Abstract
Pollen tube growth is a polarized growth process whereby the tip-growing tubes elongate within the female reproductive tissues to deliver sperm cells to the ovules for fertilization. Efficient and regulated membrane trafficking activity incorporates membrane and deposits cell wall molecules at the tube apex and is believed to underlie rapid and focused growth at the pollen tube tip. Rab GTPases, key regulators of membrane trafficking, are candidates for important roles in regulating pollen tube growth. We show that a green fluorescent protein–tagged Nicotiana tabacum pollen-expressed Rab11b is localized predominantly to an inverted cone-shaped region in the pollen tube tip that is almost exclusively occupied by transport vesicles. Altering Rab11 activity by expressing either a constitutive active or a dominant negative variant of Rab11b in pollen resulted in reduced tube growth rate, meandering pollen tubes, and reduced male fertility. These mutant GTPases also inhibited targeting of exocytic and recycled vesicles to the pollen tube inverted cone region and compromised the delivery of secretory and cell wall proteins to the extracellular matrix. Properly regulated Rab11 GTPase activity is therefore essential for tip-focused membrane trafficking and growth at the pollen tube apex and is pivotal to reproductive success.
INTRODUCTION
Upon pollination, pollen grains rapidly germinate, and each develops the polarized outgrowth of a pollen tube that penetrates the pistil. Directional cues from various pistil tissues guide the pollen tubes to the ovules to deliver the sperm cells to the embryo sac for fertilization (Cheung, 1996; Johnson and Preuss, 2002; Lord and Russell, 2002). Pollen tubes elongate by tip growth, whereby the pollen cytoplasm is confined to the most proximal region of the tube, and growth is restricted to the tube apex (Hepler et al., 2001). F-actin–dependent intracellular trafficking transports organelles and vesicles tipward along the flank of the tube and recycles them distally along the center of the tube, giving rise to what is known as a reverse-fountain cytoplasmic streaming pattern typical of elongating pollen tubes. Cytologically, an inverted cone-shaped region abutting the tube apex, referred to as the clear zone, is exclusively occupied by a high density of transport vesicles (Lancelle and Hepler, 1992; Derksen et al., 1995a, 1995b), whereas larger organelles, such as the Golgi bodies, are distributed throughout the cytoplasm distal to this region. Many of the vesicles in the clear zone fuse with the apical membrane, depositing new membrane, membrane proteins, cell wall materials, and secretory proteins to support growth at the tube tip, and some may interact with female-derived molecules. Some of these vesicles, together with endocytosed vesicles that originate from membrane around the apical and subapical regions, are retrotransported along the center of pollen tubes. Endocytic vesicles are likely to have packaged within them molecules from the extracellular matrix and, for pollen tubes elongating in the pistil, contain molecules secreted by female tissues, some of which have been localized inside pollen tubes (Lind et al., 1996; Luu et al., 2000). To date, little is understood about how anterograde and retrograde vesicle trafficking, fusion with the plasma membrane, and budding from the plasma membrane are regulated during pollen tube tip growth.
Rab GTPases are important regulators for endomembrane trafficking, regulating exocytosis, endocytosis, and membrane recycling, processes that are essential for maintaining normal cellular functions (e.g., see Geldner et al., 2001; Molendijk et al., 2004; Surpin and Raikhel, 2004) and amplified in dividing (e.g., see Waizenegger et al., 2000) and rapidly growing cells such as pollen tubes. Different Rab GTPases associate with distinct endomembrane compartments. They regulate membrane budding from donor compartments, determine the specificity of vesicle transport to target compartments, and facilitate vesicle and target membrane fusion (Schimmoller et al., 1998; Sanderfoot and Raikhel, 1999; Armstrong, 2000; Zerial and McBride, 2001; Tamm et al., 2003; Pfeffer and Aivazian, 2004; Seabra and Wasmeier, 2004). In so doing, they are essential for the transport of proteins and membrane through the endomembrane system to their destination. Like other members of the RAS superfamily of small GTP binding proteins, Rab proteins cycle between two distinct conformations, a GDP-bound, mainly cytosolic, and inactive form and a GTP-bound and membrane-associated active form. The overall activity of an individual Rab GTPase is modulated by shuttling between these two activity states in response to regulatory factors. For instance, negative regulators like the guanine nucleotide dissociation inhibitors or GTPase-activating proteins maintain these small GTPases in the GDP-bound inactive form, whereas positive regulators, like the guanine nucleotide exchange factors in mammalian cells, would shift the equilibrium toward the activated GTP-bound form.
The Rab GTPase family is by far the most complex among all subfamilies of RAS proteins (Pereira-Leal and Seabra, 2000, 2001; Stenmark and Olkkonen, 2001), reflecting the multiplicity of endomembrane trafficking pathways. Numerous Rab homologs have been identified from different plant species, including 57 RABs in the Arabidopsis thaliana genome. Based on sequence homology, the Arabidopsis RABs are predicted to encode eight functional families, which may be further divided into 18 different structural subclasses (Rutherford and Moore, 2002; Vernoud et al., 2003). Although many T-DNA insertional mutants of these Arabidopsis genes are available, functional analyses based on these mutants have not yet been reported. On the other hand, studies of plant Rab GTPases, like Rabs from other organisms and other Ras-related small G proteins (e.g., see Tisdale et al., 1992; Bar-Sagi and Hall, 2000), have taken considerable advantage of their GDP-GTP exchange regulatory mechanism, which allows the equilibrium between the activated and inactive forms of these proteins be easily manipulated. Constitutive active (CA) and dominant negative (DN) mutations at conserved positions in Ras-related GTPases render the mutant proteins predominantly in the GTP-bound form or locked in the GDP-bound state, respectively. Expression of CA or DN Rab GTPases perturbs the endogenous activity derived from their wild-type counterparts, revealing their functional significance. For a number of plant Rab GTPases, such as Arabidopsis Rab1b (At RABD2a), ARA-6 (At RABF1), ARA-7 (At RABF2b), At RAB4b, and tobacco (Nicotiana tabacum) Rab2, studies largely based on protein localization and expression of their CA and DN variants in transformed plants have shown that they perform functions homologous to those of their yeast and mammalian counterparts (Batoko et al., 2000; Cheung et al., 2002; Grebe et al., 2003; Preuss et al., 2004; Ueda et al., 2004), consistent with the Rab regulatory pathway being highly conserved among eukaryotes.
The Rab11 GTPase family is probably the most complex among all Rab protein families (e.g., with 26 Rab11 homologs, At RABAs, in the Arabidopsis genome) (Rutherford and Moore, 2002; Vernoud et al., 2003). Some Rab11 GTPases in animals and yeast are known to play important roles in membrane recycling, in particular the transport of receptor proteins between endosomes, trans-Golgi network, and the plasma membrane (Ullrich et al., 1996; Schlierf et al., 2000; Wilcke et al., 2000; Band et al., 2002; Hales et al., 2002; Volpicelli et al., 2002). They have also been associated with functions in various steps of exocytosis (Benli et al., 1996; Jedd et al., 1997; Chen et al., 1998; Cheng et al., 2002; Ortiz et al., 2002) and phagocytosis (Cox et al., 2000) or in the mobility of vesicles along actin bundles (Schott et al., 1999; Lapierre et al., 2001; Band et al., 2002; Hales et al., 2002; Volpicelli et al., 2002). In plants, green fluorescent protein (GFP)-tagged Rab11 homologs from pea (Pisum sativum), Pra-2 and Pra-3, have been localized to Golgi bodies and endosomes, respectively, when expressed in tobacco cells (Inaba et al., 2002), whereas GFP-Pra2 localized to the endoplasmic reticulum when expressed in Arabidopsis (Kang et al., 2001). Cytoimmunodetection using a monoclonal antibody specific for an Arabidopsis Rab11, ARA4 (AtRABA5c), revealed a Golgi and post-Golgi vesicle localization (Ueda et al., 1996). Another Arabidopsis Rab11 homolog, AtRABA4b, cofractionated with a non-trans-Golgi network membrane fraction (Preuss et al., 2004). These AtRABA4b-tagged membranes most probably had derived from transport vesicles since yellow fluorescent protein–AtRABA4b was localized to the apical region of root hairs (Preuss et al., 2004), another tip growth cell type with an apex enriched in transport vesicles similar to that seen in pollen tubes. Functionally, the root hair apical localization of yellow fluorescent protein–AtRABA4b correlates with normal root hair elongation (Preuss et al., 2004). Rab11 activity has also been implicated to be important for the secretion of cell wall modifying enzymes in ripening tomato (Lycopersicon esculentum) (Zainal et al., 1996; Lu et al., 2001). Other Rab11 homologs have been shown to be involved in light signal transduction and the biosynthesis of brassinosteroid (Yoshida et al., 1993; Nagano et al., 1995; Kang et al., 2001). Therefore, each plant Rab11 homolog or subsets of closely related homologs may have a highly specialized function that cannot be derived by analogy with their counterparts from other organisms but needs to be experimentally determined.
While the importance of membrane trafficking and spatially regulated exocytosis and endocytosis to polarized pollen tube growth is evident, the molecular mechanisms behind these processes are largely unknown. Specific members within the Rab GTPase family are presumably critical for the regulation of this tip growth process. To date, only one pollen-expressed Rab GTPase, a Rab2 homolog from tobacco, has been shown to be important for pollen tube growth and active in regulating vesicle trafficking between ER and Golgi bodies (Cheung et al., 2002). Using GFP-labeled Rab11b (Figure 1), we present evidence that a tobacco pollen Rab11 homolog, Rab11b, localizes predominantly to the apical clear zone of elongating pollen tubes. Its localization is intimately linked to the targeting of cell wall and secretory proteins and recycled membranes to the pollen tube clear zone and is crucial for efficient secretory activity, pollen tube growth rate, directionality, and male fertility.
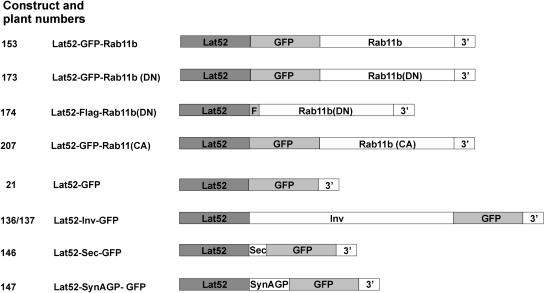
Figure 1.
A List of Constructs.
DN and CA mutations have S28N and Q73L substitutions, respectively, in Rab11b (see Supplemental Figure 1 online for the deduced Rab11b amino acid sequence). The 3′ in the sketches for the constructs indicates an added polyadenylation signal.
RESULTS
GFP-Rab11b Localized Predominantly to the Apical Clear Zone of Elongating Pollen Tubes
A cDNA for Rab11b (see Haizel et al., 1995) was readily isolated by RT-PCR from tobacco pollen mRNA but not amplified from seedling mRNA (data not shown; see Supplemental Figure 1 online for sequence alignment with other Rab11 GTPases), consistent with its being preferentially expressed in the male gametophyte. The expression profile of Rab11b mRNA (Figure 2A) conforms to a pattern of increasing accumulation during anther development, reaching a maximum at pollen maturity typical of many pollen mRNAs (Guyon et al., 2000; V. Di Stilio and A.Y. Cheung, unpublished data), a strategy thought to ready the pollen grains for germination and the early tube growth process (Mascarenhas, 1989).
Figure 2.
Expression and Localization of NtRab11b.
