Abstract
Transcriptional coactivators implicated in gene activation by the thyroid hormone receptor (TR) include members of the p160/steroid receptor coactivator (SRC) family of proteins, p300, and the multisubunit TR-associated protein (TRAP)/Mediator complex. We investigated the temporal recruitment of these cofactors to mammalian thyroid hormone (T3)-responsive promoters in vivo. We show that upon T3 treatment, TR recruits all three types of coactivators to specific promoters in at least two sequential steps: p160/SRC proteins and p300 are recruited first and rapidly induce histone acetylation, followed by the recruitment of the TRAP/Mediator complex. Interestingly, inhibition of histone deacetylase activity with trichostatin A elicited a more rapid promoter recruitment of the TRAP/Mediator complex but not p160/SRC proteins. T3-dependent gene expression assays indicate that all three coactivators are targeted to a promoter before significant activation occurs. These findings thus suggest that histone acetylation may be a prerequisite for TRAP/Mediator recruitment and function at specific T3-responsive mammalian promoters.
Thyroid hormone receptors (TRs) are members of the nuclear receptor (NR) superfamily and regulate transcription by recruiting specific coregulatory protein complexes to target gene promoters (reviewed in refs. 1 and 2). TR binds to target promoters as a heterodimer with the retinoid X receptor (RXR) and recruits transcriptional coactivators in the presence of thyroid hormone (T3) and corepressors in the absence of T3. Coactivators that directly interact with liganded TR and facilitate transactivation include members of the p160/steroid receptor coactivator (SRC) family (reviewed in refs. 3 and 4). These proteins are thought to function in part by associating with potent histone acetyltransferases (HATs) such as p300/CREB binding protein and ultimately target the HAT activity to promoter-bound TR resulting in acetylation of nucleosomes. Additionally, some p160/SRC family members contain intrinsic HAT activity (5, 6), further supporting a functional role for these factors in chromatin modification.
A second type of TR coactivator is the multisubunit TR-associated protein (TRAP)/Mediator complex (7, 8), which appears to bind liganded TR through a single subunit termed TRAP220 (9, 10). In vitro transcription assays show that TRAP/Mediator significantly enhances TR-dependent transcription on nonchromatin DNA templates (7, 11, 12), thus suggesting that the complex functions by positively influencing the basal transcription machinery, possibly by directly facilitating the recruitment of RNA polymerase II. Consistent with this view, several TRAP/Mediator subunits share homology with proteins in yeast Mediator, a large coactivator complex that directly associates with both transcriptional activators and the yeast RNA polymerase II holoenzyme (13). Most TRAP/Mediator subunits also have been identified in other large transcriptional coregulatory complexes, including NAT, DRIP, ARC, and CRSP (reviewed in ref. 14).
Although recent in vitro transcription studies clearly demonstrate a functional requirement for p160/SRC proteins, p300/CREB binding protein, and TRAP/Mediator in NR-mediated gene activation (11, 15, 17), the question remains as to how these distinct cofactors might temporally cooperate with one another at specific target promoters. Here we examine the recruitment of TR, p160/SRC proteins, p300, and TRAP/Mediator at mammalian T3-responsive promoters in vivo. Using chromatin immunoprecipitation (ChIP) assays, we show that TR is associated with these promoters in the absence of ligand. After hormone addition, TR rapidly recruits coactivators with HAT-associated activity (SRC-1, GRIP-1, p300), concomitantly triggering pronounced histone acetylation within the promoter region. Interestingly, we show that recruitment of the TRAP/Mediator complex to the same promoters occurs notably later but can be induced to bind more rapidly if histone deacetylase activity is inhibited. A time-course analysis of T3-dependent gene expression further suggests that both types of complexes must be recruited to the promoters before transcription is significantly activated. In sum, our results are consistent with the idea that HATs and TRAP/Mediator are required sequentially on distinct mammalian promoters for TR-mediated gene activation in vivo.
Materials and Methods
Antibodies.
Antibodies against AR (441), SRC-1 (S-19), GRIP-1 (C-20), TR (FL408), and p300 (C-20) were obtained from Santa Cruz Biotechnology. Antibodies against histone H4 (06–866) were obtained from Upstate Biotechnology, Lake Placid, NY. Antibodies against TRAP220 have been described (18).
ChIP.
