Abstract
The Alzheimer's disease (AD)-associated presenilin (PS) proteins are required for the γ-secretase cleavages of the β-amyloid precursor protein and the site 3 (S3) protease cleavage of Notch. These intramembrane cleavages release amyloid-β peptide (Aβ), including the pathogenic 42-aa variant (Aβ42), as well as the β-amyloid precursor protein and the Notch intracellular domains (AICD, NICD). Whereas Aβ is generated by endoproteolysis in the middle of the transmembrane domain, AICD and NICD are generated by cleavages at analogous positions close to the cytoplasmic border of the transmembrane domain. Numerous mutations causing familial AD (FAD) that all cause increased production of Aβ42 have been found in the PS1 gene. Here we have investigated the previously uncharacterized, very aggressive FAD mutation L166P that causes onset of AD in adolescence. Strikingly, the PS1 L166P mutation not only induces an exceptionally high increase of Aβ42 production but also impairs NICD production and Notch signaling, as well as AICD generation. Thus, FAD-associated PS mutants cannot only affect the generation of NICD, but also that of AICD. Moreover, further analysis with artificial L166 mutants revealed that the γ-secretase cleavage at position 40/42 and the S3-like γ-secretase cleavage at position 49 of the Aβ domain are both differentially affected by PS1 L166 mutants. Finally, we show that PS1 L166 mutants affect the generation of NICD and AICD in a similar manner, supporting the concept that S3 protease and S3-like γ-secretase cleavages are mediated by identical proteolytic activities.
Deposition of the amyloid-β peptide (Aβ) in senile plaques is a pathological hallmark of Alzheimer's disease (1). Aβ is derived by proteolytic processing from the β-amyloid precursor protein (APP) through the combined action of β-secretase and γ-secretase. Whereas β-secretase has recently been identified as the membrane bound aspartyl protease BACE (β site APP cleaving enzyme; ref. 2), the identity of γ-secretase has remained elusive. Recent evidence suggests that γ-secretase is a large protein complex with an aspartyl protease activity that may consist of either of the two presenilin (PS) proteins PS1 and PS2 as catalytic subunit (3) and other probably regulatory proteins such as Nicastrin (4, 5). Consistent with a role as catalytic subunit of γ-secretase, PSs contain two highly conserved aspartates in the putative transmembrane domains (TM) 6 and 7. Mutagenesis of these aspartates in PS1 or PS2 reduces Aβ production (6–8). Moreover, aspartyl protease transition-state analogue inhibitors that block γ-secretase can be directly cross-linked to PSs (9, 10). Finally, PSs share considerable sequence homology around the critical active site aspartate in TM7 with polytopic bacterial aspartyl proteases (11).
PSs are also critically required for the site 3 (S3) intramembrane cleavage of Notch1 (12) and the related Notch2–4 proteins (13, 14). S3 cleavage of Notch1 leads to the liberation of the Notch intracellular domain (NICD), a key molecule in cellular differentiation (15) that translocates to the nucleus where it acts as a transcriptional coactivator. A role in nuclear signaling has also recently been suggested for C-terminal fragment (CTF)-γ (16), the C-terminal cleavage product of γ-secretase (17), now generally termed AICD (APP intracellular domain; ref. 18). AICD results from predominant cleavage after leucine 49 of the Aβ domain of APP, a position that is analogous to the S3 cleavage site of Notch (18–21). This additional S3-like γ-secretase cleavage of APP is PS-dependent and can be blocked by γ-secretase inhibitors, including those that have been shown to bind to PSs (18, 20, 21).
Rare mutations have been identified within the APP gene that are associated with familial Alzheimer's disease (FAD). Almost all of these mutations cause the increased generation of the highly amyloidogenic 42-aa-long variant of Aβ (Aβ42) that aggregates faster than the shorter Aβ40 and is predominantly deposited in senile plaques (1). However, by far the highest numbers of mutations linked with FAD have been identified in the PS1 gene. In addition, rare mutations causing FAD have also been found in the homologous PS2 gene. Like most of the FAD-associated APP mutations, FAD mutations in PS1 and PS2 cause the increased generation of Aβ42. Interestingly, several FAD-associated PS1 mutations have been shown to impair Notch signaling (22) and consistent with this observation showed reduced rescuing activity of the egg-laying defect of a mutant Caenorhabditis elegans sel-12 PS homologue (23, 24). However, the FAD-associated PS1 A246E mutant has been shown to rescue the phenotype of PS1−/− mice (25, 26), a discrepancy that is currently not understood.
Several of the FAD-linked mutations in the PS genes are found to cluster within the predicted α-helical TMs. Among other TMs, TM3 has been identified as a critical site where several FAD mutations of PS1 are located. Here we studied the effects of a previously uncharacterized FAD-associated mutation in TM3 (PS1 L166P) on APP and Notch endoproteolysis. Surprisingly, this mutation not only caused an exceptional increase of pathological Aβ42 generation but also reduced proteolytic production of NICD and AICD, demonstrating that FAD-associated PS mutants cannot only affect the generation of NICD, but also that of AICD. Interestingly, data resulting from additional mutagenesis demonstrate that pathogenic Aβ42 production and proteolytic generation of AICD or NICD are differentially affected by PS1 L166 mutants. Finally, we show that PS1 L166 mutants affect NICD and AICD generation in a similar manner, suggesting that S3 protease cleavage and the S3-like γ-secretase cleavage are mediated by the same PS-dependent γ-secretase activity.
Materials and Methods
Antibodies.