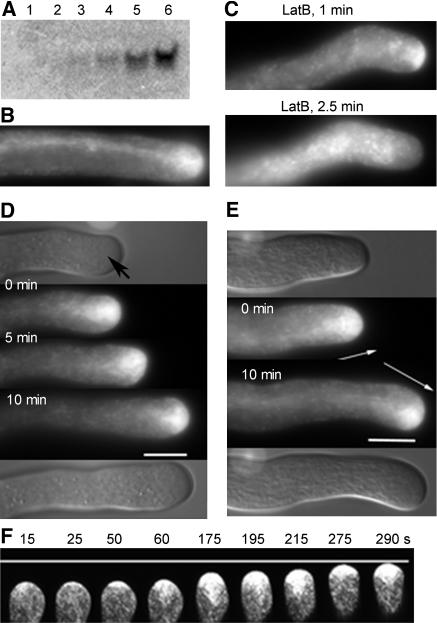
(A) Gel blot analysis for Rab11b mRNA. Lane 1, young leaves; lanes 2 to 6, developing anthers at stages 3 to 5, 6 and 7, 8 and 9, at anther maturity (stages 10 to 12), and in mature dry pollen, respectively. Twenty micrograms of total RNA was examined for each sample. Anthers were staged according to Kultonow et al. (1990). A faint NtRab11b signal was observed in lane 1 only after prolonged exposure (data not shown).
(B) to (F) Transformed pollen grains from plant 153-12 (see Table 1 and Supplemental Figure 2 online) were cultured in vitro for observation.
(B) Localization of GFP-Rab11b in an elongating pollen tube.
(C) A GFP-Rab11b–expressing pollen tube observed almost immediately (1 and 2.5 min) after addition of latrunculin B (12.5 nM).
(D) and (E) Localization of GFP-Rab11b in a pollen tube following a stable growth trajectory (D) and in a pollen tube reorienting its growth trajectory (arrows) (E). Pollen tubes were followed for 10 min as indicated. The top and bottom panels of each figure are differential interference contrast images of the same tubes taken at the beginning and end of the imaging periods. Arrow in (D) points to the apical clear zone. Bars = ∼10 μm.
(F) Selected confocal images from a time series (taken at 5 s apart) of an elongating GFP-NtRab11b–expressing pollen tube. Numbers indicate time (s) from the beginning of the time series. The entire time series is shown in Supplemental Movie 1 online.
When GFP-Rab11b (Figure 1) was expressed in transformed pollen, it localized most prominently in the apical clear zone of in vitro–grown pollen tubes (Figure 2B). A low level of punctuate, but not highly discrete, structures was also weakly labeled by GFP-Rab11b throughout the tube (see also Figure 3). The apical zone of green fluorescence was always trailed by a grainward stream of fluorescence extending along the center of the tube (Figures 2B and 3A). Accumulation of GFP-Rab11b in the inverted cone region abutting the apex was also observed in pollen tubes that had elongated through the pistil. The apical clear zone is known to be densely and exclusively occupied by transport vesicles (Lancelle and Hepler, 1992; Derksen et al., 1995a, 1995b). The apical concentration of GFP-NtRab11b thus strongly supports a transport vesicle localization of this fusion protein. The accumulation of GFP-Rab11b in the clear zone was rapidly dissipated by treatment with the actin polymerization inhibitors latrunculin B (Figure 2C) or cytochalasin D (data not shown). This observation is also consistent with GFP-Rab11b being associated with transport vesicles, which are known to track along actin filaments in plant cells (see Hepler et al., 2001). Moreover, the apical localization of GFP-Rab11b mimics those shown by GFP-tagged proteins destined for the pollen tube cell surface or secretion (Cheung et al., 2002; see Figure 7), suggesting a functional link between Rab11b to the trafficking and delivery of these proteins to the growing tube tip.
Figure 3.
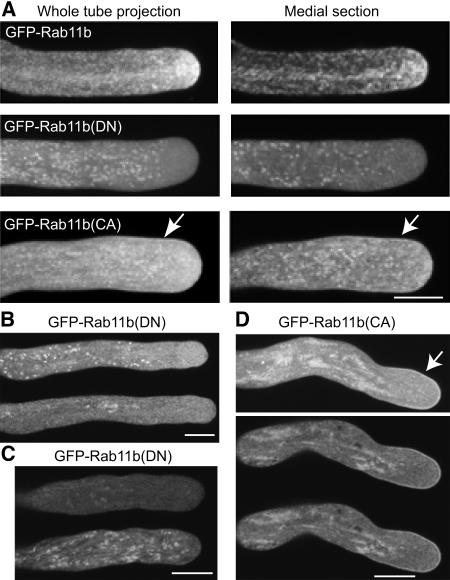
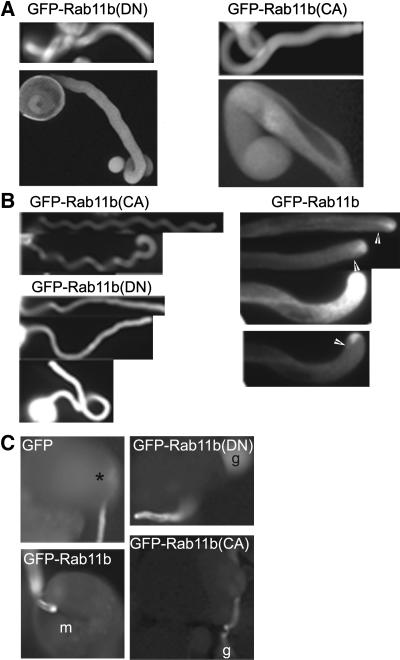
Localization of GFP-Rab11b(DN) and GFP-Rab11b(CA).
Pollen from transgenic plants 153-12, 173-8, and 207-2 (see Table 1 and Supplemental Figure 2 online) were cultured in vitro.
(A) Whole tube projection and a medial section from confocal imaging of GFP-Rab11b–, GFP-Rab11b(DN)–, and GFP-Rab11b(CA)–expressing pollen tubes.
(B) Confocal images of a medial section of a BFA-treated (1 μg/mL) GFP-Rab11b(DN)–expressing pollen tube at 2 min (top panel) and 15 min (bottom panel) after BFA addition.
(C) Confocal images of a medial section of a GFP-Rab11b(DN)–expressing pollen tube at the end of BFA treatment (1 μg/mL, 15 min) (top panel) and 30 min after BFA washout (bottom panel).
(D) Confocal images of a GFP-Rab11b(CA)–expressing pollen tube at 15 min after BFA (1 μg/mL) addition. Top panel shows a whole tube projection of all optical images; bottom panel shows two contiguous medial optical sections where the plasmalemma association of GFP-Rab11b(CA) was most evident.
Plasma membrane localization of GFP-Rab11b(CA) ([A] and [D]) is indicated by arrows. Bars = 10 μm ([A] to [D]).
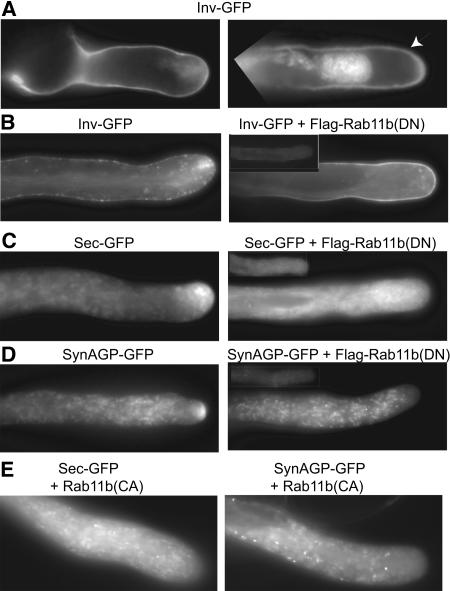
Figure 7.
Rab11b(DN) and Rab11b(CA) Affect Delivery of Cell Wall and Secreted Proteins.
Stable transformed pollen grains were used in (A) to (D); microprojectile bombardment transformed pollen tubes are shown in (E). Pollen grains were germinated in vitro. Images in the main panels of (B) to (D) were taken by autoexposure, revealing the predominant localization of the cargo proteins. To compare the level of cargo retention within the tube, images in the inset of each of the right panels were control tubes expressing cargo molecules only but imaged with the same exposure time as that for the double-transformed tube shown in the main image.
(A) Transformed pollen tubes expressing Inv-GFP during initial pollen tube growth period (0.5 h after plating) showing cell surface and apical clear zone accumulation of the fusion protein (left panel) or cell wall localization after plasmolysis (right panel). Arrowhead indicates cell wall.
(B) A Lat52-Inv-GFP transformed tube (left panel) and a Lat52-Inv-GFP and Lat52-flag-Rab11b(DN) cotransformed tube (right panel) at 4 h after plating.
(C) A Lat52-Sec-GFP transformed tube (left panel) and a Lat52-Sec-GFP and Lat52-flag-Rab11b(DN) cotransformed tube (right panel) at 4 h after plating.
(D) A Lat52-SynAGP-GFP transformed tube (left panel) and a Lat52-SynAGP-GFP and Lat52-Rab11b(DN) cotransformed tube at 6 h after plating.
(E) A Lat52-Sec-GFP and Lat52-Rab11b(CA) (left panel) and a Lat52-SynAGP-GFP and Lat52-Rab11b(CA) (right panel) cotransformed tube at 6 h after plating.
The domain occupied by GFP-Rab11b within the apical dome was highly dynamic both spatially and temporally (Figure 2F; see Supplemental Movie 1 online). The GFP-Rab11b accumulation zone cycled rapidly from spanning just a narrow distance against the apex to filling the entire clear zone and then retracting to a region more closely abutting the apex (Figure 2F, compare the 25, 175, and 275 s images; see Supplemental Movie 1 online). It seems plausible that the temporal and spatial fluctuation of GFP-Rab11b–labeled vesicles at the tube apex may correlate with the periodic fast and slow growth phases of elongating pollen tubes (see Parton et al., 2001). When observed over a broader time frame over which distances covered and growth trajectories could be clearly resolved, the apical zone of GFP-Rab11b was always maintained parallel to the pollen tube growth axis, even when these cells were reorienting to a new growth trajectory (Figures 2D and 2E). With these observations, we speculate that Rab11b is important for both apical growth and directionality of elongating pollen tubes.
Apical Localization of GFP-Rab11b Depends on Proper Regulation of Its Activity
The ability for Rab11b to regulate the shuttling between the active and inactive forms by GTP-GDP exchange is apparently critical for the targeting of Rab11b-tagged vesicles to the pollen tube apical clear zone. As shown in Figure 3, a DN mutation (S28N) completely abolished apical accumulation of GFP-Rab11b(DN), while a CA mutation (Q73L) caused more subtle effects and rendered GFP-Rab11b(CA) to be more evenly distributed within the apical dome. Moreover, GFP-Rab11b(DN) accentuated labeling of the punctuate structures, whereas GFP-Rab11b(CA) showed a plasmalemma localization not observed for its wild-type or DN counterparts.
The size, abundance, and streaming pattern of the punctuate structures labeled by GFP-Rab11b, its DN and CA derivatives were most reminiscent to pollen tube Golgi bodies (Cheung et al., 2002). Treatment by Brefeldin A (BFA), which reversibly disrupts Golgi stacks in many cell types, including pollen tubes (Rutten and Knuiman, 1993; Cheung et al., 2002; Ritzenthaler et al., 2002), resulted in the dissociation of these discrete structures and appearance of membrane patches, which were especially evident in GFP-Rab11b(DN)- and Rab11b(CA)-expressing pollen tubes (Figures 3B and 3D). As with the reversible effect of BFA on the disruption of Golgi stacks, discrete GFP-labeled organelles reappeared upon BFA removal and were most readily observed among GFP-NtRab11b(DN)–expressing tubes (Figure 3C), further supporting that this Rab11b variant has a prominent Golgi association. Moreover, BFA treatment, in addition to abolishing the GFP-Rab11b(CA)–labeled organelles, also accentuated its cell membrane association (Figure 3D).
