Abstract
TAK-220 is a CCR5 antagonist, part of the new class of anti-human immunodeficiency virus type 1 (anti-HIV-1) entry inhibitors. We evaluated the anti-HIV-1 interactions between TAK-220 and various antiretrovirals in vitro. Synergy was observed with all drugs at the 90 and 95% inhibitory concentrations. The favorable drug interactions observed suggest that further clinical evaluation is warranted.
New antiretroviral drug regimens are needed, especially for subjects who have failed two or three previous regimens (17). Among potential sites in the human immunodeficiency virus type 1 (HIV-1) replicative cycle, HIV-1 attachment and entry is a particularly promising target (3, 10).
TAK-220 is a small molecule that targets the binding of gp120 to the CCR5 coreceptor, is orally bioavailable, and has potent anti-HIV-1 activity in vitro at nanomolar concentrations (9). As with other classes of antiretroviral drugs, attachment-entry inhibitors are likely to be used in combination with other antiretrovirals. Combinations have many potential advantages over monotherapy, including a delay in the emergence of resistant viral variants, increased potency, and broadened coverage against variants that exist in the population (15). Previous studies have shown that the use of combinations in vitro may lead to various outcomes, ranging from synergy to antagonism, and that these outcomes may be predictive of subsequent clinical results (8, 11-14). Therefore, we studied TAK-220 in combination with various other antiviral compounds in vitro.
Peripheral blood mononuclear cells (PBMCs) from HIV-1-seronegative donors were obtained by Ficoll-Hypaque density gradient centrifugation of heparinized venous blood. After 3-day photohemagglutinin assay stimulation, PBMCs were resuspended at a concentration of 1 × 106 cells/ml in RPMI 1640 culture medium (Sigma, St Louis, MO) supplemented with 20% heat-inactivated fetal calf serum (Sigma), penicillin (50 U/ml), streptomycin (50 μg/ml), l-glutamine (2 mM), HEPES buffer (10 mM), and 10% interleukin-2 in 24-well tissue culture plates (Becton Dickinson, San Jose, CA). Single drugs or combinations of two or three drugs were added to each well by using a fixed ratio among the drugs and the serial dilutions. The drugs were dissolved by using dimethyl sulfoxide for TAK-220 and efavirenz and phosphate-buffered saline for the other drugs. They were added simultaneously with the HIV-1 inoculum (800 to 1,000 50% tissue culture infective doses/106 cells), and the plates were incubated at 37°C in a humidified 5% CO2 atmosphere. Each condition was tested in duplicate, and each experiment was repeated at least twice. Cell-free culture supernatants were harvested and analyzed by an enzyme-linked immunosorbent assay (Du Pont, Wilmington, DE) for HIV-1 p24 antigen production on day 7 of culture. In addition, uninfected drug-treated cytotoxicity controls were maintained at the highest concentration of each agent tested, singly or in combination. No toxicity was observed at concentrations up to 10 μM. Cell proliferation and viability were assessed by the trypan blue dye exclusion method.
Three clinical HIV-1 isolates (R5-08, R5-06, and R5-18) were derived from PBMCs of subjects with acute HIV-1 primary infection syndrome and were shown to be CCR5 users (R5) by replication in U87 MG-CD4 cell lines expressing CCR5 and the absence of replication in U87 MG-CD4 cell lines expressing CXCR4. They were also non-syncytium inducing in MT-2 assays. R5-08 and R5-06 have the wild-type reverse transcriptase (RT) and protease (PR) genotypes. R5-18 has mutations at the following codons: PR codons 10V, 20R, 36I, 63P, 71T, 77I, and 90M and RT codons 41L, 98G, 184V, and 215Y. Various combinations of drugs were tested against R5-08 and R5-06; only enfuvirtide and TAK-220 combinations were tested against R5-18.
