Abstract
The mechanism(s) by which Mycobacterium tuberculosis crosses the alveolar wall to establish infection in the lung is not well known. In an attempt to better understand the mechanism of translocation and create a model to study the different stages of bacterial crossing through the alveolar wall, we established a two-layer transwell system. M. tuberculosis H37Rv was evaluated regarding the ability to cross and disrupt the membrane. M. tuberculosis invaded A549 type II alveolar cells with an efficiency of 2 to 3% of the initial inoculum, although it was not efficient in invading endothelial cells. However, bacteria that invaded A549 cells were subsequently able to be taken up by endothelial cells with an efficiency of 5 to 6% of the inoculum. When incubated with a bicellular transwell monolayer (epithelial and endothelial cells), M. tuberculosis translocated into the lower chamber with efficiency (3 to 4%). M. tuberculosis was also able to efficiently translocate across the bicellular layer when inside monocytes. Infected monocytes crossed the barrier with greater efficiency when A549 alveolar cells were infected with M. tuberculosis than when A549 cells were not infected. We identified two potential mechanisms by which M. tuberculosis gains access to deeper tissues, by translocating across epithelial cells and by traveling into the blood vessels within monocytes.
Infection caused by M. tuberculosis represents one of the great tragedies in world history. Approximately 3 million people die annually of the disease (7), despite the availability of cheap, efficacious, and curative therapy for tuberculosis.
Once more, it seems clear that the improvement of the knowledge about the mechanisms employed by M. tuberculosis to infect the host will certainly offer new opportunities for the development of both effective therapy and vaccine.
M. tuberculosis is inhaled into the respiratory tract, eventually reaching the alveolar space. It has been assumed that the bacterium is ingested by alveolar macrophages and subsequently gains access to the bloodstream by being transported by the alveolar macrophages and blood monocytes across the alveolar wall (10). Recently, however, it was demonstrated by several groups that M. tuberculosis invades and survives within human type II alveolar epithelial cells in vitro (3, 14, 17), and a possible role for alveolar epithelial cells in vivo has been postulated. In fact, the chance that M. tuberculosis would encounter an alveolar epithelial cell (the average human male has 1,500 type II and 28,000 type I alveolar epithelial cells [22]) is significantly greater than encountering an alveolar macrophage (50 macrophages per alveolus [8]). Therefore, the participation of type II alveolar epithelial cells, alveolar macrophages, and blood monocytes in the translocation of M. tuberculosis across the alveolar wall is currently poorly understood. Previous work has established the use of an in vitro model with a bilayer with alveolar epithelial cells and human lung endothelial cells (6). Using this model, it was shown that M. tuberculosis does not cross the bilayer with great efficiency and that monocytes migrate from the lower chamber to the upper chamber of the epithelial cell bilayer, following the addition of M. tuberculosis to the upper chamber.
In this work, we investigated (i) if M. tuberculosis invades endothelial cells, (ii) if M. tuberculosis is able to cross a polarized bilayer of epithelial cells and endothelial cells, (iii) if alveolar macrophages (and/or monocytes) translocate across the epithelial-endothelial bilayer when infected with M. tuberculosis, (iv) if the infection of alveolar epithelial cells has any influence on the translocation of mononuclear phagocytes across the alveolar wall, and (v) the role of receptors such as CD11a, CD11b, very late antigen 4 (VLA-4), and intercellular adhesion molecule 1 (ICAM-1), among others, in the migration of phagocytic cells across the epithelial-endothelial bilayer.
MATERIALS AND METHODS
Bacterial strains.
M. tuberculosis H37Rv was obtained from American Tissue Culture Collection (Rockville, Md.). Mycobacterium avium strain 101 was isolated from the blood of an AIDS patient (13). Mycobacterium bovis BCG strain Pasteur was a gift from Brigitte Gicquel (Institut Pasteur, Paris, France). All the strains were grown on Middlebrook 7H11 agar, and after selection of pure colonies they were transferred to 7H9 broth for 7 days at 37°C in 5% CO2. Cultures were passed through a 23-gauge needle 10 times, and then the tube containing the suspension was kept on the bench for 5 min. The top half of the suspension was obtained and stained for viability using the LIVE-DEAD assay (Molecular Probes, Eugene, Oreg.) and used in the experiments (5). The suspension was constituted of dispersed bacteria.
Purification of monocytes.
Blood from purified protein derivative-negative donors was collected with heparin-containing tubes and submitted to a process of purification as previously described (2). Monocytes were then enriched by adherence to the plastic and subsequently resuspended by treatment of the monolayer with 0.1 M EDTA for 15 min. Monocytes were washed and resuspended in RPMI 1640 supplemented with 10% fetal bovine serum (FBS) and 2 mM L-glutamine. Monocytes in suspension in RPMI 1640 (Gibco BRL, Grand Island, N.Y.) in a Teflon jar were examined for viability by using trypan blue exclusion as described (2). Cells were approximately 95% viable.
Tissue culture.
