Abstract
Dengue virus (DEN) is the most prevalent cause of arthropod-borne viral illness in humans. We determined the influence of cellular growth state on DEN type 2 (DEN2) replication in mosquito and human cells, based on the hypothesis that manipulation of cellular growth state will facilitate identification of viral and cellular determinants of productive infection. Comparison of density-arrested and cycling C6/36 Aedes albopictus cells infected with a low-passage DEN2 isolate revealed that cycling cells generated higher virus titers per cell. When C6/36 cells were stalled in S-phase via a thymidine (THY) block, titers of low-passage DEN2 isolates and a high-passage strain, 16681, were increased approximately 30-fold and 10-fold, respectively. Moreover, virus release was earlier in THY-treated cells than in asynchronously cycling cells. Adsorption, entry, genome uncoating, and translation were not responsible for increased titers of virus from S-phase C6/36 cells. In contrast to the 30-fold increase in virus titers, intracellular levels of viral RNA were increased approximately 2-fold, suggesting that the S-phase-responsive step is late in the DEN2 replication cycle. Analysis of viral RNA and protein released from the cells indicated that enhanced DEN2 assembly is largely responsible for increased virus titers produced during S-phase. In contrast to C6/36 cells, DEN2 titers from S-phase human hepatoma cells or primary human fibroblasts were not increased. These results demonstrate a differential response of DEN2 to the mosquito and human cell cycle and provide a framework for detailed studies into the mechanisms mediating virus assembly.
Dengue virus (DEN) is a member of the Flaviviridae, a family of positive-strand RNA viruses that includes hepatitis C virus (HCV), West Nile virus, and yellow fever virus, among others. DEN is a widespread public health problem, with an estimated 50 million cases per year of dengue fever and 250,000 to 500,000 cases of more severe, life-threatening forms of the disease (29). The expanding distribution of the mosquitoes that transmit DEN, Aedes aegypti and Aedes albopictus, places nearly half of the world's population at risk for infection with one of the four serotypes (DEN1 to -4), yet there are no antiviral treatments or vaccines available against DEN. Moreover, the viral and host determinants governing DEN replication are not well understood in either the human or mosquito hosts.
Upon infection of the host cell and uncoating of the viral genome, translation of the DEN polyprotein proceeds (48). The polyprotein is cleaved co- and posttranslationally by viral and host proteases into three structural and seven nonstructural (NS) proteins. Viral RNA replication, directed by newly generated NS proteins, then ensues. Immature virions are assembled in the endoplasmic reticulum, or in endoplasmic reticulum-derived vesicles, and virion maturation occurs as the virus is secreted (48).
Mosquitoes become infected with DEN by feeding on an infected human, and the mosquito remains infected during its entire lifetime. If a sufficient titer of DEN is ingested, the virus infects midgut epithelial cells and then disseminates from the gut to secondary sites of replication that include the fat body, hemocytes, brain and other nervous tissue, and salivary glands. Finally, DEN is secreted into the salivary gland lumen and can be transmitted to a human during the next blood meal (8, 32, 63). The mechanisms governing infection, dissemination, and transmission are not well understood, but both viral and mosquito genetics are thought to influence these processes (8, 32). Upon transmission to the human host, DEN may replicate initially in dendritic cells (DC) of the skin (68); the important secondary targets and the mechanism of viral dissemination have not been definitively shown. Based on the presence of DEN antigen and/or viral RNA, possible sites of replication include peripheral blood mononuclear cells, spleen, lymph nodes, bone marrow, liver, and thymus (6, 7, 29, 39, 40). In particular, virus has been recovered from monocytes, macrophages, and B cells (9, 42, 64).
Viral replication is governed by cellular characteristics, such as species, cell type, differentiation state, cell cycle status, and immune status, as well as by viral genetics (65). For example, myeloid differentiation has been proposed to influence dissemination of DEN. Differentiation of monocytes into macrophages and of DC precursors into DC resulted in release of higher DEN titers (45), and virus entry was shown to be more efficient into macrophages than into monocytes (16, 53). In addition, internalization of DEN into mammalian cells has been reported to be influenced by the G2- and M-phases of the cell cycle (41, 67). Cell cycle status was shown to modulate the adhesion molecule expression induced by West Nile virus and Kunjin virus, possibly influencing cell-to-cell adhesion to facilitate dissemination (59). Finally, using reporter constructs and replicons, it was shown that cell cycle status influences translation and RNA replication of HCV (36, 58, 66). These studies indicate that members of the Flaviviridae are responsive to cellular growth state.
Employing different cell types and DEN strains has revealed significant insight into DEN replication (see, for example, references 2, 15, 17, and 21), while few studies have assessed the contribution of cellular growth state to DEN replication (1, 41, 53, 67). How the growth state of mosquito cells influences DEN replication has not been explored. In this report, we demonstrate host cell-specific differences in the response of DEN2 to cell cycle. DEN2 titers were increased in S-phase mosquito cells, but not in S-phase human cells, relative to titers from asynchronously cycling control cells. Moreover, viral progeny were detectable several hours earlier from S-phase mosquito cells than from asynchronously cycling cells. Increased titers were also observed when virus adsorption, entry, and uncoating were bypassed via transfection with infectious DEN2 RNA. Despite an approximately 30-fold increase in titers of low-passage DEN2 isolates, the amount of viral RNA was only about 2-fold higher and viral translation was not impacted in S-phase mosquito cells. Rather, virion assembly appears to be enhanced during S-phase. The responsiveness of DEN replication to cellular growth state may play a role in dissemination and/or the transmission potential of the virus and represents a useful tool for investigating virus and host determinants of productive infection.
MATERIALS AND METHODS
Cells and viruses.
Cells were maintained in medium supplemented with 10% fetal calf serum, 10 mM HEPES, 100 U/ml penicillin, and 100 μg/ml streptomycin (complete medium). C6/36 Aedes albopictus cells (American Type Culture Collection [ATCC]) and BHK21 clone 15 cells (a gift of R. Childs, Davis, Calif.) were grown in L15 and α-minimal essential medium, respectively. Huh-7 cells (a gift of M. G. Katze, Seattle, Wash.) and primary human foreskin fibroblasts (HFF) and 293T cells (both gifts of D. A. Galloway, Seattle, Wash.) were maintained in Dulbecco's modified Eagle's medium. Mammalian cells were grown at 37°C in 5% CO2, and mosquito cells were grown at 28°C without added CO2. The prototype Thai DEN2 strain, 16681 (passage number unknown), was obtained from the Centers for Disease Control and Prevention, Ft. Collins, Colo. Isolation of Nicaraguan and Thai DEN2 strains has been described elsewhere (4, 47). Briefly, both viruses were isolated by seeding patient serum samples onto C6/36 cells, and viral stocks were produced in C6/36 cells as previously described (4). The Nicaraguan strain (N1042; a gift of A. Balmaseda, Managua, Nicaragua) was employed after three passages in mosquito cells, except for stocks produced in human cells (see below), and the Thai strain (C0477; a gift of R. Rico-Hesse, San Antonio, Tex.), was used after four passages. Virus stock titers were determined via plaque assay using BHK21 cells as previously described (17), and stocks were stored at −80°C. For production of DEN2 in human cells, monolayers of 293T human embryonic kidney cells were inoculated with N1042, previously passaged twice in mosquito cells. Cells were infected in Dulbecco's modified Eagle's medium supplemented with 2% fetal bovine serum for 2 h, and serum concentration was then brought up to 10%. The medium was collected and clarified by centrifugation at 2,000 × g at days 3, 4, and 5 postinfection, and virus titers were determined by plaque assay using BHK cells. Day 5 supernatants were used for subsequent infections.
Cell infection.
Duplicate wells of cells were used for each infection. Infection of C6/36 mosquito cells was carried out at a multiplicity of infection (MOI) of 0.5 or 1 as indicated. Huh-7 cells and HFF were infected at an MOI of 1. Cell number at the time of infection was estimated based on the number of cells plated, the plating efficiency, and the doubling time, which we have calculated for C6/36 cells, Huh-7 cells, and HFF. Cells were exposed to virus in growth medium containing 2% fetal bovine serum for 2 h. Virus was removed, and cells were washed five times with complete medium. Cells were then incubated in complete medium under the indicated conditions for manipulation of cell cycle status (see below).
Cell cycle manipulation.
For comparison of DEN2 titers from density-arrested and cycling mosquito cells, 6 × 105 C6/36 cells were plated in 12-well plates and grown for 2 days. To ensure that cells were viable and not nutrient deprived at the time of infection, the growth medium was replaced the day after plating. Cells were infected with DEN2 N1042 the subsequent day, as described in “Cell infection” above. At this point, cells were greater than 90% confluent. After infection, cells were either maintained at confluence or were dislodged from the plate by pipetting. Cells recovered from one well were diluted into 6 new wells of a 12-well plate.
For analysis of DEN replication in S-phase cells, asynchronously cycling C6/36 cells, Huh-7 cells, or HFF in 12- or 24-well plates were infected with DEN2 N1042, C0477, or 16681 as described above in “Cell infection,” followed by incubation with complete medium containing either phosphate-buffered saline (PBS), 2 mM thymidine (THY; Sigma, St. Louis, MO), or 0.2 mM hydroxyurea (HU; Sigma). To ensure that cells remained subconfluent for the duration of the experiment, 1.5 × 105 C6/36 cells or 4 × 104 Huh-7 cells or HFF were seeded into 12-well plates for initial experiments. The human cells are significantly larger than C6/36 cells; hence, a smaller number were seeded to prevent them from reaching confluence. Subsequently, experiments were scaled down to 24-well plates (by a factor of 2) to conserve the limited stocks of low-passage DEN2. Cells were infected the day after plating unless otherwise noted. After the indicated time for all cell cycle experiments, the medium was collected for plaque assay analysis and cells were removed from the plate with trypsin (Invitrogen, Carlsbad, CA) for assessment of cell number via a hemocytometer and for flow cytometric analysis of DEN envelope (E) expression and cell cycle position (see below). Viability of C6/36 cells, Huh-7 cells, and HFF was confirmed by trypan blue exclusion.
Flow cytometry.
