Abstract
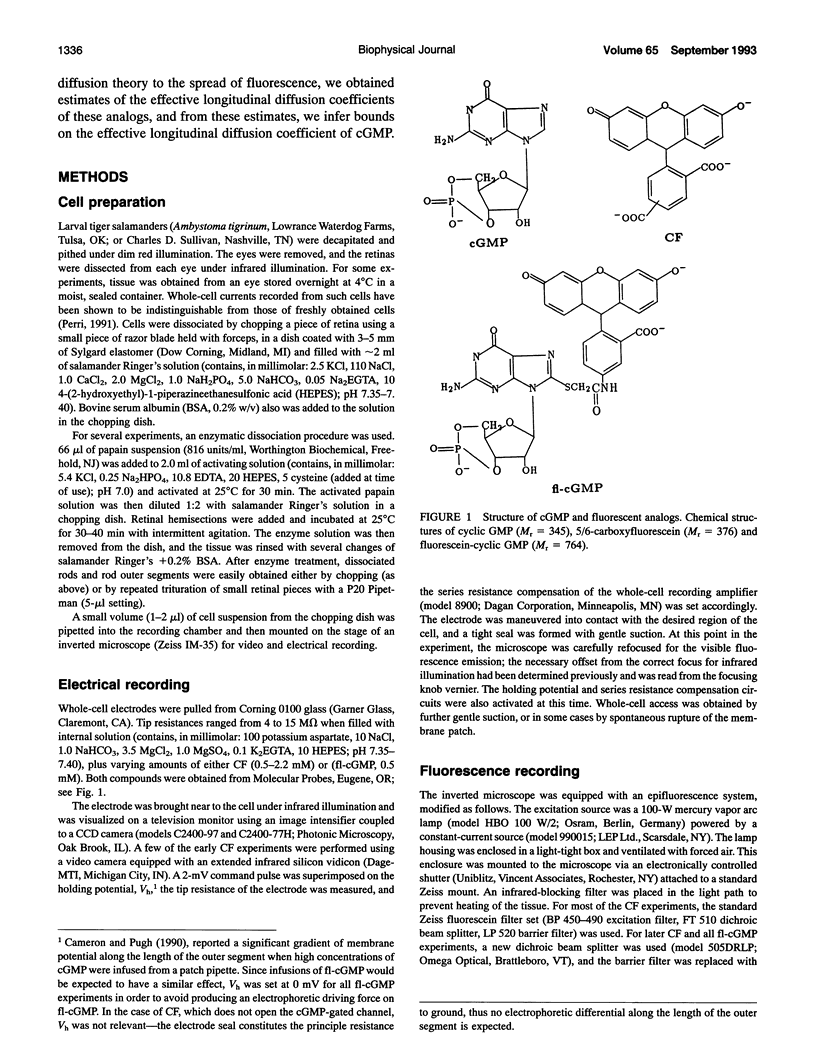
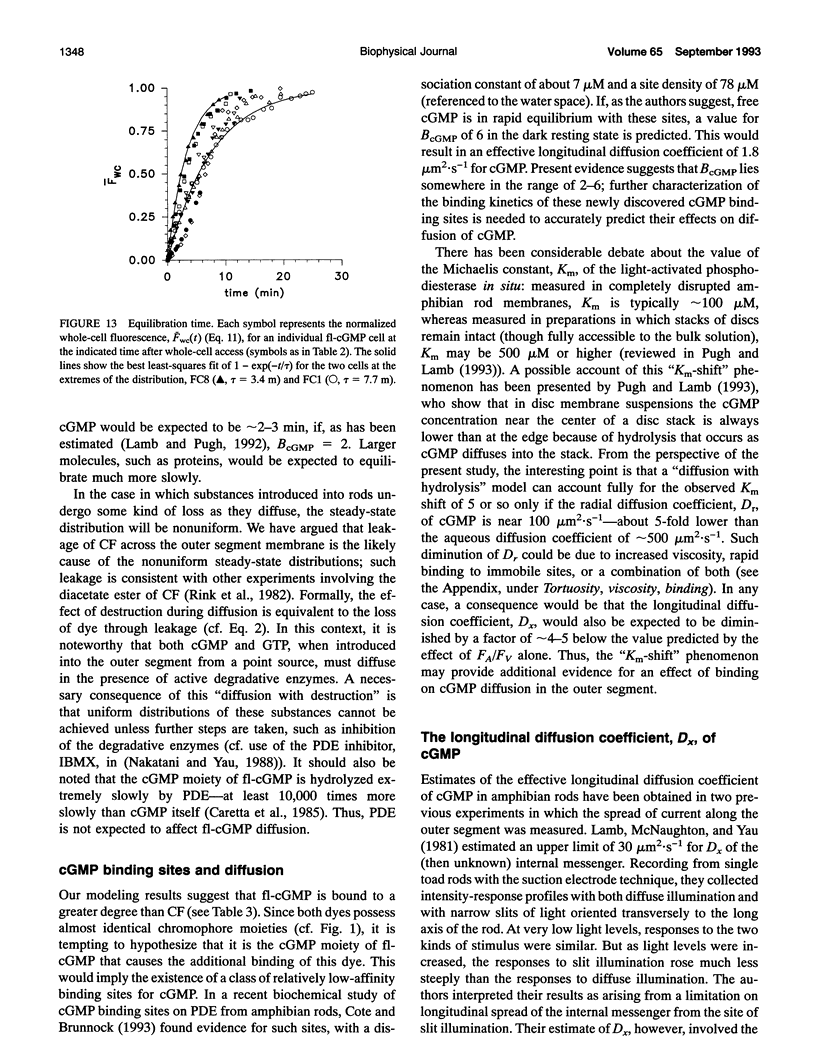
Experiments have demonstrated that single photoisomerizations in amphibian and primate rods can cause the suppression of 3-5% of the dark circulating current at the response peak (Baylor, D. A., T. D. Lamb, and K. W. Yau. 1979. J. Physiol. (Lond.). 288:613-634; Baylor, D. A., B. J. Nunn, and J. L. Schnapf. 1984. J. Physiol. (Lond.). 357:575-607). These results indicate that the change in [cGMP] effected by a single isomerization must spread longitudinally over at least the corresponding fractional length of the outer segment. The effective longitudinal diffusion coefficient, Dx, of cGMP is thus an important determinant of rod sensitivity. We report here measurements of the effective longitudinal diffusion coefficients, Dx, of two fluorescently labeled molecules: 5/6-carboxyfluorescein and 8-(fluoresceinyl)thioguanosine 3',5'-cyclic monophosphate, introduced into detached outer segments via whole-cell patch electrodes. For these compounds, the average time for equilibration of the entire outer segment with the patch pipette was approximately 6 min. Fluorescence images of rods were analyzed with a one-dimensional diffusion model that included limitations on transfer between the electrode and outer segment and the effects of intracellular binding of the dyes. The analyses yielded estimates of Dx of 1.9 and 1.0 microns 2.s-1 for the two dyes. It is shown that these results place an upper limit on Dx for cGMP of 11 microns2.s-1. The actual value of Dx for cGMP in the rod will depend on the degree of intracellular binding of cGMP. Estimates of the effective buffering power for cGMP in the rod at rest range from two to six (Lamb and Pugh, 1992; Cote and Brunnock, 1993). When combined with these estimates, our results predict that for cGMP itself, Dx falls within the range of 1.4-5.5 microns 2.s-1.
