Abstract
Although several studies have suggested that insulin-secreting cells can be generated in vitro from cells residing in adult exocrine pancreas, neither the origin of these cells nor their precise insulin secretory properties was obtained. We show here that insulin-secreting cells can be derived from adult mouse pancreatic exocrine cells by suspension culture in the presence of EGF and nicotinamide. The frequency of insulin-positive cells was only 0.01% in the initial preparation and increased to ≈5% in the culture conditions. Analysis by the Cre/loxP-based direct cell lineage tracing system indicates that these newly made cells originate from amylase/elastase-expressing pancreatic acinar cells. Insulin secretion is stimulated by glucose, sulfonylurea, and carbachol, and potentiation by glucagon-like peptide-1 also occurs. Insulin-containing secretory granules are present in these cells. In addition, we found that the enzymatic dissociation of pancreatic acini itself leads to activation of EGF signaling, and that inhibition of EGF receptor kinase blocks the transdifferentiation. These data demonstrate that pancreatic acinar cells can transdifferentiate into insulin-secreting cells with secretory properties similar to those of native pancreatic β cells, and that activation of EGF signaling is required in such transdifferentiation.
Keywords: Cre/loxP, diabetes, EGF, transdifferentiation
Pancreatic β cells play an indispensable role in the maintenance of systemic glucose homeostasis, and β cell turnover slowly occurs throughout the lifetime of a mammal (1). Although it has been reported that replication is the major mechanism of the generation of new β cells in adult mice (2, 3), various studies have shown that β cells also are generated by neogenesis (4–10), suggesting stem/progenitor cells in the adult pancreas yet to be identified. On the other hand, many attempts have been made recently to generate new insulin-producing β cells in vitro from non-β cells (11–15). Pancreatic β cells are characterized by well regulated insulin secretion in response to various stimuli required in the maintenance of blood glucose levels within a narrow physiological range. Although glucose responsiveness (16), metabolism-electrical activity coupling (17), and regulated exocytosis of insulin granules (18) are the primary features of the β cell, they have been overlooked or underemphasized in studies of in vitro-generated insulin-producing cells (15, 19).
Recent studies have revealed unexpected high plasticity of adult cells in their differentiation capacity (20, 21). For example, differentiated cells can convert into cells of a different phenotype by transdifferentiation (22–24). Several lines of evidence suggest that pancreatic acinar and duct cells can transdifferentiate into endocrine cells in vivo (5–10). In certain experimental models and pathological conditions, pancreatic lesion leads to transformation of acinar cells into duct-like structures (acinoductal metaplasia), followed by islet neogenesis (5–10). Previous studies (10, 25) have shown that pancreatic acinar cells can transdifferentiate in vitro into cells with a ductal phenotype that express Pdx1, a transcription factor critical in pancreatic development and insulin gene expression, but those transdifferentiated cells failed to produce insulin. Very recently, studies (26, 27) have suggested that rat pancreatic acinar cells can transdifferentiate into insulin-producing cells in vitro, but direct evidence of the origin of the cells was not reported. Lineage tracing by the Cre-loxP recombination system has been used successfully to determine cell fate by following the progeny of labeled cells through the differentiation process (2, 28, 29).
In the present study, we provide direct evidence that insulin-secreting cells can be generated in vitro from pancreatic acinar cells of adult mouse, using the Cre/loxP-based cell lineage tracing system. The newly made cells are equipped with the apparatus of glucose-induced insulin secretion and its potentiation, the principal mechanisms of insulin secretion. Furthermore, EGF signaling is essential for this transdifferentiation. These data clearly show that pancreatic acinar cells possess sufficient plasticity to transdifferentiate into pancreatic endocrine cells in vitro.
Materials and Methods
Culture of Isolated Pancreatic Exocrine Cells. Isolation and purification of pancreatic exocrine cells from male C57BL/6 mice are described in Supporting Text, which is published as supporting information on the PNAS web site. Purified acinar-enriched exocrine cells were cultured in RPMI medium 1640 (Sigma) with 10% FCS. After 6–8 h of culture, floating cells were collected and replated on dishes treated with 2-methacryloyloxyethyl phosphorylcholine (Nalge Nunc) in RPMI medium 1640 supplemented with 0.5% FCS/20 ng/ml EGF (R & D Systems)/10 mM nicotinamide. The culture medium was not renewed throughout the experiment. All animal experiments were approved by the animal research committees of the Kyoto University Graduate School of Medicine and the Kobe University Graduate School of Medicine.
Adenoviral Infection. ROSA26 reporter mice, in which enhanced cyan fluorescent protein (ECFP) expression is activated upon removal of the loxP site-f lanked stop cassette by Crerecombinase (R26R-ECFP) (30), were provided by F. Costantini (Columbia University, New York). Cre-recombinase expressing adenoviruses under control of mouse amylase-2 promoter (31) and rat elastase-1 promoter (32) were generated by using an adenovirus expression vector kit (Takara Bio, Tokyo), according to the manufacturer's instructions. Pancreatic acinar cell-specific activation of these promoters has been established (31–33) and also confirmed by us (see Fig. 6, which is published as supporting information on the PNAS web site). Immediately after enzymatic dissociation, pancreatic exocrine cells were infected with the adenoviruses at a multiplicity of infection of 100 for 6 h. Thereafter, the cells were cultured for 4 days in suspension as described.
