Abstract
Several cytoplasmic polyadenylation element (CPE)-containing mRNAs that are repressed in Xenopus oocytes become active during meiotic maturation. A group of factors that are anchored to the CPE are responsible for this repression and activation. Two of the most important are CPEB, which binds directly to the CPE, and Maskin, which associates with CPEB. In oocytes, Maskin also binds eukaryotic translation initiation factor 4E (eIF4E), an interaction that excludes eIF4G and prevents formation of the eIF4F initiation complex. When the oocytes are stimulated to reenter the meiotic divisions (maturation), CPEB promotes cytoplasmic polyadenylation. The newly elongated poly(A) tail becomes bound by poly(A) binding protein (PABP), which in turn binds eIF4G and helps it displace Maskin from eIF4E, thereby inducing translation. Here we show that Maskin undergoes several phosphorylation events during oocyte maturation, some of which are important for its dissociation from eIF4E and translational activation of CPE-containing mRNA. These sites are T58, S152, S311, S343, S453, and S638 and are phosphorylated by cdk1. Mutation of these sites to alanine alleviates the cdk1-induced dissociation of Maskin from eIF4E. Prior to maturation, Maskin is phosphorylated on S626 by protein kinase A. While this modification has no detectable effect on translation during oocyte maturation, it is critical for this protein to localize on the mitotic apparatus in somatic cells. These results show that Maskin activity and localization is controlled by differential phosphorylation.
All nuclear-encoded eukaryotic mRNAs contain a 7m-guanosine residue at their 5′ ends. Translation initiation at this “cap” is promoted by eukaryotic translation initiation factor 4F (eIF4F), a trimeric complex consisting of eIF4E, eIF4A, and eIF4G. eIF4E binds directly to the cap; it interacts with eIF4G, the protein that recruits eIF3 and, indirectly, the 40S ribosomal subunit to the 5′ end of the mRNA. eIF4A is a helicase that unwinds RNA secondary structure as the 40S subunit and several associated factors scan in the 3′ direction in search of the proper codon to initiate translation (5, 16, 17). Because initiation is rate limiting for translation, it is the step that is most often subject to regulation. One factor that controls the initiation is 4E-BP, which contains a peptide motif that structurally is similar to the one in eIF4G that binds eIF4E. Thus, a competition between eIF4G and 4E-BP for binding eIF4E regulates eIF4F assembly. Phosphorylation of 4E-BP modulates its interaction with eIF4E; hypophosphorylated 4E-BP stably interacts with eIF4E while hyperphosphorylated 4E-BP does not. Initiation can therefore be controlled by kinases (and phosphatases) that act on 4E-BP (10-12, 30).
Another factor with an eIF4F-disrupting activity is Maskin, a protein that coimmunoprecipitates with the RNA binding protein CPEB from extracts derived from Xenopus oocytes (34). These cells contain dormant mRNAs that have relatively short poly(A) tails, usually less than 20 to 40 nucleotides. When the oocytes are stimulated to reenter the meiotic divisions (oocyte maturation) by progesterone, the poly(A) tails on certain mRNAs are elongated and translation ensues. The specificity cis sequence that is necessary for cytoplasmic polyadenylation is the CPE (cytoplasmic polyadenylation element); the AAUAAA polyadenylation element is also required, although it is present in nearly all mRNAs irrespective of whether they undergo cytoplasmic polyadenylation. CPEB binds the CPE, and when it is phosphorylated soon after oocytes are treated with progesterone, polyadenylation ensues (23, 24). While polyadenylation is a complex process involving a number of factors (1), it is the newly elongated poly(A) tail that induces translation. Maskin is the key factor that regulates this translation. In immature oocytes, CPE-containing mRNAs, which have short poly(A) tails, are bound by CPEB, which in turn is bound by Maskin. Maskin also binds eIF4E at the same region as eIF4G, thereby preventing eIF4F formation on CPE-containing mRNAs (34). Once the polyadenylation occurs, the poly(A) tail is bound by poly(A) binding protein (PABP), which in turn binds eIF4G. PABPs help eIF4Gs displace Maskin from and bind itself to eIF4E, thereby stimulating initiation (2).
CPEB and Maskin not only act in concert to regulate translation during oocyte maturation but do so as well during the early embryonic cell cycles (14). In early Xenopus embryos, both CPEB and Maskin are localized to the mitotic apparatus, as is cyclin B1 mRNA (13). Because CPEB binds microtubules, it is probably this protein that anchors the CPE-dependent polyadenylation and translation factors to spindles and centrosomes. The injection of neutralizing CPEB or Maskin antibody into embryos prevents cell division and disrupts the integrity of the mitotic apparatus. While having little effect on the cyclin B1 synthesis, the injection of a CPEB mutant protein is unable to bind microtubules but prevents this synthesis from occurring on spindles and centrosomes, which results in inhibited cell division (13).
During this investigation of cell cycle-regulated polyadenylation and translation, we noticed that Maskin had a reduced electrophoretic mobility in embryos compared to oocytes (13). Cao and Richter (2) further noted that this mobility shift occurred during progesterone-induced oocyte maturation. Because a mobility shift often indicates phosphorylation, we were intrigued by the possibility that Maskin might undergo this modification, and that if it did, it might influence CPE-dependent translation, as in the case of 4E-BP, or centrosome and spindle localization. Here we show that Maskin is phosphorylated on a single site in immature oocytes by protein kinase A (PKA) and is phosphorylated on at least six additional sites during maturation by cdk1. The cdk1 phosphorylations influence not only the interaction between Maskin and eIF4E but also regulate cyclin B1 mRNA translation during oocyte maturation. The PKA phosphorylation is essential for Maskin localization to the mitotic apparatus. These data demonstrate that Maskin activity and localization are mediated by multiple phosphorylation events.
MATERIALS AND METHODS
Oocyte collection and injection.
Stage VI Xenopus oocytes were dispersed from ovaries by treatment with collagenase. They were injected with mos sense (ATCTAGTACAGTATCTCAATGTCCA) or antisense (complementary sequence) oligonucleotides (∼3 mg/ml, ∼70 nl/oocyte) as described by deMoor and Richter (3). Other oocytes were injected with mRNAs encoding wild-type (WT) or mutant Maskin (∼25 ng/oocyte) or Escherichia coli-expressed protein and incubated for 16 h at 20°C before Western blotting for Maskin and cyclin B1. Some of the oocytes were also cultured in the presence of progesterone for varying times before they were processed for Western blotting (probed for Maskin and cyclin B1) or two-dimensional phosphopeptide mapping. Some oocytes were also used to create extracts for H1 kinase assays, as described previously (3), and Maskin in vitro phosphorylation assays. Briefly, oocytes were lysed in H1 kinase buffer (80 mM β-glycerophosphate, 20 mM EGTA, 15 mM MgCl2, 0.5 mM NaVaO4, and protease inhibitors) at 20 μl per oocyte, centrifuged to remove insoluble material, and stored on ice.
Construction of Maskin mutants.
Full-length Maskin cDNA cloned into pET 30a (34) was used as a template for PCR-generated deletion mutants which were subsequently cloned into the BamHI and XhoI sites of the pET30a vector, with the exception of the S338-K754 mutant, which was created by digestion of the Maskin cDNA with HindIII and insertion into pET40. Point mutations of specific residues to alanine and aspartic acid were carried out with a Quick Change site-directed mutagenesis kit (Stratagene).