Full text
PDF



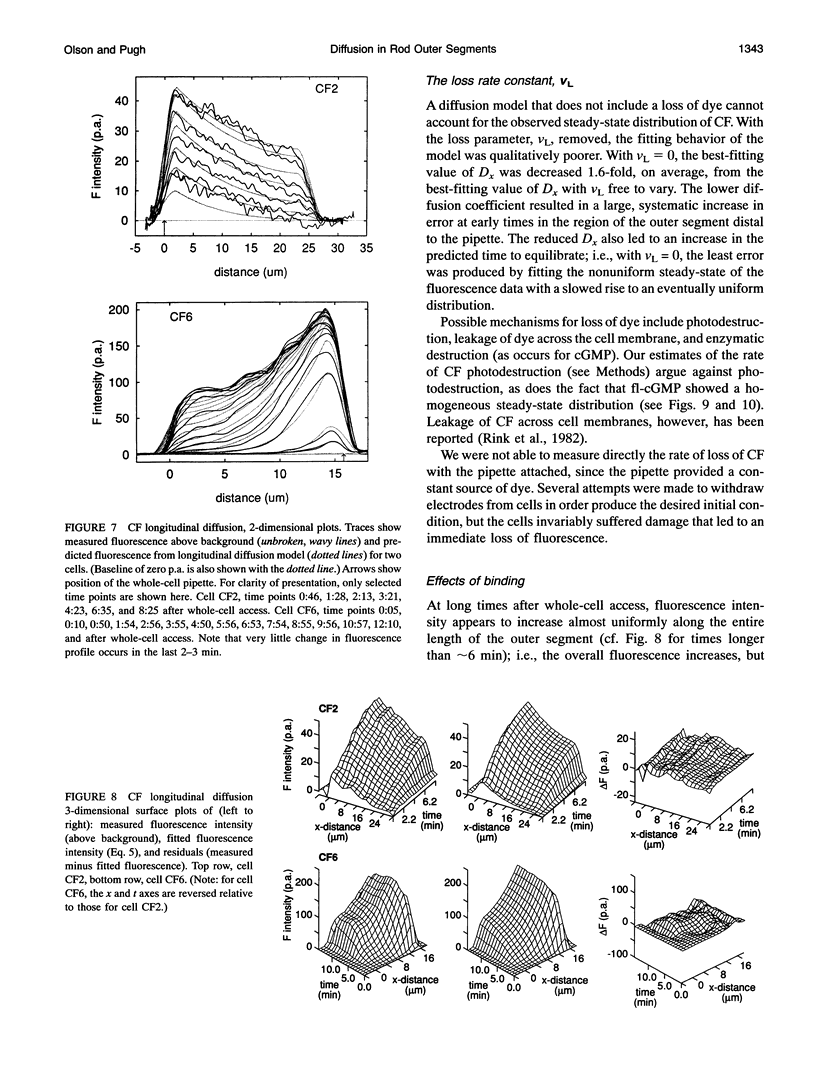
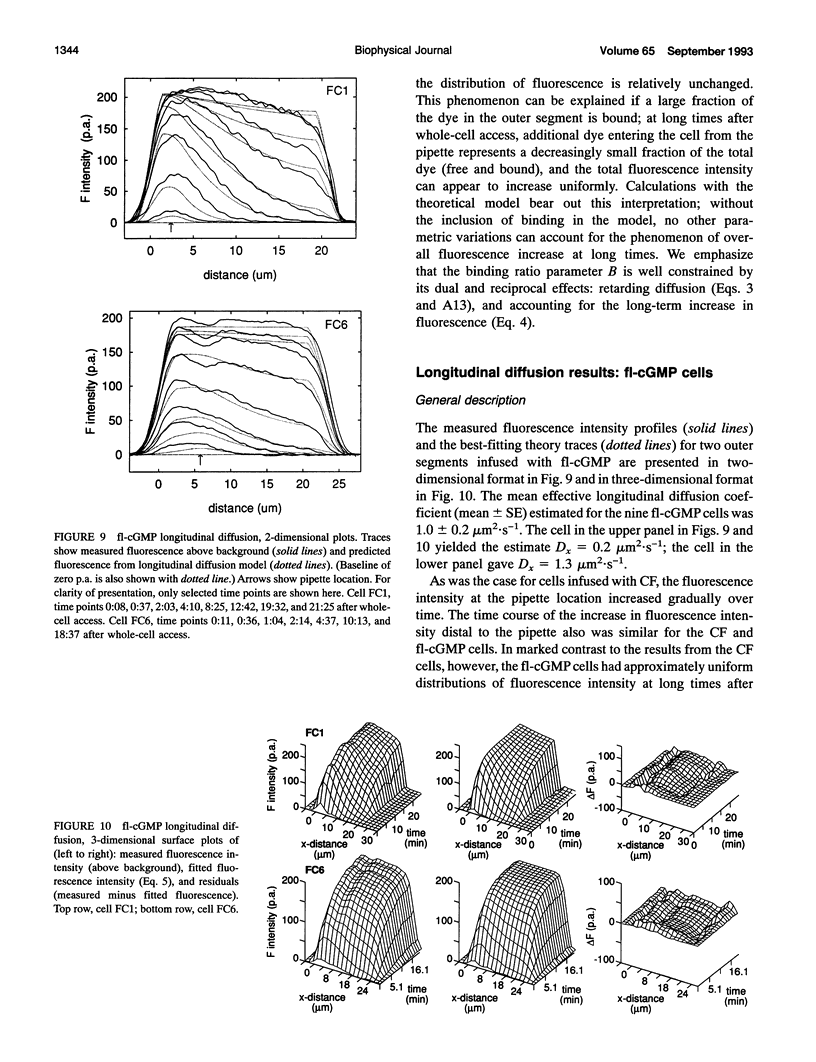
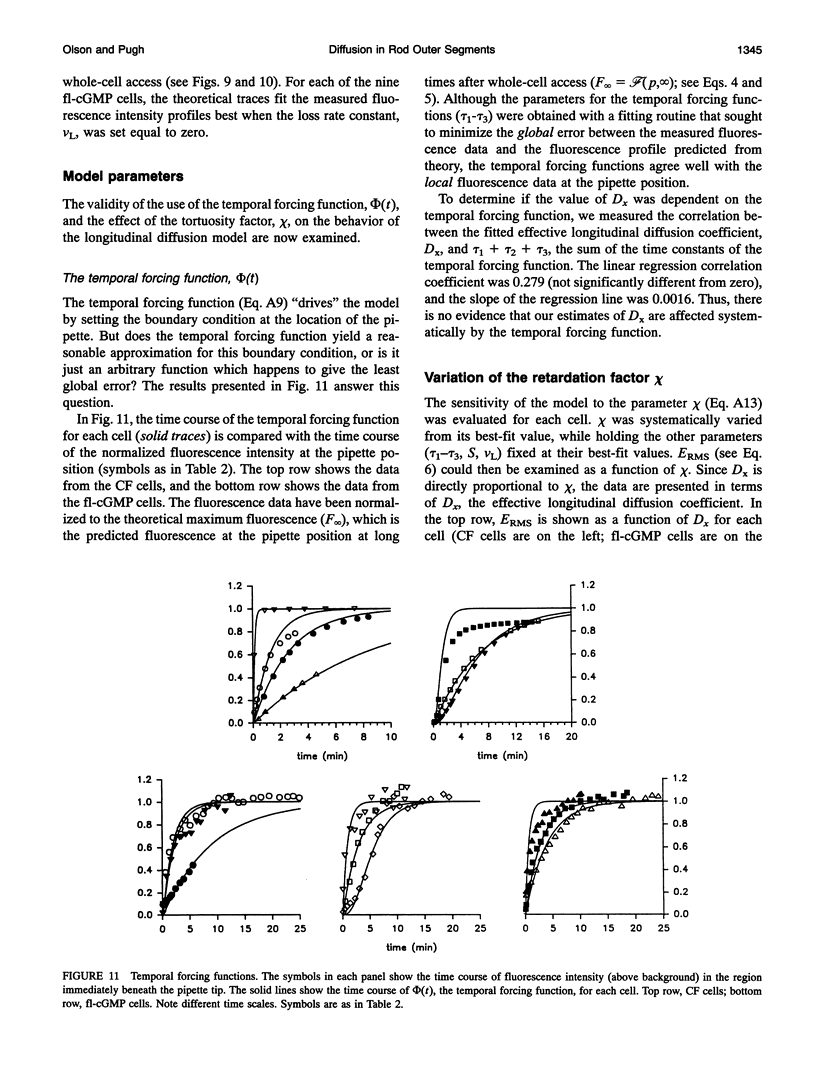
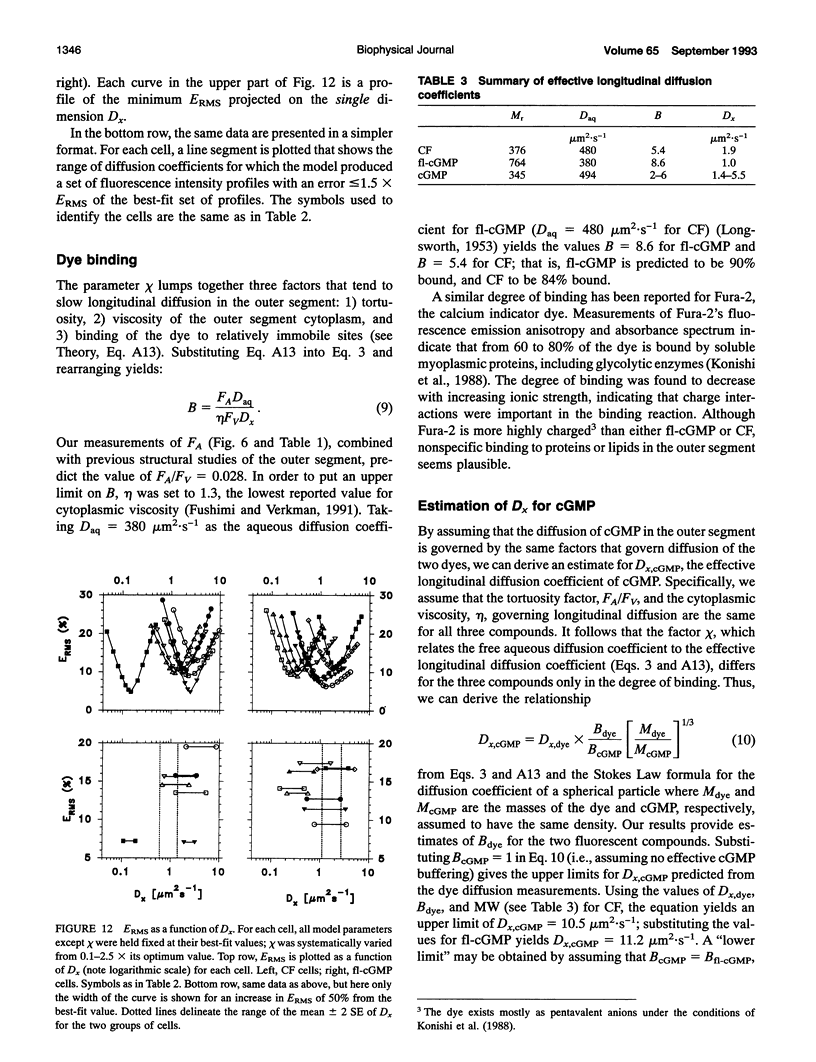





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baylor D. A., Lamb T. D., Yau K. W. Responses of retinal rods to single photons. J Physiol. 1979 Mar;288:613–634. [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Nunn B. J., Schnapf J. L. The photocurrent, noise and spectral sensitivity of rods of the monkey Macaca fascicularis. J Physiol. 1984 Dec;357:575–607. doi: 10.1113/jphysiol.1984.sp015518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaurock A. E., Wilkins M. H. Structure of frog photoreceptor membranes. Nature. 1969 Aug 30;223(5209):906–909. doi: 10.1038/223906a0. [DOI] [PubMed] [Google Scholar]
- Cameron D. A., Pugh E. N., Jr The magnitude, time course and spatial distribution of current induced in salamander rods by cyclic guanine nucleotides. J Physiol. 1990 Nov;430:419–439. doi: 10.1113/jphysiol.1990.sp018299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caretta A., Cavaggioni A., Sorbi R. T. Binding stoichiometry of a fluorescent cGMP analogue to membranes of retinal rod outer segments. Eur J Biochem. 1985 Nov 15;153(1):49–53. doi: 10.1111/j.1432-1033.1985.tb09265.x. [DOI] [PubMed] [Google Scholar]
