Abstract
Neurotransmitter release involves the assembly of a heterotrimeric SNARE complex composed of the vesicle protein synaptobrevin (VAMP 2) and two plasma membrane partners, syntaxin 1 and SNAP-25. Calcium influx is thought to control this process via Ca2+-binding proteins that associate with components of the SNARE complex. Ca2+/calmodulin or phospholipids bind in a mutually exclusive fashion to a C-terminal domain of VAMP (VAMP77–90), and residues involved were identified by plasmon resonance spectroscopy. Microinjection of wild-type VAMP77–90, but not mutant peptides, inhibited catecholamine release from chromaffin cells monitored by carbon fibre amperometry. Pre-incubation of PC12 pheochromocytoma cells with the irreversible calmodulin antagonist ophiobolin A inhibited Ca2+-dependent human growth hormone release in a permeabilized cell assay. Treatment of permeabilized cells with tetanus toxin light chain (TeNT) also suppressed secretion. In the presence of TeNT, exocytosis was restored by transfection of TeNT-resistant (Q76V, F77W) VAMP, but additional targeted mutations in VAMP77–90 abolished its ability to rescue release. The calmodulin- and phospholipid-binding domain of VAMP 2 is thus required for Ca2+-dependent exocytosis, possibly to regulate SNARE complex assembly.
Keywords: neuroendocrine cells/secretory vesicle/SNARE/tetanus toxin
Introduction
Neurones and neuroendocrine cells release transmitters and neuropeptides by calcium-dependent exocytosis of the contents of vesicles docked at the plasma membrane. This process requires assembly of trimeric SNARE complexes formed by the vesicle-associated membrane protein synaptobrevin (VAMP 2) and two partners that are expressed mainly in the plasma membrane, syntaxin 1 and synaptosome-associated protein of 25 kDa (SNAP-25) (reviewed by Jahn and Sudhof, 1999; Lin and Scheller, 2000; Mayer, 2001). Analysis of a minimal complex composed uniquely of the interacting domains of these three proteins has revealed a parallel bundle of four α-helices (one from VAMP 2, one from syntaxin 1 and two from SNAP-25) twisted into a superhelical structure (Sutton et al., 1998). Extrapolation of these data to a situation in which VAMP 2 and syntaxin 1/SNAP-25 are anchored in distinct lipid bilayers (i.e. docked vesicle membranes and plasma membranes, respectively) led to a proposal for trans SNARE complex function. The zipping-up of SNARE complexes from the N-terminus to the C-terminus would pull the opposing C-terminal transmembrane anchors towards each other and promote membrane fusion at the vesicle–plasma membrane interface.
Abundant evidence from the use of botulinum and tetanus toxins (BoNT and TeNT, respectively), which inhibit transmitter release by cleaving SNARE proteins (Xu et al., 1998; Hua and Charlton, 1999), as well as mutagenesis in invertebrates and mice (Fergestad et al., 2001; Schoch et al., 2001; Washbourne et al., 2002), have consolidated the view that SNARE proteins are required for exocytosis. However, the precise role of SNARE complex assembly in membrane fusion is still a matter for debate, and two distinct models are currently proposed. (i) SNARE complex assembly acts mechanically on the two lipid bilayers, generating a force that leads to hemi-fusion or drives full fusion. The fact that the reconstitution of appropriate SNARE partners into two liposome populations promotes their fusion upon mixing provides support for this model (Weber et al., 1998; McNew et al., 2000). (ii) Alternatively, the SNARE complex may align two halves of a protein channel, one in the vesicle membrane and the other in the plasma membrane. This gap junction-like structure would open subsequently to form a pore and then expand to allow full fusion. Dissection of homotypic vesicle fusion in yeast is consistent with the second model in which SNARE pairing is required to prepare, but not to accomplish, the final step in membrane fusion (Mayer, 2001). It also suggests that an oligomer formed by small hydrophobic subunits of the vacuolar H+-ATPase (V-ATPase) constitutes a hemi-fusion pore (Peters et al., 2001). Moreover, this mechanism may not be restricted to yeast. A proteolipid from Torpedo synaptosomes, implicated in acetylcholine release and originally designated mediatophore, is identical to the hydrophobic V-ATPase subunit (Morel et al., 2001).
Another important issue concerns the mechanisms by which calcium ions control the fusion machinery. There is no compelling evidence that SNARE proteins are intrinsically sensitive to calcium, suggesting that associated calcium-binding proteins confer this property. Attention has focused principally on the synaptotagmins, which interact with SNARE proteins and phospholipids via their C2 domains, in a calcium-dependent manner (Sudhof, 2002; Sugita et al., 2002). Mutations in the genes encoding synaptotagmins in Drosophila (Littleton et al., 2001a,b) and mice (Fernandez-Chacon et al., 2001; Voets et al., 2001), followed by functional analysis, have indicated that they play a central role in evoked neurotransmitter release.
SNARE-mediated vacuole fusion in yeast is activated by calcium efflux from the vacuole lumen and uses an alternative calcium sensor, calmodulin (Peters and Mayer, 1998). In mammalian cells, vesicular calcium and calmodulin have also been implicated in intra-Golgi and endosome fusion (Colombo et al., 1997; Porat and Elazar, 2000; Pryor et al., 2000). Calmodulin is essential for calcium-dependent exocytosis in Paramecium (Kerboeuf et al., 1993). Furthermore, studies in permeabilized chromaffin and PC12 pheochromocytoma cells support the view that calcium-activated calmodulin acts as a positive effector during the triggering step of regulated exocytosis, although its molecular mode of action remains obscure (Chamberlain et al., 1995; Kibble and Burgoyne, 1996; Chen et al., 1999a). However, evidence suggesting that calmodulin is the calcium receptor for rapid endocytosis but not exocytosis has also been reported (Artalejo et al., 1996).
These findings led us to examine whether calmodulin interacts directly with synaptic SNARE proteins. We have recently identified a calcium-dependent calmodulin-binding site located in a C-terminal domain of VAMP 2 (Quetglas et al., 2000). Calmodulin binding is supported by a consensus 1-8-14 type A motif (Rhoads and Friedberg, 1997) that is evolutionarily conserved in VAMP 2 homologues, including Nvy1p, which functions in yeast vacuole fusion. Intriguingly, in VAMP 2, this motif (residues 77–90) starts precisely downstream of the peptide bond (Q76–F77) cleaved by TeNT and BoNT/B. It is thus strategically located between the coiled-coil domain of the SNARE complex and the membrane anchors. Finally, VAMP77–90 also interacts with negatively charged phospholipids (Quetglas et al., 2000), which may be significant given its juxtamembrane location.
In this report, we have addressed the functional relevance of calmodulin and lipid interactions with VAMP 2. Using synthetic peptides and surface plasmon resonance (SPR) spectroscopy, mutations were designed that perturbed the binding properties of VAMP77–90. Two complementary approaches were employed to explore these mutations. First, wild-type or mutant peptides were microinjected in chromaffin cells. In this paradigm, the acute effects of competing for the endogenous partner(s) of VAMP77–90 were evaluated by monitoring evoked catecholamine release by carbon fibre amperometry. The second approach used a human growth hormone (hGH) release assay in permeabilized PC12 cells. Endogenous VAMP was inactivated with TeNT and secretion was restored by transfecting toxin-resistant VAMP (i.e. with mutations in the TeNT cleavage site). Additional mutations were introduced in VAMP77–90 to probe whether the calmodulin/phospholipid-binding domain is necessary to rescue secretion. Both data sets are entirely consistent with the conclusion that the C-terminal segment of VAMP 2 plays a critical role in calcium-dependent exocytosis.
