Abstract
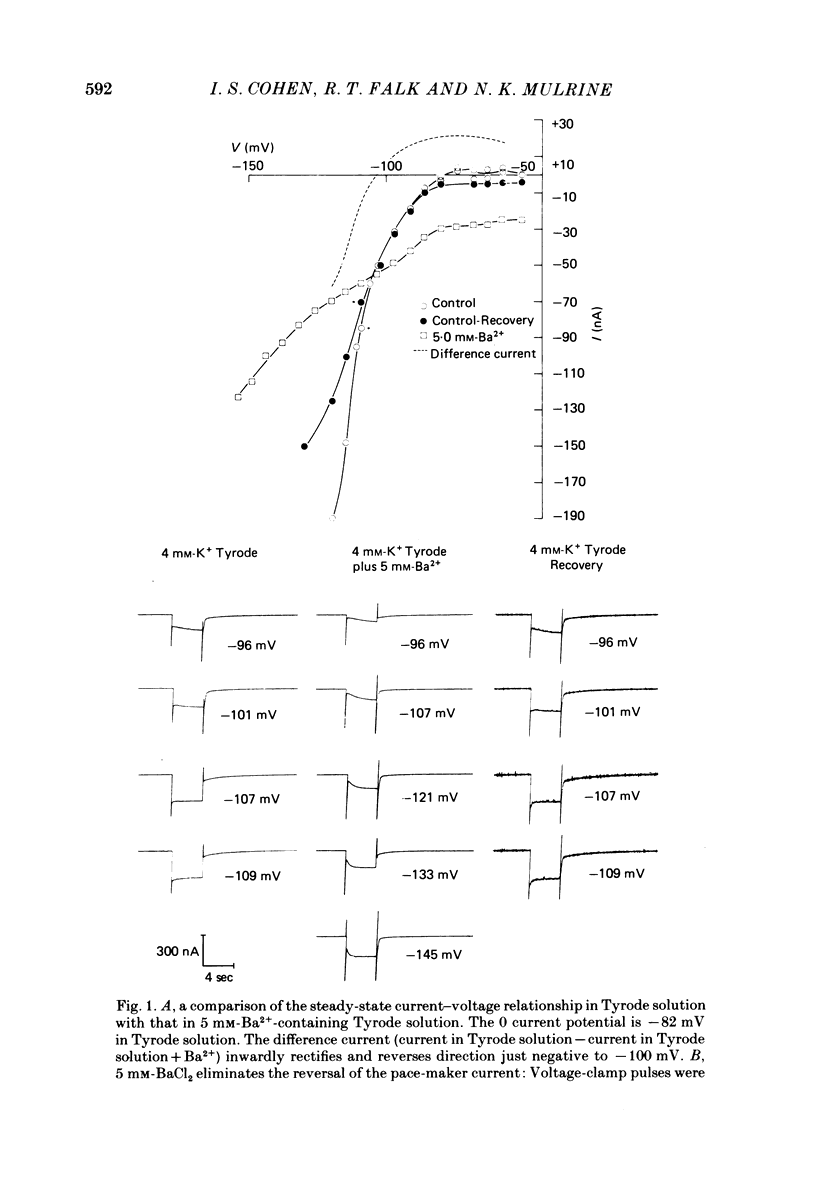
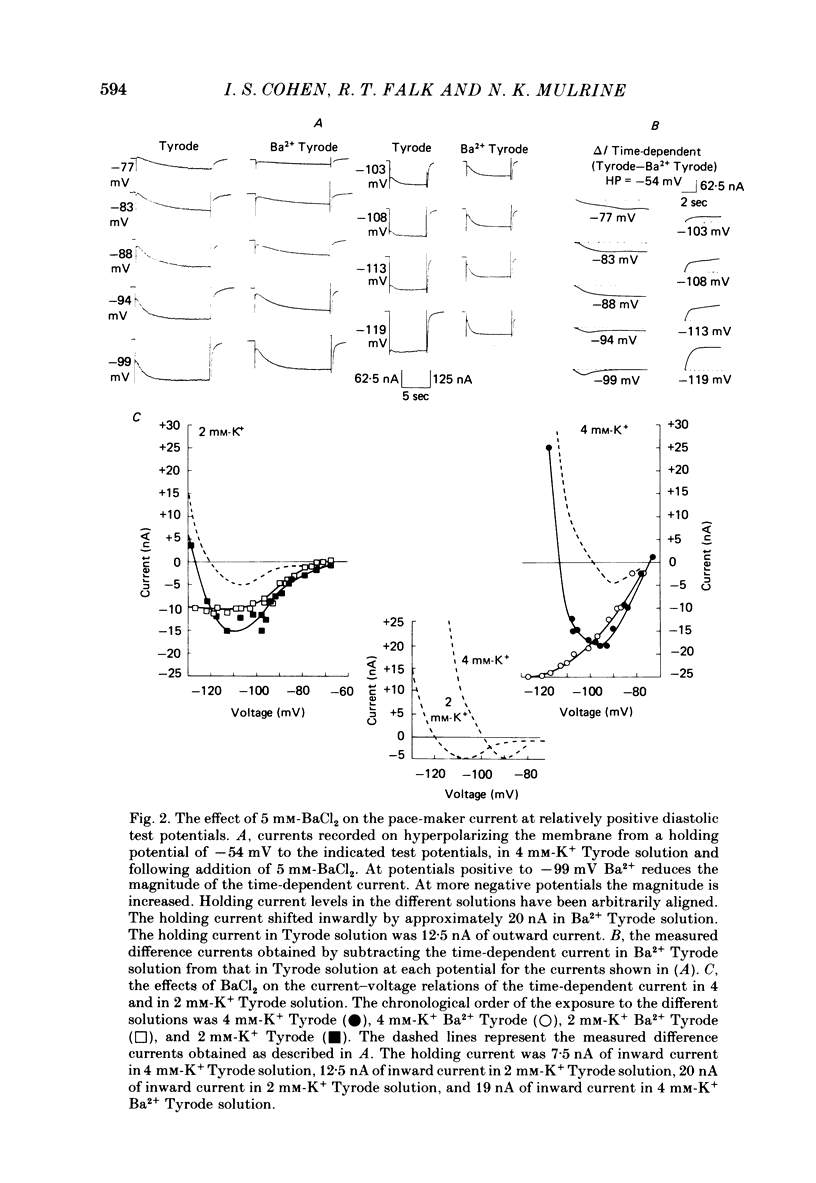
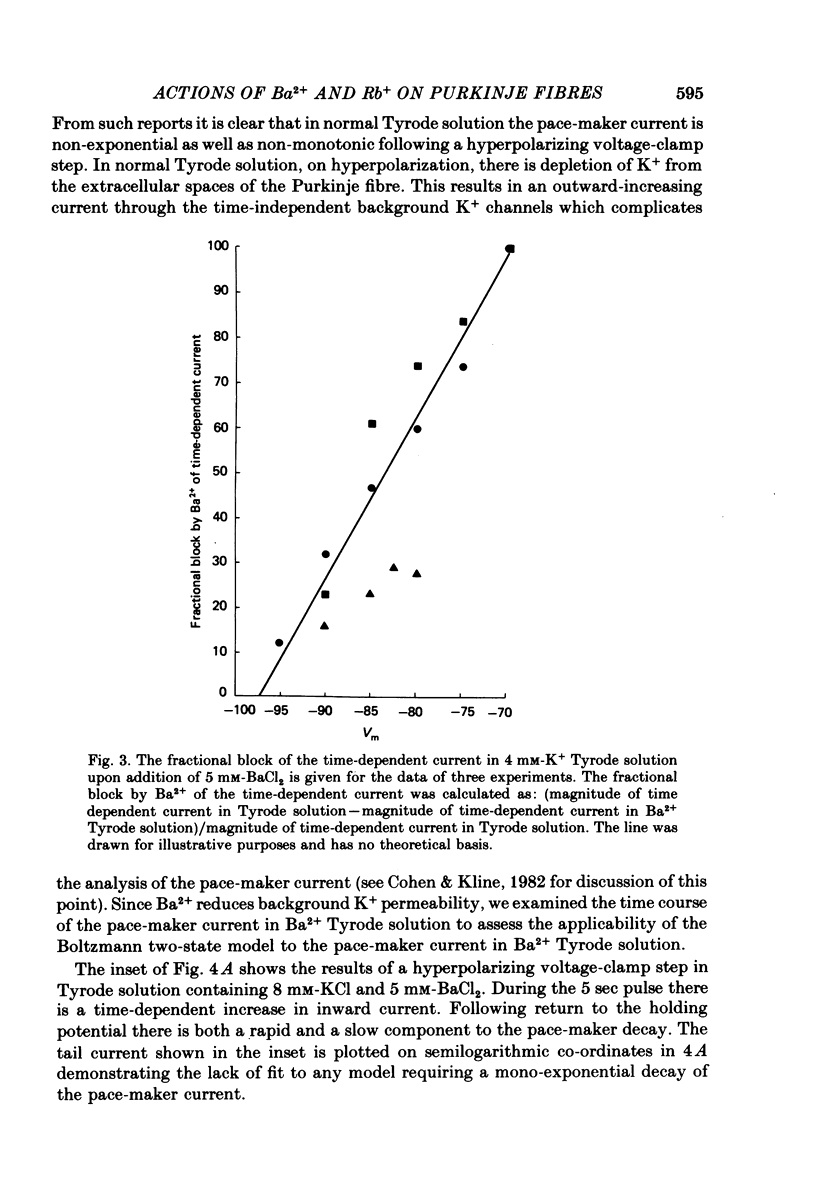
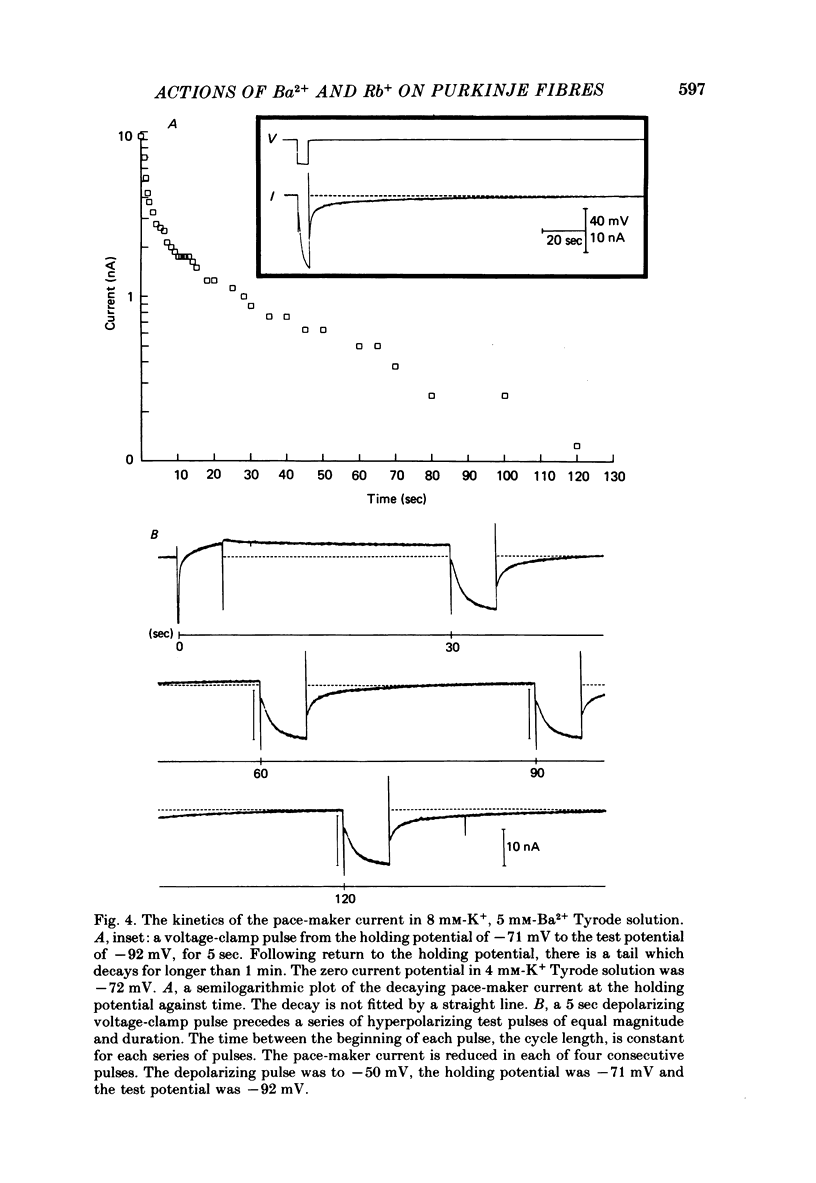
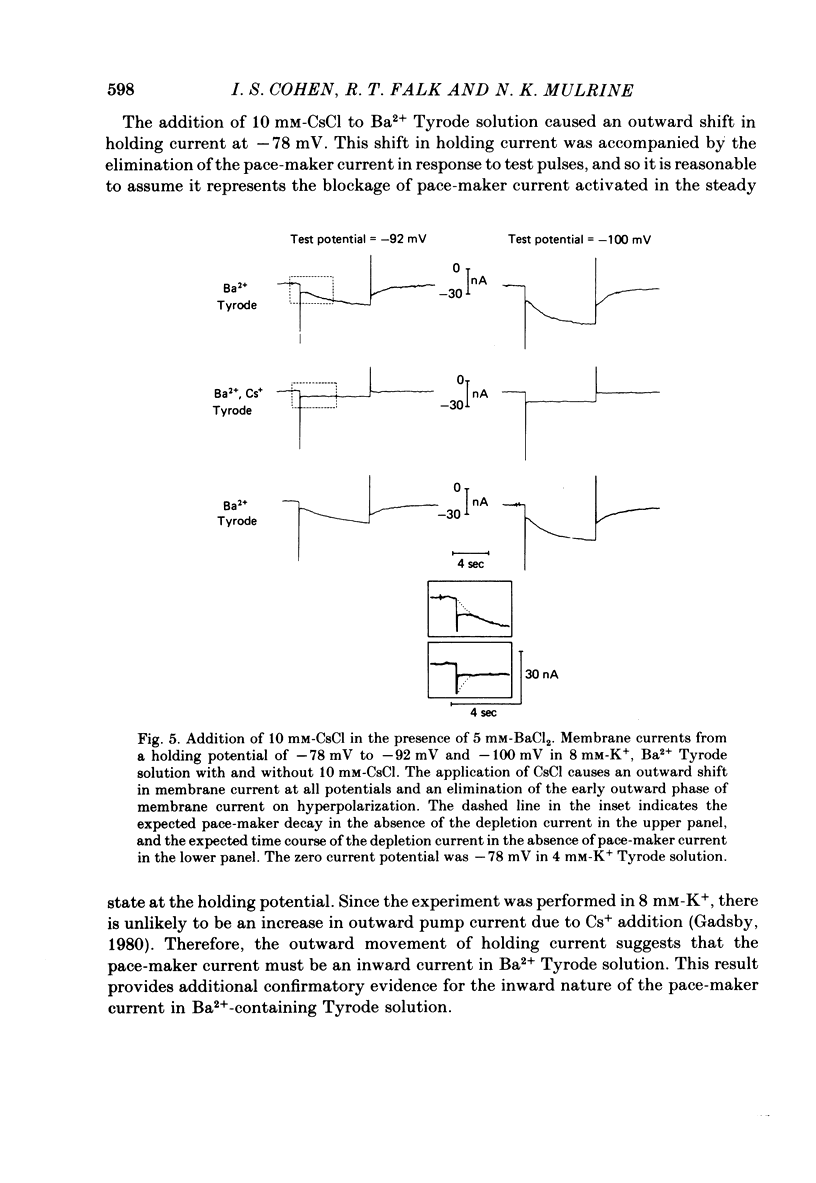
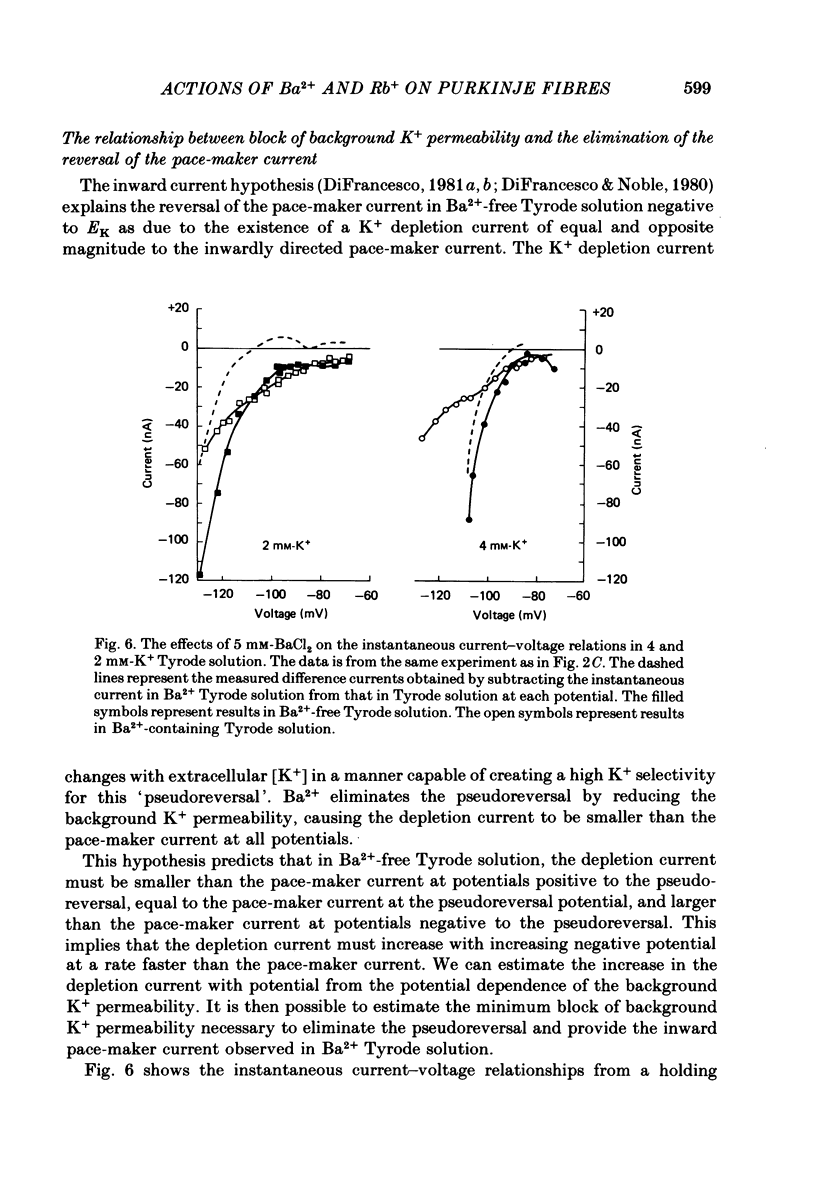
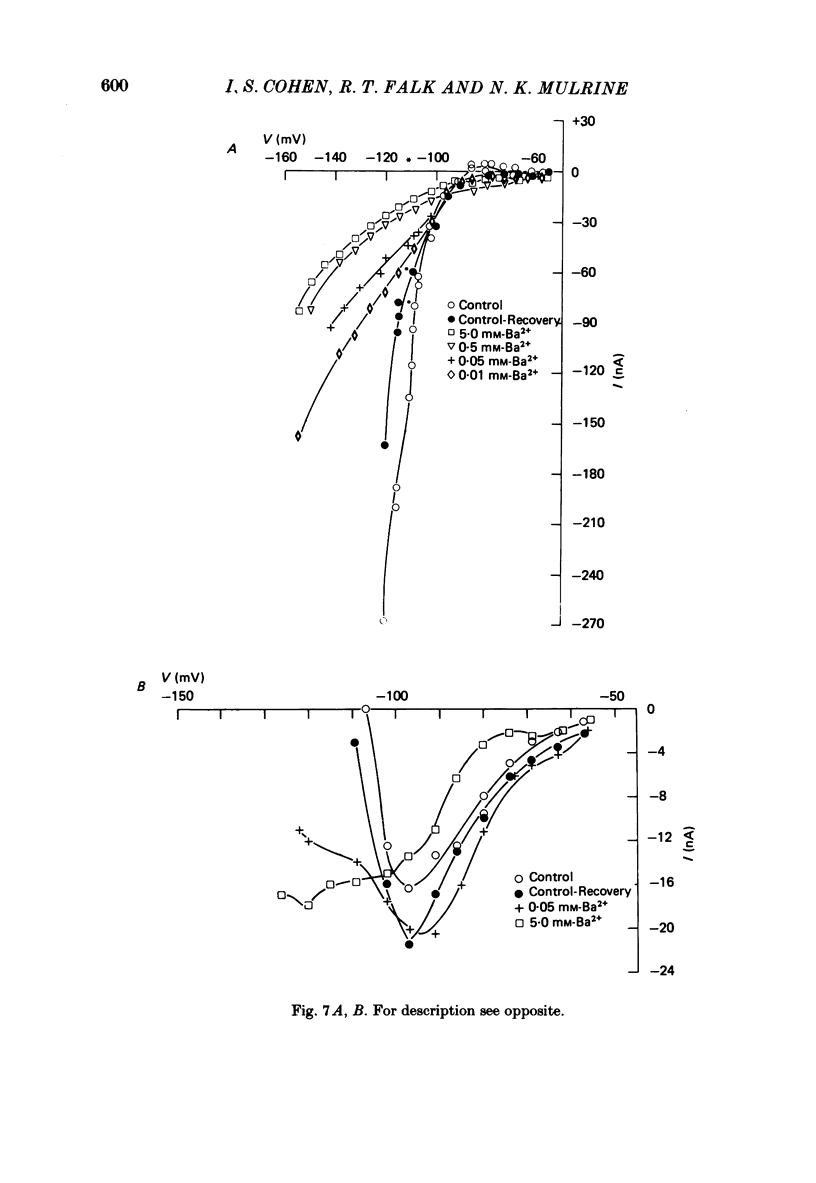
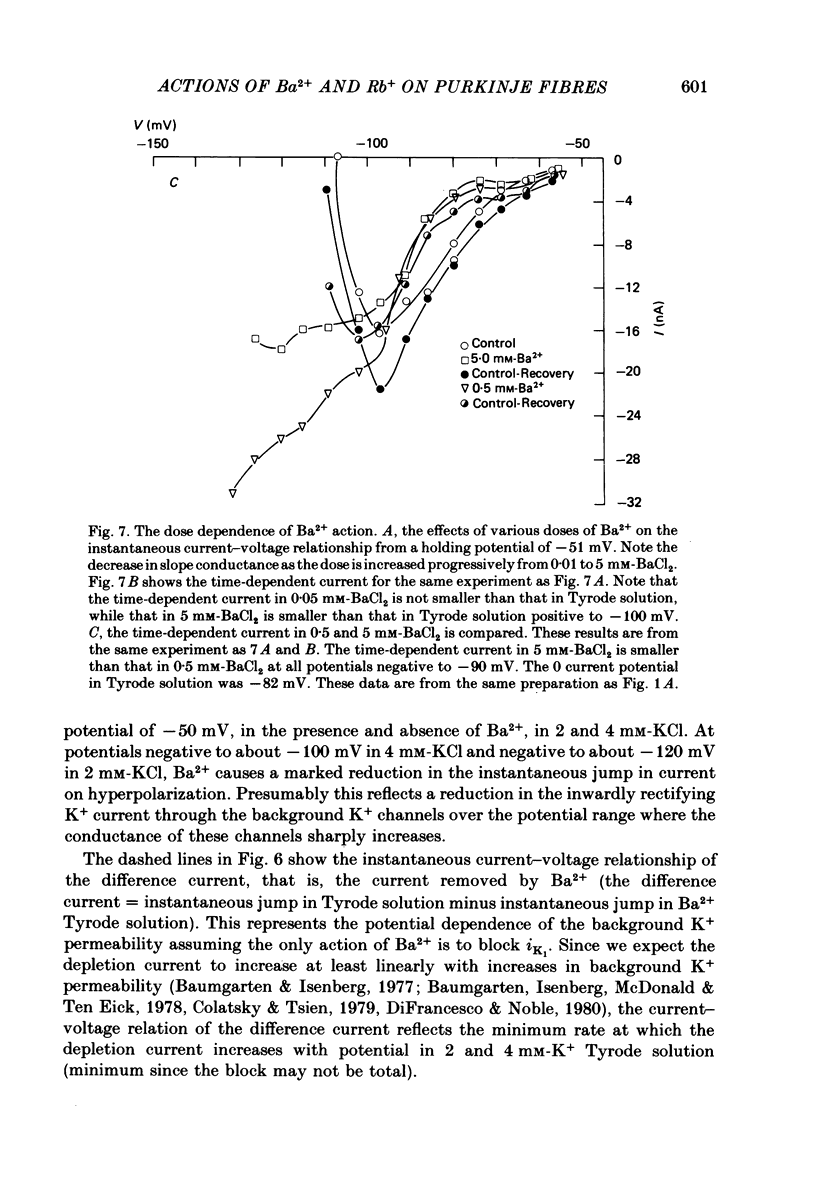
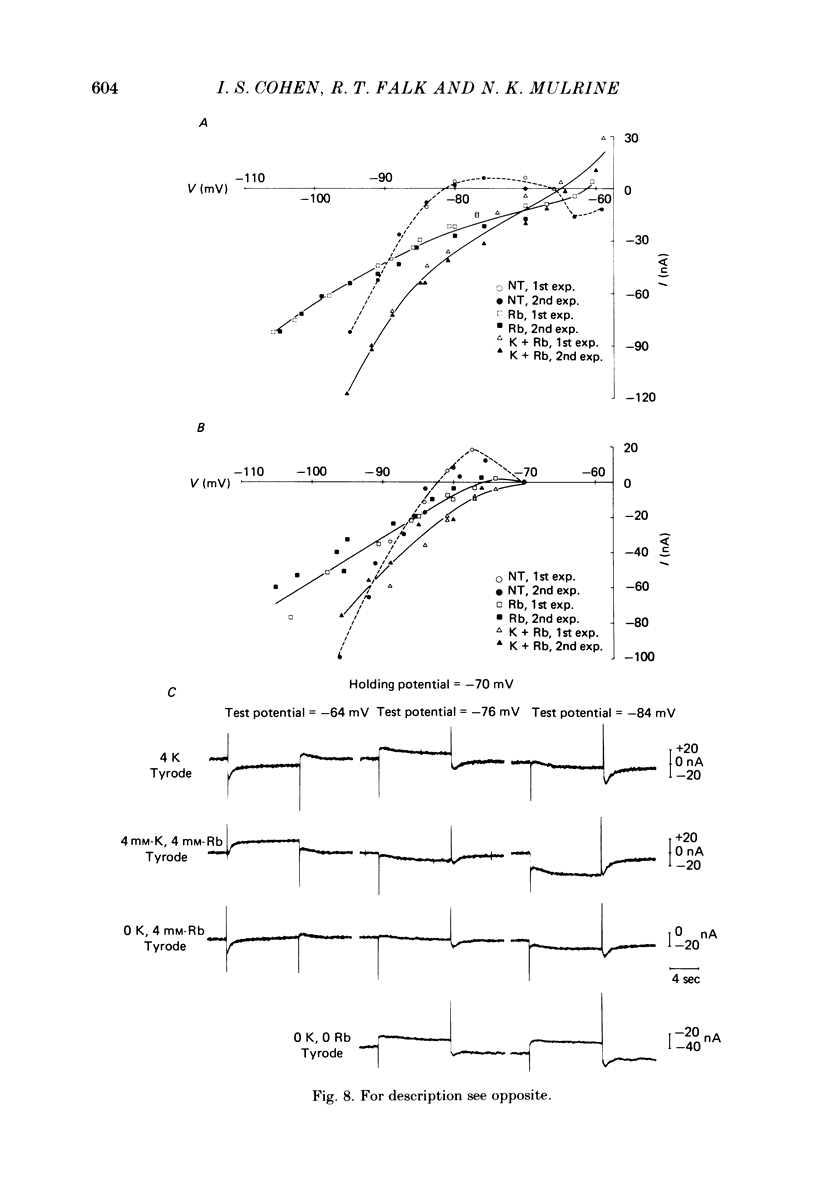
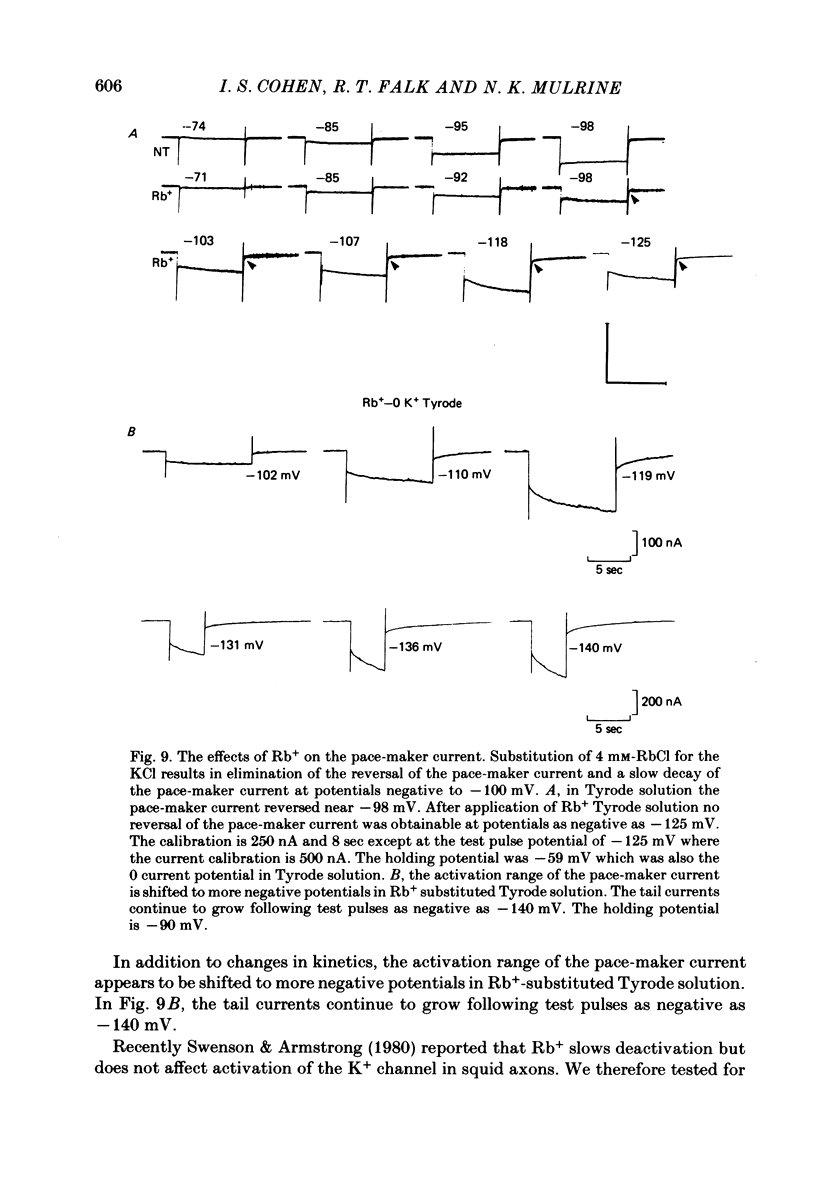