Cell Analyses. After culture, the cells were collected and subjected to analyses for immunocytochemistry, immunoblotting, RT-PCR (see Table 1, which is published as supporting information on the PNAS web site), and insulin secretion (see Supporting Text).
Results
Generation of Insulin-Producing Cells from Exocrine Pancreas. The protocol for induction of insulin-producing cells from exocrine pancreas is outlined in Fig. 7, which is published as supporting information on the PNAS web site. By Ficoll gradient centrifugation, the pancreatic acinar cell-enriched exocrine fraction was recovered as a pellet. Although the pellet contained only a small amount of native pancreatic β cells (≈0.5% of total cells recovered), we further removed native β cells by staining the pancreatic islets and their fragments with dithizone (DTZ) (34, 35), followed by handpicking under a dissecting microscope. After this procedure, the frequency of insulin-positive cells was ≈0.01%, as assessed by immunostaining and quantitative real-time RT-PCR for insulin genes. Almost no insulin response to high glucose, high KCl, or the sulfonylurea glibenclamide was detectable in the DTZ (–) fraction (data not shown).
We then cultured the acinar cell-enriched DTZ (–) fraction at a low concentration (0.5%) of FCS and 20 ng/ml EGF. Under these conditions, the cells readily formed aggregates and became smooth spheroids. The cells began to adhere within 3 days and formed small monolayer colonies between days 5 and 7. We found that a subset of the cells in these colonies expressed insulin on day 7 (Fig. 7). However, insulin secretion was undetectable under these culture conditions.
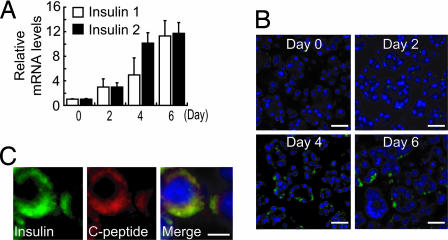
In the course of the study, we found that suspension culture using dishes treated with 2-methacryloyloxyethyl phosphorylcholine (MPC), which interferes with cell attachment (36), in combination with 10 mM nicotinamide increases the cellular insulin content (Fig. 7). Nicotinamide is known to prevent β cell damage due to activation of poly(ADP-ribose) synthethase/polymerase (37) and induce endocrine differentiation in cultured human fetal pancreatic islet cells and an increase in insulin gene expression (38). When cultured on MPC dishes, the cells did not adhere to the surface of the dish, and smooth spheroids were maintained throughout the experiments. Quantitative analysis by real-time RT-PCR showed that the expression of the insulin genes was gradually increased by the culture (Fig. 1A). The frequency of insulin-positive cells was also increased (Fig. 1B), to ≈5% of total cells on day 4. The insulin-positive cells also expressed C-peptide (Fig. 1C), indicating insulin biosynthesis in these cells. Cells with proliferating ability, assessed by cell proliferating marker Ki67-staining, were rare (<1% of total cells) in the culture. Insulin/Ki67 double-positive cells were not found (Fig. 8, which is published as supporting information on the PNAS web site).
Fig. 1.
Generation of insulin-producing cells from adult mouse exocrine pancreas. (A) Quantitative real-time RT-PCR analysis for insulin genes. Insulin expression in the culture was gradually increased. (B) Immunostaining of exocrine pancreas-derived cells. Insulin-positive cells are increased by the culture. (Scale bars, 50 μm.) (C) Double immunostaining of insulin and C-peptide. The insulin-positive cells are also positive for C-peptide. (Scale bar, 20 μm.)
Because insulin content in native mouse islets is 252 ± 8 μg/mg protein, that in the exocrine pancreas-derived cells (0.617 ± 0.083 μg/mg protein, including both insulin-positive and -negative cells) is 1/400th that of native pancreatic islets. Because the frequency of insulin-positive cells is ≈5% of total cells in the culture (Fig. 1B), the insulin content of the newly made exocrine pancreas-derived insulin-positive cells is ≈1/20th that of a native β cell.
