Abstract
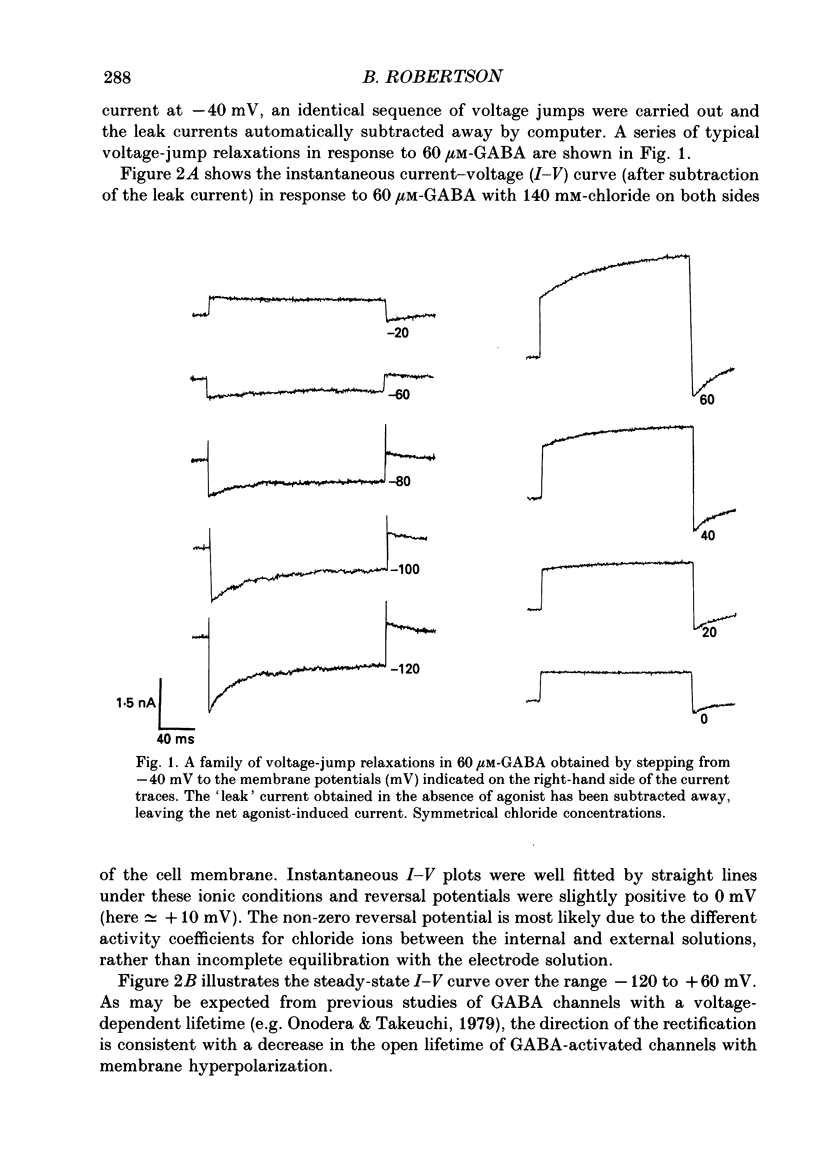
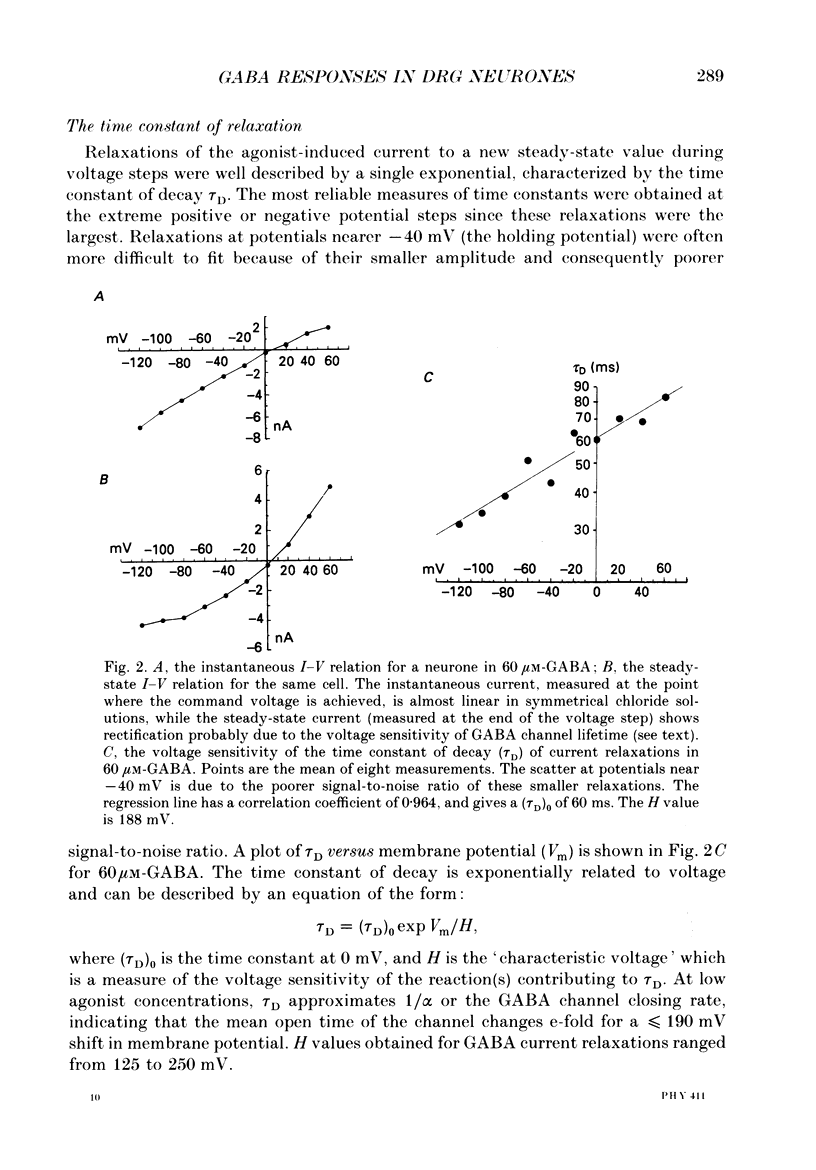
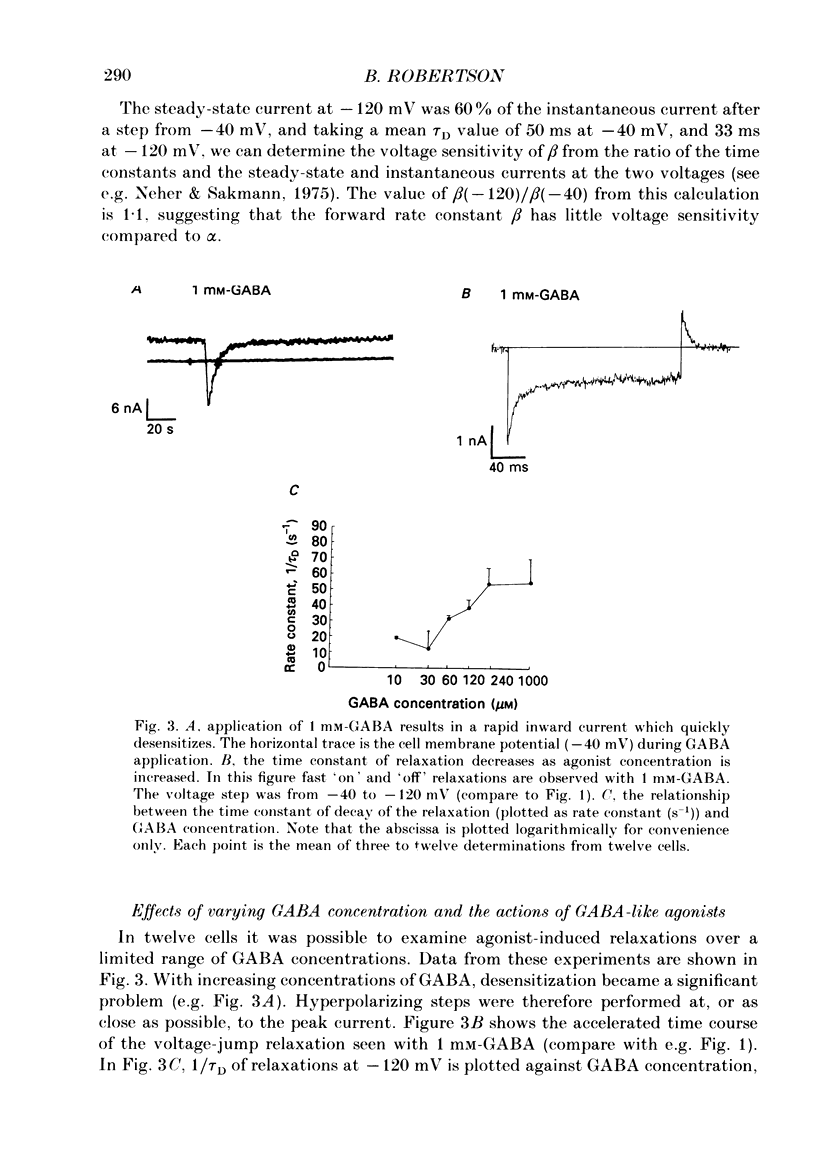
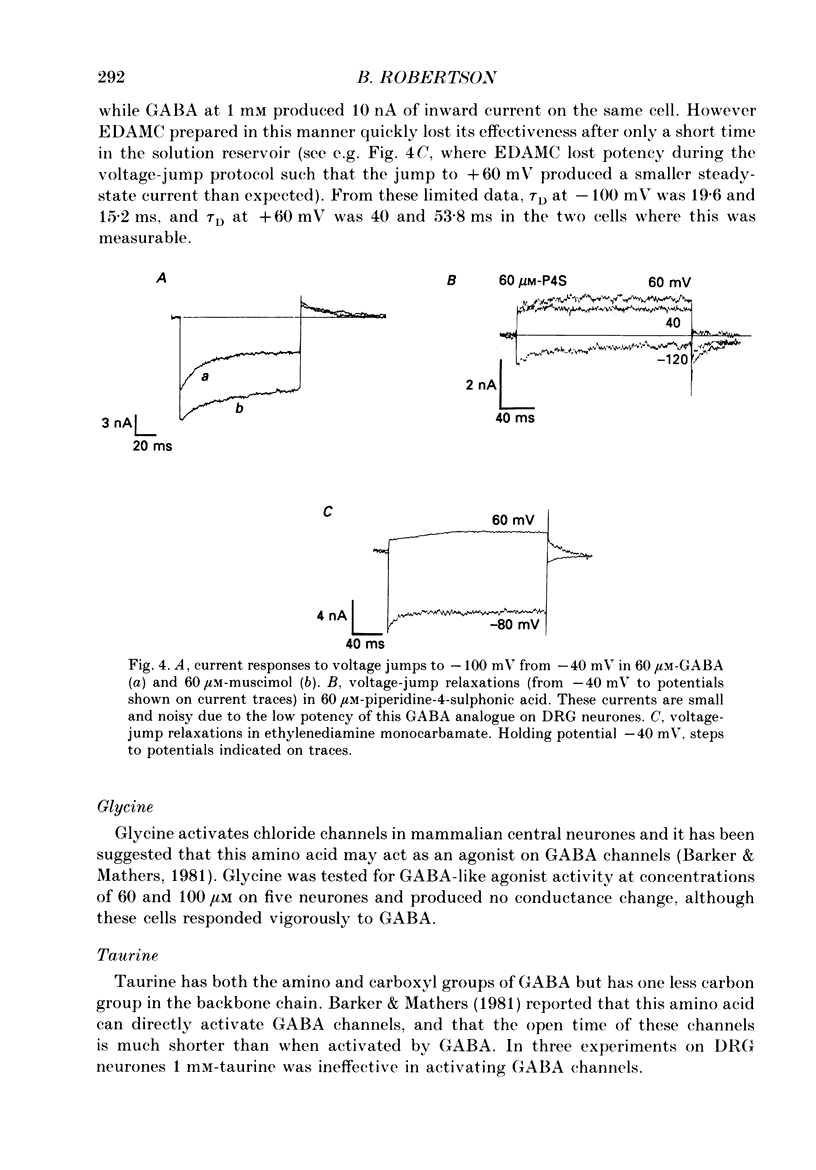
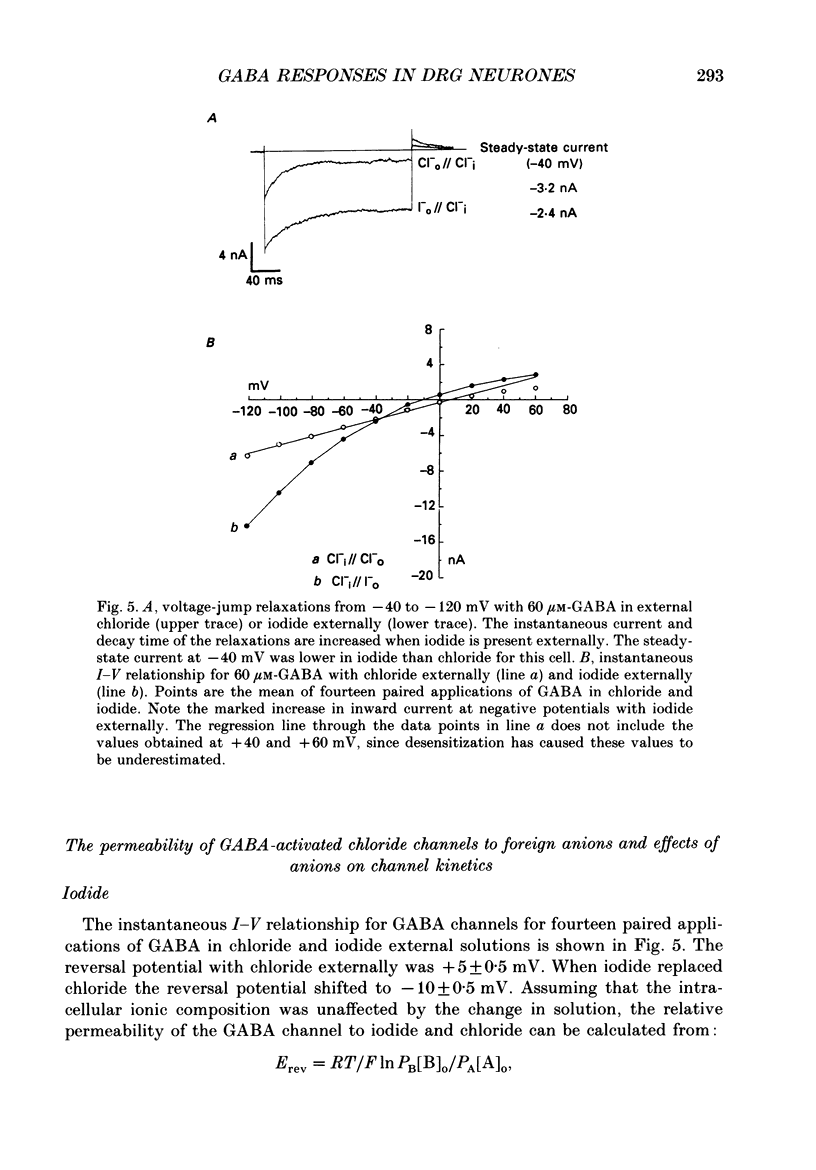
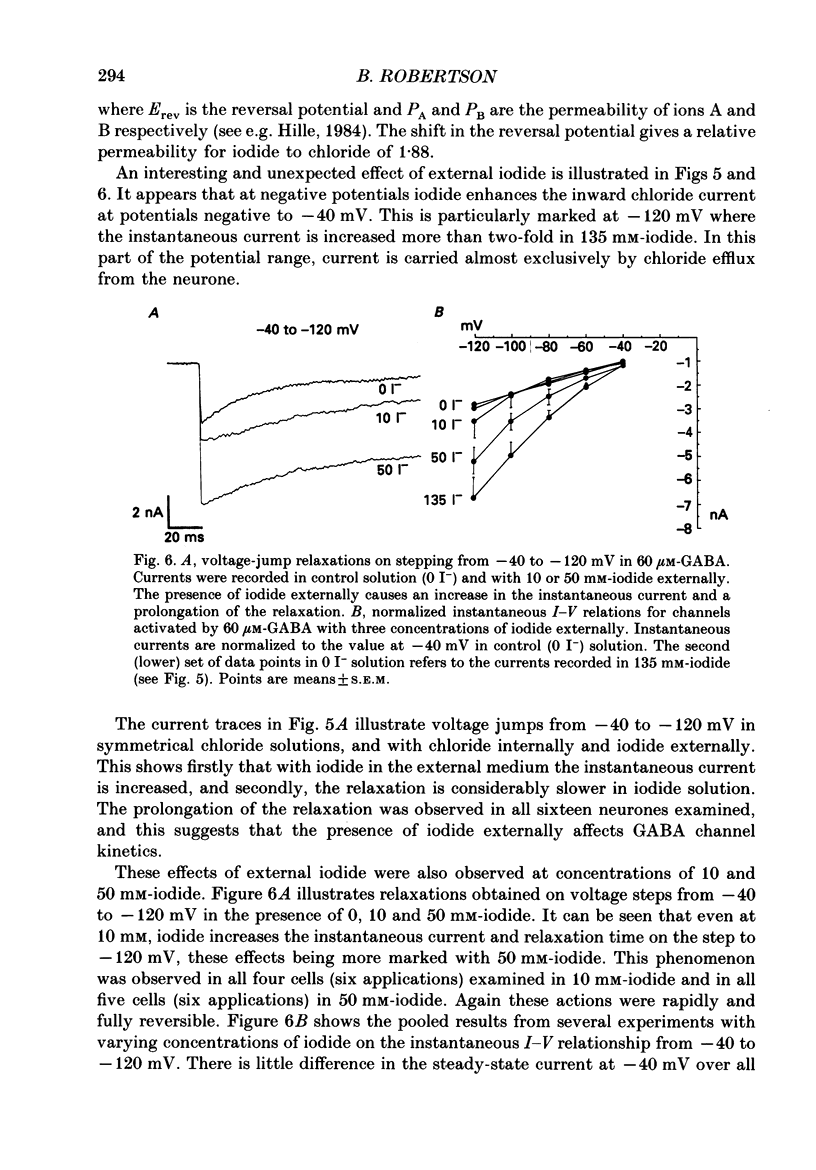
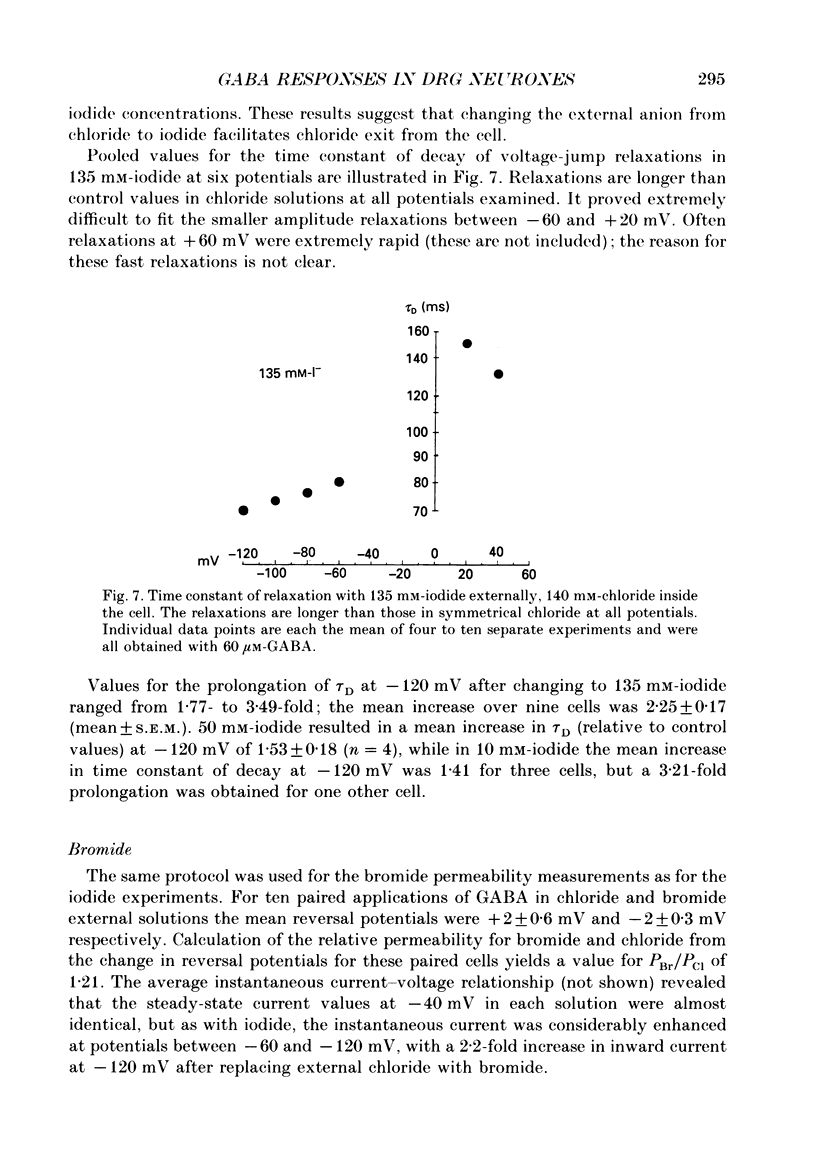
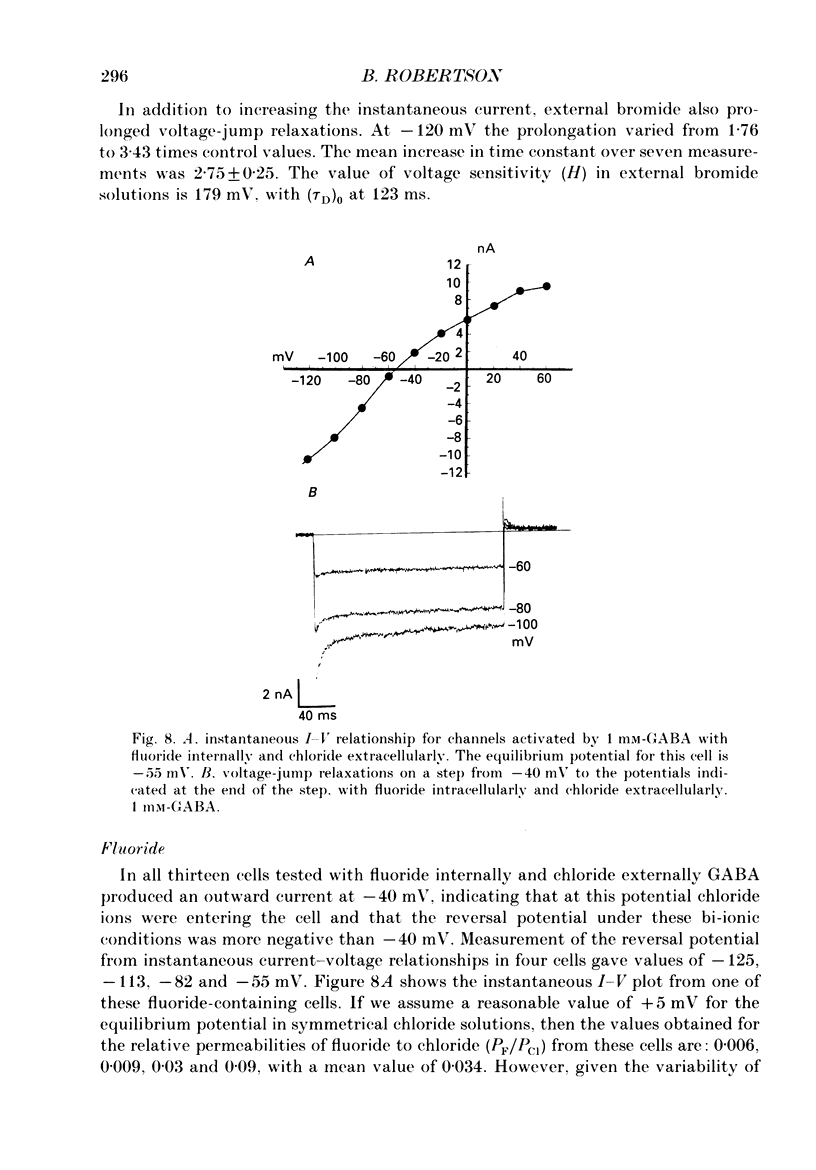
1. The properties of gamma-aminobutyric acid (GABA)-activated chloride channels in dorsal root ganglion (DRG) neurones obtained from rats and cats were examined using the single-electrode voltage clamp in conjunction with suction-electrode techniques. 2. GABA-evoked currents showed voltage-sensitive kinetics. Time constants (tau D) were measured from voltage-jump relaxations and tau D became briefer with membrane hyperpolarization. tau D was 33 ms at -120 mV with 60 microM-GABA and changed e-fold for 188 mV. tau D decreased as GABA concentration was increased - the extrapolated tau D at 'zero' GABA concentration was approximately