To assess cell cycle distribution, cells were fixed in ice-cold 70% ethanol, treated with 100 μg/ml RNase A (Sigma) for 20 min at 37°C, and stained with 50 μg/ml propidium iodide (Sigma) in PBS. DNA content was measured by flow cytometry using a Beckman-Coulter EPICS XL cytometer (Beckman-Coulter, Inc., Fullerton, CA) and Flow Jo software (Tree Star, Inc., Ashland, OR). G0/G1-phase, S-phase, and G2/M-phase populations were delineated using the cell cycle feature of Flow Jo. Profiles of HU-treated C6/36 cells could not be fit to a model using the cell cycle feature and were therefore measured using gates drawn manually in Flow Jo.
Analysis of intracellular DEN2 envelope (E) protein expression was carried out as previously described (17), with some modifications. Briefly, mock- or DEN2-infected cells were removed from the plate by trypsinization, washed in PBS, and fixed for 10 min at room temperature with 4% paraformaldehyde (Fisher Scientific, Pittsburg, PA). Cells were permeabilized in HHSN (Hanks balanced salt solution, 10 mM HEPES, 0.1% saponin, and 0.02% sodium azide) for 15 min at 37°C and blocked in 10% fetal calf serum at 37°C for 30 min. Labeling of DEN E protein was carried out using the supernatant fraction from the 3H5 mouse hybridoma (ATCC) diluted 1:4 in HHSN plus 1% bovine serum albumin for 1 h at room temperature. Cells were washed and then incubated for 30 min at room temperature with Alexa 488-conjugated anti-mouse immunoglobulin G (IgG) (Molecular Probes, Eugene, OR) diluted 1:1,000 in HHSN-1% BSA. Cells were postfixed in 0.5% paraformaldelyde, and E expression was measured by flow cytometry and analysis with Flow Jo software.
Titers of cell-associated virus.
C6/36 cells infected with DEN2 N1042 as described above were harvested at 40 h postinfection (hpi). This time point was chosen to allow accumulation of progeny virions, particularly of the low-passage isolates, but to avoid multiple complete rounds of virus infection. The medium was collected for determination of extracellular virus titers. To obtain intracellular virus, cells were washed once in PBS, incubated for 3 min on ice with an alkaline/high-salt solution of 1 M NaCl plus 50 mM Na bicarbonate, pH 9.5, to remove surface-bound virus (18), washed two more times in PBS, and then lysed by freeze-thawing. Growth medium containing extracellular virus was also subjected to one cycle of freeze-thawing. Virus titers in the cell lysates and growth medium were measured by plaque assay.
Transfection of infectious DEN2 RNA.
Full-length DEN2 16681 RNA was generated by in vitro transcription as previously described (21) from a cDNA clone, pD2/IC-30P (39) (a gift of R. Kinney, Fort Collins, CO). Briefly, the cDNA construct was linearized with XbaI (New England Biolabs, Beverly, MA), and 1 μg was used for transcription. Transcription reactions were carried out using a Ribomax T7 in vitro transcription kit (Promega, Madison, WI) as per the manufacturer's instructions. Reactions were treated with RQ DNase I (Promega), and the RNA was passed through Nucaway spin columns (Ambion, Inc., Austin, TX) prior to transfection. RNA was transfected into C6/36 cells using Lipofectamine 2000 (Invitrogen). Each 50-μl reaction mixture was used for transfecting 6 wells of asynchronously cycling C6/36 cells (in 24-well plates). To ensure that treatment with THY did not impact transfection efficiency, cells were transfected in the absence of THY and then washed and treated with complete medium containing PBS or 2 mM THY at 3 h posttransfection. Viral supernatants were collected for plaquing, and cells were collected for propidium iodide staining and flow cytometry at 40 h posttransfection.
Reporter assays.
The DEN untranslated region (UTR) reporter construct has been previously described and contains the firefly luciferase (luc) gene flanked by the 5′ and 3′ UTRs of DEN2 (strain C0477) (21, 34). A reporter construct representing a cellular housekeeping gene, actin, was constructed by replacing the β-globin 5′ UTR in the 5′Bg-LUC-3′V268-A60 construct (34) with the 5′ UTR of actin. The actin 5′ UTR was generated using four overlapping oligonucleotides, Actin 1, 5′-CGCGTAAAATTTAATACGACTCACTATAACCGCCGAGACCGCGTCCGCCCCGCGA-3′; Actin 2, 5′-GCACAGAGCCTCGCCTTTGCCGATCCGCCGCCCGTCCACACCCGCCGCCAGCTCAC-3′; Actin 3, 5′-TCTGTGCTCGCGGGGCGGACGCGGTCTCGGCGGTTATAGTGAGTCGTATTAAATTTTA-3′; and Actin 4, 5′-CATGGTGAGCTGGCGGCGGGTGTGGACGGGCGGCGGATCGGCAAAGGCGAGGC-3′, that were annealed and then ligated into pGL3-5′Bg-LUC-3′V268-A60 that had been digested with MluI and NcoI (New England Biolabs). DEN and actin reporter mRNAs were transcribed from XbaI- and AseI-digested templates, respectively, using the Ribomax T7 in vitro transcription kit per the manufacturer's instructions. C6/36 cells were plated in 24-well plates and transfected the next day with 0.3 μg of reporter mRNA with Lipofectamine 2000 transfection reagent according to the manufacturer's instructions. After 3 h, the cells were washed and treated with complete medium containing either PBS or 2 mM THY. Cells were harvested in 1× cell culture lysis reagent (Promega) for luciferase assays, per the manufacturer's instructions, at 26 h posttransfection. This time point was determined empirically to detect luciferase activity before it plateaued. In parallel, RNA was harvested in TRIzol (Invitrogen) for assessment of reporter RNA levels by real-time reverse transcription-PCR (RT-PCR) as described previously (34).
Western blot assays. (i) Anti-NS1 blot assays.
Levels of DEN proteins were assessed in single-cycle replication assays. C6/36 cells were infected with DEN2 N1042 at an MOI of 1 and treated with THY as described above. At 24 hpi, prior to significant levels of virus secretion and spread to neighboring cells, cells were washed with PBS, detached from the plate with 3 mM EDTA, washed again in PBS, and counted. Approximately 2.5 × 105 cells were pelleted and lysed in 50 mM Tris Cl, pH 8.0, 250 mM NaCl, 0.1% sodium dodecyl sulfate (SDS), 1% NP-40, 1 mM dithiothreitol, and protease inhibitor cocktail on ice for 15 min. Cellular debris was pelleted by microcentrifugation at 15,000 × g for 10 min at 4°C. Supernatants were boiled in Laemmli sample buffer, resolved by SDS-polyacrylamide gel electrophoresis (SDS-PAGE), and transferred to polyvinylidene difluoride membranes. Blots were probed with a cocktail of mouse hybridoma supernatants against DEN2 NS1 (clones 8H7, 3A5, and 2E9; P. R. Beatty and E. Harris, unpublished data) followed by rabbit-anti-mouse (IgG plus IgM)-horseradish peroxidase (HRP) conjugate (Jackson Immunochemicals, West Grove, PA) or with goat-anti-actin-HRP conjugate (Santa Cruz Biotechnology, Inc., Santa Cruz, CA). NS1 was chosen because it is not contained in the incoming viral particles and, thus, indicates viral replication. Bands were visualized by Supersignal West Dura ECL substrate (Pierce, Rockford, IL) and exposure to Kodak BioMax XAR film (Eastman Kodak Co., Rochester, NY). For some experiments, the centrifugation step was excluded, and this had no impact on the levels of viral protein observed (data not shown).
(ii) Anti-E blot assays.
Infected C6/36 cells were washed at 24 hpi with an alkaline/high-salt solution to remove any surface-bound input virus, followed by two washes in PBS. Cells were lysed in buffer containing 20 mM HEPES, pH 7.4, 150 mM NaCl, 1% Triton X-100 (Fisher), 10% glycerol, and 1 mM EDTA. The samples, which were not boiled, were resolved by SDS-PAGE under nonreducing conditions. Blotting and probing were carried out as described for NS1, but with 3H5 hybridoma supernatant against DEN2 E protein as the primary antibody. Chemiluminescent bands were detected in the linear range using a Bio-Rad Chemidoc XRS imager with Quantity One software (Bio-Rad Laboratories, Hercules, CA).
Real-time RT-PCR. (i) Intracellular DEN2 RNA.
DEN RNA levels were assessed in single-cycle infection assays. C6/36 cells were infected with DEN2 N1042 (MOI = 0.5) and treated with PBS or THY as described above. At 24 hpi, prior to significant levels of virus secretion and spread to neighboring cells, cells were washed in PBS, treated with alkaline/high salt as described above, and washed two more times in PBS. Total RNA was harvested in TRIzol, purified, and analyzed by real-time RT-PCR using the Superscript III Platinum One-Step qRT-PCR system (Invitrogen) with primers to DEN2 NS1 and to actin (the internal positive control) (Invitrogen). Primer sequences were as follows: 6-carboxyfluorescein (FAM) fluorophore-labeled NS1 forward primer, 5′-cacaaCCATGAAGAGGGCATTTG-FAM-G-3′; unlabeled NS1 reverse primer, 5′-TTTGTTTCCACATCAGATTCCCA-3′. Nucleotides indicated in lowercase letters are non-DEN sequence. Reactions were carried out in duplicate on an ABI Prism 7700 sequence detection system (Applied Biosystems, Foster City, CA). A four-point standard curve was generated using DEN2 RNA isolated from a known quantity of virus as previously described (60), using SDS 1.9.1 software (Applied Biosystems) for the analysis. The amount of total DEN2 RNA from THY-treated cells was determined relative to that from PBS-treated cells.
(ii) Extracellular DEN2 RNA.
C6/36 cells were infected with DEN2 N1042 (MOI = 0.5). At 2 hpi, virus was removed and, in addition to washes in complete medium, cells were subjected to an alkaline/high-salt wash before treatment with PBS or THY. At 40 hpi the growth medium was collected for analysis of virus titers by plaque assay and for viral RNA by real-time RT-PCR as described above (except that actin primers were not used). Extracellular virus titers and RNA levels were displayed relative to those of the PBS-treated controls.
Analysis of purified virions.