Results
Binding properties of VAMP77–90
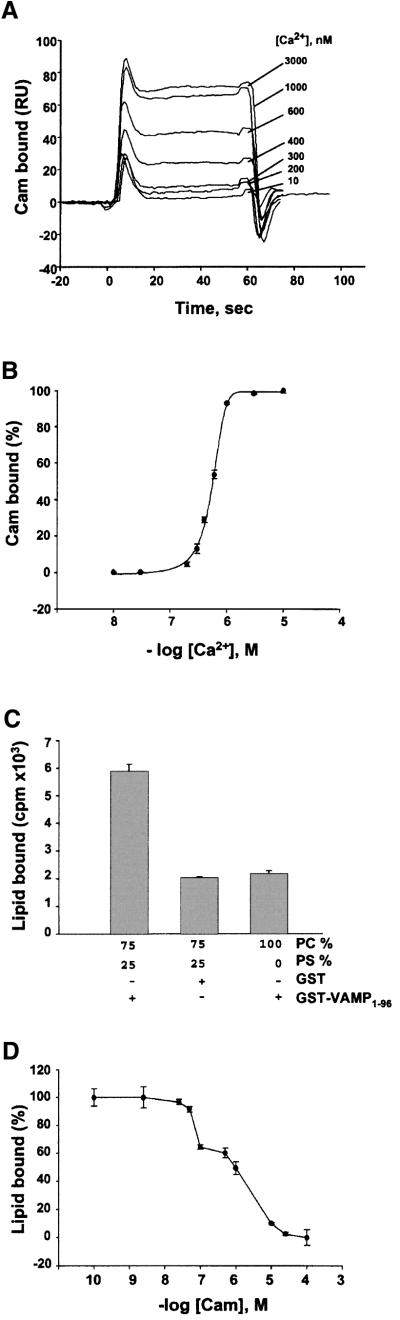
In a previous study, we demonstrated Ca2+/calmodulin binding to a consensus sequence located close to the transmembrane region of VAMP 2. The same domain also constitutes a phospholipid interaction site (Quetglas et al., 2000). These findings were extended by evaluating calcium dependency, and competitive interactions between calmodulin and phospholipids (Figure 1). GST fused to the cytoplasmic domain of VAMP 2 (GST– VAMP1–96) was immobilized on the sensor chip of an SPR apparatus via anti-GST antibodies. Calmodulin was injected in the running buffer with a range of free calcium concentrations. SPR sensorgrams (Figure 1A) indicated that calmodulin binding increased steeply with increasing concentrations of free Ca2+ within a 100 nM to 1 µM range, yielding an apparent KD of 570 nM (Figure 1B). We have shown previously that the calmodulin-binding motif (VAMP77–90) also constitutes an interaction site specific for negatively charged phospholipids (Quetglas et al., 2000). We therefore examined whether Ca2+/calmodulin could displace phospholipid binding. GST–VAMP1–96 was immobilized on glutathione–agarose beads, and incubated with [3H]liposomes [25% phosphatidylserine (PS), 75% phosphatidylcholine (PC) with tracer amounts of [3H]PC)]. Figure 1C demonstrates binding of liposomes containing PS to immobilized GST–VAMP1–96. The non-specific component, defined as the binding either of PS/PC to GST alone, or of pure PC liposomes to GST–VAMP, was ∼30% of total binding. In the presence of Ca2+, increasing concentrations of calmodulin effectively displaced the specific component of lipid binding (Figure 1D). The displacement curve was biphasic with a distinct intermediate plateau at 0.1–1 µM. It is not clear at present whether this results from heterogeneity in calmodulin or phospholipid interactions with VAMP. However, the high affinity component was consistent with the KD for calmodulin binding to VAMP1–96 (Quetglas et al., 2000) or to a synthetic VAMP77–90 peptide (see Figure 2A). Furthermore, displacement was practically complete at physiological calmodulin concentrations (∼10 µM). Thus, calmodulin inhibits the binding of acidic phospholipids to VAMP in a calcium-dependent manner.
Fig. 1. Calmodulin and phospholipid binding to VAMP. (A) GST– VAMP1–96 was immobilized on the sensor chip of an SPR (BIAcore) apparatus, and 2.5 µM calmodulin was injected into the running buffer in the presence of the indicated concentrations of free Ca2+. Calmodulin binding sensorgrams are shown in resonance units (RU). (B) Calcium dependency of calmodulin binding to VAMP determined at plateau levels in (A). (C) [3H]Liposomes containing PS and/or PC as indicated were incubated with GST–VAMP1–96 or GST immobilized on agarose beads. After washing and centrifugation, bound lipid was evaluated by scintillation counting. Mean of assays in triplicate from three independent experiments. (D) [3H]Liposomes (25% PS, 75% PC) were incubated with GST–VAMP1–96 in the presence of the indicated concentrations of calmodulin and 1 mM Ca2+. 100% = binding in the absence of calmodulin, 0% = binding to GST. Representative of two independent experiments with assays in triplicate.
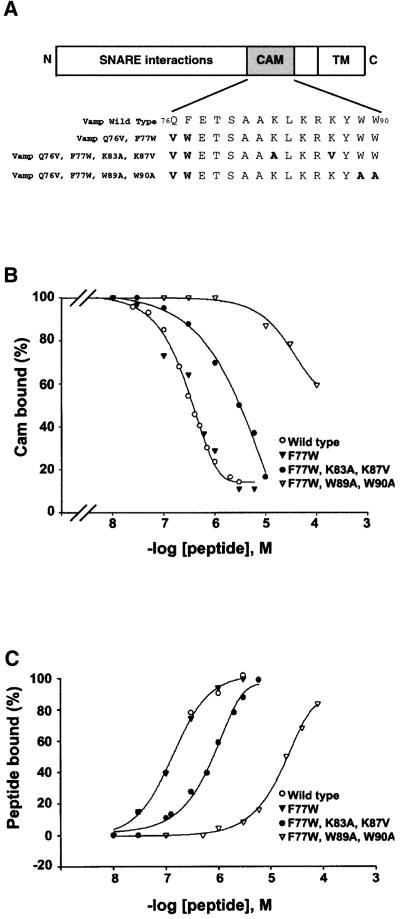
Fig. 2. Effect of mutations in VAMP77–90 on calmodulin and lipid binding. (A) Domain structure of VAMP indicating the mutations used throughout this study (CAM, calmodulin-binding domain; TM, transmembrane region). Mutations Q76V,F77W confer resistance to TeNT. The effects of mutations (in bold) within the consensus calmodulin-binding domain (i.e. VAMP residues 77–90) were evaluated in (B) and (C) by SPR using synthetic peptides. (B) Calmodulin was immobilized on the sensor chip, and the ability of peptides to inhibit binding to GST–VAMP1–96 was measured. The figure represents the mean of data from between two and five independent experiments with each peptide (for KI values, see text). (C) Liposomes (25% PS, 75% PC) were immobilized on the sensor chip, and direct binding of VAMP77–90 peptides was evaluated. The figure represents a typical experiment from several independent assays (for mean data, see text).
