Abstract
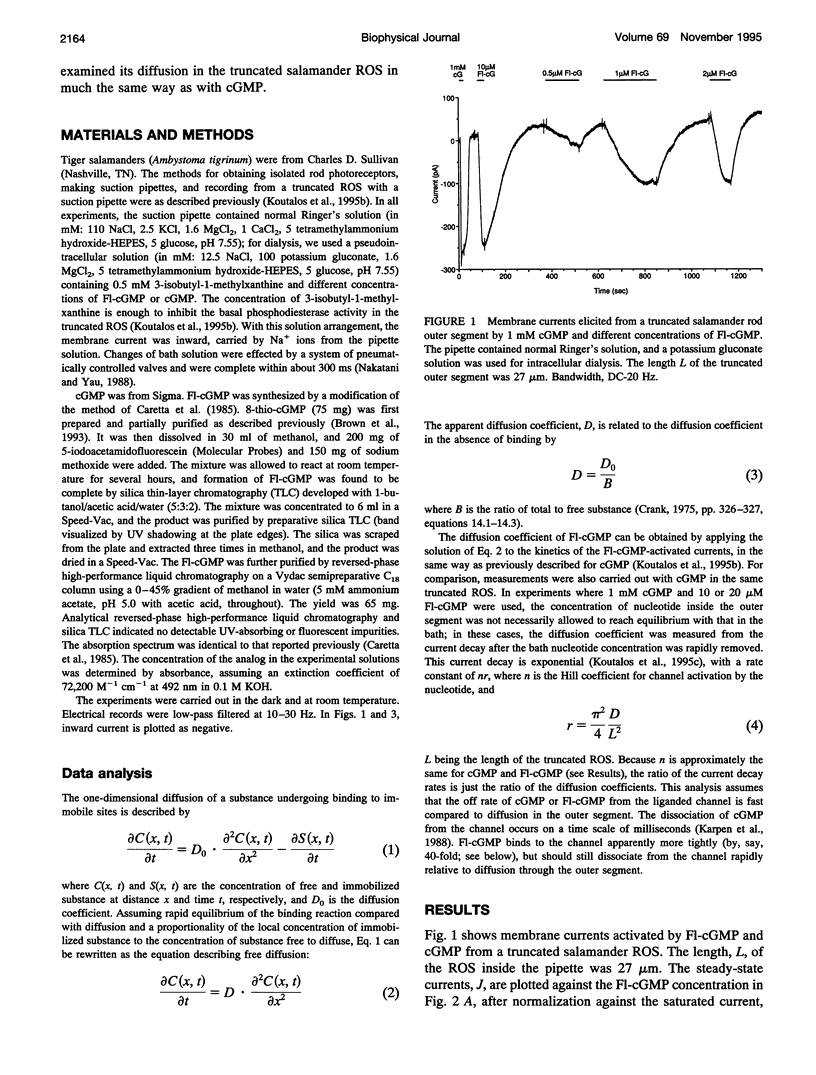
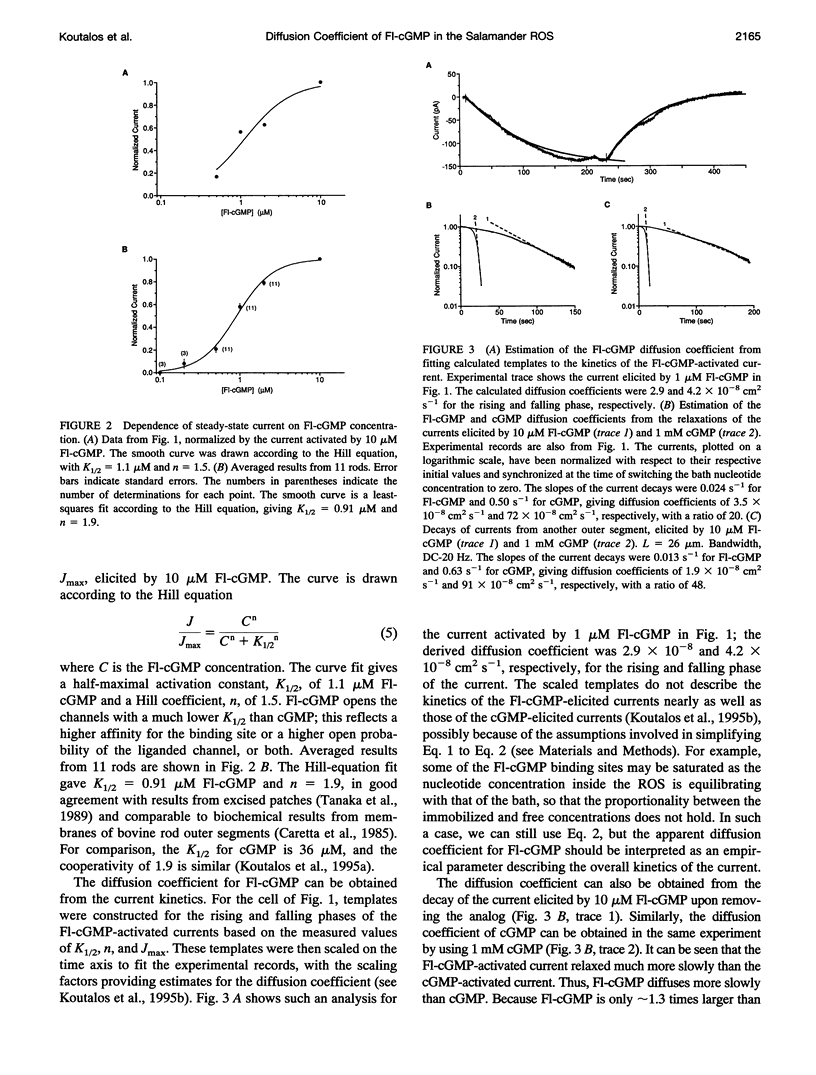
Cyclic GMP (cGMP) is the intracellular messenger mediating phototransduction in retinal rods, with its longitudinal diffusion in the rod outer segment (ROS) likely to be a factor in determining light sensitivity. From the kinetics of cGMP-activated currents in the truncated ROS of the salamander (Ambystoma tigrinum), the cGMP diffusion coefficient was previously estimated to be approximately 60 x 10(-8) cm2 s-1. On the other hand, fluorescence measurements in intact salamander ROS using 8-(fluoresceinyl)thioguanosine 3',5'-cyclic monophosphate (Fl-cGMP) led to a diffusion coefficient for this compound of 1 x 10(-8) cm2 s-1; after corrections for differences in size and in binding to cellular components between cGMP and Fl-cGMP, this gave an upper limit of 11 x 10(-8) cm2 s-1 for the cGMP diffusion coefficient. To properly compare the two sets of measurements, we have examined the diffusion of Fl-cGMP in the truncated ROS. From the kinetics of Fl-cGMP-activated currents, we have obtained a diffusion coefficient of 3 x 10(-8) cm2 s-1 for this analog; the cGMP diffusion coefficient measured from the same truncated ROSs was approximately 80 x 10(-8) cm2 s-1. Thus, a factor of 27 appears appropriate for correcting differences in size and intracellular binding between cGMP and Fl-cGMP. Application of this correction factor to the Fl-cGMP diffusion coefficient measurements by Olson and Pugh (1993) gives a cGMP diffusion coefficient of approximately 30 x 10(-8) cm2 s-1, in reasonable agreement with the value measured from the truncated ROS.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOWEN W. J., MARTIN H. L. THE DIFFUSION OF ADENOSINE TRIPHOSPHATE THROUGH AQUEOUS SOLUTIONS. Arch Biochem Biophys. 1964 Jul;107:30–36. doi: 10.1016/0003-9861(64)90265-6. [DOI] [PubMed] [Google Scholar]
- Baylor D. A., Lamb T. D., Yau K. W. Responses of retinal rods to single photons. J Physiol. 1979 Mar;288:613–634. [PMC free article] [PubMed] [Google Scholar]
- Blaurock A. E., Wilkins M. H. Structure of frog photoreceptor membranes. Nature. 1969 Aug 30;223(5209):906–909. doi: 10.1038/223906a0. [DOI] [PubMed] [Google Scholar]