Infected C6/36 cell supernatants were harvested at 40 hpi and clarified at 1,000 × g for 3 min at 4°C. Cells were harvested for Western blot analysis as described above. Virus was pelleted at 64,000 × g for 2 h at 4°C in an SW60Ti rotor. Pellets were resuspended in 20 mM Tris-Cl, pH 7.4, 150 mM NaCl, and 1 mM EDTA (TNE) and loaded onto 15-to-55% sucrose-TNE (wt/wt) gradients, which were centrifuged in an SW60Ti rotor for 17 h at 144,000 × g. The bottom of the tube was punctured with a needle to collect 700-μl fractions. Aliquots were taken for measurement of PFU/ml by plaque assay, and the remaining fractions were trichloroacetic acid precipitated and resolved by nonreducing SDS-PAGE as described above for analysis of E protein.
Statistical analysis.
Calculations of the means, standard deviations, P values, and 95% confidence intervals were carried out using Microsoft Excel (Microsoft, Redmond, WA). P values were determined by Student's t test.
RESULTS
DEN2 infection does not alter the cell cycle status of mosquito cells, but cell cycle status influences virus titer.
To determine if the growth state of mosquito cells influences DEN2 titers, C6/36 Aedes albopictus cells were grown to greater than or equal to 90% confluence but maintained in fresh growth medium to induce a G0/G1 arrest via contact inhibition rather than by nutrient deprivation. Cells were then infected with DEN2 strain N1042, a clinical isolate from Nicaragua with a short tissue culture passage history. At 2 hpi, cells were washed extensively and were either maintained in density arrest with the addition of fresh growth medium or released into the cell cycle by replating at a lower density. To allow enough time for one viral replication cycle but fewer than two complete replication cycles, the experiment was harvested at 40 hpi. Cultures were trypsinized to ensure collection of all of the cells for accurate counting. Cells were washed in PBS, counted via hemocytometer, and analyzed by flow cytometry for intracellular expression of DEN2 E protein and for cell cycle status. In parallel, the growth medium was collected, centrifuged to remove cellular debris, and analyzed by plaque assay for virus titer. As comparisons were between arrested and cycling cell populations, measurements of virus output were adjusted for cell number.
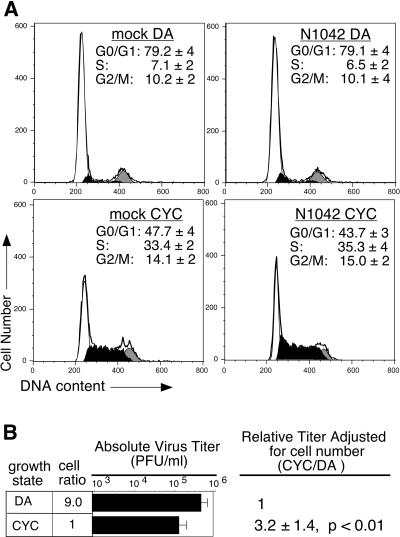
Greater than half of the density-arrested and cycling populations expressed E protein by the time of harvest (data not shown); however, DEN2 replication had no impact on C6/36 cell cycle distribution compared to mock-infected cells (Fig. 1A, compare left and right panels of each set). Flow cytometric analysis of propidium iodide-stained cells demonstrated that, regardless of DEN2 infection, nearly 80% of the density-arrested population were in G0/G1-phase (Fig. 1A, top panels), with approximately 6 to 7% and 10% of cells in S-phase and G2/M-phase, respectively. In contrast, less than half of the cycling populations were in G0/G1 phase, while approximately 33 to 35% and 14 to 15% were in S-phase and G2/M-phases, respectively (Fig. 1A, bottom panels). Overall production of low-passage DEN2 N1042 varied somewhat among experiments; however, adjustment of virus titer for population size revealed a consistent threefold increase (P < 0.01, 95% CI = 2.0 to 4.4) in virus output from cycling C6/36 cells compared to density-arrested cells (Fig. 1B). Moreover, this increase was also apparent when titers were adjusted for the number of cells expressing E protein, with a fourfold difference in virus output from cycling cells (P < 0.001) (data not shown). These data indicate that the growth state of C6/36 cells can modulate DEN2 titers but that DEN2 appears not to alter the cell cycle of C6/36 cells under our experimental conditions.
FIG. 1.
DEN2 production in density-arrested and cycling mosquito cells. C6/36 cells grown to ≥90% confluence were infected with DEN2 strain N1042 (MOI of 0.5), washed, and released into the cell cycle or maintained in density arrest. Cells and growth medium were harvested at 40 hpi for analysis of cell cycle status (A) and virus production (B). (A) Cell cycle profiles. DNA content of fixed, propidium iodide-stained cells was analyzed by flow cytometry. Profiles from a representative experiment are shown, with the G0/G1-phases (white), S-phase (black), and G2/M-phase (gray) populations indicated. The percentages of cells in G0/G1-, S-, and G2/M-phases, averaged from five experiments, are shown ± the standard deviation (SD). DA, density-arrested cells; CYC, cycling cells. (B) Virus titers. Titers were measured by plaque assay. The number of cells in DA populations was approximately ninefold greater than that in CYC populations at harvest, and this is indicated by the cell ratio. Cell number was accounted for when calculating the relative titers for DA and CYC populations. An average of five experiments, each done in duplicate, ± the SD is shown.
DEN2 titers are increased in S-phase mosquito cells.
The previous results demonstrated that cycling C6/36 populations produced higher DEN2 titers per cell than did density-arrested C6/36 populations (Fig. 1). Given that the largest change in cell cycle position between cycling and density-arrested cultures was the percentage of cells in S-phase (Fig. 1A, compare top and bottom panels), it followed that the effect on DEN2 titers may occur during S-phase. Cycling mosquito cells spend 4 to 6 h in S-phase during each round of the cell cycle (3, 27) (data not shown). If virus production were enhanced during S-phase, then a cell that is held in S-phase over the course of the experiment should produce more virus than an actively cycling cell that spends several shorter intervals in S-phase over the course of the experiment. This prediction was tested by infecting asynchronously cycling C6/36 cells with DEN2, followed by imposition of a THY-induced S-phase block. Treatment with excess THY is commonly employed to synchronize mammalian cells in S-phase (10, 11); however, the efficacy of THY in arresting mosquito cells was unknown. Therefore, a dose-response experiment was carried out with C6/36 cells, and all THY doses tested were effective (1 to 10 mM THY) (data not shown). THY was used at a 2 mM final concentration for subsequent experiments. Based on trypan blue exclusion and synchronization and release experiments, this dose did not impact cell viability over the duration of the experiment (data not shown).
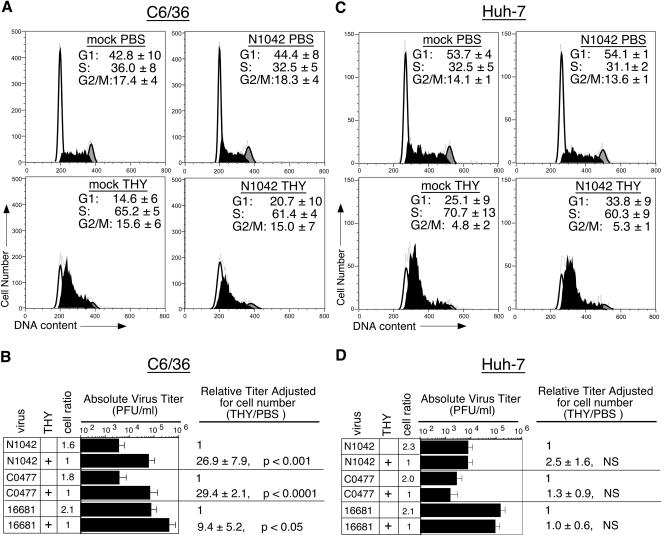
To determine the influence of S-phase on DEN2 titers, asynchronously cycling C6/36 cells were infected with DEN2 strain N1042, with a low-passage DEN2 Thai isolate (C0477), or with a high-passage DEN2 strain (16681), washed at 2 hpi, and then treated with PBS or THY. The low-passage isolates were predicted to be more sensitive to cellular growth state than a highly tissue culture-passaged virus whose replication is less restricted in cell culture (17, 21). Cells and medium were harvested at 40 hpi for measurement of cell number, cell cycle distribution, and virus titers and for assessment of E protein expression (see below). Flow cytometric analysis of propidium iodide-stained cells revealed that approximately 35% of PBS-treated cells were in S-phase at the time of harvest, while over 60% of THY-treated cells were stalled in S-phase (Fig. 2A, top and bottom panels, respectively). In response to the THY-imposed S-phase block, titers of DEN2 N1042 were increased by approximately 27-fold (P < 0.001) and DEN2 C0477 increased by 29-fold (P < 0.0001), after accounting for cell number (Fig. 2B). Normalization of DEN2 N1042 and C0477 titers to the number of cells expressing E protein also revealed over a log increase in virus output from THY-treated C6/36 cells (data not shown). The response of the more highly tissue culture-adapted DEN2 strain, 16681, to arrest of C6/36 cells in S-phase was less robust, with a ninefold increase, on average, in titers relative to the PBS-treated control (P < 0.05) (Fig. 2B). Finally, similar increases in virus production were observed when virus stocks were produced in the human cell line 293T, indicating that the results were not dependent upon the cellular source of DEN2 stocks (data not shown).
FIG. 2.
Effect of S-phase on DEN2 titers. Asynchronously cycling C6/36 cells or Huh-7 cells were mock infected or infected with DEN2 strains N1042, C0477, or 16681 for 2 h, washed, and then cultured in growth medium containing either PBS or 2 mM THY for an additional 38 h (C6/36) or 28 h (Huh-7). (A and C) Cell cycle profiles. Cell cycle analysis was carried out as described for Fig. 1. Representative profiles are shown. Percentages of cells in G1-, S-, and G2/M-phases were averaged from at least three experiments ± the standard deviation (SD). (B and D) Virus titers. Titers were measured by plaque assay. Cell number was accounted for when calculating relative virus output for PBS- and THY-treated populations. An average of at least three experiments, each done in duplicate, ± the SD is shown. NS, not significantly different than 1.