Two-dimensional phosphopeptide mapping.
To examine endogenous Maskin phosphorylation, ∼300 oocytes were incubated overnight in Barth's medium containing 0.1 mCi/ml [32P]orthophosphate. The oocytes were further incubated with progesterone until maturation. They were lysed and centrifuged, and the supernatant was subjected to Maskin immunoprecipitation as previously described (34). Briefly, oocytes were washed in Barth's medium to remove free [32P]orthophosphate, and then 100 labeled oocytes were resuspended in 500 μl phospoimmunoprecipiation (phospho-IP) buffer (100 mM KCl, 50 mM Tris [pH 7.5], 50 mM NaF, 80 mM β-glycerolphosphate, 5 mM EDTA, 5 mM Na pyrophosphate, 0.1 mM NaVaO4, 0.1% NP-40, and protease inhibitors). The lysate was centrifuged to remove insoluble material and then cleared by incubation with a 100-μl bed volume of Sepharose at 4°C for 30 min. Fifteen microliters of anti-Maskin serum was added to the lysate with 100-μl bed volume protein A Sepharose and placed on a rocker at 4°C for 2 h. The beads were washed extensively with IP buffer (five times with 5 to 10× volume). The precipitated proteins were resuspended in sodium dodecyl sulfate (SDS) sample buffer, resolved by SDS-polyacrylamide gel electrophoresis (PAGE), and blotted onto polyvinylidene difluoride membrane. One side of the blot was subjected to immunoblotting to confirm that the labeled protein was indeed Maskin, while the majority of the labeled protein was digested with tosylsulfonyl phenylalanyl chloromethyl ketone-treated trypsin and the phosphopeptides were analyzed by thin-layer electrophoresis followed by thin-layer chromatography and phosphorimager analysis (23). H1 kinase extracts (see above) derived from noninjected or mos sense or antisense oligonucleotide-injected oocytes, in vitro-matured oocytes, or ovulated eggs were used for Maskin in vitro phosphorylation assays. The reactions were carried out in a final volume of 30 μl containing E. coli-expressed full-length or mutant histidine-tagged Maskin (1 to 3 μg), 8 μl extract, 50 mM Tris (pH 7.5), 10 mM MgCl2, 1 mM EGTA, 1 mM dithiothreitol, 0.1 mM ATP, and 20 μCi [32P-γ]ATP. Following incubation for 30 min at 30°C, Maskin was processed for two-dimensional phosphopeptide mapping as described above. Maskin was also phosphorylated in vitro with mitogen-activated protein (MAP) kinase (Calbiochem), cdk1/cyclin B (Calbiochem), or PKA (Sigma). In these reactions, oocyte extract was replaced with H1 kinase extract buffer. In some experiments, a PKA inhibitor, PKI (Calbiochem), was also added to the extracts. The resulting [32P]phosphopeptides were processed as described above.
Analysis of eIF4E-Maskin interaction.
Cap (7mGTP) column chromatography has been described previously (2, 34). Briefly, mRNAs encoding Maskin (WT or A6 mutant) as well as eIF4E were translated in rabbit reticulocyte lysates in the presence of [35S]methionine. Prior to chromatography, the lysates were supplemented with 0.25 mM GTP and in some cases with 0.25 mM 7mGTP as well. Some of the lysates also contained cdk1/cyclin B1 or PP2A (protein phosphatase 2A), the latter of which reduced or eliminated Maskin phosphorylation by lysate kinase(s). The protein that was retained on the cap columns was analyzed by SDS-PAGE and autoradiography.
Analysis of Maskin localization on spindles and centrosomes.
Xenopus egg extracts were primed with rhodamine-labeled tubulin as well as demembranated sperm DNA as described previously (4). The resulting spindles were fixed, centrifuged through a glycerol cushion onto glass slides (4), and immunostained with CPEB or Maskin antibody followed by fluorescein-conjugated secondary antibody. The DNA was stained with 4′,6′diamidino-2-phenylindole (DAPI). The spindles were visualized with a Nikon E600 Eclipse microscope. Centrosome-bound Maskin was also visualized in Xenopus XTC cells, which were immunostained with Maskin antibody (1:5,000) or nonimmune serum (1:500) as well as tubulin antibody. WT, S626A, S626D, and A6 Maskin molecules were fused to green fluorescent protein (GFP) and transfected using Lipofectamine (Invitrogen) into HeLa cells (18). GFP fluorescence demonstrated that WT, but not S626A Maskin, was associated with spindles and centrosomes. The GFP moiety was also used for Maskin coimmunoprecipitation experiments from transfected HeLa cells. Plates of transfected HeLa cells (60 mm) were washed with phosphate-buffered saline and then lysed in 0.333 ml IP buffer (100 mM KCl, 50 mM Tris [pH 7.5], 1 mM EDTA, 0.5% NP-40, 200 μg/ml RNase A, and protease inhibitors) for 30 min on ice. Lysates were centrifuged and cleared by incubation with nonimmune rabbit serum and protein A Sepharose for 1 h at 4°C. Cleared lysates were incubated with 2 μl anti-GFP (guinea pig) and protein A Sepharose for 2 h at 4°C and then washed extensively in IP buffer. The precipitates were immunoblotted for Maskin and MAP-215.
RESULTS
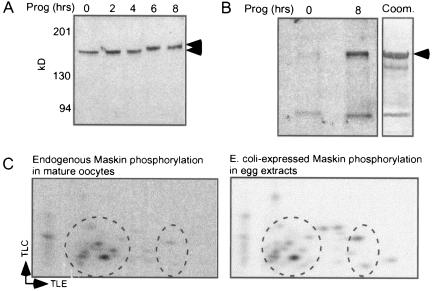
To investigate a possible relationship between the Maskin electrophoretic mobility shift and phosphorylation, we performed a Western blot for Maskin as oocytes mature. Figure 1A shows the mobility shift of this protein, which was coincident with germinal vesicle breakdown (GVBD) and MPF (maturation promoting factor, a heterodimer of cdk1 and cyclin B1) activation. [32P]orthophosphate labeling of untreated and progesterone-treated (mature) oocytes followed by Maskin immunoprecipitation demonstrates that not only does this protein become heavily phosphorylated during maturation but that it is also weakly phosphorylated in untreated oocytes (arrow; the lower band is likely a nonspecific protein since it was not detected on Maskin Western blots; data not shown) (Fig. 1B). The Maskin band in panel B was excised, trypsin digested, and subjected to two-dimensional phosphopeptide mapping, which shows that the protein was phosphorylated on several sites (Fig. 1C). However, the in vivo labeling of Maskin was very inefficient, probably because oocytes contain a large ATP pool. Because our goal was to identify the sites of Maskin phosphorylation and then determine their function(s), we required a method more efficient than in vivo labeling. Consequently, we supplemented egg (mature oocyte) extracts with E. coli-expressed Maskin (see panel B) as well as [32P-γ]ATP; Maskin was then resolved by SDS-PAGE and the band was excised and processed for two-dimensional mapping. This in vitro labeling procedure yielded a Maskin phosphorylation pattern that was very similar to the in vivo pattern (Fig. 1C, compare circled regions of middle and bottom panels). Because of this similarity, we decided to use exogenous Maskin to map phosphorylation sites; a similar procedure helped identify CPEB (23) and Aurora A (32) phosphorylation sites.
FIG. 1.