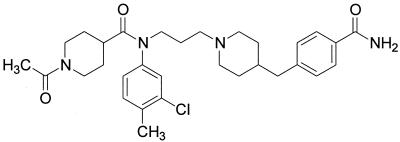
TAK-220 was provided by Takeda Chemical Industries (Osaka, Japan); the chemical structure is shown in Fig. 1. Enfuvirtide was provided by Trimeris Inc. (Durham, NC); zidovudine and lamivudine were provided by GlaxoSmithKline, Inc. (Research Triangle Park, NC); efavirenz was provided by Du Pont Pharmaceuticals Co. (Wilmington, DE); and indinavir was provided by Merck & Co, Inc. (West Point, PA).
FIG. 1.
Structure of TAK-220 (reprinted from reference 9).
The multiple-drug-effect analysis of Chou and colleagues (1, 2, 7) was used to analyze the effects of the drugs in combination. A mutually exclusive model of analysis was used. Combination indices of <0.9 indicate synergy (i.e., greater than the expected additive effect when two agents are combined), 0.9 < combination indices < 1.1 indicate nearly additive effects, and combination indices >1.1 indicate antagonism (i.e., less than the expected additive effect).
TAK-220 had potent inhibitory activity against the R5 isolates studied. The 50% inhibitory concentrations (IC50s) were 3.12 nM against HIV-1 R5-08, 13.47 nM against HIV-1 R5-06, and 2.26 nM against HIV-1 R5-18. No toxicity was observed in uninfected PBMCs at TAK-220 concentrations up to 100 nM.
TAK-220 was evaluated in two- and three-drug combinations by using representative drugs from each of the currently Food and Drug Administration-approved antiretroviral classes: nucleoside reverse transcriptase inhibitors (zidovudine and lamivudine), nonnucleoside reverse transcriptase inhibitors (efavirenz), protease inhibitors (indinavir), and fusion inhibitors (enfuvirtide). The combination indices are shown in Table 1. Combinations with reverse transcriptase and protease inhibitors showed interactions ranging from low-level antagonism at low inhibitory concentrations to synergy at higher inhibitory concentrations (IC90 and IC95). Combinations of TAK-220 with the fusion inhibitor enfuvirtide showed highly synergistic interactions against all viral isolates tested at every inhibitory concentration used.
TABLE 1.
Combination indices for TAK-220 and other anti-HIV drugs against HIV-1 clinical isolates
| Drug used in combination with TAK-220 | Virus | Mean combination indexa at various inhibitory concn
|
|||
|---|---|---|---|---|---|
| IC50 | IC75 | IC90 | IC95 | ||
| Zidovudine | R5-08 | 0.93 ± 0.13 | 0.64 ± 0.07 | 0.47 ± 0.07 | 0.40 ± 0.07 |
| R5-06 | 0.68 ± 0.06 | 0.56 ± 0.06 | 0.46 ± 0.04 | 0.42 ± 0.03 | |
| Lamivudine | R5-08 | 0.86 ± 0.14 | 0.64 ± 0.09 | 0.48 ± 0.09 | 0.40 ± 0.09 |
| R5-06 | 0.65 ± 0.17 | 0.47 ± 0.12 | 0.34 ± 0.08 | 0.28 ± 0.05 | |
| Zidovudine and lamivudine | R5-08 | 0.73 ± 0.10 | 0.53 ± 0.08 | 0.39 ± 0.09 | 0.32 ± 0.09 |
| R5-06 | 0.58 ± 0.03 | 0.45 ± 0.00 | 0.35 ± 0.01 | 0.31 ± 0.02 | |
| Indinavir | R5-08 | 1.26 ± 0.05 | 0.94 ± 0.10 | 0.78 ± 0.13 | 0.71 ± 0.13 |
| R5-06 | 0.70 ± 0.13 | 0.61 ± 0.07 | 0.60 ± 0.05 | 0.63 ± 0.05 | |
| Efavirenz | R5-08 | 0.73 ± 0.10 | 0.58 ± 0.14 | 0.49 ± 0.17 | 0.47 ± 0.20 |
| R5-06 | 0.67 ± 0.21 | 0.58 ± 0.17 | 0.55 ± 0.18 | 0.56 ± 0.19 | |
| Indinavir and efavirenz | R5-08 | 1.17 ± 0.15 | 0.94 ± 0.03 | 0.81 ± 0.02 | 0.77 ± 0.04 |
| R5-06 | 0.83 ± 0.17 | 0.73 ± 0.10 | 0.70 ± 0.06 | 0.70 ± 0.03 | |
| Enfuvirtide | R5-08 | 0.82 ± 0.12 | 0.60 ± 0.14 | 0.47 ± 0.21 | 0.42 ± 0.24 |
| R5-06 | 0.68 ± 0.00 | 0.48 ± 0.00 | 0.39 ± 0.00 | 0.37 ± 0.00 | |
| R5-18 | 0.66 ± 0.14 | 0.53 ± 0.06 | 0.48 ± 0.08 | 0.48 ± 0.12 | |
Combination index <0.9 = synergy; 0.9 < combination index < 1.1 = near additivity; combination index >1.1 = antagonism. The data are the means of two to three experiments ± standard deviations. The ranges were as follows: concentration TAK-220, 0.0375 to 40.5 nM; zidovudine, 0.75 to 48 nM; lamivudine, 0.01 μM to 0.8 μM; efavirenz, 0.25 to 13.5 nM; indinavir, 3.0 to 160 nM; enfuvirtide, 2.25 to 90 nM.