It was possible that the increased DEN2 titers in Fig. 2B were due to more efficient egress from S-phase cells, rather than an increase in virus production. To address this question, titers of both intracellular and extracellular virus were measured from DEN2 N1042-infected C6/36 cells treated with either PBS or THY. At 40 hpi, the growth medium was collected for quantifying extracellular virus titers, and the cells were harvested for measuring intracellular virus titers. To remove surface-bound virions, the cells were washed and then incubated in an alkaline/high-salt solution (18, 21). Cells were then lysed by freeze-thawing. Extracellular virus was also subjected to a cycle of freeze-thawing. Titers of intracellular and extracellular DEN2 from THY-treated cultures were both increased by approximately 30-fold relative to titers from the PBS-treated control cells (data not shown). These data indicate that increased DEN2 titers during S-phase were due to increased virus production, not to more efficient viral egress from S-phase cells.
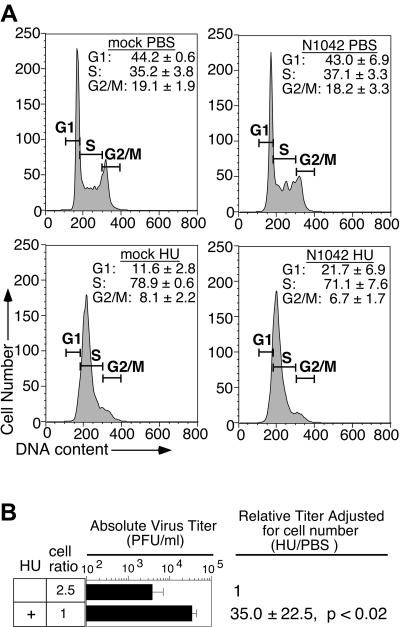
To address the possibility that the increased virus titers were related to unanticipated cell cycle-independent effects of THY treatment, a different reagent was employed to stall C6/36 cells in S-phase following infection with DEN2 strain N1042. Treatment of C6/36 cells with 0.2 mM HU, previously shown to synchronize mosquito cells in S-phase (26), resulted in an accumulation of cells in early S-phase (Fig. 3A) and a concomitant increase in titers of progeny virions (Fig. 3B). The increase in titers ranged from 10- to 70-fold, with an average of 35-fold, and was statistically significant (P < 0.02).
FIG. 3.
Effect of hydroxyurea on DEN2 titers. C6/36 cells were infected with DEN2 N1042, treated, and harvested as described for Fig. 2, except that 0.2 mM HU was used instead of THY to stall cells in S-phase. (A) HU-imposed S-phase block. Cell cycle profiles were generated using manually drawn gates with Flow Jo software, as the profiles of HU-treated cells were difficult to fit to the cell cycle models provided by Flow Jo. (B) Virus titers. Titers were measured by plaque assay. Cell number was accounted for when calculating the relative virus production from PBS- and HU-treated cultures. The average of four independent experiments ± the standard deviation is shown.
DEN2 production is not enhanced in S-phase human cells.
Experiments were also carried out with human cells to determine the effect of cell cycle on DEN2 titers in the other natural DEN host. Asynchronously cycling Huh-7 human hepatoma cells were infected with DEN2 strain N1042, C0477, or 16681 at an MOI of 1. Hepatoma cells were employed because the liver is thought to be a site of DEN replication (6) and liver damage has been observed in association with severe dengue (50). At 2 hpi, cells were washed and then incubated with complete medium containing PBS or 2 mM THY. The experiment was harvested at 30 hpi to allow enough time for one complete round of viral replication but not for two complete rounds and because virus titers from the PBS-treated control Huh-7 cells were similar at this time point to titers from the PBS-treated C6/36 cells in the previous experiment (Fig. 2B). Cells were collected via trypsinization for assessment of cell number by hemocytometer and for flow cytometric analysis of DNA content and intracellular E protein expression. In parallel, virus titers in the growth medium were determined by plaque assay. Analysis of DNA content revealed that the cell cycle distribution of PBS-treated Huh-7 cells (Fig. 2C, top panels) was similar to that of C6/36 cells (Fig. 2A, top panels). In addition, a similar accumulation of cells in S-phase resulted following THY treatment (60 to 70% of the population) (Fig. 2C, bottom panels, and A). In contrast to results in C6/36 cells, S-phase arrest of Huh-7 cells did not result in increased titers of any of the DEN2 strains (Fig. 2D). To determine if this lack of effect was due to the time of harvest, additional experiments were harvested at 20 and 45 hpi and also revealed no increase of DEN2 titers in S-phase hepatoma cells (data not shown). Moreover, no S-phase-dependent increase in titers occurred in an alternative cell type, primary human fibroblasts, infected with DEN2 strain 16681(data not shown). Thus, the S-phase-dependent enhancement of DEN2 production in mosquito cells was not recapitulated in either of the human cell types tested.
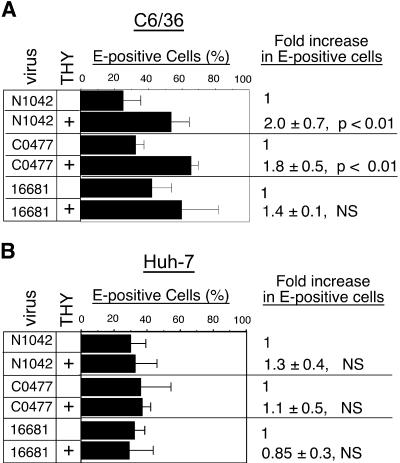
To compare the percentage of cells infected in PBS- and THY-treated mosquito and human cell cultures, flow cytometric analysis of E protein expression was performed with cells from the above experiments. DEN2 N1042- or C0477-infected C6/36 cells treated with THY exhibited an approximately twofold increase in the number of E-positive cells relative to the infected, PBS-treated controls at 40 hpi (Fig. 4A). There was a smaller, non-statistically significant increase in the percentage of E-positive C6/36 cells in 16681 infections (Fig. 4A). Similar experiments with human cells revealed no difference in the percentage of cells expressing DEN E protein (Fig. 4B and data not shown). Because cells were treated with THY after infection was initiated, no difference in the number of infected cells between PBS- and THY-treated populations was expected. Two potential explanations for the increased number of E-positive C6/36 cells observed following THY treatment were that (i) THY-treated cells were more permissive for an early step of infection, such as genome uncoating or initial translation, or that (ii) virus was released earlier from the THY-treated cells than from the PBS-treated cells, with DEN undergoing translation in the newly infected neighboring cells.
FIG. 4.
Effect of S-phase on DEN2 antigen expression. Mock- or DEN2-infected cells from the experiments shown in Fig. 2 were fixed, permeabilized, and labeled with anti-DEN2 envelope (E) antibodies. The percentage of E-positive PBS- or THY-treated cells was measured by flow cytometry. At least three experiments with C6/36 (A) and Huh-7 cells (B) are shown ± the standard deviation. NS, not significantly different than 1.
The DEN2 replication cycle is accelerated in S-phase mosquito cells.
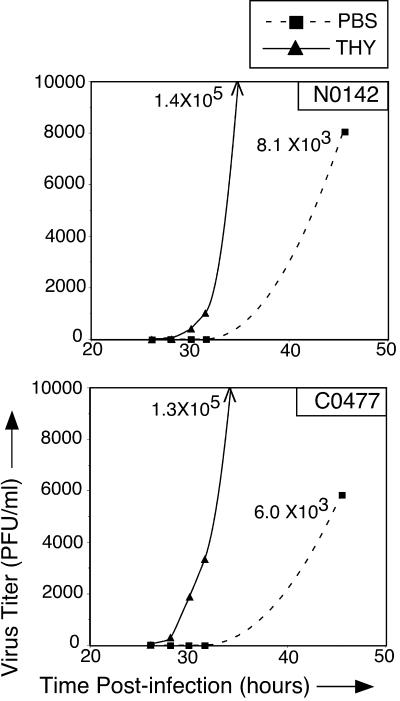
To determine if the DEN2 replication cycle is accelerated in S-phase mosquito cells, as suggested by the twofold increase in E-expressing C6/36 cells at 40 hpi in THY-treated cultures (Fig. 4A), C6/36 cells were infected with DEN2 N1042 or C0477 and treated with PBS or THY as in the previous experiments. Medium was collected at various hpi, and virus titers were measured by plaque assay. For both DEN2 strains, progeny virus was first detectable by 28 hpi in THY-treated cultures but was not consistently detectable until after 31 hpi in PBS-treated cultures (Fig. 5). Titers of DEN2 N1042 and C0477 in THY-treated cultures in the representative experiments shown were 1.4 × 105 and 1.3 × 105 PFU/ml, respectively, at the last time point. In comparison, titers of N1042 and C0477 from PBS control cultures were approximately 8 × 103 and 6 × 103 PFU/ml, respectively (Fig. 5).
FIG. 5.
Kinetics of DEN2 replication in S-phase mosquito cells. C6/36 cells were infected with DEN2 N1042 or C0477, washed, and treated with PBS or THY. The growth medium was collected periodically, and virus titers were measured by plaque assay. Dashed line, titers from PBS-treated cells; solid line, titers from THY-treated cells. Titers from the last THY time point are off the scale of the y axis and are indicated in the graphs. Representative experiments of two are shown.
To further test the hypothesis that the greater number of E-expressing cells in THY-treated cultures observed at 40 hpi resulted from more rapid virus production and spread, C6/36 cells were infected with DEN2 N1042 (MOI = 1) and treated with PBS or 2 mM THY as in previous experiments, and the number of E-expressing cells was determined in single-cycle infections harvested at 24 hpi. This time point was chosen because it is late during the first cycle of virus replication, but before detectable viral egress (Fig. 5). Flow cytometric analysis revealed little difference in the percentage of E-expressing cells at 24 hpi, with a fold increase of 1.18 ± 0.15 following THY treatment, relative to PBS-treated cells. Thus, our results demonstrate an increased rate of virus replication in S-phase mosquito cells that is not due to an increase in the number of cells initially infected with DEN2.
Early events in DEN2 replication are not responsible for increased titers during S-phase.