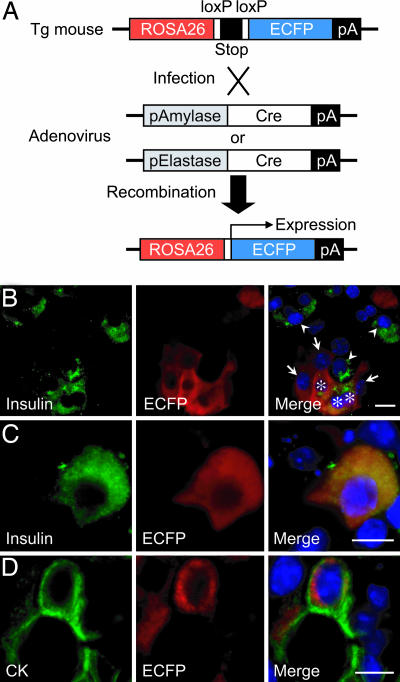
Origin of Insulin-Producing Cells. Analysis of pancreatic cell markers during culture suggests that the insulin-producing cells in the culture were derived from mature pancreatic acinar cells (see Fig. 9, which is published as supporting information on the PNAS web site). To confirm this directly, we generated adenoviruses in which either the amylase-2 (Ad-pAmy-Cre) or the elastase-1 (Ad-pEla-Cre) promoter drives the expression of Cre recombinase for lineage tracing of amylase/elastase-expressing mature pancreatic acinar cells. In cells from the R26R-ECFP reporter mouse (30), the expression of ECFP is activated through the action of Cre recombinase, removing a transcriptional “stop” sequence (Fig. 2A). When amylase/elastase-expressing cells are infected with these adenoviruses, the cells are labeled permanently with ECFP, even if amylase/elastase expression is lost afterward, because the ROSA26 promoter conveys ubiquitous expression to the downstream gene. Pancreatic acinar cells from R26R-ECFP were labeled by infection of Ad-pAmy-Cre (multiplicity of infection, 100) at 43.7% efficiency (483 ECFP-positive cells of 1,106 cells counted). ECFP-expressing insulin-positive cells were frequently found (46.4%: 77 insulin/ECFP double-positive cells of 166 insulin-positive cells counted) (Fig. 2 B and C), demonstrating that the insulin-positive cells originate from amylase-expressing cells. In addition, ECFP/cytokeratin (CK) double-positive cells were found (Fig. 2D), indicating that the CK-positive cells also are derived from acinar cells. Similar results were obtained by labeling with Ad-pEla-Cre, although the efficiency was slightly less than with Ad-pAmy-Cre (see Fig. 10, which is published as supporting information on the PNAS web site). It is noted that neither Ad-pAmy-Cre nor Ad-pEla-Cre caused nonspecific recombination in islet cells from R26R-ECFP (Fig. 6). These results demonstrate that amylase/elastase-expressing pancreatic acinar cells can transdifferentiate into insulin-producing cells.
Fig. 2.
Cell lineage tracing by the Cre/loxP-based system. (A) The scheme of pancreatic acinar cell specific cell marking. In cells from the R26R-ECFP mouse, expression of the fluorescent protein (ECFP) is activated through the action of Cre recombinase to remove a transcriptional “stop” sequence. When amylase/elastase-expressing acinar cells are infected with adenovirus expressing Cre recombinase under control of either amylase or elastase promoter, the cells are labeled permanently with ECFP. (B–D) Lineage tracing of labeled acinar cells. Pancreatic acinar cells from R26R-ECFP were labeled by infection of Ad-pAmy-Cre at ≈50% efficiency (B). Because fluorescence of ECFP is diminished after fixation, ECFP expression was detected by using anti-GFP antibody. Cells positive for insulin (arrowheads), ECFP (arrows), and both insulin and ECFP (asterisks) are observed (B). Photographs of higher magnification of insulin/ECFP double-positive cells are shown (C). CK/ECFP double-positive cells are also observed (D). (Scale bars, 20 μm.)
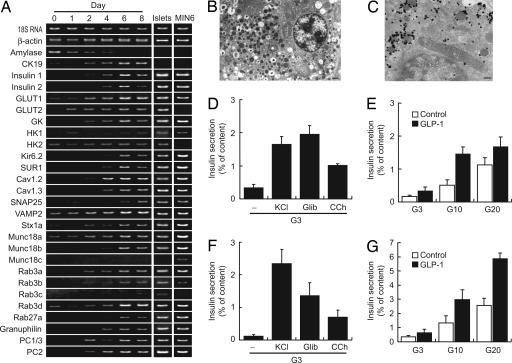
Changes in Gene Expression Profile. We then investigated gene expression of the cells by RT-PCR analysis (Fig. 3A). In native β cells, several key molecules are responsible for glucose-induced insulin secretion, the most important function of the pancreatic β cell. Glucose-sensing molecules (glucose transporters and glucokinase) were induced in the acinar cells by the culture. Expressions of Kir6.2 and SUR1, the pore-forming and regulatory subunits of pancreatic β cell type KATP channels, respectively, were also induced. The expressions of the poreforming subunits of voltage-dependent calcium channels (VDCCs), Cav1.2 and Cav1.3, which regulate Ca2+ influx into the β cell, are evident. The expressions of genes involved in regulated exocytosis were increased by the culture. PC1/3 and PC2, both of which are required for processing proinsulin to mature insulin (39), were also induced. The expression profile of pancreatic acinar cell-derived insulin-producing cells became similar to those of the pancreatic islets and clonal mouse β cell line MIN6-m9 (40) (Fig. 3A).
Fig. 3.
