimen from patient VI-1 disclosed a severe depletion of myelinated fibers (estimated value of density <1,000 fibers/mm2) and numerous extensive concentric Schwann-cell proliferation with multiple small onion bulbs isolated or surrounding the rare remaining myelinated fibers. These data indicated a severe axonal loss, following an initial process of chronic demyelination-remyelination.
After familial investigations, EDTA blood samples were collected for genetic studies. Informed consent was obtained from all adults and from parents of children included in the study. DNA was extracted from lymphocytes by use of standard methods (Miller et al. 1988). A pooling strategy (for review, see Sheffield et al. [1995]) was used in the first genomewide screening. Three separate DNA pools—the “affected” pool, the “obligate-carrier” pool, and the “unaffected” pool—were prepared by mixing equimolar quantities of individual DNAs. The screening set comprised 400 highly polymorphic fluorescently labeled markers from the ABI PRISM Linkage Map Set, version 2.0 (PE Biosystems), that had an average spacing of 10–20 cM and were chosen from the Généthon linkage map (Dib et al. 1996). Amplifications were performed in a 7.5-μl final volume on 40 ng pooled DNA. The cycling conditions used were those recommended by the manufacturer (PE Biosystems). Pooled PCR products were separated on a 5% Long Ranger denaturing urea-polyacrylamide gel and were analyzed on an ABI 377 DNA sequencer (PE Biosystems). Peaks were analyzed with GENESCAN, version 3.1, and GENOTYPER, version 2.1 (PE Biosystems).
Several zones of homozygosity, which showed reduction to homozygosity in the affected pool, were selected for genotyping of individual samples. Nevertheless, only marker D19S420 (AFM326xh9) on 19q13.2 was found to be informative in the parents and homozygous in all the affected individuals and was selected for further genotyping. To determine the size of the shared homozygous region, 10 new microsatellite markers surrounding D19S420 were selected from the Généthon human linkage map and were genotyped in all individuals. Recombination events restricted the homozygous candidate region to an 8.5-cM interval distal to D19S220 (AFM214yf12) and proximal to D19S412 (AFM284yg) (fig. 1).
Parametric linkage analysis was performed with an optimized version of the LINKAGE package, version 5.2 (Lathrop et al. 1985). Pairwise LOD scores and multipoint LOD scores were calculated using programs from FASTLINK, version 3.0P. The disease was assumed to be caused by a fully penetrant autosomal recessive gene with a frequency of .002. Allele frequencies for microsatellite markers were chosen from the Genome Database and were assumed to be the same as those defined by Généthon for the Caucasian population (Lefranc et al. 1978).
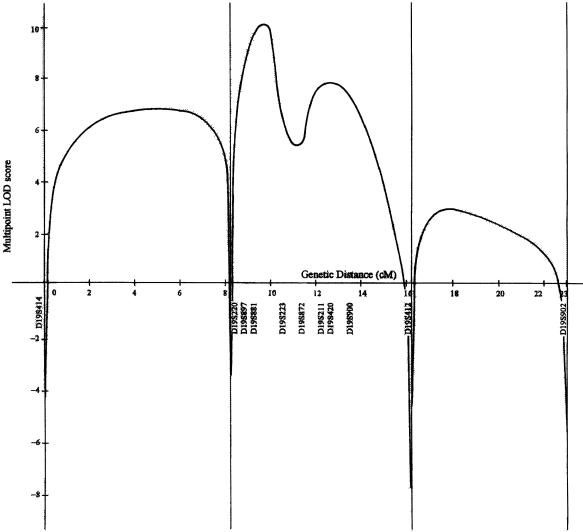
A maximal pairwise LOD score of 5.37 was obtained for D19S420 (table 1), and multipoint LOD-score analysis gave a maximum of 10.3 for D19S881, at θ = .00 (fig. 2). Markers D19S872 and D19S211 showed poor LOD-score values (table 1) as a result of their lack of informativity. The other markers were informative in all matings, and all affected individuals were homozygous for the disease allele.
Table 1.
