Abstract
Ca2+-triggered exocytosis of synaptic vesicles is controlled by the Ca2+-binding protein synaptotagmin (syt) I. Fifteen additional isoforms of syt have been identified. Here, we compared the abilities of three syt isoforms (I, VII, and IX) to regulate soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE)-mediated membrane fusion in vitro in response to divalent cations. We found that different isoforms of syt couple distinct ranges of Ca2+, Ba2+, and Sr2+ to membrane fusion; syt VII was ∼400-fold more sensitive to Ca2+ than was syt I. Omission of phosphatidylserine (PS) from both populations of liposomes completely abrogated the ability of all three isoforms of syt to stimulate fusion. Mutations that selectively inhibit syt·target-SNARE (t-SNARE) interactions reduced syt stimulation of fusion. Using Sr2+ and Ba2+, we found that binding of syt to PS and t-SNAREs can be dissociated from activation of fusion, uncovering posteffector-binding functions for syt. Our data demonstrate that different syt isoforms are specialized to sense different ranges of divalent cations and that PS is an essential effector of Ca2+·syt action.
INTRODUCTION
Synaptotagmin (syt) I was identified as an abundant constituent of synaptic and large dense core vesicles (Matthew et al., 1981; Perin et al., 1990; Chapman and Jahn, 1994). Sequence analysis revealed that the cytoplasmic domain of syt I is largely composed of two conserved motifs, C2-domains, which were thought to confer Ca2+ and phosphatidylserine (PS) binding activity to “typical” isoforms of protein kinase C (Perin et al., 1990). Biochemical studies demonstrated that syt was in fact a Ca2+ and PS binding protein (Brose et al., 1992) and that these interactions were mediated by its tandem C2-domains, designated C2A and C2B (Davletov and Sudhof, 1993; Chapman and Jahn, 1994; Bai et al., 2002, 2004a). The Ca2+-binding activity of syt I prompted the idea that it may function as a Ca2+ sensor during regulated exocytosis (Brose et al., 1992). This idea has been addressed using a variety of approaches, ranging from acute interference to genetic manipulations (Augustine, 2001; Chapman, 2002; Koh and Bellen, 2003). Results from these studies were largely consistent with the idea that syt I may function as a Ca2+ sensor during exocytosis. However, in many cases, aspects of the syt I null phenotype are mimicked by changes in other proteins, many of which do not bind Ca2+ (Koh and Bellen, 2003).
An alternative approach to address the question of whether syt I is a Ca2+ sensor that regulates membrane fusion was based on a reconstituted fusion assay. Rothman and coworkers established a system to monitor soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE)-mediated membrane fusion (Weber et al., 1998; McNew et al., 1999; Nickel et al., 1999). The vesicular SNARE (v-SNARE) synaptobrevin (syb), and the target membrane SNAREs (t-SNAREs) syntaxin and SNAP-25, are reconstituted into distinct populations of proteoliposomes. When mixed, the cytoplasmic domains of the SNARE proteins assemble, in trans, into a four helix bundle (Sutton et al., 1998) that pulls the bilayers together, resulting in membrane fusion (Weber et al., 1998; McNew et al., 2000). Fusion is monitored via the dequenching of a fluorescence donor from a donor-acceptor pair incorporated into the bilayer of the v-SNARE proteoliposomes. Using this reduced system, the cytoplasmic domain of syt I (sometimes referred to as C2AB-I) was shown to markedly enhance the rate and extent of SNARE catalyzed membrane fusion in a strictly Ca2+-dependent manner; in the absence of Ca2+, C2AB-I slightly inhibited SNARE-mediated fusion (Tucker et al., 2004). Mutations that abolished the Ca2+-binding activity of C2AB-I completely disrupted Ca2+-triggered stimulation of fusion, demonstrating that in the reduced system, syt directly functions as a Ca2+ sensor for fusion.
Fifteen additional isoforms of syt have been identified in vertebrates (Craxton, 2004). One possibility for this variety of isoforms is that syts have diverged to sense distinct ranges of intracellular calcium concentration ([Ca2+]i), thus making it possible for different kinds of cells, or even distinct organelles within the same cell, to fuse with target membranes at different [Ca2+]i (Burgoyne and Morgan, 1998). For example, the threshold for exocytosis in goldfish retinal bipolar neurons is >20 μM Ca2+ (Heidelberger et al., 1994), whereas at the rat calyx of Held reliable release occurs at <1 μM Ca2+ (Bollmann et al., 2000; Schneggenburger and Neher, 2000). Here, we make use of the reconstitution assay to directly address the question of whether different isoforms of syt couple distinct ranges of [Ca2+] to membrane fusion. We focus on three isoforms of syt: I, VII, and IX. We note that syt IX (Fukuda et al., 2002b; Zhang et al., 2002; Tucker et al., 2003; Hui et al., 2005) is sometimes referred to as syt V (Craxton and Goedert, 1995; Hudson and Birnbaum, 1995).
A second goal of this study is to determine whether syts can couple divalent cations, other than Ca2+, to fusion. In many cases, Sr2+ and Ba2+ can replace Ca2+ as the trigger for exocytosis in neuronal and neuroendocrine cells (Tomsig and Suszkiw, 1996; Capogna et al., 1997; Xu-Friedman and Regehr, 2000; Kishimoto et al., 2001; Neves et al., 2001; Searl and Silinsky, 2002). Thus, if syts are general divalent cation sensors for secretion then at least some isoforms would be expected to couple these alternative cations to membrane fusion. Sr2+, in particular, has emerged as a useful tool to study synaptic transmission, as this divalent cation gives rise to release with altered kinetics (Goda and Stevens, 1994; Xu-Friedman and Regehr, 2000; Searl and Silinsky, 2002). As such, the molecular identity of the “Sr2+-sensor” has garnered considerable interest (Li et al., 1995a; Searl and Silinsky, 2002; Shin et al., 2003). Using this reduced system, it should be possible to determine directly whether specific isoforms of syt are indeed capable of coupling not only Ca2+ but also Sr2+ and Ba2+ to SNARE-catalyzed membrane fusion.
Finally, we take advantage of the reduced fusion assay to determine whether Ca2+·syt operates by interacting with anionic lipids and/or t-SNAREs. Loss-of-function syt mutants reveal that syts act, at least in part, by binding t-SNAREs. By omitting PS, we demonstrated that PS is an essential effector of Ca2+·syt action. In addition, using alterative divalent cations, we find that the binding of syt to both t-SNAREs and to PS can be dissociated from activation of the fusion complex. Thus, the action of syt on SNARE proteins and PS during fusion seems to involve an additional step that occurs after the initial binding reaction.
MATERIALS AND METHODS
Plasmids and Protein Purification
cDNA encoding rat syt I (Perin et al., 1990) was provided by T. C. Südhof (University of Texas Southwestern Medical Institute, Dallas, TX). cDNA encoding mouse syts VII and IX (Fukuda et al., 1999) was provided by M. Fukuda (Institute of Physical and Chemical Research, Saitama, Japan). Syt I C2AB (amino acids 96–421), syt VII C2AB (amino acids 134–403), and syt IX C2AB (amino acids 104–386) were subcloned into pTrc-His vector (Invitrogen, Carlsbad, CA), expressed in Escherichia coli, and purified as described previously (Chapman et al., 1996) with modifications. Briefly, bacterial extracts were mixed with Ni-NTA agarose (0.8 ml of a 50% slurry; QIAGEN, Valencia, CA) for 30 min at 4°C. Beads were washed two times in wash buffer (25 mM HEPES-KOH, 400 mM KCl, 10% glycerol, 20 mM imidazole, and 5 mM β-mercaptoethanol) plus 10 μg/ml DNase and RNase (Roche Diagnostics, Indianapolis, IN). Two more washes were carried out in the same buffer lacking nucleases. Proteins were eluted from the beads in wash buffer with 500 mM imidazole and dialyzed overnight against buffer A (25 mM HEPES-KOH, pH 7.4, 100 mM KCl, 10% glycerol, 1 mM dithiothreitol).
Syt linker mutants (Bai et al., 2004b), which have either two (2X-linker) or three (3X-linker) copies of the linker sequence between C2A and C2B (residues 264–272), and Ca2+ ligand mutants, C2AMB I and C2ABM I, in which the subscript M corresponds to mutations that disrupt Ca2+-binding to either C2A (D230,232N) or C2B (D363,365N) (Earles et al., 2001; Bai et al., 2002), were expressed and purified as glutathione S-transferase (GST) fusion proteins and cleaved from the GST tag using thrombin, as described previously (Tucker et al., 2003).