equal to 50 ms at -120 mV. 3. The steady-state current in GABA was curvilinear, rectifying at negative potentials. The instantaneous current was linear with symmetrical chloride concentrations (140 mM) on both sides of the cell membrane. 4. Muscimol was a more effective agonist than GABA, while piperidine-4-sulphonic acid and ethylenediamine monocarbamate were only weakly effective agonists. Taurine and glycine had no detectable agonist activity. 5. Ion substitution experiments revealed the permeability sequence I- greater than Br- greater than Cl- greater than F- greater than propionate (1.88 greater than 1.21 greater than 1.0 approximately equal to 0.1 approximately equal to 0.1). 6. The presence of iodide and bromide ions externally caused an increase in chloride efflux at membrane potentials more negative than -40 mV, and caused a prolongation of voltage-jump relaxations. Relaxations in fluoride and propionate solutions were faster than those seen in chloride.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. J., Gage P. W., Hamill O. P. Inhibitory postsynaptic currents at Aplysia cholinergic synapses: effects of permeant anions and depressant drugs. Proc R Soc Lond B Biol Sci. 1982 Feb 22;214(1196):335–350. doi: 10.1098/rspb.1982.0015. [DOI] [PubMed] [Google Scholar]
- Adams P. R. Kinetics of agonist conductance changes during hyperolarization at frog endplates. Br J Pharmacol. 1975 Feb;53(2):308–310. doi: 10.1111/j.1476-5381.1975.tb07364.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akaike N., Hattori K., Inomata N., Oomura Y. gamma-Aminobutyric-acid- and pentobarbitone-gated chloride currents in internally perfused frog sensory neurones. J Physiol. 1985 Mar;360:367–386. doi: 10.1113/jphysiol.1985.sp015622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akaike N., Inoue M., Krishtal O. A. 'Concentration-clamp' study of gamma-aminobutyric-acid-induced chloride current kinetics in frog sensory neurones. J Physiol. 