The polyclonal and monoclonal antibodies against PS1 (3027, BI.3D7; ref. 27) and PS2 (3711, BI.HF5c; ref. 27), against the C terminus of APP (6687; ref. 11) and against Aβ1–42 (3926; ref. 28) have been described. The monoclonal anti-myc antibody 9E10 was obtained from the Developmental Studies Hybridoma Bank, University of Iowa, or from Santa Cruz Biotechnology for use in immunofluorescence (1:300).
cDNA Constructs, Cell Culture, and Cell Lines.
cDNA constructs encoding PS1 L166 mutants were generated as described (11) and cloned into the pcDNA3.1/zeo(+) expression vector (Invitrogen). Human embryonic kidney 293 (K293) cells were transfected and cultured as described (11). K293/sw cells stably expressing Swedish mutant APP (swAPP) have been described (29).
Analysis of PSs, APP, and Notch Endoproteolysis.
PSs (27), APP, APP CTFs, secreted Aβ species (11), and NICD generation (7, 30) were analyzed as described. AICD generation was analyzed in vitro from membrane preparations of stably transfected K293/sw cells. Equal amounts of cells were resuspended (at least 0.5 ml per 10-cm dish) in homogenization buffer [10 mM Mops (pH 7.0)/10 mM KCl] containing 1× protease inhibitors (PI; Complete, Roche Molecular Biochemicals) and homogenized using a freeze–thaw procedure. Following swelling on ice for 10 min, cells were frozen in liquid N2 for 5 min and allowed to thaw 28 min on ice and 2 min at RT. Equal volumes of the homogenate were centrifuged for 10 min at 1,000 × g at 4°C to prepare a post nuclear supernatant (PNS) fraction. In parallel, aliquots of the homogenate were solubilized in STEN-lysis buffer [50 mM Tris (pH 7.6)/150 mM NaCl/2 mM EDTA/1% Nonidet P-40] and, after a clarifying spin, subjected to protein determination. Membranes were pelleted from the PNS by centrifugation for 20 min at 16,000 × g at 4°C and resuspended (at least 75 μl per 10-cm dish) in assay buffer [150 mM sodium citrate (pH 6.4)/1 mM EDTA] according to the protein content determined in the aliquots of the homogenates above. To allow for AICD generation, aliquots of the membranes were incubated at 37°C for 1 h and analyzed for AICD as described (18). Aliquots of the membranes that were not used for AICD generation were analyzed for APP and APP CTF levels. To facilitate detection of recombinant AICD variants, cells were treated with 1.66 mM 1,10-phenanthroline for 90 min before the preparation of cell lysates.
PS1−/− Rescue Assays.
PS1−/− fibroblast cells were cotransfected with equal amount of either wild-type (wt) or L166 mutant PS1 cDNA and CBF1-luciferase reporter construct (31) by using Lipofectamine 2000 (GIBCO). The EGFP construct was also added at 1/25 molar ratio to each transfectant to normalize the transfection efficiency. Luciferase activity was measured from cell lysates 24 h post-transfection according to manufacturer's suggested method (Promega).
Confocal Microscopy.
Immunofluorescence was carried out as described (32) by using Alexa Fluor 488 (1:1,000, Molecular Probes) for detection. To detect cell nuclei of fixed cells, monomeric cyanine nucleic acid stain TO-PRO-3 iodide (1:1,000, Molecular Probes) was used. Confocal images were obtained with a Leica TCS SP confocal laser scanning microscope (Leica, Deerfield, IL). Single optical sections from each cell were chosen and processed using photoshop software (Adobe Systems, Mountain View, CA).
Results
The PS1 L166P FAD Mutation Associated with Disease Onset in Adolescence Causes Greatly Increased Aβ42 Generation and Impairs Notch Signaling.
Several FAD mutations have been identified in TM3 of PS1, suggesting that this TM is an important site for PS function. Here we report a previously uncharacterized FAD-associated mutation in TM3 of PS1 that changes the leucine residue at codon 166 to proline. This mutation is associated with an onset of AD in adolescence. In the female proband, secondary generalized seizures began at age 15, major depression occurred at 19, memory was clearly impaired by 24, ataxia and spastic paraparesis were recorded by 27, and moderate stage dementia by 28. Dementia, ataxia, and spasticity progressed until death at age 35. Numerous Aβ-immunopositive neuritic and cotton wool plaques were seen throughout the cerebral cortex and Aβ-immunopositive amyloid cores were abundant in the cerebellar cortex (J.M. and B.G., unpublished results).