Plasmids to generate recombinant synaptobrevin 2 (also called vesicle-associated membrane protein [VAMP] II) (pTW2) and the t-SNARE heterodimer (syntaxin 1A and SNAP-25) (pTW34) were kindly provided by J. E. Rothman (Columbia University, New York, NY), and proteins were expressed and purified as described previously (Weber et al., 1998; Tucker et al., 2004). The cytoplasmic domain of synaptobrevin 2 (cd VAMP, amino acids 1–94) was purified as described previously (Tucker et al., 2004). Full-length GST-SNAP-25B and GST-syntaxin 1A were purified as described previously (Earles et al., 2001). The syt IX-c-myc construct used for human embryonic kidney (HEK)-293 cell transfection was generated by cloning full-length syt IX into the pcDNA 3.1/c-myc-His vector (Invitrogen), resulting in a tag at the C terminus.
Preparation of v-SNARE- and t-SNARE-reconstituted Vesicles
All lipids were obtained from Avanti Polar Lipids (Alabaster, AL). Reconstitution of v-SNARE and t-SNARE vesicles was carried out as described previously (Tucker et al., 2004). v-SNARE vesicles were reconstituted using a lipid mix composed of 82% 1-palmitoyl, 2-oleoyl phosphatidylcholine (PC), 15% 1,2-dioleoyl phosphatidylserine, 1.5% N-(7-nitro-2–1,3-benzoxadiazol-4-yl)-1,2-dipalmitoyl phosphatidylethanolamine (NBD-PE, donor), and 1.5% N-(lissamine rhodamine B sulfonyl)-1,2-dipalmitoyl phosphatidylethanolamine (Rhodamine-PE, acceptor). t-SNARE vesicles were reconstituted in 85% PC and 15% PS (mole/mole). When PS was omitted from the vesicles, PC was increased to 97 and 100% for v-SNARE and t-SNARE vesicles, respectively. v-SNARE and t-SNARE vesicles were reconstituted to give ∼100–150 copies or ∼80 copies per vesicle, respectively, as described previously (Tucker et al., 2004). At ∼80 copies/vesicle, the t-SNARE concentration in the fusion assay is ∼2.7–3 μM.
Fusion Assays
Fusion assays were conducted as described previously (Tucker et al., 2004). Briefly, each reaction consisted of 45 μl of purified t-SNARE vesicles and 5 μl of purified, NBD/Rhodamine-PE labeled v-SNARE vesicles along with 0.2 mM EGTA, the indicated concentrations of divalent cation and syt, and buffer A in a total volume of 75 μl. The v- and t-SNARE vesicles stocks had equal concentrations of phospholipids; hence, t-SNARE vesicles were added in excess. After 2 h, 0.5% n-dodecylmaltoside (Roche Diagnostics) was added to dequench the NBD fluorescence. The raw fluorescence was converted to rounds of fusion as described previously (Parlati et al., 1999; Tucker et al., 2004). [Divalent cation]free was calculated using WebMaxC version 2.22 software (C. Patton, Stanford University, Stanford, CA). Curve fitting (for Figure 1C) was carried out using Prism 4.02 software (GraphPad Software, San Diego, CA) with the variable slope sigmoidal dose-response function. For the syt VII/Ca2+ curve, data up to the plateau (at 100 μM Ca2+) was used for fitting to calculate EC50 values.
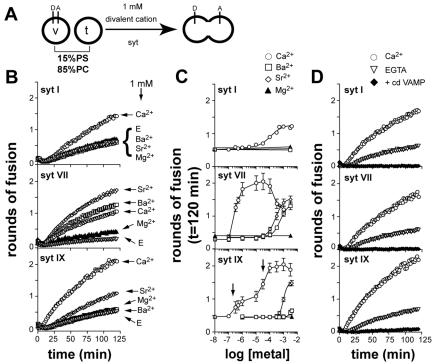
Figure 1.
Syt I, VII, and IX stimulate SNARE-mediated membrane fusion in response to divalent cations. (A) Illustration showing the different components of the in vitro fusion assay. Fusion of v-SNARE (v) vesicles, containing a donor (D) and acceptor (A) FRET pair, with unlabeled t-SNARE vesicles (t), results in an increase in donor fluorescence. The cytoplasmic domain of syt was assayed for its ability to stimulate fusion in the presence of different divalent cations. (B) Syt I, VII, and IX differentially stimulate fusion in response to divalent cations. The indicated syt isoform (7 μM) was incubated with v- and t-SNARE vesicles in the presence of 1 mM Ca2+ (open circles), Ba2+ (open squares), Sr2+ (open diamonds), or Mg2+ (closed triangles), or in the presence of 0.2 mM EGTA (open triangles). Rounds of fusion were plotted as a function of time. (C) Divalent cation dependency of syt I-, VII-, and IX-stimulated membrane fusion. v-SNARE and t-SNARE vesicles were incubated in the presence of the indicated syt isoforms (7 μM) and [divalent cation]. The total amount of fusion (t = 120 min) was plotted as a function of [divalent cation]. The [divalent cation]1/2 values are listed in Table 1. (D) Ca2+·syt-stimulated membrane fusion was efficiently blocked by cd VAMP. The indicated syt isoform (7 μM) was mixed with v- and t-SNARE vesicles in the presence of 0.2 mM EGTA (open triangles), 1 mM Ca2+ (open circles), or 1 mM Ca2+ plus cd VAMP (10 μM; closed diamonds). Rounds of fusion were plotted as a function of time.
Flotation Assays
Flotation assays were carried out as described previously (Tucker et al., 2004). For binding of syt cytoplasmic domains to protein-free vesicles shown in Figure 2C, 100-nm vesicles were prepared using an Avanti Polar lipids extruder according to the manufacturers instructions (Davis et al., 1999).
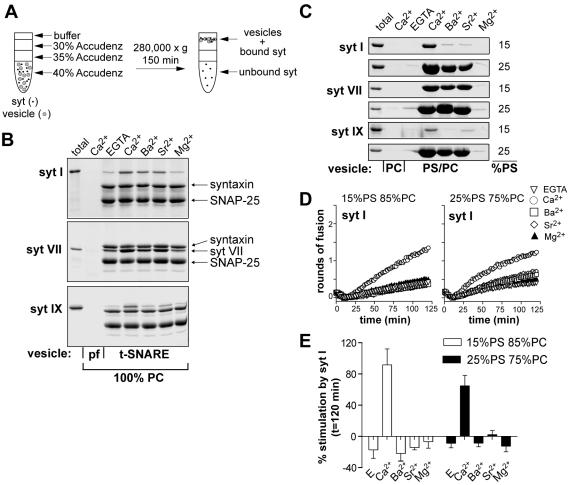
Figure 2.
Coflotation of syt I, VII, and IX with reconstituted t-SNARE vesicles and PS-harboring protein-free vesicles. (A) The cytoplasmic domain of syt was incubated with vesicles before mixing with the Accudenz density media. The mixture was overlaid with decreasing concentrations of Accudenz. After centrifugation, the vesicles floated to the 0/30% Accudenz interface along with any bound syt. Samples collected from the 0/30% Accudenz interface were analyzed by SDS-PAGE and stained with Coomassie Blue. Divalent cation (1 mM) was present throughout the gradient. Unbound syt remained in the bottom portion of the tube. (B) The indicated syt isoforms (10 μM) were incubated with t-SNARE vesicles (100% PC) in the presence of 1 mM [divalent cation] and applied to gradients as described in A. Syt can bind to these proteoliposomes via interactions with membrane embedded t-SNAREs. (C) The indicated syt isoforms (10 μM) were incubated with protein-free vesicles composed of either 15% PS and 85% PC or 25% PS and 75% PC in the presence of 1 mM [divalent cation] and applied to gradients as described in A. syt can bind to these liposomes via interactions with PS. (D) Syt I does not stimulate fusion in Ba2+ or Sr2+ even when PS is increased to 25%. Syt I (7 μM) was incubated with v- and t-SNARE vesicles harboring either 15% PS (left) or 25% PS (right), in the presence of 1 mM Ca2+ (open circles), Ba2+ (open squares), Sr2+ (open diamonds), Mg2+ (closed triangles), or in the presence of 0.2 mM EGTA (open triangles). Rounds of fusion were plotted as a function of time. (E) The amount of stimulation obtained after 2 h compared with control (-syt) was plotted for each condition. Samples contained either 0.2 mM EGTA or 1 mM divalent cation. Percent stimulation by syt was determined using the following equation: (fusion with syt - fusion without syt) × 100/fusion without syt. Error bars represent the SD (n = 4).