Pairwise LOD Scores between the CMT4F Locus and 11 Marker Loci on 19q13.1-13.3
|
LOD Score at θ = |
|||||||||
| Marker | .0001 | .01 | .05 | .1 | .2 | .3 | .4 | Maximum Pairwise LOD Score | Maximum θ |
| D19S414 | −∞ | 1.67 | 2.67 | 2.75 | 2.27 | 1.51 | .69 | 2.77 | .084 |
| D19S220 | −∞ | 3.12 | 3.41 | 3.20 | 2.42 | 1.49 | .57 | 3.42 | .043 |
| D19S897 | 2.81 | 2.76 | 2.54 | 2.25 | 1.66 | 1.04 | .47 | 2.81 | .001 |
| D19S881 | 5.14 | 5.04 | 4.63 | 4.09 | 2.99 | 1.84 | .75 | 5.14 | .001 |
| D19S223 | 4.88 | 4.78 | 4.37 | 3.86 | 2.80 | 1.71 | .65 | 4.88 | .001 |
| D19S872 | .19 | .18 | .16 | .12 | .07 | .03 | .01 | .19 | .001 |
| D19S211 | .38 | .37 | .31 | .25 | .15 | .07 | .02 | .38 | .001 |
| D19S420 | 5.37 | 5.26 | 4.83 | 4.28 | 3.12 | 1.92 | .77 | 5.37 | .001 |
| D19S900 | 2.05 | 2.01 | 1.84 | 1.62 | 1.18 | .75 | .33 | 2.05 | .001 |
| D19S412 | −∞ | 2.37 | 2.68 | 2.49 | 1.78 | .92 | .13 | 2.68 | .045 |
| D19S902 | −∞ | −1.81 | −.08 | .40 | .46 | .17 | −.06 | .51 | .154 |
Figure 2.
Multipoint LOD-score analysis of the CMT4F locus in the interval from D19S414 (centromeric) to D19S902 (telomeric). The Y-axis represents the multipoint LOD score (log10 value), and the X-axis represents the genetic distance (in cM) along the interval.
Of all the different genes already mapped to 19q13.1-13.3, the most likely candidate gene for this particular form of CMT was the MAG gene on 19q13.1. Sequencing of its coding sequence was consequently performed in two affected children (patients VI-2 and VI-4) and a control individual. Total RNA was extracted from lymphocytes by use of the total RNA isolation kit (PROMEGA), and the MAG cDNA was sequenced after reverse transcription (M-MLV Reverse Transcriptase [Gibco BRL]) in illegitimate transcripts (Chelly et al. 1991). Fragments used for sequencing were obtained by means of a two-step PCR amplification process using reverse-transcribed cDNA as a template. However, as a result of the high GC content of the amplified products (55%–75%), amplification was obtained only when the PCRx Enhancer System (Gibco BRL) was used, and the 5′ extremity of the cDNA fragment (684 bp) could not be synthesized. Exons 5–7, which corresponded to the unamplified 5′ cDNA sequence, were consequently amplified from genomic DNA. PCR primers and annealing conditions are described in table 2.
Table 2.
Primer Sequences for PCR Amplification and Sequencing of the MAG Coding Sequence
| Reaction and Primer Sequence | Position (5′)a | Amplification- Product Size (bp) | Annealing Temperature (°C) |
| RT+PCR1b: | |||
| 1: | |||
| Forward: 5′-AGGTGCAGAAGCAACTGAGT-3′ | −108 | 1,212 | 58 |
| Reverse: 5′-CGTGGACAGGATCTGCTTCT-3′ | 1225 | ||
| 2: | |||
| Forward: 5′-GGGACAATGGTGGCCGTA-3′ | 996 | 1,084 | 64 |
| Reverse: 5′-AAGGAGGTTAGAGAGGAGCC-3′ | 2100 | ||
| Nested PCR2sb: | |||
| 1a: | |||
| Forward: 5′-ATGGTGCCGGACAACTGC-3′ | 477 | 684 | 56 |
| Reverse: 5′-GCTTCTCCTTGAAGATGGTGA-3′ | 1090 | ||
| 2a: | |||
| Forward: 5′-GGGGGAGACGGTCTCTAT-3′ | 1016 | 568 | 56 |
| Reverse: 5′-GGCAATCAGGATGGCAAAGG-3′ | 1584 | ||
| 2b: | |||
| Forward: 5′-TTCCAGGGAGCCCATCGA-3′ | 1506 | 505 | 64 |
| Reverse: 5′-CCTCACTGTCACCTGCCT-3′ | 2011 | ||
| 2b′: | |||
| Forward: 5′-AGAAGTACGAGAGAGCGAGAGG-3′ | 1705 | 305 | 60 |
| Reverse: 5′-CCTCACTGTCACCTGCCT-3′ | 2011 | ||
| Genomic PCRsc: | |||
| Exon 4: | |||
| Forward: 5′-GTGTCACTGCATTCCAGCCC-3′ | IVS3-207 | 375 | 56 |
| Reverse: 5′-GTCCTACCCGATCACCCC-3′ | IVS4+120 | ||
| Exon 5: | |||
| Forward: 5′-CTGAGCAGGTTGGAGGTGGG-3′ | IVS4-117 | 578 | 66 |
| Reverse: 5′-TGCTGGGGAAGGTGCGGG-3′ | IVS5+92 | ||
| Exon 6: | |||
| Forward: 5′-GGAGGCGATACCGTTGAGGAG-3′ | IVS5-128 | 502 | 56 |