CCR5 antagonists are part of a new class of antiretrovirals that act at an early step of the HIV replicative cycle, i.e., attachment and entry. As with other antiretroviral agents, resistance will likely prove to be a problem for CCR5 inhibitors (16). Thus, the best strategy to prevent resistance from occurring is to use them in combination with other potent antiretroviral drugs.
In vitro studies of drug interactions have proven to be beneficial in predicting which drug combination regimens should be evaluated in a clinical setting (5, 6, 8). In the present study, we have evaluated in vitro the interactions between TAK-220 and representatives of each class of currently available antiretroviral agents. We have found that, in the nanomolar range, TAK-220 had excellent antiviral activity against three HIV-1 R5 clinical isolates, including one that had high-level resistance to reverse transcriptase inhibitors and protease inhibitors. This in vitro antiviral activity is comparable to those of the other CCR5 inhibitors currently being evaluated. TAK-220 had favorable drug interactions with all of the agents tested, particularly at drug concentrations that are likely to be used in the clinic, i.e., the IC90 and the IC95. The interactions of TAK-220 with the fusion inhibitor enfuvirtide were highly synergistic at these concentrations, as we have observed with combinations of some other coreceptor inhibitors and enfuvirtide in previous studies (12, 13). It has been hypothesized that the blocking of viral interactions with coreceptors alters the kinetics of the fusion process, thus allowing more time for a fusion inhibitor to intervene (4). Combinations of two drugs that target the entry process could provide valuable alternatives to current regimens, both in subjects with early infections and in highly antiretroviral agent-experienced subjects. These results suggest that TAK-220 combination therapy should be further evaluated in clinical trials.
REFERENCES
- 1.Chou, T. C., and P. Talalay. 1984. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv. Enzyme Regul. 22:27-55. [DOI] [PubMed] [Google Scholar]
- 2.Chou, T. C. 1991. The median-effect principle and the combination index for quantitation of synergism and antagonism, p. 61-102. In T. C. Chou and D. C. Rideout, (ed.) Synergism and antagonism in chemotherapy. Academic Press, Inc., San Diego, Calif.
