Abstract
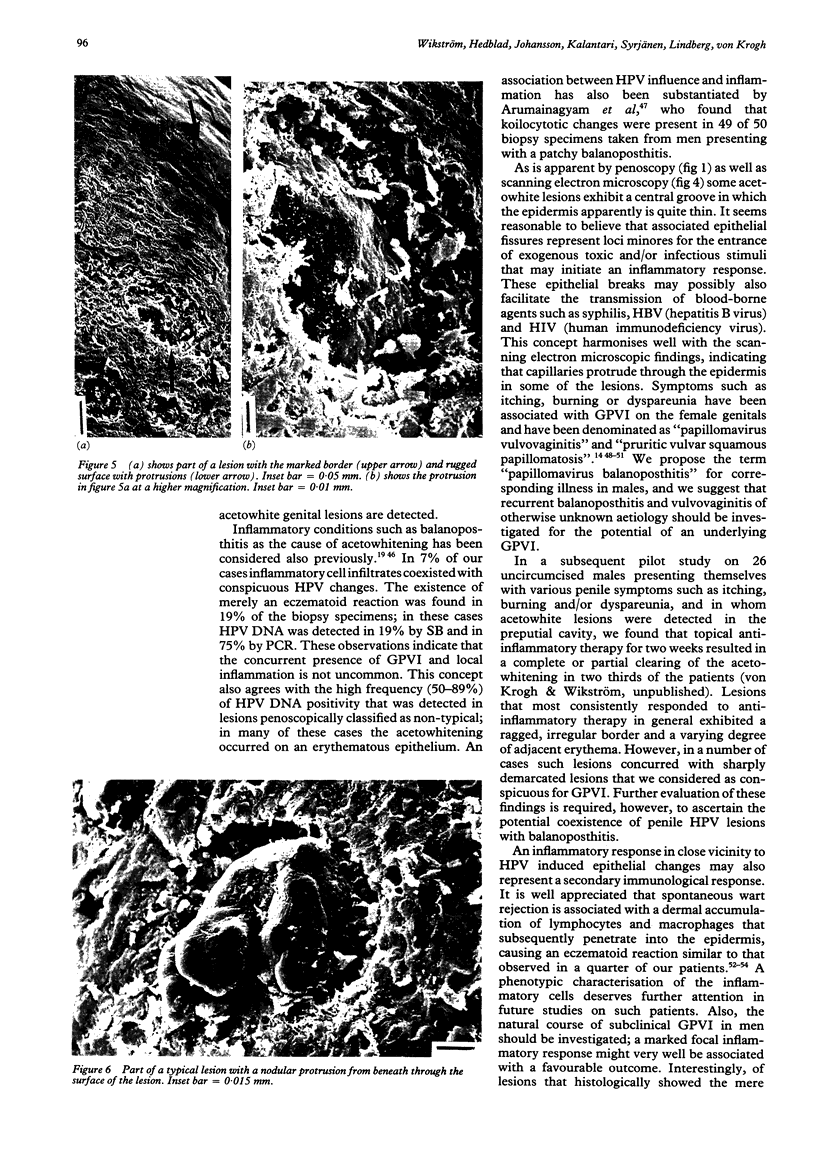
OBJECTIVES--To evaluate colposcopic criteria in acetowhite lesions of the penis ("penoscopy") for the diagnosis of subclinical genitoanal papillomavirus infection (GPVI) compared with histopathological criteria of HPV involvement and to various hybridisation assays for HPV DNA detection, and to depict typical lesions by scanning electron microscopy. DESIGN--The study included 101 randomly selected male partners of females with known GPVI, or with penile symptoms such as itching, burning and dyspareunia who did not exhibit overt genital warts but appeared to be afflicted with acetowhite penile lesions after topical application of 5% acqueous acetic acid. Lesions were judged by penoscopy as either typical, conspicuous or nontypical for underlying HPV infection. Biopsy specimens from 91 men were examined by light microscopy and by either Southern blot (SB), polymerase chain reaction (PCR) and/or in situ hybridisation (ISH) assays for the presence of HPV DNA of the HPV types 6, 11, 16, 18, 31, 33 and 42 (Group A). From another ten men lesions clinically typical for GPVI were also examined topographically by scanning electronic microscopy (Group B). SETTING--The STD out-patient clinic of the Department of Dermatovenereology of Karolinska Hospital, Stockholm, Sweden. RESULTS--Group A Seventy eight (86%) of the biopsied lesions met the penoscopy criteria of being either typical of or conspicuous for GVPI. The agreement between penoscopy and histopathology was fairly good, as HPV diagnosis was made by both methods in 56 (62%) of the cases. The reliability of applying strict colposcopic hallmarks was further substantiated by the finding that 55 (60%) of the biopsy specimens taken from penoscopically typical/conspicuous lesions contained HPV DNA. However, there are diagnostic pitfalls for the acetic acid test. Coexistence of an eczematoid reaction with changes indicative of HPV influence was detected in six (7%) of the cases, while an inflammatory response only occurred in 17 (19%) of the specimens. Additional histopathological diagnoses (normal epithelium, lichen sclerosus et atrophicus, balanitis circinata parakeratotica, verruca plana) were established in another eight (9%) of the cases. Among the HPV DNA positive cases, all of the HPV types tested for were detected with the exception of HPV 18. A severe penile intraepithelial neoplasia (PIN III) was revealed in five (5%) of biopsies; HPV 16 was present in two and HPV 42 in one of these biopsy specimens. GROUP B--Scanning electron microscopy depiction harmonised with the penoscopy findings showing that subclinical GPVI characteristically exhibits a well demarcated, slightly elevated border and that the central area of lesions often displays a "groove" in which the epithelium appears to be thin with protrusions from beneath that probably represent capillaries. CONCLUSION--Use of the acetic acid test for evaluation of GPVI should be combined with a colposcopic evaluation based on strict topographic hallmarks, followed by a directed biopsy for light microscopic evaluation. We found that