- Brown R. L., Bert R. J., Evans F. E., Karpen J. W. Activation of retinal rod cGMP-gated channels: what makes for an effective 8-substituted derivative of cGMP? Biochemistry. 1993 Sep 28;32(38):10089–10095. doi: 10.1021/bi00089a026. [DOI] [PubMed] [Google Scholar]
- Caillé J. P., Hinke J. A. The volume available to diffusion in the muscle fiber. Can J Physiol Pharmacol. 1974 Aug;52(4):814–828. doi: 10.1139/y74-107. [DOI] [PubMed] [Google Scholar]
- Caretta A., Cavaggioni A., Sorbi R. T. Binding stoichiometry of a fluorescent cGMP analogue to membranes of retinal rod outer segments. Eur J Biochem. 1985 Nov 15;153(1):49–53. doi: 10.1111/j.1432-1033.1985.tb09265.x. [DOI] [PubMed] [Google Scholar]
- Detwiler P. B., Gray-Keller M. P. Some unresolved issues in the physiology and biochemistry of phototransduction. Curr Opin Neurobiol. 1992 Aug;2(4):433–438. doi: 10.1016/0959-4388(92)90176-l. [DOI] [PubMed] [Google Scholar]
- Kao H. P., Abney J. R., Verkman A. S. Determinants of the translational mobility of a small solute in cell cytoplasm. J Cell Biol. 1993 Jan;120(1):175–184. doi: 10.1083/jcb.120.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpen J. W., Zimmerman A. L., Stryer L., Baylor D. A. Gating kinetics of the cyclic-GMP-activated channel of retinal rods: flash photolysis and voltage-jump studies. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1287–1291. doi: 10.1073/pnas.85.4.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korenbrot J. I., Brown D. T., Cone R. A. Membrane characteristics and osmotic behavior of isolated rod outer segments. J Cell Biol. 1973 Feb;56(2):389–398. doi: 10.1083/jcb.56.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koutalos Y., Nakatani K., Yau K. W. Cyclic GMP diffusion coefficient in rod photoreceptor outer segments. Biophys J. 1995 Jan;68(1):373–382. doi: 10.1016/S0006-3495(95)80198-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koutalos Y., Yau K. W. A rich complexity emerges in phototransduction. Curr Opin Neurobiol. 1993 Aug;3(4):513–519. doi: 10.1016/0959-4388(93)90049-5. [DOI] [PubMed] [Google Scholar]
