Abstract
Vesicle fusion in eukaryotic cells is mediated by SNAREs (soluble N-ethylmaleimide-sensitive factor attachment protein receptors). In neurons, the t-SNARE SNAP-25 is essential for synaptic vesicle fusion but its exact role in this process is unknown. We have isolated a SNAP-25 temperature-sensitive paralytic mutant in Drosophila, SNAP-25ts. The mutation causes a Gly50 to Glu change in SNAP-25’s first amphipathic helix. A similar mutation in the yeast homologue SEC9 also results in temperature sensitivity, implying a conserved role for this domain in secretion. In vitro-generated 70 kDa SNARE complexes containing SNAP-25ts are thermally stable but the mutant SNARE multimers (of ∼120 kDa) rapidly dissociate at 37°C. The SNAP-25ts mutant has two effects on neurotransmitter release depending upon temperature. At 22°C, evoked release of neurotransmitter in SNAP-25ts larvae is greatly increased, and at 37°C, the release of neurotransmitter is reduced as compared with controls. Our data suggest that at 22°C the mutation causes the SNARE complex to be more fusion competent but, at 37°C the same mutation leads to SNARE multimer instability and fusion incompetence.
Keywords: Drosophila/exocytosis/mutant/SNAP-25/SNARE
Introduction
Secretion in all eukaryotic cells involves a family of proteins known as SNAREs (soluble N-ethylmaleimide-sensitive factor attachment protein receptors). The assembly of target membrane SNAREs (t-SNAREs) with vesicle membrane SNAREs (v-SNAREs) is believed to mediate the fusion of vesicles in all trafficking steps of the secretory pathway (reviewed in Jahn and Sudhof, 1999; Lin and Scheller, 2000). In the nervous system, neurotransmitter release occurs when docked synaptic vesicles undergo Ca2+-dependent fusion with the plasma membrane at active zones.
Several lines of evidence point to the importance of the neuronal t-SNARE (also called Q-SNARE) proteins, syntaxin and SNAP-25, and the v-SNARE (also called R-SNARE) protein, synaptobrevin, in the fusion of synaptic vesicles. First, clostridial neurotoxins block synaptic transmission by cleaving these proteins (reviewed in Montecucco and Schiavo, 1994). Secondly, an in vitro complex has been shown to form between synaptobrevin, SNAP-25 and syntaxin (Sollner et al., 1993), and such complexes appear to be sufficient for membrane fusion in vitro (Weber et al., 1998). SNARE complexes are also present in vivo and have been isolated directly from Drosophila head extracts (Littleton et al., 1998; Tolar and Pallanck, 1998). Thirdly, severe mutations in syntaxin and synaptobrevin reduce or eliminate evoked release of neurotransmitter (Schulze et al., 1995; Deitcher et al., 1998; Nonet et al., 1998; Saifee et al., 1998; Yoshihara et al., 1999). Recently, hypomorphic alleles of Drosophila syntaxin and neuronal synaptobrevin (n-syb) have been shown to alter the Ca2+ cooperativity of neurotransmitter release (Stewart et al., 2000). Though no genetic mutations in SNAP-25 have yet been characterized, studies have indicated an important role for the second α-helix of SNAP-25 in neurotransmitter release (Chen et al., 1999; Wei et al., 2000). In addition, inhibition of the function of the first α-helix of SNAP-25 by an antibody has been shown to prevent SNARE complex assembly and greatly reduce the sustained component of exocytosis (Xu et al., 1999).
X-ray crystallography of the SNARE complex core revealed that it is composed of four parallel amphipathic α-helices: one syntaxin helix, one synaptobrevin helix and two SNAP-25 helices. This complex is a coiled-coil structure, stabilized by the hydrophobic faces of the α-helices (Sutton et al., 1998). Spin labeling electron pair resonance (EPR) spectroscopy studies on the SNARE complex identified a highly flexible region in the vicinity of Gly43 (mouse numbering) in SNAP-25 that was predicted to be involved in SNARE complex conformational changes (Poirier et al., 1998).
In order to understand the function of SNAP-25 in vivo, a mutagenesis of the Drosophila SNAP-25 gene was undertaken. SNAP-25 protein is very concentrated in synaptic regions of the nervous system (Risinger et al., 1997; Niemeyer and Schwarz, 2000) including the larval neuromuscular junction (NMJ) (this paper). Two related genes have been identified in Drosophila. SNAP-24 was reported recently (Niemeyer and Schwarz, 2000) and is expressed strongly in salivary glands. Weaker expression was also detected in selected neuronal cell bodies and in synaptic regions. A more distantly related rat protein, SNAP-29, is expressed predominantly in liver, heart and skeletal muscle and weakly in brain (Steegmaier et al., 1998) and is hypothesized to be involved in multiple steps of vesicle trafficking. Drosophila contains a gene homologous to SNAP-29, Dm SNAP-31 (Littleton, 2000), which is likely to play a similar role to that observed in mammals. Thus, SNAP-25’s expression pattern is consistent with it being the relevant SNARE protein involved in the release of neurotransmitter. Here, we report the isolation of a temperature-sensitive paralytic allele of SNAP-25, SNAP-25ts. At the larval NMJ of SNAP-25ts mutants, evoked neurotransmitter release is surprisingly enhanced 2-fold at the permissive temperature of 22°C. However, neurotransmitter release is reduced by 60% at the non-permissive temperature of 37°C.
Results
SNAP-25ts is a recessive mutation
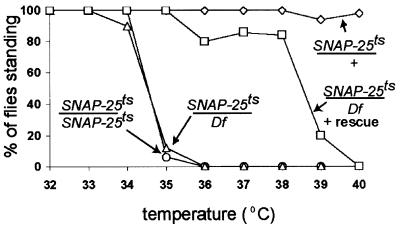
To study the role of SNAP-25 in vivo, we generated a mutation in the Drosophila gene by employing a large scale F1 temperature-sensitive (ts) paralytic screen as described (Watson et al., 2001). F1 progeny were tested at 38°C and from this screen, one allele that was paralytic over a deficiency that removes SNAP-25, Df(3L)1-16, was selected for further study. We will refer to this mutant allele as SNAP-25ts. At 22°C, SNAP-25ts flies can crawl, fly and mate but at 38°C, they are paralyzed. To further define the paralytic behavior, SNAP-25ts flies were examined over a range of temperatures. SNAP-25ts heterozygotes (SNAP-25ts/TM3, Sb), homozygotes and hemizygotes (SNAP-25ts/Df(3L)1-16) were tested between 32 and 40°C (Figure 1). Both SNAP-25ts homozygotes and hemizygotes are paralyzed within seconds at 35°C, while heterozygotes were indistinguishable from wild type and are not paralyzed at temperatures up to 40°C. These data indicate that SNAP-25ts is recessive, and since the homozygote and hemizygote behaved similarly, this indicates that SNAP-25ts behaves as an apparent loss-of-function mutation above 35°C. Moreover, the transgenic expression of SNAP-25 in the nervous system with the P-element vector P[nsyb–SNAP-25] in the mutant background rescued the paralytic phenotype as indicated by an upward shift of the paralytic temperature to 38°C. This is consistent with the paralysis being due to a mutation in SNAP-25.
Fig. 1. Behavioral effects of the SNAP-25 mutation. Fifty 4-day-old adult flies of the genotype indicated were treated for 10 min at the temperature indicated and tested for paralysis. Df(3L)1-16 is a deficiency that removes the SNAP-25 gene and P[nsyb–SNAP-25] is a P-element vector that expresses SNAP-25 pan-neurally (L.Vosshall, personal communication). SNAP-25ts/+ (diamond), P[nsyb–SNAP-25]; SNAP-25ts/Df(3L)1-16 (square), SNAP-25ts/SNAP-25ts (circle) and SNAP-25ts/Df(3L)1-16 (triangle). Both SNAP-25ts/Df(3L)1-16 and SNAP-25ts/SNAP-25ts flies are paralyzed at 35°C whereas SNAP-25ts/Df(3L)1-16 flies that contain the P-element rescue construct are not significantly paralyzed until 39°C.