Expression of GFP-Rab11b(DN) and GFP-Rab11b(CA) Inhibits in Vitro Pollen Tube Growth and Directionality
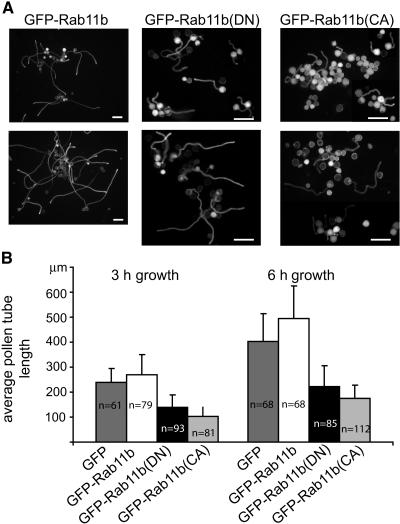
To determine whether altering the overall pollen tube Rab11 activity would affect the pollen tube growth process, we examined pollen from Lat52-GFP-Rab11, Lat52-GFP-Rab11(DN), or Lat52-GFP-Rab11b(CA) transformed plants (Figure 1, Table 1; see also Supplemental Figure 2 online for the level of fusion proteins in these transformed pollen populations). When germinated in vitro, transformed pollen tolerated a broad range of GFP-Rab11b accumulation and showed comparable germination frequency and growth rate as control nontransformed (data not shown) and transformed GFP-expressing pollen (Figure 4). The effect of GFP-Rab11b(DN) on germination was moderate; only grains that accumulated very high levels of this protein, as judged by their green fluorescence level, were inhibited for germination. However, pollen tube growth was reduced significantly by the expression of GFP-Rab11b(DN) (Figure 4). Expression of GFP-Rab11b(CA), on the other hand, was highly inhibitory to pollen germination. When pollen grains from transgenic plants that expressed different levels of pollen GFP-Rab11b(CA) (see Supplemental Figure 2B online) were cultured, tubes that developed from all of these pollen populations consistently showed very low levels of green fluorescence (see Figure 5), and they were significantly shorter than control pollen tubes (Figure 4). Moreover, in repeated experiments, the average lengths attained by GFP-Rab11b(CA)–expressing tubes were also noticeably shorter than those observed for GFP-Rab11b(DN)–expressing tubes (Figure 4).
Table 1.
Segregation Analysis of Transformed Plants Expressing Various Forms of Rab11b
| Transgenic Line (Plant Number)
|
Self-Pollination
|
Transgenic Plant as Pollen Donor
|
||||
|---|---|---|---|---|---|---|
| Kmr | Kms | (Ratio)a | Kmr | Kms | (Ratio) | |
| Lat52-GFP-Rab11b (153-12)b | 72 | 28 | (∼2.5:1) | Not determined | ||
| Lat52-GFP-Rab11b(DN) (173-4)c | 48 | 52 | (∼1:1) | 4 | 96 | (1:24) |
| Lat52-GFP-Rab11b(DN) (173-8)c | 66 | 34 | (∼2:1) | 21 | 79 | (∼1:4) |
| Lat52-GFP-Rab11b(CA) (207-2)d | 94 | 81 | (∼1:1) | 3 | 194 | (∼1:64) |
| Lat52-GFP-Rab11b(CA) (207-16)d | 105 | 18 | (∼6:1) | 0 | ∼200 | |
| Lat52-Flag-Rab11b(DN) (174-6)e | 56 | 44 | (∼1:1) | 11 | 89 | (∼1:8) |
Kmr, kanamycin resistant; Kms, kanamycin sensitive.
Line 153-12 was typical of Lat52-GFP-Rab11b transformed plants. Seed yields by self-pollination were not noticeably affected.
Lines 173-4 and 173-8 were representative of Lat52-GFP-Rab11b(DN) transformed plants with high or moderate levels, respectively, of expression of these fusion proteins (see Supplemental Figure 2A online).
Lines 207-2 and 207-16 had low and very high levels, respectively, of GFP-Rab11b(CA) protein accumulation in their pollen (see Supplemental Figure 2B online). When observed microscopically, half of the pollen grains from 207-2 showed weak GFP fluorescence, and virtually all of the pollen grains from 207-16 expressed the fusion protein, supporting a single T-DNA insertion locus in line 2 and multiple ones in line 16. Nineteen of the 22 Lat52-GFP-Rab11b(CA) transgenic lines observed were compromised in seed yields; many of them were severely reduced in male fertility. These properties correlated with GFP-Rab11b(CA) expression levels (see Supplemental Table 1 online).
Line 174-6 was used in crosses with cargo lines.
Figure 4.
Rab11b(DN) and Rab11b(CA) Inhibit in Vitro Pollen Tube Growth Efficiency.
(A) Comparison of in vitro–grown Lat52-GFP-Rab11b, Lat52-GFP-Rab11b(DN), and Lat52-GFP-Rab11b(CA) transformed pollen tubes at 3 h (top row of images) and 6 h (bottom row of images) after plating. Routinely, between 60 and 75% of the Lat52-GFP-Rab11b transformed pollen grains germinated, a level comparable to that observed among control nontransformed or Lat52-GFP transformed pollen grains. At least 50% level of the transformed pollen from Lat52-GFP-Rab11b(DN)–transformed plants germinated efficiently. Only grains that showed the strongest green fluorescence failed to germinate. At most 10 to 20% of transformed pollen from most of the Lat52-GFP-Rab11b(CA) transformed plants examined germinated, and these were among grains that showed the lowest level of green fluorescence. Bars = 100 μm.
(B) Histogram shows average pollen tube length attained by pollen tubes at 3 and 6 h after plating. Student's t tests show that data from GFP-Rab11b(DN)– and GFP-Rab11b(CA)–expressing tubes were significantly different from those of the controls (GFP and GFP-Rab11b; P < <0.01). They also show that data from GFP-Rab11b(CA)–expressing and GFP-Rab11b(DN)–expressing pollen tubes were significantly different from each other (with P < 0.05). Error bars indicate averages from the number of pollen tubes measured (n).
Pollen from transgenic plants 21, 153-12, 173-8, and 207-2 were used for these experiments, and the data shown were representative of multiple experiments. Pollen grains transiently cotransformed by Lat52-GFP and Lat52 driven untagged versions of these Rab11bs produced qualitatively similar results, except that the inhibitory effects by NtRab11b(DN) and NtRab11b(CA) were more pronounced (data not shown).
Figure 5.
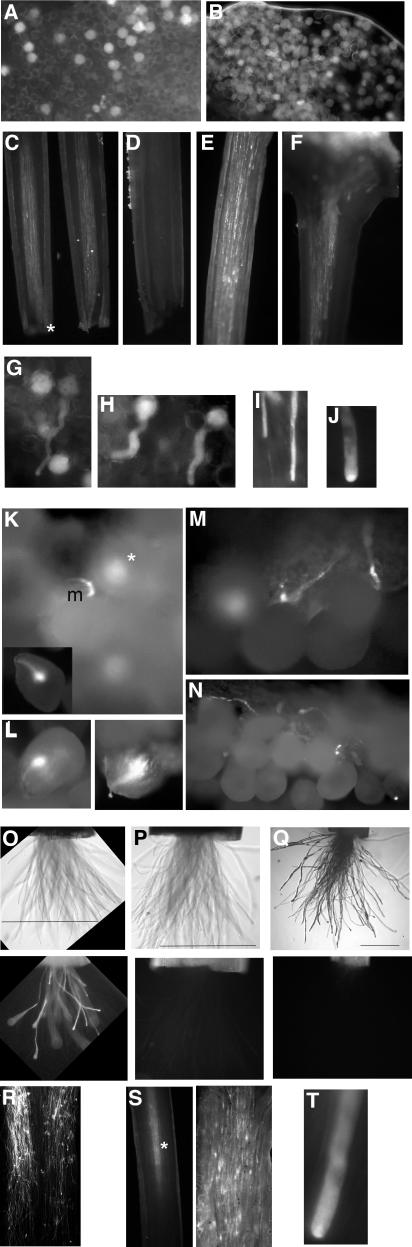
Rab11b(DN) and Rab11b(CA) Inhibit in Vitro Pollen Tube Growth Directionality.
(A) Representative meandering GFP-Rab11b(DN)– and GFP-Rab11b(CA)–expressing pollen tubes. The top panels are epifluorescence images; the bottom panels are confocal images.
(B) Correlation of the meandering pollen tube phenotype with the expression levels of GFP-Rab11b, GFP-Rab11b(DN), and GFP-Rab11b(CA). Images for the GFP-Rab11b(CA)– and GFP-Rab11b(DN)–expressing tubes were 1-s exposures. For the GFP-Rab11b–expressing tubes, the top three images were taken at 0.6-s exposures; the bottom image was the same tube as in the image above except that it was taken by autoexposure and revealed the apical inverted cone localization of the GFP-Rab11b protein. Pollen tube width at the location marked by arrowheads is 8.1, 9.4, and 11.1 μm, from top to bottom, respectively.
(C) In vitro pollen tube targeting of ovule micropyle. After pollen grains were cultured in germination medium for ∼3 h, freshly excised mature ovules from unpollinated pistils were distributed among the elongating pollen tubes. Pollen tube growth was allowed to proceed for another 3 h for GFP- and GFP-Rab11b–expressing tubes, 6 h for GFP-Rab11b(DN)– and GFP-Rab11b(CA)–expressing tubes. Pollen tubes [∼50 for GFP- and GFP-Rab11b–expressing ones and >100 for GFP-Rab11b(DN)– and GFP-Rab11b(CA)–expressing ones] growing in close proximity to the ovules were observed for their ability to target the micropyle or to penetrate the ovules. m, micropyle; g, pollen grain. The asterisk in the GFP panel indicates the signal from the penetrated tube.
Pollen from lines 21, 153-12, 173-8, and 207-2 were used in these experiments.
In vitro–grown pollen tubes have a high propensity to follow a stable growth trajectory, giving rise to tubes with a relatively straight morphology. Notably, pollen tubes expressing GFP-Rab11b(DN) or GFP-Rab11b(CA) showed dramatic meandering phenotypes (Figure 5). Some of them zigzagged along the entire length of the tube, and this property was most prevalent among the Lat52-GFP-Rab11b(CA) transformed tubes (Figure 5B). Others showed a period of straight growth interrupted by abrupt and continuous turning, resulting in a knotted morphology along their lengths or at the tube tip (Figures 5A and 5B). When pollen tubes were examined under identical imaging conditions, it became apparent that GFP-Rab11b(CA) was considerably more active in inducing pollen tube zigzagging than GFP-NtRab11(DN) (Figure 5B). Using the level of green fluorescence as a measure for the level of these fusion proteins in individual tubes, we observed that pollen tubes accumulating very low levels of GFP-Rab11b(CA) already showed severe zigzagging, whereas it took substantially higher levels of GFP-Rab11b(DN) accumulation to induce moderate twists and turns. Furthermore, pollen tubes that developed from grain populations that accumulated higher levels of GFP-Rab11b(DN) produced more meandering tubes (data not shown), consistent with a dose-dependent phenomenon. Moreover, pollen tubes apparently could tolerate relatively high levels of GFP-Rab11b(DN) as strongly fluorescent tubes with twists and turns (Figure 5B) were quite prevalent. On the other hand, few pollen tubes with green fluorescence levels higher than those seen in Figure 5B existed among GFP-Rab11b(CA)–expressing tubes. Apparently, pollen grains with even moderate levels of this fusion protein had not germinated.