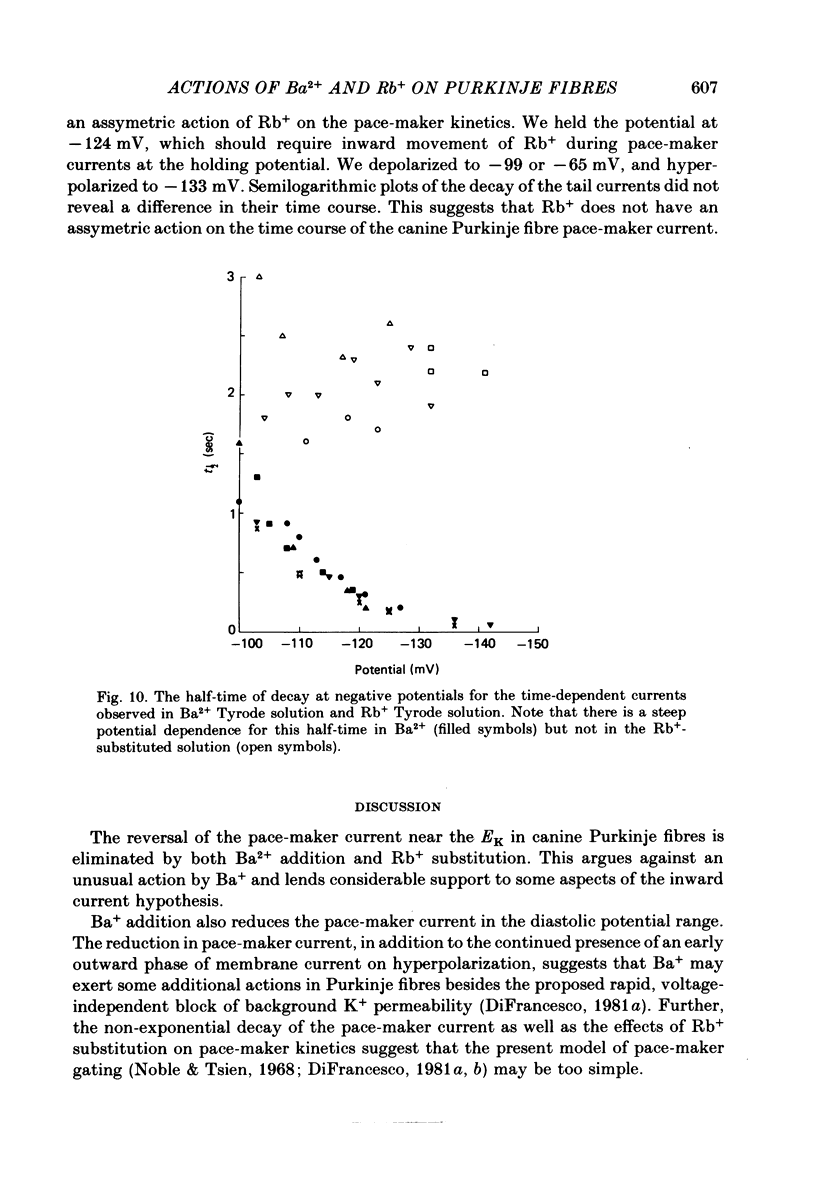
The actions of Ba2+ and Rb+, two blockers of background K+ conductance, were investigated. Recent studies performed on ungulate Purkinje fibres have suggested that the pace-maker current is an inward current activated by hyperpolarization. This hypothesis is based on the assumption that Ba2+ reduces the inwardly rectifying background K+ conductance without affecting the pace-maker