The HeLa-derived α-2 cell line (stably expressing FLAG-hTRα) and rat pituitary GH3 cells were routinely maintained in DMEM supplemented with 10% dialyzed FBS (Life Technologies, Grand Island, NY). LNCaP cells were maintained in RPMI 1640 containing 10% charcoal/dextran-stripped FBS (HyClone). ChIP analyses were performed by using a modified procedure based on two previously described protocols (19, 20). After addition of either T3, cortisol, R1881 (10−7 M for durations indicated in the figures), and/or 100 nM trichostatin A (TSA; Upstate Biotechnology), cells (2 × 107) were treated with the cross-linking reagent formaldehyde (1% final concentration) for 10 min at room temperature; cross-linking was terminated upon addition of glycine (0.125 M final concentration). Cells were then rinsed twice with cold PBS, collected by centrifugation, and washed once in cold PBS containing 0.5 mM PMSF. Cells were then swollen on ice in buffer I (5 mM Pipes, pH 8.0/85 mM NaCl/0.5% Nonidet P-40/0.5 mM PMSF/100 ng/ml aprotinin/100 ng/ml leupeptin) for 30 min. Nuclei were collected by microcentrifugation and resuspended in sonication buffer (50 mM Tris⋅Cl, pH 8.1/1% SDS/0.5 mM PMSF/10 mM EDTA/100 ng/ml aprotinin/100 ng/ml leupeptin) followed by incubation on ice for 20 min. Samples were then sonicated on ice 6–8 times for 10 s each (i.e., until the average length of sheared genomic DNA was 1–1.5 kb) followed by centrifugation for 10 min. The chromatin solution was then incubated with specific antibodies or no antibodies (see input control below) and rotated at 4°C overnight (≈12 h). Immunoprecipitated chromatin complexes were isolated by adding 10 μl (packed) protein A/G-agarose beads (Roche Molecular Biochemicals) and rotating the reactions for 1 h at 4°C. The supernatants from reactions lacking primary antibodies were saved as a control for “total input of chromatin” and processed in parallel with the eluted immunoprecipitates beginning at the cross-link reversal step (below). Immunoprecipitates were sequentially washed twice with dialysis buffer (50 mM Tris⋅Cl, pH 8.0/2 mM EDTA/0.2% sarkosyl), followed by four washes in IP wash buffer (100 mM Tris⋅Cl, pH 9.0/500 mM LiCl/1% Nonidet P-40/1% deoxycholic acid). To elute the immunoprecipitated chromatin complexes from the resin, 150 μl of elution buffer (1% SDS/50 mM NaHCO3) was added to the beads, and the tubes were vortexed for 15 min. The supernatant was then collected, and the elution was repeated with a fresh 150 μl of elution buffer. After combining the eluants in one tube, the protein-DNA cross-linking was reversed by adding 5 M NaCl to a final concentration of 200 mM. RNA was removed from the samples by adding 10 μg RNase A (Roche Molecular Biochemicals) followed by incubation at 65°C for 4 h. The DNA in each sample was ethanol-precipitated and resuspended in 100 μl of Tris⋅Cl (pH 7.5), 25 μl of 5× proteinase K buffer (50 mM Tris⋅Cl, pH 8.5/1.25% SDS/25 mM EDTA), and 15 μg proteinase K (GIBCO/BRL) and subsequently incubated at 42°C for 2 h. Samples were then extracted with phenol/chloform/isoamylalcohol (25:24:1), and the DNA ethanol was precipitated and subsequently resuspended in 25 μl of H2O. Total input samples were resuspended in 100 μl of H2O and diluted 1:100 before PCR analysis. PCRs contained 5 μl of immunoprecipitate or total input (see above), 50 μM of each primer, 1.5 mM MgCl2, 2 mM dNTP mixture, 1× thermophilic buffer (Promega), and 1.25 units of TaqDNA polymerase (Promega) in a total volume of 100 μl. The primers for the human iodothyronine deiodinase type 1 (dio1) promoter were: forward, 5′-TCG AGC CTG TAA TCC CAG CAC-3′ and reverse, 5′-GCC AGA GTA AGC TCT GAG TTC-3′; for the rat sarcoplasmic endoplasmic reticulum calcium-ATPase (SERCA) promoter: forward, 5′-GGC TAA GGA GTG ATG AGG CCT AAG-3′ and reverse, 5′-ATC CAC CTG CCT GTT AAC CTG G-3′. Initially, PCR was performed with a serial dilution of input DNA to determine the linear range of the amplification for each gene. PCR was performed for 25 cycles, and all PCR signals were quantified by using FluorImager SI (Vistra Fluorescence Systems, New York) and National Institutes of Health image software.
Immunoprecipitation.
To verify the monospecificity of the ChIP antibodies under ChIP conditions, α-2 cells (107) were treated with the cross-linking reagent formaldehyde (1% final concentration) for 10 min at room temperature; cross-linking was terminated upon addition of glycine (0.125 M final concentration). Chromatin solution was then prepared as described above for the ChIP assay. An aliquot of this sample was incubated with specific antibodies (see Fig. 1D) and rotated at 4°C overnight (≈12 h). Immunoprecipitated complexes were isolated by adding 10 μl (packed) protein A/G-agarose beads (Roche Molecular Biochemicals) and rotating the reactions for 1 h at 4°C. Precipitated proteins were resolved on SDS-polyacrylamide gels, transferred to nitrocellulose membranes, and probed with the same antibodies used for the initial immunoprecipitation. Immuno-detection was performed as described below in Western blot analysis.