| Reverse: 5′-CCGCATCCCCAGGTCCAC-3′ | IVS6+77 | ||
| Exon 7: | |||
| Forward: 5′-GACAGTGTTGGGGGCGGG-3′ | IVS6-91 | 558 | 56 |
| Reverse: 5′-AAGGAGGATGAGGCAGAGCAGCAGG-3′ | IVS7+208 |
Amplification products were sequenced in both directions, with use of the Big Dye Terminator Cycle Sequencing Ready Reaction Kit (PE Biosystems) on an ABI 377 sequencer (PE Biosystems). Electrophoregrams were analyzed using Sequence Analysis software, version 3.3 (PE Biosystems). Sequences were compared with reference sequences, by use of Sequence Navigator, version 1.0.1 (PE Biosystems). The reference sequences for cDNA (GenBank accession number M29273) and genomic DNA (GenBank accession number AC002132) were chosen from the GenBank sequence library.
The results of sequencing of the MAG coding region in two affected patients and a control individual showed neither a base change leading to an amino acid change nor a deletion, but some discrepancies were observed between the originally published cDNA sequence (Sato et al. 1989) and the cDNA and genomic DNA sequences used as references. A 399C→T change in exon 5, a 1140C→G change in exon 8, and a 1981C→G change in the 3′ UTR region were not taken into consideration, considering that 399T, 1140G, and 1981G were correct in any of the three sequences. Finally, 1989insG was observed in the 3′ UTR region in the affected patients as well as in the control individual, suggesting a polymorphism or an error in the originally published cDNA sequence. Alternative splicing of the 45-bp exon 12 (GenBank accession number X98405) was observed in the two affected patients as well as in the control individual. A 1209C/T polymorphism was detected in patients VI-2 and VI-4 and in the control individual. Since patient VI-4 was heterozygous for the polymorphism, the affected sibs (patients VI-1, VI-3, and VI-6), the unaffected brother (patient VI-5), and the parents (patients V-1 and V-2) were also tested for the polymorphism. The other branch of the family was not tested for this polymorphism, since mRNA was not available. Patients VI-1–VI-3 and patient VI-6 were homozygous for the change, whereas the affected sister (patient VI-4), her unaffected brother (patient VI-5), and the parents were heterozygous for the change (fig. 1). Assuming identity by descent and considering that patient VI-4 presented a recombination at marker D19S220, we were able to place MAG outside the homozygous candidate region because of the heterozygosity for this polymorphism in patient VI-4.
In the present study, we report a large inbred Lebanese family in whom affected members present the heterogeneous neurological defects commonly seen in autosomal recessive CMT4. Five pathological forms, CMT4A–E, are now recognized (Ben Othmane et al. 1993; Bolino et al. 1996; Kalaydjieva et al. 1996; Leguern et al. 1996; Warner et al. 1998; Ben Othmane et al. 1999). Clinical descriptions of these different forms and of the form CMT4F presented here are summarized in table 3. Although the clinical symptoms of the affected sibs in this report are similar to—if somewhat more severe than—those in the other forms of CMT4, there is enough genetic evidence to suggest a new CMT4F subtype. Furthermore, the results of a genomewide search excluded linkage to previously described autosomal recessive CMT4 loci and placed the gene of interest distal to D19S220 and proximal to D19S412, over an 8.5-cM interval at 19q13.1-13.3. The positive pairwise LOD score of 5.37 for D19S420, at θ=.00, and the maximum multipoint LOD score of 10.3 for D19S881, at θ=.00, strongly supported linkage of the disease gene to the aforementioned region.