In order to address the functional relevance of these interactions, we designed mutations to perturb calmodulin binding, using synthetic peptides and an SPR binding assay. Four peptides were analysed (Figure 2A): (i) wild-type VAMP77–90 and peptides containing the mutations; (ii) F77W; (iii) F77W + K83A,K87V; or (iv) F77W + W89A,W90A. For reasons that will become evident later (see Figures 4–6), our subsequent strategy involved mutations (Q76V,F77W) that confer resistance to cleavage by TeNT (Regazzi et al., 1996). The conservative substitution F77W modifies the first aromatic residue in the calmodulin-binding sequence but in principle maintains a consensus site. We therefore checked that it did not perturb the binding properties. The mutation K83A,K87V, designed by analogy to mutations reported in the calmodulin-binding site of rab3A (Coppola et al., 1999), was aimed at eliminating basic charges that contribute to calmodulin binding. Alternatively, the mutation W89A, W90A modifies aromatic amino acids, reducing the hydrophobicity of the last residue in the consensus (W90) while retaining the net charge. To evaluate these mutations, we assayed the ability of the four peptides to compete with GST–VAMP1–96 for binding to immobilized calmodulin (Figure 2B). The wild-type peptide displayed a KI = 350 nM (mean, n = 4) and, as predicted, the conservative F77W substitution did not modify the interaction. In contrast, the K83A,K87V (KI = 3 µM, n = 3) and W89A, W90A (KI >100 µM, n = 2) mutations reduced Ca2+/calmodulin binding ∼10- and 300-fold, respectively. The effects of mutations on peptide binding to phospholipids were also assayed by SPR. Liposomes (PS/PC) were layered onto a hydrophobic sensor chip, and direct binding of the injected peptides to immobilized lipids was monitored in resonance units (Figure 2C). Again the binding properties of wild-type and F77W peptides were practically identical (KD = 189 ± 65 nM, n = 5, and 194 ± 77 nM, n = 3, respectively). Removal of two basic charges (K83A,K87V) led to a 5-fold reduction in interaction (KD = 884 ± 115 nM, n = 4), while substitution of the aromatic residues (W89A,W90A) had a more profound effect (KD = >30 µM, n = 2). Thus, mutations of basic or aromatic residues affect both calmodulin and phospholipid binding to a similar degree, which is consistent with the fact that both types of interaction are generally supported by basic amphipathic motifs.
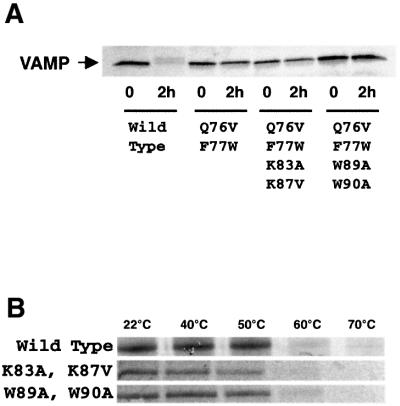
Fig. 4. Effect of mutations on tetanus toxin cleavage and SNARE complex stability. (A) In vitro translated [35S]VAMP with the indicated mutations was incubated with 100 nM TeNT light chain for 0 or 2 h. The band corresponding to intact VAMP was monitored by SDS–PAGE and autoradiography. (B) SNARE complexes were assembled on glutathione–agarose beads using in vitro translated [35S]SNAP-25, bacterially expressed syntaxin 1A and GST–VAMP1–96 with the indicated mutations. After washing, 3% SDS was added and the stability of the SDS-resistant SNARE complex (120 kDa) during a 2 min incubation at the indicated temperatures was monitored by SDS–PAGE and autoradiography.
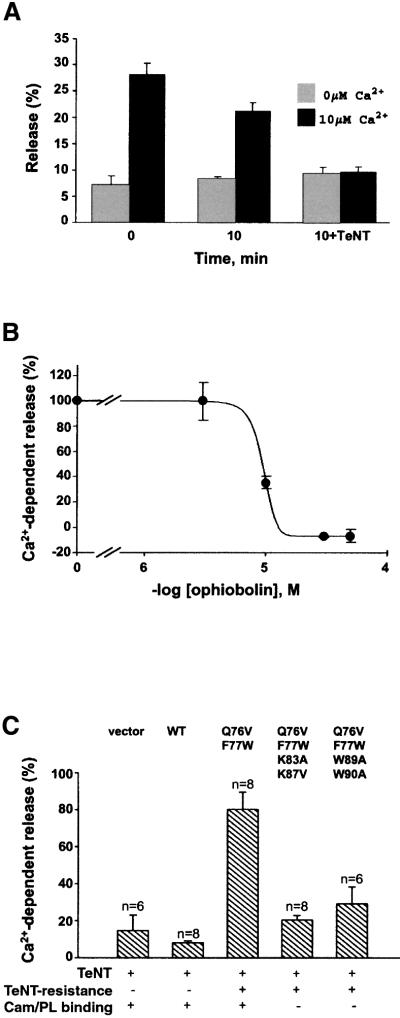
Fig. 6. Effects of a calmodulin antagonist and mutations in VAMP77–90 on calcium-dependent exocytosis in PC12 cells. (A) PC12 cells, transfected with a plasmid encoding hGH, were permeabilized with digitonin. Following the indicated pre-incubation times (±300 nM TeNT light chain), Ca2+-dependent hGH release was evoked by 10 µM Ca2+, and compared with basal release (0 µM Ca2+). The hGH released over 4 min was assayed and is represented as a percentage of total cellular hGH content. The graph is representative of three independent experiments with duplicate data points. (B) Intact cells were treated with the indicated concentrations of the calmodulin antagonist ophiobolin A. After washing and permeabilization, hGH release was measured as in (A) and plotted as Ca2+-dependent release (per cent evoked minus basal release). The graph is representative of three independent experiments with duplicate data points. (C) PC12 cells were transfected with a plasmid encoding hGH (vector) or a single plasmid encoding both hGH and VAMP (wild-type or with the indicated mutations). After permeabilization, cells were treated for 10 min with or without TeNT (as in A) and hGH release was assayed. Results are presented as the percentage of Ca2+-dependent release that persists after TeNT treatment. Data were pooled from three or four independent experiments with duplicate assays.
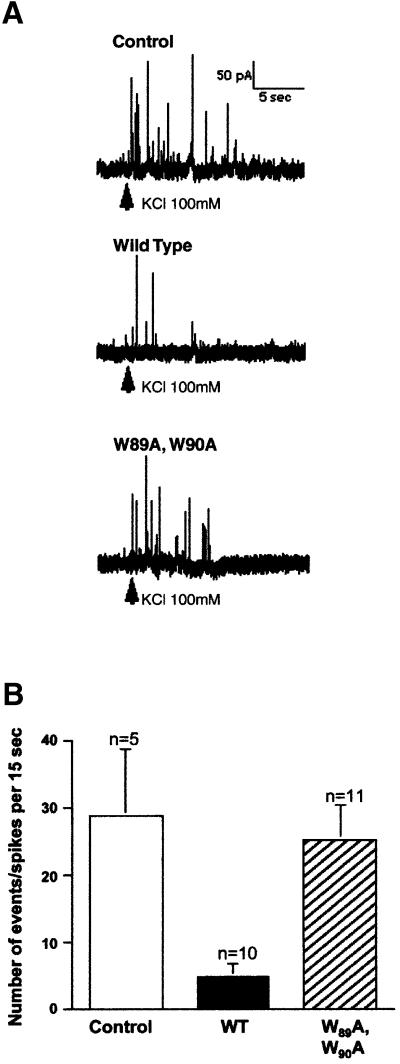
Microinjected VAMP77–90 peptide inhibits catecholamine release from chromaffin cells
A mimetic peptide approach was used to determine whether acute depletion of endogenous molecules that bind to VAMP77–90 affects exocytosis in intact cells (Figure 3). Cultured bovine chromaffin cells were microinjected with vehicle alone or with VAMP77–90 peptides. The effects on exocytosis were determined using carbon fibre amperometry to monitor catecholamine release evoked by a puff of 100 mM KCl. When cells were injected with vehicle alone, typical amperometric traces displayed multiple spikes within the 15 s that followed stimulation (Figure 3A). Injection of wild-type VAMP77–90 (in fact containing the F77W substitution that conserves the consensus sequence and binding properties) resulted in a strong reduction in the number of spikes (Figure 3A) at an estimated cytosolic concentration of 50 µM. In contrast, traces were comparable to controls when peptides carried the additional W89A,W90A mutations. Accumulated results from several cells (Figure 3B, representative of three independent experiments) demonstrated that VAMP77–90 peptide reduced the number of spikes by ∼80%, while the mutant peptide produced an inhibition of ∼15%. This is unlikely to be due to a direct effect of the peptide on SNARE complex assembly. Furthermore, 100 µM VAMP77–90 peptide had no effect on complex assembly assayed in vitro using recombinant SNARE proteins (data not shown). In contrast, the results are consistent with the hypothesis that the peptide inhibits exocytosis by competing with endogenous VAMP for binding to another partner such as calmodulin. However, it is also conceivable that the VAMP77–90 peptide acts as a general calmodulin sink, depleting cellular levels and preventing calmodulin from mediating an essential process unrelated to interactions with VAMP. For this reason, we developed a second complementary approach. In order to determine whether calmodulin binding to VAMP77–90 was required for exocytosis, the effects of a calmodulin antagonist and the VAMP mutations described above were explored using a hGH release assay in transfected PC12 cells.