current. Addition of 5 mM-BaCl2 to the bathing Tyrode solution decreases background K+ permeability and eliminates the reversal of the pace-maker current. The reversal reappears on return to Ba2+-free Tyrode solution. 5 mM-BaCl2 also reduces the time-dependent current at pace-maker potentials positive to about -95 mV in 4 mM-K+ Tyrode solution. The pace-maker current in Ba2+ Tyrode solution usually does not have an exponential time course, and often decays non-monotonically. It can take more than two minutes to reach a steady state. The fast initial component of membrane current, which is observed on hyperpolarizing in the pace-maker potential range in Purkinje fibres and which has been called the 'depletion current', is still present in Ba2+ Tyrode solution, but is reduced or eliminated if 10 mM-CsCl is added to the Ba2+ Tyrode solution. The addition of Cs+ is accompanied by an outward shift in membrane current in Ba2+ Tyrode solution. Ba2+ reduces the background K+ permeability in a dose-dependent manner. Addition of between 0.5 and 1 mM-BaCl2 achieves a maximum effect. Raising the amount of BaCl2 above this level reduces the time-dependent current even when no further effect on background permeability is observed. Rb+ substitution for K+ reduces the magnitude of the pace-maker current at potentials positive to -100 mV, eliminates the reversal of the pace-maker current, shifts the activation range to more negative potentials, and decreases the voltage dependence of pace-maker current kinetics. Rb+ addition to Tyrode solution has little effect on pace-maker current magnitude or time course positive to -90 mV, but does shift the reversal to more negative potentials. The available evidence suggests that the pace-maker current in Ba2+ Tyrode solution is an inward current activated by hyperpolarization. However, Ba2+ blocks an unknown fraction of the pace-maker current in a dose-dependent, and possibly voltage-dependent manner. Also, the presence of a slow component of pace-maker decay suggests that the standard Hodgkin-Huxley formalism for the analysis of pace-maker currents is inappropriate.