Maskin is phosphorylated during oocyte maturation. A. Protein from oocytes that were incubated with progesterone (Prog) for various times was immunoblotted and probed for Maskin (top panel). The arrows denote the Maskin electrophoretic mobility shift that occurred 6 h after progesterone addition, which was coincident with germinal vesicle breakdown. B. Oocytes were incubated with [32P]orthophosphate overnight before some of them were stimulated with progesterone. Maskin was immunoprecipitated and analyzed by SDS-PAGE and autoradiography. The lower band represents a nonspecific protein that does not react with Maskin antibody on Western blots. The right portion of the panel shows a Coomassie (Coom.) blue-stained SDS gel loaded with E. coli-expressed Maskin. C. The Maskin band in panel B (8 h in progesterone) was blotted onto a polyvinylidene difluoride membrane, digested with trypsin, and analyzed by two-dimensional phosphopeptide mapping (middle panel). Mature oocyte (egg) extracts were supplemented with E. coli-expressed Maskin (as in panel B, right) and [32P-γ]ATP; Maskin was then isolated and processed for two-dimensional phosphopeptide mapping as above. The circled regions in the two panels show areas with similar phosphopeptides. TLC refers to thin-layer chromatography, and TLE refers to thin-layer electrophoresis.
Several Maskin phosphorylation sites are developmentally regulated.
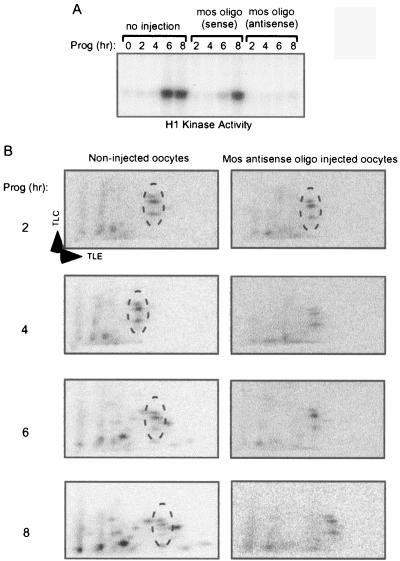
Because Maskin is phosphorylated on several sites, we sought to determine which, if any, were maturation specific; this might indicate those that would be the most likely to regulate translation. Oocytes were injected with an antisense oligonucleotide directed against mos mRNA; the ablation of this mRNA prevents the progesterone-induced activation of the MAP kinase cascade and the resulting activation of MPF and serves as a convenient method for distinguishing early (pre-mos) and late (post-mos) events of maturation (3, 32). As expected, the mos antisense oligonucleotide prevented progesterone-induced MPF activation as determined by histone H1 phosphorylation. Compared to noninjected oocytes, the mos sense oligonucleotide had little effect, since in both groups of oocytes MPF activity was detected at 6 h after progesterone addition (Fig. 2A). Next, noninjected or mos antisense oligonucleotide-injected oocytes were cultured for 2 to 8 h with progesterone before extracts were prepared and supplemented with [32P]ATP and E. coli-expressed Maskin. Extracts prepared from noninjected oocytes cultured for 2 h in progesterone yielded three obvious Maskin phosphopeptides (Fig. 2B, left panels, circled). As maturation proceeded, the three phosphopeptides remained but other phosphopeptides began to appear, particularly at 6 to 8 h when the oocytes contained robust MPF activity. In extracts derived from mos antisense oligonucleotide-injected oocytes, the same three phosphopeptides were observed at 2 or up to 8 h after progesterone addition (Fig. 2B, right panels, circled). These results show that Maskin undergoes a complex series of developmentally controlled phosphorylation events.
FIG. 2.
Time course of Maskin phosphorylation. A. Oocytes, some of which were injected with mos antisense or sense oligonucleotides, were incubated with progesterone (Prog) for up to 8 h. They were then examined for H1 kinase activity, which is an indicator of MPF (cdk1/cyclin B) activation. Note that injected mos antisense oligonucleotide (oligo) prevented MPF activation. B. E. coli-expressed Maskin and [32P]ATP were added to extracts derived from noninjected oocytes or oocytes that were injected with mos antisense oligonucleotide. Following an incubation period, Maskin was processed for two-dimensional phosphopeptide mapping. The circled areas denote Maskin phoshopeptides that were present throughout oocyte maturation, irrespective of whether MPF was activated.
Identification of maturation-specific Maskin phosphorylation sites.
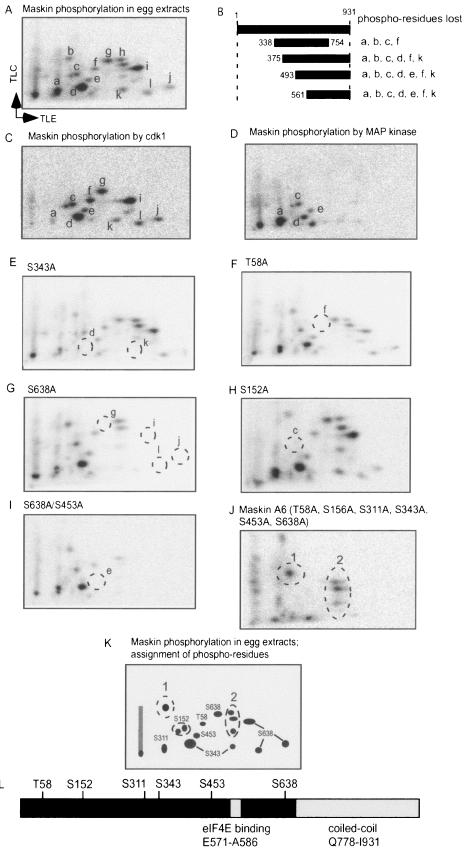
Because the maturation-specific phosphorylations of Maskin correlate with CPE-dependent mRNA translation (2, 34), we thought that they would be the most likely ones to influence eIF4E binding, and accordingly they were the first ones we sought to identify. Because our initial attempts to combine two-dimensional mapping and mass spectrometry or amino acid sequencing were not successful, we employed two other approaches. First, we made several deletions in Maskin and determined which phosphopeptides were lost when the E. coli-expressed proteins were phosphorylated in egg extracts. Figure 3A and B show that with this method, several phosphopeptides were tentatively mapped to various regions of Maskin. In combination with phosphoamino acid analysis that revealed a large amount of phosphoserine, some phosphothreonine, and no phosphotyrosine (data not shown), we were further able to ascertain which phosphopeptides (i.e., a-l) were likely to reside in certain regions of Maskin. However, because this approach could not identify specific phosphoamino acids, we addressed whether kinases that are known to become active during oocyte maturation would phosphorylate Maskin in vitro, and, if so, whether the phosphoresidues were the same as those observed when Maskin was phosphorylated in egg extracts. Because such kinases have preferred phosphorylation motifs, this would aid us in determining which amino acids were phosphorylated. The first kinase we used was cdk1 (cdc2), a component of MPF. This kinase, which usually recognizes SP and TP pairs, phosphorylated many of the same peptides in vitro as was detected in egg extracts (compare Fig. 3C with A). MAP kinase, which also recognizes SP and TP pairs, phosphorylated many fewer residues, but phosphopeptides a, c, d, and e were detected (Fig. 3D). Note that while cdk1 and MAP kinase both phosphorylated peptides a, c, d, and e, they did so to different extents. Taken together, these studies indicated the amino acids that were the most likely to be phosphorylated in egg extracts; indeed, when these residues were changed to alanine individually, specific phosphopeptides were lost (Fig. 3E to J, circled phosphopeptides were lost, compare with panel A). In combination, the substitution of six residues for alanine led to the loss of almost all the phosphopeptides (Fig. 3J, in this case the circled phosphopeptides were those that remained); some of the other peptides (Fig. 3J, circled and labeled “2”) were detected in immature oocytes (see Fig. 2 and 4). Figure 3K shows the assignment of several phosphoamino acids and Fig. 3L shows their relationship to the eIF4E binding domain and the coiled domain of Maskin.