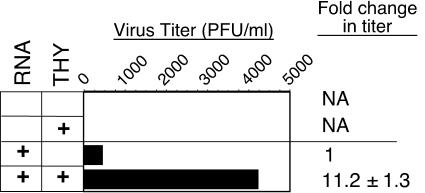
Because infection of C6/36 cells in the previous experiments was initiated prior to THY treatment, the increased DEN2 titers were not likely due to increased virus adsorption. To formally test whether increased virus titers were due to enhanced entry or genome uncoating, these processes were bypassed by transfection of full-length, positive-strand DEN2 RNA in vitro transcribed from pD2/IC-30P, a cDNA construct of strain 16681 (43). Infectious clones of DEN2 N1042 and C0477 are not yet available. Asynchronously cycling C6/36 cells were transfected, washed at 3 h posttransfection, and treated with PBS or THY. Growth medium was collected at 40 h posttransfection, and virus titers were assessed by plaque assay. Cell cycle status was determined in parallel to confirm the THY-induced S-phase arrest (data not shown). DEN2 titers from the THY-treated transfected cells were about 11-fold higher than from PBS-treated cells (Fig. 6), similar to the DEN2 16681 results when infection was initiated with intact virus (Fig. 2B). Similar results were obtained when experiments were analyzed at an earlier time posttransfection (27 h [data not shown]). These results demonstrate that the S-phase-responsive step of DEN2 replication follows genome uncoating.
FIG. 6.
Effect of S-phase following transfection of C6/36 cells with a DEN2 infectious clone. C6/36 cells were transfected with positive-sense DEN2 16681 RNA in vitro transcribed from the cDNA clone, pD2/IC-30P (43). Cells were treated with growth medium containing PBS or THY. Virus production (shown) and cell cycle distribution (not shown) were assessed at 40 h posttransfection by plaque assay and flow cytometry, respectively. Shown is the fold increase in PFU per ml in THY-treated cultures relative to PBS-treated cultures, averaged from three experiments ± the standard deviation.
S-phase responsiveness of DEN2 is not due to enhanced viral translation.
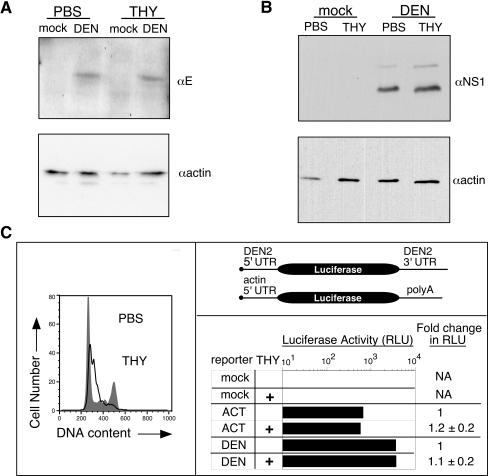
The efficiency of DEN2 translation has been shown to regulate the extent of virus replication, with translation efficiency being dependent upon both virus strain and cell type (20, 21). However, initial results suggested that DEN2 translation was not substantially enhanced during S-phase. Measurements of mean fluorescence intensity (MFI) of DEN2-infected, E-positive C6/36 cells were carried out with the flow cytometric data shown in Fig. 4A, and the MFI of THY-treated cells was, on average, less than twofold greater than that of PBS-treated cells at 40 hpi (fold increases for DEN2 N1042, C0477, and 16681 were 1.7 ± 0.4, 1.6 ± 0.2, and 1.3 ± 0.1, respectively). Based on the time of viral egress from C6/36 cells (Fig. 5), viral proteins detected at 40 hpi were likely derived from both the initial round of DEN infection as well as initiation of a second round of infection, particularly in THY-treated cultures, which exhibited more rapid production of viral progeny. Therefore, to examine viral protein expression in single-cycle infections, C6/36 cells were infected with DEN2 N1042 (MOI = 1), treated with PBS or THY as in previous experiments, and harvested at 24 hpi. Flow cytometric analysis demonstrated that the MFI of E-positive PBS- and THY-treated cells was similar, with a fold increase following THY treatment of 1.24 ± 0.20, relative to PBS-treated controls. Similar results were obtained by Western blot analysis of E (Fig. 7A) and NS1 (Fig. 7B), the latter being present only after the incoming virus undergoes translation (48). Thus, while differences in virus titers from PBS- and THY-treated cultures were apparent at the earliest times of detectable viral egress (Fig. 5), levels of viral protein appeared to be similar.
FIG. 7.
DEN translation in S-phase C6/36 cells. (A and B) Western blot analysis of viral proteins. C6/36 cells were infected with DEN2 N1042 (MOI = 1), washed at 2 hpi, and treated with PBS or THY. Cells were harvested for Western blot analysis at 24 hpi. For anti-E blot assays, cells were washed with an alkaline/high-salt solution prior to harvest to remove surface-bound virions. Blots were probed with anti-E (A) or anti-NS1 (B) antibodies, followed by an HRP-conjugated secondary antibody. Anti-actin-HRP was used for the loading control. Bands were visualized by enhanced chemiluminescence. Shown are representative experiments of three. (C) Luciferase assays. Top right panel, reporter constructs; left panel, representative cell cycle profiles from PBS- or THY-treated cells; bottom right panel, luciferase assays. The DEN reporter construct contained the luciferase gene flanked by the 5′ and 3′ UTRs of DEN2 strain C0477 (21). The control construct contained luciferase under control of the 5′ UTR from a housekeeping gene (actin) and a 3′ poly(A) tail. Cells were transfected with in vitro-transcribed reporter mRNA and treated with THY as described in Materials and Methods. Luciferase activity was measured at 26 h posttransfection, and RLU were corrected for the amount of reporter RNA present in the cells as determined by real-time RT-PCR. Luciferase data from a representative experiment of three are shown. The fold change in RLU relative to PBS-treated cells was averaged from three experiments, ± the standard deviation.
As a final means of testing whether DEN2 translation is increased in S-phase C6/36 cells, the translational activity of the regulatory viral 5′ and 3′ UTRs was measured using RNA-based UTR-reporter gene chimeras (Fig. 7C, top right panel) (21, 34). Asynchronously cycling C6/36 cells were transfected with in vitro-transcribed reporter mRNA, washed after 3 h, and treated with PBS or THY. Cells were harvested for measurement of luciferase activity, for real-time RT-PCR analysis of reporter RNA levels, and for cell cycle analysis at 26 h posttransfection, when luciferase expression from our reporter constructs in C6/36 cells was still increasing (data not shown). Cells arrested in S-phase in response to treatment with THY (Fig. 7C, left panel). However, luciferase activity from neither the DEN2 reporter nor a control reporter containing the β-actin 5′ UTR and a polyadenylated tail changed in response to THY treatment (Fig. 7C, bottom right panel). Together, these results indicate that the S-phase-responsive step of DEN2 replication follows viral translation.
Viral RNA levels do not account for increased DEN2 titers during S-phase.
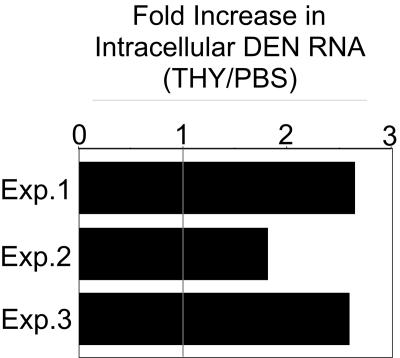
Real-time RT-PCR was carried out on total RNA isolated from N1042-infected or PBS- or THY-treated C6/36 cells in single-cycle infections (24 hpi) to determine if the THY-induced increase in DEN2 titers was due to higher levels of viral RNA, resulting either from increased RNA synthesis or from increased RNA stability. Primers to amplify both positive- and negative-strand RNA were directed at the NS1 region of the genome. The overall level of viral RNA was approximately twofold higher in THY-treated C6/36 cultures compared to PBS-treated cultures (Fig. 8). This result suggests that the level of viral RNA in S-phase mosquito cells may contribute to, but does not fully account for, the increase in DEN2 titers.
FIG. 8.
DEN2 RNA levels in S-phase mosquito cells. C6/36 cells were infected with DEN2 N1042 and treated with THY as described for Fig. 2. At 24 hpi, cells were washed with PBS and with an alkaline/high-salt solution to remove surface-bound particles. RNA was TRIzol extracted, purified, and analyzed via real-time RT-PCR using primers directed against NS1 and the internal positive control, actin. Total levels of DEN RNA (+ and − strand) in THY-treated cells were measured and graphed relative to the levels in PBS-treated cells (set at 1). Results from three independent experiments, each done in duplicate, are shown.
Viral assembly is enhanced in S-phase mosquito cells.
Given our results indicating that increased production of DEN2 from S-phase C6/36 cells is not attributable to early steps of infection (Fig. 6), to enhanced viral translation (Fig. 7), or to higher intracellular levels of viral RNA (Fig. 8), we hypothesized that increased production of DEN2 could result from an S-phase-dependent enhancement in virion assembly and/or infectivity. To test this hypothesis, we compared the amount of viral RNA and E protein secreted into the growth medium to the amount of PFU released. If viral assembly is facilitated in S-phase mosquito cells, then higher levels of E protein and viral RNA should be released into the growth medium in THY-treated cultures. Alternatively, if virions produced in S-phase cells are more infectious, due either to more efficient virion maturation or to other factors influencing infectivity, then levels of E protein and viral RNA released into the medium should be similar in PBS- and THY-treated cultures.
To compare the amount of viral RNA released to PFU, C6/36 cells were infected with DEN2 N1042 (MOI = 0.5) for 2 h, followed by stringent washing, which included incubation with an alkaline/high-salt solution to remove residual surface-bound particles (18). Cells were then treated with PBS or THY. Clarified viral supernatants were generated at 40 hpi, a time point chosen to facilitate analysis of secreted virions. DEN2 RNA in the supernatants was measured by real-time RT-PCR using primers recognizing the NS1 region. In parallel, virus titers in the medium were measured by plaque assay. Extracellular levels of viral RNA were approximately 10-fold higher, and titers were 20-fold higher, in the THY-treated cultures compared to PBS-treated cultures (Fig. 9A). Relative to PBS-treated cells, THY-treated cells released approximately 10-fold-higher levels of DEN RNA and 20-fold-higher virus titers (Fig. 9A). This result suggests an enhancement of DEN2 assembly in S-phase mosquito cells. However, based on the discrepancy between increases in RNA level and increases in PFU, the data do not eliminate the possibility that there is a small enhancement in overall virion infectivity.
FIG. 9.