- Fushimi K., Verkman A. S. Low viscosity in the aqueous domain of cell cytoplasm measured by picosecond polarization microfluorimetry. J Cell Biol. 1991 Feb;112(4):719–725. doi: 10.1083/jcb.112.4.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gras W. J., Worthington C. R. X-ray analysis of retinal photoreceptors. Proc Natl Acad Sci U S A. 1969 Jun;63(2):233–238. doi: 10.1073/pnas.63.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagins W. A., Penn R. D., Yoshikami S. Dark current and photocurrent in retinal rods. Biophys J. 1970 May;10(5):380–412. doi: 10.1016/S0006-3495(70)86308-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura S., Murakami M. Regulation of cGMP levels by guanylate cyclase in truncated frog rod outer segments. J Gen Physiol. 1989 Oct;94(4):649–668. doi: 10.1085/jgp.94.4.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konishi M., Olson A., Hollingworth S., Baylor S. M. Myoplasmic binding of fura-2 investigated by steady-state fluorescence and absorbance measurements. Biophys J. 1988 Dec;54(6):1089–1104. doi: 10.1016/S0006-3495(88)83045-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushmerick M. J., Podolsky R. J. Ionic mobility in muscle cells. Science. 1969 Dec 5;166(3910):1297–1298. doi: 10.1126/science.166.3910.1297. [DOI] [PubMed] [Google Scholar]
- Lamb T. D., McNaughton P. A., Yau K. W. Spatial spread of activation and background desensitization in toad rod outer segments. J Physiol. 