Mutation in SNAP-25ts affects amphipathic helix I
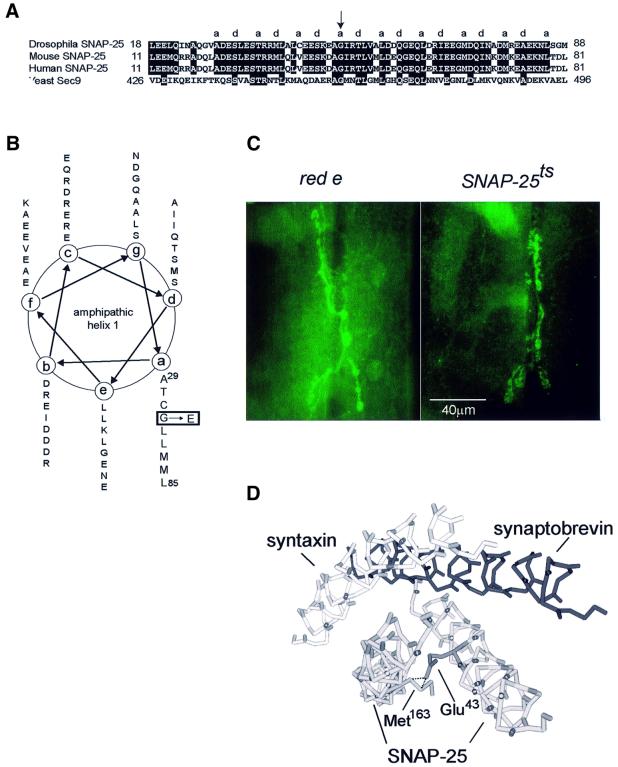
The Drosophila SNAP-25 gene is composed of eight exons (Risinger et al., 1997). DNA sequencing of the SNAP-25ts allele revealed a single base pair change of a G to A in exon 4 of the gene that resulted in a Gly50 to Glu substitution in the hydrophobic face of amphipathic helix I (Figure 2A and B). This Gly residue is highly conserved in members of the SNAP-25 family and related proteins. A temperature-sensitive allele of the yeast homologue of SNAP-25, SEC9, changes the analogous Gly to Asp, resulting in a loss of protein secretory function at 37°C (Brennwald et al., 1994). In order to see whether the mutation affected SNAP-25 localization, we stained larvae with an antibody against SNAP-25. We observed no difference in distribution of SNAP-25 at the third instar larval NMJ of wild-type and mutant animals (Figure 2C).
Fig. 2. Site of the SNAP-25ts mutation and its effect on SNAP-25 localization. (A) Sequence alignment of SNAP-25 and homologues from the species indicated. Amino acids from the hydrophobic face of amphipathic helix I are designated a and d as described previously (Weimbs et al., 1997) and the arrow indicates the position of the mutation at Gly50 (in the mouse this is Gly43). (B) α-helical wheel projection of amphipathic helix I from Ala29 to Leu85 with the position of mutated Gly50, with the corresponding amino acid change to Glu, shown boxed. (C) Anti-SNAP-25 staining of red e (control) third instar larval NMJ 6/7. Right panel: anti-SNAP-25 staining of mutant third instar larval NMJ 6/7. No change in SNAP-25 localization was seen in the mutant NMJ. (D) Predicted three-dimensional structure of SNARE complex with Gly43 (mouse numbering) to Glu change. Steric hindrance with Met163 is indicated by the dashed line.
Because exon 4 is also alternatively spliced in Drosophila (H.Bellen, personal communication), it was possible that the other splice form could substitute for the ts allele. However, when we examined the level of splice variant mRNA we found that it accounts for <15% of the total SNAP-25 transcript. Furthermore, staining with an antiserum specific for the splice variant revealed that, unlike the predominant form of SNAP-25 protein, the splice variant was not concentrated in synaptic regions and was not detectable at the larval NMJ (our unpublished results). Thus, this splice variant was unlikely to play a significant role in the synaptic release of neurotransmitter.
To understand the effect of this mutation on the structure of the SNARE complex, we used Swiss Protein Database Viewer (Guex and Peitsch, 1997) to simulate the Gly to Glu substitution in the mouse SNARE complex structure. We found that the Glu side chain was predicted to sterically hinder Met163 (mouse numbering) in SNAP-25’s second amphipathic helix (Figure 2D). We anticipated that this steric hindrance may reduce the stability of the SNARE complex and alter its conformation.
SNAP-25ts does not affect steady state levels of SNARE complex in vivo
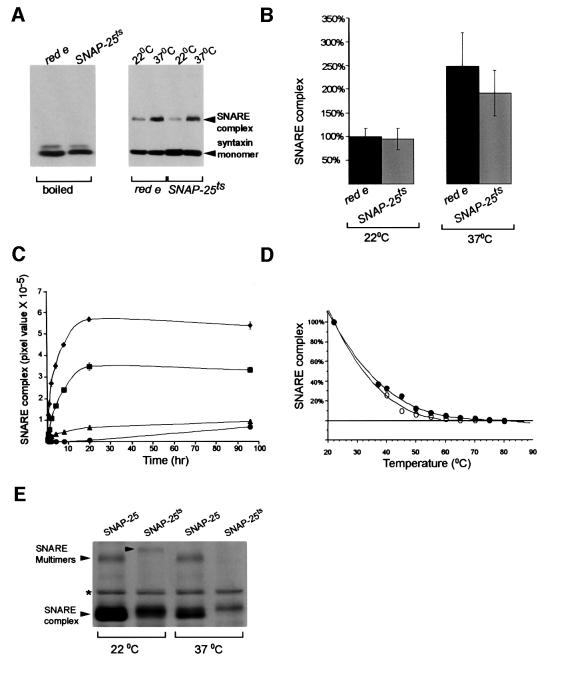
To test whether the SNAP-25ts protein affects SNARE complex stability in vivo, we examined the steady state levels of SNARE complex from adult head extracts. We predicted that the Gly50 to Glu change would affect SNARE complex levels in the SNAP-25ts flies. An SDS-resistant 73 kDa SNARE complex consisting of SNAP-25, syntaxin and n-syb had been characterized previously from unboiled adult fly head extracts with an anti-syntaxin antibody on western blots (Tolar and Pallanck, 1998). A 73 kDa band was detected in head extracts from control and SNAP-25ts lines (Figure 3A) at both 22 and 37°C. The 73 kDa band disappeared if mutant or control (red e) extracts were boiled and only syntaxin monomer was detected. The amounts of detectable complex at 22°C from SNAP-25ts and control extracts were not significantly different from each other (Figure 3B). At 37°C, both control and mutant lines show a temperature-dependent increase in SNARE complex levels. The temperature-dependent increase in SNARE complex levels in control flies is in agreement with a previous study (Tolar and Pallanck, 1998). Thus, the mutation in SNAP-25 had virtually no effect on SNARE complex levels at 22 or 37°C.