the positive predictive value of colposcopy was as high when correlated with histopathological findings (72%) as when virological methods were used, whether HPV DNA hybridisation testing was performed with the well established SB and ISH assays (45%), or by applying the newly introduced and highly sensitive PCR assay as well (71%). False positivity from the acetic acid test occurs and is mainly due to inflammatory conditions but also to the presence of other conditions. Epithelial fissures are evidently associated with some subclinical GPVI lesions and may potentially represent loci minores for infectious stimuli and perhaps facilitate the transmission of some blood-borne STDs. We prose that the term "papillomavirus balanoposthitis" should be used for penile HPV infection associated with inflammatory responses. Our study indicates that PIN III frequently occurs in a subclinical form and may be associated with not only previously identified "high-risk" HPV types such as type 16, but also with the HPV type 42 that has not previously been considered as oncogenic.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arumainayagam J. T., Sumathipala A. H., Smallman L. A., Shahmanesh M. Flat condylomata of the penis presenting as patchy balanoposthitis. Genitourin Med. 1990 Aug;66(4):251–253. doi: 10.1136/sti.66.4.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrasso R., De Brux J., Croissant O., Orth G. High prevalence of papillomavirus-associated penile intraepithelial neoplasia in sexual partners of women with cervical intraepithelial neoplasia. N Engl J Med. 1987 Oct 8;317(15):916–923. doi: 10.1056/NEJM198710083171502. [DOI] [PubMed] [Google Scholar]
- Beaudenon S., Kremsdorf D., Obalek S., Jablonska S., Pehau-Arnaudet G., Croissant O., Orth G. Plurality of genital human papillomaviruses: characterization of two new types with distinct biological properties. Virology. 1987 Dec;161(2):374–384. doi: 10.1016/0042-6822(87)90130-9. [DOI] [PubMed] [Google Scholar]
- Bergeron C., Ferenczy A., Shah K. V., Naghashfar Z. Multicentric human papillomavirus infections of the female genital tract: correlation of viral types with abnormal mitotic figures, colposcopic presentation, and location. Obstet Gynecol. 1987 May;69(5):736–742. [PubMed] [Google Scholar]
- Bishop P. E., McMillan A., Fletcher S. An immunohistological study of spontaneous regression of condylomata acuminata. Genitourin Med. 1990 Apr;66(2):79–81. doi: 10.1136/sti.66.2.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodén E., Eriksson A., Rylander E., von Schoultz B. Clinical characteristics of papillomavirus-vulvovaginitis. A new entity with oncogenic potential. Acta Obstet Gynecol Scand. 1988;67(2):147–151. doi: 10.3109/00016348809004188. [DOI] [PubMed] [Google Scholar]
- Bodén E., Rylander E., Evander M., Wadell G., von Schoultz B. Papilloma virus infection of the vulva. Acta Obstet Gynecol Scand. 1989;68(2):179–184. doi: 10.3109/00016348909009908. [DOI] [PubMed] [Google Scholar]
- Butler S., Molinari J. A., Plezia R. A., Chandrasekar P., Venkat H. Condyloma acuminatum in the oral cavity: four cases and a review. Rev Infect Dis. 1988 May-Jun;10(3):544–550. doi: 10.1093/clinids/10.3.544. [DOI] [PubMed] [Google Scholar]