FIG. 3.
Identification of maturation-specific Maskin phosphoamino acids. A. E. coli-expressed Maskin was phosphorylated in egg extracts, and the resulting phosphopeptides were identified arbitrarily. B. Several Maskin deletion proteins were added to egg extracts and processed for two-dimensional phosphopeptide mapping. The phoshopeptides that were lost when each deletion mutant was analyzed are noted. C. Maskin was phosphorylated in vitro with cdk1/cyclin B1 (MPF) and processed for two-dimensional phosphopeptide mapping. D. Maskin was phosphorylated in vitro with MAP kinase and processed for phosphopeptide mapping. E to I. Phosphorylation of Maskin containing an S343A, T58A, S638A, S152A, or S638A and S453A mutations in egg extracts. J. Phosphorylation of Maskin containing T58A, S152A, S311A, S343A, S453A, and S638A mutations (A6) in egg extracts. K. Representation of a Maskin phosphopeptide map showing the positions of peptides containing phosphorylated amino acids. Phosphopeptides 1 and 2 were not identified in these assays; phosphopeptide 2 was also detected in oocytes without progesterone stimulation (see Fig. 4). L. Positions of phosphorylated residues of Maskin relative to the eIF4E binding domain and the coiled-coil domain. TLC, thin-layer chromatography; TLE, thin-layer electrophoresis.
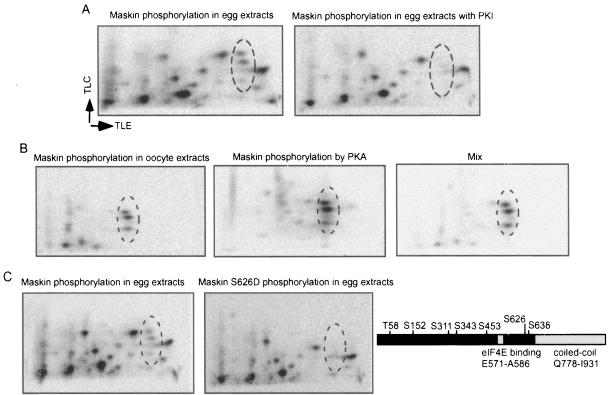
FIG. 4.
Maskin is phosphorylated by PKA in oocyte extracts. A. E. coli-expressed Maskin was phosphorylated in egg extracts in the presence or absence of PKI, a specific inhibitor of PKA, and then processed for two-dimensional phosphopeptide mapping. The circled areas show phosphopeptides that were eliminated by PKI. B. Maskin was phosphorylated in oocyte extracts or by pure PKA and processed as described previously. The phosphopeptides from these two reactions were also mixed and analyzed on a two-dimensional phosphopeptide map. C. Wild-type or S626D Maskin proteins were phosphorylated in egg extracts and analyzed as described above. The circled area shows phosphopeptides that were eliminated when the mutant Maskin was used. The right portion shows all the Maskin phosphoamino acids in relation to the eIF4E binding and coiled-coil domains. TLC, thin-layer chromatography; TLE, thin-layer electrophoresis.
We also note that differential trypsin digestion could yield multiple phosphopeptide spots that disappear with a single alanine substitution. For example, S638 is found in the following context: RES*PKK. Digestion with trypsin could give up to four peptides, since the E residue makes the first R a poor trypsin site, thus upstream K would sometimes be used. In addition, the tandem K residues could result in two possible carboxy end sites of the same phosphopeptide.
Protein kinase A (PKA) phosphorylates Maskin in oocyte extracts.
Oocytes contain a kinase(s) that phosphorylates Maskin prior to and after the synthesis of mos (Fig. 2). As before, we considered what kinases(s) might be active before mos synthesis in progesterone-treated oocytes and even in immature (unstimulated) oocytes. The most obvious possibility was PKA, not only because Maskin contains a motif that is potentially recognized by this enzyme (RKQS626L) but also because Maller and Krebs (21) showed that it was active in oocytes. While this result has been questioned (33), a number of other studies strongly indicate that PKA is an important maturation-controlling enzyme in oocytes (6, 22, 38). To investigate the possible involvement of PKA, egg extracts were supplemented with PKI, a specific inhibitory peptide of this enzyme. Extracts were then supplemented with E. coli-expressed Maskin, which was then examined by two-dimensional phosphopeptide mapping. Figure 4A shows that the three phosphopeptides (circled) that were observed in extracts from eggs as well as from mos antisense oligonucleotide-injected oocytes (Fig. 2B) were eliminated, suggesting that they were products of PKA activity. Next, Maskin was phosphorylated in extracts derived from untreated oocytes; the same three phosphopeptides were observed (Fig. 4B). They were also observed when Maskin was phosphorylated in vitro with commercial PKA, which was confirmed by a mixing experiment (Fig. 4B). Maskin contains a PKA consensus site at S626. When this residue was mutated and the protein again phosphorylated in extracts, none of the three phosphopeptides was detected (Fig. 4C). Therefore, we surmise that Maskin is a substrate for PKA in oocytes prior to and after maturation and that the three phosphopeptides contain a single phosphorylated amino acid (S626); they most probably arose through partial trypsin digestion.
Maskin phosphorylation regulates eIF4E binding and mRNA translation.
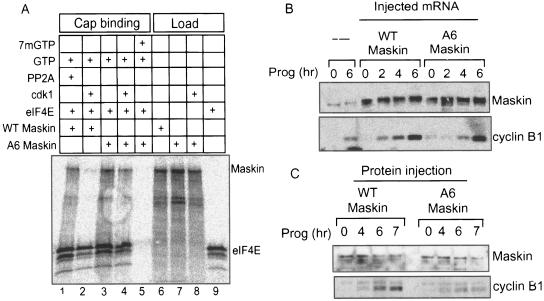
During oocyte maturation, PABP-bound eIF4G displaces Maskin from eIF4E, thereby promoting the translation of cyclin B1 mRNA. Because the cdk1 phosphorylations of Maskin residues T58, S156, S311, S343, S453, and S638 also coincide with this event, we thought that they might contribute to the dissociation of this protein from eIF4E. To assess this possibility, mRNAs encoding wild-type (WT) or mutant Maskin proteins where the six residues noted above were changed to alanine (henceforth referred to as Maskin A6) as well as eIF4E were translated in a reticulocyte lysate in the presence of [35S]methionine (note that while newly synthesized eIF4E contributes very little to the overall pool size of this protein in the lysate, the radiolabeling obviates the need for immunoblotting). The lysates were then supplemented with 0.2 mM GTP and in one case with 0.25 mM 7mGTP as well. Some of the lysates were further supplemented with the kinase cdk1/cyclin B1 (i.e., MPF) or phosphatase PP2A (to eliminate or reduce possible Maskin phosphorylation by reticulocyte kinases). The lysates were then applied to cap columns, and the material that was retained was analyzed by SDS-PAGE and phosphorimaging. The load fraction (1/20th the amount that was applied to the columns) shows that WT and A6 Maskin mRNAs were translated to the same extent and that the level of A6 Maskin was unaffected by cdk1 (Fig. 5A, lanes 6 to 8). While WT Maskin (plus PP2A) was retained on the cap column, WT Maskin that was phosphorylated by cdk1 was not; in both cases, however, eIF4E was retained on the column to the same extent (lanes 1 and 2). In contrast, A6 Maskin was retained on the column irrespective of whether cdk1 was added to the lysate (lanes 3 and 4); again in both cases, similar levels of eIF4E were retained. When free cap (7mGTP) was added to the lysate, neither Maskin nor eIF4E was retained on the cap column (lane 5). Thus, cdk1-mediated phosphorylation of Maskin controls its interaction with eIF4E.