Fig. 3. Effect of VAMP77–90 peptides on catecholamine release from chromaffin cells. (A) Cultured bovine chromaffin cells were injected with H2O (Control), VAMP77–90 (wild-type) or mutated VAMP77–90 (W89A,W90A) peptides. Evoked catecholamine release was measured by carbon fibre amperometry following local application of 100 mM KCl. (B) Individual spikes in the 15 s following KCl stimulation were counted in 26 cells injected with the indicated peptides. The illustrated experiment is representative of three totally independent experiments.
Properties and expression pattern of VAMP mutants
Expression vectors were constructed encoding the sequences of hGH and VAMP in a single plasmid to direct the co-expression of both proteins in the same cells. VAMP 2 sequences were wild-type or contained the mutations described above that confer TeNT resistance and deficient calmodulin binding. An extended C-terminal spacer sequence was included ending in a myc tag. In order to confirm that the Q76V,F77W mutation effectively reduced VAMP cleavage by TeNT, mutant proteins were generated by coupled transcription and translation in vitro in the presence of [35S]methionine. They were then incubated in the presence of recombinant TeNT light chain for 2 h and processed for analysis by SDS–PAGE and autoradiography. Figure 4A demonstrates that full-length wild-type VAMP was not detected after TeNT treatment. In contrast, the Q76V,F77W mutation conferred resistance to proteolysis in the VAMP mutants translated from the other three constructs.
Although the mutated residues in VAMP77–90 do not appear to contribute to direct interactions with syntaxin and SNAP-25, we examined whether these mutations compromise the thermostability of SNARE complexes in the presence of SDS (Figure 4B). GST–VAMP1–96 (wild-type or mutant) was immobilized on glutathione– agarose beads and incubated with syntaxin1–261 and [35S]SNAP-25. Following assembly and washing to remove free [35S]SNAP-25, complexes were incubated in 3% SDS at a range of temperatures and then analysed by SDS–PAGE and autoradiography. High molecular weight (∼120 kDa) SNARE complexes were generated with wild-type and mutant VAMP, and dissociated in a similar temperature range (50–60°C). These data indicate that the mutations do not significantly compromise interactions between SNARE proteins.
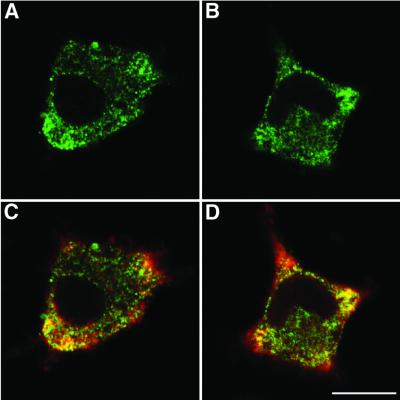
Experiments were then performed to determine whether mutations in VAMP77–90 affect the subcellular trafficking of VAMP (Figure 5). Transfected PC12 cell cultures were permeabilized and the distribution of VAMP was analysed by immunostaining with a polyclonal antibody directed against the myc epitope located at the C-terminus of the VAMP constructs (green). Typical confocal fluorescence images obtained after transfection with wild-type (Figure 5A) and TeNT-resistant VAMP with the mutation W89A,W90A (Figure 5B) illustrate a similar distribution. Double labelling with a monoclonal anti-synaptotagmin I antibody (red, Figure 5C and D) indicated co-localization (yellow) of VAMP with the endogenous secretory granule protein, notably at the base of cell processes. All four VAMP constructs used in the hGH release assays displayed similar expression patterns (data not shown). Thus, mutations in the TeNT cleavage site and in VAMP77–90 did not prevent appropriate targeting of heterologously expressed VAMP 2 to secretory granules. Furthermore, similar protocols established that hGH was only detected in cells expressing VAMP-myc (data not shown).
Fig. 5. Subcellular distribution of VAMP-myc expressed in PC12 cells. The distribution of transfected VAMP-myc was examined in PC12 cells by confocal immunofluorescence microscopy. (A) Wild-type VAMP and (B) W89A,W90A mutants stained with a polyclonal anti-myc antibody (green). (C and D) Staining of the cells illustrated in (A) and (B), respectively, with a monoclonal anti-synaptotagmin antibody (red). Co-localization is shown in yellow. Scale bar = 10 µm.
Effects of a calmodulin antagonist and mutations in VAMP77–90 on calcium-dependent exocytosis
PC12 cells were transfected with the hGH construct then permeabilized with digitonin to allow the introduction of Ca2+ and TeNT light chain into the cytosol. The addition of 10 µM Ca2+ immediately after permeabilization elicited robust hGH release (Figure 6A). When permeabilized cells were incubated for an additional 10 min in the presence of ATP, Ca2+-dependent hGH release was slightly reduced, presumably due to leaking of cytoplasmic factors, while basal release was stable. The addition of TeNT light chain during the 10 min pre-incubation period strongly inhibited Ca2+-dependent secretion. Thus, permeabilization allowed the introduction of exogenous ions and proteins into cells, and TeNT treatment abolished Ca2+-dependent release, demonstrating that it is a vesicular process requiring intact VAMP.
Previous reports have indicated that the addition of exogenous calmodulin stimulates release, suggesting that it is required for exocytosis (Chamberlain et al., 1995; Chen et al., 1999a). To address this issue, we treated intact PC12 cells with the irreversible membrane-permeable calmodulin antagonist ophiobolin A, and then analysed release in permeabilized cells. Ophiobolin A completely inhibited (KI = ∼10 µM) calcium-dependent hGH release (Figure 6B), without affecting basal release or total hGH content. Incubation of permeabilized cells with the calmodulin-binding peptides calmodulin-dependent kinase II290–309 and VAMP77–90, or ophiobolin A (all at 50 µM) for 10 min before calcium triggering produced a more moderate 25–35% inhibition, possibly due to incomplete equilibration with the antagonist (data not shown). Thus, calmodulin is required for exocytosis in PC12 cells, but multiple potential targets exist.
One of the possible sites of calmodulin action is the consensus binding domain of VAMP 2. In order to evaluate more directly the role of VAMP77–90, we analysed the effects of transfecting VAMP mutants. Permeabilized cells were treated in the presence or absence of TeNT light chain, and exocytosis was quantified for each transfected construct as the percentage of the Ca2+-dependent component of hGH release that remained after TeNT treatment (Figure 6C). When cells were transfected with hGH alone or a plasmid encoding hGH and wild-type VAMP, Ca2+-dependent secretion was profoundly decreased (10–15% of control) by TeNT. The expression of TeNT-resistant VAMP (Q76V,F77W) clearly restored secretion (80% of control) in the presence of TeNT. However, when TeNT-resistant VAMP carried additional mutations of basic or aromatic residues in VAMP77–90, the construct no longer rescued secretion (20% release with K83A,K87V; 25% release with W89A,W90A). These data indicate that calmodulin and/or phospholipid binding to VAMP77–90 is required for calcium-dependent exocytosis.
Discussion
A general role for Ca2+/calmodulin in SNARE-mediated membrane fusion?