Few meandering tubes were observed among pollen that expressed GFP-Rab11b. When zigzagging did occur, the amount of GFP-Rab11b in these tubes was much higher than those observed in wavy GFP-Rab11b(CA)–expressing pollen tubes (Figure 5B, right panel). However, unlike GFP-RAb11b(DN) and GFP-Rab11b(CA), the ability of GFP-Rab11b to accumulate in the apical inverted cone region had not been compromised, though pollen tube broadening was apparently induced by increasing levels of this fusion protein (Figure 5B).
In order to enter the ovules for fertilization, pollen tubes respond to guidance cues that emanate from the ovule and target growth toward and into the ovule via an aperture known as the micropyle (Hulskamp et al., 1995; Higashiyama et al., 1998, 2001; Shimizu and Okada, 2000; Huck et al., 2003; Palanivelu et al., 2003; Marton et al., 2005). Pollen tubes from a number of plant species grown in vitro retain the capacity to grow toward and penetrate ovules (Higashiyama et al., 1998, 2001), though at efficiency substantially lower than what occurs in vivo. When ovules were included in in vitro pollen tube growth cultures, GFP- and GFP-Rab11b–expressing pollen tubes that extended close to an ovule were often (∼25%) seen to approach or have entered the ovule (Figure 5C). On the other hand, of the >100 GFP-Rab11b(DN) and GFP-Rab11b(CA) pollen tubes growing in close proximity to ovules that were observed, none penetrated the ovules. Instead, the growth path of some of these pollen tubes suggests that even when elongating in the micropylar vicinity, they would elongate past it and continued their growth onto the medium (Figure 5C). The tendency for GFP-Rab11b(DN)– and GFP-NtRab11b(CA)–expressing pollen tubes to twist, turn, and meander had apparently compromised their ability to aim their growth trajectory at the micropyle.
Expression of GFP-Rab11b(DN) or GFP-Rab11b(CA) Inhibits Pollen Tube Growth in Vivo and Reduces Male Fertility
Upon pollination on the stigma, wild-type tobacco pollen grains germinate efficiently, and a majority of their tubes reach the style-ovary junction ∼30 h after pollination (Cheung et al., 1995; Wang et al., 1996). Direct examination of pollinated pistils (Figures 6A to 6N) showed that most GFP-expressing (data not shown) and GFP-Rab11b–expressing grains germinated (Figure 6A), and many of these tubes reached the basal stylar region by 30 h after pollination (Figure 6C). As for GFP-Rab11b(DN)–expressing pollen grains, most of them germinated, except the most highly fluorescent grains (data not shown). However, by 30 h after pollination, a majority of GFP-Rab11b(DN) pollen tubes only reached one-half to two-thirds down the style (Figures 6D and 6E), and a few short wavy tubes remained in the stigmatic region (Figure 6G). Germination of GFP-Rab11b(CA)–expressing pollen grains were severely inhibited (Figure 6B). Tubes that germinated were still almost exclusively within the upper half of the style (Figure 6F) even by 48 h after pollination. Short wavy GFP-Rab11b(CA)–expressing pollen tubes could be observed on the stigmatic surface (Figure 6H).
Figure 6.
Effect of DN and CA Rab11bs on Pollen Tube Growth in the Pistil.
(A) to (N) In vivo pollen germination and tube growth. Pistils were observed at 30 h ([A], [C] to [E], [G], and [I] to [L]) or 48 h ([B], [F], [H], [M], and [N]) after pollination. Stylar segments shown here were from bisected pollinated styles; ovules were from excised placenta tissues.
(A) and (C) Stigmatic (A) and basal (C) stylar region of a pistil pollinated by Lat52-GFP-Rab11b transformed pollen grains (from plant 153-12). A majority of pollen grains have germinated, and a large number of the transformed pollen tubes have reached the stylar/ovary junction (indicated by an asterisk) by 30 h after pollination.
(B) and (F) Stigmatic (B) and substigmatic (F) region of a pistil pollinated by Lat52-GFP-Rab11b(CA) (plant 207-2). Even at 48 h after pollination, most transformed pollen grains remained ungerminated, and tubes from the germinated grains only reached at most half way down the style.
(D) and (E) Basal (D) and mid-stylar (E) region of a pistil pollinated with Lat52-GFP-Rab11b(DN) transformed pollen (from plant 173-8) at 30 h after pollination.
(G) and (H) Meandering Lat52-GFP-Rab11b(DN) (G) and Lat52-GFP-Rab11b(CA) (H) transformed pollen tubes on pollinated stigmas.
(I) and (J) Lat52-GFP (I) and Lat52-GFP-Rab11b (J) transformed pollen tubes elongating within the transmitting tissue. GFP-Rab11b predominantly localized to the apical region, contrasting the even distribution of GFP throughout the tube cell.
(K) Ovules from pistils pollinated with Lat52-GFP transformed pollen. Ovules penetrated by these pollen tubes showed green fluorescence from within (asterisk). A pollen tube was seen penetrating a micropyle (m). Inset shows fluorescence in the embryo sac of an ovule.
(L) Ovules representative of those penetrated by Lat52-GFP-Rab11b transformed pollen tubes.
(M) and (N) Ovules from a pistil pollinated with Lat52-GFP-Rab11b(DN) at 48 h after pollination.
(O) to (T) Pollen tubes from semi–in vivo pollen tube cultures.
(O) Pistil was pollinated by pollen from Lat52-GFP-Rab11b transformed plant 153-12.
(P) and (R) Pistil was pollinated by pollen from Lat52-Rab11b(DN) transformed plant 173-8.
(Q) and (S) Pistil was pollinated by pollen from Lat52-Rab11b(CA) transformed line 207-2. Plants used were segregating 1:1 transformed and nontransformed pollen. The top two-thirds of the pollinated pistils were excised at 6 h ([O], [P], and [R]) and 30 h ([Q] and [S]) after pollination and transferred to in vitro pollen tube growth medium and cultured for 14 h ([O], [P], and [R]) and 30 h ([Q] and [S]).
(O) to (Q) Top row: bright-field images showed that nontransformed pollen tubes have emerged from the cut stylar ends. Bottom row: fluorescent images showed that GFP-Rab11b–expressing pollen tubes have also emerged (O) but not GFP-Rab11b(DN)– (P) or GFP-Rab11b(CA)–expressing (Q) pollen tubes. Bars indicate stylar diameter.
(R) Mid-stylar segment of the pistil shown in (P), where most GFP-Rab11b(DN)–expressing pollen tubes were located.
(S) Upper-stylar region of the pistil shown in (Q), where GFP-Rab11b(CA)–expressing pollen tubes were located. The region with the asterisk is shown enlarged in the right-hand panel.
(T) A GFP-Rab11b–expressing pollen tube that has emerged from a pollinated pistil. Localization of this fusion protein to the apical clear zone was evident.
When the placenta from pistils pollinated with Lat52-GFP, transformed pollen was excised at 30 h after pollination, GFP-expressing pollen tubes could be seen penetrating micropyles, and green fluorescence was already observed in ∼10% of the ovules, reflecting pollen tube penetration (Figure 6K). Similarly, GFP-NtRab11b–expressing pollen tubes have also penetrated ovules (Figure 6L), but the number of green fluorescent ovules was slightly lower than that seen in pistils pollinated by GFP-expressing pollen. However, we have not observed GFP-Rab11b–expressing pollen tubes in the middle of entering an ovule, possibly the restricted apical localization of this fusion protein in in vivo elongating pollen tubes (Figure 6J) had made it very difficult to capture the moment of micropyle entrance. A considerable number of Lat52-GFP-Rab11b(DN) transformed pollen tubes reached the ovary by 48 h after pollination. Ovules with green fluorescence signal were observed (Figures 6M and 6N), but they were fewer than that achieved by GFP- or GFP-Rab11b–expressing pollen tubes. The ability of GFP-Rab11b(CA)–expressing pollen tubes to penetrate ovules was not examined. Their slow growth rate would have precluded their arrival at the ovary before ovules have lost their receptivity, ∼72 h after anther dehiscence (see Cheung et al., 1995; Wang et al., 1996).
Semi–in vivo pollen tube growth assays (Cheung et al., 1995; Higashiyama et al., 1998) were used to further illustrate the differences in pollen tube growth rate within the pistil for pollen that expressed the wild-type or mutant forms of GFP-Rab11b (Figures 6O to 6T). In these experiments, pollen from heterozygous transformed plants with a single T-DNA insertion locus was used to pollinate wild-type pistils. For pistils pollinated by pollen from a Lat52-GFP-Rab11b transformed plant, both wild-type and GFP-Rab11b–expressing tubes emerged at the cut stylar end at a comparable time after culturing, although the number of green fluorescent tubes was consistently lower than half of the entire pollen tube population (Figure 6O). It is possible that the strongest fusion protein-expressing tubes were slightly retarded during in vivo pollen tube growth. On the other hand, for pistils that had been pollinated with pollen from a Lat52-GFP-Rab11b(DN) or a Lat52-GFP-Rab11b(CA) transgenic plant, no transformed pollen tube emerged even when wild-type tubes had grown some distance after their emergence from the cut styles (Figures 6P and 6Q). Instead, most of the transformed pollen tubes were in the middle or upper part of the excised pistils (Figures 6R and 6S).
Observations on pollen tube growth in the pistil described above support that pollen tube growth in the stigma and stylar transmitting tissue was substantially retarded by the expression of GFP-tagged DN or CA Rab11b mutant variants. This conclusion was further supported by segregation analysis of transformed plants (Table 1). Lat52-GFP-Rab11b transformed plants showed close to normal male fertility. The slightly reduced male inheritance observed in some of these transgenic plants (Table 1) was most probably due to highly expressed GFP-Rab11b in a minor population of the transformed pollen grains, undermining their germination and growth efficiency. On the contrary, expression of DN or CA variants of the GFP-Rab11b considerably reduced male fertility, and this effect was especially drastic among Lat52-GFP-Rab11b(CA) transformed plants (Table 1). The extent of reduced male inheritance observed in Lat52-GFP-Rab11b(DN) transformed plants correlated with the level of expression of the fusion protein (see Supplemental Figure 2 online). On the other hand, a majority of the >20 Lat52-GFP-Rab11b(CA) transformed plants examined showed a comparably strong reduced male fertile property irrespective of the level of GFP-Rab11b(CA) observed among their pollen grains (Table 1; see Supplemental Figure 2 online). Seed yield was significantly reduced among these Lat52-GFP-Rab11b(CA) transformed plants (see Supplemental Table 1 online). Together with the observations that pollen germination and tube growth were highly sensitive to GFP-Rab11b(CA) (Figures 4 to 6), the uniformly observed reduced male fertility was consistent with the possibility that in every Lat52-GFP-Rab11b(CA) transformed line, only pollen grains that expressed very low and below inhibitory levels of this fusion protein managed to contribute to fertilization. Moreover, fusion with GFP apparently had modulated the activity of these small GTPases as expression of untagged versions of DN and CA Rab11bs consistently showed stronger inhibitory effects than their GFP-tagged counterparts and significantly reduced male fertility (e.g., see line 174-6 in Table 1).
Rab11 Regulates Delivery of Cell Wall and Secreted Proteins and Membrane Recycling
Three GFP-labeled cargo proteins (Figure 1) and the fluorescent lipophilic dye FM4-64 (Bolte et al., 2004) were used to examine the functional significance of Rab11 activity in membrane trafficking. The pollen tube wall-located invertase (Inv)-GFP (Figure 7A) was derived from a tobacco pollen-expressed invertase (Greiner et al., 1995). Sec-GFP was a secreted form of GFP, whereas SynAGP-GFP was a secreted fusion protein of a synthetic arabinogalactan protein and GFP (Shpak et al., 1999) (see Supplemental Figure 2 online for a protein blot for these pollen-expressed fusion proteins). Similar to several other proteins destined to the pollen tube cell surface observed (e.g., see Cheung et al., 2002; Cheung and Wu, 2004), Inv-GFP, Sec-GFP, and SynAGP-GFP showed prominent accumulation at the inverted cone region of the pollen tube apex (Figures 7A to 7D, left-hand panels). Some intracellular labeling was also noticeable, reflecting passage of these proteins through the endomembrane system. Organelles reminiscent of Golgi bodies were more prevalent in SynAGP-GFP–expressing tubes (Figure 7D) then in tubes expressing the other two cargo molecules, probably because arabinogalactan modification takes place mainly within the Golgi compartments. Cell surface labeling was never observed for Sec-GFP or SynAGP-GFP, indicating that they were efficiently released to the medium upon vesicle fusion with the apical membrane.