A549 human type II alveolar epithelial cells were purchased from American Type Culture Collection. Approximately 105 cells were suspended in RPMI 1640 medium supplemented with 10% FBS, and 104 cells were added to each well of a 24-well tissue culture plate (Costar Corp., Cambridge, Mass.). Cells were allowed to grow to 100% confluence or, in some experiments, 80% confluence. The EAhy926 human endothelial cell line (a permanent endothelial cell line established by the hybridization of human umbilical vein endothelial cells and A549 cells) was kindly provided by Cora-Jean Edgell (University of North Carolina) (9). EAhy926 cells were grown in Dulbecco’s minimum essential medium (Difco, Detroit, Mich.) supplemented with 10% FBS, endothelial growth factor (Sigma Chemicals Co., St. Louis, Mo.), and 10 U of heparin per ml. The cells were cultured to complete confluence or, in some experiments, to 80% confluence. EAhy926 expresses human factor VIII-related antigen and produces prostacyclin.
Transwell-polarized monolayer.
A transwell insert (Costar) with 3.0-μm pores was placed in each well of a 24-well tissue culture plate (Costar). To construct a polarized monolayer, the transwell insert was inverted (i.e., the bottom part was placed towards the top), and 105 EAhy926 cells were placed on the bottom side of the membrane and allowed to be established (usually 48 h). The cells were then allowed to grow to confluence (approximately 5 × 108 A549 cells and 7 × 108 EAhy926 cells). The transmembrane resistance was measured every 2 days until it reached approximately 520 ± 63 Ω/cm2 for A549 cells and 486 ± 35 Ω/cm2 for EAhy926 cells, and the integrity of the monolayers was determined with 125I-inulin (ICN, Costa Mesa, Calif.) and, in some experiments, 0.1 ml of 1% blue dextran 2000 (Pharmacia, San Diego, Calif.). The content of the lower chamber after 2 h was retrieved, and the amount of radioactive material was measured in a gamma radiation counter or the optical density was determined.
To construct a bilayer culture, filters were seeded both in upside-down (top up) and upright positions. The A549 alveolar epithelial cells seeded in the upper part and EAhy926 cells were seeded in the bottom. Briefly, filters were placed in inverted position (upside down) and 105 EAhy926 cells were seeded first in F-12 medium on the bottom side of the filter, supplemented with 10% FBS and endothelial growth factor (0.5%) (Sigma), and allowed to establish for 48 h. Then, the filter was inverted back to the upright position, and 105 A549 cells were seeded in F-12 medium supplemented with 10% FBS. Endothelial growth factor was added to the bottom chamber every time the medium was changed (every 4 days). The confluence of the monolayers and the resistance were monitored daily. The bilayer was considered ready for use when the transmembrane resistance achieved approximately 546 ± 33 Ω/cm3, which took an average of 10 to 12 days. The permeability of the bilayer was measured as previously reported (21) and as described above.
Infection of monolayers and bilayers.
Monolayers were infected with 107 bacteria (approximate ratio, 10 bacteria to one cell) for different periods of time. Then, the supernatant was removed, and the wells were extensively washed with Hank’s buffered salt solution (HBSS). Afterward, amikacin (200 μg/ml) was added for 2 h at 37°C to kill the extracellular bacteria remaining in the well. Amikacin at a concentration of 200 × the MIC kills the majority of bacteria in the wells and inhibits the adherence of the microorganisms that survive (3, 17). The monolayer was then lysed with 1% Triton X-100 in HBSS for 15 min, and the suspension was plated onto 7H11 agar after dilution.
In the case of bilayers, the medium was removed, the cells were washed once with HBSS, and medium and 105 bacteria were added to the top chamber. Passage of the bacteria across the layers of cells was monitored by collecting the supernatant in the bottom chamber. Transmembrane resistance was also monitored during the course of the experiment.
In some assays, M. tuberculosis was used after passage in either A549 epithelial cells or human monocytes for 3 days. To obtain M. tuberculosis, A549 cells or human monocyte monolayers were infected with H37Rv or H37Ra (100 bacteria to one cell) for 1 h, and the extracellular bacteria were removed afterwards by two consecutive washings. The intracellular bacteria were allowed to replicate and were obtained after 3 days by a previously described method (5). The viability of the bacteria was determined by using the LIVE-DEAD assay (5), and the number of organisms and the purity of preparation were determined by plating bacteria on 7H11 agar and microscopic examination, as described (5). Bacteria obtained this way were kept at 4°C for 24 h before the assay. This period at 4°C did not change the characteristics of the inoculum (data not shown).
Translocation of monocytes.
To evaluate the role of blood monocytes and alveolar macrophages in the transport of M. tuberculosis from the alveolar space to the blood, we used infected and uninfected blood monocytes and measured their translocation across the bilayer (epithelial and endothelial cells) with the transwell system. Monocytes were used uninfected or infected with bacteria for 1 h (10 bacteria per monocyte) in suspension under constant rotation at 37°C. After 1 h, monocytes were centrifuged at 400 × g for 5 min, and the pellet was examined for viability and the approximate percentage of intracellular bacteria by trypan blue exclusion and acid-fast staining by phase-contrast microscopy, respectively. Only preparations that contained more than 90% viable monocytes were used in the experiment. In addition, only preparations that contained at least 60% of the monocytes infected (as determined by counting 300 cells in 10 fields) by one or more bacteria were used in the assays (as evidenced by phase-contrast microscopy with an image-enhancing system).
Infected monocytes were added to the top chamber in the transwell, and translocation was measured as the number of monocytes that crossed the bilayer over time. In some experiments, A549 epithelial cells in the transwell were infected with 105 bacteria for 1 h, and then the supernatant containing extracellular bacteria was changed by the addition of fresh medium before monocytes were added.