Fig. 3. The effect of the SNAP-25ts mutation on in vivo and in vitro SNARE complexes. (A) SNARE complexes detected on western blots from head extracts of red e (control) and SNAP-25ts flies at different temperatures. Extracts were either boiled or loaded directly onto a gel without boiling (right). The positions of the SNARE complex and syntaxin monomer are indicated. Heating of red e (control) and SNAP-25ts heads to 37°C resulted in an increase in SNARE complex levels. (B) Quantitation of SNARE complexes levels from red e and SNAP-25ts flies at different temperatures. Data were normalized to red e SNARE complex levels at 22°C. At 37°C, a significant increase in SNARE complex levels was observed in red e flies (control) and a modest, but statistically insignificant, increase in SNARE complex levels was observed in SNAP-25ts flies. Error bars are ± SEM. (C) Kinetics of SNARE complex (∼70 kDa) and SNARE multimer (122–127 kDa) assembly at 4°C; wild-type ∼70 kDa SNARE complex (diamond), ∼70 kDa mutant SNARE complex (square), wild-type 122 kDa SNARE multimer (triangle) and mutant 127 kDa SNARE multimer (circle). SNARE levels are reported as the pixel value (the product of mean pixel intensity and number of selected pixels). Error bars are ± SEM. (D) A regression curve of the melting temperatures of wild-type (open circle) and mutant (closed circle) 70 kDa SNARE complexes. Mutant and wild-type SNARE complex reactions were heated with 0.67% SDS, separated by SDS–PAGE and detected by Coomassie Blue. Quantities were normalized to the amount of SNARE complex present at 22°C. (E) Wild-type and mutant SNARE complexes detected on a Coomassie Blue-stained gel after heating to 22 or 37°C. Note that 120 kDa SNARE multimers are seen at 22°C for both wild-type and mutant SNAP-25, although the multimers run at different sizes (122 kDa for wild type and 127 kDa for mutant). Mutant SNARE multimers dissociate completely after a 5 min incubation at 37°C. Wild-type SNARE multimers are stable to at least 45°C (data not shown). The asterisk denotes the position of a bacterial contaminant that does not disappear upon boiling.
SNAP-25ts affects the mobility and temperature stability of SNARE multimers
In order to further examine the nature of the mutant complex and its temperature-dependent phenotype, SNARE complex formation and temperature stability were examined in vitro using recombinant proteins. Recombinant syntaxin and neuronal synaptobrevin (minus their transmembrane domains) were mixed with either wild-type SNAP-25 or SNAP-25ts and the amount of SNARE complex was assayed at different time intervals by SDS–PAGE and Coomassie Blue staining. We saw the expected SNARE complexes of ∼70 kDa and higher order SNARE complexes or SNARE multimers of ∼120 kDa in the unboiled assembly reactions. No SNARE complexes were detected if any of the three components were omitted from the reaction mixture or if the sample was boiled. Binary complexes between SNAP-25 and syntaxin were seen in unboiled reactions lacking n-syb, but these differed in size from SNARE complexes and SNARE multimers. Although SNARE complex formation rates were similar in both wild-type SNAP-25- and SNAP-25ts-containing mixtures with maximal formation occurring by 20 h, the total amount of SNAP-25ts complexes formed was reduced by 39% (p <0.001) as compared with wild type (Figure 3C). Mutant SNARE multimer formation at 20 h was reduced by 90% as compared with wild type (p <0.001, Figure 3C) but by only 28% at 96 h (p = 0.02).
We also examined the mutant complexes’ stability upon heating. Mutant and wild-type SNARE complexes were heated from temperatures of 22 to 80°C as described (Chen et al., 1999). Little difference was observed in the melting temperature curves of mutant and wild-type SNARE complexes (Figure 3D). However, mutant SNARE multimers were very unstable upon heating. After only a 5 min incubation at 37°C, mutant SNARE multimers were undetectable by Coomassie Blue staining or by western blotting whereas wild-type multimers were detectable up to 45°C (Figure 3E). This suggests that temperature-sensitive paralysis in the mutant may result from temperature instability of SNARE multimers. We also observed that mutant SNARE complexes had lower mobility than wild-type SNARE complexes on SDS–PAGE gels. These mobility changes were even more pronounced in SNARE multimers, with mutant SNARE multimers running significantly larger in size than the corresponding wild-type multimer (Figure 3E). The increased size of the mutant complex is consistent with the notion that its shape is altered. Mobility changes were also observed in 73 kDa SNARE complexes derived from some preparations of head extracts but these shifts were not found as consistently as those in vitro (data not shown). SNARE multimers were not routinely detected from head extracts, thus their mobility changes could not be assessed (data not shown).
In an effort to further define the structural change in the in vitro mutant complex, both mutant and wild-type SNARE complexes were treated with trypsin to look for any differences in digestion pattern. However, no difference was seen in the SNARE complex digestion pattern between the wild-type and mutant complexes (data not shown).
Evoked release is increased at 22°C and decreased at 37°C
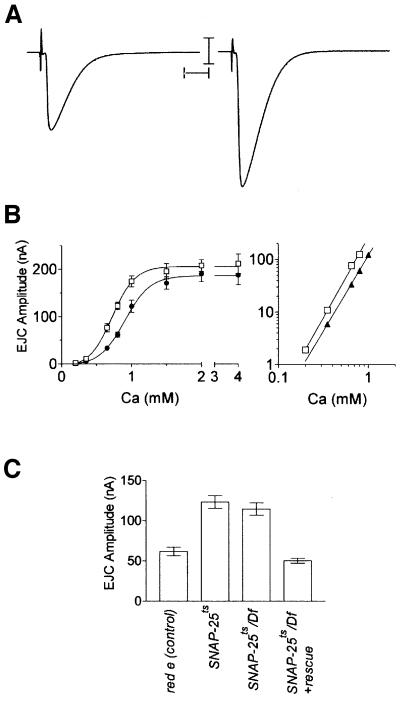
To examine how the SNAP-25ts mutation affects neurotransmitter release we recorded synaptic events from the NMJ of third instar larvae. At the permissive temperature, nerve stimulation surprisingly resulted in larger excitatory junctional currents (EJCs) in SNAP-25ts homozygotes as compared with controls (Figure 4A and C). An ∼2-fold increase in EJC amplitude was observed at subsaturating concentrations of external calcium (e.g. p <0.01, ANOVA at 0.8 mM Ca2+).
Fig. 4. Evoked release of neurotransmitter from red e (control) and SNAP-25ts third instar larval NMJs at 22°C. (A) Two-electrode voltage clamp recordings of evoked release from red e (left) and SNAP-25ts (right) third instar larvae NMJ at 22°C. Representative traces are the average of 20 individual stimuli. Scale bars are 10 nA, 10 ms. (B) Linear and log–log plot of EJC amplitude versus Ca2+ concentration at 22°C. SNAP-25ts shows enhanced neurotransmitter release. Twenty stimuli, delivered at 1 Hz, were recorded from each cell and each data point represents the average obtained from five to nine cells. Error bars, where visible, are ± SEM: red e (closed circle), SNAP-25ts (open circle). (C) Quantification of evoked release from the genotypes indicated at 22°C in 0.8 mM Ca2+. The data are the average result obtained from six to nine cells.
To examine whether this increase resulted from a change in the sensitivity of evoked release to external Ca2+, recordings were performed on SNAP-25ts and control larvae over a range of Ca2+ concentrations. A linear plot of EJC amplitude versus Ca2+ concentration revealed that the SNAP-25ts mutant was indeed more sensitive to external Ca2+ than were controls (Figure 4B, left). Examination of log–log plots of EJC amplitude versus Ca2+ concentration showed that, though the curve is clearly shifted to the left, cooperativity of transmitter release was not different in the mutant as compared with the control, with both having cooperativity coefficients of ∼3 (Figure 4B, right). We further examined the synaptic phenotype of SNAP-25ts/Df(3L)1-16 hemizygotes at 0.8 mM Ca2+ and found that it was identical to that of SNAP-25ts homozygotes (Figure 4C), indicating that the SNAP-25ts allele is recessive and acts as an apparent loss-of-function mutation at 22°C. Expression of a SNAP-25 transgene in the hemizygote background rescued the synaptic phenotype (Figure 4C).