In order to examine the effect of downregulated Rab11b activity on protein trafficking, Lat52-Flag-Rab11b(DN) transformed plants (see Table 1) were crossed with pollen from plants transformed by each of the GFP-labeled cargo transgenes described above. Accumulation of the GFP-labeled cargo proteins in these double transformed pollen tubes was abolished (Figures 7B to 7D, right-hand panels). Furthermore, a considerably higher level of these proteins was retained intracellularly in these double transformed pollen tubes relative to that observed in control tubes expressing only the cargo protein (cf. Figures 7B to 7D, right-hand panels and their insets), consistent with reduced efficiency in secreting these cargo molecules. Because of the highly inhibitory effect of Rab11(CA) on pollen germination, its effect on cargo trafficking was examined in transiently transformed pollen that would have germinated before an inhibitory level of this protein had accumulated. In the double transformed pollen tubes, apical targeting of SecGFP and SynAGP-GFP was also abolished, and intracellular retention of these cargo molecules was prominent (Figure 7E). Together, these observations indicate that pollen tubes with misregulated Rab11 activity were compromised in their exocytic activity.
FM dyes are taken up efficiently from the growth medium by elongating pollen tubes (O'Driscoll et al., 1993; Camacho and Malho, 2003; Parton et al., 2003). The temporal pattern of FM4-64 labeling suggests an initial internalization by endocytosis followed by membrane recycling from the endosomal system to the apex, resulting in the accumulation of FM4-64–labeled transport vesicles in the clear zone (Figure 8A; Camacho and Malho, 2003; Parton et al., 2003). When GFP-Rab11b–expressing pollen tubes were treated with FM4-64, the localization of the fusion protein in the apical clear zone and in the retrograde cytoplasmic stream overlapped with that of the fluorescent dye (Figures 8B and 8C), consistent with exocytic vesicles and vesicles derived from endocytic membranes co-occupying the apical clear zone. Expression of GFP-Rab11b(DN) or GFP-Rab11b(CA) abolished the accumulation of these FM4-64–labeled vesicles at the tip (Figures 8D and 8E), suggesting probable participation of Rab11 in the endocytic and recycling processes.
Figure 8.
Rab11b(DN) and Rab11b(CA) Affect Endocytic Membrane Recycling.
(A) Epifluorescence image of a wild-type pollen tube treated with FM4-64 (3.2 μM, 10 min). The accumulation of labeled transport vesicles in the apical clear zone was as previously reported (Camacho and Malho, 2003; Parton et al., 2003).
(B) Epifluorescence images of a GFP-Rab11b–expressing pollen tube treated with FM4-64 (3.2 μM, 10 min) taken in the green and red channels (top and middle panels). The bottom panel shows a merged image of the green and red images.
(C) to (E) Confocal images of a GFP-Rab11b– (C), a GFP-Rab11b(DN)– (D), and a GFP-Rab11(CA)–expressing (E) and FM4-64 treated (3.2 μM, 10 min) pollen tube. In (C), the panel on the left shows a whole tube projection and that on the right shows a single medial optical section from the same tube. The top to bottom images in each of the panels in (C) to (E) show the green, red, and merged images of the two, respectively.
In vitro–germinated stable transformed pollen grains were used in these analyses.
Misregulating Rab11b Activity Affects Actin Organization in the Apical and Subapical Regions of Transformed Pollen Tubes
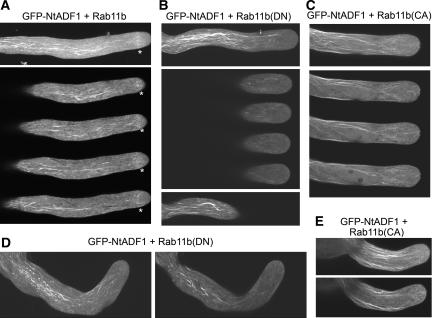
Elongating pollen tubes characteristically maintain an elaborate actin organization (see Hepler et al., 2001 and Figure 10). Transformed pollen tubes that coexpressed Rab11b and GFP-NtADF1, an actin binding reporter protein for live cell imaging (Chen et al., 2002), showed a prominent actin mesh at the subapical region subtended by long actin cables in the shank (Figure 9A), similar to that typically seen in control GFP-NtADF1–expressing pollen tubes (Figure 10; see also Chen et al., 2002; Cheung and Wu, 2004). The subapical actin mesh is a highly dynamic structure (Chen et al., 2002). It is highly sensitive to pollen tube growth property and considerably more easily dissipated when growth is perturbed (C.Y. Chen, A.Y. Cheung, and H.-m. Wu, unpublished data; see also Cheung and Wu, 2004) than the long actin cables in the shank. When Rab11b(DN) or Rab11b(CA) were coexpressed with GFP-NtADF1, actin cables along the shank of these transformed pollen tubes were by and large maintained, but the subapical actin mesh was consistently abolished (Figures 9B to 9E). On the other hand, short actin cables could be seen in the apical region, some abutting the cell membrane and especially evident when single optical sections were examined, but which were rarely observed in control pollen tubes that maintained a vibrant actin mesh (Figure 9A).
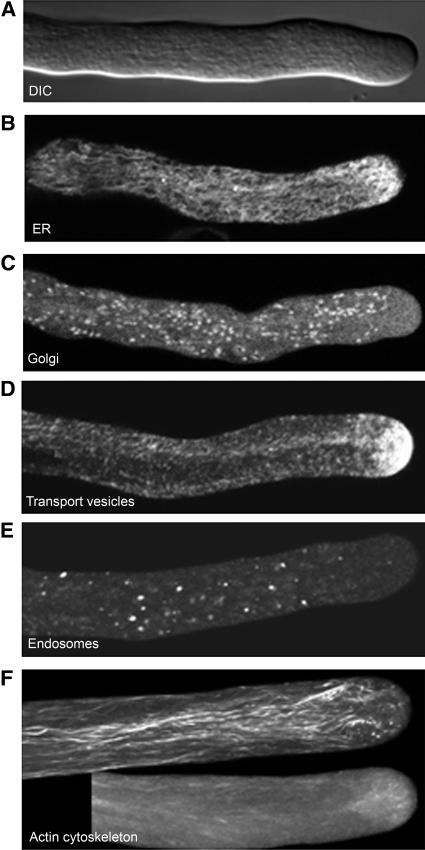
Figure 10.
Compartmentalization of Endomembrane Components in Elongating Pollen Tubes.
(A) A differential interference contrast image showing a granular cytoplasm in the shank of the tube and the apical clear zone, where the cytoplasm gives a smooth appearance.
(B) A confocal image from the medial section of a pollen tube expressing GFP-HDEL, revealing the endoplasmic reticulum system. The endoplasmic reticulum membranes are extensive and present throughout the entire pollen tube except in the extreme apex (see also Parton et al., 2003). The densest network can be seen at the subapical region.
(C) A confocal image from the medial section of a pollen tube expressing GFP-Rab2, revealing the Golgi bodies (see also Cheung et al., 2002).
(D) A whole tube projection of confocal images of a GFP-Rab11b–expressing pollen tube showing apical concentration of transport vesicles.
(E) A confocal image from the medial section of a GFP-Rab5–expressing pollen tube, revealing the endosomes.
(F) Whole tube projection of confocal images of the pollen tube actin cytoskeleton as revealed by GFP-NtADF1 (Chen et al., 2002). GFP-NtADF1 associates with the subapical actin mesh most prominently (bottom). When expression level is higher, long actin cables are also efficiently decorated (top).
Micrographs are used to provide a more vivid representation not achievable by a schematic diagram. Normaski optics reveal the reverse fountain streaming pattern with organelles mobilized tipward along the cortex, reversing directionality at the subapical region and moving grainward along the center of the tube (see also Cheung, 2001). Golgi bodies (Cheung et al., 2002) and endosomes (B.H.J. de Graaf, A.Y. Cheung, and H.-m. Wu, unpublished data) follow the reverse fountain streaming pattern but rarely invade the clear zone. Transport vesicles are likely to be distributed throughout the tube cytoplasm but are maintained at very high density in apical clear zone. Exclusive occupation by transport vesicles in the apical clear zone was initially shown by transmission electron microscopy (Lancelle and Hepler, 1992; Derksen et al., 1995a, 1995b) and is evident when vesicles and organelles are tagged by GFP. Long actin cables along the tube reaching the subapical region are responsible for mobilizing pollen tube organelles, and the dense actin mesh at the subapical region may serve as a sieve to only permit vesicles reaching the tube apex, along with being a structure that is tightly coupled to growth (see Vidali et al., 2001; Chen et al., 2002).
Figure 9.
Rab11b(DN) and Rab11(CA) Expression Is Associated with a Disrupted Actin Organization in the Pollen Tube Apex.
Confocal images of pollen tubes cotransformed by Lat52-GFP-NtADF1 and Lat52-Rab11b, its DN or CA variant. Pollen grains were transformed by microprojectile bombardment.
(A) A pollen tube cotransformed by Lat52-GFP-NtADF1 and Lat52-Rab11b. Asterisks indicate the location of the subapical actin mesh. The top panel shows a whole tube projection, and the bottom panel shows contiguous optical sections where the subapical actin mesh was prominently observed.
(B) and (D) Pollen tubes cotransformed by Lat52-GFP-NtADF1 and Lat52-Rab11b(DN). These pollen tubes had elongated for ∼350 μm (B) and 70 μm (D) at the time of observation. The top and middle panels in (B) show a whole tube projection and serial optical sections in the medial region of the tube apex, respectively. The bottom panel in (B) shows a medial section of the slightly distal region of the pollen tube. The Rab11b(DN)-induced bent was evident in this region of the pollen tube. The left and right panels of (D) show a whole tube projection and the medial section of the same pollen tube.
(C) and (E) Pollen tubes cotransformed by Lat52-GFP-NtADF1 and Lat52-Rab11b(CA). These pollen tubes had elongated ∼150 μm (C) and 50 μm (E) at the time of observation. The top and bottom panels of (C) show a whole tube projection and serial optical sections in the medial region of the same pollen tube. The top and bottom panels of (E) show a whole tube projection and the medial section of the same pollen tube.
DISCUSSION
Rapid tip growth and a highly dynamic intracellular trafficking system that targets transport vesicles to the apical clear zone are hallmark characteristics of elongating pollen tubes. Results reported here show that apical targeting of transport vesicles is crucial for rapid, tip-focused pollen tube growth and male fertility. They demonstrate that Rab11 GTPase-regulated membrane trafficking activity underlies the targeted delivery of exocytic and recycling vesicles to the pollen tube apex and efficient transport of cell wall and secretory proteins to its cell surface. As with most functional studies in Ras-related GTPases, expression of DN or CA variants of Rab11b to perturb the Rab11 activity in transformed pollen tubes was instrumental in revealing its functional contribution to this tip growth process. Twenty-six of the 57 Arabidopsis RAB genes encode Rab11 homologs; at least 10 of them have comparable levels of mRNA expression levels in pollen, and none predominate (Pina et al., 2005; see also www.cbs.umn.edu/arabidopsis/). Observations of several T-DNA knockouts in some of these pollen-expressed RABAs did not reveal noticeable reproductive phenotypes (A.Y. Cheung, unpublished data). Functional redundancy or overlapping functions among closely related and coexpressed RABAs could have compensated for the loss of function of any single gene. Moreover, other Rab GTPases (e.g., Rab8 GTPases) may also participate in post-Golgi vesicle trafficking, alleviating the problem adequately so male fertility is not observably affected in Arabidopsis.