FIG. 5.
Phosphorylation controls Maskin association with eIF4E and translation of CPE-containing mRNA. A. mRNAs encoding wild-type (WT) Maskin, A6 Maskin (T58A, S152A, S311A, S343A, S453A, S638A), and eIF4E were translated in reticulocyte lysates in the presence of [35S]methionine. The lysates also contained 0.2 mM GTP and in some cases 0.25 mM 7mGTP (i.e., free cap), cdk1/cyclin B1, or the phosphatase PP2A. One-twentieth of each lysate was analyzed for Maskin and eIF4E synthesis (load), while the rest was applied to cap columns. The material that was retained on the columns was analyzed by SDS-PAGE and autoradiography. B. mRNAs encoding WT or A6 Maskin proteins were injected into oocytes, which were then stimulated with progesterone (Prog) for various times. Protein from the oocytes was extracted, Western blotted, and probed for Maskin and cyclin B1. C. E. coli-expressed WT and A6 Maskin proteins were injected into oocytes, some of which were treated with progesterone. At the indicated times, the oocytes were harvested; the protein was extracted and probed on Western blots for Maskin and cyclin B1.
To determine whether the cdk1 phosphorylations of Maskin influence translation, mRNA encoding WT or A6 Maskin proteins was injected into oocytes, some of which were then incubated with progesterone. At several time points, the oocytes were harvested and the extracted protein was probed on Western blots for Maskin and cyclin B1. All the oocytes synthesized about the same amount of Maskin, which was ∼8-fold greater than endogenous Maskin. As expected, cyclin B1 levels accumulated in response to progesterone even when excess WT Maskin mRNA was injected (Fig. 5B). In contrast, the accumulation of cyclin B1 was somewhat slower when the A6 Maskin mRNA was injected. These results were confirmed in Fig. 5C, where E. coli-expressed WT and A6 Maskin proteins were injected. In a more dramatic fashion, the A6 Maskin protein prevented cyclin B1 accumulation compared to WT Maskin (overall [35S]methionine incorporation was unaffected, indicating that general cap-dependent translation is not regulated by Maskin; data not shown). We infer from these results that the A6 Maskin protein, which is not phosphorylated by cdk1 and thus not readily dissociated from eIF4E, acted as a dominant-negative mutant protein and inhibited cyclin B1 mRNA translation.
S626 phosphorylation controls Maskin association with the mitotic apparatus.
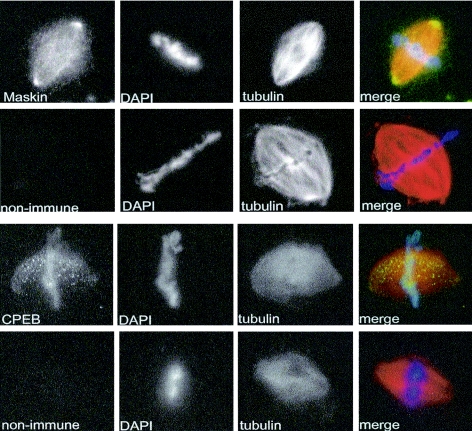
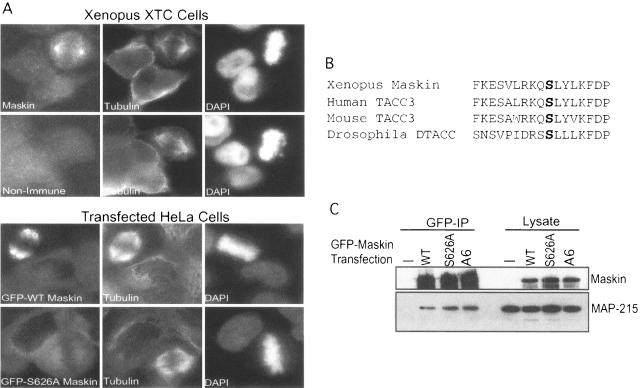
We could detect no change in cyclin B1 mRNA translation in oocytes injected with Maskin S626A (data not shown). To assess the possible function of this phosphoresidue, we considered another aspect of Maskin, which is its association with the mitotic apparatus (13). Maskin contains a coiled-coil domain that resembles those in the TACC (transforming acidic coiled-coil) family of proteins (7, 8, 9). Because these proteins are often associated with centrosomes and spindle microtubules and contain conserved putative PKA phosphorylation sites in the same region as Maskin (e.g., human TACC3, Drosophila melanogaster DTACC; see below), we thought that the S626 phosphorylation might mediate Maskin localization on the mitotic apparatus. To first confirm the data of Groisman et al. (13) that Maskin indeed is associated with spindles and centrosomes, rhodamine-labeled tubulin and demembranated sperm DNA were added to egg extracts; the resulting spindles were immunostained for Maskin as well as for CPEB and then collected on glass coverslips by centrifugation and visualized by fluorescence microscopy. Figure 6 demonstrates that both of these proteins were associated with the mitotic apparatus; Maskin was detected on spindles but was particularly concentrated at centrosomes. CPEB, on the other hand, was not particularly concentrated at centrosomes but appeared as puncta on the spindles. We next added E. coli-expressed Maskin (WT and S626A mutant) to egg extracts together with rhodamine-tubulin to assess whether the two proteins would be differentially localized to the mitotic apparatus. Unfortunately, neither protein was detected on spindles or centrosomes, perhaps denoting a foible of the extract (data not shown). We therefore turned to living cells to examine Maskin localization. In XTC cells, a Xenopus somatic cell line, Maskin again was detected strongly at centrosomes (Fig. 7A). To investigate the function of the S626 phosphorylation, we generated GFP-Maskin fusion proteins to distinguish between endogenous and exogenous Maskin. However, because these cells were transfected with a very low efficiency, we could not use them to test the function of S626 phosphorylation. Consequently, HeLa cells, which contain no protein that reacts with our anti-Xenopus Maskin antibody, were efficiently transfected with constructs encoding GFP-Maskin fusion proteins, in one case containing an S626A alteration. While WT Maskin strongly localized to the mitotic apparatus, the GFP-S626A Maskin remained dispersed throughout the cytoplasm (Fig. 7A). Conversely, cdk1 phosphorylation of Maskin appears to have no effect on localization as GFP-Maskin A6 was detected on spindles and centrosomes (data not shown).
FIG. 6.
Maskin and CPEB are associated with mitotic spindles and centrosomes. Xenopus egg extracts were supplemented with rhodamine-labeled tubulin and demembranated frog sperm DNA. Following incubation, the spindles were fixed and centrifuged onto glass coverslips, where they were immunostained for Maskin or CPEB and were costained with DAPI to detect DNA.