Chemokine production and neutralization.
Previous work has shown that A549 cells produce interleukin 8 (IL-8) when infected with M. tuberculosis. To determine if two chemokines important for phagocytic cell migration, IL-8 and macrophage chemoattractant protein 1 (MCP-1), had a role in the chemotaxis of infected monocytes across the cell bilayer, we performed the assays described above in the presence and absence of anti-IL-8 (Biosource International, Camarillo, Calif.) and anti-MCP-1 (R & D Systems Minneapolis, Minn.) antibodies. In addition, the concentration of both chemokines in both the upper and bottom chamber supernatants was measured by assays purchased from Biosource International. Irrelevant antibodies, mouse anti-human immunoglobulin G (IgG) and rat anti-human IgG, were used as controls. Enzyme-linked immunosorbent assays had a sensitivity of 20 (MCP-1) and 5 (IL-8) pg/ml.
Role of receptors in the translocation of monocytes.
To determine the receptors used by monocytes to cross the alveolar wall, we carried out translocation assays with bilayer A549 epithelial cells and endothelial cells in the presence of mouse anti-human antibodies to CD11a (clone 12101, mouse IgG; Biosource), CD11b (clone L-MO-1, mouse IgG; Biosource), ICAM-1 (clone 84A6, mouse IgG; Biosource), anti-CD11c (clone 3.9, mouse IgG; Biosource), CD29 (clone PD-15, mouse IgG; Biosource), anti-VLA-4 (clone VD-46, Upstate Biotechnology, Inc., Lake Placid, N.Y.), anti-CD51 (clone 23C6, mouse IgG; Biosource), anti-CD26 (clone SMO, mouse IgM; Biosource), anti-CD14 (clone LO-MD-1, rat IgG; Biosource), and anti-CD47 (clone BRIC 126, mouse IgG; Biosource). Antibodies at three different concentrations were added 30 min before infection, and cells were incubated at 37°C. Then, the monolayers were infected for different periods of time, and translocation was measured as described above.
Statistical analysis.
Each experiment was repeated at least three times, and the results were analyzed using Student’s t test.
RESULTS
Does M. tuberculosis cross an A549 polarized monolayer?
To examine whether M. tuberculosis crosses a polarized intact A549 monolayer, we performed the assay in comparison to M. bovis BCG and measured translocation after different periods of time. As shown in Table 1, M. tuberculosis was capable of crossing the intact monolayer with greater efficiency than M. bovis BCG. Crossing by M. tuberculosis was significant only after 4 h of incubation. The transmembrane resistance was constantly measured and showed to be unaltered at 4 h. Five days after infection, 4% of the inoculum had translocated across the A549 membrane; however, the fall in the transmembrane resistance suggested that the integrity of the monolayer was compromised. In fact, by using blue dextran added to the upper chamber as a marker for membrane integrity, we were able to determine that the polarized membrane allowed the passage of a small amount of dextran.
TABLE 1.
Translocation of M. tuberculosis H37Rv and M. bovis BCG across the A549 polarized monolayera
| Bacteria | Time point | Resistance (Ω/cm2) | % Bacterial inoculum |
|---|---|---|---|
| M. bovis BCG Pasteur | 1 h | 520 | 0 |
| 4 h | 523 | 0 | |
| 24 h | 526 | 0.02 | |
| 48 h | 524 | 0.03 | |
| 5 d | 520 | 0.5 ± 0.01 | |
| M. tuberculosis H37Rv | 1 h | 535 | 0 |
| 2 h | 523 | 0.001 ± 0.0006 | |
| 4 h | 527 | 0.36 ± 0.04 | |
| 24 h | 511 | 0.86 ± 0.06 | |
| 48 h | 491 | 1.6 ± 0.3b | |
| 5 d | 460 | 5.3 ± 0.4b |
A ratio of 10 bacteria to one cell (107 bacteria) was used. Monolayers were constructed as described in Materials and Methods. Experiments were repeated three times; the results shown represent the mean ± standard deviation of the data. d, days.
Monolayers showed evidence of loss of integrity.
M. tuberculosis invasion of endothelial cells.
The ability of M. tuberculosis to invade endothelial EAhy926 cells was first evaluated using a endothelial cell monolayer seeded on plastic. As shown in Table 2, M. bovis BCG and M. tuberculosis strains H37Rv and H37Ra invaded EAhy926 poorly, in agreement with the results reported with human lung endothelial cells (6). However, if M. tuberculosis was passed in A549 cells or human macrophages for 3 days, the efficiency of the invasion increased by more than 10-fold. The same behavior was not observed with M. bovis BCG (data not shown).
TABLE 2.
Ability of M. bovis BCG and M. tuberculosis to invade EAhy926 endothelial cells
| Bacteriaa | Invasion after 1 h (% of inoculum) |
|---|---|
| BCG | 0.08 ± 0.004 |
| H37Rv | 0.13 ± 0.03 |
| H37Ra | 0.08 ± 0.003 |
| H37Rv (A549)b | 12.1 ± 1.2c |
| H37Rv (Mo)b | 18.4 ± 1.6c |
Bacteria were added at a ratio of approximately 10 bacteria to one cell.