In yeast, evidence suggests that calmodulin functions as a calcium sensor during the final steps that culminate in vacuolar fusion. Yeast homotypic vacuole fusion involves membrane attachment, trans SNARE pairing and calcium efflux from the vacuole lumen, which activates calmodulin. Ca2+/calmodulin binds to components of the vacuole membrane, including a protein phosphatase and the hydrophobic c subunit of Vo, the membrane sector of the V-ATPase (Peters and Mayer, 1998; Peters et al., 2001). It has been proposed that hexamers of the c subunit form hemi-channels that, guided via an association with the SNARE Vam3, meet at the membrane contact point forming a gap junction-like trans channel, linking the vacuole lumens (Peters et al., 2001). Thus Ca2+/calmodulin may be involved in the assembly or activation of a fusion pore. The fact that calcium efflux from vesicles and calmodulin are required for SNARE-mediated intra-Golgi transport, endosome fusion and endosome– lysosome fusion (Colombo et al., 1997; Porat and Elazar, 2000; Pryor et al., 2000) has prompted the suggestion that this may be a general theme in intracellular membrane transport (Mayer, 2001).
Ca2+/calmodulin and neuroexocytosis
Is the scheme of events outlined above relevant to neurotransmitter release? Calmodulin can potentially regulate neurotransmitter release by interacting with a variety of target proteins in the steps upstream from exocytosis, including voltage-dependent calcium channels, calmodulin-dependent kinase II and rab3A (Coppola et al., 1999; Lee et al., 2000). A role for the c subunit of the V-ATPase (mediatophore) as a fusion pore component has also been proposed in Torpedo cholinergic synaptosomes (Morel et al., 2001). However, evidence that calmodulin controls fusion pore opening in mammalian nerve terminals has not been forthcoming. The presynaptic application of antagonists at the calyx of Held synapse has shown that calmodulin is involved in the refilling of a rapidly releasable vesicle pool, but not in the calcium-triggering step itself (Sakaba and Neher, 2001). Thus, there appears to be significant divergence between the roles of calmodulin in yeast vacuole fusion versus synaptic vesicle fusion.
Calcium-dependent exocytosis of secretory granules in chromaffin and PC12 cells might constitute an intermediate case, although the basic molecular machinery which supports membrane fusion in neuroendocrine cells and nerve terminals is widely held to be equivalent. We have shown that when intact PC12 cells were treated with the irreversible calmodulin antagonist ophiobolin A just before permeabilization, calcium-dependent hGH release was totally blocked. The IC50 of 10 µM was consistent with its reported potency as an inhibitor of cyclic nucleotide phosphodiesterase (Leung et al., 1984) and of yeast vacuole fusion (Peters and Mayer, 1998). These experiments are consistent with the conclusion that calmodulin plays a role, but do not define the target at a molecular level.
A requirement for VAMP77–90 in Ca2+-dependent exocytosis from neuroendocrine cells
We have addressed specifically the hypothesis that the C-terminal domain of VAMP 2 constitutes a functional calmodulin-binding site. The C-terminal domains of SNARE proteins in proximity to the membrane anchors appear to be involved in coupling proteins that confer calcium regulation. Synaptotagmin interacts with syntaxin 1 close to its transmembrane region (Davis et al., 1999), and the C-terminus of SNAP-25 is also essential for synaptotagmin binding to the SNARE complex (Gerona et al., 2000). BoNT/A inhibits transmitter release by cleaving a C-terminal fragment of SNAP-25. However, increased calcium concentrations can reverse inhibition by this toxin, consistent with the notion that cleavage at this site perturbs coupling to a calcium sensor (Capogna et al., 1997; Xu et al., 1998).
Our data indicate that VAMP77–90, a juxtamembrane motif in strikingly close proximity to the domains described above, is involved in calcium-dependent regulation of exocytosis. VAMP77–90 binds Ca2+/calmodulin or negatively charged phospholipids in a mutually exclusive fashion. The microinjection of VAMP77–90 into chromaffin cells inhibited calcium-dependent catecholamine release, an effect that was suppressed by the mutation W89A,W90A. SNARE complex formation in vitro was not affected by 100 µM VAMP77–90. Thus, the inhibitory effects of the peptide in intact chromaffin cells are unlikely to be mediated by interactions with syntaxin or SNAP-25 but by acute depletion of other endogenous molecules that bind to VAMP77–90. A potential pitfall of mimetic peptide methodology in general is that while it confirms a requirement for the depleted partner molecule, it does not demonstrate unequivocally the importance of the target binding sequence. For example, the VAMP77–90 peptide conceivably could act as a general calmodulin antagonist, preventing the action of calmodulin on a target other than VAMP. To eliminate this possibility, a complementary strategy was developed involving mutagenesis of VAMP and an hGH release assay in PC12 cells.
Data from experiments in which mutated proteins are simply overexpressed can be difficult to interpret as a functional contribution of endogenous native proteins may persist. For this reason, we used TeNT to inactivate native VAMP and monitored the rescue of secretion by transfection with TeNT-resistant VAMP (Regazzi et al., 1996). This approach allowed the analysis of mutations in VAMP77–90 in conditions in which exocytosis was entirely dependent on transfected VAMP, and confirmed a crucial role for VAMP77–90. The structural determinants that support calmodulin and phospholipid binding are similar. Interactions with these two partners were inhibited to a comparable extent by mutation of basic or aromatic residues, consistent with the observation that many peptides bind calmodulin and lipids via similar basic amphipathic motifs. Thus mutagenesis did not allow us to determine unequivocally whether inhibition of exocytosis results primarily from a reduction in calmodulin rather than in phospholipid binding. However, we cannot formally eliminate the possibility that mutations also perturb the binding of an additional and as yet unidentified regulatory protein to VAMP77–90.
Calmodulin is a positive effector of homotypic vesicle fusion in yeast (Peters and Mayer, 1998), endosomal fusion in mammalian cells (Colombo et al., 1997) and calcium-dependent exocytosis in neuroendocrine cells (Chamberlain et al., 1995; Chen et al., 1999a), and the consensus binding motif we have identified is conserved in the VAMP homologues that mediate these processes. These observations predict that mutations that perturb calmodulin binding are likely to be inhibitory. In contrast, recent studies using reconstitution into liposomes suggested that cis interactions of VAMP with phospholipids prevent trans SNARE complex assembly (Hu et al., 2002). This inhibitory action is presumably mediated via VAMP77–90, which represents the only lipid-binding domain within the cytosolic region (Quetglas et al., 2000). SNARE complex formation in PC12 cells is calcium dependent (Chen et al., 1999b). The fact that calmodulin effectively displaces phospholipid binding to this domain suggests that it could promote trans SNARE complex assembly in a calcium-dependent manner, although synaptotagmin may also be required (Hu et al., 2002). However, our data indicate that mutations which diminish both phospholipid and calmodulin binding still inhibit exocytosis. Thus, VAMP77–90 is probably required for an additional downstream function yet to be defined.
An interesting possibility is that SNARE complex assembly leads to trans interactions of VAMP77–90 with negatively charged lipid domains in the plasma membrane. We may speculate that a Ca2+ transient in the 0.1–1 µM range, insufficient in itself to trigger release, could activate calmodulin, removing cis inhibition by phospholipid binding to VAMP. Subsequent initiation of SNARE complex assembly would pull VAMP77–90 towards the plasma membrane. As Ca2+i falls, calmodulin would dissociate, allowing trans interactions with phospholipids. Calmodulin could thus act as a calcium-dependent shuttle that conveys VAMP77–90 between two alternative sites of phospholipid binding. Trans interactions of VAMP with phospholipids would bring together the inner leaflet of the plasma membrane (bound to VAMP77–90) and the outer leaflet of the vesicle membrane (vesicle membrane anchor at VAMP98–113), and could contribute to stabilizing intermediate conformations of the SNARE complex or inducing hemi-fusion. The proposed mode of action suggests a role for calmodulin in preparing rather than triggering fusion. Several lines of argument support this hypothesis. Ca2+ in the 0.1–1 µM concentration range enhances ATP-dependent priming of secretion in chromaffin cells (Bittner and Holz, 1992). Calmodulin antagonists suppress the refilling of a readily releasable vesicle pool in nerve terminals (Sakaba and Neher, 2001). In contrast, 100 µM Sr2+ does not significantly activate calmodulin binding to GST–VAMP 2 (our unpublished results) but nevertheless triggers robust release in permeabilized PC12 cells (Sugita et al., 2002). Thus, C2 domain proteins such as the synaptotagmins that bind Sr2+ are likely to be involved in the triggering step.