Insulin secretory properties of pancreatic acinar-derived cells. (A) Temporal gene expression analysis by RT-PCR. Representative photographs from four independent experiments are shown. Genes involved in glucose-induced insulin secretion were induced by the culture. Note that the expression pattern becomes similar to that of the pancreatic islets and the clonal β cells line MIN6-m9. GK, glucokinase; HK, hexokinase, Stx1a, syntaxin 1a. (B) Electron microscopic analysis of pancreatic acinar-derived cells. Cells containing small secretory granules with crystalline structure were often found. (Scale bar, 500 nm.) (C) Immunoelectron microscopic analysis for insulin. Insulin immunoreactivities were detected in the secretory granules. (Scale bar, 200 nm.) (D and E) Insulin secretion in pancreatic acinar-derived cells. Insulin secretion was stimulated by 30 mM KCl/0.1 μM glibenclamide (Glib)/0.1 mM carbachol (CCh), or increased concentrations of glucose (G3, 3 mM; G10, 10 mM; G20, 20 mM) for 60 min. Potentiation by glucagon-like peptide-1 (7–36 amide) (100 nM) is also shown. Data are means ± SE of three to seven independent experiments. (F and G) Insulin secretion in native mouse pancreatic islets.
Insulin Secretion from Pancreatic Acinar Cell-Derived Cells. Regulated insulin secretion requires the formation of insulin-containing secretory granules. We performed ultrastructural analysis to determine whether insulin granules are present in the insulin-producing cells derived from pancreatic acinar cells. Cells containing small secretory granules having crystalline structure, a characteristic of the insulin granules in native pancreatic β cells, were found frequently (Fig. 3B), and insulin immunoreactivity was localized in the granules (Fig. 3C). Together with the RT-PCR analysis, this strongly suggests that regulated insulin secretion occurs in these cells.
We then examined the insulin secretory properties of the cells 5–6 days after culture using batch incubation method. In the presence of 3 mM glucose, high KCl, glibenclamide, and carbachol stimulated insulin secretion (Fig. 3D). Importantly, glucose stimulated insulin secretion in a concentration-dependent manner (Fig. 3E). In addition, glucagon-like peptide-1 (7–36 amide), an incretin, potentiated insulin secretion in the presence of relatively high concentrations of glucose (Fig. 3E), indicating that the cAMP-mediated potentiation system is also present in the cells. These results show that pancreatic acinar cell-derived insulin-producing cells are endowed with insulin secretory properties similar to those of native pancreatic islets (Fig. 3 F and G).
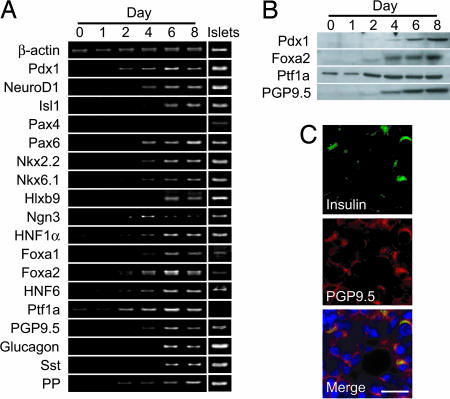
Expression of Molecules Involved in Pancreatic Development. There are several transcription factors known to be critical for pancreatic development and the maintenance of β cell function. Pdx1, a master regulator of pancreatic development restricted to β cells in adults (41), was induced by the culture at both the mRNA and protein levels (Fig. 4 A and B). Other transcription factors characteristic of endocrine pancreas (NeuroD1, Isl1, Pax6, Nkx2.2, Nkx6.1, Hlxb9, HNF1α, and Foxa1) also were induced (Fig. 4A). Ngn3, which is expressed in pancreatic progenitor cells during embryogenesis, was induced by the culture (Fig. 4A). Transcription factors seen early in the developing pancreas, such as Foxa2 (42), HNF6 (43), and Ptf1a (44, 45), were also increased (Fig. 4 A and B), suggesting that pancreatic acinar cells regain the properties of immature pancreatic cells.
Fig. 4.
Expressions of molecules involved in pancreatic development. (A) Temporal gene expression analysis by RT-PCR. Transcription factors involved in pancreas development and β cell functions were induced in the acinar-derived cells, but Pax4 was not induced. Expression of PGP9.5, a potential endocrine progenitor marker, was detected. Glucagon, somatostatin (Sst), and pancreatic polypeptide (PP) was induced by the culture. (B) Protein expression determined by immunoblotting. Pdx1, Foxa2, Ptf1a, and PGP9.5 were induced or increased by the culture. (C) Double immunostaining for insulin and PGP9.5. Almost all of the insulin-positive cells were also positive for PGP9.5. PGP9.5-positive cells negative for insulin were frequently found. (Scale bars, 50 μm.)
In addition to these transcription factors, the expression of PGP9.5, a potential marker of endocrine progenitors (46), also was induced in the acinar-derived cells (Fig. 4 A and B). PGP9.5 was expressed in almost all of the insulin-positive cells (Fig. 4C) but was not found in adult mouse pancreatic β cells (see Fig. 11, which is published as supporting information on the PNAS web site), suggesting that the insulin-positive cells were not mature β cells but were in the process of differentiation. PGP9.5-positive but insulin-negative cells were observed frequently (Fig. 4C) and may well be the precursors of endocrine cells. The expressions of glucagon, somatostatin, and pancreatic polypeptide were also induced in the acinar-derived cells (Fig. 4A).