Immunoprecipitation Assays
Rat brain detergent extracts were prepared as described, and all incubations and binding reactions were carried out at 4°C (Chapman et al., 1995). One milligram of total protein extract was incubated with the indicated [divalent cation] for 1 h before the addition of 1.5 μl of the α-syntaxin 1A monoclonal antibody (mAb) HPC-1 (Barnstable et al., 1985). After incubating for an additional 2 h, 30 μl of protein-G Fast-Flow beads (of a 50% slurry; GE Healthcare, Piscataway, NJ) was added and incubated for another hour. Samples were then washed three times in buffer B (50 mM HEPES-NaOH, pH 7.4, 100 mM NaCl, 0.2 mM EGTA, and 1 mM [divalent cation]free). Bound protein was eluted from the beads by boiling in SDS sample buffer; 25% of the bound material was subjected to SDS-PAGE and immunoblot analysis. Native syt I was probed using mAb 41.1 (Chapman and Jahn, 1994) and syntaxin was probed using HPC-1. For all blots in this study, immunoreactive bands were detected using enhanced chemiluminescence; signals were quantified by densitometry and bar graphs depict averages from at least three independent experiments. Error bars represent the SD. Significance was determined using an unpaired student t test with a 95% confidence interval. HPC-1 and 41.1 were provided by R. Jahn (Max Planck Institute for Biophysical Chemistry, Gottingen, Germany).
Transfection of HEK-293 Cells and Coimmunoprecipitation
Twelve micrograms of syt IX-c-myc (p-CDNA3.1) and syntaxin 1A (p-IRES-GFP) were cotransfected into HEK-293 cells using calcium phosphate (Jordan and Wurm, 2004). Four plates (100 mm) of confluent cells were harvested and lysed 48–72 h posttransfection using 0.5% cholate and 1% Triton X-100. The protein concentration was determined using the BCA assay and bovine serum albumin as a standard (Pierce Chemical, Rockford, IL). Coimmunoprecipitation (CoIP) assays were carried out as described above with 75 μg of protein from the detergent extract and 1.5 μl of α-c-myc antibody (9E-10; obtained from Harlan Bioproducts for Science, Indianapolis, IN; Evan et al., 1985). Fifty percent of the bound material was subjected to SDS-PAGE and immunoblot analysis. Syntaxin was probed using HPC-1, and syt IX was detected using an α-c-myc antibody.
GST-Pull-Down Assays
GST-binding assays were carried out in a total volume of 150 μl using 15 μg of GST-syntaxin or GST-SNAP-25 immobilized on 25 μl of glutathione-Sepharose beads (GE Healthcare) and 1.5 μM cytoplasmic domain of syt (Earles et al., 2001). Binding reactions were carried out for 1 h at 4°C in buffer B and beads were washed three times. Five percent of the bound material was subjected to SDS-PAGE and immunoblot analysis. Syt I was detected using mAb 41.1. For experiments with the cytoplasmic domain of syt IX, 35% of the bound material was subjected to SDS-PAGE and syt IX was detected using a polyclonal antibody directed against the cytoplasmic domain of the protein (Tucker et al., 2003).
RESULTS
Divalent Cation Dependency of syt I-, VII-, and IX-regulated Membrane Fusion
In a recent study, we demonstrated that the cytoplasmic domain of syt I stimulated SNARE-mediated membrane fusion in the presence of Ca2+ (Tucker et al., 2004). Here, we asked whether cytoplasmic domains derived from other members of the syt family can also stimulate SNARE-mediated membrane fusion in response to Ca2+ (Figure 1A). For these studies, we compared syt I, VII, and IX, because these are the major isoforms expressed in PC12 cells, which are a common model system to study secretion (Kishimoto et al., 2001; Zhang et al., 2002; Tucker et al., 2003). In addition, we selected syt VII because it has been suggested to be a high-affinity Ca2+ sensor in comparison with syt I (Sugita et al., 2002, but see Li et al., 1995a,b). We note that all experiments reported in this study use the cytoplasmic domain of either syt I, VII, or IX, because the full-length proteins have a tendency to aggregate.
We found that the cytoplasmic domains of all three syt isoforms tested stimulated SNARE-mediated membrane fusion in response to Ca2+ (Figure 1B). The [syt] dependence for stimulation was nearly identical for each of the three isoforms tested, with maximal stimulation occurring at ∼7 μM protein (Supplemental Figure 1). Although different syt isoforms stimulated membrane fusion to different extents, it is clear that stimulation of SNARE-mediated fusion is not limited to syt I but rather extends to other members of the syt family.
In these experiments, syt I-stimulated membrane fusion with a [Ca2+]1/2 of 116 μM, in agreement with previous work (Tucker et al., 2004). In contrast, syt VII stimulated fusion with a [Ca2+]1/2 of 0.30 μM, which is roughly 400-fold lower than for syt I. The Ca2+ dose response for syt IX-stimulated membrane fusion was more complex, yielding two components with [Ca2+]1/2 values of 0.40 and 32 μM (Figure 1C and Table 1). The major conclusion drawn for these experiments is that the syt family is capable of sensing a wide range of Ca2+ concentrations. It should be noted that these [Ca2+]1/2 values were obtained using liposomes containing 15% PS. For syt I, increasing the PS to 25% significantly reduced the [Ca2+]1/2 for fusion by enhancing the ability of syt to bind Ca2+ (Brose et al., 1992; Tucker et al., 2004). Thus, all of the values reported here are likely to be modulated by changes in lipid composition.
Table 1.
EC50 of [divalent cation] stimulated membrane fusion
| Ca2+
|
Ba2+
|
Sr2+
|
||||
|---|---|---|---|---|---|---|
| syt | EC50 (μM) | Hill slope | EC50 (μM) | Hill slope | EC50 (μM) | Hill slope |
| syt I | 116 | 1.0 | n.a. | n.a. | n.a. | n.a. |
| syt VII | 0.30 | 2.0 | 563 | 1.5 | 260 | 1.5 |
| syt IX* | 1st 0.40 | 0.20 | n.a. | n.a. | 983 | 3.0 |
| 2nd 32 | 2.0 | |||||
n.a., not applicable.
The asterisk (*) indicates syt IX-stimulated fusion in Ca2+ yielded two inflection points denoted as 1st and 2nd (also see Figure 1).
The cytoplasmic domain of synaptobrevin 2 (cd VAMP) effectively blocked Ca2+·syt-stimulated membrane fusion for all isoforms tested, as shown previously for syt I (Tucker et al., 2004; Figure 1D). This suggests that trans pairing of SNAREs is required for syt-stimulated membrane fusion. Furthermore, the cytoplasmic domain of each syt isoform had no effect on NBD dequenching when either v- or t-SNAREs were omitted from the vesicles (Tucker et al., 2004; our unpublished observations), suggesting that both SNARE populations are required for syt action during fusion.
As outlined in Introduction, other alkaline metals such as Ba2+ and Sr2+ can substitute for Ca2+ in triggering release from numerous cell types, including PC12 cells (Kishimoto et al., 2001). One question that has arisen is whether syt I can couple these alternative metals to membrane fusion or whether other syt isoforms selectively serve as Sr2+ and Ba2+ sensors. We therefore assayed the ability of Sr2+ and Ba2+ to regulate the activity of syt I, VII, and IX in the reconstituted fusion assay (Figure 1, B and C, and Table 1). We found that Sr2+ activated only syt VII and IX ([Sr2+]1/2 values of 260 and 983 μM, respectively) and Ba2+ activated only syt VII in the fusion assay ([Ba2+]1/2 value of 563 μM). Thus, although all syt isoforms tested responded to Ca2+, only a subset of syts are capable of coupling Sr2+ and Ba2+ to membrane fusion under the conditions of our assay system. These data suggest that in PC12 cells, syt VII and IX might function as Sr2+ sensors and that syt VII might also function as a Ba2+ sensor.
In all cases, syt stimulation of SNARE-mediated membrane fusion was strictly regulated by Ca2+, Ba2+, and/or Sr2+; in the absence of these divalent cations, none of the syt isoforms stimulated fusion (Tucker et al., 2004). The slight increase in fusion seen in the presence of Mg2+ (Figure 1B) is within error of the assay, and raising the [Mg2+] from 10 nM to 3 mM (Figure 1C) did not result in significant increases in fusion. Furthermore, none of the divalent cations tested affected fusion in the absence of syt (our unpublished observations).