FIG. 7.
Serine 626 directs Maskin to the mitotic apparatus. A. Xenopus tissue culture cells (XTC) were immunostained for Maskin and tubulin and costained with DAPI. HeLa cells were also transfected with plasmids encoding GFP fused to wild-type or S626A mutant Maskin molecules. The cells were immunostained for tubulin and costained with DAPI. B. Limited sequence alignment among Xenopus Maskin, human and mouse TACC3, and Drosophila DTACC denoting a conserved serine corresponding to Maskin serine 626. C. HeLa cells were transfected with the same constructs, as well as GFP-Maskin A6, as noted for panel A (bottom). Antibody directed against GFP was use to coimmunoprecipitate MAP-215, a protein that links TACC family proteins to microtubules; this protein and the Maskin fusion proteins were detected by Western blotting.
TACC family member proteins are linked to microtubules through an association with MAP-215 (7, 8, 9, 28). Because Maskin has several structural similarities to vertebrate and invertebrate TACC proteins (34), including a conserved serine corresponding to S626 (Fig. 7B), we thought that the phosphorylation of this residue might mediate an interaction between Maskin and MAP-215. To test this possibility, HeLa cells transfected with WT, S626A, or A6 Maskin proteins fused to GFP were subjected to immunoprecipitation with GFP antibody and immunoblotted for Maskin and MAP-215 (Fig. 7C). Nearly equal amounts of MAP-215 were coimmunoprecipitated with GFP antibody irrespective of whether the Maskin moiety was WT or contained amino acid substitutions. Thus, Maskin S626 phosphorylation does not affect binding to MAP-215.
DISCUSSION
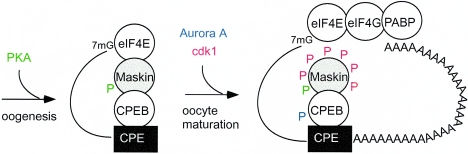
This study demonstrates that Maskin undergoes a complex series of phosphorylation events both prior to and during oocyte maturation. In immature oocytes, Maskin is phosphorylated on S626 by protein kinase A; while this modification has no clear function in oocytes, it is necessary to localize Maskin on centrosomes and spindles, presumably during embryogenesis and in adult somatic cells. As oocytes mature, Maskin is phosphorylated on several sites that generate nearly 10 phosphopeptides; one or more of the six major sites, and likely targets of cdk1 (T58, S152, S311, S343, S453, S638), help Maskin dissociate from eIF4E, which results in translational activation of the CPE-containing cyclin B1 mRNA (Fig. 8).
FIG. 8.
Phosphorylation of Maskin. During oogenesis, when oocytes are arrested at the diplotene stage of prophase I, PKA phosphorylates (P) Maskin residue S626. When the oocytes are stimulated by progesterone to reenter the meiotic divisions (oocyte maturation), Aurora A is activated (23, 32) and phosphorylates CPEB S174; this event stimulates polyadenylation (1). The newly elongated poly(A) tail is bound by poly(A) binding protein (PABP), which in turn binds eIF4G and helps it displace Maskin from eIF4E, thereby initiating translation (2). cdk1, whose activity is detected after that of Aurora A (23), phosphorylates Maskin residues T58, S152, S311, S343, S453, and S638. These phosphorylations help Maskin dissociate from eIF4E. The S626 phosphorylation regulates the association of Maskin with the mitotic apparatus during embryogenesis and in adult somatic cells (13). Green, red, and blue P’s indicate phosphorylation catalyzed by PKA, cdk1, and Aurora A, respectively.
In maturing oocytes, Aurora A-catalyzed phosphorylation of CPEB stimulates polyadenylation (1, 23). Not only does the inhibition of polyadenylation prevent translation of CPE-containing mRNAs in response to progesterone stimulation, the injection of free poly(A) into oocytes does so as well (2). Poly(A) very likely inhibited translation by titrating an essential factor, which was confirmed by experiments showing that injected PABP restored robust translation to the PABP-injected oocytes. Because the effect of PABP on translation is thought to occur through an interaction with eIF4G (35-37), Cao and Richter (2) determined that PABP-eIF4G interactions were important for the dissociation of Maskin from eIF4E. These investigators injected a peptide derived from eIF4G that disrupted the eIF4G-PABP interaction; this peptide prevented both Maskin dissociation from eIF4E and translation of CPE-containing RNA (i.e., cyclin B1 RNA). Thus, it appeared that PABP-bound eIF4G competed with Maskin for binding eIF4E; when this occurred, translation ensued (30). Here we show that cdk1-catalyzed Maskin phosphorylation is also important for dissociating this protein from eIF4E. The Maskin association with eIF4E is relatively weak, at least as judged by yeast two-hybrid analysis (34). However, Maskin resides in a multifactor RNP complex, which could help stabilize its association with eIF4E. Perhaps both phosphorylation and PABP help ensure that eIF4G displaces Maskin from and binds itself to eIF4E.
The phosphorylation control of Maskin binding to eIF4E resembles that of 4E-BP1. This protein undergoes a complex series of major and minor phosphorylation events, the most important of which appear to occur on T37, T46, S65, T70, S83, and S112. At least in 293 cells, a hierarchical phosphorylation is necessary to abrogate eIF4E binding. T37 and T46 are the first to be modified and prime subsequent phosphorylations on T70 and S65. However, phosphorylation of T70 or S65 is insufficient to prevent interaction with eIF4E (10). Using different experimental approaches, Karim et al. (19) and Mothe-Satnay et al. (25) found that phosphorylation of S65 is sufficient to strongly inhibit 4E-BP-eIF4E binding. While there is no clear consensus of the relative importance of individual phosphorylations, there is general agreement that mTOR is the kinase responsible for these modifications (15). Nonetheless, it should be borne in mind that the kinase ATM, the product of the ataxia telangiectasia gene, has been shown to phosphorylate 4E-BP S111, which causes the protein to at least partially dissociate from eIF4E (40).
Another Maskin-like molecule is Cup, a protein in Drosophila that interacts with eIF4E as well as the sequence-specific RNA binding proteins Smaug and Bruno (26, 27, 39, 41); such configurations of factors resemble the CPEB-Maskin-eIF4E complex. To date, Cup has been shown only to be a negative regulator of translation; there is no evidence that it can dissociate from eIF4E and allow translation to proceed. On the other hand, keeping mRNAs repressed in the cytoplasm without the possibility of being translated is energetically wasteful, and thus we speculate that some Cup mRNA substrates might be translated at some point during development. If true, then cytoplasmic polyadenylation (31) as well as Cup phosphorylation may be responsible.
