Soon after the widespread availability of computerized x-ray tomography (CT) in clinical medicine in the 1970s, reports began to appear of the changes in the brain in patients with Alzheimer's disease (AD). Atrophy of brain tissue was a common finding, but a critical review in 1990 concluded that “at present there is little definite evidence for clear anatomic brain changes that accurately predict the cognitive dysfunction within a group of patients suffering with AD” (1). Since then, convincing evidence that selective atrophy of particular brain regions is highly correlated with cognitive deficits characteristic of AD has been obtained. What has caused this revolution? First, it has been recognized that the diagnosis of AD was not sufficiently accurate in many early neuroimaging studies. Second, until pioneering studies from New York University (2), most early work did not examine the medial temporal lobe (MTL), the part of the brain with the highest density of AD histopathological markers (amyloid plaques and neurofibrillary tangles) (Fig. 1). In 1992 a CT study of patients with AD, whose diagnosis was later confirmed by histopathology, showed marked atrophy of the MTL (3). Third, there were few longitudinal studies to reveal changes in the same patients over time. One of these, however, showed rapid enlargement of the fluid-filled ventricles in the brain in patients with AD (4). Enlargement of the ventricles is usually caused by loss of brain tissue. A dramatic loss of tissue from the MTL, part of which shrunk at a rate of 15% per year, was found in serial CT studies on patients with AD (5). Because the MTL in controls only shrunk at one-tenth of this rate, it was concluded that AD cannot be the result of an acceleration of normal aging, but must be the consequence of a disease process. The article by Scahill et al. (6) in this issue of PNAS takes the story into a new chapter by using serial MRI scans in patients with AD to show how the disease process, revealed by regional atrophy, spreads in a highly specific way from the MTL to other parts of the brain.
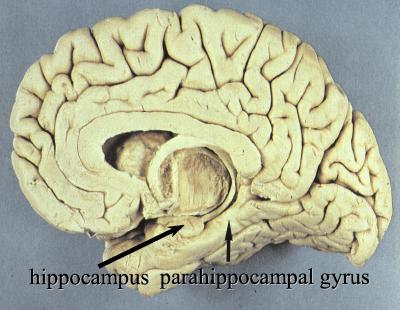
Figure 1.
Photograph of the median aspect of the human brain (front to left) showing two parts of the MTL, the hippocampus, and parahippocampal gyrus. The third part is the amygdala.
How do we know that the atrophy revealed by the structural neuroimaging reflects the progression of the disease process? AD is defined by its histopathology, so it might be assumed that the only way to track the spread of pathology would be devise a way of revealing the plaques and tangles in the living brain by neuroimaging. However, the clinical symptoms are not directly caused by the deposition of amyloid or the formation of intracellular tangles, but rather by the loss of neurons and, in particular, loss of their connections with other neurons made through synapses (7, 8).
We can follow a trail back from the symptoms, through regional atrophy, through loss of neurons and fibers, and finally to the histopathological markers of AD. Cognitive deficits, in particular of memory, are associated with atrophy of the MTL in AD as revealed by CT (9) or MRI (10). This association is specific because deficits in verbal memory correlate with atrophy of the left hippocampus and deficits in the nonverbal memory correlate with atrophy of the right hippocampus (11, 12). Atrophy of brain tissue can be caused by shrinkage or death of neurons, loss of the neuropil (the axons and dendrites of neurons), or shrinkage of tracts of nerve fibers. All of these have been implicated in the atrophy of the MTL that occurs in AD. The numbers are striking: the cornu Ammonis of one hippocampus in the normal elderly occupies about 1.5 ml and contains some 9 million neurons but in end-stage AD the volume has shrunk by 66% and the number of neurons has dropped by 84% (13). The MTL atrophy revealed by CT (9) and MRI (13–15) not only correlates with progression of the disease and the progressive loss of neurons but also with the increasing numbers of tangles. However, until the end stage of the disease, only a small proportion of neurons contains tangles, and the number of neurons that have died far exceeds the number containing tangles (14, 16). Thus, although the density of tangles correlates with cognitive deficits in many studies, it is very likely that this correlation reflects the relationship between tangle deposition and neuronal loss in the vicinity of the tangle-bearing neurons. We can say, therefore, that atrophy of a brain region probably does reflect the classical histopathology of AD but that the atrophy is a better marker for damage to the functioning of a brain region than the deposits of insoluble proteins in plaques and tangles.