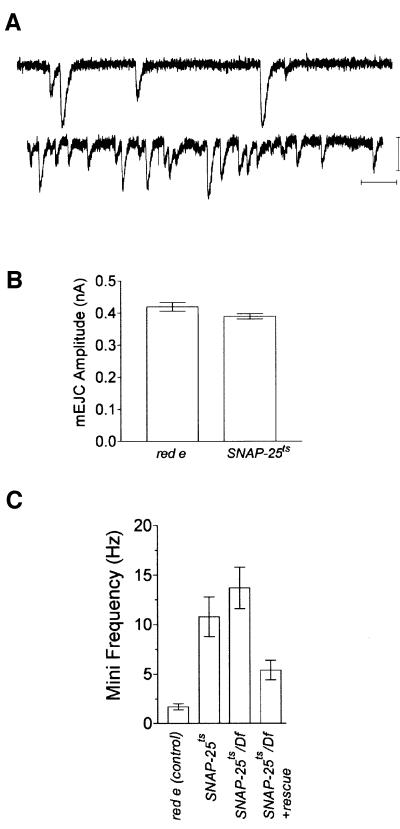
Why is there enhanced evoked release in the SNAP-25ts mutant? One possibility is that quantal size is larger in the mutant. However, recordings of miniature EJCs (mEJCs) from the mutant and control animals revealed that mEJC amplitude was unaltered in the mutant, indicating that quantal size was not changed (Figure 5A and B). Interestingly, we additionally found that the SNAP-25ts mutant showed a 6-fold increase in mEJC frequency (p <0.01, ANOVA) as compared with controls (Figure 5C). Expression of a SNAP-25 transgene partially rescued the elevated mEJC frequency (Figure 5C). The measured mEJC frequency was not dependent on extracellular Ca2+ since we recorded similar frequencies for control and SNAP-25ts in both 1 mM Ca2+ and 0 mM Ca2+ plus 2 mM EGTA [SNAP-25ts, 0 mM Ca2+, 9.0 ± 0.9 Hz (n = 15); red e, 0 mM Ca2+, 1.4 ± 0.2 Hz (n = 6)].
Fig. 5. Spontaneous release from SNAP-25ts larvae at 22°C. (A) Two-electrode voltage clamp recording of spontaneous release (mEJC) from red e (upper) and SNAP-25ts (lower) at 22°C. Representative traces are shown. Scale bars are 1 nA, 100 ms. (B) Average mEJC amplitude of red e and SNAP-25ts at 22°C. No significant difference in mEJC amplitude was observed between the two genotypes. The data are the average results obtained from three cells representing 300 individual mEJCs for each genotype. (C) Quantification of spontaneous release frequency from the genotypes indicated at 22°C. SNAP-25ts has a 6-fold increase in mEJC frequency as compared with red e at 22°C and this effect was partially rescued by the expression of a SNAP-25 transgene. The frequency was calculated as the average value obtained from six to seven cells. Error bars, where visible, are ± SEM.
Evoked release could also be elevated if the mutation caused an increase in the number of neuromuscular synapses. SNAP-25ts and control larvae were stained with anti-synaptotagmin antiserum to determine whether the number of synaptic boutons was altered in the mutant. We counted the number of synaptic boutons per muscle in SNAP-25ts (113.9 ± 7.3, mean ± SEM) and control (92.8 ± 5.5) larvae and found no significant difference.
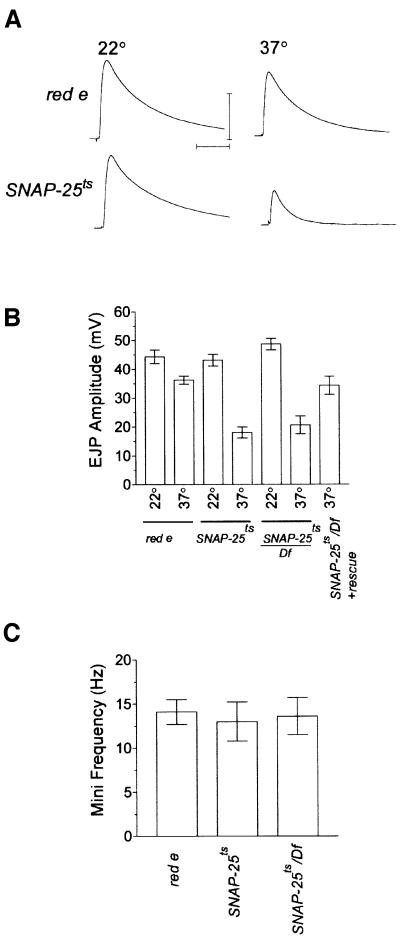
From the behavioral data, we know that SNAP-25ts flies are paralyzed at 35°C. Is this paralysis due to synaptic failure? To address this question, single electrode intracellular recordings were performed on SNAP-25ts larvae at 37°C, since two electrode voltage clamp recordings proved deleterious to the preparation. In addition, we found that the high temperature caused a high rate of synaptic failures in HL3 saline with the standard 0.8 mM Ca2+, even in control animals. To counter the failure rate, we chose to carry out the elevated temperature recordings at 2 mM Ca2+, a concentration at which transmitter release is saturated for both genotypes at 22°C (Figure 6A, left, also see Figure 4B, left). These recording conditions would have the tendency to minimize the difference between control and SNAP-25ts since this Ca2+ level yields the same amplitude of the excitatory junctional potentials (EJPs) from both genotypes at 22°C. None the less, we found that at 37°C, evoked release in the SNAP-25ts mutant was significantly reduced, by 60% (p <0.001, ANOVA) as compared with SNAP-25ts at 22°C, whereas controls showed no significant temperature-dependent decrease (Figure 6A and B). As we found at the permissive temperature, the phenotype at the elevated temperature of SNAP-25ts/Df(3L)1-16 hemizygotes was identical to that of SNAP-25ts homozygotes and the phenotype of the hemizygote was rescued by the SNAP-25 transgene (Figure 6B). We next analyzed spontaneous transmitter release at 37°C (Figure 6C) and found that the frequency of release in controls was increased to a level comparable to that of SNAP-25ts at 22°C. Interestingly, despite the reduction in evoked release, there was no detectable difference in the frequency of spontaneous release at 37°C in SNAP-25ts mutants as compared with controls. The spontaneous frequency in SNAP-25ts/Df(3L)1-16 hemizygotes was also similar to that of SNAP-25ts homozygotes at 37°C.
Fig. 6. Evoked and spontaneous release from SNAP-25ts at 37°C. (A) Intracellular recordings from red e and SNAP-25ts third instar larvae NMJ at 22 and 37°C in 2 mM Ca2+. Representative traces, each the average of 20 stimuli, are shown. SNAP-25ts EJP amplitude is reduced as compared with red e at 37°C. However, the EJP amplitude for both genotypes is equal at 22°C due to saturating levels of extracellular Ca2+ used for these recordings. (B) Quantification of EJPs from the indicated genotypes at 22°C or 37°C. These data are the average results obtained from 8–13 cells. (C) Quantification of spontaneous release frequency from the indicated genotypes at 37°C. At 37°C all genotypes had similarly elevated release frequencies. The frequency was calculated as the average value obtained from six to seven cells. Error bars, where visible, are ± SEM.
SNAP-25ts alters the structure of the active zone in larvae
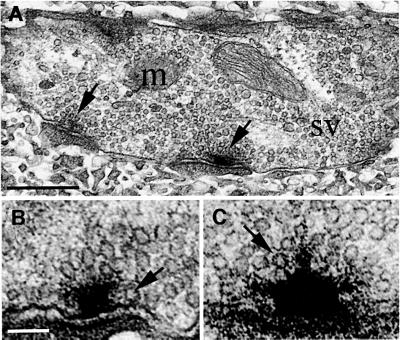
To identify morphological effects of the mutation, we examined the synaptic ultrastructure of the larval NMJ. The synapses of SNAP-25ts larvae appeared to be grossly normal at 22 and 37°C. The nerve terminals were filled with synaptic vesicles (30 nm in diameter), with many vesicles clustered near the active zone, known as the T-bar; vesicles were also found in morphologically docked positions near the T-bar (Figure 7A).
Fig. 7. Synaptic ultrastructure of larval NMJ in control and mutant at 22°C. (A) Typical larval bouton in control containing mitochondria (m), synaptic vesicles (sv) and active zones (arrows) is shown here. Scale bar is 0.5 µm. (B) Control (red e). A section through the T-bar shows a single vesicle docked at the membrane (arrow). Scale bar for (B) and (C) is 100 nm. (C) SNAP-25ts. T-bars in the mutant appear to be more dense and filamentous than T-bars in control. Note the unusual three-pronged appearance of the top of the T-bar. Vesicles appear to be tethered to the T-bar by electron dense material (arrow).
