Abstract
MicroRNAs (miRNAs) are a class of small RNAs that silence gene expression. In animal cells, miRNAs bind to the 3′ untranslated regions of specific mRNAs and inhibit their translation. Although some targets of a handful of miRNAs are known, the number and identities of mRNA targets in the genome are uncertain, as are the developmental functions of miRNA regulation. To identify the global range of miRNA-regulated genes during oocyte maturation of Drosophila, we compared the proteome from wild-type oocytes with the proteome from oocytes lacking the dicer-1 gene, which is essential for biogenesis of miRNAs. Most identified proteins appeared to be subject to translation inhibition. Their transcripts contained putative binding sites in the 3′ untranslated region for a subset of miRNAs, based on computer modeling. The fraction of genes subject to direct and indirect repression by miRNAs during oocyte maturation appears to be small (4%), and the genes tend to share a common functional relationship in protein biogenesis and turnover. The preponderance of genes that control global protein abundance suggests this process is under tight control by miRNAs at the onset of fertilization.
Keywords: translation control
Small RNAs, including microRNAs (miRNAs) and short interfering RNAs, are components of a RNA-based mechanism of gene silencing (1, 2). The miRNA branch of RNA-based gene regulation is found in plants and animals (3, 4). miRNAs have a specific size of ≈22 nt, and they are processed from hairpin-loop RNA precursors by endoribonucleases of the Dicer class. These precursors are transcribed from genes within plant and animal genomes, with the number of miRNA genes found in different species roughly corresponding to 0.5–1% of the total number of genes in their genomes (5). Most miRNA genes are conserved between related species, and ≈30% of miRNA genes are highly conserved, with orthologs found in vertebrate and invertebrate genomes. This suggests that a significant fraction of miRNAs have evolutionarily conserved biological functions.
miRNAs specifically repress gene expression by negatively regulating complementary mRNAs. Plant miRNAs generally cause degradation of complementary mRNAs by near-perfect basepairing (5). Conversely, most characterized miRNAs from animals repress gene expression by blocking the translation of complementary mRNAs into protein. They interact with their targets by imperfectly basepairing to mRNA sequences within the 3′ UTR. The exact mechanism of translation inhibition is unknown, although miRNAs have been found not to interfere with translation initiation (5).
A question of great interest concerns the functions of miRNAs in animals. Although the functions of only a few miRNAs are known, available evidence suggests that miRNAs play diverse and important roles in development. This conclusion is based on the mutant phenotypes of individual miRNA genes and on the identification of genes that are direct targets of miRNA regulation. In Caenorhabditis elegans, the genes lin-14, lin-28, lin-41, and hbl-1 are translationally repressed by interaction with lin-4 or let-7 miRNAs (6–10). Neuronal development is regulated by sequential action of lsy-6 and miR-273 on effectors of neuron differentiation (11). In Drosophila, translation of the apoptosis effector gene hid is down-regulated by the bantam miRNA (12). A different approach to finding gene targets of miRNAs has been to scan sequence databases for conserved sequences within 3′ UTRs that are favored to interact with miRNAs (13–18). Several predicted targets have been experimentally validated, suggesting that these computational methods can predict genome-wide targets. However, it is unclear how well these theoretical analyses identify genes that are directly regulated by miRNAs.
In this study, we have undertaken a proteomic screen for genes that are regulated by miRNAs within late-stage Drosophila oocytes. We find a small percentage (≈4%) of expressed genes are derepressed when miRNA function is lost. Identification of those genes by MS indicates that many of these genes function in protein biogenesis and turnover. Thus, miRNAs selectively regulate related functional groups of genes at this stage of development.
Materials and Methods
Genetics. To generate null dicer-1 (dcr-1) oocytes, FRT82B dcr-1Q1147X/TM6B females were crossed with hsFLP122;FRT82B ovoD1/TM6B males, and progeny were heat-shocked twice at 37°C for 2 h during early larval stages, as described (19). F1 females were induced to lay mature oocytes that were deposited onto egg-laying plates. Wild-type oocytes were collected from hsFLP122;FRT82B/FRT82B ovoD1 females treated in the same manner as above. Oocytes were collected, pooled in batches of 200, and frozen as described (20).
Difference Gel Electrophoresis (DIGE) and MS. Seventy to 100 μg of protein homogenate from a particular genotype was conjugated with either Cy3 or Cy5 dye, mixed with equal protein from the other genotype that had been coupled with the opposite dye, and the mixture subjected to DIGE analysis, as described (20). Positive protein spots were excised from five gels, and proteins were extracted, digested with trypsin, and analyzed by a PerSpective Biosystems Voyager STR MALDI-TOF, as described (20). Data were analyzed by using mascot (21). Details of DIGE analysis and imaging are in Supporting Text, which is published as supporting information on the PNAS web site.