The idea that the pathological changes in AD spread through the cerebral cortex during the course of the disease was clearly expressed in 1985 in a seminal article in this journal (17). These authors noticed that the tangles were not randomly distributed in neurons within a cortical area but were selectively deposited within the pyramidal neurons that give rise to pathways that connect an area with other cortical areas and/or with subcortical regions. They suggested “that the pathological changes in AD affect regions that are interconnected by well defined groups of connections and that the disease process may extend along the connecting fibers.” The selective distribution of the pathology in the association areas of the cortex was thus accounted for because these areas have strong connections with neurons in the MTL (Fig. 2). This discovery not only provides an explanation for the specific distribution of pathology at the end stage of AD, it also helps us to understand how certain symptoms can develop quite early in the disease before extensive pathological involvement of these cortical regions. The MTL comprises little more than 2% of the total volume of the cerebral cortex, but it is a crucial nodal point in the entire neural network. As pointed out by Hyman et al. (7), the destruction of neurons in this region “isolates the hippocampal formation by disconnecting major input and output pathways, and it is difficult to believe that the hippocampal formation in the brains of Alzheimer patients is functionally useful.”
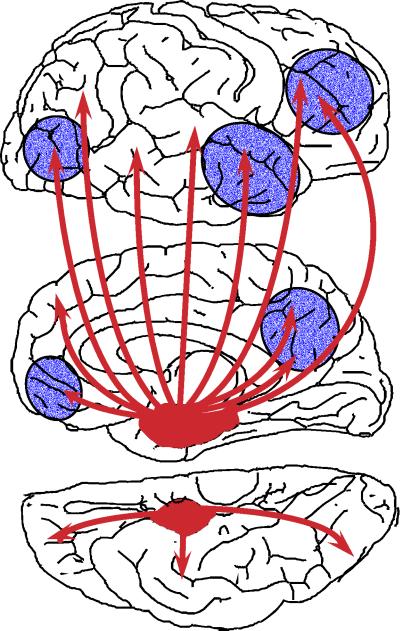
Figure 2.
Diagrammatic representation of the neural projections from the medial temporal lobe (red) to the neocortex (Top, lateral aspect; Middle, medial aspect; Bottom, ventral aspect). Areas shown in blue are those where reduced metabolism or reduced blood flow are found in AD on functional imaging. Several of these areas showed atrophy in the study by Scahill et al. (6).
What can we expect to happen in the target regions of the cortex (Fig. 2) when they become disconnected from the hippocampus? First, we would expect that the number of synapses in the target region would decrease, as found by staining for a synaptic marker (8). Second, we might expect that this loss of synaptic input would lead to reduced activity in the target neurons and a decrease in blood flow in the region. This disconnection hypothesis was invoked (18) to explain the characteristic finding of reduced blood flow in the parietotemporal areas of the neocortex in AD at a stage when it is unlikely that atrophy of this region could account for the changed blood flow. The disconnection hypothesis may also account for metabolic (19) and blood flow reductions (20) found in this and several other regions that also receive input from neurons in the MTL. The disconnection hypothesis can account for the variety and the progression of symptoms in AD, reflecting the different cortical areas that become disconnected, usually starting with memory deficits, followed by semantic and executive deficits, then deficits in praxis, then behavioral problems, ending with the full-blown symptoms of AD.
The characteristic MTL atrophy in presymptomatic subjects reported by Scahill et al. (6) in this issue of PNAS has been found in several studies on people with mild cognitive impairment (21–23). These findings are consistent with the idea that the pathological changes in the brain are initiated several years before the symptoms of AD become apparent. Clues about how early the pathology might occur have come from a more detailed analysis of the brain areas affected. The region of the MTL showing greatest atrophy in mild cognitive impairment is the entorhinal cortex, part of the parahippocampal gyrus (21, 23). This is the same region that has been postulated by Braak and Braak (24) to be the site where AD pathology is first expressed. The Braaks have proposed a sequence for the spread of the pathology, based on the distribution of tangles found in unselected brains obtained at autopsy (Fig. 3). This sequence is consistent with the known connections of the MTL and with the original hypothesis of Pearson et al. (17). Clinical studies on patients who have died at different stages in this sequence have described the cognitive decline (25, 26). It has been found, however, that the mild cognitive deficit at early stages is worsened by the presence of vascular pathology (27) and thus it is fortunate that the patients studied by Scahill et al. (6) were relatively young and therefore less likely to have cerebrovascular disease. A rough estimate of the time scale of the overall progression (Fig. 3) was provided by Ohm et al. (28). The findings of Scahill et al. are broadly consistent with the scheme shown in Fig. 3. Thus, their results can fairly be said to confirm the postulated spread of tangle pathology through the brain in AD, first proposed in 1991 (24).