Full text
PDF












Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baumgarten C. M., Isenberg G. Depletion and accumulation of potassium in the extracellular clefts of cardiac Purkinje fibers during voltage clamp hyperpolarization and depolarization. Pflugers Arch. 1977 Mar 11;368(1-2):19–31. doi: 10.1007/BF01063450. [DOI] [PubMed] [Google Scholar]
- Baumgarten C. M., Isenberg G., McDonald T. F., Ten Eick R. E. Depletion and accumulation of potassium in the extracellular clefts of cardiac Purkinje fibers during voltage clamp hyperpolarization and depolarization: experiments in sodium-free bathing media. J Gen Physiol. 1977 Aug;70(2):149–169. doi: 10.1085/jgp.70.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmeliet E. Induction and removal of inward-going rectification in sheep cardiac Purkinje fibres. J Physiol. 1982 Jun;327:285–308. doi: 10.1113/jphysiol.1982.sp014232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen I., Daut J., Noble D. The effects of potassium and temperature on the pace-maker current, iK2, in Purkinje fibres. J Physiol. 1976 Aug;260(1):55–74. doi: 10.1113/jphysiol.1976.sp011504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen I., Falk R., Kline R. Voltage-clamp studies on the canine Purkinje fibre [proceedings]. J Physiol. 1979 Nov;296:72P–72P. [PubMed] [Google Scholar]
- Cohen I., Kline R. K+ fluctuations in the extracellular spaces of cardiac muscle. Evidence from the voltage clamp and extracellular K+ - selective microelectrodes. Circ Res. 1982 Jan;50(1):1–16. [PubMed] [Google Scholar]
- Colatsky T. J., Tsien R. W. Electrical properties associated with wide intercellular clefts in rabbit Purkinje fibres. J Physiol. 1979 May;290(2):227–252. doi: 10.1113/jphysiol.1979.sp012769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFrancesco D. A new interpretation of the pace-maker current in calf Purkinje fibres. J Physiol. 1981 May;314:359–376. doi: 10.1113/jphysiol.1981.sp013713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFrancesco D. A study of the ionic nature of the pace-maker current in calf Purkinje fibres. J Physiol. 1981 May;314:377–393. doi: 10.1113/jphysiol.1981.sp013714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFrancesco D., Ohba M., Ojeda C. Measurement and significance of the reversal potential for the pace-maker current (iK2) in sheep Purkinje fibres. J Physiol. 1979 Dec;297(0):135–162. doi: 10.1113/jphysiol.1979.sp013032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisner D. A., Lederer W. J. Characterization of the electrogenic sodium pump in cardiac Purkinje fibres. J Physiol. 1980 Jun;303:441–474. doi: 10.1113/jphysiol.1980.sp013298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadsby D. C. Activation of electrogenic Na+/K+ exchange by extracellular K+ in canine cardiac Purkinje fibers. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4035–4039. doi: 10.1073/pnas.77.7.4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gay L. A., Stanfield P. R. Cs(+) causes a voltage-dependent block of inward K currents in resting skeletal muscle fibres. Nature. 1977 May 12;267(5607):169–170. doi: 10.1038/267169a0. [DOI] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952 Aug;117(4):500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Miyazaki S., Moody W., Patlak J. Blocking effects of barium and hydrogen ions on the potassium current during anomalous rectification in the starfish egg. J Physiol. 1978 Jun;279:167–185. doi: 10.1113/jphysiol.1978.sp012338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Takahashi K. The anomalous rectification and cation selectivity of the membrane of a starfish egg cell. J Membr Biol. 1974;18(1):61–80. doi: 10.1007/BF01870103. [DOI] [PubMed] [Google Scholar]
- Hellam D. C., Studt J. W. A core-conductor model of the cardiac Purkinje fibre based on structural analysis. J Physiol. 1974 Dec;243(3):637–660. doi: 10.1113/jphysiol.1974.sp010770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isenberg G. Cardiac Purkinje fibers: cesium as a tool to block inward rectifying potassium currents. Pflugers Arch. 1976 Sep 30;365(2-3):99–106. doi: 10.1007/BF01067006. [DOI] [PubMed] [Google Scholar]
- McAllister R. E., Noble D. The time and voltage dependence of the slow outward current in cardiac Purkinje fibres. J Physiol. 1966 Oct;186(3):632–662. doi: 10.1113/jphysiol.1966.sp008060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura D. S., Hoffman B. F., Rosen M. R. The effect of extracellular potassium on the intracellular potassium ion activity and transmembrane potentials of beating canine cardiac Purkinje fibers. J Gen Physiol. 1977 Apr;69(4):463–474. doi: 10.1085/jgp.69.4.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobley B. A., Page E. The surface area of sheep cardiac Purkinje fibres. J Physiol. 1972 Feb;220(3):547–563. doi: 10.1113/jphysiol.1972.sp009722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble D., Tsien R. W. The kinetics and rectifier properties of the slow potassium current in cardiac Purkinje fibres. J Physiol. 1968 Mar;195(1):185–214. doi: 10.1113/jphysiol.1968.sp008454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperelakis N., Schneider M. F., Harris E. J. Decreased K+ conductance produced by Ba++ in frog sartorius fibers. J Gen Physiol. 1967 Jul;50(6):1565–1583. doi: 10.1085/jgp.50.6.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standen N. B., Stanfield P. R. A potential- and time-dependent blockade of inward rectification in frog skeletal muscle fibres by barium and strontium ions. J Physiol. 1978 Jul;280:169–191. doi: 10.1113/jphysiol.1978.sp012379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standen N. B., Stanfield P. R. Rubidium block and rubidium permeability of the inward rectifier of frog skeletal muscle fibres. J Physiol. 1980 Jul;304:415–435. doi: 10.1113/jphysiol.1980.sp013333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vereecke J., Isenberg G., Carmeliet E. K efflux through inward rectifying K channels in voltage clamped Purkinje fibers. Pflugers Arch. 1980 Apr;384(3):207–217. doi: 10.1007/BF00584555. [DOI] [PubMed] [Google Scholar]