M. tuberculosis used was passed in A549 cells or human macrophages for 3 days as described in Materials and Methods.
P < 0.001 compared with the other experimental groups.
We then evaluated if the same sort of results were observed with monolayers of polarized EAhy926 cells. Cells were seeded upside down to mimic the contact between M. tuberculosis in the alveolar space and the basolateral surface of endothelial cells. As shown in Table 3, only M. tuberculosis previously passed in A549 cells was able to cross EAhy926 cells with significant efficiency.
TABLE 3.
Translocation of M. tuberculosis, M. avium, and M. bovis BCG across EAhy926 endothelial cell polarized monolayers
| Bacteriaa | Time point (h) | % Inoculumc | Transmembrane resistance (Ω/cm2) |
|---|---|---|---|
| M. bovis BCG | 1 | 0 | 495 |
| 2 | 0 | 489 | |
| 4 | 0 | 493 | |
| 24 | 0 | 493 | |
| M. tuberculosis | |||
| H37Rv | 1 | 0 | 497 |
| 2 | 0 | 497 | |
| 4 | 0 | 486 | |
| 24 | 0.01 | 490 | |
| H37Rv (A549)b | 1 | 4 ± 2* | 494 |
| 2 | 6 ± 1* | 494 | |
| 4 | 7 ± 2* | 492 | |
| 24 | 9 ± 3* | 491 | |
| M. avium 101 | 1 | 0 | 491 |
| 2 | 0 | 487 | |
| 4 | 0 | 492 | |
| 24 | 0 | 495 |
Ratio of bacteria to cells of 10:1.
M. tuberculosis was passed in A549 cells for 3 days.
*, P < 0.05 compared with the translocation of M. bovis BCG, M. tuberculosis H37Rv, and M. avium.
Translocation across bilayer.
Translocation of M. tuberculosis across the bilayer was significantly greater than the translocation of M. bovis BCG. When M. tuberculosis used in the bilayer assay was derived from macrophages or A549 cells, the efficiency of translocation was significantly higher than with bacteria that were not passed in macrophages or A549 cells (Table 4), suggesting that an invasive phenotype might have emerged when the bacterium was exposed to the intracellular environment.
TABLE 4.
Translocation of bacteria across polarized bilayer of epithelial-endothelial cells
| Bacteriaa | % Inoculum atb:
|
|||
|---|---|---|---|---|
| 2 h | 4 hc | 24 hc | 48 hc | |
| BCG (Pasteur) | 0 | 0 | 0 | 0.01 ± 0.0003 |
| H37Ra | 0 | 0 | 0.4 ± 0.02* | 0.6 ± 0.002 |
| H37Rv | 0 | 0.03 ± 0.002 | 0.5 ± 0.004* | 0.7 ± 0.004* |
| H37Ra (A549)d | 0 | 0.03 ± 0.01 | 0.6 ± 0.03* | 0.5 ± 0.05* |
| H37Rv (A549)d | 3.2 ± 0.1 | 6.4 ± 0.3** | 10.7 ± 0.6** | 15.1 ± 0.7** |
| H37Rv (Mo)d | 2.3 ± 0.3 | 4.9 ± 0.5** | 10.4 ± 0.4* | 14.8 ± 0.9** |
Bacteria were added to the culture at a ratio of 10:1 (bacteria to cells). Mo, monocytes.
Transmembrane resistance as well as permeability to dextran was evaluated during the experiment and was shown not to vary significantly from the baseline (546 ± 33 Ω/cm2).
*, P < 0.05 compared with M. bovis BCG as the same time point; **, P < 0.05 compared with H37Rv, H37Ra, and H37 RA (A549) at the same time point.
Bacteria were retrieved from A549 cells and macrophages as described in Materials and Methods.
Mononuclear phagocytes translocate across the A549-EAhy926 bilayer.
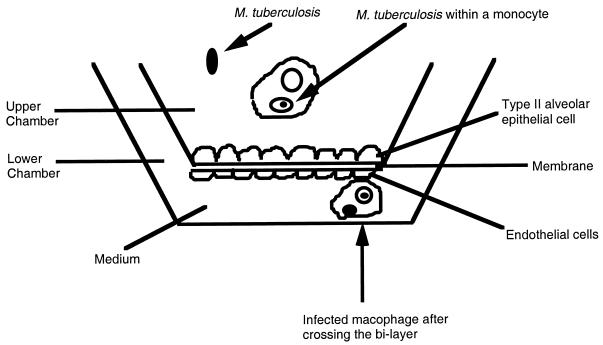
To determine if mononuclear phagocytes would carry M. tuberculosis across the alveolar wall (a bilayer of epithelial and endothelial cells), we infected or did not infect human monocytes and added them to the upper chamber. Figure 1 schematically shows the model used. As shown in Table 5, infected monocytes cross the bilayer barrier very efficiently in contrast to uninfected ones. In some assays, A549 cells were infected with M. tuberculosis prior to the addition of the monocytes. Infection of A549 cells significantly increased monocyte translocation across the bilayer membrane (Table 5). Preliminary data (not shown) established that the passage of M. bovis BCG in A549 cells did not alter its ability to translocate.
FIG. 1.
Schematic representation of the bilayer model used.
TABLE 5.