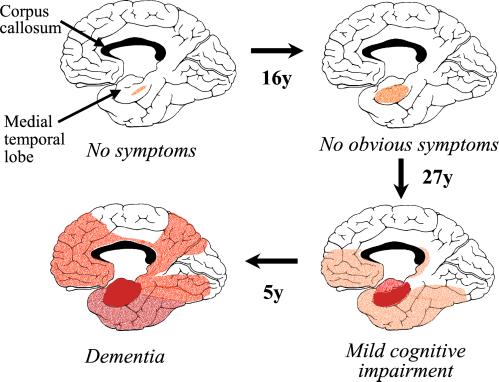
Figure 3.
Postulated sequence of spread of neurofibrillary pathology in AD, showing the medial aspect of the cerebral cortex. The depth of the red color is in proportion to the density of tangles (based on refs. 24 and 28). Several of the red areas showed atrophy in the study by Scahill et al. (6).
We can now be more confident in formulating a hypothesis to describe likely events in the brain of a person who develops AD. Many years (up to 50) before the symptoms occur, neurofibrillary tangles start to form in neurons in the parahippocampal gyrus. At some stage, this process is exacerbated, and many projection neurons in the MTL then start to die, leading to atrophy of the lobe and to early signs of memory deficits. Once denuded of their input from the MTL, neurons in the target areas of neocortex show reduced activity, leading to slower metabolism and a fall in local blood flow. They will no longer function properly in the neural networks underlying higher cognition. Perhaps as a consequence of their loss of synaptic input, some of these neocortical neurons then begin to develop tangles and many of them die, repeating the processes that began in the MTL. As the neurons die, their axons that connect the area to other cortical regions disintegrate. This axonal loss may be one of the reasons why the corpus callosum (Fig. 3), the white matter tract that carries these cortico-cortical axons, also shows rapid atrophy (up to 12% per year) in AD (29). Finally, the whole process of denervation, followed by induction of pathology and atrophy, occurs in the next cortical region in the chain. Because most of these cortical regions are reciprocally connected, we can imagine that an anatomically driven cascade process builds up.
At least three key questions arise from these findings. First, by what mechanism does the pathology spread along neural pathways from one brain region to another? Second, what initiates the process in the MTL that converts a benign deposition of neurofibrillary tangles into a rampant neurodegenerative event? Third, what are the implications of the long time course of the development and spread of the pathological changes?
I have already suggested that the loss of synaptic input might be one way in which the pathology spreads from one region to another in the cortex. Denervation is known to evoke many different responses in the target areas, which include remodeling of dendrites and the compensatory sprouting of axons originating from other areas. It has been suggested that such remodeling may underlie the cellular pathology of AD (30).
By the age of 85 virtually everyone will have some neurofibrillary tangles in their cerebral cortex (28) and yet not everyone develops AD. Except for the autosomal dominant familial variants of AD, tangles and/or amyloid alone are clearly not sufficient: other factors have to be present to initiate the pathological cascade that leads to rapid atrophy of the MTL that, in turn, sets off the march of the pathology through the cortex. It seems as though the MTL is a vulnerable part of the brain, because many things can cause it to atrophy, including hypoxia, posttraumatic stress, head injury, high blood pressure, and adrenal steroids. In patients with AD, elevated blood levels of the amino acid homocysteine are associated with a more rapid rate of MTL atrophy (31), and in the normal elderly the size of the MTL is inversely related to the level of homocysteine (32). These findings become more significant in view of the discovery that elevated levels of homocysteine many years before the onset of symptoms increase the risk of developing AD (33). As well as environmental factors, there are likely to be genetic risk factors that predispose to atrophy of this brain region; thus, the presence of the apoE ɛ4 allele seems to favor atrophy in the normal elderly (34) and in AD (35).
The long time course of the spread of AD pathology through the brain raises the question whether neuroimaging could reveal these changes even earlier than in the study by Scahill et al. (6). It is striking that minor deficits in learning and retention and abstract reasoning occur up to 22 years before the development of AD (36). Such a long time course is similar to that of other common diseases, such as atherosclerosis. Interventions against known risk factors for atherosclerosis have had a dramatic effect on deaths from heart disease and stroke. There is every expectation that modifiable risk factors for the initiation and spread of AD pathology will be discovered, and therefore we can trust that it will be possible before too long to prevent or delay the development this terrible disease.
Footnotes
See companion article on page 4703.