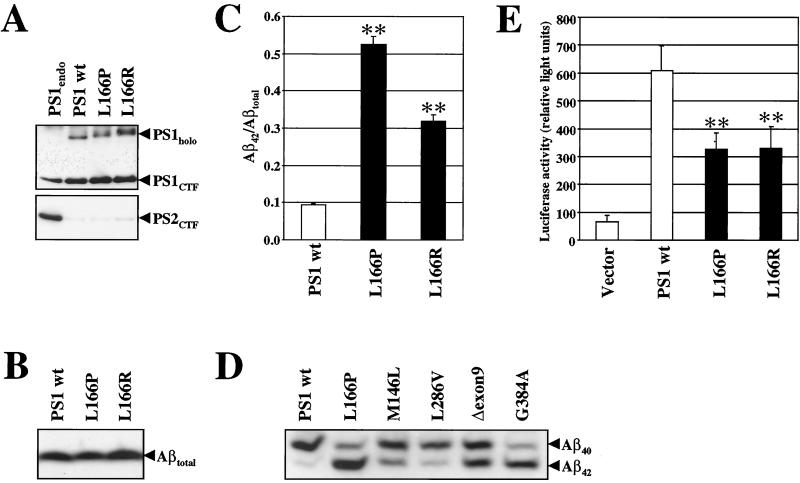
Leucine 166 appears to be a rather important residue for PS function because it is conserved in all PSs, the only exceptions being the C. elegans PS homologue hop-1 and the Arabidopsis thaliana and Oryza sativa PSs. In addition, a FAD mutation changing leucine 166 to arginine has been identified in a Spanish family (33). To investigate this critical site of PS1 in more detail, we examined the effects of the L166 FAD mutations, as well as of several artificial mutations at this residue on APP and Notch endoproteolysis. We first investigated the pathological activity of the two FAD-associated PS1 L166 mutants on Aβ production by using a tissue culture system that has previously been proven to be very sensitive for the detection of abnormal Aβ42 generation (ref. 34 and references therein). cDNAs encoding wt PS1, PS1 L166P, and PS1 L166R were stably transfected into K293/sw cells (29) and analyzed for PS expression. As shown in Fig. 1A, expression of wt PS1 and the L166 mutants allowed the generation of robust amounts of PS1 CTFs, demonstrating that the L166 mutants did not affect endoproteolysis of PS1. Functional expression of the PS1 variants was confirmed by the replacement of endogenous PS2 (ref. 35; Fig. 1A). We next analyzed the pathological function of the L166P and L166R mutation on total Aβ, Aβ40, and Aβ42 production. Conditioned media from metabolically labeled cells stably expressing wt PS1 or the two PS1 L166 FAD mutants were collected and analyzed for levels of total Aβ (Fig. 1B) or Aβ40 and Aβ42 species (Fig. 1C). Although the levels of total Aβ were similar (Fig. 1B), quantitation of Aβ40 and Aβ42 species revealed that the PS1 L166P caused a striking 5- to 6-fold increase of the Aβ42/Aβtotal ratio (Fig. 1C and Table 1). Although not as high as observed for the L166P mutation, the L166R mutation showed a 3-fold increase of the Aβ42/Aβtotal ratio (Fig. 1C and Table 1). Comparison of the PS1 L166P mutant with the PS1 M146L, L286V, Δexon9, and G384A FAD mutants underlined its exceptional Aβ42 increase (Fig. 1D). This increase was comparable to that found for the PS1 G384A mutant (refs. 11 and 36; Fig. 1D) and considerably higher than that found for the large majority of the PS1 mutations (37).
Figure 1.
The FAD-associated PS1 L166P and PS1 L166R mutants cause pathological Aβ42 production and impair Notch signaling. (A Upper) Cell lysates from K293/sw cells expressing endogenous PSs or overexpressing the indicated PS1 variants were immunoprecipitated with antibody 3027 and PS1 CTFs and holoproteins were detected by immunoblotting using the monoclonal antibody BI.3D7. Endoproteolytic cleavage occurs in cell lines stably expressing wt PS1, PS1 L166P and PS1 L166R. (Lower) Cell lysates from the cell lines in A were immunoprecipitated with antibody 3711, and PS2 CTFs were detected by immunoblotting using the monoclonal antibody BI.HF5c. Overexpression of wt PS1, L166P, or L166R results in efficient replacement of endogenous PS2. (B) Total Aβ levels were analyzed in conditioned media of metabolically labeled K293/sw cells stably expressing wt PS1, PS1 L166P, or PS1 L166R by immunoprecipitation with antibody 3926. (C) Aβ species were analyzed as in B, but separated on a Tris-bicine-urea gel system that allows the specific identification of Aβ40 and Aβ42 (42). Aβ42/Aβtotal ratios (Aβtotal = Aβ40 + Aβ42) were quantified by phosphorimaging. Bars represent the mean ± SE of three independent experiments. The asterisks indicate the significance (two-tailed Student's t test) relative to wt PS1 (**, P < 0, 001). (D) Levels of Aβ40 and Aβ42 species were analyzed in conditioned media of K293/sw cells stably expressing wt PS1, PS1 L166P, PS1 M146L, PS1 L286V, PS1 Δexon9, and PS1 G384A as in C. (E) Notch downstream signaling assay. PS1−/− cells were cotransfected with CBF1-luciferase construct along with empty vector, PS1 wt, PS1 L166P, or PS1 L166R cDNA constructs. PS1 activity in Notch signaling was quantified by measuring the luciferase activity. Bars represent the mean ± SE of seven independent experiments. The asterisks indicate the significance (two-tailed Student's t test) relative to wt PS1 (**, P < 0.001).
Table 1.
Effects of FAD-associated and artificial L166 mutants on Aβ42 production
| PS1 | Ratio Aβ42/Aβtotal ± SE | P value |
|---|---|---|
| wt | 1.00 | |
| L166P | 5.61 ± 0.15 | <0.001 |
| L166R | 3.40 ± 0.06 | <0.001 |
| L166E | 4.55 ± 0.31 | <0.01 |
| L166A | 3.47 ± 0.17 | <0.001 |
| L166S | 3.92 ± 0.13 | <0.001 |
| L166C | 2.01 ± 0.19 | <0.01 |
| L166W | 1.55 ± 0.05 | <0.001 |
Levels of Aβ40 and Aβ42 were analyzed in conditioned media of metabolically labeled K293/sw cells stably expressing wt PS1, PS1 FAD (L166P and L166R), or artificial PS1 L166 mutants as described in Fig. 1C. Means ± SE represent three independent experiments. Ratios are expressed relative to those in cells expressing wt PS1 that was set 1.00. P values indicate the significance (two-tailed Student's t test) relative to wt PS1.
We next investigated whether the L166P and L166R mutants also affect Notch signaling and assessed the activation of the Notch-dependent downstream transcription factor CBF1 (C-promoter binding factor 1), using luciferase as a reporter gene (31). PS1−/− fibroblast cells were transiently transfected with wt PS1 or the PS1 L166P or L166R FAD mutants along with the CBF1-luciferase construct and activation of luciferase was measured. Robust luciferase activity was detected in PS1−/− cells transfected with wt PS1 (Fig. 1E). In contrast, significantly reduced luciferase activity was observed with the L166P and L166R mutants. Thus, these data demonstrate that in addition to producing aberrant amounts of Aβ42, the L166P and L166R mutants are also partially defective in Notch signaling.