To determine whether the enhanced evoked release at 22°C and the reduced release at 37°C is due to changes in the distribution or number of synaptic vesicles, the number of vesicles clustered within 200 nm of the T-bar was counted in SNAP-25ts animals and in controls at both temperatures. No difference in vesicle density was detected in SNAP-25ts as compared with controls at 22°C (188 ± 10 versus 185 ± 11 vesicles/µm2). While a slight decrease in vesicle density was observed in SNAP-25ts as compared with controls at 37°C (205 ± 19 versus 229 ± 8 vesicles/µm2, respectively), this decrease was not statistically significant (Mann–Whitney test, p >0.05). The vesicle densities reported here are consistent with previous reports where vesicle density in wild-type animals ranged from 155 to 200 vesicles/µm2 (Jia et al., 1993; Renger et al., 2000). We also determined whether the number of morphologically docked vesicles was different in the mutant as compared with control at both 22 and 37°C. As described in Reist et al. (1998), morphologically docked vesicles are defined as those vesicles that are within 200 nm of the T-bar and whose centers are within 12–18 nm (one vesicle radius ± 3 nm) of the presynaptic membrane. No difference in vesicle docking was observed at either temperature with SNAP-25ts having 0.79 ± 0.15 and 0.67 ± 0.21 docked vesicles at 22 and 37°C, respectively, and control having 1.09 ± 0.21 and 0.93 ± 0.18 docked vesicles at 22 and 37°C, respectively (Mann–Whitney test; p >>0.05).
Although the vesicle distribution near the T-bar was unchanged at the SNAP-25ts larval NMJ, some qualitative changes in the structure of the synapse were evident. In particular, the T-bar in the SNAP-25ts larvae at 22°C appeared to be more dense and filamentous as compared with controls (Figure 7B and C). Such T-bars were rarely seen in control animals at both 22 and 37°C and were less frequently observed in SNAP-25ts at 37°C. Because docked vesicles very near the central beam of the T-bar were difficult to discern due to the filamentous nature of the T-bar, this may have resulted in an underestimate of vesicle docking in SNAP-25ts at 22°C.
Discussion
We have isolated a temperature-sensitive paralytic allele of SNAP-25. The SNAP-25ts mutation results in the replacement of glycine with glutamate on the hydrophobic face of amphipathic helix I, altering the structure of SNAP-25 and its interaction with the other SNARE proteins, synaptobrevin and syntaxin. This mutation has two very distinct phenotypes depending upon temperature. At 22°C, the mutation enhanced evoked and spontaneous fusion of synaptic vesicles in the larva. At 35°C or above, mutant flies are paralyzed and evoked neurotransmitter release at the larval NMJ is reduced relative to control.
The similarity of the mutation in SNAP-25ts to that of the yeast mutant, sec9-4ts, indicates a highly conserved function for this region of the protein in secretion. Heating of the sec9-4ts strain to 37°C leads to inviability due to inability to form a stable SNARE complex (Lustgarten and Gerst, 1999). Like the sec9-4 mutation, the SNAP-25ts mutant results in a substantial decrease in synaptic vesicle fusion at 37°C. In contrast to the sec9-4 allele, SNARE complexes were still detectable in SNAP-25ts extracts at 37°C. SNAP-25ts also has a distinct phenotype at 22°C, that of elevated neurosecretion. This phenotype may not be apparent in the sec9-4 mutant as the yeast secretory pathway is constitutive.
Role of SNARE complex in neurotransmitter release
Neurotransmitter release is affected in two opposing ways in SNAP-25ts mutants depending upon temperature. How could these divergent phenotypes arise from a single amino acid substitution in SNAP-25? The region around Gly50, which is mutated in SNAP-25ts, is one of the most mobile in the SNARE complex and conformational changes in this complex are likely to be centered within this region (Poirier et al., 1998). CD spectroscopy and EPR studies have indicated that SNARE proteins undergo dramatic conformational changes during complex assembly in vitro (Fasshauer et al., 1997; Margittai et al., 2001). After the complex assembles, different conformations of the trans-SNARE complex (loose and tight) have been proposed to explain changes in the kinetics of release and suggest that relaxed conformations may be more fusion competent (Xu et al., 1999). Our in vitro electrophoretic mobility data suggest that the mutant SNARE multimer exists in an altered, more fusion-ready conformation, since the evoked release of neurotransmitter was increased 2-fold at 22°C. The in vitro data also suggest an explanation for the decrease in neurotransmitter release at 37°C. Mutant SNARE multimers are very unstable at 37°C. Two recent reports indicate that SNARE multimers, specifically trimers, may be the form responsible for vesicle fusion (Stewart et al., 2000; Hua and Scheller, 2001). Thus, decreased levels of mutant SNARE multimers at 37°C could lead to the blockade in evoked release of neurotransmitter observed in the SNAP-25ts mutant.
We also observed that the mutation in SNAP-25 reduced its ability to form SNARE complexes in vitro but not in vivo. How might this discrepancy be explained? The in vitro SNARE assembly reactions used syntaxin and synaptobrevin without their transmembrane domains. Transmembrane domains have been shown to stabilize in vitro-generated SNARE complexes (Poirier et al., 1998). It is presumed that other cellular factors aid in the very rapid process of SNARE assembly in vivo but these, too, are lacking in in vitro systems. The increased level of SNAREs in heated flies also differs from in vitro results. This effect appears to be due to an accumulation of misfolded, SDS-resistant SNARE complexes that are not effectively resolved by N-ethylmaleimide-sensitive factor (NSF) (Tolar and Pollanck, 1998; Mohtashami et al., 2001; this study) and this form of the SNARE complex is unlikely to be involved in any fusion events.
What is the role of Ca2+ in the phenotype of the mutant? The SNAP-25ts mutant shows increased evoked release at a given external Ca2+ concentration. One possibility is that the SNARE complex may act in Ca2+ sensing and this function may be enhanced by the mutation. Classical studies have indicated that the blockage of neurotransmitter release by botulinum toxin A treatment and SNAP-25 cleavage could be partially reversed by increasing Ca2+ levels (Simpson, 1978). Treatment with toxins that cleave syntaxin or synaptobrevin cannot be overcome with Ca2+, suggesting that SNAP-25 in particular is important in Ca2+ sensing. The crystal structure of the SNARE complex revealed Sr2+ binding sites, which may also bind Ca2+ (Fasshauer et al., 1998; Sutton et al., 1998). In addition, recent evidence indicates that hypomorphic mutations of n-syb and syntaxin in Drosophila reduce the Ca2+ cooperativity of release (Stewart et al., 2000). These data collectively suggest a Ca2+ sensing role for SNARE proteins.
It is possible that the enhanced Ca2+ sensitivity in the mutant could also be mediated by other proteins. It has recently been shown that there is Ca2+-dependent interaction between the C-terminus of SNAP-25 and synaptotagmin (Gerona et al., 2000). However, the site of the mutation in SNAP-25ts is near the N-terminus not the C-terminus, arguing against this mechanism of action. Recent work also suggests that synaptotagmin may mediate SNARE complex formation or oligomerization (Littleton et al., 2001). It is possible that synaptotagmin may more favorably assemble the mutant SNARE complex. Another hypothesis is that enhanced Ca2+ sensitivity could also result from alteration of resting levels of Ca2+ in the nerve terminal. Elevated levels of resting Ca2+ could explain both the elevated evoked and spontaneous release in the mutant. Our data argue against this hypothesis as removal of extracellular Ca2+ has no effect on the frequency of spontaneous release in the mutant. How ever, the enhanced evoked release of neurotransmitter could result from increased Ca2+ entry through Ca2+ channels during stimulation. Although SNAREs interact with Ca2+ channels through the conserved synprint peptide in mammals (Sheng et al., 1996), this sequence does not appear to be present in the major Drosophila Ca2+ channel (Kawasaki et al., 2000). Another Ca2+ channel modulatory site has been found to interact with syntaxin (Bezprozvanny et al., 2000) so it is possible that this site may cause greater Ca2+ entry during stimulation.