References
- 1.DeCarli C, Kaye J A, Horwitz B, Rapoport S I. Neurology. 1990;40:872–883. doi: 10.1212/wnl.40.6.872. [DOI] [PubMed] [Google Scholar]
- 2.De Leon M J, George A E, Stylopoulos L A, Smith G, Miller D C. Lancet. 1989;ii:672–673. doi: 10.1016/s0140-6736(89)90911-2. [DOI] [PubMed] [Google Scholar]
- 3.Jobst K A, Smith A D, Szatmari M, Molyneux A, Esiri M M, King E, Smith A, Jaskowski A, McDonald B, Wald N. Lancet. 1992;340:1179–1183. doi: 10.1016/0140-6736(92)92890-r. [DOI] [PubMed] [Google Scholar]
- 4.Luxenberg J S, Haxby J V, Creasey H, Sundaram M, Rapoport S I. Neurology. 1987;37:1135–1140. doi: 10.1212/wnl.37.7.1135. [DOI] [PubMed] [Google Scholar]
- 5.Jobst K A, Smith A D, Szatmari M, Esiri M M, Jaskowski A, Hindley N, McDonald B, Molyneux A J. Lancet. 1994;343:829–830. doi: 10.1016/s0140-6736(94)92028-1. [DOI] [PubMed] [Google Scholar]
- 6.Scahill R I, Schott J M, Stevens J M, Rossor M N, Fox N C. Proc Natl Acad Sci USA. 2002;99:4703–4707. doi: 10.1073/pnas.052587399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hyman B T, van Hoesen G W, Damasio A R, Barnes C L. Science. 1984;225:1168–1170. doi: 10.1126/science.6474172. [DOI] [PubMed] [Google Scholar]
- 8.Terry R D, Masliah E, Salmon D P, Butters N, DeTeresa R, Hill R, Hansen L A, Katzman R. Ann Neurol. 1991;30:572–580. doi: 10.1002/ana.410300410. [DOI] [PubMed] [Google Scholar]
- 9.Nagy Z, Jobst K A, Esiri M M, Morris J H, King E M-F, MacDonald B, Litchfield S, Barnetson L, Smith A D. Dementia. 1996;7:76–81. doi: 10.1159/000106857. [DOI] [PubMed] [Google Scholar]
- 10.Deweer B, Lehericy S, Pillon B, Baulac M, Chiras J, Marsault C, Agid Y, Dubois B. J Neurol Neurosurg Psychiatry. 1995;58:590–597. doi: 10.1136/jnnp.58.5.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Petersen R C, Jack C R, Jr, Xu Y C, Waring S C, O'Brien P C, Smith G E, Ivnik R J, Tangalos E G, Boeve B F, Kokmen E. Neurology. 2000;54:581–587. doi: 10.1212/wnl.54.3.581. [DOI] [PubMed] [Google Scholar]
- 12.de Toledo-Morrell L, Dickerson B, Sullivan M P, Spanovic C, Wilson R, Bennett D A. Hippocampus. 2000;10:136–142. doi: 10.1002/(SICI)1098-1063(2000)10:2<136::AID-HIPO2>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 13.Bobinski M, Wegiel J, Wisniewski H M, Tarnawski M, Bobinski M, Reisberg B, de Leon M J, Miller D C. Neurobiol Aging. 1996;17:909–919. doi: 10.1016/s0197-4580(97)85095-6. [DOI] [PubMed] [Google Scholar]
- 14.Bobinski M, Wegiel J, Tarnawski M, Bobinski M, Reisberg B, de Leon M J, Miller D C, Wisniewski H M. J Neuropathol Exp Neurol. 1997;56:414–420. doi: 10.1097/00005072-199704000-00010. [DOI] [PubMed] [Google Scholar]
- 15.Bobinski M, de Leon M J, Wegiel J, Desanti S, Convit A, Saint Louis L A, Rusinek H, Wisniewski H M. Neuroscience. 2000;95:721–725. doi: 10.1016/s0306-4522(99)00476-5. [DOI] [PubMed] [Google Scholar]
- 16.Gomez-Isla T, Hollister R, West H, Mui S, Growdon J H, Petersen R C, Parisi J E, Hyman B T. Ann Neurol. 1997;41:17–24. doi: 10.1002/ana.410410106. [DOI] [PubMed] [Google Scholar]