Divalent Cations Trigger Binding of syt to t-SNAREs and PS
Syt I binds anionic lipids and the t-SNAREs, syntaxin and SNAP-25, in a Ca2+-promoted manner (Zhang et al., 2002; Bai et al., 2004b). These interactions have been suggested to couple Ca2+ influx to regulated secretion, although the role of SNARE binding activity in Ca2+-regulated secretion has been questioned (Shin et al., 2003). We therefore carried out experiments to determine whether there is a correlation between stimulation of fusion and divalent cation-induced binding of syt to t-SNAREs and/or to anionic lipids.
A coflotation assay was used to monitor the binding of syt to t-SNARE liposomes (Figure 2A). This assay is unique because it makes it possible to measure binding of syt to t-SNAREs that are embedded into membranes rather than to t-SNAREs in detergent micelles or soluble t-SNARE fragments. In the first series of experiments, we assayed for binding of syt to t-SNARE liposomes composed of 100% PC; under these conditions (i.e., in the absence of PS) all of the proteoliposome binding activity is mediated by interactions with t-SNAREs and not membranes, because syt does not bind to PC (Tucker et al., 2004). We observed that all three divalent cations were able to trigger binding of syt I to t-SNAREs (Figure 2B), which is consistent with previous results (Chapman et al., 1995; Tucker et al., 2004, but see also Shin et al., 2003). For syt VII, Ca2+, Sr2+, and Ba2+ promoted binding to t-SNAREs and also stimulated membrane fusion. Syt IX exhibited strong t-SNARE binding activity in the presence of Ca2+; weaker, but significant levels of binding in response to Sr2+; and little binding in Ba2+, similar to the pattern of stimulation seen in the reduced fusion assay. Thus, in all cases where divalent cations stimulated fusion, they also triggered syt·t-SNARE interactions. However, in some cases, binding was not sufficient to regulate fusion (e.g., Ba2+/Sr2+, and syt I). We propose that syt operates by first binding to effectors and then driving subsequent conformational changes/rearrangements that result in the regulation of fusion. Apparently, some metals can drive binding but fail to drive the subsequent steps that regulate fusion.
Next, we examined the interaction of the cytoplasmic domains of syt I, VII, and IX with protein free PS-harboring liposomes in response to different divalent cations. Under these conditions, syt associates with the liposomes by virtue of its high-affinity interaction with PS (Tucker et al., 2004). Syt I bound protein-free vesicles containing 15% PS in the presence of Ca2+ but weakly in the presence of Ba2+ and Sr2+ (Figure 2C); these results correlate well with syt I stimulation of membrane fusion (Figure 1, B and C). Likewise, there was a tight correlation between stimulation of membrane fusion and the PS binding activity of syt VII and IX in response to each divalent cation. Specifically, syt VII stimulated membrane fusion in response to Ca2+, Ba2+, and Sr2+ and also bound to PS in the presence of all three divalent cations. Syt IX stimulated membrane fusion in the presence of Ca2+, weakly in response to Sr2+, and not at all in Ba2+—the same pattern of behavior as seen for lipid binding.
Binding of syt I to vesicles in response to Ba2+ and Sr2+ could be induced by increasing the mole fraction of PS from 15 to 25% (Figure 2C). However, reconstitution of v- and t-SNAREs into vesicles that harbored 25% PS did not result in syt I stimulation of membrane fusion in response to Ba2+ and Sr2+ (Figure 2, D and E). Thus, in at least some cases, Sr2+- and Ba2+-triggered binding of syt to PS, and to t-SNAREs (discussed above), can be uncoupled from membrane fusion. These data indicate that syt regulation of membrane fusion may involve additional divalent cation-induced activation steps that occur after binding of syt to these effector molecules.
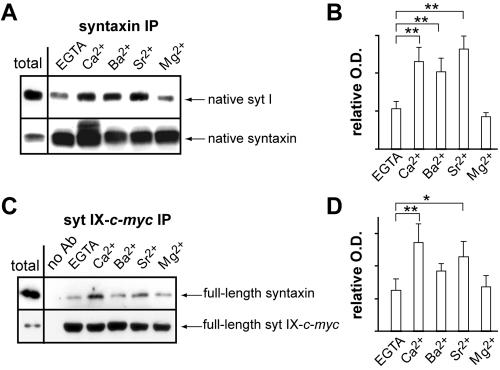
Recent studies suggested that syt I·t-SNARE interactions are not essential for the fast component of divalent cation regulated secretion. This was largely based on the observation that syt I did not bind t-SNAREs in response to Sr2+ (Shin et al., 2003), and native syt IX was reported not to coimmunoprecipitate (IP) with t-SNAREs in a Ca2+-dependent manner (Shin et al., 2004). However, we found that recombinant syt I and IX bind reconstituted t-SNAREs in the presence of Ca2+ and Sr2+, although syt IX bound t-SNAREs somewhat weakly in the presence of Sr2+ (Figure 2B). To clarify this issue, we performed coIP experiments from rat brain detergent extracts. Native syt I coIPed with the t-SNARE syntaxin to a small extent in EGTA; binding was significantly increased in response to Ca2+ as well as Ba2+ and Sr2+ (Figure 3, A and B). As a control, we probed for SNAP-25 in the immunoprecipitates and found the levels of SNAP-25 were unaffected by divalent cations (our unpublished observations). Because syt IX is expressed at >10-fold lower levels in rat brain than syt I (Chicka, Edwardson, Bhalla, and Chapman, unpublished observations), we cannot reliably detect syt IX in t-SNARE IPs. This observation might explain why Shin et al. (2004) failed to detect syt IX·t-SNARE interactions from rat brain. To further explore this issue, full-length syt IX was tagged with a c-myc epitope and coexpressed with full-length syntaxin in HEK-293 cells. CoIP experiments were then performed using an α-c-myc antibody. Low levels of syntaxin bound to syt IX in EGTA with a significant increase in binding in the presence of both Ca2+ (p < 0.05) and Sr2+ (p = 0.05; Figure 3, C and D). These data are in agreement with data obtained using recombinant proteins (Figure 2B). As a final confirmation, pull-down experiments were carried out using the cytoplasmic domain of syt and immobilized GST-tagged SNAP-25 and GST-tagged syntaxin. The pull-down data confirmed that syt I and IX bind t-SNAREs in a Ca2+, Ba2+, and Sr2+ promoted manner (Supplemental Figure 2) and are also in agreement with previous studies showing that in PC12 cell membranes, native syts I and IX interact with the other t-SNARE, SNAP-25, in a Ca2+-promoted manner (Zhang et al., 2002). Although there are some differences in the extent of binding, the interaction of syt I and IX with t-SNAREs was always stimulated by divalent cations that promote fusion.
Figure 3.
Full-length syt I and IX bind syntaxin in a divalent cation promoted manner. (A) Ca2+, Ba2+, and Sr2+, but not Mg2+, promote the coIP of native syt I with the t-SNARE syntaxin. Syntaxin was IPed from a rat brain detergent extract using HPC-1 as described in Materials and Methods in either 1 mM [divalent cation] or 0.2 mM EGTA. Samples were subjected to SDS-PAGE and immunoblot analysis using α-syt I (41.1) or α-syntaxin (HPC-1) antibodies. Total represents 3 μg of the rat brain extract; 25% of the IPed material was analyzed. (B) Bound syt I from A is quantified by densitometry. Error bars represent the SD (n = 5); **p < 0.05. (C) Full-length syntaxin and syt IX coIP in response to Ca2+ and Sr2+. syt IX-c-myc and syntaxin were cotransfected into HEK-293 cells. Detergent extracts from transfected cells were incubated with a α-c-myc antibody to IP syt IX. Samples (50% of the IPed material) were subjected to SDS-PAGE and immunoblot analysis using α-c-myc (syt IX) or α-syntaxin antibodies. Total represents 3 μg of the extract. (D) Syntaxin bound to syt IX (C) was quantified by densitometry. Error bars represent the SD (n = 3); **p < 0.05, *p = 0.05.
