Abstract
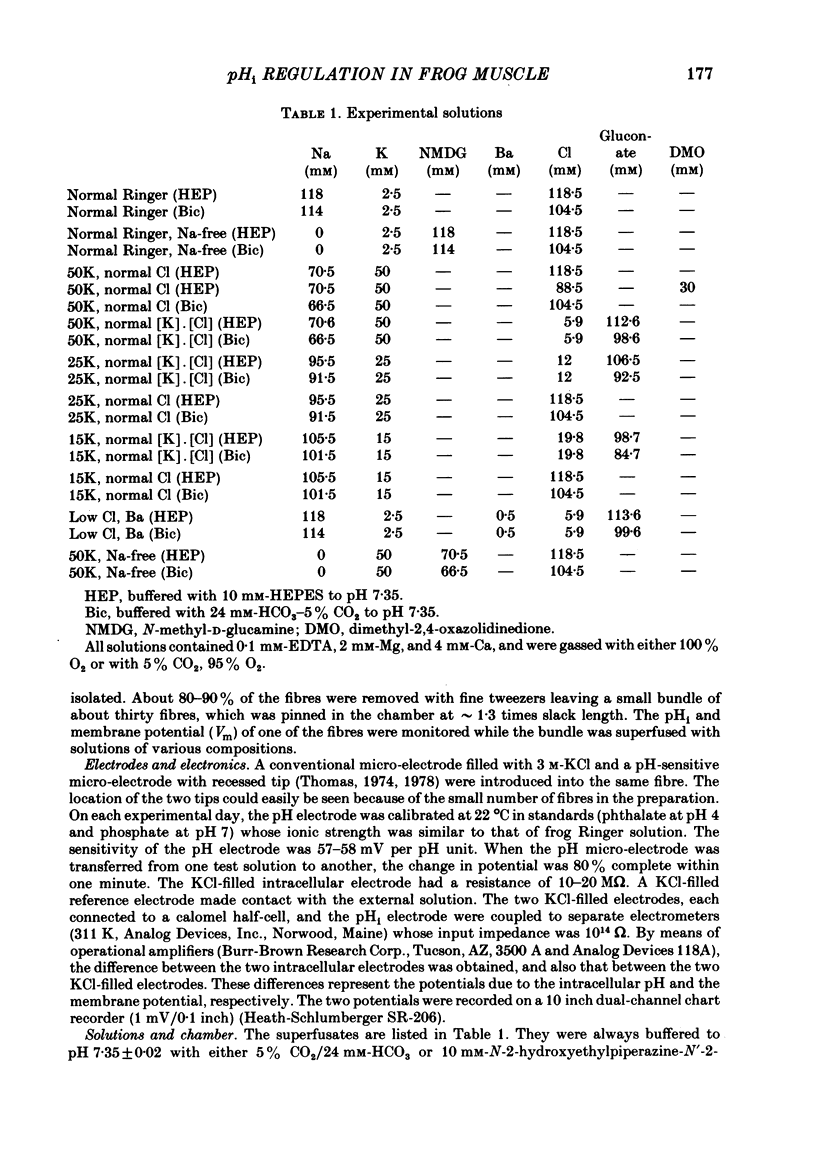
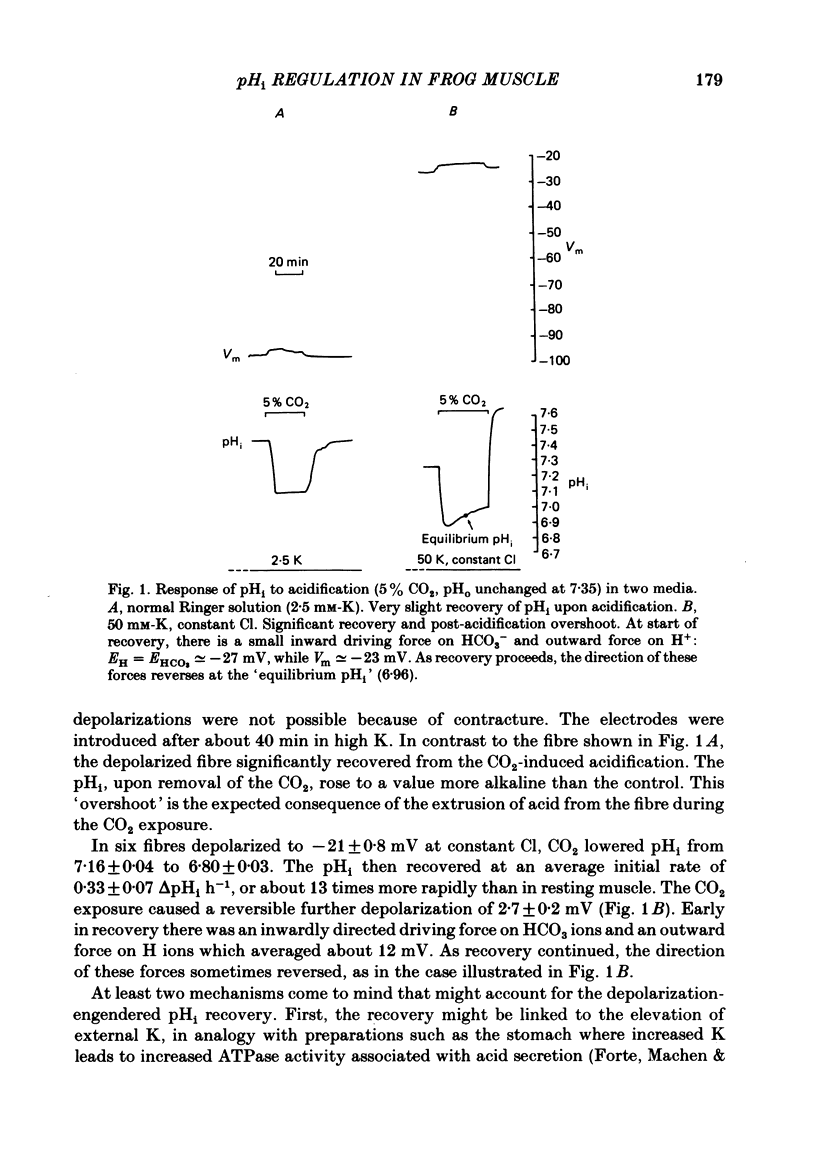
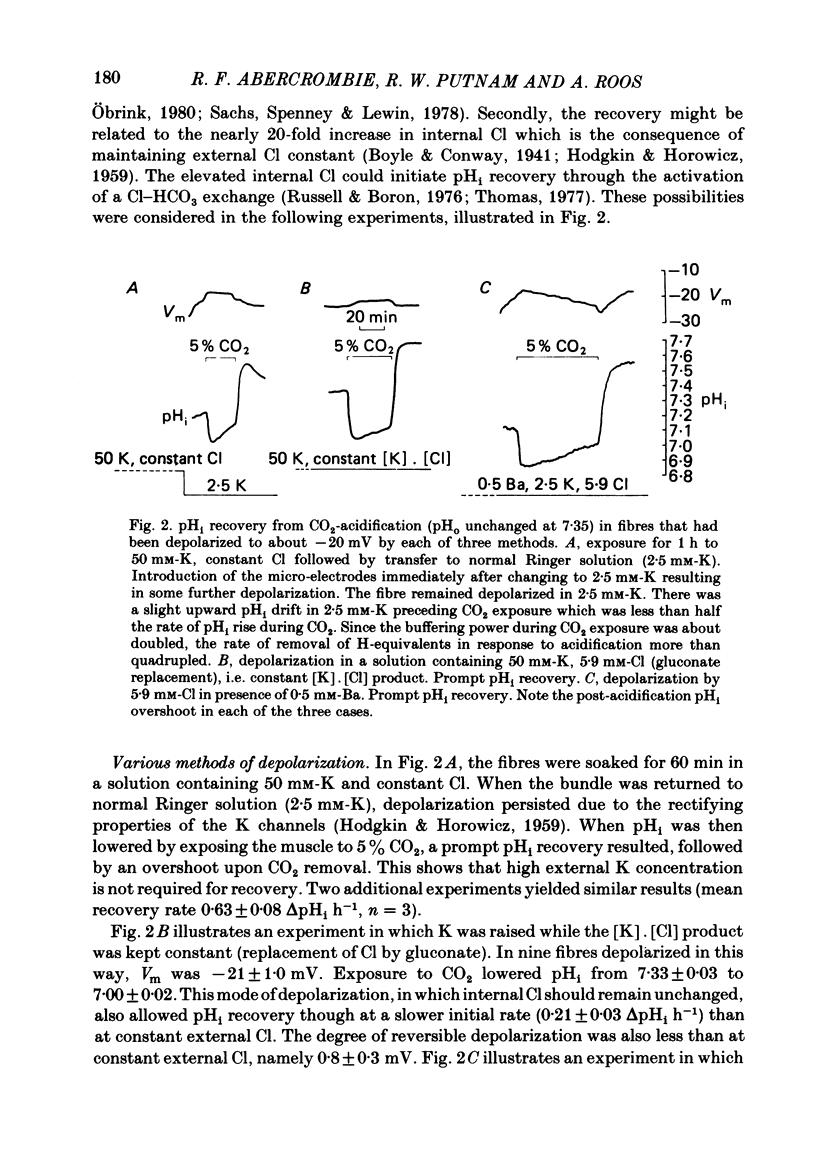
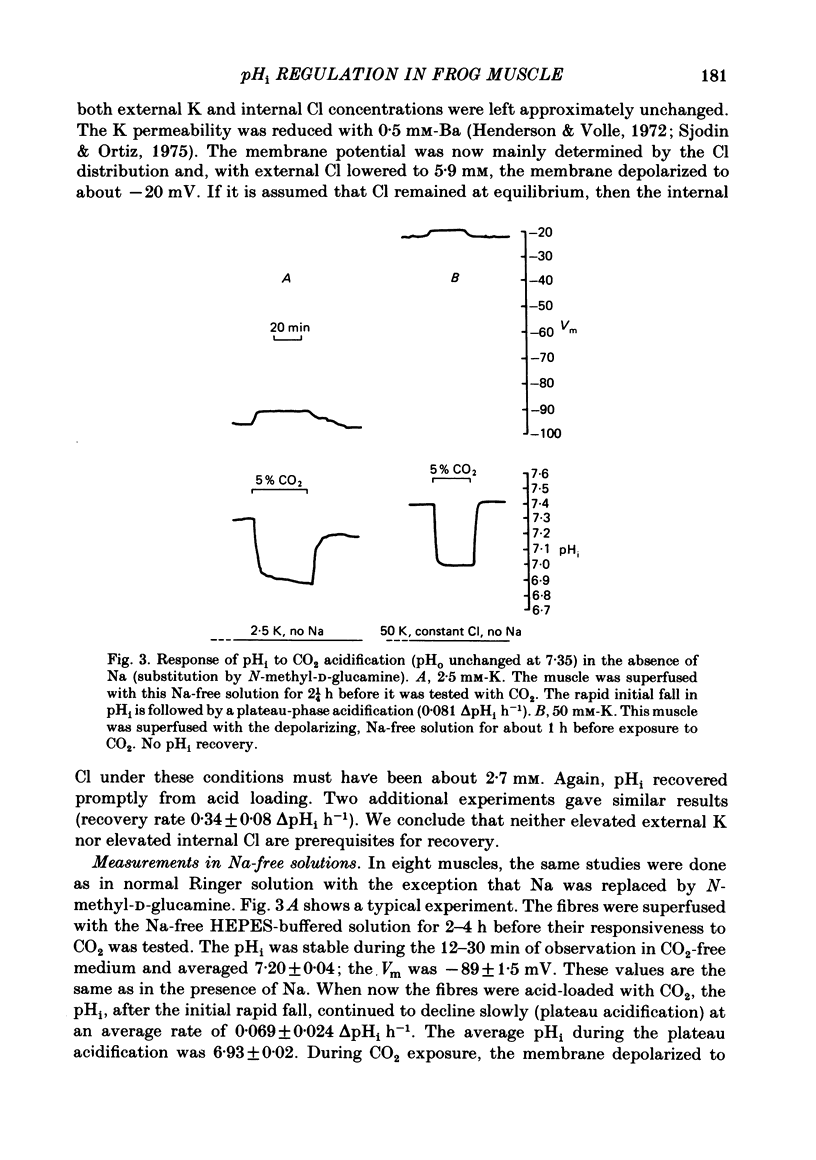
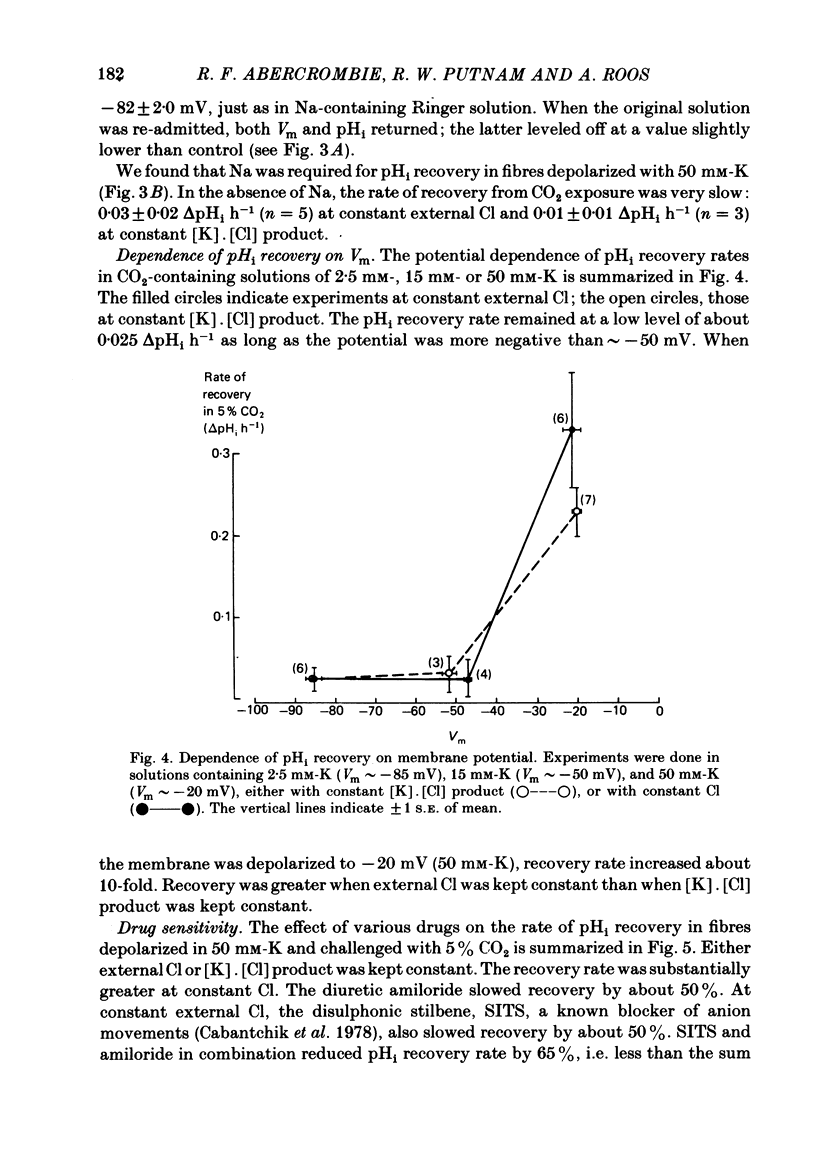
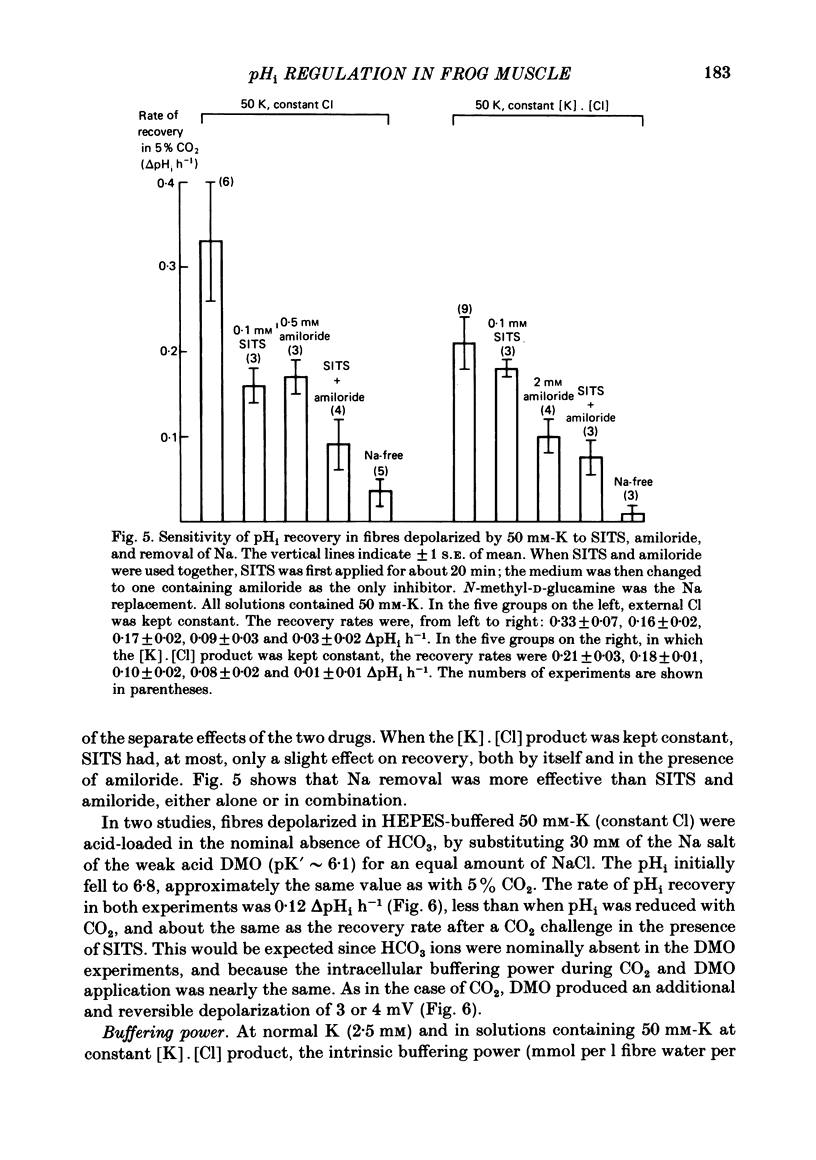
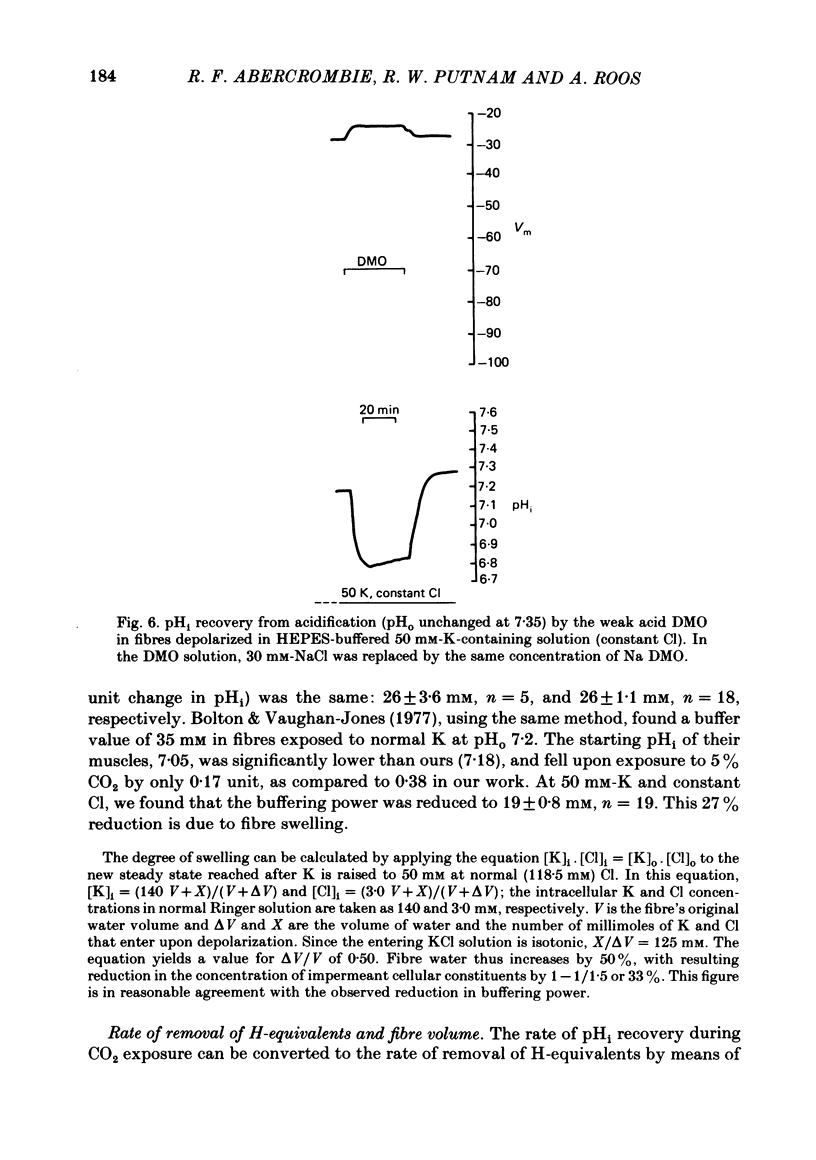
The behaviour of intracellular pH (pHi) was studied with micro-electrodes in frog semitendinosus muscle which was superfused with Ringer solution and with depolarizing solutions. The electrodes were introduced into the depolarized muscle about 40 min after contracture had subsided. All studies were done at external pH (pHo) of 7.35 and at 22 degrees C. The pHi in normal Ringer solution buffered with HEPES was 7.18 +/- 0.03 (S.E. of mean) (n = 10); the membrane potential, Vm, was -88 +/- 1.8 mV. When pHi was lowered to about 6.8 by replacing the HEPES by 5% CO2, 24 mM-HCO3 (constant pHo), it recovered at a very slow rate of 0.025 +/- 0.011 delta pHi h-1 (n = 6). When all the Na was replaced by N-methyl-D-glucamine (initial pHi 7.20 +/- 