RT-PCR. RNA was isolated from dcr-1Q1147X and wild-type oocytes, and cDNA was generated as described (22). The primers used to amplify ribosomal protein (rp)49 cDNA were described (23), and primers positioned in the 3′ UTR of each positive target gene were used to amplify their cDNAs (see Supporting Primer Data Set, which is published as supporting information on the PNAS web site, for primer sequences). Each reaction was performed as described (23). Signals were normalized to the control rp49 levels in each sample.
3′ UTR Sequence Analysis. The annotated 3′ UTR sequence for each gene was collated as described (14). Details of algorithms, sequences, and statistical analysis are given in Supporting Text.
Results
We performed comparative proteomics to identify genes that are regulated by miRNAs. We assumed that the protein products of such genes would be more abundant in the absence of mature miRNAs. To identify this population of proteins, we used DIGE (20, 24) and compared the proteome of normal individuals with the proteome of those depleted of miRNAs. It was not possible to genetically ablate all miRNAs from the genome, given their number. Therefore, we examined the proteome of dcr-1 null mutants. The dcr-1 gene encodes for the Dicer enzyme essential for miRNA biogenesis in Drosophila (22). A dcr-1 null mutant is blocked for miRNA processing, leading to an absence of mature miRNAs. The mutant, however, is not defective for short interfering RNA processing, because another Dicer gene carries out this role (22).
An important issue in proteomic analysis of any mutant is the possible impact of the mutation on cell composition at any given stage of development. For example, mutant dcr-1 oocytes are ventralized (Fig. 1A), which make it likely that resulting embryos develop with a greater proportion of ventral cell types such as neurons and muscle. The proteome profile of such embryos would reflect this altered cell composition in addition to proteins that might be autonomously controlled by miRNAs. For this reason, we chose to perform our analysis on mature oocytes. These constitute a homogeneous cell population, which reduces the likelihood that any proteome differences are due to heterogeneity in cell composition (25). Moreover, dcr-1 mutant oocytes could be fertilized by wild-type sperm and undergo embryonic development, demonstrating that mutant oocytes reached this developmental stage (data not shown).
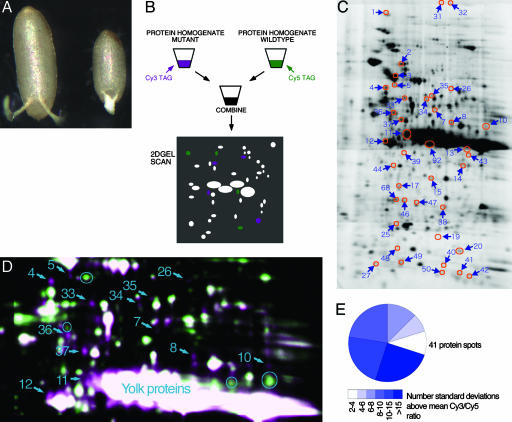
Fig. 1.
DIGE analysis of dcr-1 eggs. (A) Dorsal view of mature wild-type (Left) and dcr-1 mutant oocytes (Right). Anterior is down. The mutant oocyte is reduced in size and has a partial fusion of dorsal appendages, indicating a ventralized phenotype. (B) Schematic of DIGE. In this example, proteins from dcr-1 mutants are coupled to Cy3 and wild-type are coupled to Cy5. Cy3 fluorescence is imaged purple and Cy5 is imaged green. Protein spots that fluoresce equally with both dyes appear white. Spots that fluoresce more intensely from one dye than the other appear either purple or green. These represent difference proteins. (C) A 2D gel scanned for Cy5-tagged proteins that had been isolated from dcr-1 mutants. Circled are the 41 protein spots, referred to as dcr-1-enriched proteins, which are reproducibly more fluorescent for proteins from the mutant than wild-type. Numbers refer to spot identities as listed in Table 1. (D) Magnified view of a portion of a scanned 2D gel showing protein spots containing dcr-1 mutant proteins tagged with Cy3 (purple) and wild-type proteins tagged with Cy5 (green). Spots that exhibit greater Cy3 fluorescence appear purple, and those spots that reproducibly exhibited greater Cy3 fluorescence are numbered. Some of the spots that appear purple in this gel were not reproducible and so are not numbered. Numbers refer to spot identities as listed in Table 1. (E) Ratio of fluorescence from dcr-1 to wild-type spot samples normalized to the background variation. The ratio is expressed as the number of standard deviations above the background mean.