Syt Stimulates Membrane Fusion by Engaging t-SNAREs
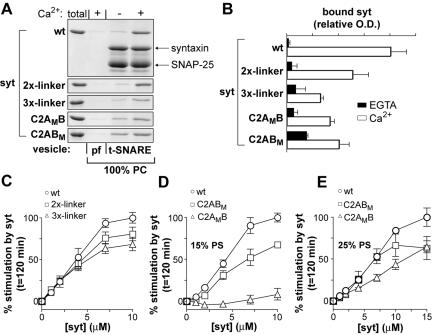
The data mentioned above suggest that t-SNARE binding is not sufficient for syt stimulation of membrane fusion. To address more directly whether syt·t-SNARE interactions do in fact regulate fusion, we determined whether mutations in syt I, that selectively disrupt t-SNARE binding activity, also disrupt stimulation in the reduced fusion assay. In a previous study, we showed that doubling or tripling the length of the linker that connects the C2A and C2B domains of syt I results in a graded reduction in Ca2+-dependent t-SNARE binding activity (Bai et al., 2004b). This is a selective loss-of-function mutation; increasing the linker length does not affect the interaction of syt I with anionic lipids (Bai et al., 2004b). Coflotation of the linker mutants with reconstituted t-SNAREs confirmed the loss of t-SNARE binding activity with increased linker length, although some degree of Ca2+-dependent binding persisted even with the 3X-linker construct (Figure 4, A and B) (Bai et al., 2004b).
Figure 4.
Role of Ca2+ and t-SNARE binding activity in syt regulation of fusion. (A) Increasing the length of the linker that connects the C2-domains of syt I results in graded reductions in Ca2+-triggered binding to membrane embedded t-SNAREs. Wt, linker mutants, and Ca2+-ligand mutant forms of syt I, (10 μM) were incubated with protein-free (pf; 100% PC) or t-SNARE vesicles (100% PC) in the presence (+) or absence (-; 0.2 mM EGTA) of 1 mM Ca2+ as described in Figure 2A. (B) Bound protein from A was quantified by densitometry (n = 3, error bars represent the SD). (C) Increasing the length of the linker between the C2A and C2B domain of syt reduces syt-stimulated membrane fusion. Wild-type and linker mutant forms of syt I (10 μM) were incubated with v- and t-SNARE vesicles in the presence of 1 mM Ca2+ (open symbols). Error bars represent the SD (n = 4). (D) Role of Ca2+ binding to the C2A and C2B domain of syt in syt-stimulated membrane fusion. Fusion assays were carried out as described in C using a mutant forms of syt I that fail to bind Ca2+ via the C2A (C2AMB) or C2B (C2ABM) domain. These experiments were carried out using liposomes that contain 15% PS. Error bars represent the SD (n = 3). (E) Fusion assays were carried out as described in D except that the amount of PS in both vesicle populations was increased to 25%. Error bars represent the SD (n = 3). In C–E, the data obtained in EGTA were omitted for clarity; in EGTA, slight inhibition of fusion by syt was observed in all experiments. Percent stimulation by syt was determined as defined in Figure 2E.
Increasing the length of the linker that connects the C2A and C2B domains of syt I resulted in a graded loss of Ca2+·syt stimulated SNARE-catalyzed membrane fusion (Figure 4C). For example, the extent of stimulation was reduced 32% for the 3X-linker mutant compared with wild-type (wt) syt I. These data indicate that reduction of t-SNARE binding activity correlates with a loss of Ca2+-dependent stimulation of fusion. Thus, syt regulates membrane fusion, at least in part, via interactions with t-SNAREs. In the absence of t-SNAREs or v-SNAREs, Ca2+·syt is without effect in the fusion assay (Tucker et al., 2004; our unpublished observations).
Disruption of the Ca2+-binding sites in both C2 domains of syt I abolished Ca2+-stimulated fusion of v- and t-SNARE vesicles (Tucker et al., 2004). However, it is not known whether Ca2+ binding to the C2A domain, C2B domain, or both domains is necessary for stimulation. We therefore analyzed two mutant versions of syt I; one in which the Ca2+-binding activity of C2A was disrupted (C2AMB), and one in which the Ca2+-binding activity of C2B was disrupted (C2ABM). These mutations reduce t-SNARE and PS/PC liposome binding activity (Figure 4, A and B; Earles et al., 2001). Disruption of Ca2+-binding to either C2A or C2B reduced the ability of syt to stimulate fusion (Figure 4D). Similar reductions in activity for the C2A and C2B mutants were observed when liposomes contained 25% PS and 15 μM syt was added to the reaction (Figure 4E). These data indicate that both C2-domains of syt I play roles in Ca2+ triggered, SNARE-mediated membrane fusion. However, at lower levels of PS (e.g., 15%; Figure 4D) and soluble syt I, the mutations in C2A had a greater effect than mutations in C2B (Figure 4, D compared with E).
PS Is Required for Syt Stimulation of Fusion
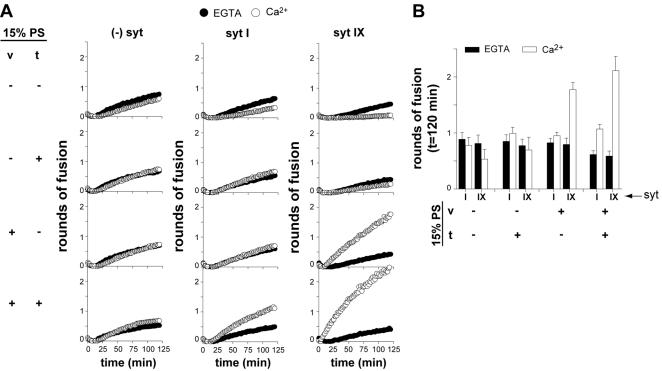
Finally, we addressed the role of syt·PS interactions during membrane fusion. We had previously shown that changes in the PS content of reconstituted v- and t-SNARE vesicles results in a shift in the Ca2+ dependence of syt-stimulated membrane fusion, indicating that syt executes its function via interactions with anionic lipids in addition to interactions with t-SNAREs (Tucker et al., 2004). Here, we asked whether PS is essential for Ca2+·syt-stimulated fusion by selectively omitting this lipid from the v- and/or t-SNARE vesicles. Removal of PS from both vesicle populations did not affect SNARE catalyzed fusion but abolished Ca2+·syt stimulation of fusion (Figure 5, A and B). Thus, PS is an essential effector for the action of Ca2+·syt.
Figure 5.
PS is an essential effector of Ca2+·syt action. (A) The indicated isoforms of syt (7 μM) were mixed with v-SNARE (v) and t-SNARE (t) vesicles in either 0.2 mM EGTA (open triangles) or 1 mM Ca2+ (open circles). Vesicles that harbored PS are indicated by (+) and vesicles that lacked PS are indicated by (-); all combinations were tested. Rounds of fusion were plotted as a function of time. (B) Data from A were quantified (n ≥4; error bars represent SD). Data obtained in the absence of syt were omitted for clarity. Total rounds of fusion at 120 min were plotted for each condition.
Removal of PS from the v-SNARE membranes also resulted in a loss of syt-stimulated membrane fusion. However, we observed that syt VII and IX, but not syt I, could tolerate removal of PS from the t-SNARE vesicles (Figure 5, A and B; our unpublished observations). Thus, PS is essential for the ability of all isoforms of syt studied here to stimulate membrane fusion. However, the precise localization of PS needed for syt activity differs among syt isoforms. Although the reason for these latter differences is unclear, these experiments nevertheless demonstrate that PS is an essential effector for Ca2+·syt action.
DISCUSSION
Recent studies have established that syt I is a Ca2+ sensor that regulates neuronal exocytosis (Augustine, 2001; Koh and Bellen, 2003; Tucker et al., 2004). Since the identification of syt I, 15 additional isoforms have been identified in vertebrates (Craxton, 2004). Thus, a major question that has emerged is: Why have so many distinct isoforms of syt evolved? One idea is that syts have diverged to sense distinct ranges of Ca2+. In the current study, we addressed this hypothesis using a reduced reconstituted system.
We found that syt I, VII, and IX are each able to directly couple Ca2+ to SNARE-catalyzed membrane fusion, but the [Ca2+]1/2 values for each isoform were markedly distinct (Table 1). For example, the [Ca2+]1/2 for syt VII (0.30 μM) was ∼400-fold lower than for syt I (116 μM). The Ca2+ dose response for syt IX was more complex, with two inflection points at 0.40 and 32 μM. These findings establish that syts have diverged to couple different ranges of [Ca2+] to membrane fusion.