0.04, initial Vm -89 +/- 1.5 mV, n = 8), this slow alkalinization was converted into a slow acidification at a rate of 0.069 +/- 0.024 delta pHi h-1. In muscle depolarized in 15 mM-K (Vm approximately -50 mV), the rate of recovery from CO2 acidification was not increased above that in normal Ringer solution (2.5 mM-K). When, however, the muscle was depolarized in 50 mM-K to about -20 mV, the rate of recovery increased to 0.33 +/- 0.07 delta pHi h-1 (n = 6) when external Cl was kept constant, or to 0.21 +/- 0.03 (n = 9) when [K]. [Cl] product was kept constant. In the absence of Na, pHi recovery rate in 50 mM-K was reduced by at least 90%. Enhanced recovery from CO2-induced acidification was also observed in 2.5 mM-K when the fibres were depolarized to about -20 mV in one of two ways: (a) by previous exposure for 60 min to 50 mM-K at constant Cl, or (b) by reduction of external Cl to 5.9 mM in the presence of 0.5 mM-Ba. When pHi of depolarized fibres (50 mM-K) was lowered to about 6.8 by the weak acid dimethyl-2,4-oxazolidinedione (DMO), it recovered at a rate of 0.12 delta pHi h-1 in two experiments. In fibres depolarized in 50 mM-K and constant Cl, either 0.1 mM-SITS or 0.5 mM-amiloride slowed pHi recovery from CO2 exposure by about 50%. When the depolarization was achieved at constant [K]. [Cl] product, amiloride slowed pHi recovery by about 50%, while SITS had, at most, only a slight effect.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aickin C. C., Thomas R. C. An investigation of the ionic mechanism of intracellular pH regulation in mouse soleus muscle fibres. J Physiol. 1977 Dec;273(1):295–316. doi: 10.1113/jphysiol.1977.sp012095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton T. B., Vaughan-Jones R. D. Continuous direct measurement of intracellular chloride and pH in frog skeletal muscle. J Physiol. 1977 Sep;270(3):801–833. doi: 10.1113/jphysiol.1977.sp011983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boron W. F., De Weer P. Active proton transport stimulated by CO2/HCO3-, blocked by cyanide. Nature. 1976 Jan 22;259(5540):240–241. doi: 10.1038/259240a0. [DOI] [PubMed] [Google Scholar]