Effect of S-phase on DEN2 assembly. C6/36 cells were infected with DEN2 N1042 as described for Fig. 2, only an additional wash in an alkaline/high-salt solution was included to remove surface-bound virus prior to treatment with PBS- or THY-containing medium. At 40 hpi, the growth medium was harvested and clarified by centrifugation. (A) Comparison of DEN2 titers to extracellular levels of viral RNA. Fractions of clarified viral supernatants were analyzed by plaque assay or were extracted with TRIzol for RNA isolation. Levels of viral RNA were measured by real-time RT-PCR with probes recognizing NS1. Displayed are the fold increases in both virus titer and amounts of viral RNA from THY-treated cultures relative to PBS-treated cultures. Three independent experiments are shown. (B) Comparison of DEN2 titers to extracellular levels of E protein. Clarified viral supernatants were concentrated and then purified on 15%-to-55% (wt/wt) sucrose gradients. Fractions were analyzed by plaque assay and by quantitative Western blotting for virus titers and E protein levels, respectively. Shown are titers and E protein levels from the fraction containing peak infectivity from a representative experiment of three (fraction number 3 of 7; numbering from bottom to top of gradient).
To compare the amount of E protein and PFU released into the growth medium, C6/36 cells were infected with DEN2 N1042, washed, and treated as described for the previous experiment. Virions were concentrated from clarified supernatants and then purified via sucrose gradient centrifugation. Fractions were collected from the bottom of the tube and analyzed by plaque assay for infectious titers and by quantitative Western blot for levels E protein. Consistent with an earlier study of DEN assembly in mosquito cells (61), E protein was detected only in gradient fractions containing infectious particles (data not shown). In contrast to the similar intracellular levels of E observed in PBS- and THY-treated cultures, levels of extracellular E increased in parallel with virus titers following THY treatment, with a 40-fold increase in titers and an approximately 30-fold increase in E protein compared to that from PBS-treated cultures (Fig. 9A). Shown are the gradient fractions containing peak virus titers (60% of the total virus from either PBS- or THY-treated cultures). However, similar results were obtained when all fractions were included in the measurements and when virus was not purified over sucrose gradients before analysis (data not shown). These results indicate that more efficient virion assembly accounts for the majority of increased virus production in S-phase mosquito cells.
DISCUSSION
Manipulation of cellular growth state is an important tool for uncovering aspects of viral replication not apparent under steady-state cellular conditions. We employed this approach and have shown that DEN2 replication is influenced by cellular growth state in mosquito cells. DEN2 titers were dramatically increased in S-phase mosquito cell cultures but in not S-phase human cells. In contrast, DEN2 infection did not alter the cell cycle status of mosquito or human cells under our experimental conditions. Clinical DEN2 isolates responded more robustly than the prototype strain 16681 to S-phase, suggesting that viral genetic determinants may contribute to this response. In addition, progeny virions were detectable earlier from S-phase mosquito cells than from asynchronously cycling cells, indicating an accelerated rate of viral replication. The S-phase-responsive step of DEN2 replication was subsequent to genome uncoating and viral translation, but previous to virion release, as higher titers of intracellular virus were observed following THY treatment. Moreover, in contrast to a 30-fold increase in titers of a clinical DEN2 isolates, levels of viral RNA were increased only about 2-fold in S-phase mosquito cells. Further experiments indicate that enhanced virion assembly is largely responsible for higher DEN2 titers in S-phase mosquito cells, although a small increase in overall virion infectivity may also contribute to increased titers. Our findings highlight differences in the influence of cellular growth/metabolic state on DEN2 replication in cells of the two natural host species. Furthermore, identification of conditions that enhance DEN2 assembly will facilitate investigation of viral and host-specific cellular factors involved in this important viral process.
The possibility that DEN was responding not to S-phase but to an unanticipated effect of THY was tested by employing a different reagent, HU, to impose cell cycle arrest. HU treatment trapped C6/36 cells in S-phase, with a concomitant increase in virus production (Fig. 3), indicating that increased DEN titers are S-phase-specific and not due to THY itself. Cell cycle analysis revealed an approximately twofold increase in the proportion of C6/36 cells in S-phase following THY or HU treatment, while virus output was enhanced significantly more than twofold (Fig. 2 and 3). This twofold difference in S-phase population is somewhat misleading, as it does not account for the fact that cells in the PBS-treated cultures enter and exit S-phase, while THY- or HU-treated cultures spend much more cumulative time in S-phase. Hence, virus in a THY- or HU-treated cell would be exposed to the S-phase cellular milieu from the time of S-phase entry until the end of the experiment, while virus in a cycling, PBS-treated cell would be exposed to the S-phase environment over several shorter intervals.
The reasons underlying the difference in DEN2 responsiveness to S-phase in mosquito and human cells are under investigation. C6/36 and Huh-7 cells responded similarly to THY treatment (Fig. 2A and C), and neither virus titers (Fig. 2B and D) nor percent infection (Fig. 4A and B) in the PBS-treated control cultures differed substantially. Rather, the disparate response of DEN2 to cellular growth state in mosquito and human cells may reflect differences between the two species with respect to the availability of cellular factors during the cell cycle. Differences in viral protein processing, assembly, and virion maturation in mosquito and mammalian cells have been reported (51, 54, 61, 62). For example, cleavage of capsid protein (C) from premembrane protein (prM) and production of mature M protein, two important steps in virion assembly and maturation, are less efficient in mosquito cells (51). Preliminary experiments suggest that processing of prM to M is not enhanced in S-phase mosquito cells (A.-M. Helt and E. Harris, unpublished results). Differences in processing of NS4A and NS4B have also been demonstrated (54). In fact, a mutation in NS4B destroyed the ability of DEN to replicate in mosquito cells, while enhancing replication in mammalian cells (31), and a potential role for NS4B in virion assembly has been proposed (55). Alterations in the viral 5′ and 3′ UTRs have differential effects on replication in cells of the two host species (12, 69). Moreover, insect and mammalian cell membranes, integral to each step of DEN replication, differ in composition (reviewed in reference 52). While DEN2 titers did not increase in S-phase human cells, preliminary data indicate that DEN2 translation and virus titers are enhanced in M-phase human cells (Helt and Harris, unpublished), and others have reported an impact of cell cycle on DEN entry into mammalian cells (41, 67). Thus, the cell cycle responsiveness of DEN replication is not restricted to mosquito cells, and future work will investigate both host cell and viral determinants contributing to the responsiveness of DEN2 to cellular growth/metabolic state.
Indeed, relative to the clinical DEN2 isolates, the prototype DEN2 strain, 16681, contains alterations in prM, E, NS1, NS3, NS4B, NS5, and the 3′ UTR (21) (data not shown). Because DEN2 16681 replicates more efficiently in cell culture (Fig. 2B) (17, 21), it may be less susceptible to changes in cellular physiology resulting from cell cycle manipulation. The difference in S-phase responsiveness between 16681 and the low-passage DEN2 strains indicates that viral genetics influences the response of DEN to cell cycle. To more precisely identify the viral components responsive to S-phase and, thus, contributing either directly or indirectly to virion assembly, DEN2 variants with increased or reduced responsiveness to S-phase are under selection. Knowledge of the viral components involved will, in turn, facilitate identification of the interacting cellular factors that participate in assembly. Furthermore, genetic mapping of the S-phase-responsive viral determinants will allow the importance of this response to cellular growth/metabolic state to be studied in mosquitoes through the use of recombinant viruses.
Our data do not eliminate the possibility that an inhibitor of DEN replication is present in other cell cycle phases. However, because DEN2 replicates under all cell cycle conditions, we propose that the presence of a rate-limiting cellular factor whose availability increases during S-phase regulates viral replication. Importantly, DNA synthesis inhibitors that stall cells in S-phase do not inhibit other cellular metabolic processes, such as translation and membrane biogenesis (38, 49). Thus, this putative rate-limiting factor could be proteinaceous; for example, a cell cycle-regulated chaperone that also acts in viral protein processing or assembly. Alternatively, cellular membrane biosynthesis could enhance DEN2 production in S-phase cells. Membrane biosynthesis is cell cycle regulated in both eukaryotic and prokaryotic cells (37, 49). Accumulation of membrane phospholipids is highest during S-phase in mammalian cells (30, 38), although whether membrane biosynthesis is increased in S-phase mosquito cells has not been reported. Cellular membranes are integral to DEN replication by providing a surface on which viral translation, RNA replication, and assembly occur and by supplying the lipid envelope (48). The induction of membrane accumulation and rearrangements by DEN (48) may be augmented in S-phase mosquito cells, thereby facilitating viral assembly. Moreover, differences in cellular lipid content during the cell cycle could also influence the composition of the viral envelope, thereby contributing to the small increase in infectivity suggested by our results (Fig. 9).
The influence of cellular growth/metabolic state on DEN2 replication in vivo should be a fruitful area of investigation both in mosquitoes and in mouse models of infection. Indeed, studies of RNA viruses in the context of cellular growth state have shed light on mechanisms governing viral replication, dissemination and pathogenesis in vivo. For example, human immunodeficiency virus type 1 (HIV-1) was found to arrest infected T cells in the G2-phase of the cell cycle (22), resulting in upregulation of viral transcription and increased virus titers (28). This arrest is mediated by viral protein R (vpr), which is not required for viral replication in cell culture but is selected for in vivo (22, 28). T cells are normally short-lived and are rapidly killed by HIV; therefore, a delay in G2 may maximize virus production (28). Coxsackievirus (CV) 3B was shown to replicate preferentially in G1/S-phase cells in culture but established a latent infection in quiescent (G0) cells (25). The association of CV with both acute and chronic inflammatory diseases in humans and the evidence for persistence of picornavirus RNA in vivo long after initial infection led to the proposal that cell cycle status of the host cell may influence CV persistence, replication, and disease manifestations (25). This proposal was supported in a mouse model of infection (23, 24). Finally, RNA replication (58) and translation of HCV (36, 66) were shown to be modulated by cellular growth state through the use of reporter constructs and replicons. It has been proposed that a cycle may exist between HCV expression and the liver damage and regeneration that occur during chronic HCV infection (36).