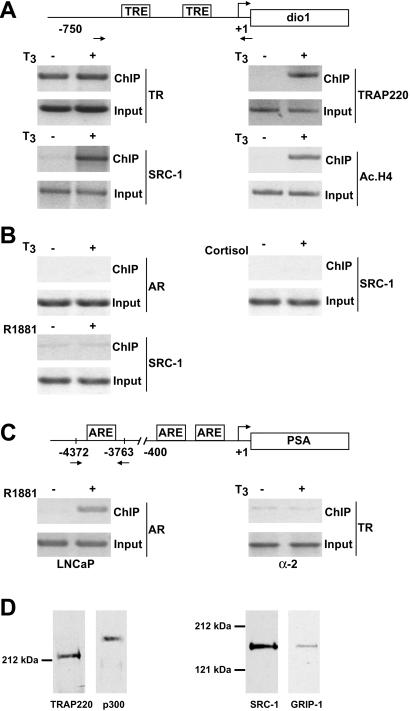
Figure 1.
TR-mediated cofactor recruitment and histone acetylation at the human dio1 promoter in vivo. (A) T3-dependent recruitment of TR cofactor complexes to the dio1 promoter. Cross-linked chromatin prepared from human α-2 cells, treated with or without T3 for 1 h, was immunoprecipitated with antibodies indicated on the right. The immunoprecipitates were subjected to PCR analysis using primer pairs spanning the human dio1 promoter (depicted schematically). Aliquots of chromatin taken before immunoprecipitation were used as PCR controls (Input). TREs and primer pairs (arrows) are indicated. (B) TR cofactor recruitment to the dio1 promoter is receptor and ligand specific. ChIP analyses were performed exactly as in A, except that α-2 cells were stimulated with the steroid hormones R1881 or cortisol (in place of T3) or antibodies against the androgen receptor (AR) were used. (C) TR fails to bind at the prostate-specific antigen (PSA) promoter. ChIP analyses were performed exactly as in A and B, except that both prostate LNCaP and α-2 cells were used and immunoprecipitates were subjected to PCR analysis using primer pairs spanning the human PSA promoter (shown schematically). (D) Antibody monospecificity under ChIP conditions. Chromatin extracts made from formaldehyde-treated α-2 cells were incubated with specific antibodies against either TRAP220, p300, SRC-1, or GRIP-1. Immune complexes were precipitated, boiled, resolved by SDS/PAGE, and probed by Western blot using the same antibodies initially used for the immunoprecipitation (indicated below each lane). SDS/PAGE molecular mass markers are shown.
Western Blot.
Equal numbers of α-2 or GH3 cells (107) were treated with T3 (10−7 M) for the duration indicated in the figures. Whole-cell lysate preparation was performed by scraping the cells in 1 ml of ice-cold buffer A (50 mM Tris⋅Cl, pH 7.4/150 mM NaCl/5 mM EDTA/0.5% Nonidet P-40/1 mM PMSF/10 μg/ml aprotinin/10 μg/ml leupeptin). The lysate was rotated 360o for 1 h at 4°C followed by centrifugation at 12,000 × g for 10 min at 4°C to clear the cellular debris. Proteins were resolved directly on SDS-polyacrylamide gels and subsequently transferred to nitrocellulose membranes. Immuno-detection was performed with enhanced chemiluminescence (ECL system, Amersham Pharmacia) according to the manufacturer's instructions. Antibodies were as described earlier except that anti-SRC-1 (MA1–840) was obtained from Affinity Bioreagents, Neshanic Station, NJ.
Ribonuclease Protection Assay (RPA).
The template for an antisense RNA probe complementary to rat SERCA mRNA was generated by PCR by incorporating a T7 promoter sequence into one of the PCR primers. The primers used were: forward, 5′-TCA TGG TGG CTC TTG GAT CAG-3′ and reverse, 5′-TAA TAC GAC TCA CTA TAG GTT CTC TAC CAG GCA CGT GAG A-3′. The T7 promoter in the PCR product was used to generate a 523-nt antisense SERCA RNA probe whereas the template for the antisense β-actin probe (pTRI-Actin; Ambion) yields a 304-nt transcript. Both antisense RNA probes were synthesized and 32P-labeled with the MAXIscript in vitro transcription kit (Ambion) and subsequently gel-purified. For the RPAs, total RNA was extracted from T3-treated GH3 cells by using the TRIZOL Reagent kit (Life Technologies) and quantified by UV absorption. RPA was carried out with the RPA III kit (Ambion) following the protocol provided. Briefly, 10 μg of total RNA was hybridized overnight at 42°C with 105 cpm of both antisense probes. Unhybridized RNA was then digested with a mixture of RNase T1 and RNase A. The products were resolved on a 6% acrylamide gel and visualized by autoradiography. Images were recorded and quantified with a PhosphorImager (STORM 840; Molecular Dynamics).
Results
We recently used a human HeLa-derived cell line (termed α-2) stably expressing epitope-tagged human TRα (7) to investigate the assembly kinetics of distinct types of TR coactivator complexes by coimmunoprecipitation (21). Interestingly, we found that TR formed complexes with HAT-containing cofactors almost immediately after T3 stimulation whereas precipitation of the TR-TRAP/Mediator complex occurred significantly later. Here, in an effort to better understand the ordered molecular events involved in ligand-dependent transcription by TR, we used ChIP analyses to examine the temporal recruitment of distinct TR coactivator complexes to specific T3-responsive gene promoters in vivo.