- Boron W. F., De Weer P. Intracellular pH transients in squid giant axons caused by CO2, NH3, and metabolic inhibitors. J Gen Physiol. 1976 Jan;67(1):91–112. doi: 10.1085/jgp.67.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boron W. F. Intracellular pH transients in giant barnacle muscle fibers. Am J Physiol. 1977 Sep;233(3):C61–C73. doi: 10.1152/ajpcell.1977.233.3.C61. [DOI] [PubMed] [Google Scholar]
- Boron W. F., McCormick W. C., Roos A. pH regulation in barnacle muscle fibers: dependence on intracellular and extracellular pH. Am J Physiol. 1979 Sep;237(3):C185–C193. doi: 10.1152/ajpcell.1979.237.3.C185. [DOI] [PubMed] [Google Scholar]
- Boyle P. J., Conway E. J. Potassium accumulation in muscle and associated changes. J Physiol. 1941 Aug 11;100(1):1–63. doi: 10.1113/jphysiol.1941.sp003922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabantchik Z. I., Knauf P. A., Rothstein A. The anion transport system of the red blood cell. The role of membrane protein evaluated by the use of 'probes'. Biochim Biophys Acta. 1978 Sep 29;515(3):239–302. doi: 10.1016/0304-4157(78)90016-3. [DOI] [PubMed] [Google Scholar]
- Cala P. M. Volume regulation by Amphiuma red blood cells. The membrane potential and its implications regarding the nature of the ion-flux pathways. J Gen Physiol. 1980 Dec;76(6):683–708. doi: 10.1085/jgp.76.6.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forte J. G., Machen T. E., Obrink K. J. Mechanisms of gastric H+ and Cl- transport. Annu Rev Physiol. 1980;42:111–126. doi: 10.1146/annurev.ph.42.030180.000551. [DOI] [PubMed] [Google Scholar]
- HODGKIN A. L., HOROWICZ P. The influence of potassium and chloride ions on the membrane potential of single muscle fibres. J Physiol. 1959 Oct;148:127–160. doi: 10.1113/jphysiol.1959.sp006278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson E. G., Volle R. L. Ion exchange in frog sartorius muscle treated with 9-aminoacridine or barium. J Pharmacol Exp Ther. 1972 Nov;183(2):356–369. [PubMed] [Google Scholar]