The prominent localization of GFP-Rab11b in the exclusively transport vesicle-occupied apical clear zone of elongating pollen tubes (Lancelle and Hepler, 1992; Derksen et al., 1995a, 1995b) provides strong support for a predominant vesicle membrane association for Rab11b. Where exactly in the endomembrane system Rab11b acts is more difficult to decipher. For most Rab GTPases, prenylated GDP-bound proteins are escorted from the cytosol to their respective donor membrane system whereupon GDP-GTP exchange occurs in response to cellular regulatory signals (Alory and Balch, 2003; Sivars et al., 2003; Pfeffer and Aivazian, 2004). Membrane fragments tagged with GTP-bound Rabs bud off, and interactions with their corresponding effectors guide these vesicles to their respective target membrane system. Not being capable of undergoing GDP-GTP exchange, DN Rabs would have prolonged association with the donor membranes. The prominent localization of GFP-Rab11b(DN) to Golgi bodies (Figure 3) therefore suggests that wild-type NtRab11b is most predominantly escorted to these organelles, tagging Golgi-derived vesicles as they bud off. That GFP-Rab11b and GFP-Rab11b(CA) also associated weakly with Golgi is consistent with rapid activation of GFP-Rab11b upon insertion into the donor membranes, budding, and release of vesicles tagged by the activated forms of Rab11b variants.
After vesicle docking with the target membrane, GTP hydrolysis and SNARE-mediated vesicle and target membrane fusion occurs. The GDP-bound inactivated Rabs are then extracted from the target membrane and recycled to the cytosol (Zerial and McBride, 2001; Pfeffer and Aivazian, 2004; Seabra and Wasmeier, 2004). With GTP hydrolysis substantially inhibited in CA mutants, their release from target membranes would be considerably retarded. Thus, the pollen tube cell membrane association for GFP-Rab11b(CA) is consistent with defective recycling of this protein from the plasma membrane. These observations are consistent with Rab11b-tagged vesicles being targeted to the pollen tube apical membrane. These and observations showing that efficient secretion of cell wall and secretory proteins depends on proper targeting of Rab11b-tagged vesicles to the apical clear zone (Figure 7) provide support for a major functional role for Rab11b in regulating the trafficking of Golgi-derived vesicles to the cell membrane.
Members of the Rab11 family are also known to play prominent roles in the recycling of receptor proteins from endosomes directly to the plasma membrane or through the Golgi network (Ullrich et al., 1996; Chen et al., 1998; Schlierf et al., 2000; Wilcke et al., 2000; Band et al., 2002; Cheng et al., 2002; Volpicelli et al., 2002; Molendijk et al., 2004). The temporal pattern of FM4-64 uptake into pollen tubes, initial internalization and labeling of small organelles, followed by signal redistribution to a pronounced accumulation in the apical clear zone showed that endocytic membrane recycling also actively contributes to the apical collection of vesicles (Parton et al., 2001; Camacho and Malho, 2003). That altering Rab11b activity abolished the apical accumulation of FM4-64 (Figures 8D and 8E) is consistent with the participation by Rab11 in regulating the transport of recycled vesicles to the pollen tube apex. Future studies should dissect the functional specificity that most likely exists among the pollen-expressed Rab11 isoforms in the overall exocytic and recycling processes and how the functional specialization is achieved.
The normal Rab11 activity level in pollen tubes apparently supports growth rates optimum for reproductive success. The inhibited tube elongation observed in GFP-Rab11b(DN)– and GFP-Rab11b(CA)–expressing pollen tubes (Figures 4 and 6) could largely be the consequence of retarded exocytic activities. Observations that pollen tube width increased with increasing GFP-Rab11b expression level (Figure 5B) indicate that cell expansion had occurred at a broader apical region, resulting in lateral cell expansion and, thus, wider tubes. That this coincided with a broader distribution of GFP-Rab11b at the apical dome also supports a tight linkage between Rab11b-mediated secretory activity and cell growth.
Tip-focused pollen tube growth, on the other hand, is believed to have resulted from spatially regulated exocytosis and endocytosis around the apical dome, so peak cell expansion is maintained at the tube apex; when this spatial regulation is relaxed, depolarized growth occurs (Camacho and Malho, 2003). Therefore, the meandering growth observed for GFP-Rab11b(DN)– and GFP-Rab11b(CA)–expressing tubes (Figure 5) has apparently resulted from spatial disruption of the tip-focused vesicle delivery system. Frequent bents, prevalently seen in GFP-Rab11b(CA)–expressing tubes, suggest that the focal point of vesicular activity around the apical dome had made frequent changes, resulting in shifting growth trajectories that oscillated around an overall forward growth direction. On the other hand, the less frequent twists and turns seen in GFP-Rab11b(DN)–expressing tubes reflect their ability to maintain tip growth along a stable trajectory for a period of time. However, once a subapical focus of peak secretory activity was organized, subapical growth was sustained, giving rise to pronounced turns that resulted in major changes in growth direction, sometimes ending in a knotted phenotype (Figure 5). These observations strongly support that tube growth direction is tightly coupled to the spatial distribution of vesicular activities focused at the growing tip membrane.
The reduced male fertility observed for plants expressing the DN variant of Rab11b in their pollen was likely to be the combined consequence of reduced tube growth rates and compromised tube capacity to penetrate ovules (Figures 4 to 6). On the other hand, since pollen germination was inhibited in grains that expressed even moderate levels of GFP-Rab11b(CA), this and the considerably slower growth among the germinated tubes must have been the major factors underlying the severely reduced male fertility observed in the Lat52-GFP-Rab11b(CA) transformed plants (Table 1; see Supplemental Table 1 online). The meandering property of these pollen tubes undoubtedly would have compromised their ability to target ovules (e.g., see Figure 5), although the pollination process was never completed for ovule entrance in vivo to be analyzed. Whether the localization of receptors for female factors was affected in these pollen tubes thus contributing to their compromised targeting ability is an interesting and important question to be explored in future studies.
Kinky pollen tubes have previously been correlated with compromised male fertility in an Arabidopsis mutant, kinky pollen (Procissi et al., 2003). Although where these pollen tubes failed in vivo is not known, KINKY POLLEN encodes a putative secretory protein. This and the kinky phenotype were used to suggest that defects in membrane trafficking might have contributed to the reproductive phenotypes observed in this Arabidopsis mutant plant. Studies reported here on Rab11b clearly show that properly regulated membrane trafficking activity is key to successful sexual reproduction. That expression of either the DN or CA Rab11b variant both negatively impacted pollen tube growth properties and male fertility implies that a proper ratio of active to inactive forms of Rab11b and its closely related isoforms that share common interacting proteins must be stringently regulated in order to maintain an optimum vesicular transport activity targeted at the pollen tube tip. The less severe pollen tube growth and male fertility phenotypes induced by the expression of the DN variant of NtRab11b relative to those by the CA variant suggest that perturbing the exit of vesicles from the Golgi and their transport along the exocytic path is less disruptive than inhibiting fusion of the docked vesicles with the cell membrane. It is possible that post-Golgi vesicle trafficking to the plasmalemma is supported by multiple Rab GTPase-regulated pathways, whereas persistent docking on the cell membrane and occupation of effectors by CA NtRab11b could have impeded overall vesicle delivery and membrane recycling, thus more severely impacting cell growth.
A most notable feature for elongating pollen tubes is the distinct intracellular organization whereby various functional systems are differentially distributed in the tube cytoplasm (Figure 10). Growth defects invariably accompany disruption of this organization, and the subapical actin mesh is most sensitive to growth perturbation, as was observed in pollen tubes with misregulated Rab11b activity (Figure 9). That the subapical actin mesh, tip-focused secretory activity, and growth at the pollen tube apex are functionally intertwined and Rab11b contributes importantly to maintaining this relationship are obvious from these observations. However, whether a direct functional relationship exists between Rab11b and the mechanisms that maintain the organization and regulate the dynamics of the actin cytoskeleton in pollen tubes needs to be further explored. The demonstration here that pollen tube growth and reproductive success is compromised when apical targeting of transport vesicles is disrupted underscores the importance of regulated vesicular transport in these tip growth cells. Revealing how various Rab GTPases participate in the different steps of vesicular transport in pollen tubes should contribute to the general understanding of the cellular machinery that underlies this tip growth process.
METHODS
cDNA Isolation, Mutagenesis, Construction of GFP Fusion Proteins, and Gene Expression Cassettes
cDNAs for a pollen-expressed Rab11 (Rab11b) (Haizel et al., 1995) and Inv (Greiner et al., 1995) were obtained from tobacco (Nicotiana tabacum) pollen mRNA by RT-PCR. Mutation S28N and Q73L were introduced individually into Rab11b by PCR-based mutagenesis (Ho et al., 1989; Ito et al., 1991) to produce Rab11b(DN) and Rab11b(CA), respectively. N-terminal fusion of all three forms of Rab11b with GFP (Chiu et al., 1996) or a FLAG tag and C-terminal fusion of Inv with GFP (Inv-GFP) were constructed by PCR-based methodology. Inv-GFP, Sec-GFP (with a signal peptide sequence fused to the N terminus of GFP), and SynAGP-GFP (with a signal peptide and a synthetic arabinogalactan protein fused to GFP) (Shpak et al., 1999) were used as cargo molecules for the secretory pathway. All chimeric genes were fused with the pollen-active promoter LAT52 (Twell et al., 1990) either in pBluescript (pKS+) for microprojectile bombardment to transiently transform pollen (Chen et al., 2002) or in a T-DNA vector for Agrobacterium tumefaciens–mediated transformation to obtain stable transgenic plants (Delebrese et al., 1986). Figure 1 shows a list of constructs used in this study.
Transformation, Genetic Crosses, and Analyses
Transformed tobacco plants were generated by Agrobacterium Ti plasmid transformation (Delebrese et al., 1986). Stable transformed pollen grains from these plants were used for analyses unless indicated otherwise. They were also used in genetic crosses to obtain double transformed plants that expressed various forms of Rab11b and GFP-labeled cargo molecules in their pollen. Transgene segregation analyses were performed by germinating seeds in tissue culture medium supplemented with kanamycin to score for the transformation marker gene. Transient transformation of tobacco pollen by microprojectile bombardment was performed as previously described (Chen et al., 2002).
Pollen Tube Growth
In vivo, semi–in vivo, and in vitro pollen tube growth assays follow previously described procedures (Cheung et al., 1995; Chen et al., 2002). Pollen from transformed plants lines 21a, 153-12, 173-4, 173-8, 207-2, and 207-3 (Figure 1, Table 1) were used for analyses. Treatment of in vitro–grown pollen tubes by FM4-64, BFA, and latrunculin B followed published conditions (Parton et al., 2001; Cheung et al., 2002). For in vivo and semi–in vivo pollen tube growth assays, pistils from nontransformed plants were hand-pollinated. For semi in vivo growth assays, emasculated tobacco flowers were pollinated with segregating populations of pollen from Lat52-GFP-Rab11b, Lat52-GFP-Rab11b(DN), or Lat52-GFP-Rab11b(CA) transformed plants with a single T-DNA insertion locus (lines 153-12, 173-8, and 207-2). Pollinated stigma-styles were excised at specified hours (see figure legends) after pollination, and the cut stylar ends were immersed in germination medium to allow further growth of pollen tubes through the style and onto the medium. These cut pistils were examined periodically between 8 and 24 h after culturing to observe the emergence of pollen tubes at the stylar end. Pollen tubes within the transmitting tissue were observed after bisecting the styles. All pollen tube growth experiments were repeated at least three times with comparable observations.