1986 Oct;379:171–185. doi: 10.1113/jphysiol.1986.sp016246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker J. L., Mathers D. A. GABA analogues activate channels of different duration on cultured mouse spinal neurons. Science. 1981 Apr 17;212(4492):358–361. doi: 10.1126/science.6259733. [DOI] [PubMed] [Google Scholar]
- Bormann J., Hamill O. P., Sakmann B. Mechanism of anion permeation through channels gated by glycine and gamma-aminobutyric acid in mouse cultured spinal neurones. J Physiol. 1987 Apr;385:243–286. doi: 10.1113/jphysiol.1987.sp016493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CURTIS D. R., WATKINS J. C. The excitation and depression of spinal neurones by structurally related amino acids. J Neurochem. 1960 Sep;6:117–141. doi: 10.1111/j.1471-4159.1960.tb13458.x. [DOI] [PubMed] [Google Scholar]
- Collingridge G. L., Gage P. W., Robertson B. Inhibitory post-synaptic currents in rat hippocampal CA1 neurones. J Physiol. 1984 Nov;356:551–564. doi: 10.1113/jphysiol.1984.sp015482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D., Sakmann B. Fast events in single-channel currents activated by acetylcholine and its analogues at the frog muscle end-plate. J Physiol. 1985 Dec;369:501–557. doi: 10.1113/jphysiol.1985.sp015912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enna S. J., Snyder S. H. Influences ions, enzymes, and detergents on gamma-aminobutyric acid-receptor binding in synaptic membranes of rat brain. Mol Pharmacol. 1977 May;13(3):442–453. [PubMed] [Google Scholar]
- Gallagher J. P., Higashi H., Nishi S. Characterization and ionic basis of GABA-induced depolarizations recorded in vitro from cat primary afferent neurones. J Physiol. 1978 Feb;275:263–282. doi: 10.1113/jphysiol.1978.sp012189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Bormann J., Sakmann B. Activation of multiple-conductance state chloride channels in spinal neurones by glycine and GABA. 1983 Oct 27-Nov 2Nature. 305(5937):805–808. doi: 10.1038/305805a0. [DOI] [PubMed] [Google Scholar]