To compare proteomes of wild-type and dcr-1 individuals, we covalently labeled protein homogenates with Cy3 or Cy5 dyes that did not significantly alter protein charge or mass (Fig. 1B). The Cy3- and Cy5-labeled homogenates were combined and electrophoresed on the same 2D gel. Fluorescence imaging of the gel with Cy3 and Cy5 excitation light generated images of the two protein samples in perfect register. Proteins common to both samples appeared as spots that fluoresced with both dyes. Proteins that were more abundant in one sample appeared as spots that fluoresced more intensely with one dye than the other. DIGE can detect protein differences as low as ±1.2-fold (24). Master DIGE gels revealed a total of 94 difference proteins of 1,003 detected proteins with changed abundance between wild-type and dcr-1 samples. Dye reversal of protein homogenates revealed the same spectrum of difference proteins. Two difference proteins (spots 51a and -b) located close to each other changed reciprocally (Fig. 3, which is published as supporting information on the PNAS web site), and MS analysis confirmed these were related isoforms of the same gene product encoded by CG1516. Of the remaining 92 difference proteins, 51 were more abundant in wild-type samples (Fig. 3), and 41 difference proteins were more abundant in dcr-1 mutant samples (Fig. 1 C and D). We concentrated further analysis on the latter category, the dcr-1-enriched proteins, because these difference proteins were repressed in the presence of Dcr-1.
We quantified protein abundance by digital imaging of the Cy3 and Cy5 fluorescence of each relevant spot, using a charge-coupled device camera that detects linear light intensity over 4 orders of magnitude (20). The fluorescence ratios of the dcr-1-enriched proteins were compared with the normalized ratio, and all were found to be >2 SD (±50%) different from the normalized factor (Fig. 1E). Thus, the difference proteins exhibit significantly greater abundance in dcr-1 than wild-type samples (P < 0.05). The ratios of dcr-1 vs. wild-type fluorescence were expressed as a fold difference and are listed for each difference protein (Table 1). The fold difference ranged from 1.6- to 69-fold; the average was 3.5-fold. The relative abundance of difference proteins varied over 3 orders of magnitude, as determined by the fluorescence intensity of wild-type proteins (Table 1).
Table 1. dcr-1-enriched proteins detected in Drosophila oocytes by DIGE.
| Protein spot | CG identifier | Fluorescence ratio* (dcr-1/wild type) | Relative abundance† | mascot score‡ |
|---|---|---|---|---|
| 1 | CG1782 | 3.6 | 2.6E-2 | 194 |
| 2 | CG5355 | 2.6 | 6.7E-2 | 209 |
| 3 | CG4264 | 3.7 | 1.9E-1 | 244 |
| 4 | CG12101 | 14.0 | 1.3E-2 | 82 |
| 5 | CG10602 | 4.2 | 1.2E-2 | 67 |
| 7 | CG5384 | 2.8 | 1.0E-2 | 91 |
| 8 | CG17654 | 34.0 | 1.3E-3 | 82 |
| 10 | CG4916 | 7.0 | 1.0E-2 | 63 |
| 11 | CG2985 | 2.7 | 1.0 | 100 |
| 12 | CG4027 | 4.3 | 1.4E-1 | 124 |
| 13 | — | 3.3 | 9.5E-2 | |
| 14 | CG6084 | 5.6 | 3.8E-3 | 116 |
| 15 | CG4904 | 3.2 | 2.6E-2 | 153 |
| 17 | — | 3.5 | 1.5E-2 | |
| 19 | — | 3.3 | 9.0E-3 | |
| 20 | — | 2.0 | 4.0E-1 | |
| 25 | CG4463 | 1.7 | 2.3E-2 | 100 |
| 26 | — | 3.5 | 3.2E-3 | |
| 27 | — | 4.0 | 1.1E-2 | |
| 31 | — | 2.5 | 3.0E-3 | |
| 32 | CG7052 | 3.2 | 5.0E-3 | 69 |
| 33 | CG7033 | 1.6 | 6.5E-2 | 160 |
| 34 | — | 2.3 | 2.5E-2 | |
| 35 | — | 2.4 | 1.2E-2 | |
| 36 | CG4535 | 4.0 | 3.7E-2 | 90 |
| 37 | CG4422 | 3.3 | 4.1E-2 | 139 |
| 38 | — | 2.0 | 5.7E-2 | |
| 39 | — | 5.4 | 1.6E-3 | |
| 40 | — | 6.0 | 1.6E-2 | |
| 41 | — | 2.9 | 3.5E-2 | |
| 42 | — | 3.2 | 2.1E-2 | |
| 43 | CG3416 | 10.1 | 1.3E-3 | 104 |
| 44 | CG4634 | 69.0 | 5.0E-4 | 62 |
| 46 | — | 4.0 | 2.2E-2 | |
| 47 | — | 3.2 | 1.9E-2 | |
| 48 | — | 3.4 | 1.7E-2 | |
| 49 | — | 3.8 | 2.2E-2 | |
| 50 | CG17820 | 52.0 | 1.0E-3 | 83 |
| 68 | CG14792 | 3.8 | 4.8E-2 | 83 |
| 92 | CG2979 | 2.9 | 6.7E-1 | 201 |
| 93 | — | 2.2 | 3.1E-1 | |
| M = 3.5§ | M = 2.2E-2§ |
Sum of pixel values in spot from one channel over sum from other channel, corrected for background.