There is a certain degree of functional and topological convergence between synaptotagmins and calmodulin. Both are involved in Ca2+-dependent regulation of phospholipid binding at the base of the SNARE complex. Certain clostridial toxins appear to target these two processes specifically. BoNT/A cleaves a C-terminal fragment of SNAP-25 implicated in calcium sensing and interactions with synaptotagmin. TeNT and BoNT/B clip VAMP at the peptide bond Q76–F77, precisely separating the C-terminal calmodulin/phospholipid-binding domain (VAMP77–90) from the N-terminal superhelical region. Our present data in neuroendocrine cells indicate a fundamental role for a calmodulin- and phospholipid-binding domain of VAMP in preparing exocytosis. Conservation of the consensus sequence in VAMP homologues throughout evolution (Quetglas et al., 2000) is consistent with the hypothesis that calmodulin regulates both yeast vacuole and synaptic vesicle fusion via the same binding domain. We may speculate that calmodulin functions as a conserved archetypal calcium sensor playing a ubiquitous role in intracellular membrane transport. It is possible that the machinery for regulated exocytosis in neurones and neuroendocrine cells has been refined in the metazoans by the convergent evolution of the synaptotagmins, relegating calmodulin to a permissive regulatory role.
Materials and methods
Materials
Calmodulin and biotinylated calmodulin were purchased from Calbiochem. The plasmid encoding His6-TeNT light chain was kindly donated by the late Dr H.Niemann (University of Hanover, Germany). GST–VAMP and syntaxin 1A fusion proteins and synthetic VAMP peptides were prepared as described previously (Quetglas et al., 2000).
Surface plasmon resonance (SPR) spectroscopy
SPR was performed at 25°C on a BIAcore X or 3000 biosensor system (Pharmacia Biosensor AB, Uppsala, Sweden). Approximately 25 fmol of GST–VAMP1–96 and GST were immobilized on sensor chips in experimental and control cells, respectively, via anti-GST antibodies as previously described (Quetglas et al., 2000). Ca2+ buffers were prepared in 2 mM EGTA, 140 mM NaCl, 10 mM MgCl2, 10 mM HEPES adjusted to pH 7.4 with NaOH, and free Ca2+ concentrations were calculated (Mouton et al., 2001). A 40 µl aliquot of running buffer containing 2.5 µM calmodulin was injected at 5 µl/min. After measuring calmodulin binding, the chip was regenerated with 1 mM EDTA.
The ability of synthetic VAMP77–90 and mutant peptides to compete with GST–VAMP for binding to calmodulin was assayed as follows. Biotinylated calmodulin was immobilized on a streptavidin sensor chip which was then saturated with biotin (Quetglas et al., 2000). The running buffer contained Tris-buffered saline (TBS; 25 mM Tris, 150 mM NaCl pH 7.4), 0.05% Tween-20 and 1 mM CaCl2. GST–VAMP (200 nM) was injected at 20 µl/min for 2 min in the presence of the indicated peptide concentrations, and the resonance signal was measured 10 s after terminating injection. Regeneration was achieved by injecting 5 mM EDTA, 0.4% CHAPS in TBS after each measurement. Experiments with large unilammellar liposomes are described in the Supplementary data available at The EMBO Journal Online.
Binding assays with [3H]liposomes
Synthetic PS and PC (Sigma, St Louis, MO) and [3H]PC (Amersham) were dissolved in 66% chloroform, 33% methanol. Lipid solutions were mixed to obtain 99.5% PC, 0.5% [3H]PC, or 25% PS, 74.5% PC, 0.5% [3H]PC, then evaporated under N2. Lipid films were heated to 60°C, then 50 mM HEPES, 100 mM NaCl, adjusted to pH 7.4 with NaOH, was added to give a lipid concentration of 900 µM. Samples were agitated for 5 min, sonicated for 30 s and then extruded through 100 nm filters. Binding assays were carried out with 0.4 µM GST–VAMP1–96 or GST immobilized on glutathione–agarose beads and 30 µM [3H]liposome in 50 mM HEPES, 100 mM NaCl, 1 mM CaCl2, 0.04 mg/ml bovine serum albumin (BSA), adjusted to pH 7.4 with NaOH for 3 h at 4°C. Beads were recovered by centrifugation and washed with TBS. Bound [3H]liposome was quantified by liquid scintillation counting.
Microinjection and amperometric recording in single chromaffin cells
Bovine chromaffin cells were cultured on a collagen-coated 35 mm glass-based culture dish (IWAKI, Chiba, Japan). Microinjection experiments were conducted on 5- to 7-day-old cultures. In each experiment, 50–150 cells were selected and peptides or vehicle were microinjected into the cytosol using a Transjector 5246 (Eppendorf, Hamburg, Germany) injection system and glass micropipettes (Femtotips, Eppendorf) with an outlet diameter of 0.5 ± 0.2 µm. The injection time was 0.2 s at a pressure of 100 hPa; thus, the calculated volume injected was 50–100 fl (Graessmann and Graessmann, 1983). Peptides were dissolved in distilled water at 5 mg/ml, and the final cytosolic concentration was estimated to be 60–120 µg/ml. Amperometric measurement of catecholamine release was performed 30 min after microinjection.
Culture dishes were washed with Locke’s solution and placed on the stage of an inverted microscope with a heated stage maintained at 25°C. A carbon fibre electrode (ProCFE; 5 µm diameter, Dagan Corporation, NM) was positioned in tangential contact with a single chromaffin cell using a three-dimensional micromanipulator (model 5171, Eppendorf). The reference electrode was a silver wire coated with AgCl immersed in the medium bathing the cells. A two-electrode potentiostat (CHEM-CLAMP; Dagan Corporation) was used to apply +0.65 V to the carbon fibre electrode versus the reference electrode and to record the current passing through them. The amplified signal was digitized at 4 kHz by a Mac Lab/4s system (AD instruments, Castle Hill, Australia) coupled to a Macintosh computer. Catecholamine release was evoked by applying 100 mM KCl in Locke’s solution for 4 s to single cells by pressure (1000 hPa) ejection through a glass micropipette (Femtotips, Eppendorf) positioned at a distance of ∼50 µm from the cell. Exocytotic events were quantified as the number of spikes recorded in the 15 s following KCl application.
VAMP constructs
A 13 residue spacer (KGVEPKTYCYYSS) was added by PCR to the 3′ extremity of the full-length VAMP 2 coding sequence, which was then inserted into pcDNa3MycHis (Invitrogen). Mutagenesis was performed with the Quik Change™ Site-Directed Mutagenesis kit (Stratagene). cDNAs encoding VAMPspacerMyc (with and without mutations) were then incorporated into a vector with two promoters (psI with the pcDNa3 expression unit): the cDNA encoding hGH under the control of SV40 and that of VAMPspacerMyc driven by CMV (i.e. psI-hGH-pcDNa3-VAMPMyc.) The GST–VAMP1–96 K83A,K87V and W89A,W90A constructs were made by directed mutagenesis of GST–VAMP1–96 in pGex-4T.