Translocation of monocytes across a bilayer of A549 and EAhy926 cells
| Experimental groupsa | No. of cells atb:
|
|||
|---|---|---|---|---|
| 1 h | 2 h | 4 h | 24 h | |
| Mo (U) + A549 (U) | 0 | 0 | 1 ± 1 | 14 ± 6 |
| Mo (U) + A549 (I) | 0 | 0 | 8 ± 2c | 76 ± 12c |
| Mo (I) + A549 (U) | 0 | 0 | 38 ± 9cd | 384 ± 29cd |
| Mo (I) + A549 (I) | 0 | 3 ± 1 | 61 ± 11cde | 481 ± 37cd |
A total of 104 monocytes (Mo) were added to the upper chamber. U, uninfected; I, infected (105 bacteria).
Transmembrane resistance from infected cultures did not vary significantly from the uninfected bilayer (526 ± 21 Ω/cm2) at any time point.
P < 0.05 compared with uninfected cells.
P < 0.05 compared with Mo (U) and A549 (I).
P < 0.05 compared with Mo (I) and A549 (U).
Production of IL-8 and MCP-1.
To examine if infected A549 cells produced chemokines that induced monocyte migration, we determined the concentrations of IL-8 and MCP-1 in the supernatants of upper and lower chambers of a bilayer of A549 and EAhy926 cells (Fig. 1). As shown in Table 6, there was a significant increase of the chemokines in both chambers following infection. The lower chamber supernatant showed a greater augmentation of chemokine concentrations than the upper chamber.
TABLE 6.
IL-8 and MCP-1 production in the supernatant of bilayers of A549 and EAhy926 cells after infection with M. tuberculosisa
| Location of supernatant | Time (h) after infection and production (pg/mL)
|
|||
|---|---|---|---|---|
| 4
|
24
|
|||
| IL-8 | MCP-1 | IL-8 | MCP-1 | |
| Lower chamber | 763 ± 70(1) | 932 ± 47 | 1,576 ± 140 | 1,886 ± 126 |
| Upper chamber | 342 ± 31 | 471 ± 44 | 671 ± 82 | 1,063 ± 170 |
Cells were infected with 105 bacteria as described in Materials and Methods. Concentrations (in picograms per milliliter) before infection were as follows: IL-8, 29 ± 6 (upper chamber) and 46 ± 9 (lower chamber); MCP-1, 22 ± 6 (upper chamber) and 34 ± 4 (lower chamber). Considering transmembrane resistance and permeability to dextran, the bilayer did not leak during the course of the experiment.
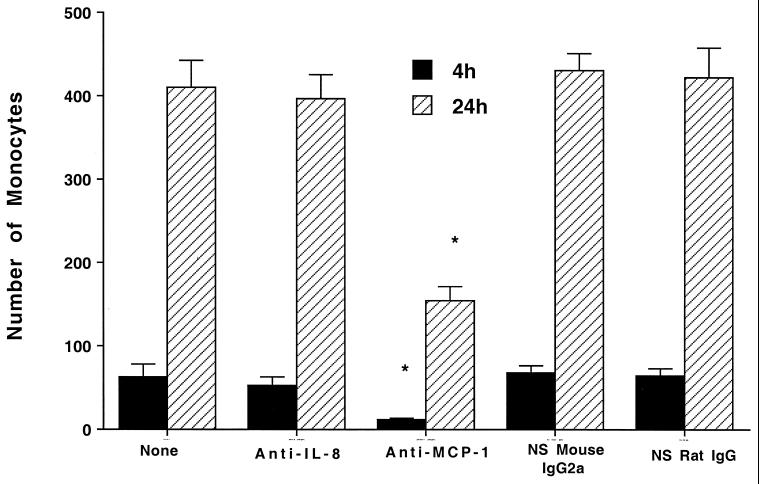
To investigate the relative importance of both chemokines in the migration of monocytes across the bilayer, the bilayer was infected as described in Materials and Methods, followed by the addition of anti-IL-8 and anti-MCP-1 to both chambers, and then 105 infected monocytes were added to the upper chamber and their translocation was determined. Figure 2 shows that the use of neutralizing concentrations of anti-MCP-1 but not anti-IL-8 significantly inhibited the migration of monocytes across the bilayer membrane.
FIG. 2.
Effect of neutralizing antibodies against chemokines on mononuclear cell migration. A concentration of 104 bacteria was added to the monolayers (the top of a bilayer of A549 and EAhy926 cells) and allowed to infect them. Then, neutralizing antibodies were added to both the top and bottom chambers. The concentration of 10 μg of anti-IL-8/ml is known to neutralize 10 ng of IL-8, and 10 μg of anti-MCP-1/ml is known to neutralize 5 ng of MCP-1. After 30 min, 105 infected monocytes were added as described in Materials and Methods. The number of monocytes translocating across the layer was determined over time. *, P < 0.05 compared with control.
Role of cell receptors in monocyte migration.
To identify surface molecules on A549 cells, EAhy926 cells, and monocytes that participate in the process of translocation across the bilayer, we used monoclonal antibodies against several molecules known to be expressed on the host cells and known to be involved in cell translocation. As shown in Table 7, monoclonal antibodies against CD11a, CD11b, and CD47 partially blocked monocyte translocation in a dose-response fashion (only a higher concentration is shown in Table 7). Use of anti-VLA-4 and anti-ICAM-1 had a limited but still significant effect on the crossing of monocytes.