Many cells in the adult mosquito are nondividing, and DEN infects a number of tissues throughout the mosquito. In addition, DEN2 replicates in both resting and cycling mosquito cells in culture (Fig. 1B). These observations suggest that cell cycle status does not restrict viral tropism in the mosquito. However, the mosquito gut epithelia and other tissues in which DEN replicates undergo dramatic metabolic activation upon ingestion of a blood meal (56, 57). In fact, S-phase induction occurs in tissues such as the fat body and ovaries (19, 46). Regardless of whether cell division is involved, metabolic events induced by a blood meal share features found in cycling cells, including increased lipid metabolism, membrane biogenesis, and rearrangements (35, 56, 57), DNA and RNA synthesis (19), protein synthesis (35), and polyamine synthesis (44). Furthermore, cellular activation in vivo is not limited to blood meal-induced activation. Both hemocyte proliferation and markers of proliferation in abdominal tissues have been observed in immune-activated mosquitoes (14, 33). These observations, together with our results, suggest the potential for cellular growth or activation state to influence the course of DEN infection in the mosquito.
Our results indicate that the mosquito cell environment during S-phase facilitates DEN2 assembly, either directly or, perhaps, via effects on processing or trafficking of the structural proteins, possibilities under investigation. Interestingly, inefficient virion assembly and/or maturation has been associated with a dissemination barrier in the mosquito (5, 8). Thus, a responsiveness of DEN to cellular activation could influence the timing of dissemination and/or the transmission potential of the virus. Coupling of arbovirus replication to the metabolic state of mosquito tissues has been reported for La Crosse virus, a member of the Bunyaviridae family (13). Whether the timing of DEN infection and spread in the mosquito overlaps with the examples of activated cellular metabolism noted above remains to be determined. Regardless, investigation of DEN2 in the context of mosquito and human cell growth state has set the stage for characterizing the role of viral and host cell components in DEN replication.
Acknowledgments
We are grateful to Sondra Schlesinger, Milt Schlesinger, and Christopher Meiering for critical reading of the manuscript and to members of the Harris laboratory for helpful discussions.
This work was supported by grant 2617SC from the Pew Charitable Trusts to E.H. A.-M.H. was supported by both the Pew Charitable Trusts and by NIH training grant AI07641.
REFERENCES
- 1.Andrews, B. S., A. N. Theofilopoulos, C. J. Peters, D. J. Loskutoff, W. E. Brandt, and F. J. Dixon. 1978. Replication of dengue and junin viruses in cultured rabbit and human endothelial cells. Infect. Immun. 20:776-781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Armstrong, P. M., and R. Rico-Hesse. 2003. Efficiency of dengue serotype 2 virus strains to infect and disseminate in Aedes aegypti. Am. J. Trop. Med. Hyg. 68:539-544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baim, A. S., and A. B. Mukherjee. 1978. The cell cycle of an established cell line of the mosquito Aedes aegypti. Can. J. Genet. Cytol. 20:373-376. [DOI] [PubMed] [Google Scholar]
- 4.Balmaseda, A., E. Sandoval, L. Perez, C. M. Gutierrez, and E. Harris. 1999. Application of molecular typing techniques in the 1998 dengue epidemic in Nicaragua. Am. J. Trop. Med. Hyg. 61:893-897. [DOI] [PubMed] [Google Scholar]
- 5.Beaty, B. J., B. R. Miller, R. E. Shope, E. J. Rozhon, and D. H. L. Bishop. 1982. Molecular basis of bunyavirus per os infection of mosqutoes: role of the middle-sized RNA segment. Proc. Natl. Acad. Sci. USA 79:1295-1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhamarapravati, N. 1989. Hemostatic defects in dengue hemorrhagic fever. Rev. Infect. Dis. 11(Suppl. 4):S826-S829. [DOI] [PubMed] [Google Scholar]
- 7.Bhamarapravati, N., P. Tuchinda, and V. Boonyapaknavik. 1967. Pathology of Thailand hemorrhagic fever: a study of 100 autopsy cases. Ann. Trop. Med. Parasitol. 61:500-510. [DOI] [PubMed] [Google Scholar]
- 8.Black, W. C., IV, K. E. Bennett, N. Gorrochotegui-Escalante, C. V. Barillas-Mury, I. Fernandez-Salsa, M. de Lourdes Munoz, J. A. Farfan-Ale, K. E. Olson, and B. J. Beaty. 2002. Flavivirus susceptibility in Aedes aegypti. Arch. Med. Res. 33:379-388. [DOI] [PubMed] [Google Scholar]
- 9.Blok, J., B. H. Kay, R. A. Hall, and B. M. Gorman. 1988. Isolation and characterization of dengue viruses serotype 1 from an epidemic in northern Queensland, Australia. Arch. Virol. 100:213-220. [DOI] [PubMed] [Google Scholar]
- 10.Bootsma, D., L. Budke, and O. Vos. 1964. Studies on synchronized division of tissue culture cells initiated by excess thymidine. Exp. Cell. Res. 33:301-304. [DOI] [PubMed] [Google Scholar]
- 11.Bostock, C. J., D. M. Prescott, and J. B. Kirkpatrick. 1971. An evaluation of the double thymidine block for synchronizing mammalian cells at the G1-S border. Exp. Cell. Res. 68:163-168. [DOI] [PubMed] [Google Scholar]
- 12.Cahour, A., A. Pletnev, M. Vazielle-Falcoz, L. Rosen, and C. J. Lai. 1995. Growth-restricted dengue virus mutants containing deletions in the 5′ noncoding region of the RNA genome. Virology 207:68-76. [DOI] [PubMed] [Google Scholar]
- 13.Chandler, L. J., L. P. Wasieloski, C. D. Blair, and B. J. Beaty. 1996. Analysis of La Crosse virus S-segment RNA and its positive-sense transcripts in persistently infected mosquito tissues. J. Virol. 70:8972-8976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Christensen, B. M., B. M. Huff, G. S. Miranpuri, K. L. Harris, and L. A. Christensen. 1989. Hemocyte population changes during the immune response of Aedes aegypti to inoculated microfilariae of Dirofilaria immitis. J. Parasitol. 75:119-123. [PubMed] [Google Scholar]
- 15.Cologna, R., and R. Rico-Hesse. 2003. American genotype structures decrease dengue virus output from human monocytes and dendritic cells. J. Virol. 77:3928-3938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Daughaday, C. C., W. E. Brandt, J. M. McCown, and P. K. Russell. 1981. Evidence for two mechanisms of dengue virus infection of adherent human monocytes: trypsin-sensitive virus receptors and trypsin-resistant immune complex receptors. Infect. Immun. 32:469-473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Diamond, M. S., D. Edgil, T. G. Roberts, B. Lu, and E. Harris. 2000. Infection of human cells by dengue virus is modulated by different cell types and viral strains. J. Virol. 74:7814-7823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Diamond, M. S., and E. Harris. 2001. Interferon inhibits dengue virus infection by preventing translation of viral RNA through a PKR-independent mechanism. Virology 289:297-311. [DOI] [PubMed] [Google Scholar]
- 19.Dittmann, F., P. H. Kogan, and H. H. Hagedorn. 1989. Ploidy levels and DNA synthesis in fat body cells of the adult mosquito, Aedes aegypti: the role of juvenile hormone. Arch. Insect Biochem. Physiol. 12:133-143. [Google Scholar]
- 20.Edgil, D., and E. Harris. Submitted for publication.
- 21.Edgil, D., and E. Harris. 2003. Translation efficiency determines differences in cellular infection among dengue virus type 2 strains. Virology 317:275-290. [DOI] [PubMed] [Google Scholar]
- 22.Emerman, M. 1996. HIV-1, Vpr and the cell cycle. Curr. Biol. 6:1096-1103. [DOI] [PubMed] [Google Scholar]
- 23.Feuer, R., I. Mena, R. Pagarigan, S. Harkins, D. E. Hassett, and J. L. Whitton. 2003. The roles of stem cells, developing neurons, and apoptosis in infection, viral dissemination and disease. Am. J. Pathol. 163:1379-1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feuer, R., I. Mena, R. Pagarigan, D. E. Hassett, and J. L. Whitton. 2003. Coxsackievirus replication and the cell cycle: a potential regulatory mechanism for viral persistence/latency. Med. Microbiol. Immunol. 193:83-90. [DOI] [PubMed] [Google Scholar]
- 25.Feuer, R., I. Mena, R. Pagarigan, M. K. Slifka, and J. L. Whitton. 2002. Cell cycle status affects coxsackievirus replication, persistence, and reactivation in vitro. J. Virol. 76:4430-4440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gerenday, A., T. S. Blauwkamp, and A. M. Fallon. 1997. Synchronization of Aedes albopictus mosquito cells using hydroxyurea. Insect Mol. Biol. 6:191-196. [DOI] [PubMed] [Google Scholar]
- 27.Gerenday, A., and A. M. Fallon. 1996. Cell cycle parameters in Aedes albopictus mosquito cells. In Vitro Cell Dev. Biol. Anim. 32:307-312. [DOI] [PubMed] [Google Scholar]
- 28.Goh, W. C., M. E. Rogel, C. M. Kinsey, S. F. Michael, P. N. Fultz, M. A. Nowak, B. H. Hahn, and M. Emerman. 1998. HIV-1 Vpr increases viral expression by manipulation of the cell cycle: a mechanism for selection of Vpr in vivo. Nat. Med. 4:65-71. [DOI] [PubMed] [Google Scholar]
- 29.Gubler, D. J. 1998. Dengue and dengue hemorrhagic fever. Clin. Microbiol. Rev. 11:480-496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Habenicht, A. J. R., J. A. Glomset, M. Goerig, R. Gronwald, J. Grulich, U. Loth, and G. Schettler. 1985. Cell cycle-dependent changes in arachidonic acid and glycerol metabolism in Swiss 3T3 cells stimulated by platelet-derived growth factor. J. Biol. Chem. 260:1370-1373. [PubMed] [Google Scholar]
- 31.Hanley, K. A., L. R. Manlucu, L. E. Gilmore, J. E. Blaney, C. T. Hanson, B. R. Murphy, and S. S. Whitehead. 2003. A trade-off in replication in mosquito versus mammalian systems conferred by a point mutation in the NS4B protein of dengue virus type 4. Virology 312:222-232. [DOI] [PubMed] [Google Scholar]
- 32.Hardy, J. L. 1988. Susceptibility and resistance of vector mosquitoes, p. 87-126. In T. Monath (ed.), The arboviruses: epidemiology and ecology, vol. 2. CRC Press, Boca Raton, Fla. [Google Scholar]
- 33.Hernandez-Martinez, S. 2003. Presented at the EMBO Workshop: Molecular and Population Biology of Mosquitoes, Crete, Greece.