Artificial L166 Mutants Cause Increased Aβ42 Levels.
To examine the consequences of L166 mutations on γ-secretase cleavage in more detail, we changed leucine 166 to glutamate, alanine, serine, cysteine, and tryptophan. The respective cDNAs were stably transfected into K293/sw cells. All mutants were expressed at similar levels and replaced endogenous PSs (data not shown). We then analyzed whether the various artificial L166 mutants affect Aβ42 production. Conditioned media were collected from metabolically labeled cells expressing wt PS1 or the artificial L166 mutants and analyzed for Aβ40 and Aβ42 production. Quantitation revealed that all mutations increased the Aβ42/Aβtotal ratio to various extents, demonstrating that codon 166 is very sensitive to amino acid changes (Table 1). Introduction of the negatively charged amino acid glutamate led to the strongest elevation (4- to 5-fold) of the Aβ42/Aβtotal ratio among the artificial L166 mutants. A rather strong increase in the Aβ42/Aβtotal ratio was also observed for the serine and alanine substitutions. In contrast, the cysteine and tryptophan substitutions elevated the Aβ42/Aβtotal ratio only slightly, similar to the majority of the known PS1 mutations (37). Thus, all artificial L166 mutants described here induce pathological Aβ42 production and may therefore probably lead to AD if they occur in humans.
Differential Effects of PS1 L166 Mutants on Notch Endoproteolysis.
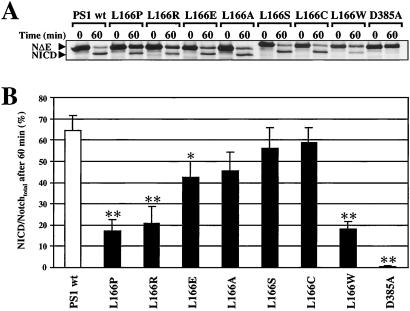
The results shown above suggest that the L166 mutants cause structural alterations of TM3 that result in an altered γ-secretase cleavage and lead to variable production of Aβ42. We therefore next asked whether the various L166 mutants would also affect endoproteolysis of Notch. The above described cell lines expressing either wt PS1, FAD-linked, or artificial L166 mutations were transfected with the NotchΔE (NΔE) cDNA (38) and NICD formation was monitored in pulse–chase experiments as described (7). As a control, K293/sw cells stably co-expressing NΔE with the PS1 D385A mutant were used. NICD production was observed in all cell lines with the exception of cells expressing the PS1 D385A mutant that led to an almost complete inhibition of NICD generation, consistent with previous results (7, 11, 30). Consistent with impaired CBF1-mediated Notch signaling (Fig. 1E), the FAD-associated mutations L166P and L166R showed a significantly impaired conversion of NΔE to NICD (Fig. 2A). Marked impairment of NICD generation was also observed for the artificial L166W mutation, whereas the other L166 mutants displayed moderate or very little impairment in NICD formation. Quantitation confirmed the differential effects of the various L166 mutants on the conversion of NΔE to NICD (Fig. 2B).
Figure 2.
Differential NICD production in cells expressing PS1 L166 mutants. (A) K293/sw cells stably expressing wt PS1, PS1 L166 mutant derivatives, or PS1 D385A were transfected with the NotchΔE cDNA. NICD formation was analyzed in pulse chase experiments as described (7). Note the significant change in the ratio of NΔE:NICD in cells expressing the PS1 L166P, L166R, and L166W mutants as compared with cells expressing wt PS1 or PS1 D385A. Consistent with previous results, NICD formation was blocked in cell lines expressing PS1 D385A. (B) NICD/Notchtotal ratios (Notchtotal = NICD + NΔE) obtained after 60 min of chase were quantified by phosphorimaging. Bars represent the mean ± SE of three independent experiments. The asterisks indicate the significance (two-tailed Student's t test) relative to wt PS1 (*, P < 0.01; **, P < 0.001).
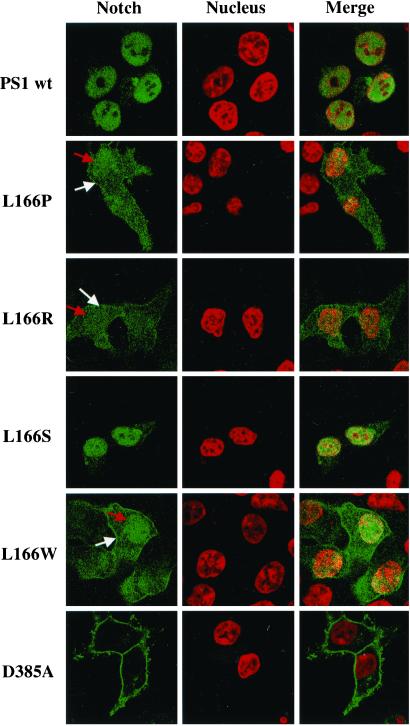
To further confirm these results we followed the cellular distribution of Notch in confocal immunofluorescence experiments. Cells co-expressing NΔE and wt PS1, the two FAD-linked L166 mutants, and the artificial PS1 L166S and L166W mutants were permeabilized and co-stained with antibody 9E10 to the myc-tag of NΔE and with TO-PRO-3 iodide to visualize cell nuclei. Consistent with the results described above, cells expressing wt PS1 or the artificial PS1 L166S mutant showed a prominent nuclear Notch staining, suggesting efficient generation and nuclear translocation of NICD (Fig. 3). In contrast, cells expressing the PS1 L166P or L166R FAD mutants or the artificial L166W mutant showed much weaker staining of nuclear Notch, confirming the impaired generation and consequently reduced nuclear translocation of NICD (Fig. 3). Concomitant with reduced staining of nuclear Notch, increased staining of Notch on the cell surface was observed, indicating an inefficient conversion of cell-surface-located NΔE to NICD. In agreement with previous results (30), cell-surface staining of Notch, representing accumulated uncleaved NΔE precursor, was observed in cells expressing the PS1 D385A mutant (Fig. 3). In these cells, very little if any staining of nuclear Notch was observed (Fig. 3), consistent with an almost complete inhibition of NICD formation (see Fig. 2).