- 17.Pearson R C A, Esiri M M, Hiorns R W, Wilcock G K, Powell T P S. Proc Natl Acad Sci USA. 1985;82:4531–4534. doi: 10.1073/pnas.82.13.4531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jobst K A, Smith A D, Barker C S, Wear A, King E M, Smith A, Anslow P A, Molyneux A J, Shepstone B J, Soper N, et al. J Neurol Neurosurg Psychiatry. 1992;55:190–194. doi: 10.1136/jnnp.55.3.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meguro K, LeMestric C, Landeau B, Desgranges B, Eustache F, Baron J C. J Neurol Neurosurg Psychiatry. 2001;71:315–321. doi: 10.1136/jnnp.71.3.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnson K A, Jones K, Holman B L, Becker J A, Spiers P A, Satlin A, Albert M S. Neurology. 1998;50:1563–1571. doi: 10.1212/wnl.50.6.1563. [DOI] [PubMed] [Google Scholar]
- 21.Bobinski M, deLeon M J, Convit A, DeSanti S, Wegiel B, Tarshish C Y, SaintLouis L A, Wisniewski H M. Lancet. 1999;353:38–40. doi: 10.1016/s0140-6736(05)74869-8. [DOI] [PubMed] [Google Scholar]
- 22.Jack C R, Jr, Petersen R C, Xu Y, O'Brien P C, Smith G E, Ivnik R J, Boeve B F, Tangalos E G, Kokmen E. Neurology. 2000;55:484–490. doi: 10.1212/wnl.55.4.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Killiany R J, Gomez-Isla T, Moss M, Kikinis R, Sandor T, Jolesz F, Tanzi R, Jones K, Hyman B T, Albert M S. Ann Neurol. 2000;47:430–439. [PubMed] [Google Scholar]
- 24.Braak H, Braak E. Acta Neuropathol. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 25.Grober E, Dickson D, Sliwinski M J, Buschke H, Katz M, Crystal H, Lipton R B. Neurobiol Aging. 1999;20:573–579. doi: 10.1016/s0197-4580(99)00063-9. [DOI] [PubMed] [Google Scholar]
- 26.Nagy Z, Hindley N J, Braak H, Braak E, YilmazerHanke D M, Schultz C, Barnetson L, King E M F, Jobst K A, Smith A D. Dementia Geriatr Cogn Disord. 1999;10:115–120. doi: 10.1159/000017111. [DOI] [PubMed] [Google Scholar]
- 27.Esiri M M, Nagy Z, Smith M Z, Barnetson L, Smith A D. Lancet. 1999;354:919–920. doi: 10.1016/S0140-6736(99)02355-7. [DOI] [PubMed] [Google Scholar]
- 28.Ohm T G, Muller H, Braak H, Bohl J. Neuroscience. 1995;64:209–217. doi: 10.1016/0306-4522(95)90397-p. [DOI] [PubMed] [Google Scholar]
- 29.Teipel S J, Bayer W, Alexander G E, Zebuhr Y, Teichberg D, Kulic L, Schapiro M B, Moller H J, Rapoport S I, Hampel H. Arch Neurol (Chicago) 2002;59:243–248. doi: 10.1001/archneur.59.2.243. [DOI] [PubMed] [Google Scholar]
- 30.Arendt T. Neuroscience. 2001;102:723–765. doi: 10.1016/s0306-4522(00)00516-9. [DOI] [PubMed] [Google Scholar]
- 31.Clarke R, Smith A D, Jobst K A, Refsum H, Sutton L, Ueland P M. Arch Neurol (Chicago) 1998;55:1449–1455. doi: 10.1001/archneur.55.11.1449. [DOI] [PubMed] [Google Scholar]
- 32. Williams, J. H., Pereira, E. A. C., Budge, M. M. & Bradley, K. M. (2002) Age Ageing, in press. [DOI] [PubMed]
- 33.Seshadri S, Beiser A, Selhub J, Jacques P F, Rosenberg I H, D'Agostino R B, Wilson P W, Wolf P A. N Engl J Med. 2002;346:476–483. doi: 10.1056/NEJMoa011613. [DOI] [PubMed] [Google Scholar]
- 34.Moffat S D, Szekely C A, Zonderman A B, Kabani N J, Resnick S M. Neurology. 2000;55:134–136. doi: 10.1212/wnl.55.1.134. [DOI] [PubMed] [Google Scholar]
- 35.Juottonen K, Lehtovirta M, Helisalmi S, Riekkinen P J, Soininen H. J Neurol Neurosurg Psychiatry. 1998;65:322–327. doi: 10.1136/jnnp.65.3.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Elias M F, Beiser A, Wolf P A, Au R, White R F, D'Agostino R B. Arch Neurol (Chicago) 2000;57:808–813. doi: 10.1001/archneur.57.6.808. [DOI] [PubMed] [Google Scholar]