Sum of pixel values in spot from wild-type sample relative to most abundant spot (11).
A mascot score value > 58 is considered significant (P < 0.05) (38).
The median average for the 41 values listed.
Identification of Difference Proteins. Each of the dcr-1-enriched proteins was excised, subjected to in-gel tryptic digestion, and analyzed by MALDI-TOF MS. Twenty-two of the 41 proteins were identified by MS (Table 1). Many dcr-1-enriched proteins function in protein metabolism (Table 2), with two playing direct roles in protein synthesis. stubarista encodes rp-S2, whereas me31B encodes a DEAD-box ATPase regulator of translation (26, 27). One highly represented category functions in protein folding. Four protein chaperones were identified, and the FKBP59 proline isomerase, which is active in the embryo, was detected as well (28). The GDP dissociation inhibitor is a factor important for intracellular protein transport (29). Another highly represented category functions in protein turnover. Uba1 encodes an E1 ubiquitin-activating enzyme, whereas Pros35 and Mov34 encode subunits of the 26S proteasome (30). A protein with putative ubiquitin-specific protease activity (31) and an M1-class metallopeptidase were also identified. In addition to those proteins that function in protein metabolism, a few proteins are enzymes involved in carbohydrate and lipid metabolism. Enolase and aldehyde reductase are involved in glucose metabolism, and Nurf-38 encodes a pyrophosphatase. Yp1 and Yp2 are yolk-associated lipases that catabolize yolk lipids. Other difference proteins included actin, TepII [a α-2-macroglobulin protein (32)], and Fit, a protein of unknown function.
Table 2. Functional and regulatory annotation of identified dcr-1-enriched proteins.
| CG identifier | Gene* | Function | Transcript ratio† (dcr-1/wild type) | Protein/transcript (dcr-1/wild type)‡ |
|---|---|---|---|---|
| CG4264 | Hspc4 | Chaperone | 0.88 | 3.6 |
| CG12101 | Hsp60 | Chaperone | 0.59 | 24.1 |
| CG4463 | Hsp23 | Chaperone | 0.95 | 1.8 |
| CG7033 | — | GroEL chaperone | 0.63 | 2.6 |
| CG4535 | FKBP56 | Prolyl cis-trans isomerase | 0.75 | 5.4 |
| CG4422 | GDI | Rab-GDP regulator | 0.58 | 5.6 |
| CG14792 | stub | rpS2 ribosomal subunit | 0.19 | 31.7 |
| CG4916 | Me31B | DEAD-box ATPase | 0.97 | 8.6 |
| CG1782 | Uba1 | Ubiquitin activating enzyme | 0.54 | 6.6 |
| CG5384 | — | Ubiquitin specific protease | 0.82 | 3.4 |
| CG4904 | Pros35 | Proteasome subunit | 0.80 | 5.2 |
| CG3416 | Mov34 | Proteasome subunit | 0.08 | 125.0 |
| CG5355 | — | Serine protease | 2.10 | 1.2 |
| CG10602 | — | M1 metalloprotease | 0.84 | 5.1 |
| CG7052 | TepII | Toll pathway protease inh. | 0.20 | 15.6 |
| CG6084 | — | Aldchyde reductase | 0.40 | 10.8 |
| CG17654 | Enolase | Pyruvate hydratase | 0.88 | 38.4 |
| CG4634 | Nurf38 | Inorganic pyrophosphatase | 1.38 | 50.4 |
| CG4027 | Actin5C | Cytoskeleton protein | 0.66 | 5.6 |
| CG2985 | Yp1 | Yolk protein | — | — |
| CG2979 | Yp2 | Yolk protein | — | — |
| CG17820 | fit | Unknown | — | — |
FLYBASE entry (http://flybase.bio.indiana.edu).