Microscopy
Bright-field and epifluorescence imaging were performed on a Nikon Eclipse E800 microscope equipped with a SPOT camera (Nikon). Most images were taken by autoexposure. When the level of GFP fusion protein expression was compared microscopically between individual pollen tubes, identical exposure conditions were used among the samples. Confocal microscopy was performed on a Zeiss 510 Meta system.
RNA and Protein Analyses
RNA and protein isolation and gel blot analyses followed previous procedures (Wang et al., 1993, 1996), except that chemiluminescent detection was used for some of the protein blots.
Accession Numbers
Sequence data from this article can be found in the EMBL/GenBank data libraries under the following accession numbers: L29269 (Rab11b) and X81834 (Inv). Accession numbers for genes in the alignment are shown in Supplemental Figure 1 online.
Supplementary Material
Acknowledgments
This work was supported by grants from the USDA (2001-01936 and 2003-35304-13241) and from the National Science Foundation to the University of Massachusetts Central Microscopy Facility. K.L. was a recipient of a Research Experience for Undergraduates summer fellowship from the National Science Foundation to the University of Massachusetts Plant Biology Program. The Lat52 promoter was a gift from S. McCormick.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantcell.org) is: Alice Y. Cheung (acheung@biochem.umass.edu).
Online version contains Web-only data.
Article, publication date, and citation information can be found at www.plantcell.org/cgi/doi/10.1105/tpc.105.033183.
References
- Alory, C., and Balch, W.E. (2003). Molecular evolution of the Rab-escort-protein/guanine-nucleotide-dissociation-inhibitor superfamily. Mol. Biol. Cell 14, 3857–3867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong, J. (2000). How do Rab proteins function in membrane traffic? Int. J. Biochem. Cell Biol. 32, 303–307. [DOI] [PubMed] [Google Scholar]
- Band, A.M., Ali, H., Vartiainen, M.K., Welti, S., Lappalainen, P., Olkkonen, V.M., and Kuismanen, E. (2002). Endogenous plasma membrane t-SNARE syntaxin 4 is present in rab11 positive endosomal membranes and associates with cortical actin cytoskeleton. FEBS Lett. 20, 513–519. [DOI] [PubMed] [Google Scholar]
- Bar-Sagi, D., and Hall, A. (2000). Ras and Rho GTPases: A family reunion. Cell 103, 227–238. [DOI] [PubMed] [Google Scholar]
- Batoko, H., Zheng, H.-Q., Hawes, C., and Moore, I. (2000). A Rab1 GTPase is required for transport between the endoplasmic reticulum and Golgi apparatus and for normal Golgi movement in plants. Plant Cell 12, 2201–2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benli, M., Doring, F., Robinson, D.G., Yang, X., and Gallwitz, D. (1996). Two GTPase isoforms, Ypt31p and Ypt32p, are essential for Golgi function in yeast. EMBO J. 15, 6460–6475. [PMC free article] [PubMed] [Google Scholar]
- Bolte, S., Talbot, C., Boutte, Y., Catrice, E., Read, N.D., and Satiat-Jeunemaitre, B. (2004). FM-dyes as experimental probes for dissecting vesicle trafficking in living plant cells. J. Microsc. 214, 159–173. [DOI] [PubMed] [Google Scholar]
- Camacho, L., and Malho, R. (2003). Endo/exocytosis in the pollen tube apex is differentially regulated by Ca2+ and GTPases. J. Exp. Bot. 54, 83–92. [DOI] [PubMed] [Google Scholar]
- Chen, C.Y., Wong, E.I., Vidali, L., Estavillo, A., Hepler, P.K., Wu, H.-M., and Cheung, A.Y. (2002). The regulation of actin organization by actin-depolymerizing factor in elongating pollen tubes. Plant Cell 14, 2175–2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, W., Feng, Y., Chen, D., and Wandinger-Ness, A. (1998). Rab11 is required for trans-Golgi network-to-plasma membrane transport and a preferential target for GDP dissociation inhibitor. Mol. Biol. Cell 9, 3241–3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng, H., Sugiura, R., Wu, W., Fujita, M., Lu, Y., Sio, S.O., Kawai, R., Takegawa, K., Shuntoh, H., and Kuno, T. (2002). Role of the Rab GTP-binding protein Ypt3 in the fission yeast exocytic pathway and its connection to calcineurin function. Mol. Biol. Cell 13, 2963–2976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung, A.Y. (1996). Pollen-pistil interactions during pollen tube growth. Trends Plant Sci. 1, 45–51. [Google Scholar]
- Cheung, A.Y. (2001). Imaging elongating pollen tubes by the green fluorescence protein. Sex. Plant Reprod. 14, 9–14. [Google Scholar]
- Cheung, A.Y., Chen, C.Y., Glaven, R.H., de Graaf, B.H.J., Vidali, L., Hepler, P.K., and Wu, H.M. (2002). Rab2 GTPase regulates vesicle trafficking between the endoplasmic reticulum and the Golgi bodies and is important to pollen tube growth. Plant Cell 14, 945–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung, A.Y., Wang, H., and Wu, H.-M. (1995). A floral transmitting tissue-specific glycoprotein attracts pollen tubes and stimulates their growth. Cell 11, 383–393. [DOI] [PubMed] [Google Scholar]
- Cheung, A.Y., and Wu, H.-M. (2004). Over expression of an Arabidopsis formin stimulates supernumerary actin cable formation from pollen tube cell membrane. Plant Cell 16, 257–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu, W., Niwa, Y., Zeng, W., Hirano, T., Kobayashi, H., and Sheen, J. (1996). Engineered GFP as a vital reporter in plants. Curr. Biol. 6, 325–330. [DOI] [PubMed] [Google Scholar]
- Cox, D., Lee, D.J., Dale, B.M., Calafat, J., and Greenberg, S. (2000). A Rab11-containing rapidly recycling compartment in macrophages that promotes phagocytosis. Proc. Natl. Acad. Sci. USA 97, 680–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delebrese, R., Reynaert, A., Hofte, H., Hernalsteen, H.P., Leemans, J., and Van Montagu, M. (1986). Vectors for cloning in plant cells. Methods Enzymol. 153, 277–290. [Google Scholar]
- Derksen, J., Rutten, T., Lichtscheidl, I.K., de Win, A.H.N., Pierson, E.S., and Rongen, G. (1995. b). Quantitative analysis of the distribution of organelles in tobacco pollen tubes: Implication for exocytosis and endocytosis. Protoplasma 188, 267–276. [Google Scholar]
- Derksen, J., Rutten, T., van Amstel, T., de Win, A., Doris, F., and Steer, M. (1995. a). Regulation of pollen tube growth. Acta Bot. Neerl. 44, 93–119. [Google Scholar]
- Geldner, N., Friml, J., Stierhof, Y.D., Jurgens, G., and Palme, K. (2001). Auxin transport inhibitors block PIN1 cycling and vesicle trafficking. Nature 27, 425–428. [DOI] [PubMed] [Google Scholar]
- Grebe, M., Xu, J., Mobius, W., Ueda, T., Nakano, A., Geuze, H.J., Rook, M.B., and Scheres, B. (2003). Arabidopsis sterol endocytosis involves actin-mediated trafficking via ARA6-positive early endosomes. Curr. Biol. 19, 1378–1387. [DOI] [PubMed] [Google Scholar]
- Greiner, S., Weil, M., Krausgrill, S., and Rausch, T. (1995). A tobacco cDNA coding for cell wall invertase. Plant Physiol. 108, 825–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyon, V.N., Astwood, J.D., Garner, E.C., Dunker, A.K., and Taylor, L.P. (2000). Isolation and characterization of cDNAs expressed in the early stages of flavonol-induced pollen germination in petunia. Plant Physiol. 123, 699–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haizel, T., Merkle, T., Turck, F., and Nagy, F. (1995). Characterization of membrane-bound small GTP-binding proteins from Nicotiana tabacum. Plant Physiol. 108, 59–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hales, C.M., Vaerman, J.P., and Goldenring, J.R. (2002). Rab11 family interacting protein 2 associates with Myosin Vb and regulates plasma membrane recycling. J. Biol. Chem. 277, 50415–50421. [DOI] [PubMed] [Google Scholar]
- Hepler, P.K., Vidali, L., and Cheung, A.Y. (2001). Polarized cell growth in higher plants. Annu. Rev. Cell Dev. Biol. 17, 159–187. [DOI] [PubMed] [Google Scholar]
- Higashiyama, T., Kuroiwa, H., Kawano, S., and Kuroiwa, T. (1998). Guidance in vitro of the pollen tube to the naked embryo sac of Torenia fournieri. Plant Cell 10, 2019–2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashiyama, T., Yabe, S., Sasaki, N., Nishimura, Y., Miyagishima, S., Kuroiwa, H., and Kuroiwa, T. (2001). Pollen tube attraction by the synergid cell. Science 24, 1480–1483. [DOI] [PubMed] [Google Scholar]
- Ho, S.N., Hunt, H.D., Horton, R.M., Pullen, J.K., and Pease, L.R. (1989). Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene 77, 51–59. [DOI] [PubMed] [Google Scholar]
- Huck, N., Moore, J.M., Federer, M., and Grossniklaus, U. (2003). The Arabidopsis mutant feronia disrupts the female gametophytic control of pollen tube reception. Development 130, 2149–2159. [DOI] [PubMed] [Google Scholar]
- Hulskamp, M., Schneitz, K., and Pruitt, R.E. (1995). Genetic evidence for a long-range activity that directs pollen tube guidance in Arabidopsis. Plant Cell 7, 57–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inaba, T., Nagano, Y., Nagasaki, T., and Sasaki, Y. (2002). Distinct localization of two closely related Ypt3/Rab11 proteins on the trafficking pathway in higher plants. J. Biol. Chem. 277, 9183–9188. [DOI] [PubMed] [Google Scholar]
- Ito, W., Ishiguro, H., and Kurosawa, Y. (1991). A general method for introducing a series of mutations into cloned DNA using the polymerase chain reaction. Gene 102, 67–70. [DOI] [PubMed] [Google Scholar]
- Jedd, G., Mulholland, J., and Segev, N. (1997). Two new Ypt GTPases are required for exit from the yeast trans-Golgi compartment. J. Cell Biol. 137, 563–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson, M.A., and Preuss, D. (2002). Plotting a course: Multiple signals guide pollen tubes to their targets. Dev. Cell 2, 273–281. [DOI] [PubMed] [Google Scholar]
- Kang, J.G., Yun, J., Kim, D.H., Chung, K.S., Fujioka, S., Kim, J.I., Dae, H.W., Yoshida, S., Takatsuto, S., Song, P.S., and Park, C.M. (2001). Light and brassinosteroid signals are integrated via a dark-induced small G protein in etiolated seedling growth. Cell 105, 625–636. [DOI] [PubMed] [Google Scholar]
- Kultonow, A.M., Truettner, J., Cox, K.H., Wallroth, M., and Goldberg, R.B. (1990). Different temporal and spatial gene expression patterns occur during anther development. Plant Cell 2, 1201–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancelle, S.A., and Hepler, P.K. (1992). Ultrastructure of the cytoskeleton in freeze substituted pollen tubes of Nicotiana alata. Protoplasma 140, 141–150. [Google Scholar]