Using antibodies against either TR, the p160/SRC protein SRC-1, or TRAP220 (the protein that anchors TRAP/Mediator to TR), formaldehyde cross-linked protein-chromatin complexes were immunoprecipitated from α-2 cells cultured with or without T3. The resulting precipitated genomic DNA was then analyzed by PCR using primers spanning the T3 response elements (TREs) in the promoter region of the dio1 gene (22). As shown in Fig. 1A, ChIP analysis with anti-TR antibodies revealed that TR is associated with the dio1 promoter in both the presence and absence of T3, consistent with the observance of promoter-specific gene silencing by unliganded RXR/TR heterodimers (reviewed in ref. 3). As expected, TR recruitment of TRAP220 and SRC-1 to the dio1 promoter was observed only in T3-treated cells (≈10.5- and 12-fold, respectively) but not in cells treated with steroid hormones (Fig. 1B). Moreover, T3 treatment caused a significant increase in histone acetylation at the dio1 promoter (≈14.5-fold) as evidenced by ChIP analysis using anti-acetylated histone H4 antibodies (Fig. 1A). By contrast, T3 treatment failed to induce androgen receptor binding at the dio1 promoter (Fig. 1B) nor TR binding at the androgen-responsive prostate-specific antigen promoter (Fig. 1C). The monospecificity of the anti-TRAP220, -p300, -SRC-1, and -GRIP-1 antibodies under ChIP conditions was verified by cofactor immunoprecipitation from formaldehyde-treated α-2 cells followed by Western blotting (Fig. 1D). The monospecificity of the antiacetylated histone H4 antibody has been demonstrated (23, 24).
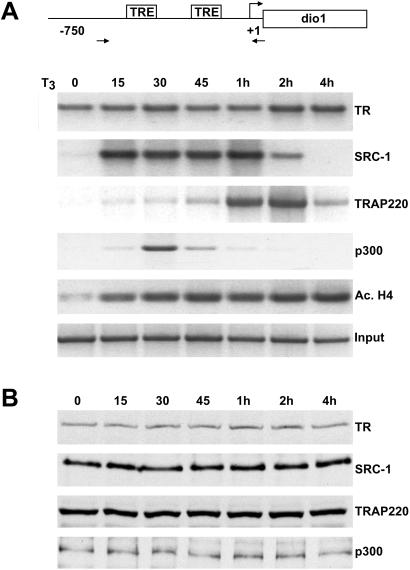
Although our findings clearly demonstrate T3-dependent occupancy of distinct TR coactivator complexes at the human dio1 promoter in vivo, we further sought to determine both the time course and the order of specific TR cofactor recruitment. Toward this end, α-2 cells were cultured with T3 for different lengths of time and again submitted to ChIP analyses. As before, TR was associated with the dio1 promoter in both the presence and absence of T3 (Fig. 2A). Examination of the recruitment kinetics of specific TR coactivator complexes revealed rapid association of cofactors possessing HAT activity (i.e., SRC-1 and p300) within 15–30 min post-T3 treatment. Maximal levels of SRC-1 binding (≈9.5-fold) were achieved within 15 min of T3 stimulation followed by a decline in promoter occupancy 2–4 h post-T3 treatment. Similarly, recruitment of p300 increased ≈8-fold within 30 min post-T3 treatment and rapidly returned to a basal level 1 h post-T3 treatment. The decline in SRC-1/p300 occupancy is consistent with an earlier study that proposed that acetylation of P160 by p300/CREB binding protein might conceivably disrupt NR coactivator interactions (24). In agreement with the rapid T3-induced recruitment of HAT cofactors, histones associated with the dio1 promoter undergo significant acetylation (≈10-fold) within 15 min of T3 treatment and appear to remain acetylated up to 4 h post-ligand exposure (Fig. 2A). Given that the HAT activity associated with p160 coactivators is thought to be relatively weak (3), the significant levels of histone acetylation observed at 15 min post-T3 treatment is likely accounted for by the low, yet apparently potent, levels of p300 associated with the promoter 15 min post-T3 exposure (Fig. 2A).
Figure 2.
Ordered recruitment of HAT cofactors and TRAP/Mediator at the dio1 promoter in vivo. (A) T3-dependent temporal recruitment of TR cofactors at the dio1 promoter. ChIP assays were performed as in Fig. 1, except that α-2 cells were treated with T3 for varying lengths of time (indicated above the lanes). Antibodies used for ChIP are indicated on the right. (B) Protein expression of TR and TR cofactors in α-2 cells. Western blot analyses comparing the relative protein expression levels of TR and TR coactivators in α-2 cells at different times after T3 treatment (indicated above the lanes). Antibodies used for Western blotting are indicated on the right.
In contrast to the rapid T3-induced recruitment of HAT cofactors, recruitment of TRAP220 to dio1 promoter displayed considerably slower kinetics. Moderate TRAP220 occupancy of the dio1 promoter did not occur until 45 min post-T3 induction, with maximal levels (≈13.5-fold) occurring 2 h post-T3 treatment (Fig. 2A). Interestingly, TRAP220 occupancy declined 2–4 h post-T3 treatment, becoming only marginal at 4 h post-T3 exposure. As shown in Fig. 2B, no significant changes in protein expression levels were observed up to 4 h post-T3 treatment for SRC-1, TRAP220, TR, and p300. In sum, the data in Fig. 2 suggest that TR-mediated transactivation at the human dio1 gene involves sequential recruitment of distinct TR coactivator complexes.