- 3.Doms, R. W. 2004. Viral entry denied. N. Engl. J. Med. 351:743-744. [DOI] [PubMed] [Google Scholar]
- 4.Doms, R. W., and S. C. Peiper. 1997. Unwelcomed guests with master keys: how HIV uses chemokine receptors for cellular entry. Virology 235:179-190. [DOI] [PubMed] [Google Scholar]
- 5.Havlir, D. V., C. Tierney, G. H. Friedland, R. B. Pollard, L. Smeaton, J.-P. Sommadossi, L. Fox, H. Kessler, K. H. Fife, and D. D. Richman. 2000. In vivo antagonism with zidovudine plus stavudine combination therapy. J. Infect. Dis. 182:321-325. [DOI] [PubMed] [Google Scholar]
- 6.Hoggard, P. G., G. J. Veal, M. J. Wild, M. G. Barry, and D. J. Back. 1995. Drug interactions with zidovudine phosphorylation in vitro. Antimicrob. Agents Chemother. 39:1376-1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnson, V. A., M. A. Barlow, D. P. Merrill, T.-C. Chou, and M. S. Hirsch. 1990. Three-drug synergistic inhibition of HIV-1 replication in vitro by zidovudine, recombinant soluble CD4 and recombinant interferon-alpha. J. Infect. Dis. 161:1059-1067. [DOI] [PubMed] [Google Scholar]
- 8.Merrill, D. P., M. Moonis, T.-C. Chou, and M. S. Hirsch. 1996. Lamivudine or stavudine in two- and three-drug combinations against human immunodeficiency virus type 1 replication in vitro. J. Infect. Dis. 173:355-364. [DOI] [PubMed] [Google Scholar]
- 9.Takashima, K., H. Miyake, N. Kanzaki, Y. Tagawa, X. Wang, Y. Sugihara, Y. Iizawa, and M. Baba. 2005. Highly potent inhibition of human immunodeficiency virus type 1 replication by TAK-220, an orally bioavailable small-molecule CCR5 antagonist. Antimicrob. Agents Chemother. 49:3481-3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tremblay, C. 2004. Effects of HIV-1 entry inhibitors in combination. Curr. Pharm. Des. 10:1861-1865. [DOI] [PubMed] [Google Scholar]
- 11.Tremblay, C., D. P. Merrill, T. C. Chou, and M. S. Hirsch. 1999. Interactions among combinations of two and three protease inhibitors against drug-susceptible and drug-resistant HIV-1 isolates. J. Acquir. Immune Defic. Syndr. 22:430-436. [DOI] [PubMed] [Google Scholar]
- 12.Tremblay, C., C. Kollmann, F. Giguel, T. C. Chou, and M. S. Hirsch. 2000. Strong in vitro synergy between the fusion inhibitor T-20 and the CXCR4 blocker AMD-3100. J. Acquir. Immune Defic. Syndr. 25:99-102. [DOI] [PubMed] [Google Scholar]
- 13.Tremblay, C. L., F. Giguel, C. Kollmann, Y. Guan, T. C. Chou, B. M. Baroudy, and M. S. Hirsch. 2002. Anti-human immunodeficiency virus interactions of SCH-C (SCH 351125), a CCR5 antagonist, with other antiretroviral agents in vitro. Antimicrob. Agents Chemother. 46:1336-1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tremblay, C. L., D. L. Poulin, J. L. Hicks, S. Selliah, A. Chamberland, F. Giguel C. S. Kollmann, T. C. Chou, H. Dong, and M. S. Hirsch. 2003. Favorable interactions between enfuvirtide and 1-β-d-2,6-diaminopurine dioxolane in vitro. Antimicrob. Agents Chemother. 47:3644-3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tremblay, C. L., J. C. Kaplan, and M. S. Hirsch. 2001. Combination antiretroviral therapy, p. 313-337. In E. DeClercq (ed.). Antiretroviral therapy. American Society for Microbiology, Washington, D.C.
- 16.Trkola, A., S. E. Kuhmann, J. M. Strizki, E. Maxwell, T. Ketas, T. Morgan, P. Pugach, S. Xu, L. Wojcik, J. Tagat, A. Palani, S. Shapiro, J. W. Clader, S. McCombie, G. R. Reyes, B. M. Baroudy, and J. P. Moore. 2002. HIV-1 escape from a small molecule, CCR5-specific entry inhibitor does not involve CXCR4 use. Proc. Natl. Acad. Sci. USA 99:395-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yeni, P. G, S. M. Hammer, M. S. Hirsch, M. S. Saag, M. Schechter, C. C. Carpenter, M. A. Fischl, J. M. Gatell, B. G. Gazzard, D. M. Jacobsen, D. A. Katzenstein, J. S. Montaner, D. D. Richman, R. T. Schooley, M. A. Thompson, S. Vella, and P. A. Volberding. 2004. Treatment for adult HIV infection: 2004 recommendations of the International AIDS Society-USA Panel. JAMA 292:251-265. [DOI] [PubMed] [Google Scholar]