1981;319:463–496. doi: 10.1113/jphysiol.1981.sp013921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb T. D., Pugh E. N., Jr A quantitative account of the activation steps involved in phototransduction in amphibian photoreceptors. J Physiol. 1992 Apr;449:719–758. doi: 10.1113/jphysiol.1992.sp019111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luby-Phelps K., Taylor D. L., Lanni F. Probing the structure of cytoplasm. J Cell Biol. 1986 Jun;102(6):2015–2022. doi: 10.1083/jcb.102.6.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastro A. M., Babich M. A., Taylor W. D., Keith A. D. Diffusion of a small molecule in the cytoplasm of mammalian cells. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3414–3418. doi: 10.1073/pnas.81.11.3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatani K., Yau K. W. Guanosine 3',5'-cyclic monophosphate-activated conductance studied in a truncated rod outer segment of the toad. J Physiol. 1988 Jan;395:731–753. doi: 10.1113/jphysiol.1988.sp016943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugh E. N., Jr, Lamb T. D. Amplification and kinetics of the activation steps in phototransduction. Biochim Biophys Acta. 1993 Mar 1;1141(2-3):111–149. doi: 10.1016/0005-2728(93)90038-h. [DOI] [PubMed] [Google Scholar]
- Pugh E. N., Jr, Lamb T. D. Cyclic GMP and calcium: the internal messengers of excitation and adaptation in vertebrate photoreceptors. Vision Res. 1990;30(12):1923–1948. doi: 10.1016/0042-6989(90)90013-b. [DOI] [PubMed] [Google Scholar]