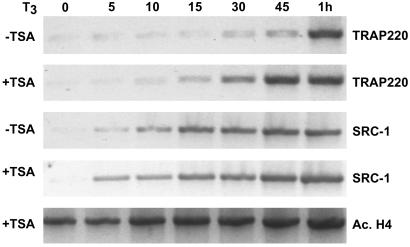
The recruitment of SRC-1 and p300 to the dio1 promoter before the binding of TRAP/Mediator suggests that histone acetylation may be a prerequisite for TRAP/Mediator recruitment at specific promoters. To address this hypothesis more closely, α-2 cells were pretreated with the histone deacetylase inhibitor TSA for 24 h and then assayed for TRAP/Mediator binding at the dio1 promoter after T3 treatment. Consistent with a decrease in histone deacetylase activity, enhanced levels of histone acetylation were observed in α-2 cells pretreated with TSA even in the absence of T3 (Fig. 3). Intriguingly, TRAP220 was recruited to the dio1 promoter more rapidly in TSA-treated cells than in nontreated cells with significant TRAP220 occupancy at 30–45 min post-T3 exposure (Fig. 3). In contrast to the accelerated TRAP220 recruitment, the kinetics of SRC-1 recruitment remained essentially unchanged in TSA-treated versus nontreated α-2 cells (Fig. 3). These data thus suggest that acetylation of nucleosomes within the dio1 promoter region may generate a chromatin structure that favors the recruitment and binding of TRAP/Mediator.
Figure 3.
Inhibition of histone deacetylase activity accelerates T3-dependent recruitment of TRAP/Mediator complex to the dio1 promoter in vivo. α-2 Cells were treated with or without 100 nM TSA (+ or − TSA as indicated) for 24 h followed by treatment with T3 for different intervals of time (indicated above the lanes). ChIP analysis of the dio1 promoter was then performed as in Fig. 2 by using the antibodies indicated on the right.
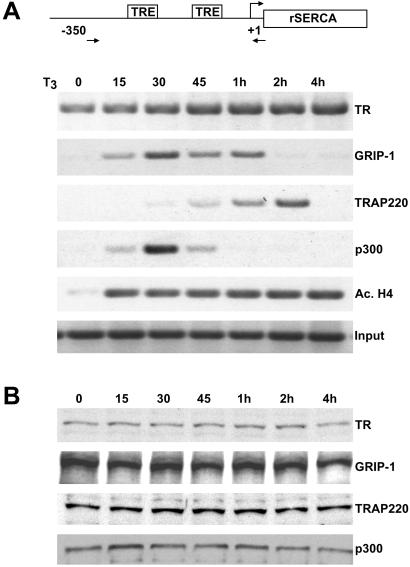
To investigate whether the observed temporal recruitment of different coactivator complexes at the human dio1 promoter might reflect a more common mechanism of TR-mediated activation in other mammalian cells, we examined TR cofactor recruitment at T3-responsive promoters in the rat pituitary GH3 cell line. Given their significant endogenous TR expression, GH3 cells can serve as effective model systems to study the molecular mechanism of T3-mediated gene expression (25). One well-characterized T3-responsive promoter in rat cells is found upstream of the rat SERCA gene (26). To investigate TR cofactor recruitment in GH3 cells, primer pairs specific for the rat SERCA promoter were used to perform ChIP analyses on cells cultured with T3 for different lengths of time. Remarkably, the temporal pattern of TR coactivator recruitment at the rat SERCA promoter was nearly identical to the pattern observed at the human dio1 promoter (compare Fig. 2A with Fig. 4A). Indeed, TR coactivators associated with HAT activity (i.e., the rat p160/SRC protein GRIP-1 and p300) again displayed rapid and transient association with the rat SERCA promoter and concomitantly triggered a pronounced acetylation of histones (Fig. 4A). Importantly, recruitment of TRAP220 again displayed considerably slower kinetics with maximum recruitment at ≈2 h post-T3 treatment followed by virtually no binding at 4 h post-T3 exposure (Fig. 4A). ChIP analyses using primers specific for the T3-responsive CYP7 rat gene promoter (27) gave similar results (data not shown). As observed earlier with the α-2 line, there was no significant change in the protein expression levels of either TR or TR coactivators in GH3 cells up to 4 h post-T3 treatment (Fig. 4B).
Figure 4.
Ordered recruitment of HAT cofactors and TRAP/Mediator at the rat SERCA promoter. (A) T3-dependent temporal recruitment of TR cofactors at the rat SERCA promoter. A T3-dependent ChIP time course was performed as in Fig. 2 except that rat GH3 cells were used and the immunoprecipitates were subjected to PCR analysis using primer pairs spanning the rat SERCA promoter (depicted schematically at the top). Primers (arrows) and TREs are indicated. (B) Protein expression of TR and TR cofactors in GH3 cells. Western blot analysis comparing the relative cellular protein levels of TR and TR coactivators in GH3 cells at different times after T3 treatment (indicated above the lanes).