In vitro cleavage by tetanus toxin and SNARE complex thermostability
Wild-type and mutant VAMP sequences were inserted in the pcDNa3 MycHis vector and translated in vitro in the presence of [35S]methionine (NEN) using the TNT T7 Quick Coupled Transcription/Translation System (Promega). 35S-labelled proteins were incubated for 2 h at 37°C with 100 nM TeNT light chain in 4 mM HEPES, 100 mM NaCl, 3.5 mM CaCl2, 3.5 mM MgCl2, adjusted to pH 7.4 with NaOH. Samples were analysed by SDS–PAGE and autoradiography.
[35S]SNAP-25 was produced by in vitro translation in the presence of [35S]methionine. GST was removed from GST–syntaxin1A1–261 by thrombin cleavage and monitored by SDS–PAGE and protein staining. GST–VAMP1–96 (1.6 µM) with and without mutations was immobilized on glutathione–agarose beads and incubated in TBS, 0.1% BSA, 0.1% Triton X-100 with 2 µM syntaxin 1A and [35S]SNAP-25. After 4 h at 4°C, samples were washed by centrifugation, adjusted to 3% SDS, incubated for 2 min at different temperatures and analysed by SDS–PAGE and autoradiography.
Immunofluorescence microscopy
PC12 cells were fixed in 4% paraformaldehyde for 30 min, blocked with 50 mM NH4Cl for 15 min and permeabilized with 0.066% saponin, 0.2% gelatin in 0.1 M phosphate buffer for 15 min at room temperature. Primary antibodies (4 µg/ml rabbit anti-myc, Upstate Biotechnology; 4 µg/ml monoclonal anti-synaptotagmin mAb1D12) were applied for 1 h, and secondary antibodies (3 µg/ml goat anti-rabbit Alexa 488, 2.5 µg/ml goat anti-mouse Alexa 594, Molecular Probes) for 45 min at room temperature. After washing in 0.1 M phosphate buffer, samples were mounted in Mowiol 4-88, and observed using sequential confocal image acquisition (0.48 µM set step size, Leica TCS 4D Leitz DMIRB).
PC12 cell transfection and hGH release assays
PC12 cells were cultured in Dulbecco’s modified Eagle’s medium with 5% horse serum and 5% fetal calf serum (Gibco) at 37°C and 10% CO2. Cells were seeded on a poly-l-lysine-coated support (molecular weight 70 000–150 000, Sigma). One day after seeding, cells were transfected with Lipofectamine 2000 (Invitrogen). For immunofluorescence experiments, 2 µg of DNA (psI-hGH-pcDNa3VAMPMyc with the indicated mutations) were used per 18 mm coverslip. For pharmacological assays of hGH release, transfection was performed with 0.25–0.8 µg of DNA (psI-hGH-pcDNa3), and for studies with TeNT-resistant VAMP mutants with 0.1 µg of DNA (psI-hGH-pcDNa3 or psI-hGH-pcDNA3-VAMPMyc encoding the indicated mutations) in 4-well dishes.
Experiments were carried out at 37°C. Cells were washed five times with 156 mM NaCl, 5.6 mM KCl, 5.6 mM glucose, 0.2 mM EGTA and 5 mM HEPES pH 6.8, then incubated for 5 min with 8 µM digitonin (Calbiochem) in 140 mM K-glutamate, 5 mM glucose, 5 mM EGTA and 20 mM PIPES at pH 6.8 (KGEP). Cells were then incubated for 10 min in KGEP containing 5 mM MgATP in the presence or absence of 300 nM TeNT light chain. Release was triggered for 4 min in KGEP containing 5 mM MgATP, 4.76 mM CaCl2 (10 µM free Ca2+), and basal release was assayed in the absence of CaCl2. The buffer was recovered and centrifuged for 10 min at 4°C for assay of released hGH. Triton X-100 (1%) in TBS was added to the wells for 10 min at 4°C and then centrifuged for assay of the remaining cellular hGH content. Triton X-100 was added to the released sample to the same final concentration. Assays were performed with an hGH IRMA kit (Beckman Coulter) in a linear response range. In some experiments, intact cells were treated with ophiobolin A (Calbiochem) in a serum-free culture medium for 30 min at 37°C.
Supplementary data
Supplementary data are available at The EMBO Journal Online.
Acknowledgments
Acknowledgements
We thank Hiroshi Ohnishi for assistance with setting up release assays, Makoto Itakura for the expression vector, and Masami Takahashi for many helpful discussions. We are grateful to Raymond Miquelis for his contribution to SPR assays. Additional financial support was provided by the Centre National de la Recherche Scientifique, the Association Française contre les Myopathies and the Japanese Society for the Promotion of Science (Accord INSERM/JSPS).
References
- Artalejo C.R., Elhamdani,A. and Palfrey,H.C. (1996) Calmodulin is the divalent cation receptor for rapid endocytosis, but not exocytosis, in adrenal chromaffin cells. Neuron, 16, 195–205. [DOI] [PubMed] [Google Scholar]
- Bittner M.A. and Holz,R.W. (1992) A temperature-sensitive step in exocytosis. J. Biol. Chem., 267, 16226–16229. [PubMed] [Google Scholar]
- Capogna M., McKinney,R.A., O’Connor,V., Gahwiler,B.H. and Thompson,S.M. (1997) Ca2+ or Sr2+ partially rescues synaptic transmission in hippocampal cultures treated with botulinum toxin A and C, but not tetanus toxin. J. Neurosci., 17, 7190–7202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain L.H., Roth,D., Morgan,A. and Burgoyne,R.D. (1995) Distinct effects of α-SNAP, 14-3-3 proteins and calmodulin on priming and triggering of regulated exocytosis. J. Cell Biol., 130, 1063–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y.A., Duvvuri,V., Schulman,H. and Scheller,R.H. (1999a) Calmodulin and protein kinase C increase Ca2+-stimulated secretion by modulating membrane-attached exocytic machinery. J. Biol. Chem., 274, 26469–26476. [DOI] [PubMed] [Google Scholar]
- Chen Y.A., Scales,S.J., Patel,S.M., Doung,Y.C. and Scheller,R.H. (1999b) SNARE complex formation is triggered by Ca2+ and drives membrane fusion. Cell, 97, 165–174. [DOI] [PubMed] [Google Scholar]
- Colombo M.I., Beron,W. and Stahl,P.D. (1997) Calmodulin regulates endosome fusion. J. Biol. Chem., 272, 7707–7712. [DOI] [PubMed] [Google Scholar]
- Coppola T., Perret-Menoud,V., Luthi,S., Farnsworth,C.C., Glomset,J.A. and Regazzi,R. (1999) Disruption of Rab3–calmodulin interaction, but not other effector interactions, prevents Rab3 inhibition of exocytosis. EMBO J., 18, 5885–5891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis A.F., Bai,J., Fasshauer,D., Wolowick,M.J., Lewis,J.L. and Chapman,E.R. (1999) Kinetics of synaptotagmin responses to Ca2+ and assembly with the core SNARE complex onto membranes. Neuron, 24, 363–376. [DOI] [PubMed] [Google Scholar]
- Fergestad T., Wu,M.N., Schulze,K.L., Lloyd,T.E., Bellen,H.J. and Broadie,K. (2001) Targeted mutations in the syntaxin H3 domain specifically disrupt SNARE complex function in synaptic transmission. J. Neurosci., 21, 9142–9150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Chacon R. et al. (2001) Synaptotagmin I functions as a calcium regulator of release probability. Nature, 410, 41–49. [DOI] [PubMed] [Google Scholar]