SNAP-25ts may affect the tethering of vesicles to the active zone material
The SNAP-25ts mutation caused the active zone material to have a thickened and filamentous appearance. Three-dimensional reconstructions using EM tomography of frog NMJs have discerned ‘ribs’ that attach vesicles to the active zone material (Harlow et al., 2001). The filamentous projections that we observed at T-bars in SNAP-25ts may correspond to an extended version of these ribs and they may be involved in either the elevated mini-frequency or elevated evoked release.
In summary, our characterization of SNAP-25ts indicates that the conformation of SNAP-25 plays a key role in regulating the fusion of synaptic vesicles. Our in vitro data suggest that a less compact or ‘relaxed’ SNARE multimer may support vesicle fusion. Further studies may define how SNAP-25 more precisely carries out this function.
Materials and methods
Plasmid construction and P-element transformation
P[nsyb–SNAP-25] is a Casper2 derivative containing a pan-neural promoter driving the expression of a SNAP-25 cDNA. It was constructed as follows. A 2 kb EcoRI genomic fragment of the n-syb promoter (L.Vosshall, personal communication) was subcloned into Casper2. The resulting plasmid was digested with HpaI and a blunted HindIII fragment containing a chimeric intron from pCAT3-Basic (Promega, Madison, WI) was ligated into the plasmid. Then, a blunted BamHI–XbaI fragment of the SV40 late poly(A) region from pCAT3-Basic was subcloned into the StuI cloning site of the above construct. An SspI fragment of the SNAP-25 cDNA containing the entire ORF was then subcloned into the blunted BglII site of the above construct to yield the P-element vector, P[nsyb–SNAP-25]. P[nsyb–SNAP-25] was used to transform Drosophila.
Fly stocks and genetic crosses
Deficiency line Df(3L)1-16/TM3, Sb (Marchant and Holm, 1988), which removes SNAP-25 (our unpublished results) was obtained from the Bloomington Stock Center. Isozygotic red e males were fed a sucrose solution containing 25 mM ethylmethane sulfonate and mated en masse with Df(3L)1-16/TM3, Sb virgin females; 450 000 F1 progeny were collected and temperature tested in a heated box. A single line was identified that, upon repeated testing, was paralyzed in combination with Df(3L)1-16. A stock was established from this line. Genetic rescue experiments were performed by crossing SNAP-25ts with P[nsyb– SNAP-25]/P[nsyb–SNAP-25]; Df(3L)1-16/TM6B, Hu Tb. Larvae that were SNAP-25ts/Df(3L)1-16 were selected based on non-Tb appearance and adult flies were selected based upon those lacking Hu.
RT–PCR and DNA sequencing
RNA was prepared from heads of red e and SNAP-25ts flies using Trizol (Life Technologies, Gaithersburg, MD). The resulting total RNA was reverse transcribed using Superscript II (Life Technologies) and the entire ORF was amplified by PCR with primers 5′-GCGCGCGCACGTGTTTAAG-3′ and 5′-GGGCTAGGTTGCACTCGTATGTATC-3′, using Tfl DNA polymerase (Epicentre Technologies, Madison, WI). The PCR product was purified using Qiagen spin columns (Qiagen, Valencia, CA) and sequenced by the Cornell University Sequencing Facility.
Antibodies
The peptide MPADPSEEVAPQVPKT(norL)C containing N-terminal sequence from Drosophila SNAP-25 was synthesized (Cornell University BioResource Center), coupled to keyhole limpet hemocyanin (KLH) and used to raise antisera in rabbits by Cornell Veterinary College Animal Services. The alternative exon peptide AEVGMRSIVMLDC was synthesized by Bethyl Laboratories (Montgomery, TX), coupled to KLH, and used to raise antisera as described above. Antisera were affinity purified using the Sulfolink Kit (Pierce Chemical, Rockford, IL). The anti-synaptotagmin antiserum was a kind gift from Dr Hugo Bellen and the 8C3 anti-syntaxin monoclonal was obtained from the Developmental Studies Hybridoma Bank.
Western blotting and Coomassie staining
SNARE complexes were isolated by a modification of the method of Tolar and Pallanck (1998). Briefly, two freshly dissociated heads from SNAP-25ts or red e adults were placed in 5 µl of HL3 saline containing 1 mM Ca2+ (Stewart et al., 1994) and incubated at 22 or 37°C for 5 min. Heads were crushed on ice in 25 µl of 10% glycerol, 1% SDS, 62.5 mM Tris–HCl pH 6.8, 1.2% β-mercaptoethanol and 0.01% bromophenol blue; 20 µl was loaded on a 8% gel and blotted onto Immobilon membranes (Millipore, Bedford, MA). The blot was probed with the 8C3 anti-syntaxin monoclonal antibody at 1:1000 dilution, processed and visualized with enhanced chemiluminescence (Amersham Pharmacia Biotech, Piscataway, NJ). Blots were exposed to Kodak BioMax ML film (Kodak, Rochester, NY) within the linear range of the film and bands were scanned and quantitated using Quantity One software (Bio-Rad, Richmond, CA). The average SNARE complex levels were calculated from nine independent samples. The in vitro SNARE complex formation assay was a modification of the one used in Pevsner et al. (1994). Drosophila n-syb (minus the transmembrane domain), syntaxin (minus the transmembrane domain), SNAP-25 and SNAP-25ts were cloned into a pGEX-KG vector, expressed in XL-1 Blue, purified with glutathione–Sepharose-4B beads (Amersham Pharmacia Biotech) and cleaved by thrombin. N-syb, syntaxin and SNAP-25 or SNAP-25ts were mixed (10 µM each) in 50 mM Tris–HCl pH 8.0, 150 mM NaCl, 0.5% Tween-20, 5 mM dithiothreitol and 2 mM EDTA and incubated for the times indicated at 4°C. The amount of complex formation was calculated from four independent experiments and one-way ANOVA was used for statistical comparisons. After incubation in binding buffer, samples were mixed with an equal volume of sample loading buffer (10% glycerol, 1.34% SDS, 62 mM Tris–HCl pH 7.5, 0.17 M β-mercaptoethanol) and separated by SDS–PAGE at 4°C. Temperature stability of the complexes was tested by incubating 20 h assembly reactions for an additional 5 min at the temperatures indicated, in sample loading buffer before SDS–PAGE. Gels were stained with Coomassie Blue, scanned, and bands were quantitated by ImageQuant 5.0 (Molecular Dynamics, Sunnyvale, CA). The regression melting curve was calculated using StatView. Wild-type and mutant SNARE melting temperature regression curves had R2 values of 0.996 and 0.998, respectively. Western transfer of SNARE complexes were probed with antibodies against syntaxin (1:1000), synaptobrevin (1:750) and SNAP-25 (1:1000) to confirm the presence of each component in the SNARE complex. Controls lacking individual proteins failed to produce SNARE complexes and higher order complexes.
Electrophysiology
Electrophysiological procedures and physiological solutions are described in Stewart et al. (1994). In brief, wandering third instar larvae were dissected at room temperature in HL3 saline. For two-electrode voltage clamp recordings a holding potential of –80 mV was used. Each electrode was ∼15–20 MΩ when filled with 3 M KCl. Data were digitized at 10 kHz and low-pass filtered at 2 kHz for EJCs, or 600–800 Hz for mEJCs, with pClamp8 software (Axon Instruments, Foster City, CA). For mEJC analysis 1 min epochs of data were analyzed for amplitude and frequency using Clampfit (Axon Instruments, Foster City, CA). For recordings at restrictive temperature (37°C), larvae were dissected at room temperature, then immersed directly into the 37°C recording chamber and allowed to incubate for at least 5 min before recording. Comparisons of EJP amplitude and mEJP frequency were thus made between populations of responses recorded from independent larvae at the permissive and restrictive temperatures. The bath temperature was controlled by a Peltier device (Model PSMI) and temperature controller (Model TC-202A) from Medical Systems Research Products.