We extended our study to include Sr2+ and Ba2+, because both of these divalent cations have been shown to substitute for Ca2+ as a trigger for exocytosis in a number of cell types (Tomsig and Suszkiw, 1996; Capogna et al., 1997; Xu-Friedman and Regehr, 2000; Kishimoto et al., 2001; Shin et al., 2003). The goal of these experiments was to determine whether Sr2+ and Ba2+ act via syt I or via distinct isoforms of the protein, to regulate fusion. Under the conditions of our in vitro fusion assay, syt VII was able to couple both Sr2+ and Ba2+ to membrane fusion, syt IX was able to couple Sr2+ but not Ba2+ to fusion, and syt I was unable to couple either Sr2+ or Ba2+ to fusion. These data suggest that syt I might not function as a major Sr2+ and Ba2+ sensor in neurons or neuroendocrine cells. The levels of Ba2+ and/or Sr2+ needed to stimulate fusion in the presence of syt VII and IX are in the high micromolar range, similar to the high micromolar concentrations of these metals needed to drive release from intact PC12 cells (Kishimoto et al., 2001). In addition, the range of [Ca2+] sensed by the syt isoforms tested here (EC50 values from 0.30 to 116 μM Ca2+) lie within the range of concentrations that drive membrane fusion in living cells—from low micromolar to ∼200 μM Ca2+, depending on the cell type under study (Thomas et al., 1993; Heidelberger et al., 1994; Heinemann et al., 1994; Rodriguez et al., 1997; Bollmann et al., 2000; Schneggenburger and Neher, 2000; Beutner et al., 2001; Kishimoto et al., 2001).
We observed that in all cases where different divalent cations are able to stimulate membrane fusion, stimulation correlated with binding to both t-SNARE proteins and to PS-harboring liposomes. However, in some cases, Ba2+ and Sr2+ were able to trigger binding of syt to these effectors (e.g., syt I) without stimulating fusion. Thus, under some conditions, binding does not always result in activation of the fusion reaction. These findings indicate that although binding is critical for syt function, there are postbinding steps that are essential to regulate fusion. Understanding these steps will require structural studies to reveal differences in syt-effector complexes in the presence of various divalent cations. However, we note that the initial open state of fusion pores might correspond to a SNARE-lined channel (Han et al., 2004) that subsequently dilates, resulting in complete membrane fusion. We speculate that the posteffector binding steps mediated by Ca2+ and syt might involve assembly of SNARE complexes (Chen et al., 1999) and opening of the fusion pore. Ca2+ and syt might then also function to drive lateral separation of SNARE proteins, or intercalation of lipids between SNARE subunits, to dilate the fusion pore (Bai et al., 2004b).
Direct support for the idea that Ca2+·syt regulates fusion via interactions with t-SNAREs was provided using mutant forms of syt. We have previously characterized a series of mutations in the linker of syt I that connects the tandem C2-domains. By lengthening this linker two- and threefold, we observed a graded reduction in t-SNARE binding activity, with no effect on syt·PS interactions (Bai et al., 2004b). When full-length syt-linker mutants were expressed in PC12 cells, they resulted in a graded reduction in catecholamine secretion and in the destabilization of fusion pores. Here, we observed that the syt I linker mutants reduced the ability of syt I to couple Ca2+ to fusion in our reduced fusion assay. We note that the effect of the linker mutations was less marked in the reconstituted system compared with PC12 cells (Figure 4C compared with Bai et al., 2004b). We suggest that this difference is due to differences in the rate limiting steps in these two systems; fusion in PC12 cells is much faster than in our minimal defined system. Thus, the smaller effect of the linker mutant in the slower, reduced system might be due to a rate-limiting step that is distinct from syt·t-SNARE interactions.
It has been reported that syt IX fails to bind to t-SNAREs in response to Ca2+ (Shin et al., 2004). Thus, if syt IX is important for driving exocytosis in some cell types (Fukuda et al., 2002b), it might do so without binding t-SNAREs. However, our data—using three independent assays— clearly demonstrate that Ca2+ indeed triggers efficient binding of syt IX to t-SNAREs. A plausible explanation for why binding was not observed by Shin et al. (2004) might be because syt IX is expressed at 10-fold lower abundance in brain (Chicka, Edwardson, Bhalla, and Chapman, unpublished observations) and therefore fell below the limits of detection in the previous study (Shin et al., 2004).
Direct support for the idea that Ca2+·syt regulates fusion via interactions with PS was provided by omitting PS from either the v- or t-SNARE liposomes in the reconstituted fusion assay. Complete removal of PS from both populations of liposomes did not affect SNARE-mediated fusion in the absence of syt. This was a somewhat surprising finding, because previous studies had indicated that syb interacts with PS in a manner that prevents SNARE complex assembly (Hu et al., 2002; Quetglas et al., 2002; Kweon et al., 2003). Thus, in our assay system, either PS does not affect the ability of syb to form SNARE complexes, or, the release of PS by syb is not rate limiting. The key finding here was that removal of PS completely abolished the ability of Ca2+·syt to stimulate fusion. These data unequivocally establish that PS is an essential effector of Ca2+·syt action. For syt I, activity required the presence of PS on both sides of the membranes destined to fuse, whereas for syt VII and IX PS was needed only in the v-SNARE membrane. The reasons for these differences are unclear, but may involve the distinct abilities of syt, or syt-oligomers (Fukuda et al., 2002a; Wu et al., 2003), to draw two bilayers together.
We do not yet know, on a quantitative basis, the relative contribution of syt·t-SNARE interactions in the fusion reaction. Unlike PS, we cannot simply remove SNARE proteins from one or both membranes, because under those conditions Ca2+·syt is without effect and no fusion is observed (Tucker et al., 2004). However, syt stimulation of membrane fusion was completely blocked by the cytoplasmic domains of both v-SNAREs and t-SNAREs, supporting the idea that Ca2+·syt acts through SNARE proteins (Figure 1D; Tucker et al., 2004). Furthermore, alterations in t-SNAREs alone (i.e., a truncation of SNAP-25 that mimics cleavage by botulinum neurotoxin A) shifted the Ca2+ sensitivity of the fusion reaction (without affecting syt·membrane interactions or syt-mediated aggregation of vesicles), further supporting the idea that syt acts, at least in part, via t-SNAREs (Tucker et al., 2004). Furthermore, the concentration of syt I needed to saturate the in vitro fusion reaction was directly related to the density of t-SNAREs used in the assay, again suggesting that syt operates by interacting with t-SNAREs (Tucker et al., 2004). The syt I linker mutant data discussed above (Figure 4, A–C) provide additional evidence for a role of syt·t-SNARE interactions in fusion.
Point mutations that disrupt the Ca2+-sensing ability of the C2A or C2B domain have been expressed in syt I null neurons and their effects on synaptic transmission determined. In some of these studies, it was concluded that the C2B domain plays a more important role in synaptic transmission than the C2A domain (Fernandez-Chacon et al., 2002; Mackler et al., 2002; Nishiki and Augustine, 2004). However, other studies suggest that, like C2B, C2A also plays a critical role in transmitter release from neurons (Fernandez-Chacon et al., 2001; Stevens and Sullivan, 2003) and PC12 cells (Wang et al., 2003). In addition, it should be noted that the readout of our fusion assay is distinct from electrophysiological measurements of exocytosis that rely on postsynaptic recordings. Indeed, recent studies indicate that the majority of release events in hippocampal neurons might not be associated with complete fusion and lipid bilayer mixing (Aravanis et al., 2003; Richards et al., 2005). Moreover, our simplified fusion assay lacks many of the additional regulatory components that are present in a cellular milieu.
In summary, our data suggest that syts act via a common mechanism that involves forming complexes with both t-SNAREs and with PS. Different isoforms of syt have diverged to couple distinct ranges of [Ca2+] to fusion, and some isoforms, but not others, are Sr2+ and Ba2+ sensors, as defined by functional criteria.
Supplementary Material
Acknowledgments
We thank J. M. Edwardson, M. Jackson, J. Weisshaar, and members of the Chapman laboratory for discussions and comments. This study was supported by National Institutes of Health Grants NIGMS GM-56827 and NIMH MH-61876 and the American Heart Association (AHA) Grant 0440168N (to E.R.C.). A. B. is supported by an AHA Predoctoral Fellowship. W.C.T. is supported by a postdoctoral National Research Service Award from the National Institutes of Health.
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E05-04-0277) on August 10, 2005.
Abbreviations used: IP, immunoprecipitation; PC, phosphatidylcholine; PS, phosphatidylserine; SNARE, soluble N-ethylmaleimide-sensitive factor attachment receptor; syt, synaptotagmin; VAMP, vesicle-associated membrane protein; cd VAMP; cytoplasmic domain of VAMP.