In Xenopus embryos, CPEB and Maskin as well the CPE-containing mRNAs encoding Xbub3 and cyclin B1 are associated with spindles and centrosomes (13). At least in the case of cyclin B1 mRNA, translation on or in the vicinity of the mitotic apparatus is important for cell division. That is, CPEB appears to anchor the polyadenylation and translation machinery to spindles and centrosomes through a small domain that interacts with microtubules. When this domain is deleted, CPEB can still bind RNA and regulate translation (presumably with Maskin), it just does not do so on spindles and centrosomes. The result of this “unlocalized” translation is inhibited cell division (13). These results led us to consider whether the S626 phosphorylation, which had no detectable function in oocytes, might be important for tethering Maskin to the mitotic apparatus. To assess this, we first had to confirm our earlier observations that Maskin and CPEB were indeed associated with spindles and centrosomes. In egg extracts, Maskin and CPEB were clearly found on the mitotic apparatus (Fig. 6); however, exogenous Maskin (WT or mutant) did not similarly associate with spindles and thus the extracts were not suitable to test the function of S626 phosphorylation. Xenopus XTC cells contain Maskin that was localized to spindles and centrosomes, but the very low transfection efficiency of these cells again precluded further analysis. Consequently, we turned to HeLa cells, which while having no detectable Maskin, efficiently localized WT Maskin-GFP to spindles and centrosomes. However, Maskin-GFP containing an S626A substitution was not similarly localized but instead was distributed throughout the cytoplasm. We also tested the localization of an S626D Maskin, which like the S626A Maskin was not localized (data not shown). Of course, negatively charged amino acids do not always mimic phosphoresidues, which is the case presented here. Nonetheless, the importance of S626 phosphorylation for localization may be conserved in other proteins that share structural similarities to Maskin. Human and mouse TACC3 and Drosophila DTACC, while not containing eIF4E binding domains, have similar coiled-coil domains and are found on centrosomes (8). These proteins contain conserved serine residues in motifs that are strikingly similar to that of Maskin (Fig. 7B), and we speculate that phosphorylation at these positions modifies the localization of these proteins.
The mechanism by which S626 phosphorylation controls Maskin localization is unknown; MAP-215, a microtubule binding protein, bridges TACC proteins to centrosomes (20). However, S626A Maskin binds MAP-215 as avidly as WT Maskin, consistent with the recent finding that the C-terminal TACC domain of Maskin (amino acids 714 to 931) is sufficient for interaction with XMAP215 (28). Interestingly, Aurora A, which phosphorylates CPEB, is also involved in DTACC localization to centrosomes (9). However, we have no evidence that Aurora A phosphorylates Maskin S626; indeed, PKI, a potent and specific inhibitor of PKA, completely abolished Maskin S626 phosphorylation in oocytes (Fig. 4), strongly indicating that PKA is the in vivo kinase that phosphorylates this residue. In contrast, Pascreau et al. (29) have shown that Aurora A can phosphorylate a Maskin peptide containing S626; these investigators also suggest that this modification influences translation. In our hands, however, Aurora A does not phosphorylate S626 in the context of the full Maskin protein; we also can observe no effect of phospho-S626 on mRNA translation. Although we find that purified Aurora A does phosphorylate recombinant Maskin in vitro, the peptide map of the phosphorylated Maskin does not resemble the in vivo phosphopeptide map (data not shown). Indeed, Pascreau et al. (29) did not test directly the effect of phospho-626A on translation; they injected a peptide corresponding to this region that presumably bound endogenous Aurora A. We suspect that this peptide could have interacted with any number of kinases or other proteins and yielded a nonspecific effect on oocyte maturation. Moreover, we show clearly that S626 is phosphorylated in immature oocytes (Fig. 4) when Aurora A is not active (23).
In agreement with the results reported here, recent evidence confirms that Maskin is present on spindles and, moreover, helps mediate spindle assembly in Xenopus egg extracts (28). These observations, together with those reported here that Maskin and CPEB are associated with spindles and centrosomes formed in egg extracts suggests that other components of the polyadenylation machinery, including mRNA, could be identified though a bulk isolation of spindles. This approach is under way, and it may lead us to understanding how S626 phosphorylation controls Maskin localization on the mitotic apparatus.
Acknowledgments
We thank L. Cassimeris for the XMAP-215 antibody, J. Maller for the cyclin B1 antibody, Y.-S. Huang for GFP antibody, R. Mendez for suggestions on phosphorylation mapping, and T. Hu for instruction on spindle formation in egg extracts.
D.B. was supported by an NRSA postdoctoral fellowship (F32-GM64872). This work was supported by grants from the NIH (GM46779 and HD37267) and the G. Harold and Leila Y. Mathers Charitable Foundation. Additional support from the Diabetes Endocrinology Research Center Program Project (DK32520) is also gratefully acknowledged.
REFERENCES
- 1.Barnard, D. C., K. Ryan, J. L. Manley, and J. D. Richter. 2004. Symplekin and xGLD-2 are required for CPEB-mediated cytoplasmic polyadenylation. Cell 119:641-651. [DOI] [PubMed] [Google Scholar]
- 2.Cao, Q., and J. D. Richter. 2002. Dissolution of the Maskin-eIF4E complex by cytoplasmic polyadenylation and poly(A)-binding protein controls cyclin B1 mRNA translation and oocyte maturation. EMBO J. 21:3852-3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.DeMoor, C. H., and J. D. Richter. 1997. The Mos pathway regulates cytoplasmic polyadenylation in Xenopus oocytes. Mol. Cell. Biol. 17:6419-6426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Desai, A., A. Murray, T. J. Mitchison, and C. E. Walczak. 1999. The use of Xenopus egg extracts to study mitotic spindle assembly and function in vitro. Methods Cell Biol. 61:385-412. [DOI] [PubMed] [Google Scholar]
- 5.Dever, T. E. 2002. Gene-specific regulation by general translation factors. Cell 108:545-556. [DOI] [PubMed] [Google Scholar]
- 6.Duckworth, B. C., J. S. Weaver, and J. V. Ruderman. 2002. G2 arrest in Xenopus oocytes depends on phosphorylation of cdc25 by protein kinase A. Proc. Natl. Acad. Sci. USA 99:16794-16799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gergely, F., C. Karlsson, I. Still, J. Cowell, J. Kilmartin, and J. W. Raff. 2000. The TACC domain identifies a family of centrosomal proteins that can interact with microtubules. Proc. Natl. Acad. Sci. USA 97:14352-14357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gergely, F., D. Kidd, K. Jeffers, J. Wakefield, and J. W. Raff. 2000. D-TACC: a novel centrosomal protein required for normal spindle function in the early Drosophila embryo. EMBO J. 19:241-252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Giet, R., D. McLean, S. Descamps, M. Lee, J. W Raff, C. Prigent, and D. M. Glover. 2002. Drosophila Aurora A kinase is required to localize D-TACC to centrosomes and to regulate astral microtubules. J. Cell Biol. 156:437-451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gingras, A. C., B. Raught, S. P. Gygi, A. Niedzwiecka, M. Miron, S. K. Burley, P. D. Polakiewicz, A. Wyslouch-Cieszynska, R. Aebersold, and N. Sonenberg. 2001. Hierarchical phosphorylation of the translation inhibitor 4E-BP1. Genes Dev. 15:2852-2864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gingras, A. C., B. Raught, and N. Sonenberg. 1999. eIF4 initiation factors: effectors of mRNA recruitment to ribosomes and regulators of translation. Annu. Rev. Biochem. 68:913-963. [DOI] [PubMed] [Google Scholar]