- Byrne M. A., Taylor-Robinson D., Anderson M. A., Mason P., Harris J. R. Value of colposcopy in sexually transmitted diseases clinic based on first year's experience. Genitourin Med. 1989 Jan;65(1):42–45. doi: 10.1136/sti.65.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne M. A., Walker M. M., Leonard J., Pryce D., Taylor-Robinson D. Recognising covert disease in women with chronic vulval symptoms attending an STD clinic: value of detailed examination including colposcopy. Genitourin Med. 1989 Jan;65(1):46–49. doi: 10.1136/sti.65.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang F., Syrjänen S., Shen Q., Ji H. X., Syrjänen K. Human papillomavirus (HPV) DNA in esophageal precancer lesions and squamous cell carcinomas from China. Int J Cancer. 1990 Jan 15;45(1):21–25. doi: 10.1002/ijc.2910450106. [DOI] [PubMed] [Google Scholar]
- Crum C. P., Mitao M., Levine R. U., Silverstein S. Cervical papillomaviruses segregate within morphologically distinct precancerous lesions. J Virol. 1985 Jun;54(3):675–681. doi: 10.1128/jvi.54.3.675-681.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crum C. P., Nuovo G., Friedman D., Silverstein S. J. Accumulation of RNA homologous to human papillomavirus type 16 open reading frames in genital precancers. J Virol. 1988 Jan;62(1):84–90. doi: 10.1128/jvi.62.1.84-90.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deitch K. V., Smith J. E. Symptoms of chronic vaginal infection and microscopic condyloma in women. J Obstet Gynecol Neonatal Nurs. 1990 Mar-Apr;19(2):133–138. doi: 10.1111/j.1552-6909.1990.tb01631.x. [DOI] [PubMed] [Google Scholar]
- Dürst M., Gissmann L., Ikenberg H., zur Hausen H. A papillomavirus DNA from a cervical carcinoma and its prevalence in cancer biopsy samples from different geographic regions. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3812–3815. doi: 10.1073/pnas.80.12.3812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gissmann L., Wolnik L., Ikenberg H., Koldovsky U., Schnürch H. G., zur Hausen H. Human papillomavirus types 6 and 11 DNA sequences in genital and laryngeal papillomas and in some cervical cancers. Proc Natl Acad Sci U S A. 1983 Jan;80(2):560–563. doi: 10.1073/pnas.80.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Growdon W. A., Fu Y. S., Lebherz T. B., Rapkin A., Mason G. D., Parks G. Pruritic vulvar squamous papillomatosis: evidence for human papillomavirus etiology. Obstet Gynecol. 1985 Oct;66(4):564–568. [PubMed] [Google Scholar]
- Gupta J., Pilotti S., Rilke F., Shah K. Association of human papillomavirus type 16 with neoplastic lesions of the vulva and other genital sites by in situ hybridization. Am J Pathol. 1987 May;127(2):206–215. [PMC free article] [PubMed] [Google Scholar]
- Jenison S. A., Yu X. P., Valentine J. M., Koutsky L. A., Christiansen A. E., Beckmann A. M., Galloway D. A. Evidence of prevalent genital-type human papillomavirus infections in adults and children. J Infect Dis. 1990 Jul;162(1):60–69. doi: 10.1093/infdis/162.1.60. [DOI] [PubMed] [Google Scholar]