Immunocytochemistry
Third instar wandering larvae were dissected as described in Stewart et al. (1994), and fixed in non-alcoholic Bouin’s fluid. Preparations were stained with either 1:1000 rabbit anti-synaptotagmin or 1:100 rabbit anti-SNAP-25 diluted in PBST. For SNAP-25 staining, preparations were incubated with 1:500 dilution of a goat anti-rabbit Alexa Fluor 488 (Molecular Probes, Eugene, OR), rinsed and mounted with Vectashield (Vector Laboratories, Inc., Burlingame, CA), and viewed with a Nikon Eclipse E600-FN microscope (Nikon, Melville, NY) equipped with 40×, 0.8 NA water-immersion lens, 100 W mercury lamp and an FITC/GFP filter (Chroma Technology, Brattleboro, VT). Images from mutant and control animals were acquired with a cooled CCD SPOT2 camera using the SPOT software v2.2 (Diagnostic Instruments, Sterling Heights, MI) with identical camera settings. For synaptotagmin staining, preparations were incubated with biotinylated goat anti-rabbit IgG and processed using a Vectastain Elite ABC kit (Vector Laboratories, Inc.). We counted the number of anti-synaptotagmin stained boutons on muscle fibers 6 and 7 of the third abdominal segment (A3) in SNAP-25ts (n = 10) and red e controls (n = 8). For statistical comparison of bouton counts, 95% confidence intervals (CI) on each mean were calculated and the counts were considered significantly different when their CI did not overlap.
Electron microscopy
Third instar wandering larvae were dissected in HL3 saline as described above and incubated in HL3 saline at 22 or 37°C for at least 5 min before fixation. Dissected larvae were processed for electron microscopy as described (Jia et al., 1993) with the addition of 4–9% sucrose to the fixative. Tissue was embedded in EMbed (Electron Microscopy Sciences, Inc), and several series of transverse sections (50–150 sections/series, 75–100 nm thick) were collected.
Only type Ib boutons (Atwood et al., 1993) primarily from muscle fibers 6 and 7, and, occassionally, fibers 12 and 13, of A3 were used. NIH image software was used to measure the distance from the center of a vesicle to the presynaptic membrane. The number of docked vesicles observed in a single section through the T-bar is reported in this study. We counted the number of vesicles within a 200 nm radius of the center of the T-bar (defined as the point where the top of the bar intersects its base). The area of the sampled region was measured and the density was calculated as number of vesicles/µm2. Statistical significance was determined using a Mann–Whitney test where p <0.05 was considered significant.
Acknowledgments
Acknowledgements
We thank T.Schwarz for his support of the early phase of this work and M.Shuster and R.Burgess for help in mapping the SNAP-25 gene, L.Vosshall for sharing unpublished data on the n-syb promoter, T.Lloyd, M.Wu and H.Bellen for sending us the unpublished sequence of SNAP-25’s alternative exon 4, H.Bellen for sending synaptotagmin antiserum, L.Pallanck for advice on SNARE complexes assays and R.Ordway for sending us a manuscript prior to publication. We also thank R.Hoy, R.Harris-Warrick and E.Alani for comments on the manuscript. We dedicate this paper to the memory of Mika Salpeter, our friend and colleague. This work was supported by a grant from the American Heart Association (D.L.D.), NIH training grant 5T3GM07469 (I.V.), NIH NS09315 (M.M.S.), The Hospital for Sick Children Research Training Centre (B.A.S.) and grants from CIHR (G.L.B.).
References
- Atwood H.L., Govind,C.K. and Wu,C.F. (1993) Differential ultra structure of synaptic terminals on ventral longitudinal abdominal muscles in Drosophila larvae. J. Neurobiol., 24, 1008–1024. [DOI] [PubMed] [Google Scholar]
- Bezprozvanny I., Zhong,P., Scheller,R.H. and Tsien,R.W. (2000) Molecular determinants of the functional interaction between syntaxin and N-type Ca2+ channel gating. Proc. Natl Acad. Sci. USA, 97, 13943–13948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennwald P., Kearns,B., Champion,K., Keranen,S., Bankaitis,V. and Novick,P. (1994) Sec9 is a SNAP-25-like component of a yeast SNARE complex that may be the effector of Sec4 function in exocytosis. Cell, 79, 245–258. [DOI] [PubMed] [Google Scholar]
- Chen Y.A., Scales,S.J., Patel,S.M., Doung,Y.C. and Scheller,R.H. (1999) SNARE complex formation is triggered by Ca2+ and drives membrane fusion. Cell, 97, 165–174. [DOI] [PubMed] [Google Scholar]
- Deitcher D.L., Ueda,A., Stewart,B.A., Burgess,R.W., Kidokoro,Y. and Schwarz,T.L. (1998) Distinct requirements for evoked and spontaneous release of neurotransmitter are revealed by mutations in the Drosophila gene neuronal-synaptobrevin. J. Neurosci., 18, 2028–2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasshauer D., Otto,H., Eliason,W.K., Jahn,R., Brunger,A.T. (1997) Structural changes are associated with soluble N-ethylmaleimide-sensitive fusion protein attachment protein receptor complex formation. J. Biol. Chem., 272, 28036–28041 [DOI] [PubMed] [Google Scholar]
- Fasshauer D., Sutton,R.B., Brunger,A.T. and Jahn,R. (1998) Conserved structural features of the synaptic fusion complex: SNARE proteins reclassified as Q- and R-SNAREs. Proc. Natl Acad. Sci. USA, 95, 15781–15786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerona R.R., Larsen,E.C., Kowalchyk,J.A. and Martin,T.F. (2000) The C terminus of SNAP25 is essential for Ca2+-dependent binding of synaptotagmin to SNARE complexes. J. Biol. Chem., 275, 6328–6336. [DOI] [PubMed] [Google Scholar]
- Guex N. and Peitsch,M.C. (1997) SWISS-MODEL and the Swiss-PdbViewer: an environment for comparative protein modeling. Electrophoresis, 18, 2714–2723. [DOI] [PubMed] [Google Scholar]
- Harlow M.L., Ress,D.B., Stoschek,A., Marshall,R.M. and McMahan,U.J. (2001) The architecture of active zone material at the frog’s neuromuscular junction. Nature, 409, 479–484. [DOI] [PubMed] [Google Scholar]
- Hua Y. and Scheller,R.H. (2001) Three SNARE complexes cooperate to mediate membrane fusion. Proc. Natl Acad. Sci. USA, 98, 8065–8070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahn R. and Sudhof,T.C. (1999) Membrane fusion and exocytosis. Annu. Rev. Biochem., 68, 863–911. [DOI] [PubMed] [Google Scholar]
- Jia X.X., Gorczyca,M. and Budnik,V. (1993) Ultrastructure of neuromuscular junctions in Drosophila: comparison of wild type and mutants with increased excitability. J. Neurobiol., 24, 1025–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki F., Felling,R. and Ordway,R.W. (2000) A temperature-sensitive paralytic mutant defines a primary synaptic calcium channel in Drosophila. J. Neurosci., 20, 4885–4889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin R.C. and Scheller,R.H. (2000) Mechanisms of synaptic vesicle exocytosis. Annu. Rev. Cell Dev. Biol., 16, 19–49. [DOI] [PubMed] [Google Scholar]
- Littleton J.T. (2000) A genomic analysis of membrane trafficking and neurotransmitter release in Drosophila. J. Cell Biol., 150, F77–F82. [DOI] [PubMed] [Google Scholar]