Figure 3.
Differential distribution of Notch in cells expressing PS1 L166 mutants. Cellular distribution of Notch in cells expressing wt PS1 and the indicated PS1 mutants was assessed by confocal immunofluorescence. Fixed cells were permeabilized and co-stained with antibody 9E10 to the cytoplasmic myc-tag of NΔE (green) and with TO-PRO-3 iodide (red) to detect cell nuclei. Confocal images revealed an accumulation of Notch (NICD) in the cell nuclei in cells expressing wt PS1 and the artificial mutant PS1 L166S. In contrast, cells expressing the FAD-associated PS1 L166P or PS1 L166R or the artificial L166W mutants show reduced nuclear Notch staining (red arrows) and increased cell-surface Notch staining (white arrows). Consistent with previous results (30), cells expressing PS1 D385A show an accumulation of Notch (NΔE) at the cell surface.
PS1 L166 Mutants Differentially Affect AICD Generation.
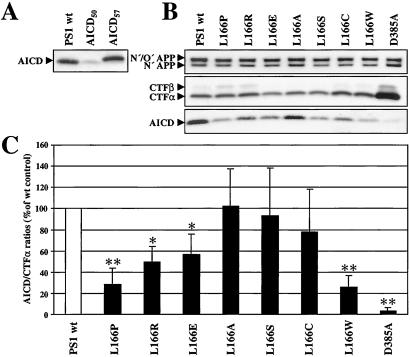
Because some L166 mutants, in particular the FAD-associated L166P mutant, drastically impaired NICD generation, we next investigated whether the S3-like γ-secretase cleavage of APP that generates AICD would also be affected by these mutants. K293/sw cells stably transfected with either wt PS1, the PS1 L166 mutants, or PS1 D385A were analyzed for AICD generation in in vitro assays that allow the production of robust amounts of AICD (16, 18, 39). We first analyzed AICD generation from cells expressing wt PS1. In agreement with previous findings, in vitro-generated AICD comigrated with a recombinant AICD variant starting at amino acid 50 of the Aβ domain (AICD50) but not with a recombinant AICD variant starting at amino acid 43 (AICD57) (Fig. 4A). Thus, these data confirm the efficient production of AICD that is generated by γ-secretase cleavage predominantly at position 49 of the Aβ domain. We next investigated whether the L166P mutant and the other L166 mutants affect AICD generation. As shown in Fig. 4B, all cell lines contained similar amounts of full-length APP and APP C-terminal fragments, CTFβ and CTFα, the substrates of γ-secretase, with the exception of the PS1 D385A mutant, which caused an expected strong accumulation of CTFβ and CTFα (6). AICD production was strongly reduced in cells expressing the L166P mutant. Interestingly, the L166W mutant also impaired AICD production consistent with its significant effect on S3 cleavage of Notch (compare Fig. 4 B and C with Fig. 2), whereas the other mutants displayed differential effects on AICD generation (Fig. 4B). Consistent with previous results (18, 20, 21), expression of the biologically inactive PS1 D385A mutant led to an almost complete block of AICD generation. To compare the effects of the L166 mutants on AICD production, we quantified AICD production relative to the amounts of CTFα, the predominant AICD precursor species in the K293/sw cells investigated (Fig. 4B). Quantitation of the AICD/CTFα ratios confirmed the differential effects of the L166 mutants on AICD production (Fig. 4C). Interestingly, comparison of the effects of the L166 mutants on NICD (Fig. 2B) and AICD production (Fig. 4C) indicated that Notch S3 and APP S3-like γ-secretase cleavage were affected by the L166 mutants in a similar manner. Indeed, statistical analysis (Spearman rank test) revealed that the effects of the L166 mutants on NICD and AICD production correlated (P < 0.02).
Figure 4.
Differential AICD production in cells expressing PS1 L166 mutants. (A) The migration behavior of in vitro generated AICD was compared with that of recombinant AICD variants starting either at amino acid 50 (AICD50) or at amino acid 43 (AICD57). Membranes of K293/sw cells stably expressing wt PS1 were incubated at 37°C for 1 h to allow AICD generation. Following ultracentrifugation, AICD was identified from the supernatant fraction by immunoblotting with antibody 6687. K293 cells stably transfected with cDNA encoding recombinant AICD variants were analyzed by combined immunoprecipitation/immunoblotting with antibody 6687. Note that in vitro-produced AICD co-migrates with recombinant AICD50 but not with recombinant AICD57. (B, Top and Middle) Membranes were prepared from K293/sw cells stably expressing wt PS1, the PS1 L166 mutants, or PS1 D385A and analyzed for levels of full-length APP and CTFβ and CTFα by immunoblotting with antibody 6687. Note that all cell lines show similar amounts of full-length APP (N′/O′-glycosylated APP and N′-glycosylated APP). The amounts of APP CTFs were similar with the exception of the PS1 D385A mutant as expected (6). (Bottom) Membranes from K293/sw cells expressing wt PS1, the PS1 L166 mutants or PS1 D385A were analyzed for the production of AICD as in A. Note the different levels of AICD that are produced in the various cell lines expressing the L166 mutants as compared with cell lines expressing wt PS1 or PS1 D385A. (C) AICD/CTFα ratios were quantified by densitometric scanning and expressed relative to the AICD/CTFα ratio generated in cells expressing wt PS1 that was set 100%. Bars represent the mean ± SE of five independent experiments. The asterisks indicate the significance (two-tailed Student's t test) relative to wt PS1 (*, P < 0.01; **, P < 0.001).