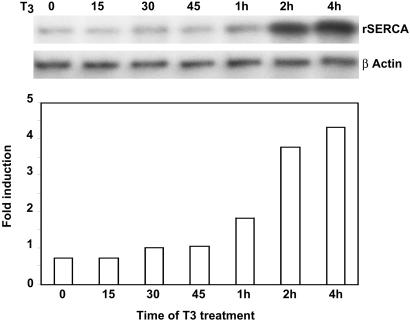
Our results indicate that at specific T3-responsive mammalian promoters, TR recruits HAT cofactors and the TRAP/Mediator complex in at least two sequential steps within a ≈0–2-h span post-T3 induction. The findings lead to a hypothesis in which both types of complexes might be functionally required before transcriptional activation. Consistent with this supposition, we previously demonstrated that T3-dependent activation of the human dio1 gene in α-2 cells is preceded by at least a 2-h lag period after T3 exposure (21). To determine whether T3-responsive genes in GH3 cells exhibit a similar delay in ligand-dependent activation, we measured the kinetics of rat SERCA gene expression after T3 exposure by using RPA. In close agreement with the dio1 expression kinetics, significant T3-dependent SERCA gene transcription did not occur until 1–2 h post-T3 induction with maximal expression occurring 2–4 h post-T3 exposure (Fig. 5). Thus, not only do both human dio1 and rat SERCA promoters exhibit a similar pattern of TR cofactor recruitment in vivo, both promoters exhibit a similar pattern of delayed T3-dependent transactivation. Taken together, these findings are consistent with the idea that ordered recruitment of HAT cofactors and TRAP/Mediator at specific T3-responsive gene promoters in mammals may be a functional requirement for TR-mediated activation.
Figure 5.
Ordered recruitment of HAT cofactors and TRAP/Mediator at the rat SERCA (rSERCA) promoter correlates with the kinetics of T3-induced SERCA gene expression. RPAs of rSERCA and β-actin expression were performed on GH3 cells treated with T3 for different lengths of time. Images were recorded and quantified with a PhosphorImager.
Discussion
In this study, we examined the binding kinetics of TR coactivator complexes at mammalian T3-responsive promoters in vivo. Our findings provide evidence for an ordered recruitment of HAT cofactors, followed by TRAP/Mediator, at distinct target promoters in mammalian cells. Relevant to our data, studies in yeast show that activation of the HO gene specifically requires the sequential recruitment of HAT cofactors, followed by the yeast Mediator complex (28); these studies concluded that HAT-mediated nucleosome destabilization is a functional prerequisite for yeast Mediator recruitment. Given the ordered recruitment of distinct TR cofactor complexes demonstrated here, our findings support a multistep model of NR-mediated gene activation (8, 29). In this scenario, TR recruitment of HAT activity to T3-responsive promoters presumably generates a chromatin structure permissible to the binding of other large multimeric coregulatory complexes. In a temporally subsequent step, TR recruits TRAP/Mediator, which in turn more directly interfaces with RNA polymerase II and potentiates transcription initiation.
How could initial recruitment of HAT activity facilitate the subsequent recruitment of the TRAP/Mediator complex on certain promoters? Several recent reports indicate that acetylated histones can act as direct binding surfaces for several different transcriptional coactivators (30–32). Hence, it is plausible that specific TRAP/Mediator subunit(s) might preferentially contact acetylated histone residues and possibly stabilize TRAP/Mediator association with higher-ordered chromatin. In addition, targeted HAT activity may simply generate a less compact chromatin structure that presumably allows TRAP/Mediator access to the promoter. Indeed, given the relatively large size of the TRAP/Mediator complex (≈1.5 MDa) and its association with the equally large RNA polymerase II holocomplex, HAT-mediated chromatin accessibility likely plays an important role in targeting these multimeric complexes to specific gene promoters. It is noteworthy in this regard that recruitment of the Drosophila Mediator complex to the hsp70 promoter, which is continuously maintained in an open nucleosome-free configuration, occurs rapidly in vivo and does not require prerequisite HAT activity (33).
Interestingly, a recent ChIP study found that p160/SRC proteins and TRAP220 (a.k.a. PBP) are simultaneously recruited to estrogen-responsive promoters by the estrogen receptor (ER) rapidly after ligand treatment (within 15–30 min postexposure) and that their binding precisely coincides with rapid ligand-dependent transactivation (34). Given that ER binds DNA cyclically in a ligand-dependent manner, whereas RXR/TR heterodimers bind DNA in the absence of ligand and are concomitantly associated with corepressors, T3-dependent transactivation by TR may require an additional chromatin derepression step that presumably necessitates a different order of cofactor recruitment and slower transcriptional activation kinetics. Indeed, our results may reveal fundamental differences between steroid and nonsteroid NR signaling pathways in terms of specific temporal requirements for coactivators. Recent studies by Hager and colleagues (35, 36) show that steroid receptors rapidly cycle on and off target genes and that a significant portion of TR exists in the cytoplasm and translocates to the nucleus after T3 stimulation. Thus, with regard to the data presented here, the continuous presence of TR throughout the duration of the ChIP assays may actually reflect a steady-state rate of TR association and dissociation with specific target promoters in vivo.