- 34.Holden, K. H., and E. Harris. 2004. Enhancement of dengue virus translation: role of the 3′ untranslated region and the terminal 3′ stem-loop domain. Virology 329:119-133. [DOI] [PubMed] [Google Scholar]
- 35.Holt, R. A., G. M. Subramanian, A. Halpern, et al. 2002. The genome sequence of the malaria mosquito Anopheles gambiae. Science 298:129-149. [DOI] [PubMed] [Google Scholar]
- 36.Honda, M., S. Kaneko, E. Matsushita, K. Kobayashi, G. A. Abell, and S. M. Lemon. 2000. Cell cycle regulation of hepatitis C virus internal ribosomal entry site-directed translation. Gastroenterology 118:152-162. [DOI] [PubMed] [Google Scholar]
- 37.Jackowski, S. 1996. Cell cycle regulation of membrane phospholipid metabolism. J. Biol. Chem. 271:20219-20222. [DOI] [PubMed] [Google Scholar]
- 38.Jackowski, S. 1994. Coordination of membrane phospholipid synthesis with the cell cycle. J. Biol. Chem. 269:3858-3867. [PubMed] [Google Scholar]
- 39.Jessie, K., M. Y. Fong, S. Devi, S. K. Lam, and K. T. Wong. 2004. Localization of dengue virus in naturally infected human tissues, by immunohistochemistry and in situ hybridization. J. Infect. Dis. 189:1411-1418. [DOI] [PubMed] [Google Scholar]
- 40.Killen, H., and M. A. O'Sullivan. 1993. Detection of dengue virus by in situ hybridization. J. Virol. Methods 41:135-146. [DOI] [PubMed] [Google Scholar]
- 41.Kimura, T., E. Tanimura, N. Yamamoto, T. Ito, and A. Ohyama. 1985. The quantitative kinetic study of dengue viral antigen by flow cytometry. I. An in vitro study. Virus Res. 2:375-389. [DOI] [PubMed] [Google Scholar]
- 42.King, A. D., A. Nisalak, S. Kalayanrooj, K. S. Myint, K. Pattanapanyasat, S. Nimmannitya, and B. L. Innis. 1999. B cells are the principal circulating mononuclear cells infected by dengue virus. Southeast Asian J. Trop. Med. Public Health 30:718-728. [PubMed] [Google Scholar]
- 43.Kinney, R. M., S. Butrapet, G. J. Chang, K. R. Tsuchiya, J. T. Roehrig, N. Bhamarapravati, and D. J. Gubler. 1997. Construction of infectious cDNA clones for dengue 2 virus: strain 16681 and its attenuated vaccine derivative, strain PDK-53. Virology 230:300-308. [DOI] [PubMed] [Google Scholar]
- 44.Kogan, P. H., and H. H. Hagedorn. 2000. Polyamines, and effects from reducing their synthesis during egg development in the yellow fever mosquito, Aedes aegypti. J. Insect Physiol. 46:1079-1095. [DOI] [PubMed] [Google Scholar]
- 45.Kwan, W. H., A.-M. Helt, C. Maranon, J. B. Barbaroux, A. Hosmalin, E. Harris, W. H. Fridman, and C. G. Mueller. 2005. Dendritic cell precursors are permissive to dengue virus and human immunodeficiency virus infection. J. Virol. 79:7291-7299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Laurence, B. R., and M. G. Simpson. 1974. Cell replication in the follicular epithelium of the adult mosquito. J. Insect Physiol. 20:703-715. [DOI] [PubMed] [Google Scholar]
- 47.Leitmeyer, K. C., D. W. Vaughn, D. M. Watts, R. Salas, I. Villalobos de Chacon, C. Ramos, and R. Rico-Hesse. 1999. Dengue virus structural differences that correlate with pathogenesis. J. Virol. 73:4738-4747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lindenbach, B. D., and C. M. Rice. 2001. Flaviviridae: the viruses and their replication, p. 991-1041. In D. M. Knipe and P. M. Howley (ed.), Field's virology, 4th ed., vol. 1. Lippincott-Williams & Wilkins Publishers, Philadelphia, Pa. [Google Scholar]
- 49.Lingwood, C. A., and D. B. Thomas. 1975. Modulation in the rates of incorporation of lipid precursors during the cell cycle. J. Cell. Physiol. 86:635-640. [DOI] [PubMed] [Google Scholar]
- 50.Lum, L. C., S. K. Lam, R. George, and S. Devi. 1993. Fulminant hepatitis in dengue infection. Southeast Asian J. Trop. Med. Public Health 24:467-471. [PubMed] [Google Scholar]
- 51.Murray, J. M., J. G. Aaskov, and P. J. Wright. 1993. Processing of the dengue virus type 2 proteins prM and C-prM. J. Gen. Virol. 74:175-182. [DOI] [PubMed] [Google Scholar]
- 52.Opekarova, M., and W. Tanner. 2003. Specific lipid requirements of membrane proteins—a putative bottleneck in heterologous expression. Biochim. Biophys. Acta 1610:11-22. [DOI] [PubMed] [Google Scholar]
- 53.O'Sullivan, M. A., and H. M. Killen. 1994. The differentiation state of monocytic cells affects their susceptibility to infection and the effects of infection by dengue virus. J. Gen. Virol. 75:2387-2392. [DOI] [PubMed] [Google Scholar]
- 54.Preugschat, F., and J. H. Strauss. 1991. Processing of nonstructural proteins NS4A and NS4B of dengue 2 virus in vitro and in vivo. Virology 185:689-697. [DOI] [PubMed] [Google Scholar]
- 55.Pugachev, K. V., F. Guirakhoo, S. W. Ocran, F. Mitchell, M. Parsons, C. Penal, S. Girakhoo, S. O. Pougatcheva, J. Arroyo, D. W. Trent, and T. Monath. 2004. High fidelity of yellow fever virus RNA polymerase. J. Virol. 78:1032-1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rudin, W., and H. Hecker. 1979. Functional morphology of the midgut of Aedes aegypti L. (Insecta, Diptera) during blood digestion. Cell Tissue Res. 200:193-203. [DOI] [PubMed] [Google Scholar]
- 57.Sanders, H. R., A. M. Evans, L. S. Ross, and S. S. Gill. 2003. Blood meal induces global changes in midgut gene expression in the disease vector, Aedes aegypti. Insect Biochem. Mol. Biol. 33:1105-1122. [DOI] [PubMed] [Google Scholar]
- 58.Scholle, F., K. Li, F. Bodola, M. Ikeda, B. A. Luxon, and S. M. Lemon. 2004. Virus-host cell interactions during hepatitis C virus RNA replication: impact of polyprotein expression on the cellular transcriptome and cell cycle association with viral RNA synthesis. J. Virol. 78:1513-1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shen, J., J. M. Devery, and N. J. King. 1995. Early induction of interferon-independent virus-specific ICAM-1 (CD54) expression by flavivirus in quiescent but not proliferating fibroblasts-implications for virus-host interactions. Virology 208:437-449. [DOI] [PubMed] [Google Scholar]
- 60.Shresta, S., J. L. Kyle, P. R. Beatty, and E. Harris. 2004. Early activation of natural killer and B cells in response to primary dengue virus infection in A/J mice. Virology 319:262-273. [DOI] [PubMed] [Google Scholar]
- 61.Sinarachatanant, P., and L. C. Olson. 1973. Replication of dengue virus type 2 in Aedes albopictus cell culture. J. Virol. 12:275-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Smith, G. W., and P. J. Wright. 1985. Synthesis of proteins and glycoproteins in dengue type 2 virus-infected vero and Aedes albopictus cells. J. Gen. Virol. 66:559-571. [DOI] [PubMed] [Google Scholar]
- 63.Sriurairatna, S., and N. Bhamarapravati. 1977. Replication of dengue-2 virus in Aedes albopictus mosquitoes. An electron microscopic study. Am. J. Trop. Med. Hyg. 26:1199-1205. [DOI] [PubMed] [Google Scholar]
- 64.Theofilopoulos, A. N., W. E. Brandt, P. K. Russell, and F. T. Dixon. 1976. Replication of dengue-2 virus in cultured human lymphoblastoid cells and subpopulations of human peripheral leukocytes. J. Immunol. 117:953-961. [PubMed] [Google Scholar]
- 65.Tyler, K. L., and B. N. Fields. 1996. Pathogenesis of viral infections, p. 173-218. In D. M. Knipe, P. M. Howley, and B. N. Fields (ed.), Fields virology, vol. 1. Lippincott-Raven, Philadelphia, Pa. [Google Scholar]
- 66.Venkatesan, A., R. Sharma, and A. Dasgupta. 2003. Cell cycle regulation of hepatitis C and encephalomyocarditis virus internal ribosome entry site-mediated translation in human embryonic kidney 293 cells. Virus Res. 94:85-95. [DOI] [PubMed] [Google Scholar]
- 67.Waranyoo, P., and D. R. Smith. 2004. Internalization of the dengue virus is cell cycle modulated in HepG2 but not Vero cells. J. Med. Virol. 74:434-441. [DOI] [PubMed] [Google Scholar]
- 68.Wu, S. J., G. Grouard-Vogel, W. Sun, J. R. Mascola, E. Brachtel, R. Putvatana, M. K. Louder, L. Filgueira, M. A. Marovich, H. K. Wong, A. Blauvelt, G. S. Murphy, M. L. Robb, B. L. Innes, D. L. Birx, C. G. Hayes, and S. S. Frankel. 2000. Human skin Langerhans cells are targets of dengue virus infection. Nat. Med. 6:816-820. [DOI] [PubMed] [Google Scholar]
- 69.Zeng, L., B. Falgout, and L. Markoff. 1998. Identification of specific nucleotide sequences within the conserved 3′-SL in the dengue type 2 virus genome required for replication. J. Virol. 72:510-522. [DOI] [PMC free article] [PubMed] [Google Scholar]