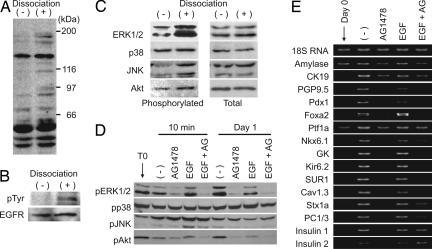
Signals Required for Transdifferentiation. We then investigated cellular signaling in the induction of transdifferentiation. Because the culture medium used included both EGF and nicotinamide, we first evaluated the effects of these agents separately. In the presence of EGF, the acinar cells began to express CK19, transcription factors including Pdx1, pancreatic β cell-specific molecules, and insulin (see Fig. 12, which is published as supporting information on the PNAS web site). Nicotinamide blocked or delayed the expression of these genes (Fig. 12). Surprisingly, even in the absence of both EGF and nicotinamide, the acinar cells exhibited a gene expression profile similar to that obtained by EGF treatment (Fig. 12). We found that intracellular signaling molecules downstream of the EGF receptor (EGFR) were activated before the addition of EGF (Fig. 12). Although EGF promoted the activation of these molecules, nicotinamide attenuated their activation (Fig. 12). However, without any supplements, the acinar cells did not survive 6 days after culture (Fig. 12). The addition of EGF and nicotinamide had a beneficial effect on cell survival, especially when used in combination. Overall, the addition of both EGF and nicotinamide in the culture was effective in inducing insulin-secreting cells from pancreatic acinar cells, in terms of both quality and quantity.
These results suggest that EGF signaling is spontaneously activated in isolated pancreatic acinar cells. Accordingly, we examined the activation (tyrosine phosphorylation) of the EGFR in intact pancreas and enzymatic-dissociated pancreatic cells. Enzymatic dissociation increased tyrosine phosphorylation of cellular proteins (Fig. 5A). The EGFR was not activated in pancreas without dissociation but was activated in dissociated pancreatic cells (Fig. 5B). Downstream signaling molecules of the EGFR examined, except for p38 mitogen-activated protein kinase (MAPK), were also activated after enzymatic dissociation (Fig. 5C). Application of AG1478, an EGFR kinase inhibitor, attenuated phosphorylation of both extracellular signal-regulated protein kinase 1/2 (ERK1/2) and Akt but neither p38 MAPK nor c-Jun N-terminal kinase (JNK) in isolated pancreatic acinar cells (Fig. 5D) and inhibited induction of the genes that are markers for transdifferentiation (Fig. 5E). These results demonstrate that activation of EGF signaling (especially the ERK1/2 and Akt pathways) is essential for transdifferentiation of pancreatic acinar cells into insulin-secreting cells.
Fig. 5.
Involvement of EGF signaling in acinar cell transdifferentiation. (A) Tyrosine phosphorylation of cellular protein. In dissociated pancreatic cells (+), tyrosine phosphorylation was increased compared with undissociated pancreas (–). Antiphosphotyrosine antibody (PY20) was used for detection. (B) Phosphorylation of EGFR. Tyrosine phosphorylation of the EGFR was detected in dissociated pancreatic cells. (C) Activation of intracellular signaling pathways. After dissociation of pancreas, phosphorylated forms of extracellular signal-regulated protein kinase 1/2 (ERK1/2), c-Jun kinase (JNK), and Akt were increased. (D) Effect of EGFR kinase inhibitor AG1478 on intracellular signaling. AG1478 attenuated activation of ERK1/2 and Akt. Effects on p38 mitogen-activated protein kinase and JNK were unclear. (E) Effect of AG1478 on acinar cell transdifferentiation. AG1478 strongly inhibited induction of genes indicating transdifferentiation.
Discussion
Several studies have suggested that pancreatic acinar cells are capable of transdifferentiating into insulin-secreting cells (26, 27). These studies were based primarily on longitudinal expression analysis of marker genes (amylase and insulin) and histological observations (existence of amylase/insulin double-positive cells), but direct evidence for the origin of the cells was not given. We show here that a suspension culture of isolated adult mouse pancreatic acinar cells with EGF and nicotinamide produces insulin-secreting cells. To identify the origin of these insulin-secreting cells, we used the Cre/loxP-based lineage tracing system by which pancreatic acinar cells can be permanently labeled, allowing detection of progeny cells that no longer express acinar cell marker genes. Nearly half of the insulin-positive cells detected were labeled with the fluorescent protein after the culture (Fig. 2). This clearly shows that almost all of the insulin-positive cells were derived from the pancreatic acinar cells in these culture conditions, because the labeling efficiency for pancreatic acinar cells was 43.7%.