- Jackson M. B., Lecar H., Mathers D. A., Barker J. L. Single channel currents activated by gamma-aminobutyric acid, muscimol, and (-)-pentobarbital in cultured mouse spinal neurons. J Neurosci. 1982 Jul;2(7):889–894. doi: 10.1523/JNEUROSCI.02-07-00889.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krogsgaard-Larsen P., Falch E., Peet M. J., Leah J. D., Curtis D. R. Molecular pharmacology of the GABA receptors and GABA agonists. Adv Biochem Psychopharmacol. 1983;37:1–13. [PubMed] [Google Scholar]
- Lee K. S., Akaike N., Brown A. M. Properties of internally perfused, voltage-clamped, isolated nerve cell bodies. J Gen Physiol. 1978 May;71(5):489–507. doi: 10.1085/jgp.71.5.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathers D. A., Barker J. L. GABA and muscimol open ion channels of different lifetimes on cultured mouse spinal cord cells. Brain Res. 1981 Jan 5;204(1):242–247. doi: 10.1016/0006-8993(81)90672-7. [DOI] [PubMed] [Google Scholar]
- Neher E., Sakmann B. Voltage-dependence of drug-induced conductance in frog neuromuscular junction. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2140–2144. doi: 10.1073/pnas.72.6.2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen R. W., Snowman A. M. Chloride-dependent enhancement by barbiturates of gamma-aminobutyric acid receptor binding. J Neurosci. 1982 Dec;2(12):1812–1823. doi: 10.1523/JNEUROSCI.02-12-01812.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onodera K., Takeuchi A. An analysis of the inhibitory post-synaptic current in the voltage-clamped crayfish muscle. J Physiol. 1979 Jan;286:265–282. doi: 10.1113/jphysiol.1979.sp012618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson B., Taylor W. R. Effects of gamma-aminobutyric acid and (-)-baclofen on calcium and potassium currents in cat dorsal root ganglion neurones in vitro. Br J Pharmacol. 1986 Dec;89(4):661–672. doi: 10.1111/j.1476-5381.1986.tb11170.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal M., Barker J. L. Rat hippocampal neurons in culture: voltage-clamp analysis of inhibitory synaptic connections. J Neurophysiol. 1984 Sep;52(3):469–487. doi: 10.1152/jn.1984.52.3.469. [DOI] [PubMed] [Google Scholar]
- Sheridan R. E., Lester H. A. Relaxation measurements on the acetylcholine receptor. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3496–3500. doi: 10.1073/pnas.72.9.3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spalding B. C. Properties of toxin-resistant sodium channels produced by chemical modification in frog skeletal muscle. J Physiol. 1980 Aug;305:485–500. doi: 10.1113/jphysiol.1980.sp013377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright E. M., Diamond J. M. Anion selectivity in biological systems. Physiol Rev. 1977 Jan;57(1):109–156. doi: 10.1152/physrev.1977.57.1.109. [DOI] [PubMed] [Google Scholar]