The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
References
- Aravanis, A. M., Pyle, J. L., and Tsien, R. W. (2003). Single synaptic vesicles fusing transiently and successively without loss of identity. Nature 423, 623-627. [DOI] [PubMed] [Google Scholar]
- Augustine, G. J. (2001). How does calcium trigger neurotransmitter release? Curr. Opin. Neurobiol. 11, 320-326. [DOI] [PubMed] [Google Scholar]
- Bai, J., Tucker, W. C., and Chapman, E. R. (2004a). PIP2 increases the speed-of-response of synaptotagmin and steers its membrane penetration activity toward the plasma membrane. Nat. Struct. Mol. Biol. 11, 36-44. [DOI] [PubMed] [Google Scholar]
- Bai, J., Wang, P., and Chapman, E. R. (2002). C2A activates a cryptic Ca2+-triggered membrane penetration activity within the C2B domain of synaptotagmin I. Proc. Natl. Acad. Sci. USA 99, 1665-1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai, J., Wang, C. T., Richards, D. A., Jackson, M. B., and Chapman, E. R. (2004b). Fusion pore dynamics are regulated by synaptotagmin*t-SNARE interactions. Neuron 41, 929-942. [DOI] [PubMed] [Google Scholar]
- Barnstable, C. J., Hofstein, R., and Akagawa, K. (1985). A marker of early amacrine cell development in rat retina. Brain Res. 352, 286-290. [DOI] [PubMed] [Google Scholar]
- Beutner, D., Voets, T., Neher, E., and Moser, T. (2001). Calcium dependence of exocytosis and endocytosis at the cochlear inner hair cell afferent synapse. Neuron 29, 681-690. [DOI] [PubMed] [Google Scholar]
- Bollmann, J. H., Sakmann, B., and Borst, J. G. (2000). Calcium sensitivity of glutamate release in a calyx-type terminal. Science 289, 953-957. [DOI] [PubMed] [Google Scholar]
- Brose, N., Petrenko, A. G., Sudhof, T. C., and Jahn, R. (1992). Synaptotagmin: a calcium sensor on the synaptic vesicle surface. Science 256, 1021-1025. [DOI] [PubMed] [Google Scholar]
- Burgoyne, R. D., and Morgan, A. (1998). Calcium sensors in regulated exocytosis. Cell Calcium 24, 367-376. [DOI] [PubMed] [Google Scholar]
- Capogna, M., McKinney, R. A., O'Connor, V., Gahwiler, B. H., and Thompson, S. M. (1997). Ca2+ or Sr2+ partially rescues synaptic transmission in hippocampal cultures treated with botulinum toxin A and C, but not tetanus toxin. J. Neurosci. 17, 7190-7202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman, E. R. (2002). Synaptotagmin: a Ca2+ sensor that triggers exocytosis? Nat. Rev. Mol. Cell. Biol. 3, 498-508. [DOI] [PubMed] [Google Scholar]
- Chapman, E. R., An, S., Edwardson, J. M., and Jahn, R. (1996). A novel function for the second C2 domain of synaptotagmin. Ca2+-triggered dimerization. J. Biol. Chem. 271, 5844-5849. [DOI] [PubMed] [Google Scholar]
- Chapman, E. R., Hanson, P. I., An, S., and Jahn, R. (1995). Ca2+ regulates the interaction between synaptotagmin and syntaxin 1. J. Biol. Chem. 270, 23667-23671. [DOI] [PubMed] [Google Scholar]
- Chapman, E. R., and Jahn, R. (1994). Calcium-dependent interaction of the cytoplasmic region of synaptotagmin with membranes. Autonomous function of a single C2-homologous domain. J. Biol. Chem. 269, 5735-5741. [PubMed] [Google Scholar]
- Chen, Y. A., Scales, S. J., Patel, S. M., Doung, Y. C., and Scheller, R. H. (1999). SNARE complex formation is triggered by Ca2+ and drives membrane fusion. Cell 97, 165-174. [DOI] [PubMed] [Google Scholar]
- Craxton, M. (2004). Synaptotagmin gene content of the sequenced genomes. BMC Genomics 5, 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craxton, M., and Goedert, M. (1995). Synaptotagmin V: a novel synaptotagmin isoform expressed in rat brain. FEBS Lett. 361, 196-200. [DOI] [PubMed] [Google Scholar]
- Davis, A. F., Bai, J., Fasshauer, D., Wolowick, M. J., Lewis, J. L., and Chapman, E. R. (1999). Kinetics of synaptotagmin responses to Ca2+ and assembly with the core SNARE complex onto membranes. Neuron 24, 363-376. [DOI] [PubMed] [Google Scholar]
- Davletov, B. A., and Sudhof, T. C. (1993). A single C2 domain from synaptotagmin I is sufficient for high affinity Ca2+/phospholipid binding. J. Biol. Chem. 268, 26386-26390. [PubMed] [Google Scholar]
- Earles, C. A., Bai, J., Wang, P., and Chapman, E. R. (2001). The tandem C2 domains of synaptotagmin contain redundant Ca2+ binding sites that cooperate to engage t-SNAREs and trigger exocytosis. J. Cell Biol. 154, 1117-1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evan, G. I., Lewis, G. K., Ramsay, G., and Bishop, J. M. (1985). Isolation of monoclonal antibodies specific for human c-myc proto-oncogene product. Mol. Cell. Biol. 5, 3610-3616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Chacon, R., Konigstorfer, A., Gerber, S. H., Garcia, J., Matos, M. F., Stevens, C. F., Brose, N., Rizo, J., Rosenmund, C., and Sudhof, T. C. (2001). Synaptotagmin I functions as a calcium regulator of release probability. Nature 410, 41-49. [DOI] [PubMed] [Google Scholar]
- Fernandez-Chacon, R., Shin, O. H., Konigstorfer, A., Matos, M. F., Meyer, A. C., Garcia, J., Gerber, S. H., Rizo, J., Sudhof, T. C., and Rosenmund, C. (2002). Structure/function analysis of Ca2+ binding to the C2A domain of synaptotagmin 1. J. Neurosci. 22, 8438-8446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda, M., Kanno, E., and Mikoshiba, K. (1999). Conserved N-terminal cysteine motif is essential for homo- and heterodimer formation of synaptotagmins III, V, VI, and X. J. Biol. Chem. 274, 31421-31427. [DOI] [PubMed] [Google Scholar]
- Fukuda, M., Katayama, E., and Mikoshiba, K. (2002a). The calcium-binding loops of the tandem C2 domains of synaptotagmin VII cooperatively mediate calcium-dependent oligomerization. J. Biol. Chem. 277, 29315-29320. [DOI] [PubMed] [Google Scholar]
- Fukuda, M., Kowalchyk, J. A., Zhang, X., Martin, T. F., and Mikoshiba, K. (2002b). Synaptotagmin IX regulates Ca2+-dependent secretion in PC12 cells. J. Biol. Chem. 277, 4601-4604. [DOI] [PubMed] [Google Scholar]
- Goda, Y., and Stevens, C. F. (1994). Two components of transmitter release at a central synapse. Proc. Natl. Acad. Sci. USA 91, 12942-12946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han, X., Wang, C. T., Bai, J., Chapman, E. R., and Jackson, M. B. (2004). Transmembrane segments of syntaxin line the fusion pore of Ca2+-triggered exocytosis. Science 304, 289-292. [DOI] [PubMed] [Google Scholar]
- Heidelberger, R., Heinemann, C., Neher, E., and Matthews, G. (1994). Calcium dependence of the rate of exocytosis in a synaptic terminal. Nature 371, 513-515. [DOI] [PubMed] [Google Scholar]
- Heinemann, C., Chow, R. H., Neher, E., and Zucker, R. S. (1994). Kinetics of the secretory response in bovine chromaffin cells following flash photolysis of caged Ca2+. Biophys. J. 67, 2546-2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu, K., Carroll, J., Fedorovich, S., Rickman, C., Sukhodub, A., and Davletov, B. (2002). Vesicular restriction of synaptobrevin suggests a role for calcium in membrane fusion. Nature 415, 646-650. [DOI] [PubMed] [Google Scholar]
- Hudson, A. W., and Birnbaum, M. J. (1995). Identification of a nonneuronal isoform of synaptotagmin. Proc. Natl. Acad. Sci. USA 92, 5895-5899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui, E., Bai, J., Wang, P., Sugimori, M., Llinas, R. R., and Chapman, E. R. (2005). Three distinct kinetic groupings of the synaptotagmin family: candidate sensors for rapid and delayed exocytosis. Proc. Natl. Acad. Sci. USA 102, 5210-5214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan, M., and Wurm, F. (2004). Transfection of adherent and suspended cells by calcium phosphate. Methods 33, 136-143. [DOI] [PubMed] [Google Scholar]