Native pancreatic β cells not only express but also secrete insulin in a highly regulated manner. However, there has been no report describing in detail the insulin secretory properties or profiles of the genes expressed in insulin-secreting cells derived from non-β cells. Glucose-induced insulin secretion is the most important mechanism of insulin secretion, involving glucose sensing, metabolism-electrical activity coupling, and regulated exocytosis (16–18, 47). Although some of the molecules associated with regulated exocytosis are present already in the acinar cell preparation, the molecules involved in glucose sensing and metabolism-electrical activity coupling are present only after culture. The acinar-derived cells secreted insulin in response to both glucose and the sulfonylurea glibenclamide, indicating that glucose sensing and metabolism-electrical activity coupling are both functional in the cells. The cAMP-mediated potentiation mechanism, which includes both protein kinase A-dependent and -independent pathways (48, 49), is important in the insulin secretory response in native β cells. Physiologically, incretins such as glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide have such potentiating effects. We also show that GLP-1 augments insulin secretion from the acinar-derived cells in a glucose concentration-dependent manner, indicating that the cAMP-mediated potentiation system is functional in these cells. These findings show that the transdifferentiated acinar-derived cells possess insulin secretory properties similar to those of native pancreatic β cells. However, the insulin content of acinar-derived insulin-secreting cells is low (1/20th of native β cell), and glucose-induced insulin secretion and its potentiation (secretion expressed as percent of content) are somewhat lower than those in native islets (Fig. 3 E and G), suggesting that the in vitro-generated insulin-secreting cells are immature in terms of insulin production and glucose responsiveness.
Although these cells possess insulin-containing secretory granules, a characteristic of native pancreatic β cells, they show a somewhat immature phenotype that includes expression of early pancreatic genes. In addition, we found that many of the acinar-derived cells expressed PGP9.5, which is known as a pan-endocrine marker (50) and has been reported recently to be a marker of endocrine progenitors (46). We find here that native mouse β cells do not express PGP9.5. In the pancreatic duct ligation model, PGP9.5-positive cells appear in the acinar cell-derived duct-like structures from which new islets are generated (46). Thus, the presence of insulin/PGP9.5 double-positive cells may indicate a transitional state of endocrine differentiation.
The present results show that EGF signaling plays a critical role in the transdifferentiation of acinar cells into insulin-secreting cells. In embryonic pancreas, EGF increases the number of undifferentiated endocrine precursor cells; upon removal of EGF, a large number of β cells are differentiated (51). Thus, EGF may be important in the proliferation of endocrine precursors and/or endow the cells with commitment to endocrine lineage. EGF signaling is thought to be involved in the process of acinoductal metaplasia and islet neogenesis in adult pancreas (8, 9). TGFα is a member of the EGF family and acts via EGFR (52). In mouse pancreas overexpressing TGFα, ductal hyperplasia and pronounced interstitial fibrosis occur without an increase in acinar cell mass (8). In these mice, numerous duct cells of the pancreas have both zymogen and mucin granules (8), suggesting that TGFα induces acinoductal metaplasia. Budding of pancreatic islets (islet neogenesis) from the metaplastic ductal epithelium is observed in the pancreas of TGFα transgenic mice (9). Accordingly, the present results in vitro may reflect in part the in vivo findings on the pancreas of these transgenic mice. Furthermore, in the pancreatic duct ligation and partial pancreatectomy models, formation of a large quantity of ductal structures is followed by islet neogenesis (4, 53). Although it is not clear whether EGF signaling is involved in the process, inflammatory cytokines might activate the intracellular signaling pathways. Thus, enzymatic dissociation of pancreatic tissue itself might generate a similar cellular response, activating EGF signaling leading to transdifferentiation of acinar cells.
In conclusion, the present study demonstrates that pancreatic acinar cells possess sufficient plasticity to transdifferentiate into pancreatic endocrine cells in vitro, and that activation of EGF signaling is essential for such transdifferentiation.
Supplementary Material
Acknowledgments
We thank Dr. Y. Kajimoto (Osaka University, Osaka) for the gift of the Pdx1 antibody, Dr. F. Costantini (Columbia University, New York) for thegift of the R26R-ECFP mice, and Dr. T. Miki (Kobe University, Kobe, Japan) for valuable suggestions for the study. We also thank JCR Pharmaceuticals (Kobe, Japan) for preparing adenoviruses. This work was supported by a Grant-in-Aid for Specially Promoted Research and Scientific Research Grants from the Ministry of Education, Culture, Science, Sports, and Technology.
Author contributions: K. Minami designed research; K. Minami, M.O., K. Miyawaki, A.O., K.I., K.O., M.K., N.I., and T.I. performed research; K. Minami and S.S. analyzed data; and K. Minami and S.S. wrote the paper.
Abbreviations: ECFP, enhanced cyan fluorescent protein; EGFR, EGF receptor.