- Kushmerick M. J., Podolsky R. J. Ionic mobility in muscle cells. Science. 1969 Dec 5;166(3910):1297–1298. doi: 10.1126/science.166.3910.1297. [DOI] [PubMed] [Google Scholar]
- Lagnado L., Baylor D. Signal flow in visual transduction. Neuron. 1992 Jun;8(6):995–1002. doi: 10.1016/0896-6273(92)90122-t. [DOI] [PubMed] [Google Scholar]
- Lamb T. D., McNaughton P. A., Yau K. W. Spatial spread of activation and background desensitization in toad rod outer segments. J Physiol. 1981;319:463–496. doi: 10.1113/jphysiol.1981.sp013921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatani K., Yau K. W. Calcium and magnesium fluxes across the plasma membrane of the toad rod outer segment. J Physiol. 1988 Jan;395:695–729. doi: 10.1113/jphysiol.1988.sp016942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson A., Pugh E. N., Jr Diffusion coefficient of cyclic GMP in salamander rod outer segments estimated with two fluorescent probes. Biophys J. 1993 Sep;65(3):1335–1352. doi: 10.1016/S0006-3495(93)81177-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugh E. N., Jr, Lamb T. D. Amplification and kinetics of the activation steps in phototransduction. Biochim Biophys Acta. 1993 Mar 1;1141(2-3):111–149. doi: 10.1016/0005-2728(93)90038-h. [DOI] [PubMed] [Google Scholar]
- Rosenkranz J. New aspects of the ultrastructure of frog rod outer segments. Int Rev Cytol. 1977;50:25–158. doi: 10.1016/s0074-7696(08)60098-4. [DOI] [PubMed] [Google Scholar]
- SIDMAN R. L. The structure and concentration of solids in photoreceptor cells studied by refractometry and interference microscopy. J Biophys Biochem Cytol. 1957 Jan 25;3(1):15–30. doi: 10.1083/jcb.3.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka J. C., Eccleston J. F., Furman R. E. Photoreceptor channel activation by nucleotide derivatives. Biochemistry. 1989 Apr 4;28(7):2776–2784. doi: 10.1021/bi00433a006. [DOI] [PubMed] [Google Scholar]
- Yarfitz S., Hurley J. B. Transduction mechanisms of vertebrate and invertebrate photoreceptors. J Biol Chem. 1994 May 20;269(20):14329–14332. [PubMed] [Google Scholar]
- Yau K. W. Phototransduction mechanism in retinal rods and cones. The Friedenwald Lecture. Invest Ophthalmol Vis Sci. 1994 Jan;35(1):9–32. [PubMed] [Google Scholar]