Discussion
To date, eleven mutations associated with FAD have been reported to be located in TM3 of PS1 (http://molgen-www.uia.ac.be/ADMutations/). Among these mutations, the PS1 L166P mutation is of particular interest because it is associated with disease onset in adolescence. Moreover, the L166P mutation causes an exceptional increase in the Aβ42/Aβtotal ratio, similar to the FAD-associated PS1 G384A mutation (11, 36). This extreme increase of Aβ42 may be due to disruption of TM3 by the helix-breaking proline. Additional mutational analysis of leucine 166 revealed that all L166 mutations investigated cause increased Aβ42 levels, suggesting that leucine 166 is critically required for the specificity of γ-secretase cleavage. However, none of the L166 mutations investigated here inhibited γ-secretase activity. This is different from a recent mutational analysis of glycine 384 that is directly adjacent to the C-terminal active site aspartate of PS1 (11). Whereas the FAD-associated G384A mutation has been shown to dramatically increase Aβ42 levels similar to the L166P mutation described here, other (artificial) mutants at position G384 lead to reduced function in APP endoproteolysis in a dominant-negative manner (11). Thus, unlike “active site” mutations that inhibit γ-secretase activity, the L166 mutants rather influence the preference of γ-secretase for Aβ42 versus Aβ40 cleavage.
It is currently not clear whether pathogenic production of Aβ42 by PS FAD mutants causes defects in Notch signaling. Studies with PS1−/− cells showed that several FAD-associated PS1 mutations impair Notch signaling (22). These observations are supported by the finding that FAD mutants show reduced rescuing activity of the egg-laying defect of a mutant C. elegans sel-12 PS homologue (23, 24). However, these findings may be in conflict with the observation that a FAD-associated mutant PS1 rescues the phenotype of PS1−/− mice (25, 26). As shown in our study, the mutations at residue 166 affect pathological Aβ42 production and NICD generation in a differential manner. Increased levels of Aβ42 did not correlate with decreased NICD production and vice versa (Spearman rank test). For example, the FAD-associated L166P and L166R mutants and the artificial mutant L166W differed drastically in pathological Aβ42 production but impaired NICD generation in a comparable manner (compare Table 1 and Fig. 2). Conversely, despite differential increases in pathological Aβ42 levels, the artificial mutations L166E, L166A, L166S, and L166C allowed NICD generation at moderately reduced or wt levels (compare Table 1 and Fig. 2). Taken together, these data demonstrate that pathologically elevated levels of Aβ42 may not necessarily be associated with impaired Notch cleavage and suggest that γ-secretase cleavage and S3 cleavage can be differentially affected by FAD mutations. Thus, it appears that FAD mutations can have dual effects on PS function. They may cause a toxic gain of misfunction, which is associated with increased production of the highly amyloidogenic Aβ42, but they can also cause a (partial) loss of function in Notch signaling. In that regard, the L166 mutations analyzed here behaved similar as some of the mutations of leucine 286 of PS1 (40). However, whereas the naturally occurring FAD mutation L286V did not interfere with Notch endoproteolysis, charged amino acid substitutions strongly inhibited NICD generation (40).
Mutations at position L166 can also affect the PS-dependent, S3-like γ-secretase cleavage at position 49 of the Aβ domain that results in liberation of AICD from the membrane. This cleavage occurs at a site almost identical to the S3 cleavage site of Notch (18–21). A strong reduction of AICD generation was observed with the L166P mutant, demonstrating that FAD-associated PS mutants cannot only affect the generation of NICD, but also that of AICD. Interestingly, AICD production and pathogenic production of Aβ42 was affected in an individual and differential manner. For example, although the L166P and L166W mutants inhibited AICD generation similarly, they affected Aβ42 production differentially (compare Table 1 with Fig. 4 B and C). This finding is further supported by the L166A mutant that did not inhibit AICD generation, despite the production of high Aβ42 levels. Indeed, statistical analysis revealed that inhibition of AICD generation is not associated with the production of high Aβ42 levels (Spearman rank test). Further analysis revealed that all L166 mutants affected AICD and NICD production in a similar manner. For example, the mutants that had the strongest inhibitory effect on NICD generation (PS1 L166P and L166W) also had the strongest effect on AICD generation. This finding suggests that S3 protease and S3-like γ-secretase cleavages are mediated by identical PS-dependent proteolytic activities.
The finding that γ-secretase cleavage at position 40/42 and the S3-like γ-secretase at position 49 of the Aβ domain are differentially affected by PS1 L166 mutants suggests that these cleavages occur either independently from each other in a single pathway or that they occur in two different independent pathways. Thus, although AICD may be principally derived from both APP CTFs, CTFβ, and CTFα, by the S3-like γ-secretase cleavage, we cannot exclude the possibility that CTFβ is the substrate of γ-secretase-mediated 40/42 cleavage, whereas AICD is predominantly derived from S3-like γ-secretase cleavage of CTFα. At present it is impossible to distinguish between these two possibilities. However, as neither p317–49 nor Aβ1–49 species could be detected (41), it appears that the S3-like γ-secretase cleavage of CTFβ and CTFα at position 49 of the Aβ domain cannot occur without initial or simultaneous γ-secretase cleavage at position 40/42. This finding suggests that the substrates for AICD generation are cleaved in a single pathway that mediates both cleavages, the γ-secretase cleavage after position 40/42 of the Aβ domain and the S3-like γ-secretase cleavage. This is in agreement with the strict PS-dependence of both cleavages (12, 18, 20, 21) and supports the hypothesis that PSs contribute the catalytic site of γ-secretase and S3-like γ-secretase, as well as of S3 protease.