References
- 1.Bonner-Weir, S. (2000) Trends Endocrinol. Metab. 11, 375–378. [DOI] [PubMed] [Google Scholar]
- 2.Dor, Y., Brown, J., Martinez, O. I. & Melton, D. A. (2004) Nature 429, 41–46. [DOI] [PubMed] [Google Scholar]
- 3.Georgia, S. & Bhushan, A. (2004) J. Clin. Invest. 114, 963–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bouwens, L. (1998) Microsc. Res. Technol. 43, 332–336. [DOI] [PubMed] [Google Scholar]
- 5.Wang, R. N., Kloppel, G. & Bouwens, L. (1995) Diabetologia 38, 1405–1411. [DOI] [PubMed] [Google Scholar]
- 6.Gu, D. & Sarvetnick, N. (1993) Development (Cambridge, U.K.) 118, 33–46. [DOI] [PubMed] [Google Scholar]
- 7.Gu, D., Lee, M. S., Krahl, T. & Sarvetnick, N. (1994) Development (Cambridge, U.K.) 120, 1873–1881. [DOI] [PubMed] [Google Scholar]
- 8.Bockman, D. E. & Merlino, G. (1992) Gastroenterology 103, 1883–1892. [DOI] [PubMed] [Google Scholar]
- 9.Song, S. Y., Gannon, M., Washington, M. K., Scoggins, C. R., Meszoely, I. M., Goldenring, J. R., Marino, C. R., Sandgren, E. P., Coffey, R. J., Jr., Wright, C. V., et al. (1999) Gastroenterology 117, 1416–1426. [DOI] [PubMed] [Google Scholar]
- 10.Gmyr, V., Kerr-Conte, J., Belaich, S., Vandewalle, B., Leteurtre, E., Vantyghem, M. C., Lecomte-Houcke, M., Proye, C., Lefebvre, J. & Pattou, F. (2000) Diabetes 49, 1671–1680. [DOI] [PubMed] [Google Scholar]
- 11.Ramiya, V. K., Maraist, M., Arfors, K. E., Schatz, D. A., Peck, A. B. & Cornelius, J. G. (2000) Nat. Med. 6, 278–282. [DOI] [PubMed] [Google Scholar]
- 12.Bonner-Weir, S., Taneja, M., Weir, G. C., Tatarkiewicz, K., Song, K. H., Sharma, A. & O'Neil, J. J. (2000) Proc. Natl. Acad. Sci. USA 97, 7999–8004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zulewski, H., Abraham, E. J., Gerlach, M. J., Daniel, P. B., Moritz, W., Muller, B., Vallejo, M., Thomas, M. K. & Habener, J. F. (2001) Diabetes 50, 521–533. [DOI] [PubMed] [Google Scholar]
- 14.Gao, R., Ustinov, J., Pulkkinen, M. A, Lundin, K., Korsgren, O. & Otonkoski, T. (2003) Diabetes 52, 2007–2015. [DOI] [PubMed] [Google Scholar]
- 15.Halban, P. A. (2004) Nat. Cell Biol. 6, 1021–1025. [DOI] [PubMed] [Google Scholar]
- 16.Newgard, C. B. & McGarry, J. D. (1995) Annu. Rev. Biochem. 64, 689–719. [DOI] [PubMed] [Google Scholar]
- 17.Seino, S. (1999) Annu. Rev. Physiol. 61, 337–362. [DOI] [PubMed] [Google Scholar]
- 18.Rorsman, P. & Renstrom, E. (2003) Diabetologia 46, 1029–1045. [DOI] [PubMed] [Google Scholar]
- 19.Halban, P. A., Kahn, S. E., Lernmark, A. & Rhodes, C. J. (2001) Diabetes 50, 2181–2191. [DOI] [PubMed] [Google Scholar]
- 20.Poulsom, R., Alison, M. R., Forbes, S. J. & Wright, N. A. (2002) J. Pathol. 197, 441–456. [DOI] [PubMed] [Google Scholar]
- 21.Cova, L., Ratti, A., Volta, M., Fogh, I., Cardin, V., Corbo, M. & Silani, V. (2004) Stem Cells Dev. 13, 121–131. [DOI] [PubMed] [Google Scholar]
- 22.Eisenberg, L. M. & Eisenberg, C. A. (2003) Birth Defects Res. C Embryo Today 69, 209–218. [DOI] [PubMed] [Google Scholar]
- 23.Shen, C. N., Horb, M. E., Slack, J. M. & Tosh, D. (2003) Mech. Dev. 120, 107–116. [DOI] [PubMed] [Google Scholar]
- 24.Meivar-Levy, I. & Ferber, S. (2003) Trends Endocrinol. Metab. 14, 460–466. [DOI] [PubMed] [Google Scholar]
- 25.Rooman, I., Heremans, Y., Heimberg, H. & Bouwens, L. (2000) Diabetologia 43, 907–914. [DOI] [PubMed] [Google Scholar]
- 26.Song, K. H., Ko, S. H., Ahn, Y. B., Yoo, S. J., Chin, H. M., Kaneto, H., Yoon, K. H., Cha, B. Y., Lee, K. W. & Son, H. Y. (2004) Biochem. Biophys. Res. Commun. 316, 1094–1100. [DOI] [PubMed] [Google Scholar]
- 27.Baeyens, L., De Breuck, S., Lardon, J., Mfopou, J. K., Rooman, I. & Bouwens L. (2005) Diabetologia 48, 49–57. [DOI] [PubMed] [Google Scholar]
- 28.Gu, G., Brown, J. R. & Melton, D. A. (2003) Mech. Dev. 120, 35–43. [DOI] [PubMed] [Google Scholar]