TABLE 7.
Role of cell membrane receptors on the translocation of mononuclear phagocytes across the bilayer of A549 and EAhy926 cells
| Antibodiesa | No. of cells atbc:
|
|||
|---|---|---|---|---|
| 1 h | 2 h | 4 hd | 24 hd | |
| None | 0 | 4 ± 1 | 53 ± 12 | 446 ± 40 |
| Anti-CD11b | 0 | 0 | 15 ± 3* | 132 ± 21* |
| Anti-CD11a | 0 | 1 ± 1 | 29 ± 11 | 163 ± 37* |
| Anti-CD11c | 0 | 4 ± 3 | 57 ± 4 | 431 ± 20 |
| Anti-VLA-4 | 0 | 0 | 43 ± 8 | 297 ± 47* |
| Anti-CD14 | 0 | 4 ± 2 | 58 ± 14 | 474 ± 28 |
| Anti-CD29 | 0 | 3 ± 2 | 64 ± 8 | 409 ± 39 |
| Anti-CD51 | 0 | 5 ± 2 | 57 ± 6 | 429 ± 52 |
| Anti-CD47 | 0 | 0 | 10 ± 2* | 91 ± 8* |
| Anti-ICAM-1 | 0 | 4 ± 1 | 31 ± 6* | 316 ± 41* |
| NR IgG | 0 | 6 ± 2 | 53 ± 16 | 399 ± 51 |
| NR IgM | 0 | 4 ± 1 | 59 ± 12 | 453 ± 32 |
Antibodies were added to the upper chamber at 5, 10, and 20 μg/ml, but only the data obtained with 20 μg/ml are shown. NR, nonrelevant.
The assays were performed using H37Rv-infected A549 cells and H37Rv-infected monocytes. A total of 104 monocytes were added, infected with 105 bacteria.
The transmembrane resistance did not vary significantly from the baseline (before infection and before adding mononuclear phagocytes) (baseline, 531 ± 16 Ω/cm2).
*, P < 0.05 compared with no control.
DISCUSSION
While a number of late events with M. tuberculosis infection of the lung are known, those related to transepithelial migration across the alveolar wall have received less attention. This study defines an in vitro model system of M. tuberculosis interaction with human type II alveolar epithelial cells and endothelial cells in polarized monolayers. In addition, it addresses the participation of mononuclear phagocytes in the process. Using this model, we demonstrated that efficient M. tuberculosis translocation is complex, involving invasion of epithelial cells and bacterial transport by infected mononuclear phagocytes.
The alveolar macrophage is generally thought to be the first line of defense against M. tuberculosis and also involved in the crossing of the alveolar wall by carrying bacilli present in the alveolar space (10, 15). However, this simplistic explanation does not take into account that there are thousands of alveolar epithelial cells to approximately 50 mononuclear phagocytes in the alveolar space. Therefore, even assuming the ability of macrophages to undergo chemostatic migration, the chances are that at least a few M. tuberculosis will establish initial interaction with alveolar epithelial cells.
Evidence during the last several years has emerged about the possible role of alveolar epithelial cells in the mechanisms of M. tuberculosis translocation across the alveolar wall (3, 14, 17). Several laboratories have shown that M. tuberculosis invades and replicates within type II alveolar cells in vitro; however, the observation that M. tuberculosis enters alveolar epithelial cells does not imply a role of the alveolar cells in M. tuberculosis translocation. Although it is possible that epithelial cells in the alveolar wall represent an “end of the line” for those bacilli that get internalized, our data suggest two mechanisms by which uptake of M. tuberculosis by alveolar epithelial cells can be important for translocation: (i) the internalized bacteria cross the epithelial-endothelial barrier, and (ii) uptake of M. tuberculosis by alveolar epithelial cells triggers the release of chemokines, creating a gradient responsible for the migration of infected mononuclear phagocytes. In addition, recent observation suggests that M. tuberculosis needs to bind and perhaps invade epithelial cells in order to disseminate (19).
The alveolar barrier is constituted of alveolar epithelial cells, a basal membrane (permeable), and a layer of endothelial cells. The wall is designed to allow exchange of oxygen and carbon dioxide. M. tuberculosis has been shown to invade alveolar epithelial cells and polarized alveolar epithelial cells (3, 6, 8, 14, 17). In this study, we confirmed that observation and showed that M. tuberculosis but not M. bovis BCG can cross the alveolar epithelial cell monolayer. However, the same is not observed when M. tuberculosis was placed in contact with polarized endothelial cells. In this case, the efficiency of both invasion and translocation was poor. This observation raised the hypothesis that efficient crossing of the alveolar wall would not occur unless the M. tuberculosis phenotype was altered by infection of alveolar epithelial cells. This hypothesis was based on previous results demonstrating that M. tuberculosis-dependent eukaryotic cell cytotoxicity was enhanced by prior infection of epithelial cells (16) and that invasion of macrophages by M. avium is significantly increased by the crossing of intestinal epithelial cells (21). The observation in this study that M. tuberculosis enters as well as translocates across endothelial cells with a severalfold increase in efficiency when it is passed through either macrophages or alveolar epithelial cells once more shows that change of phenotype following a period of intracellular life is a constant in mycobacterial infections. In fact, this phenomenon could be observed in vitro and in vivo in M. avium (4, 5) and M. tuberculosis (12) infection. Therefore, when added to a system containing a bilayer constituted of alveolar epithelial-endothelial cells, M. tuberculosis is capable of efficiently crossing the cells mimicking the alveolar wall. While M. tuberculosis develops an invasive phenotype within alveolar epithelial cells, the same is not true for M. bovis BCG (data not shown).