- Kataja V., Syrjänen K., Mäntyjärvi R., Väyrynen M., Syrjänen S., Saarikoski S., Parkkinen S., Yliskoski M., Salonen J. T., Castren O. Prospective follow-up of cervical HPV infections: life table analysis of histopathological, cytological and colposcopic data. Eur J Epidemiol. 1989 Mar;5(1):1–7. doi: 10.1007/BF00145037. [DOI] [PubMed] [Google Scholar]
- Koss L. G. Cytologic and histologic manifestations of human papillomavirus infection of the female genital tract and their clinical significance. Cancer. 1987 Oct 15;60(8 Suppl):1942–1950. doi: 10.1002/1097-0142(19901015)60:8+<1942::aid-cncr2820601504>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Koss L. G. Cytologic and histologic manifestations of human papillomavirus infection of the uterine cervix. Cancer Detect Prev. 1990;14(4):461–464. [PubMed] [Google Scholar]
- Krebs H. B., Helmkamp B. F. Does the treatment of genital condylomata in men decrease the treatment failure rate of cervical dysplasia in the female sexual partner? Obstet Gynecol. 1990 Oct;76(4):660–663. [PubMed] [Google Scholar]
- Krebs H. B., Schneider V. Human papillomavirus-associated lesions of the penis: colposcopy, cytology, and histology. Obstet Gynecol. 1987 Sep;70(3 Pt 1):299–304. [PubMed] [Google Scholar]
- Köchel H. G., Teichmann A., Eckardt N., Arendt P., Kuhn W., Thomssen R. Occurrence of human papillomavirus DNA types 16 and 18 (HPV-16/18) in cervical smears as compared to cytological findings. Int J Gynaecol Obstet. 1990 Feb;31(2):145–152. doi: 10.1016/0020-7292(90)90712-t. [DOI] [PubMed] [Google Scholar]
- McMillan A., Bishop P. E., Fletcher S. An immunohistological study of condylomata acuminata. Histopathology. 1990 Jul;17(1):45–52. doi: 10.1111/j.1365-2559.1990.tb00662.x. [DOI] [PubMed] [Google Scholar]
- Mittal K. R., Chan W., Demopoulos R. I. Sensitivity and specificity of various morphological features of cervical condylomas. An in situ hybridization study. Arch Pathol Lab Med. 1990 Oct;114(10):1038–1041. [PubMed] [Google Scholar]
- Nuovo G. J., Darfler M. M., Impraim C. C., Bromley S. E. Occurrence of multiple types of human papillomavirus in genital tract lesions. Analysis by in situ hybridization and the polymerase chain reaction. Am J Pathol. 1991 Jan;138(1):53–58. [PMC free article] [PubMed] [Google Scholar]
- Nuovo G. J., Friedman D., Richart R. M. In situ hybridization analysis of human papillomavirus DNA segregation patterns in lesions of the female genital tract. Gynecol Oncol. 1990 Feb;36(2):256–262. doi: 10.1016/0090-8258(90)90184-m. [DOI] [PubMed] [Google Scholar]
- Nuovo G. J., Hochman H. A., Eliezri Y. D., Lastarria D., Comite S. L., Silvers D. N. Detection of human papillomavirus DNA in penile lesions histologically negative for condylomata. Analysis by in situ hybridization and the polymerase chain reaction. Am J Surg Pathol. 1990 Sep;14(9):829–836. [PubMed] [Google Scholar]
- Nuovo G. J. Human papillomavirus DNA in genital tract lesions histologically negative for condylomata. Analysis by in situ, Southern blot hybridization and the polymerase chain reaction. Am J Surg Pathol. 1990 Jul;14(7):643–651. doi: 10.1097/00000478-199007000-00005. [DOI] [PubMed] [Google Scholar]