- 29.Herrera, P. L. (2000) Development (Cambridge, U.K.) 127, 2317–2322. [DOI] [PubMed] [Google Scholar]
- 30.Srinivas, S., Watanabe, T., Lin, C. S., William, C. M., Tanabe, Y., Jessell, T. M. & Costantini, F. (2001) BMC Dev. Biol. 1, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dematteo, R. P., McClane, S. J., Fisher, K., Yeh, H., Chu, G., Burke, C. & Raper, S. E. (1997) J. Surg. Res. 72, 155–161. [DOI] [PubMed] [Google Scholar]
- 32.Hammer, R. E., Swift, G. H., Ornitz, D. M., Quaife, C. J., Palmiter, R. D., Brinster, R. L. & MacDonald, R. J. (1987) Mol. Cell. Biol. 7, 2956–1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heller, R. S., Stoffers, D. A., Bock, T., Svenstrup, K., Jensen, J., Horn, T., Miller, C. P., Habener, J. F., Madsen, O. D. & Serup, P. (2001) Diabetes 50, 1553–1561. [DOI] [PubMed] [Google Scholar]
- 34.Shiroi, A., Yoshikawa, M., Yokota, H., Fukui, H., Ishizaka, S., Tatsumi, K. & Takahashi, Y. (2002) Stem Cells 20, 284–292. [DOI] [PubMed] [Google Scholar]
- 35.Latif, Z. A., Noel, J. & Alejandro, R. (1988) Transplantation 45, 827–830. [PubMed] [Google Scholar]
- 36.Ishihara, K., Ishikawa, E., Iwasaki, Y. & Nakabayashi, N. (1999) J. Biomater. Sci. Polym. Ed. 10, 1047–1061. [DOI] [PubMed] [Google Scholar]
- 37.Yamamoto, H., Uchigata, Y. & Okamoto, H. (1981) Nature 294, 284–286. [DOI] [PubMed] [Google Scholar]
- 38.Otonkoski, T., Beattie, G. M., Mally, M. I., Ricordi, C. & Hayek, A. (1993) J. Clin. Invest. 92, 1459–1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Steiner, D. F. (1998) Curr. Opin. Chem. Biol. 2, 31–39. [DOI] [PubMed] [Google Scholar]
- 40.Minami, K., Yano, H., Miki, T., Nagashima, K., Wang, C. Z., Tanaka, H., Miyazaki, J. I. & Seino, S. (2000) Am. J. Physiol. 279, E773–E781. [DOI] [PubMed] [Google Scholar]
- 41.Edlund, H. (2002) Nat. Rev. Genet. 3, 524–532. [DOI] [PubMed] [Google Scholar]
- 42.Ang, S. L., Wierda, A., Wong, D., Stevens, K. A., Cascio, S., Rossant, J. & Zaret, K. S. (1993) Development (Cambridge, U.K.) 119, 1301–1315. [DOI] [PubMed] [Google Scholar]
- 43.Rausa, F., Samadani, U., Ye, H., Lim, L., Fletcher, C. F., Jenkins, N. A., Copeland, N. G. & Costa, R. H. (1997) Dev. Biol. 192, 228–246. [DOI] [PubMed] [Google Scholar]
- 44.Krapp, A., Knofler, M., Ledermann, B., Burki, K., Berney, C., Zoerkler, N., Hagenbuchle, O. & Wellauer, P. K. (1998) Genes Dev. 12, 3752–3763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kawaguchi, Y., Cooper, B., Gannon, M., Ray, M., MacDonald, R. J. & Wright, C. V. (2002) Nat. Genet. 32, 128–134. [DOI] [PubMed] [Google Scholar]
- 46.Yokoyama-Hayashi, K., Takahashi, T., Kakita, A. & Yamashina, S. (2002) Endocr. J. 49, 61–74. [DOI] [PubMed] [Google Scholar]
- 47.Henquin, J. C. (2000) Diabetes 49, 1751–1760. [DOI] [PubMed] [Google Scholar]
- 48.Ozaki, N., Shibasaki, T., Kashima, Y., Miki, T., Takahashi, K., Ueno, H., Sunaga, Y., Yano, H., Matsuura, Y., Iwanaga, T., et al. (2000) Nat. Cell Biol. 2, 805–811. [DOI] [PubMed] [Google Scholar]
- 49.Kashima, Y., Miki, T., Shibasaki, T., Ozaki, N., Miyazaki, M., Yano, H. & Seino, S. (2001) J. Biol. Chem. 276, 46046–46053. [DOI] [PubMed] [Google Scholar]
- 50.Thompson, R. J., Doran, J. F., Jackson, P., Dhillon, A. P. & Rode, J. (1983) Brain Res. 278, 224–228. [DOI] [PubMed] [Google Scholar]
- 51.Cras-Meneur, C., Elghazi, L., Czernichow, P. & Scharfmann, R. (2001) Diabetes 50, 1571–1579. [DOI] [PubMed] [Google Scholar]
- 52.Yarden, Y. & Sliwkowski, M. X. (2001) Nat. Rev. Mol. Cell Biol. 2, 127–137. [DOI] [PubMed] [Google Scholar]
- 53.Bonner-Weir, S., Baxter, L. A., Schuppin, G. T. & Smith, F. E. (1993) Diabetes 42, 1715–1720. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.