Table 3.
Major Clinical Features of Autosomal Recessive Demyelinating CMT4
|
Clinical Signsa |
|||||||||
| CMT4 Subtype(Location) | Age at Onset | Muscle Weakness | Tendon Reflexes | Sensory Loss | Disease Progression | KS | FootDeformity | MNCVb(m/s) | Pathological Characteristicsc |
| CMT4A (8q13-21) | Early childhood | Distal | − | Mild | Incapacity, 1st decade | + | + | Reduced, ∼30 | Hypomyelination; loss of MF; presence of basal laminal onion bulbs |
| CMT4B1 (11q23) | 2–4 years | Distal and proximal | − | Moderate | Death, 4th decade | + | + | Slow, 15–17 | Loss of MF; presence of focally folded myelin sheaths |
| CMT4B2 (11p15) | <20 years | Distal and proximal | − | Moderate | Incapacity, 3d–4th decade | + | + | Reduced to slow, 15–30 | Loss of MF; presence of focally folded myelin sheaths |
| CMT4C (5q32) | Childhood and adolescence | Delayed walking | Reduced | Mild | Increased disability | + | + | Reduced, ∼24 | Loss of MF; presence of few classical onion bulbs |
| CMT4D (8q24) | Childhood | Distal | − | Moderate, deafness | Incapacity, 5th decade | + | + | Slow, 10–15 | Loss of MF; presence of onion bulbs |
| CMT4E (10q21-22) | Birth | Distal | − | Moderate | Increased disability | − | + | Slow, 9–20 | Thin or absent myelin sheaths; presence of onion bulbs |
| CMT4F (19q13.1-13.3) | Early childhood | Distal | − | Severe | Slow progression | + | + | Not detectable | Severe loss of MF; presence of onion bulbs |
A plus sign (+) denotes presence; a minus sign (−) denotes absence. KS = kyphoscoliosis.
MNCV = motor NCV.
MF = myelinated fibers.
Of all the different genes already mapped to 19q13.1-13.3, the most likely candidate gene for this particular form of CMT was the MAG gene on 19q13.1. Indeed, although the role of MAG in myelination is still unclear (Attia et al. 1989; Li et al. 1994), its gene encodes a transmembrane glycoprotein of the CNS and the peripheral nervous system that is thought to play a role in the formation and maintenance of the myelin sheaths (Quarles et al. 1983–84). Furthermore, the presence of anti-MAG antibodies in the serum of patients affected with some demyelinating sensory or sensorimotor neuropathies and the overexpression of a shorter alternatively spliced MAG mRNA in dysmyelinating mutant mice have been reported (Frail et al. 1985; Sato et al. 1986). Nevertheless, none of the abnormalities usually observed in nerve-biopsy specimens from mice, which have a knocked-out MAG gene (Montag et al. 1994), were observed in the nerve-biopsy specimen from patient VI-1. Also, sequencing of the entire MAG gene-coding region in two affected patients revealed neither a base change nor a deletion, most probably excluding a point mutation in the coding region as the cause of the disease. Finally, heterozygosity for the 1209C/T polymorphism in one of the affected children placed the MAG gene outside the homozygous candidate region and definitely excluded this gene as the gene responsible for the disease (fig. 1).
Thus, refinement of the candidate interval and identification of the gene will need further investigations in this family and in families presenting the same new CMT4F subtype. Identification of the gene will improve understanding of the pathogenesis of autosomal recessive CMT4 disease.