- Kishimoto, T., Liu, T. T., Ninomiya, Y., Takagi, H., Yoshioka, T., Ellis-Davies, G. C., Miyashita, Y., and Kasai, H. (2001). Ion selectivities of the Ca2+ sensors for exocytosis in rat phaeochromocytoma cells. J. Physiol. 533, 627-637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh, T. W., and Bellen, H. J. (2003). Synaptotagmin I, a Ca2+ sensor for neurotransmitter release. Trends. Neurosci. 26, 413-422. [DOI] [PubMed] [Google Scholar]
- Kweon, D. H., Kim, C. S., and Shin, Y. K. (2003). Regulation of neuronal SNARE assembly by the membrane. Nat. Struct. Biol. 10, 440-447. [DOI] [PubMed] [Google Scholar]
- Li, C., Davletov, B. A., and Sudhof, T. C. (1995a). Distinct Ca2+ and Sr2+ binding properties of synaptotagmins. Definition of candidate Ca2+ sensors for the fast and slow components of neurotransmitter release. J. Biol. Chem. 270, 24898-24902. [DOI] [PubMed] [Google Scholar]
- Li, C., Ullrich, B., Zhang, J. Z., Anderson, R. G., Brose, N., and Sudhof, T. C. (1995b). Ca2+-dependent and -independent activities of neural and non-neural synaptotagmins. Nature 375, 594-599. [DOI] [PubMed] [Google Scholar]
- Mackler, J. M., Drummond, J. A., Loewen, C. A., Robinson, I. M., and Reist, N. E. (2002). The C2B Ca2+-binding motif of synaptotagmin is required for synaptic transmission in vivo. Nature 418, 340-344. [DOI] [PubMed] [Google Scholar]
- Matthew, W. D., Tsavaler, L., and Reichardt, L. F. (1981). Identification of a synaptic vesicle-specific membrane protein with a wide distribution in neuronal and neurosecretory tissue. J. Cell Biol. 91, 257-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNew, J. A., Weber, T., Engelman, D. M., Sollner, T. H., and Rothman, J. E. (1999). The length of the flexible SNAREpin juxtamembrane region is a critical determinant of SNARE-dependent fusion. Mol. Cell 4, 415-421. [DOI] [PubMed] [Google Scholar]
- McNew, J. A., Weber, T., Parlati, F., Johnston, R. J., Melia, T. J., Sollner, T. H., and Rothman, J. E. (2000). Close is not enough: SNARE-dependent membrane fusion requires an active mechanism that transduces force to membrane anchors. J. Cell Biol. 150, 105-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neves, G., Neef, A., and Lagnado, L. (2001). The actions of barium and strontium on exocytosis and endocytosis in the synaptic terminal of goldfish bipolar cells. J. Physiol. 535, 809-824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickel, W., Weber, T., McNew, J. A., Parlati, F., Sollner, T. H., and Rothman, J. E. (1999). Content mixing and membrane integrity during membrane fusion driven by pairing of isolated v-SNAREs and t-SNAREs. Proc. Natl. Acad. Sci. USA 96, 12571-12576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiki, T., and Augustine, G. J. (2004). Dual roles of the C2B domain of synaptotagmin I in synchronizing Ca2+-dependent neurotransmitter release. J. Neurosci. 24, 8542-8550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parlati, F., Weber, T., McNew, J. A., Westermann, B., Sollner, T. H., and Rothman, J. E. (1999). Rapid and efficient fusion of phospholipid vesicles by the alpha-helical core of a SNARE complex in the absence of an N-terminal regulatory domain. Proc. Natl. Acad. Sci. USA 96, 12565-12570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perin, M. S., Fried, V. A., Mignery, G. A., Jahn, R., and Sudhof, T. C. (1990). Phospholipid binding by a synaptic vesicle protein homologous to the regulatory region of PKC. Nature 345, 260-263. [DOI] [PubMed] [Google Scholar]
- Quetglas, S., Iborra, C., Sasakawa, N., De Haro, L., Kumakura, K., Sato, K., Leveque, C., and Seagar, M. (2002). Calmodulin and lipid binding to synaptobrevin regulates calcium-dependent exocytosis. EMBO J. 21, 3970-3979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards, D. A., Bai, J., and Chapman, E. R. (2005). Two modes of exocytosis at hippocampal synapses revealed by rate of FM1–43 efflux from individual vesicles. J. Cell Biol. 168, 929-939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez, A., Webster, P., Ortego, J., and Andrews, N. W. (1997). Lysosomes behave as Ca2+-regulated exocytic vesicles in fibroblasts and epithelial cells. J. Cell Biol. 137, 93-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneggenburger, R., and Neher, E. (2000). Intracellular calcium dependence of transmitter release rates at a fast central synapse. Nature 406, 889-893. [DOI] [PubMed] [Google Scholar]
- Searl, T. J., and Silinsky, E. M. (2002). Evidence for two distinct processes in the final stages of neurotransmitter release as detected by binomial analysis in calcium and strontium solutions. J. Physiol. 539, 693-705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin, O. H., Maximov, A., Lim, B. K., Rizo, J., and Sudhof, T. C. (2004). Unexpected Ca2+-binding properties of synaptotagmin 9. Proc. Natl. Acad. Sci. USA 101, 2554-2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin, O. H., Rhee, J. S., Tang, J., Sugita, S., Rosenmund, C., and Sudhof, T. C. (2003). Sr2+ binding to the Ca2+ binding site of the synaptotagmin 1 C2B domain triggers fast exocytosis without stimulating SNARE interactions. Neuron 37, 99-108. [DOI] [PubMed] [Google Scholar]
- Stevens, C. F., and Sullivan, J. M. (2003). The synaptotagmin C2A domain is part of the calcium sensor controlling fast synaptic transmission. Neuron 39, 299-308. [DOI] [PubMed] [Google Scholar]
- Sugita, S., Shin, O. H., Han, W., Lao, Y., and Sudhof, T. C. (2002). Synaptotagmins form a hierarchy of exocytotic Ca2+ sensors with distinct Ca2+ affinities. EMBO J. 21, 270-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton, R. B., Fasshauer, D., Jahn, R., and Brunger, A. T. (1998). Crystal structure of a SNARE complex involved in synaptic exocytosis at 2.4 A resolution. Nature 395, 347-353. [DOI] [PubMed] [Google Scholar]
- Thomas, P., Wong, J. G., Lee, A. K., and Almers, W. (1993). A low affinity Ca2+ receptor controls the final steps in peptide secretion from pituitary melanotrophs. Neuron 11, 93-104. [DOI] [PubMed] [Google Scholar]
- Tomsig, J. L., and Suszkiw, J. B. (1996). Metal selectivity of exocytosis in alpha-toxin-permeabilized bovine chromaffin cells. J. Neurochem. 66, 644-650. [DOI] [PubMed] [Google Scholar]
- Tucker, W. C., Edwardson, J. M., Bai, J., Kim, H. J., Martin, T. F., and Chapman, E. R. (2003). Identification of synaptotagmin effectors via acute inhibition of secretion from cracked PC12 cells. J. Cell Biol. 162, 199-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker, W. C., Weber, T., and Chapman, E. R. (2004). Reconstitution of Ca2+-regulated membrane fusion by synaptotagmin and SNAREs. Science 304, 435-438. [DOI] [PubMed] [Google Scholar]
- Wang, P., Wang, C. T., Bai, J., Jackson, M. B., and Chapman, E. R. (2003). Mutations in the effector binding loops in the C2A and C2B domains of synaptotagmin I disrupt exocytosis in a non-additive manner. J. Biol. Chem. 278, 47030-47037. [DOI] [PubMed] [Google Scholar]
- Weber, T., Zemelman, B. V., McNew, J. A., Westermann, B., Gmachl, M., Parlati, F., Sollner, T. H., and Rothman, J. E. (1998). SNAREpins: minimal machinery for membrane fusion. Cell 92, 759-772. [DOI] [PubMed] [Google Scholar]
- Wu, Y., He, Y., Bai, J., Ji, S. R., Tucker, W. C., Chapman, E. R., and Sui, S. F. (2003). Visualization of synaptotagmin I oligomers assembled onto lipid monolayers. Proc. Natl. Acad. Sci. USA 100, 2082-2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu-Friedman, M. A., and Regehr, W. G. (2000). Probing fundamental aspects of synaptic transmission with strontium. J. Neurosci. 20, 4414-4422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, X., Kim-Miller, M. J., Fukuda, M., Kowalchyk, J. A., and Martin, T. F. (2002). Ca2+-dependent synaptotagmin binding to SNAP-25 is essential for Ca2+-triggered exocytosis. Neuron 34, 599-611. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.