- Gerona R.R., Larsen,E.C., Kowalchyk,J.A. and Martin,T.F. (2000) The C terminus of SNAP25 is essential for Ca2+-dependent binding of synaptotagmin to SNARE complexes. J. Biol. Chem., 275, 6328–6336. [DOI] [PubMed] [Google Scholar]
- Graessmann M. and Graessmann,A. (1983) Microinjection of tissue culture cells. Methods Enzymol., 101, 482–492. [DOI] [PubMed] [Google Scholar]
- Hu K., Carroll,J., Fedorovich,S., Rickman,C., Sukhodub,A. and Davletov,B. (2002) Vesicular restriction of synaptobrevin suggests a role for calcium in membrane fusion. Nature, 415, 646–650. [DOI] [PubMed] [Google Scholar]
- Hua S.Y. and Charlton,M.P. (1999) Activity-dependent changes in partial VAMP complexes during neurotransmitter release. Nat. Neurosci., 2, 1078–1083. [DOI] [PubMed] [Google Scholar]
- Jahn R. and Sudhof,T.C. (1999) Membrane fusion and exocytosis. Annu. Rev. Biochem., 68, 863–911. [DOI] [PubMed] [Google Scholar]
- Kerboeuf D., Le Berre,A., Dedieu,J.C. and Cohen,J. (1993) Calmodulin is essential for assembling links necessary for exocytotic membrane fusion in Paramecium. EMBO J., 12, 3385–3390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kibble A.V. and Burgoyne,R.D. (1996) Calmodulin increases the initial rate of exocytosis in adrenal chromaffin cells. Pflugers Arch., 431, 464–466. [DOI] [PubMed] [Google Scholar]
- Lee A., Scheuer,T. and Catterall,W.A. (2000) Ca2+/calmodulin-dependent facilitation and inactivation of P/Q-type Ca2+ channels. J. Neurosci., 20, 6830–6838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung P.C., Taylor,W.A., Wang,J.H. and Tipton,C.L. (1984) Ophiobolin A. A natural product inhibitor of calmodulin. J. Biol. Chem., 259, 2742–2747. [PubMed] [Google Scholar]
- Lin R.C. and Scheller,R.H. (2000) Mechanisms of synaptic vesicle exocytosis. Annu. Rev. Cell Dev. Biol., 16, 19–49. [DOI] [PubMed] [Google Scholar]
- Littleton J.T., Bai,J., Vyas,B., Desai,R., Baltus,A.E., Garment,M.B., Carlson,S.D., Ganetzky,B. and Chapman,E.R. (2001a) Synaptotagmin mutants reveal essential functions for the C2B domain in Ca2+-triggered fusion and recycling of synaptic vesicles in vivo. J. Neurosci., 21, 1421–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littleton J.T., Barnard,R.J., Titus,S.A., Slind,J., Chapman,E.R. and Ganetzky,B. (2001b) SNARE-complex disassembly by NSF follows synaptic-vesicle fusion. Proc. Natl Acad. Sci. USA, 98, 12233–12238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer A. (2001) What drives membrane fusion in eukaryotes? Trends Biochem. Sci., 26, 717–723. [DOI] [PubMed] [Google Scholar]
- McNew J.A., Weber,T., Parlati,F., Johnston,R.J., Melia,T.J., Sollner,T.H. and Rothman,J.E. (2000) Close is not enough: SNARE-dependent membrane fusion requires an active mechanism that transduces force to membrane anchors. J. Cell Biol., 150, 105–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morel N., Dunant,Y. and Israel,M. (2001) Neurotransmitter release through the V0 sector of V-ATPase. J. Neurochem., 79, 485–488. [DOI] [PubMed] [Google Scholar]
- Mouton J., Feltz,A. and Maulet,Y. (2001) Interactions of calmodulin with two peptides derived from the C-terminal cytoplasmic domain of the Ca(v)1.2 Ca2+ channel provide evidence for a molecular switch involved in Ca2+-induced inactivation. J. Biol. Chem., 276, 22359–22367. [DOI] [PubMed] [Google Scholar]
- Peters C. and Mayer,A. (1998) Ca2+/calmodulin signals the completion of docking and triggers a late step of vacuole fusion. Nature, 396, 575–580. [DOI] [PubMed] [Google Scholar]
- Peters C., Bayer,M.J., Buhler,S., Andersen,J.S., Mann,M. and Mayer,A. (2001) Trans-complex formation by proteolipid channels in the terminal phase of membrane fusion. Nature, 409, 581–588. [DOI] [PubMed] [Google Scholar]
- Porat A. and Elazar,Z. (2000) Regulation of intra-Golgi membrane transport by calcium. J. Biol. Chem., 275, 29233–29237. [DOI] [PubMed] [Google Scholar]
- Pryor P.R., Mullock,B.M., Bright,N.A., Gray,S.R. and Luzio,J.P. (2000) The role of intraorganellar Ca2+ in late endosome–lysosome heterotypic fusion and in the reformation of lysosomes from hybrid organelles. J. Cell Biol., 149, 1053–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quetglas S., Leveque,C., Miquelis,R., Sato,K. and Seagar,M. (2000) Ca2+-dependent regulation of synaptic SNARE complex assembly via a calmodulin- and phospholipid-binding domain of synaptobrevin. Proc. Natl Acad. Sci. USA, 97, 9695–9700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regazzi R., Sadoul,K., Meda,P., Kelly,R.B., Halban,P.A. and Wollheim,C.B. (1996) Mutational analysis of VAMP domains implicated in Ca2+-induced insulin exocytosis. EMBO J., 15, 6951–6959. [PMC free article] [PubMed] [Google Scholar]
- Rhoads A.R. and Friedberg,F. (1997) Sequence motifs for calmodulin recognition. FASEB J., 11, 331–340. [DOI] [PubMed] [Google Scholar]
- Sakaba T. and Neher,E. (2001) Calmodulin mediates rapid recruitment of fast-releasing synaptic vesicles at a calyx-type synapse. Neuron, 32, 1119–1131. [DOI] [PubMed] [Google Scholar]
- Schoch S., Deak,F., Konigstorfer,A., Mozhayeva,M., Sara,Y., Sudhof,T.C. and Kavalali,E.T. (2001) SNARE function analyzed in synaptobrevin/VAMP knockout mice. Science, 294, 1117–1122. [DOI] [PubMed] [Google Scholar]
- Sudhof T.C. (2002) Synaptotagmins: why so many? J. Biol. Chem., 277, 7629–7632. [DOI] [PubMed] [Google Scholar]
- Sugita S., Shin,O.H., Han,W., Lao,Y. and Sudhof,T.C. (2002) Synaptotagmins form a hierarchy of exocytotic Ca2+ sensors with distinct Ca2+ affinities. EMBO J., 21, 270–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton R.B., Fasshauer,D., Jahn,R. and Brunger,A.T. (1998) Crystal structure of a SNARE complex involved in synaptic exocytosis at 2.4 Å resolution. Nature, 395, 347–353. [DOI] [PubMed] [Google Scholar]
- Voets T., Moser,T., Lund,P.E., Chow,R.H., Geppert,M., Sudhof,T.C. and Neher,E. (2001) Intracellular calcium dependence of large dense-core vesicle exocytosis in the absence of synaptotagmin I. Proc. Natl Acad. Sci. USA, 98, 11680–11685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washbourne P. et al. (2002) Genetic ablation of the t-SNARE SNAP-25 distinguishes mechanisms of neuroexocytosis. Nat. Neurosci., 5, 19–26. [DOI] [PubMed] [Google Scholar]
- Weber T., Zemelman,B.V., McNew,J.A., Westermann,B., Gmachl,M., Parlati,F., Sollner,T.H. and Rothman,J.E. (1998) SNAREpins: minimal machinery for membrane fusion. Cell, 92, 759–772. [DOI] [PubMed] [Google Scholar]
- Xu T., Binz,T., Niemann,H. and Neher,E. (1998) Multiple kinetic components of exocytosis distinguished by neurotoxin sensitivity. Nat. Neurosci., 1, 192–200. [DOI] [PubMed] [Google Scholar]