Acknowledgments
We are grateful to the members of the family, who were always very cooperative despite their difficult health situation. We wish to address special thanks to Professor G. Lefranc, for his continuous help and encouragement. We acknowledge Nabiha Salem and Anne Mornand, for their technical help; Dr. Salim Adib, for his review; and Professor Vallat, for his comments on the histopathologic findings. We would like to acknowledge the Institut Electricité-Santé de France and Alsthom, for their donation to the Laboratoire de Génétique Moléculaire, Montpellier, France, and the Université Saint-Joseph, for financial support of V.D.’s fellowship. The clinical part of this work was supported by grants from the Jérôme Lejeune Foundation, and the technical part was supported by a grant from the Laboratoire de Génétique Moléculaire, Montpellier, France.
Electronic-Database Information
Accession numbers and URLs for data in this article are as follows:
- GenBank, http://www.ncbi.nlm.nih.gov/Genbank/index.html (for reference sequences for cDNA [accession number M29273] and genomic DNA [accession number AC002132], and for exon 12 [accession number X98405])
- Généthon, http://www.Genethon.fr/ (for human linkage map and markers' allele frequencies)
- Geneclinics: Medical Genetics Knowledge Database, http://www.geneclinics.org (for clinical and genetic information about different CMT types)
- Genome Database, http://gdbwww.gdb.org/ (for maps and markers' allele frequencies)
- National Center for Biotechnology Information, http://www.ncbi.nlm.nih.gov/ (for information on genes, expressed-sequence tags, or sequence-tagged sites assigned to a genetic locus, Genemap'99, information on genes, BLAST search, and retrieval of sequences)
- Neuromuscular/Hereditary Motor Sensory Neuropathies, http://www.neuro.wustl.edu/neuromuscular/time/hmsn.html (for information on mapped loci associated with hereditary motor and sensory neuropathies)
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for CMT [MIM 118300]; CMT4A [MIM 214400]; CMT4B1 [MIM 601382]; CMT4B2 [MIM 604563], CMT4C, known as “CMT neuropathy, demyelinating” [MIM 601596]); CMT4D, known as “neuropathy, hereditary motor and sensory, Lom type” [MIM 601455]; and the MTMR2 gene [MIM 603557])
References
- Attia J, Tropak M, Johnson PW, Newerly-Abranow W, Pawson T, Roder JC, Dunn RJ (1989) Modulated adhesion: a proposal for the role of myelin-associated glycoprotein in myelin wrapping. Clin Chem 35:717–720 [PubMed] [Google Scholar]
- Bell C, Haites N (1997) The peripheral neuropathies and their molecular genetics. Adv Genet 36:1–44 [DOI] [PubMed] [Google Scholar]
- Ben Othmane K, Hentati F, Lennon F, Ben Hamida C, Blel S, Roses AD, Pericack-Vance MA, et al (1993) Linkage of a locus (CMT4A) for autosomal recessive Charcot-Marie-Tooth disease to chromosome 8q. Hum Mol Genet 2:1625–1628 [DOI] [PubMed] [Google Scholar]
- Ben Othmane K, Johnson E, Menold M, Graham FL, Ben Hamida M, Hasegawa O, Rogala AD, et al (1999) Identification of a new locus for autosomal recessive Charcot-Marie-Tooth disease with focally folded myelin on chromosome 11p15. Genomics 62:344–349 [DOI] [PubMed] [Google Scholar]
- Bolino A, Brancolini V, Bono F, Bruni A, Gambardella A, Romeo G, Quattrone A, et al (1996) Localization of a gene responsible for autosomal recessive demyelinating neuropathy with focally folded myelin sheaths to chromosome 11q23 by homozygosity mapping and haplotype sharing. Hum Mol Genet 5:1051–1054 [DOI] [PubMed] [Google Scholar]
- Bolino A, Muglia M, Conforti FL, LeGuern E, Salih MAM, Georgiou DM, Christodoulou K, et al (2000) Charcot-Marie-Tooth type 4B is caused by mutations in the gene encoding myotubularin-related protein-2. Nat Genet 25:17–19 [DOI] [PubMed] [Google Scholar]