Once M. tuberculosis reaches the alveolar space, it can be ingested by alveolar macrophages and by blood monocytes attracted to the alveolar space by a gradient of chemokines. Previous studies have demonstrated that infection of A549 epithelial cells with M. tuberculosis H37Rv induces the release of IL-8 and MCP-1, among other chemokines (14), and Birkness and colleagues (6) have shown that adding a polarized bilayer of M. tuberculosis to the upper chamber causes migration of mononuclear phagocytes through the cellular bilayer, probably by inducing chemokine release by alveolar epithelial cells. We now extended the observation by showing that the most efficient translocation of M. tuberculosis across the alveolar wall model is when both monocytes and epithelial cells are infected. One of the reasons for this finding is that chemokine (mainly MCP-1) release by alveolar epithelial cells creates a gradient between the alveolar side and the endothelial side, resulting in stimulation of monocyte migration. This migration can be partially abrogated by the use of anti-MCP-1 antibody. Interestingly, IL-8 appears not to have any role in the translocation of M. tuberculosis-infected monocytes across the epithelial-endothelial cell bilayer. However, the presence of IL-8 might be important to the influx of neutrophils commonly observed during the early phase of lung and central nervous system infections by M. tuberculosis (11, 18).
Although EAhy926 is not an alveolar endothelial cell, our results resemble the results obtained by Birkness and colleagues (6) with an alveolar endothelial cell.
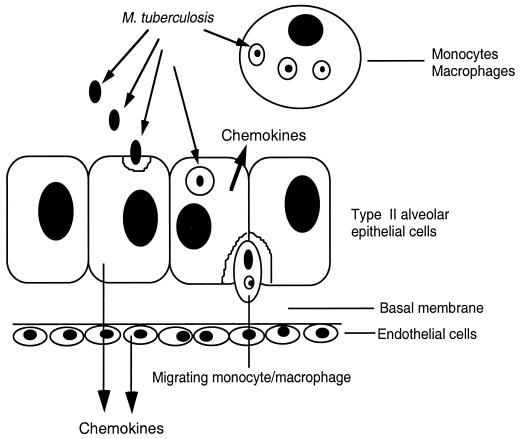
Monocyte migration across the alveolar epithelium-endothelial barrier depends not only on the production of chemokines but also on the presence of surface molecules on both alveolar epithelial cells and endothelial cells. Figure 3 shows a schematic cartoon of M. tuberculosis translocation across the alveolar wall.
FIG. 3.
Schematic representation of the stages of M. tuberculosis translocation across the alveolar wall.
It is known that infection of mononuclear phagocytes with M. tuberculosis triggers the release of tumor necrosis factor alpha (1), which has been shown to influence the regulation of the surface molecules on alveolar epithelial cells and endothelial cells (20). Even more, the increased efficiency of infected monocytes to translocate across the endothelial-epithelial cells bilayer may be dependent on the augmented expression of membrane receptors that facilitate monocyte migration. The role of M. tuberculosis infection on the expression of surface molecules in alveolar epithelial cells, endothelial cells, and monocytes is currently being investigated in the laboratory.
Together with previous work which suggests that M. tuberculosis can use bronchial M cells as a portal of entry (23), our study proposes that M. tuberculosis uses both invasion of epithelial cells and translocation through the alveolar wall and migration across the alveolar barrier within mononuclear cells. Therefore, it may turn out that M. tuberculosis uses more than one mechanism to quickly get to tissue macrophages and lung lymph nodes.
Thus, it appears likely that epithelial cells have evolved mechanisms to actively participate in the signaling loop which orchestrates inflammation and migration across the alveolar wall. Our data suggest that M. tuberculosis takes advantage of those characteristics of alveolar epithelial cells to efficiently cross the alveolar barrier and gain access to blood. Work ongoing in our laboratory is attempting to better characterize the membrane receptors involved in the translocation and based on preliminary observations that M. tuberculosis infects alveolar epithelial cells in vivo (data not shown), the relevance of this model for host infection.
Acknowledgments
We thank Lalita Ramakristma, Raúl Barletta, Jeffery McGarvey, and Lowell Young for critical reading of the manuscript. We are also in debt to Karen Allen for preparing the manuscript.
This work was supported by funds for tuberculosis research from the Kuzell Institute and the Hedco Foundation.