- Littleton J.T., Chapman,E.R., Kreber,R., Garment,M.B., Carlson,S.D. and Ganetzky,B. (1998) Temperature-sensitive paralytic mutations demonstrate that synaptic exocytosis requires SNARE complex assembly and disassembly. Neuron, 21, 401–413. [DOI] [PubMed] [Google Scholar]
- Littleton J.T., Bai,J., Vyas,B., Desai,A.E., Garment,M.B., Carlson,S.D., Ganetzky,B. and Chapman,ER. (2001) Synaptotagmin mutants reveal essential functions for the C2B domain in Ca2+-triggered fusion and recycling of synaptic vesicles in vivo. J. Neurosci., 21, 1421–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lustgarten V. and Gerst,J.E. (1999) Yeast VSM1 encodes a v-SNARE binding protein that may act as a negative regulator of constitutive exocytosis. Mol. Cell. Biol., 19, 4480–4494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchant G.E. and Holm,D.G. (1988) Genetic analysis of the heterochromatin of chromosome 3 in Drosophila melanogaster. I. products of compound-autosome detachment. Genetics, 120, 503–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margittai M., Fasshauer,D., Pabst,S., Jahn,R. and Langen,R. (2001) Homo- and heterooligomeric SNARE complexes studied by site-directed spin labeling. J. Biol. Chem., 276, 13169–13177. [DOI] [PubMed] [Google Scholar]
- Mohtashami M., Stewart,B.A., Boulianne,G.L. and Trimble,W.S. (2001) Analysis of the mutant Drosophila N-ethylmaleimide sensitive fusion-1 protein in comatose reveals molecular correlates of the behavioural paralysis. J. Neurochem., 77, 1407–1417. [DOI] [PubMed] [Google Scholar]
- Montecucco C. and Schiavo,G. (1994) Mechanism of action of tetanus and botulinum neurotoxins. Mol. Microbiol., 13, 1–8. [DOI] [PubMed] [Google Scholar]
- Niemeyer B.A. and Schwarz,T.L. (2000) SNAP-24, a Drosophila SNAP-25 homologue on granule membranes, is a putative mediator of secretion and granule–granule fusion in salivary glands. J. Cell Sci., 113, 4055–4064. [DOI] [PubMed] [Google Scholar]
- Nonet M.L., Saifee,O., Zhao,H., Rand,J.B. and Wei,L. (1998) Synaptic transmission deficits in Caenorhabditis elegans synaptobrevin mutants. J. Neurosci., 18, 70–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pevsner J., Hsu,S.C., Braun,J.E., Calakos,N., Ting,A.E., Bennett,M.K. and Scheller,R.H. (1994) Specificity and regulation of a synaptic vesicle docking complex. Neuron, 13, 353–361. [DOI] [PubMed] [Google Scholar]
- Poirier M.A., Xiao,W., Macosko,J.C., Chan,C., Shin,Y.K. and Bennett,M.K. (1998) The synaptic SNARE complex is a parallel four-stranded helical bundle. Nature Struct. Biol., 5, 765–769. [DOI] [PubMed] [Google Scholar]
- Reist N.E., Buchanan,J., Li,J., DiAntonio,A., Buxton,E.M. and Schwarz,T.L. (1998) Morphologically docked synaptic vesicles are reduced in synaptotagmin mutants of Drosophila. J. Neurosci., 18, 7662–7673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renger J.J., Ueda,A., Atwood,H.L., Govind,C.K. and Wu,C.F. (2000) Role of cAMP cascade in synaptic stability and plasticity: ultrastructural and physiological analyses of individual synaptic boutons in Drosophila memory mutants. J. Neurosci., 20, 3980–3992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risinger C., Deitcher,D.L., Lundell,I., Schwarz,T.L. and Larhammar,D. (1997) Complex gene organization of synaptic protein SNAP-25 in Drosophila melanogaster. Gene, 194, 169–177. [DOI] [PubMed] [Google Scholar]
- Saifee O., Wei,L. and Nonet,M.L. (1998) The Caenorhabditis elegans unc-64 locus encodes a syntaxin that interacts genetically with synaptobrevin. Mol. Biol. Cell., 9, 1235–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze K.L., Broadie,K., Perin,M.S. and Bellen,H.J. (1995) Genetic and electrophysiological studies of Drosophila syntaxin-1A demonstrate its role in nonneuronal secretion and neurotransmission. Cell, 80, 311–320. [DOI] [PubMed] [Google Scholar]
- Sheng Z.H., Rettig,J., Cook,T. and Catterall,W.A. (1996) Calcium-dependent interaction of N-type calcium channels with the synaptic core complex. Nature, 379, 451–454. [DOI] [PubMed] [Google Scholar]
- Simpson L.L. (1978) Pharmacological studies on the subcellular site of action of botulinum toxin type A. J. Pharmacol. Exp. Ther., 206, 661–669. [PubMed] [Google Scholar]
- Sollner T., Bennett,M.K., Whiteheart,S.W., Scheller,R.H., Rothman,J.E. (1993) A protein assembly–disassembly pathway in vitro that may correspond to sequential steps of synaptic vesicle docking, activation, and fusion. Cell, 75, 409–418. [DOI] [PubMed] [Google Scholar]
- Steegmaier M., Yang,B., Yoo,J.S., Huang,B., Shen,M., Yu,S., Luo,Y. and Scheller,R.H. (1998) Three novel proteins of the syntaxin/SNAP-25 family. J. Biol. Chem., 273, 34171–34179. [DOI] [PubMed] [Google Scholar]
- Stewart B.A., Atwood,H.L., Renger,J.J., Wang,J. and Wu,C.F. (1994) Improved stability of Drosophila larval neuromuscular preparations in haemolymph-like physiological solutions. J. Comp. Physiol. A, 175, 179–191. [DOI] [PubMed] [Google Scholar]
- Stewart B.A., Mohtashami,M., Trimble,W.S. and Boulianne,G.L. (2000) SNARE proteins contribute to calcium cooperativity of synaptic transmission. Proc. Natl Acad. Sci. USA, 97, 13955–13960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton R.B., Fasshauer,D., Jahn,R. and Brunger,A.T. (1998) Crystal structure of a SNARE complex involved in synaptic exocytosis at 2.4 Å resolution. Nature, 395, 347–353. [DOI] [PubMed] [Google Scholar]
- Tolar L.A. and Pallanck,L. (1998) NSF function in neurotransmitter release involves rearrangement of the SNARE complex downstream of synaptic vesicle docking. J. Neurosci., 18, 10250–10256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson B.O., Vilinsky,I. and Deitcher,D. (2001) Generation of a semidominant mutation with temperature sensitive effects on both locomotion and phototransduction in Drosophila melanogaster. J. Neurogenet., in press. [DOI] [PubMed] [Google Scholar]
- Weber T, Zemelman,B.V., McNew,J.A., Westermann,B., Gmachl,M., Parlati,F., Sollner,T.H. and Rothman,J.E. (1998) SNAREpins: minimal machinery for membrane fusion. Cell, 92, 759–772. [DOI] [PubMed] [Google Scholar]
- Wei S., Xu,T., Ashery,U., Kollewe,A., Matti,U., Antonin,W., Rettig,J. and Neher,E. (2000) Exocytotic mechanism studied by truncated and zero layer mutants of the C-terminus of SNAP-25. EMBO J., 19, 1279–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weimbs T., Low,S.H., Chapin,S.J., Mostov,K.E., Bucher,P. and Hofmann,K. (1997) A conserved domain is present in different families of vesicular fusion proteins: a new superfamily. Proc. Natl Acad. Sci. USA, 94, 3046–3051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu T., Rammner,B., Margittai,M., Artalejo,A.R., Neher,E. and Jahn,R. (1999) Inhibition of SNARE complex assembly differentially affects kinetic components of exocytosis. Cell, 99, 713–722. [DOI] [PubMed] [Google Scholar]
- Yoshihara M., Ueda,A., Zhang,D., Deitcher,D.L., Schwarz,T.L. and Kidokoro,Y. (1999) Selective effects of neuronal-synaptobrevin mutations on transmitter release evoked by sustained versus transient Ca2+ increases and by cAMP. J. Neurosci., 19, 2432–2441. [DOI] [PMC free article] [PubMed] [Google Scholar]