Although the findings here implicate HAT cofactors and TRAP/Mediator in facilitating important sequential steps during NR-mediated gene expression, several recent studies further demonstrate a functional requirement for chromatin remodeling complexes. For example, the ATP-dependent ISWI chromatin remodeling complex is required for retinoic acid receptor/RXR binding to chromatin templates (15) and conceivably facilitates a similar role for RXR/TR. Furthermore, the SWI/SNF chromatin remodeling complex was shown to play a critical role in several NR-mediated transactivation pathways (15, 37, 38). Paradoxically, studies in yeast implicate a temporal functional role for SWI/SNF before the recruitment of HAT cofactors (28, 39), whereas experiments with NRs and other mammalian activators suggest that SWI/SNF functions after the recruitment of HAT activity (15, 40). Future studies should reveal the temporal functional role of TRAP/Mediator in the context of promoter-specific recruitment of chromatin remodeling activity.
Acknowledgments
We thank Drs. Peggy Farnham and Sankar Mitra for sharing protocols and technical advice with the ChIP assays and Dr. Neeraj Saxena for assistance with RPA. We also thank Dr. Paul Yen for helpful discussions and critical reading of the manuscript. This work was supported by National Institutes of Health Grant DK54030-03 (to J.D.F.).
Abbreviations
- TR
thyroid hormone receptor
- TRAP
TR-associated protein
- ChIP
chromatin immunoprecipitation
- HAT
histone acetyltransferase
- dio1
iodothyronine deiodinase type 1
- SERCA
sarcoplasmic endoplasmic reticulum calcium-ATPase
- NR
nuclear receptor
- RXR
retinoid X receptor
- TSA
trichostatin A
- RPA
ribonuclease protection assay
- TRE
T3 response element
- SRC
steroid receptor coactivator
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Zhang J, Lazar M A. Annu Rev Physiol. 2000;62:439–466. doi: 10.1146/annurev.physiol.62.1.439. [DOI] [PubMed] [Google Scholar]
- 2.Yen P M. Physiol Rev. 2001;81:1097–1142. doi: 10.1152/physrev.2001.81.3.1097. [DOI] [PubMed] [Google Scholar]
- 3.Glass C K, Rosenfeld M G. Genes Dev. 2000;14:121–141. [PubMed] [Google Scholar]
- 4.McKenna N J, Lanz R B, O'Malley B W. Endocr Rev. 1999;20:321–344. doi: 10.1210/edrv.20.3.0366. [DOI] [PubMed] [Google Scholar]
- 5.Chen H, Lin R J, Schiltz R L, Chakravarti D, Nash A, Nagy L, Privalsky M L, Nakatani Y, Evans R M. Cell. 1997;90:569–580. doi: 10.1016/s0092-8674(00)80516-4. [DOI] [PubMed] [Google Scholar]
- 6.Spencer T E, Jenster G, Burcin M M, Allis C D, Zhou J, Mizzen C A, McKenna N J, Onate S A, Tsai S Y, Tsai M J, O'Malley B W. Nature (London) 1997;389:194–198. doi: 10.1038/38304. [DOI] [PubMed] [Google Scholar]
- 7.Fondell J D, Ge H, Roeder R G. Proc Natl Acad Sci USA. 1996;93:8329–8333. doi: 10.1073/pnas.93.16.8329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ito M, Roeder R G. Trends Endocrinol Metab. 2001;12:127–134. doi: 10.1016/s1043-2760(00)00355-6. [DOI] [PubMed] [Google Scholar]
- 9.Yuan C X, Ito M, Fondell J D, Fu Z Y, Roeder R G. Proc Natl Acad Sci USA. 1998;95:7939–7944. doi: 10.1073/pnas.95.14.7939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ren Y, Behre E, Ren Z, Zhang J, Wang Q, Fondell J D. Mol Cell Biol. 2000;20:5433–5446. doi: 10.1128/mcb.20.15.5433-5446.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fondell J D, Guermah M, Malik S, Roeder R G. Proc Natl Acad Sci USA. 1999;96:1959–1964. doi: 10.1073/pnas.96.5.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ito M, Yuan C X, Malik S, Gu W, Fondell J D, Yamamura S, Fu Z Y, Zhang X, Qin J, Roeder R G. Mol Cell. 1999;3:361–370. doi: 10.1016/s1097-2765(00)80463-3. [DOI] [PubMed] [Google Scholar]
- 13.Myers L C, Kornberg R D. Annu Rev Biochem. 2000;69:729–749. doi: 10.1146/annurev.biochem.69.1.729. [DOI] [PubMed] [Google Scholar]