- Chelly J, Gilgenkrantz H, Hugnot JP, Hamard G, Lambert M, Recan D, Akli S, et al (1991) Illegitimate transcription: application to the analysis of truncated transcripts of the dystrophin gene in nonmuscle cultured cells from Duchenne and Becker patients. J Clin Invest 88:1161–1166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dib C, Fauré S, Fizames C, Samson D, Drouot N, Vignal A, Millasseau P, et al (1996) A comprehensive genetic map of the human genome based on 5,264 microsatellites. Nature 380:152–154 [DOI] [PubMed] [Google Scholar]
- Dyck PJ, Chance P, Lebo R, Carney JA (1993) In: Dyck PJ, Thomas PK, Griffin JW, Low PA, Podulso JF (eds) Hereditary, motor and sensory neuropathies. Philadelphia WB Saunders, Philadelphia, pp 1094–1136 [Google Scholar]
- Frail DE, Braun PE (1985) Abnormal expression of the myelin-associated glycoprotein in the central nervous system of dysmyelinating mutant mice. J Neurochem 45:1071–1075 [DOI] [PubMed] [Google Scholar]
- Kalaydjieva L, Hallmayer J, Chandler D, Savov A, Nikolova A, Angelicheva D, King RHH, et al (1996) Gene mapping in Gypsies identifies a novel demyelinating neuropathy on chromosome 8q24. Nat Genet 14:214–217 [DOI] [PubMed] [Google Scholar]
- Lander ES, Botstein D (1987) Homozygosity mapping: a way to map human recessive traits with the DNA of inbred children. Science 236:1567–1570 [DOI] [PubMed] [Google Scholar]
- Lathrop G, Lalouel J, Julier C, Ott J (1985) Multilocus linkage analysis in humans: detection of linkage and estimation of recombination. Am J Hum Genet 37:482–498 [PMC free article] [PubMed] [Google Scholar]
- Lefranc G, Rivat G, Serre JL, Lalouel JM, Pison G, Loiselet J, Ropartz C, et al (1978) Common and uncommon immunoglobulin haplotypes among Lebanese communities. Hum Genet 41:197–209 [DOI] [PubMed] [Google Scholar]
- Leguern E, Guilbot A, Kessali M, Ravisé N, Tassin J, Maisonobe T, Grid D, et al (1996) Homozygosity mapping of an autosomal recessive form of demyelinating Charcot-Marie-Tooth disease to chromosome 5q23-q33. Hum Mol Genet 5:1685–1688 [DOI] [PubMed] [Google Scholar]
- Li C, Tropak MB, Gerlai R, Clapoff S, Abramov-Newerly W, Trapp B, Peterson A, et al (1994) Myelination in the absence of myelin-associated glycoprotein. Nature 369:747–750 [DOI] [PubMed] [Google Scholar]
- Miller SA, Dynes DD, Polesky HF (1988) A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res 16:1215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montag D, Giese KP, Bartsch U, Martini R, Lang Y, Blüthmann H, Karthigasan J, et al (1994) Mice deficient for the myelin-associated glycoprotein show subtle abnormalities in myelin. Neuron 13:229–246 [DOI] [PubMed] [Google Scholar]
- Quarles RH (1983–84) Myelin-associated glycoprotein in development and disease. Dev Neurosci 6:285–303 [DOI] [PubMed] [Google Scholar]
- Sato S, Baba H, Inuzuka T, Miyatake T (1986) Anti–myelin-associated glycoprotein antibodies in sera from patients with demyelinating disease. Acta Neurol Scand 74:115–120 [DOI] [PubMed] [Google Scholar]
- Sato S, Fujita N, Kurihara T, Kuwano R, Sakimura K, Takahashi Y, Miyatake T (1989) cDNA cloning and amino acid sequence for human myelin-associated glycoprotein. Biochem Biophys Res Commun 163:1473–1780 [DOI] [PubMed] [Google Scholar]
- Sheffield VC, Nishimura DY, Stone EM (1995) Novel approaches to linkage mapping. Curr Opin Genet Dev 5:335–341 [DOI] [PubMed] [Google Scholar]
- Skre H (1974) Genetic and clinical aspects of Charcot-Marie-Tooth disease. Clin Genet 6:98–118 [DOI] [PubMed] [Google Scholar]
- Warner E, Mancias P, Butler IJ, Mc Donald CM, Keppen L, Koob KG, Lupski JR (1998) Mutations in the early growth response 2 (EGR2) gene are associated with hereditary myelinopathies. Nat Genet 18:382–384 [DOI] [PubMed] [Google Scholar]