- Johnson J. D., Epel D. Intracellular pH and activation of sea urchin eggs after fertilisation. Nature. 1976 Aug 19;262(5570):661–664. doi: 10.1038/262661a0. [DOI] [PubMed] [Google Scholar]
- Kinsella J. L., Aronson P. S. Properties of the Na+-H+ exchanger in renal microvillus membrane vesicles. Am J Physiol. 1980 Jun;238(6):F461–F469. doi: 10.1152/ajprenal.1980.238.6.F461. [DOI] [PubMed] [Google Scholar]
- Moody W. J., Jr The ionic mechanism of intracellular pH regulation in crayfish neurones. J Physiol. 1981 Jul;316:293–308. doi: 10.1113/jphysiol.1981.sp013788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos A., Boron W. F. Intracellular pH. Physiol Rev. 1981 Apr;61(2):296–434. doi: 10.1152/physrev.1981.61.2.296. [DOI] [PubMed] [Google Scholar]
- Russell J. M., Boron W. F. Role of choloride transport in regulation of intracellular pH. Nature. 1976 Nov 4;264(5581):73–74. doi: 10.1038/264073a0. [DOI] [PubMed] [Google Scholar]
- Sachs G., Spenney J. G., Lewin M. H+ transport: regulation and mechanism in gastric mucosa and membrane vesicles. Physiol Rev. 1978 Jan;58(1):106–173. doi: 10.1152/physrev.1978.58.1.106. [DOI] [PubMed] [Google Scholar]
- Sjodin R. A., Ortiz O. Resolution of the potassium ion pump in muscle fibers using barium ions. J Gen Physiol. 1975 Sep;66(3):269–286. doi: 10.1085/jgp.66.3.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas R. C. Intracellular pH of snail neurones measured with a new pH-sensitive glass mirco-electrode. J Physiol. 1974 Apr;238(1):159–180. doi: 10.1113/jphysiol.1974.sp010516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas R. C. The effect of carbon dioxide on the intracellular pH and buffering power of snail neurones. J Physiol. 1976 Mar;255(3):715–735. doi: 10.1113/jphysiol.1976.sp011305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas R. C. The role of bicarbonate, chloride and sodium ions in the regulation of intracellular pH in snail neurones. J Physiol. 1977 Dec;273(1):317–338. doi: 10.1113/jphysiol.1977.sp012096. [DOI] [PMC free article] [PubMed] [Google Scholar]