- 14.Malik S, Roeder R G. Trends Biochem Sci. 2000;25:277–283. doi: 10.1016/s0968-0004(00)01596-6. [DOI] [PubMed] [Google Scholar]
- 15.Dilworth F J, Fromental-Ramain C, Yamamoto K, Chambon P. Mol Cell. 2000;6:1049–1058. doi: 10.1016/s1097-2765(00)00103-9. [DOI] [PubMed] [Google Scholar]
- 16.Li J, O'Malley B W, Wong J. Mol Cell Biol. 2000;20:2031–2042. doi: 10.1128/mcb.20.6.2031-2042.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kraus W L, Kadonaga J T. Genes Dev. 1998;12:331–342. doi: 10.1101/gad.12.3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang J, Fondell J D. Mol Endocrinol. 1999;13:1130–1140. doi: 10.1210/mend.13.7.0295. [DOI] [PubMed] [Google Scholar]
- 19.Boyd K E, Farnham P J. Mol Cell Biol. 1999;19:8393–8399. doi: 10.1128/mcb.19.12.8393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bhakat K K, Mitra S. J Biol Chem. 2000;275:34197–34204. doi: 10.1074/jbc.M005447200. [DOI] [PubMed] [Google Scholar]
- 21.Sharma D, Fondell J D. Mol Endocrinol. 2000;14:2001–2009. doi: 10.1210/mend.14.12.0567. [DOI] [PubMed] [Google Scholar]
- 22.Toyoda N, Zavacki A M, Maia A L, Harney J W, Larsen P R. Mol Cell Biol. 1995;15:5100–5112. doi: 10.1128/mcb.15.9.5100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eberhardy S R, D'Cunha C A, Farnham P J. J Biol Chem. 2000;275:33798–33805. doi: 10.1074/jbc.M005154200. [DOI] [PubMed] [Google Scholar]
- 24.Chen H, Lin R J, Xie W, Wilpitz D, Evans R M. Cell. 1999;98:675–686. doi: 10.1016/s0092-8674(00)80054-9. [DOI] [PubMed] [Google Scholar]
- 25.Spindler S R, Mellon S H, Baxter J D. J Biol Chem. 1982;257:11627–11632. [PubMed] [Google Scholar]
- 26.Simonides W S, Brent G A, Thelen M H, van der Linden C G, Larsen P R, van Hardeveld C. J Biol Chem. 1996;271:32048–32056. doi: 10.1074/jbc.271.50.32048. [DOI] [PubMed] [Google Scholar]
- 27.Ness G C, Lopez D. Arch Biochem Biophys. 1995;323:404–408. doi: 10.1006/abbi.1995.0061. [DOI] [PubMed] [Google Scholar]
- 28.Cosma M P, Panizza S, Nasmyth K. Mol Cell. 2001;7:1213–1220. doi: 10.1016/s1097-2765(01)00266-0. [DOI] [PubMed] [Google Scholar]
- 29.Dilworth F J, Chambon P. Oncogene. 2001;20:3047–3054. doi: 10.1038/sj.onc.1204329. [DOI] [PubMed] [Google Scholar]
- 30.Dhalluin C, Carlson J E, Zeng L, He C, Aggarwal A K, Zhou M M. Nature (London) 1999;399:491–496. doi: 10.1038/20974. [DOI] [PubMed] [Google Scholar]
- 31.Jacobson R H, Ladurner A G, King D S, Tjian R. Science. 2000;288:1422–1425. doi: 10.1126/science.288.5470.1422. [DOI] [PubMed] [Google Scholar]
- 32.Owen D J, Ornaghi P, Yang J-C, Lowe N, Evans P R, Ballario P, Neuhaus D, Filetici P, Travers A A. EMBO J. 2000;19:6141–6149. doi: 10.1093/emboj/19.22.6141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Park J M, Werner J, Kim J M, Lis J T, Kim Y-J. Mol Cell. 2001;8:9–19. doi: 10.1016/s1097-2765(01)00296-9. [DOI] [PubMed] [Google Scholar]
- 34.Shang Y, Hu X, DiRenzo J, Lazar M A, Brown M. Cell. 2000;103:843–852. doi: 10.1016/s0092-8674(00)00188-4. [DOI] [PubMed] [Google Scholar]
- 35.McNally J G, Müller W G, Walker D, Wolford R, Hager G L. Science. 2000;287:1262–1265. doi: 10.1126/science.287.5456.1262. [DOI] [PubMed] [Google Scholar]
- 36.Zhu X-G, Hanover J A, Hager G L, Cheng S-Y. J Biol Chem. 1998;273:27058–27063. doi: 10.1074/jbc.273.42.27058. [DOI] [PubMed] [Google Scholar]
- 37.Fryer C J, Archer T K. Nature (London) 1998;393:88–91. doi: 10.1038/30032. [DOI] [PubMed] [Google Scholar]
- 38.DiRenzo J, Shang Y, Phelan M, Sif S, Myers M, Kingston R, Brown M. Mol Cell Biol. 2000;20:7541–7549. doi: 10.1128/mcb.20.20.7541-7549.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cosma M P, Tanaka T, Nasmyth K. Cell. 1999;97:299–311. doi: 10.1016/s0092-8674(00)80740-0. [DOI] [PubMed] [Google Scholar]
- 40.Munshi N, Agalioti T, Lomvardas S, Merika M, Chen G, Thanos D. Science. 2001;293:1133–1136. doi: 10.1126/science.293.5532.1133. [DOI] [PubMed] [Google Scholar]