- 12.Gingras, A. C., B. Raught, and N. Sonenberg. 2001. Regulation of translation initiation by FRAP/mTOR. Genes Dev. 15:807-826. [DOI] [PubMed] [Google Scholar]
- 13.Groisman, I., Y.-S. Huang, R. Mendez, Q. Cao, W. Theurkauf, and J. D. Richter. 2000. CPEB, maskin, and cyclin B1 mRNA at the mitotic apparatus: implications for local translational control of cell division. Cell 103:435-447. [DOI] [PubMed] [Google Scholar]
- 14.Groisman, I., M.-Y. Jung, M. Sarkissian, Q. Cao, and J. D. Richter. 2002. Translational control of the embryonic cell cycle. Cell 109:473-483. [DOI] [PubMed] [Google Scholar]
- 15.Hay, N., and N. Sonenberg. 2004. Upstream and downstream of mTOR. Genes Dev. 18:1926-1945. [DOI] [PubMed] [Google Scholar]
- 16.Hentze, M. W. 1997. eIF4G: a multipurpose ribosome adapter? Science 275:500-501. [DOI] [PubMed] [Google Scholar]
- 17.Hershey, J. W. B., and W. C. Merrick. 2000. Pathway and mechanism of initiation of protein synthesis, p. 33-88. In J. W. Sonenberg, B. Hershey, and M. Mathews (ed.), Translational control of gene expression. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 18.Huang, Y.-S., J. H. Carson, E. Barbarese, and J. D. Richter. 2003. Facilitation of dendritic mRNA transport by CPEB. Genes Dev. 17:638-653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karim, M. M., J. M. Hughes, J. Warwicker, G. C. Scheper, C. G. Proud, and J. E. G. McCarthy. 2001. A quantitative molecular model for modulation of mammalian translation by the eIF4E-binding protein 1. J. Biol. Chem. 276:20750-20755. [DOI] [PubMed] [Google Scholar]
- 20.Lee, M. J., F. Gergely, K. Jeffers, S. Y. Peak-Chew, and J. W. Raff. 2001. Msps/XMAP215 interacts with the centrosomal protein D-TACC to regulate microtubule behaviour. Nat. Cell Biol. 3:643-649. [DOI] [PubMed] [Google Scholar]
- 21.Maller, J. L., and E. Krebs. 1977. Progesterone-stimulated meiotic cell division in Xenopus oocytes. Induction by regulatory subunit and inhibition by catalytic subunit of adenosine 3′:5′-monophosphate-dependent protein kinase. J. Biol. Chem. 52:1712-1718. [PubMed] [Google Scholar]
- 22.Matten, W., I. Daar, and G. F. Vande Woude. 1994. Protein kinase A acts at multiple points to inhibit Xenopus oocyte maturation. Mol. Cell. Biol. 14:4419-4426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mendez, R., L. E. Hake, T. Andresson, L. Littlepage, J. V. Ruderman, and J. D. Richter. 2000. Phosphorylation of CPE binding factor by Eg2 regulates translation of c-mos mRNA. Nature 404:302-307. [DOI] [PubMed] [Google Scholar]
- 24.Mendez, R., and J. D. Richter. 2001. Translational control by CPEB: a means to the end. Nat. Rev. Mol. Cell Biol. 2:521-529. [DOI] [PubMed] [Google Scholar]
- 25.Mothe-Satney, I., D. Yang, P. Fadden, T. A. Haystead, and J. C. Lawrence, Jr. 2000. Multiple mechanisms control phosphorylation of PHAS-I in five (S/T)P sites that govern translational repression. Mol. Cell. Biol. 20:3558-3567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakamura, A., K. Sato, and K. Hanyu-Nakamura. 2004. Drosophila cup is an eIF4E binding protein that associates with Bruno and regulates oskar mRNA translation in oogenesis. Dev. Cell 6:69-78. [DOI] [PubMed] [Google Scholar]
- 27.Nelson, M. R., A. M. Leidal, and C. A. Smibert. 2004. Drosophila Cup is an eIF4E-binding protein that functions in Smaug-mediated translational repression. EMBO J. 23:150-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O'Brien, L. L., A. J. Albee, L. Liu, W. Tai, P. Dobrzyn, A. B. Lizarraga, and C. Wiese. 2005. The Xenopus TACC homologue, Maskin, functions in spindle assembly. Mol. Biol. Cell 16:2836-2847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pascreau, G., J. G. Delcros, J. Y. Cremet, C. Pregent, and Y. Arlot-Bonnemains. 2005. Phosphorylation of maskin by aurora-A participates to the control of sequential protein synthesis during Xenopus laevis oocyte maturation. J. Biol. Chem. 280:13415-13423. [DOI] [PubMed] [Google Scholar]
- 30.Richter, J. D., and N. Sonenberg. 2005. Regulation of cap-dependent translation by eIF4E inhibitory proteins. Nature 433:477-480. [DOI] [PubMed] [Google Scholar]
- 31.Salles, F. J., M. E. Lieberfarb, C. Wreden, J. P. Gergen, and S. Strickland. 1994. Coordinate initiation of Drosophila development by regulated polyadenylation of maternal messenger RNAs. Science 266:1996-1999. [DOI] [PubMed] [Google Scholar]
- 32.Sarkissian, M., R. Mendez, and J. D. Richter. 2004. Progesterone and insulin stimulation of CPEB-dependent polyadenylation is regulated by Aurora A and glycogen synthase kinase-3. Genes Dev. 18:48-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schmitt, A., and A. R. Nebreda. 2002. Inhibition of Xenopus oocyte meiotic maturation by catalytically inactive protein kinase A. Proc. Natl. Acad. Sci. USA 99:4361-4366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stebbins-Boaz, B., Q. Cao, C. H. de Moor, R. Mendez, and J. D. Richter. 1999. Maskin is a CPEB-associated factor that transiently interacts with elF-4E. Mol. Cell 4:1017-1027. [DOI] [PubMed] [Google Scholar]
- 35.Tarun, S. Z., and A. B. Sachs. 1996. Association of the yeast poly(A) tail binding protein with translation initiation factor eIF-4G. EMBO J. 15:7168-7177. [PMC free article] [PubMed] [Google Scholar]
- 36.Tarun, S. Z., S. E. Wells, J. A. Deardorff, and A. B. Sachs. 1997. Translation initiation factor eIF4G mediates in vitro poly(A) tail-dependent translation. Proc. Natl. Acad. Sci. USA 94:9046-9051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wakiyama, M., H. Imataka, and N. Sonenberg. 2000. Interaction of eIF4G with poly(A)-binding protein stimulates translation and is critical for Xenopus oocyte maturation. Curr. Biol. 10:1147-1150. [DOI] [PubMed] [Google Scholar]
- 38.Wang, J., and X. J. Liu. 2004. Progesterone inhibits protein kinase A (PKA) in Xenopus oocytes: demonstration of endogenous PKA activities using an expressed substrate. J. Cell Sci. 117:5107-5116. [DOI] [PubMed] [Google Scholar]
- 39.Wilhelm, J. E., M. Hilton, Q. Amos, and W. J. Henzel. 2003. Cup is an eIF4E binding protein required for both the translational repression of oskar and the recruitment of Barentsz. J. Cell Biol. 163:1197-1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang, D. A., and M. B. Kastan. 2000. Participation of ATM in insulin signaling through phosphorylation of eIF4E-binding protein 1. Nat. Cell Biol. 2:893-898. [DOI] [PubMed] [Google Scholar]
- 41.Zappavigna, V., F. Piccioni, J. C. Villaescusa, and A. C. Verrotti. 2004. Cup is a nucleocytoplasmic shuttling protein that interacts with the eukaryotic translation initiation factor 4E to modulate Drosophila ovary development. Proc. Natl. Acad. Sci. USA 101:14800-14805. [DOI] [PMC free article] [PubMed] [Google Scholar]