- Nuovo G. J., Nuovo J. Should family physicians test for human papillomavirus infection? An opposing view. J Fam Pract. 1991 Feb;32(2):188–192. [PubMed] [Google Scholar]
- Nuovo G. J., Richart R. M. A comparison of slot blot, southern blot, and in situ hybridization analyses for human papillomavirus DNA in genital tract lesions. Obstet Gynecol. 1989 Oct;74(4):673–678. [PubMed] [Google Scholar]
- Rathjen F. G., Schachner M. Immunocytological and biochemical characterization of a new neuronal cell surface component (L1 antigen) which is involved in cell adhesion. EMBO J. 1984 Jan;3(1):1–10. doi: 10.1002/j.1460-2075.1984.tb01753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid R., Greenberg M., Jenson A. B., Husain M., Willett J., Daoud Y., Temple G., Stanhope C. R., Sherman A. I., Phibbs G. D. Sexually transmitted papillomaviral infections. I. The anatomic distribution and pathologic grade of neoplastic lesions associated with different viral types. Am J Obstet Gynecol. 1987 Jan;156(1):212–222. doi: 10.1016/0002-9378(87)90241-9. [DOI] [PubMed] [Google Scholar]
- Reid R., Lorincz A. T. Should family physicians test for human papillomavirus infection? An affirmative view. J Fam Pract. 1991 Feb;32(2):183–188. [PubMed] [Google Scholar]
- Richart R. M., Nuovo G. J. Human papillomavirus DNA in situ hybridization may be used for the quality control of genital tract biopsies. Obstet Gynecol. 1990 Feb;75(2):223–226. [PubMed] [Google Scholar]
- Rosemberg S. K. Subclinical papilloma viral infection of male genitalia. Urology. 1985 Dec;26(6):554–557. doi: 10.1016/0090-4295(85)90359-0. [DOI] [PubMed] [Google Scholar]
- Rosenfeld W. D., Vermund S. H., Wentz S. J., Burk R. D. High prevalence rate of human papillomavirus infection and association with abnormal papanicolaou smears in sexually active adolescents. Am J Dis Child. 1989 Dec;143(12):1443–1447. doi: 10.1001/archpedi.1989.02150240065018. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sand P. K., Bowen L. W., Blischke S. O., Ostergard D. R. Evaluation of male consorts of women with genital human papilloma virus infection. Obstet Gynecol. 1986 Nov;68(5):679–681. [PubMed] [Google Scholar]
- Schneider A., Kirchmayr R., De Villiers E. M., Gissmann L. Subclinical human papillomavirus infections in male sexual partners of female carriers. J Urol. 1988 Dec;140(6):1431–1434. doi: 10.1016/s0022-5347(17)42065-9. [DOI] [PubMed] [Google Scholar]
- Schultz R. E., Miller J. W., MacDonald G. R., Auman J. R., Peterson N. R., Ward B. E., Crum C. P. Clinical and molecular evaluation of acetowhite genital lesions in men. J Urol. 1990 May;143(5):920–923. doi: 10.1016/s0022-5347(17)40138-8. [DOI] [PubMed] [Google Scholar]
- Schultz R. E., Skelton H. G. Value of acetic acid screening for flat genital condylomata in men. J Urol. 1988 Apr;139(4):777–779. doi: 10.1016/s0022-5347(17)42633-4. [DOI] [PubMed] [Google Scholar]
- Sehgal V. N., Koranne R. V., Srivastava S. B., Gupta M. M., Luthra U. K. Clinicopathology and immunohistochemistry of genital warts. Int J Dermatol. 1988 Dec;27(10):690–694. doi: 10.1111/j.1365-4362.1988.tb01265.x. [DOI] [PubMed] [Google Scholar]