Editor: S. H. E. Kaufmann
REFERENCES
- 1.Barnes, P. F., S. Lu, J. S. Abrams, E. Wang, M. Yamamura, and R. L. Modlin. 1993. Cytokine production at the site of disease in human tuberculosis. Infect. Immun. 61: 3482–3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bermudez, L. E. 1993. Production of transforming growth factor-beta by Mycobacterium avium-infected human macrophages is associated with unresponsiveness to IFN-gamma. J. Immunol. 150: 1838–1845. [PubMed] [Google Scholar]
- 3.Bermudez, L. E., and J. Goodman. 1996. Mycobacterium tuberculosis invades and replicates within type II alveolar cells. Infect. Immun. 64: 1400–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bermudez, L. E., J. Goodman, and M. Petrofsky. 1999. Role of complement receptors in uptake of Mycobacterium avium by macrophages in vivo: evidence from studies using CD18-deficient mice. Infect. Immun. 67: 4912–4916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bermudez, L. E., A. Parker, and J. R. Goodman. 1997. Growth within macrophages increases the efficiency of Mycobacterium avium in invading other macrophages by a complement receptor-independent pathway. Infect. Immun. 65: 1916–1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Birkness, K. A., M. Deslauriers, J. H. Bartlett, E. H. White, C. H. King, and F. D. Quinn. 1999. An in vitro tissue culture bilayer model to examine early events in Mycobacterium tuberculosis infection. Infect. Immun. 67: 653–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention. 1997. Tuberculin skin test survey in a pediatric population with high BCG vaccination coverage—1996. Morb. Mortal. Wkly. Rep. 46: 846–851. [PubMed] [Google Scholar]
- 8.Crystal, R. J. 1991. Alveolar macrophages, p. 527–538. In R. J. Crystal and J. B. West (ed.), The lung: scientific foundations. Raven Press, New York, N.Y.
- 9.Edgell, C. J., C. C. McDonald, and J. B. Graham. 1983. Permanent cell line expressing human factor VIII-related antigen established by hybridization. Proc. Natl. Acad. Sci. USA 80: 3734–3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Henderson, H. J., A. M. Dannenberg, Jr., and M. B. Lurie. 1963. Phagocytosis of tubercle bacilli by rabbit pulmonary alveolar macrophages and its relation to native resistance to tuberculosis. J. Immunol. 90: 553–556. [PubMed] [Google Scholar]
- 11.Hopewell, P. C. 1994. Overview of clinical tuberculosis. In B. Bloom (ed.), Tuberculosis: pathogenesis, protection, and control. American Society for Microbiology, Washington, D.C.
- 12.Hu, C., T. Mayadas-Norton, K. Tanaka, J. Chan, and P. Salgame. 2000. Mycobacterium tuberculosis infection in complement receptor 3-deficient mice. J. Immunol. 165: 2596–2602. [DOI] [PubMed] [Google Scholar]
- 13.Inderlied, C. B., L. S. Young, and J. K. Yamada. 1987. Determination of in vitro susceptibility of Mycobacterium avium complex isolates to antimycobacterial agents by various methods. Antimicrob. Agents Chemother. 31: 1697–1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin, Y., M. Zhang, and P. F. Barnes. 1998. Chemokine production by a human alveolar epithelial cell line in response to Mycobacterium tuberculosis. Infect. Immun. 66: 1121–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McCune, R. M., and R. Tompsett. 1956. Fate of Mycobacterium tuberculosis in mouse tissues as determined by the microbial enumeration technique. J. Exp. Med. 104: 737–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McDonough, K. A., and Y. Kress. 1995. Cytotoxicity for lung epithelial cells is a virulence-associated phenotype of Mycobacterium tuberculosis. Infect. Immun. 63: 4802–4811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mehta, P. K., C. H. King, E. M. White, J. J. Murtagh, and F. D. Quinn. 1996. Comparison of in vitro models for the study of Mycobacterium tuberculosis invasion and intracellular replication. Infect. Immun. 64: 2673–2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pedrosa, J., B. M. Saunders, R. Appelberg, I. M. Orme, M. T. Silva, and A. M. Cooper. 2000. Neutrophils play a protective nonphagocytic role in systemic Mycobacterium tuberculosis infection of mice. Infect. Immun. 68: 577–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pethe, K., S. Alonso, F. Biet, G. Delogu, M. J. Brennan, C. Locht, and F. D. Menozzi. 2001. The heparin-binding haemagglutinin of M. tuberculosis is required for extrapulmonary dissemination. Nature 412: 190–194. [DOI] [PubMed] [Google Scholar]
- 20.Rosseau, S., J. Selhorst, K. Wiechmann, K. Leissner, U. Maus, K. Mayer, F. Grimminger, W. Seeger, and J. Lohmeyer. 2000. Monocyte migration through the alveolar epithelial barrier: adhesion molecule mechanisms and impact of chemokines. J. Immunol. 164: 427–435. [DOI] [PubMed] [Google Scholar]
- 21.Sangari, F. J., J. Goodman, and L. E. Bermudez. 2000. Mycobacterium avium enters intestinal epithelial cells through the apical membrane, but not by the basolateral surface, activates small GTPase Rho and, once within epithelial cells, expresses an invasive phenotype. Cell. Microbiol. 2: 561–568. [DOI] [PubMed] [Google Scholar]
- 22.Schneeberger, E. E. 1991. Alveolar type II cells, p. 736–758. In R. J. Crystal and J. B. West (ed.), The lung: scientific foundations. Raven Press, New York, N.Y.
- 23.Teitelbaum, R., W. Schubert, L. Gunther, Y. Kress, F. Macaluso, J. W. Pollard, D. N. McMurray, and B. R. Bloom. 1999. The M cell as a portal of entry to the lung for the bacterial pathogen Mycobacterium tuberculosis. Immunity 10: 641–650. [DOI] [PubMed] [Google Scholar]