Acknowledgments
We thank Olga Alexandrova for help with confocal microscopy, Raphael Kopan for the CBF1-luciferase construct, and Philipp Kahle for helpful discussions. This work was supported by the American Health Assistance Foundation (C.H. and H.S.), European Community Grant DIADEM (to C.H.), the Boehringer Ingelheim K.G. (C.H.), National Institutes of Health Grant NS 40039 (to H.Z.), and Indiana Alzheimer Disease Center Grant PHS P30 AG10133 (to B.G.).
Abbreviations
- Aβ
amyloid-β peptide
- AD
Alzheimer's disease
- APP
β-amyloid precursor protein
- AICD
APP intracellular domain
- CTF
C-terminal fragment
- FAD
familial Alzheimer's disease
- NICD
Notch intracellular domain
- PS
presenilin
- S3
site 3
- TM
transmembrane domain
- wt
wild type
- NΔE
NotchΔE
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Selkoe D J. Nature (London) 1999;399:A23–A31. doi: 10.1038/399a023. [DOI] [PubMed] [Google Scholar]
- 2.Vassar R, Citron M. Neuron. 2000;27:419–422. doi: 10.1016/s0896-6273(00)00051-9. [DOI] [PubMed] [Google Scholar]
- 3.Li Y M, Lai M T, Xu M, Huang Q, DiMuzio-Mower J, Sardana M K, Shi X P, Yin K C, Shafer J A, Gardell S J. Proc Natl Acad Sci USA. 2000;97:6138–6143. doi: 10.1073/pnas.110126897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yu G, Nishimura M, Arawaka S, Levitan D, Zhang L, Tandon A, Song Y Q, Rogaeva E, Chen F, Kawarai T, et al. Nature (London) 2000;407:48–54. doi: 10.1038/35024009. [DOI] [PubMed] [Google Scholar]
- 5.Esler W P, Kimberly W T, Ostaszewski B L, Ye W, Diehl T S, Selkoe D J, Wolfe M S. Proc Natl Acad Sci USA. 2002;99:2720–2725. doi: 10.1073/pnas.052436599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wolfe M S, Xia W, Ostaszewski B L, Diehl T S, Kimberly W T, Selkoe D J. Nature (London) 1999;398:513–517. doi: 10.1038/19077. [DOI] [PubMed] [Google Scholar]
- 7.Steiner H, Duff K, Capell A, Romig H, Grim M G, Lincoln S, Hardy J, Yu X, Picciano M, Fechteler K, et al. J Biol Chem. 1999;274:28669–28673. doi: 10.1074/jbc.274.40.28669. [DOI] [PubMed] [Google Scholar]
- 8.Kimberly W T, Xia W, Rahmati T, Wolfe M S, Selkoe D J. J Biol Chem. 2000;275:3173–3178. doi: 10.1074/jbc.275.5.3173. [DOI] [PubMed] [Google Scholar]
- 9.Li Y-M, Xu M, Lai M-T, Huang Q, Castro J L, DiMuzio-Mower J, Harrison T, Lellis C, Nadin A, Neduvelli J G, et al. Nature (London) 2000;405:689–694. doi: 10.1038/35015085. [DOI] [PubMed] [Google Scholar]
- 10.Esler W P, Kimberly W T, Ostaszewski B L, Diehl T S, Moore C L, Tsai J-Y, Rahmati T, Xia W, Selkoe D J, Wolfe M S. Nat Cell Biol. 2000;2:428–433. doi: 10.1038/35017062. [DOI] [PubMed] [Google Scholar]
- 11.Steiner H, Kostka M, Romig H, Basset G, Pesold B, Hardy J, Capell A, Meyn L, Grim M G, Baumeister R, et al. Nat Cell Biol. 2000;2:848–851. doi: 10.1038/35041097. [DOI] [PubMed] [Google Scholar]
- 12.Steiner H, Haass C. Nat Rev Mol Cell Biol. 2000;1:217–224. doi: 10.1038/35043065. [DOI] [PubMed] [Google Scholar]
- 13.Mizutani T, Taniguchi Y, Aoki T, Hashimoto N, Honjo T. Proc Natl Acad Sci USA. 2001;98:9026–9031. doi: 10.1073/pnas.161269998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saxena M T, Schroeter E H, Mumm J S, Kopan R. J Biol Chem. 2001;276:40268–40273. doi: 10.1074/jbc.M107234200. [DOI] [PubMed] [Google Scholar]
- 15.Mumm J S, Kopan R. Dev Biol. 2000;228:151–165. doi: 10.1006/dbio.2000.9960. [DOI] [PubMed] [Google Scholar]
- 16.Pinnix I, Musunuru U, Tun H, Sridharan A, Golde T, Eckman C, Ziani-Cherif C, Onstead L, Sambamurti K. J Biol Chem. 2001;276:481–487. doi: 10.1074/jbc.M005968200. [DOI] [PubMed] [Google Scholar]
- 17.Cao X, Sudhof T C. Science. 2001;293:115–120. doi: 10.1126/science.1058783. [DOI] [PubMed] [Google Scholar]
- 18.Sastre M, Steiner H, Fuchs K, Capell A, Multhaup G, Condron M M, Teplow D B, Haass C. EMBO Rep. 2001;23:835–841. doi: 10.1093/embo-reports/kve180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gu Y, Misonou H, Sato T, Dohmae N, Takio K, Ihara Y. J Biol Chem. 2001;276:35235–35238. doi: 10.1074/jbc.C100357200. [DOI] [PubMed] [Google Scholar]
- 20.Yu C, Kim S H, Ikeuchi T, Xu H, Gasparini L, Wang R, Sisodia S S. J Biol Chem. 2001;276:43756–43760. doi: 10.1074/jbc.C100410200. [DOI] [PubMed] [Google Scholar]