- Lapierre, L.A., Kumar, R., Hales, C.M., Navarre, J., Bhartur, S.G., Burnette, J.O., Provance, D.W., Jr., Mercer, J.A., Bahler, M., and Goldenring, J.R. (2001). Myosin vb is associated with plasma membrane recycling systems. Mol. Biol. Cell 12, 1843–1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lind, J.L., Bönig, I., Clarke, A.E., and Anderson, M.A. (1996). A style-specific 120 kDa glycoprotein enters pollen tubes of Nicotiana alata in vivo. Sex. Plant Reprod. 9, 75–86. [Google Scholar]
- Lord, E.M., and Russell, S.D. (2002). The mechanisms of pollination and fertilization in plants. Annu. Rev. Cell Dev. Biol. 18, 81–105. [DOI] [PubMed] [Google Scholar]
- Lu, C., Zainal, Z., Tucker, G.A., and Lycett, G.W. (2001). Developmental abnormalities and reduced fruit softening in tomato plants expressing an antisense Rab11 GTPase gene. Plant Cell 13, 1819–1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luu, D.T., Qin, X., Morse, D., and Cappadocia, M. (2000). S-RNase uptake by compatible pollen tubes in gametophytic self-incompatibility. Nature 5, 649–651. [DOI] [PubMed] [Google Scholar]
- Marton, M.L., Cordts, S., Broadhvest, J., and Dresselhaus, T. (2005). Micropylar pollen tube guidance by egg apparatus 1 of maize. Science 304, 573–576. [DOI] [PubMed] [Google Scholar]
- Mascarenhas, J.P. (1989). The male gametophyte of flowering plants. Plant Cell 1, 657–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molendijk, A.J., Ruperti, B., and Palme, K. (2004). Small GTPases in vesicle trafficking. Curr. Opin. Plant Biol. 7, 694–700. [DOI] [PubMed] [Google Scholar]
- Nagano, Y., Okada, Y., Narita, H., Asaka, Y., and Sasaki, Y. (1995). Location of light-repressible, small GTP-binding protein of the YPT/rab family in the growing zone of etiolated pea stems. Proc. Natl. Acad. Sci. USA 92, 6314–6318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Driscoll, D., Hann, C., Read, S.M., and Steer, M.W. (1993). Endocytotic uptake of fluorescent dextrans by pollen tubes grown in vitro. Protoplasma 175, 126–130. [Google Scholar]
- Ortiz, D., Medkova, M., Walch-Solimena, C., and Novick, P. (2002). Ypt32 recruits the Sec4p guanine nucleotide exchange factor, Sec2p, to secretory vesicles; evidence for a Rab cascade in yeast. J. Cell Biol. 10, 1005–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palanivelu, R., Brass, L., Edlund, A.F., and Preuss, D. (2003). Pollen tube growth and guidance is regulated by POP2, an Arabidopsis gene that controls GABA levels. Cell 11, 47–59. [DOI] [PubMed] [Google Scholar]
- Parton, R.M., Fischer-Parton, S., Trewavas, A.J., and Watahiki, M.J. (2003). Pollen tubes exhibit regular periodic membrane trafficking events in the absence of apical extension. J. Cell Sci. 116, 2707–2719. [DOI] [PubMed] [Google Scholar]
- Parton, R.M., Fischer-Parton, S., Watahiki, M.K., and Trewavas, A.J. (2001). Dynamics of the apical vesicle accumulation and the rate of growth are related in individual pollen tubes. J. Cell Sci. 114, 2685–2695. [DOI] [PubMed] [Google Scholar]
- Pereira-Leal, J.B., and Seabra, M.C. (2000). The mammalian Rab family of small GTPases: Definition of family and subfamily sequence motifs suggests a mechanism for functional specificity in the Ras superfamily. J. Mol. Biol. 301, 1077–1087. [DOI] [PubMed] [Google Scholar]
- Pereira-Leal, J.B., and Seabra, M.C. (2001). Evolution of the Rab family of small GTP-binding proteins. J. Mol. Biol. 313, 889–901. [DOI] [PubMed] [Google Scholar]
- Pfeffer, S., and Aivazian, D. (2004). Targeting Rab GTPases to distinct membrane compartments. Nat. Rev. Mol. Cell Biol. 5, 886–896. [DOI] [PubMed] [Google Scholar]
- Pina, C., Pinto, F., Feijo, J.A., and Becker, J.D. (2005). Gene family analysis of the Arabidopsis pollen transcriptome reveals biological implications for cell growth, division control and gene expression regulation. Plant Physiol. 138, 744–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preuss, M.L., Serna, J., Falbel, T.G., Bednarek, S.Y., and Nielsen, E. (2004). The Arabidopsis Rab GTPase RabA4b localizes to the tips of growing root hair cells. Plant Cell 16, 1589–1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Procissi, A., Guyon, A., Pierson, E.S., Giritch, A., Knuiman, B., Grandjean, O., Tonelli, C., Derksen, J., Pelletier, G., and Bonhomme, S. (2003). KINKY POLLEN encodes a SABRE-like protein required for tip growth in Arabidopsis and conserved among eukaryotes. Plant J. 36, 894–904. [DOI] [PubMed] [Google Scholar]
- Ritzenthaler, C., Nebenfuhr, A., Movafeghi, A., Stussi-Garaud, C., Behnia, L., Pimpl, P., Staehelin, L.A., and Robinson, D.G. (2002). Reevaluation of the effects of brefeldin A on plant cells using tobacco Bright Yellow 2 cells expressing Golgi-targeted green fluorescent protein and COPI antisera. Plant Cell 14, 237–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutherford, S., and Moore, I. (2002). The Arabidopsis Rab GTPase family: Another enigma variation. Curr. Opin. Plant Biol. 5, 518–528. [DOI] [PubMed] [Google Scholar]
- Rutten, T.L., and Knuiman, B. (1993). Brefeldin A effects on tobacco pollen tubes. Eur. J. Cell Biol. 61, 247–255. [PubMed] [Google Scholar]
- Sanderfoot, A.A., and Raikhel, N.V. (1999). The specificity of vesicle trafficking: Coat proteins and SNAREs. Plant Cell 11, 629–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimmoller, F., Simon, I., and Pfeffer, S.R. (1998). Rab GTPases, directors of vesicle docking. J. Biol. Chem. 273, 22161–22164. [DOI] [PubMed] [Google Scholar]
- Schlierf, B., Fey, G.H., Hauber, J., Hocke, G.M., and Rosorius, O. (2000). Rab11b is essential for recycling of transferrin to the plasma membrane. Exp. Cell Res. 259, 257–265. [DOI] [PubMed] [Google Scholar]
- Schott, D., Ho, J., Pruyne, D., and Bretscher, A. (1999). The COOH terminal domain of Myo2p, a yeast myosin V, has a direct role in secretory vesicle targeting. J. Cell Biol. 147, 791–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seabra, M.C., and Wasmeier, C. (2004). Controlling the location and activation of Rab GTPases. Curr. Opin. Cell Biol. 16, 451–457. [DOI] [PubMed] [Google Scholar]
- Shimizu, K.K., and Okada, K. (2000). Attractive and repulsive interactions between female and male gametophytes in Arabidopsis pollen tube guidance. Development 127, 4511–4518. [DOI] [PubMed] [Google Scholar]
- Shpak, E., Leykam, J.F., and Kieliszewski, M.J. (1999). Synthetic genes for the elucidation of hydroxyproline-O-glycosylation codes and de novo glycoprotein design. Proc. Natl. Acad. Sci. USA 96, 14736–14741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivars, U., Aivazian, D., and Pfeffer, S.R. (2003). Yip3 catalyses the dissociation of endosomal Rab-GCI complexes. Nature 425, 856–859. [DOI] [PubMed] [Google Scholar]
- Stenmark, H., and Olkkonen, V.M. (2001). The Rab GTPase family. Genome Biology 2, 3007.1–3007.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surpin, M., and Raikhel, N. (2004). Traffic jams affect plant development and signal transduction. Nat. Rev. Mol. Cell Biol. 5, 100–109. [DOI] [PubMed] [Google Scholar]
- Tamm, L.K., Crane, J., and Kiessling, V. (2003). Membrane fusion: A structural perspective on the interplay of lipids and proteins. Curr. Opin. Struct. Biol. 13, 453–466. [DOI] [PubMed] [Google Scholar]
- Tisdale, E.J., Bourne, J.R., Khosravi-Far, R., Der, C.J., and Balch, W.E. (1992). GTP-binding mutants of Rab1 and Rab2 are potent inhibitors of vesicular transport from the endoplasmic reticulum to the Golgi complex. J. Cell Biol. 119, 749–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twell, D., Yamaguchi, J., and McCormick, S. (1990). Pollen-specific gene expression of two different tomato gene promoters during microsporogenesis. Development 109, 705–713. [DOI] [PubMed] [Google Scholar]
- Ueda, T., Anai, T., Tsukaya, H., Hirata, A., and Uchimiya, H. (1996). Characterization and subcellular localization of a small GTP-binding protein (Ara-4) from Arabidopsis: Conditional expression under control of the promoter of the gene for heat-shock protein HSP81-1. Mol. Gen. Genet. 20, 533–539. [DOI] [PubMed] [Google Scholar]
- Ueda, T., Uemura, T., Sato, M.H., and Nakano, A. (2004). Functional differentiation of endosomes in Arabidopsis cells. Plant J. 40, 783–789. [DOI] [PubMed] [Google Scholar]
- Ullrich, O., Reinsch, S., Urbe, S., Zerial, M., and Parton, R.G. (1996). Rab11 regulates recycling through the pericentriolar recycling endosome. J. Cell Biol. 135, 913–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernoud, V., Horton, A.C., Yang, Z., and Nielsen, E. (2003). Analysis of the small GTPase gene superfamily of Arabidopsis. Plant Physiol. 131, 1191–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidali, L., McKenna, S.T., and Hepler, P.K. (2001). Actin polymerization is essential for pollen tube growth. Mol. Biol. Cell 12, 2534–2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpicelli, L.A., Lah, J.J., Fang, G., Goldenring, J.R., and Levey, A.I. (2002). Rab11a and myosin Vb regulate recycling of the M4 muscarinic acetylcholine receptor. J. Neurosci. 15, 9776–9784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waizenegger, I., Lukowitz, W., Assaad, F., Schwarz, H., Jurgens, G., and Mayer, U. (2000). The Arabidopsis KNOLLE and KEULLE genes interact to promote vesicle fusion during cytokinesis. Curr. Biol. 10, 1371–1374. [DOI] [PubMed] [Google Scholar]
- Wang, H., Wu, H.-M., and Cheung, A.Y. (1993). Development and pollination regulated accumulation and glycosylation of a stylar transmitting tissue-specific proline rich protein. Plant Cell 5, 1639–1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, H., Wu, H.-M., and Cheung, A.Y. (1996). Pollination induces mRNA poly(A) tail-shortening and cell deterioration in flower transmitting tissue. Plant J. 9, 715–727. [DOI] [PubMed] [Google Scholar]
- Wilcke, M., Johannes, L., Galli, T., Mayau, V., Goud, B., and Salamero, J. (2000). Rab11 regulates the compartmentalization of early endosomes required for efficient transport from early endosomes to the trans-Golgi network. J. Cell Biol. 151, 1207–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida, K., Nagano, Y., Murai, N., and Sasaki, Y. (1993). Phytochrome-regulated expression of the genes encoding the small GTP-binding proteins in peas. Proc. Natl. Acad. Sci. USA 90, 6636–6640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zainal, Z., Tucker, G.A., and Lycett, G.W. (1996). A rab11-like gene is developmentally regulated in ripening mango (Mangifera indica L.) fruit. Biochim. Biophys. Acta 12, 187–190. [DOI] [PubMed] [Google Scholar]
- Zerial, M., and McBride, H. (2001). Rab proteins as membrane organizers. Nat. Rev. Mol. Cell Biol. 2, 107–117. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.