- Soares V. R., Nieminen P., Aho M., Vesterinen E., Vaheri A., Paavonen J. Human papillomavirus DNA in unselected pregnant and non-pregnant women. Int J STD AIDS. 1990 Jul;1(4):276–278. doi: 10.1177/095646249000100409. [DOI] [PubMed] [Google Scholar]
- Spitzer M., Chernys A. E., Hirschfield L., Spiegel G., Sedlis A., Zuna R. E., Steinberg B., Brandsma J. L., Krumholz B. A. Assessment of criteria used in the histologic diagnosis of human papillomavirus-related disease of the female lower genital tract. Gynecol Oncol. 1990 Jul;38(1):105–109. doi: 10.1016/0090-8258(90)90019-h. [DOI] [PubMed] [Google Scholar]
- Syrjänen K. J. Epidemiology of human papillomavirus (HPV) infections and their associations with genital squamous cell cancer. Review article. APMIS. 1989 Nov;97(11):957–970. doi: 10.1111/j.1699-0463.1989.tb00504.x. [DOI] [PubMed] [Google Scholar]
- Syrjänen K. J. Human papillomavirus (HPV) infections of the female genital tract and their associations with intraepithelial neoplasia and squamous cell carcinoma. Pathol Annu. 1986;21(Pt 1):53–89. [PubMed] [Google Scholar]
- Syrjänen K., Syrjänen S. Epidemiology of human papilloma virus infections and genital neoplasia. Scand J Infect Dis Suppl. 1990;69:7–17. [PubMed] [Google Scholar]
- Syrjänen S. M., von Krogh G., Syrjänen K. J. Detection of human papillomavirus DNA in anogenital condylomata in men using in situ DNA hybridisation applied to paraffin sections. Genitourin Med. 1987 Feb;63(1):32–39. doi: 10.1136/sti.63.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syrjänen S., Partanen P., Mäntyjärvi R., Syrjänen K. Sensitivity of in situ hybridization techniques using biotin- and 35S-labeled human papillomavirus (HPV) DNA probes. J Virol Methods. 1988 Mar-Apr;19(3-4):225–238. doi: 10.1016/0166-0934(88)90017-1. [DOI] [PubMed] [Google Scholar]
- Turner M. L., Marinoff S. C. Association of human papillomavirus with vulvodynia and the vulvar vestibulitis syndrome. J Reprod Med. 1988 Jun;33(6):533–537. [PubMed] [Google Scholar]
- Van Cutsem J., Janssen P. A. Experimental systemic dermatophytosis. J Invest Dermatol. 1984 Jul;83(1):26–31. doi: 10.1111/1523-1747.ep12261652. [DOI] [PubMed] [Google Scholar]
- Wikström A., Lidbrink P., Johansson B., von Krogh G. Penile human papillomavirus carriage among men attending Swedish STD clinics. Int J STD AIDS. 1991 Mar-Apr;2(2):105–109. doi: 10.1177/095646249100200205. [DOI] [PubMed] [Google Scholar]
- von Krogh G. Genitoanal papillomavirus infection: diagnostic and therapeutic objectives in the light of current epidemiological observations. Int J STD AIDS. 1991 Nov-Dec;2(6):391–404. doi: 10.1177/095646249100200601. [DOI] [PubMed] [Google Scholar]
- von Krogh G., Syrjänen S. M., Syrjänen K. J. Advantage of human papillomavirus typing in the clinical evaluation of genitoanal warts. Experience with the in situ deoxyribonucleic acid hybridization technique applied on paraffin sections. J Am Acad Dermatol. 1988 Mar;18(3):495–503. doi: 10.1016/s0190-9622(88)70072-9. [DOI] [PubMed] [Google Scholar]