- Pusch M., Neher E. Rates of diffusional exchange between small cells and a measuring patch pipette. Pflugers Arch. 1988 Feb;411(2):204–211. doi: 10.1007/BF00582316. [DOI] [PubMed] [Google Scholar]
- Rink T. J., Tsien R. Y., Pozzan T. Cytoplasmic pH and free Mg2+ in lymphocytes. J Cell Biol. 1982 Oct;95(1):189–196. doi: 10.1083/jcb.95.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenkranz J. New aspects of the ultrastructure of frog rod outer segments. Int Rev Cytol. 1977;50:25–158. doi: 10.1016/s0074-7696(08)60098-4. [DOI] [PubMed] [Google Scholar]
- Sather W. A., Detwiler P. B. Intracellular biochemical manipulation of phototransduction in detached rod outer segments. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9290–9294. doi: 10.1073/pnas.84.24.9290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stryer L. Cyclic GMP cascade of vision. Annu Rev Neurosci. 1986;9:87–119. doi: 10.1146/annurev.ne.09.030186.000511. [DOI] [PubMed] [Google Scholar]
- Torre V., Matthews H. R., Lamb T. D. Role of calcium in regulating the cyclic GMP cascade of phototransduction in retinal rods. Proc Natl Acad Sci U S A. 1986 Sep;83(18):7109–7113. doi: 10.1073/pnas.83.18.7109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yau K. W., Nakatani K. Light-suppressible, cyclic GMP-sensitive conductance in the plasma membrane of a truncated rod outer segment. Nature. 1985 Sep 19;317(6034):252–255. doi: 10.1038/317252a0. [DOI] [PubMed] [Google Scholar]