- 21.Weidemann A, Eggert S, Reinhard F B, Vogel M, Paliga K, Baier G, Masters C L, Beyreuther K, Evin G. Biochemistry. 2002;41:2825–2835. doi: 10.1021/bi015794o. [DOI] [PubMed] [Google Scholar]
- 22.Song W, Nadeau P, Yuan M, Yang X, Shen J, Yankner B A. Proc Natl Acad Sci USA. 1999;96:6959–6963. doi: 10.1073/pnas.96.12.6959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baumeister R, Leimer U, Zweckbronner I, Jakubek C, Grunberg J, Haass C. Genes Funct. 1997;1:149–159. doi: 10.1046/j.1365-4624.1997.00012.x. [DOI] [PubMed] [Google Scholar]
- 24.Levitan D, Doyle T G, Brousseau D, Lee M K, Thinakaran G, Slunt H H, Sisodia S S, Greenwald I. Proc Natl Acad Sci USA. 1996;93:14940–14944. doi: 10.1073/pnas.93.25.14940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Davis J A, Naruse S, Chen H, Eckman C, Younkin S, Price D L, Borchelt D R, Sisodia S S, Wong P C. Neuron. 1998;20:603–609. doi: 10.1016/s0896-6273(00)80998-8. [DOI] [PubMed] [Google Scholar]
- 26.Qian S, Jiang P, Guan X M, Singh G, Trumbauer M E, Yu H, Chen H Y, Van de Ploeg L H, Zheng H. Neuron. 1998;20:611–617. doi: 10.1016/s0896-6273(00)80999-x. [DOI] [PubMed] [Google Scholar]
- 27.Steiner H, Romig H, Grim M G, Philipp U, Pesold B, Citron M, Baumeister R, Haass C. J Biol Chem. 1999;274:7615–7618. doi: 10.1074/jbc.274.12.7615. [DOI] [PubMed] [Google Scholar]
- 28.Wild-Bode C, Yamazaki T, Capell A, Leimer U, Steiner H, Ihara Y, Haass C. J Biol Chem. 1997;272:16085–16088. doi: 10.1074/jbc.272.26.16085. [DOI] [PubMed] [Google Scholar]
- 29.Citron M, Oltersdorf T, Haass C, McConlogue L, Hung A Y, Seubert P, Vigo-Pelfrey C, Lieberburg I, Selkoe D J. Nature (London) 1992;360:672–674. doi: 10.1038/360672a0. [DOI] [PubMed] [Google Scholar]
- 30.Capell A, Steiner H, Romig H, Keck S, Baader M, Grim M G, Baumeister R, Haass C. Nat Cell Biol. 2000;2:205–211. doi: 10.1038/35008626. [DOI] [PubMed] [Google Scholar]
- 31.Lu F M, Lux S E. Proc Natl Acad Sci USA. 1996;93:5663–5667. doi: 10.1073/pnas.93.11.5663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wacker I, Kaether C, Kromer A, Migala A, Almers W, Gerdes H H. J Cell Sci. 1997;110:1453–1463. doi: 10.1242/jcs.110.13.1453. [DOI] [PubMed] [Google Scholar]
- 33.Ezquerra M, Carnero C, Blesa R, Oliva R. Arch Neurol. 2000;57:485–488. doi: 10.1001/archneur.57.4.485. [DOI] [PubMed] [Google Scholar]
- 34.Steiner H, Romig H, Pesold B, Philipp U, Baader M, Citron M, Loetscher H, Jacobsen H, Haass C. Biochemistry. 1999;38:14600–14605. doi: 10.1021/bi9914210. [DOI] [PubMed] [Google Scholar]
- 35.Thinakaran G, Harris C L, Ratovitski T, Davenport F, Slunt H H, Price D L, Borchelt D R, Sisodia S S. J Biol Chem. 1997;272:28415–28422. doi: 10.1074/jbc.272.45.28415. [DOI] [PubMed] [Google Scholar]
- 36.De Jonghe C, Cras P, Vanderstichele H, Cruts M, Vanderhoeven I, Smouts I, Vanmechelen E, Martin J J, Hendriks L, Van Broeckhoven C. Neurobiol Dis. 1999;6:280–287. doi: 10.1006/nbdi.1999.0247. [DOI] [PubMed] [Google Scholar]
- 37.Murayama O, Tomita T, Nihonmatsu N, Murayama M, Sun X, Honda T, Iwatsubo T, Takashima A. Neurosci Lett. 1999;265:61–63. doi: 10.1016/s0304-3940(99)00187-1. [DOI] [PubMed] [Google Scholar]
- 38.Schroeter E H, Kisslinger J A, Kopan R. Nature (London) 1998;393:382–386. doi: 10.1038/30756. [DOI] [PubMed] [Google Scholar]
- 39.McLendon C, Xin T, Ziani-Cherif C, Murphy M P, Findlay K A, Lewis P A, Pinnix I, Sambamurti K, Wang R, Fauq A, et al. FASEB J. 2000;14:2383–2386. doi: 10.1096/fj.00-0286fje. [DOI] [PubMed] [Google Scholar]
- 40.Kulic L, Walter J, Multhaup G, Teplow D B, Baumeister R, Romig H, Capell A, Steiner H, Haass C. Proc Natl Acad Sci USA. 2000;97:5913–5918. doi: 10.1073/pnas.100049897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang R, Sweeney D, Gandy S E, Sisodia S S. J Biol Chem. 1996;271:31894–31902. doi: 10.1074/jbc.271.50.31894. [DOI] [PubMed] [Google Scholar]
- 42.Wiltfang J, Smirnov A, Schnierstein B, Kelemen G, Matthies U, Klafki H W, Staufenbiel M, Huther G, Ruther E, Kornhuber J. Electrophoresis. 1997;18:527–532. doi: 10.1002/elps.1150180332. [DOI] [PubMed] [Google Scholar]