RT-PCR levels from mutant and wild-type RNA, normalized to rp49 as control.
Fluorescence ratio from Table 1 adjusted using the transcript ratio.
We also excised and analyzed 31 dcr-1-depleted proteins that were more abundant in wild-type samples. MS identification of 21 of these proteins revealed that they have a variety of biochemical activities, with the majority functioning in carbohydrate metabolism and transport (Table 4, which is published as supporting information on the PNAS web site). The dcr-1-depleted proteins are presumably regulated one or more steps downstream of miRNA action, because their expression is up-regulated by the silencing machinery.
Comparison of Transcript and Protein Levels. The dcr-1-enriched proteins appear to be repressed by miRNAs. However, it was not clear whether the repression was a direct effect of miRNAs. If it is direct, oocytes should contain mRNA transcripts encoding these proteins, because transcripts are the substrates for miRNA binding. This eliminated Yp1 and Yp2 from consideration, because these genes are transcribed in somatic cells of the female, and their protein products are transported to the oocyte (33). To test the other genes, we measured levels of each transcript by quantitative RT-PCR, and we detected transcripts for 19 genes in mature wild-type eggs (Fig. 2 and Table 2). No transcript was detected for Fit, which is expressed in the maternal fat body (34).
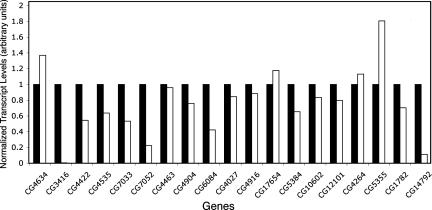
Fig. 2.
Levels of mRNA transcripts in wild-type (black bars) or dcr-1 mutant (white bars) oocytes. Shown are levels of transcripts from the indicated genes, normalized to the corresponding levels of rp49 transcript in each sample, and determined by semiquantitative RT-PCR. For each gene, the level from the wild-type sample is set to a value of 1.0. The data displayed are from one experiment, and transcript data listed in Table 2 represent averages of values from replicate experiments.
If the 19 transcripts we detected are translationally repressed by miRNAs, their abundance should not be greater in the dcr-1 mutant than in wild-type. When we compared the transcript levels in wild-type and dcr-1 individuals, 18 of the 19 transcripts were not significantly increased in the mutant (Fig. 2 and Table 2). One exception was the gene CG5355, which showed an increased transcript level in the mutant that was quantitatively similar to its increase in protein abundance. This suggests that Dcr-1 primarily represses CG5355 through transcript production or stability. For the other 18 transcripts, several were actually reduced in dcr-1 mutants, even though their protein products showed enhanced accumulation by DIGE. This might be due to the enhanced translation in the mutant resulting in greater mRNA turnover. Alternatively, reduced transcript abundance might result from disruption of other regulatory pathways in the mutant. In summary, 18 transcripts appeared to be candidate substrates for translational inhibition by miRNAs. The strength of inhibition, as measured indirectly as the ratio of protein to transcript abundance, varied between 1.8- and 125-fold, with little ranking by functional class (Table 2).
Detection of miRNA-Binding Sites. Transcripts that are directly regulated by miRNAs should contain miRNA-binding sites in their 3′ UTRs. Functional binding sites typically pair to cognate miRNAs with extensive gaps and base mismatches (5). However, a six- to eight-base “seed” of contiguous pairing exists between the 3′ end of a binding site and 5′ end of a miRNA (18). Another feature of known functional binding sites is that they are predicted to bind with greater free energy (ΔG) to a cognate miRNA than to an RNA of random sequence. Two other qualities have been noted about functional miRNA-binding sites. First, binding sites are frequently repeated within a 3′ UTR, and binding sites for multiple miRNAs can be found within a UTR. Second, conserved binding sites can be found in the 3′ UTRs of homologous genes of related species.
We developed a search algorithm to find potential miRNA-binding sites in RNA sequence. This algorithm searches for motifs based on the most recent discoveries about binding-site features. These are (i) a 7/8 base-pairing match or better between the 5′ end of a miRNA and 3′ end of the target site, with no more than one permitted GU wobble pair; (ii) an mfold (35) ΔG value for each miRNA-binding interaction that is greater than random average. We scanned the 3′ UTR sequences of the 18 dcr-1 enriched-genes for putative miRNA-binding sites. We searched only for sites recognized by a miRNA that is possibly expressed in mature oocytes or early embryos based on Northern analysis.
The results of the analysis are shown in Table 3. All but 1 of the 18 genes contains putative miRNA-binding sites in their 3′ UTRs. The average number of binding sites is 2.4 per gene, and the number of sites varies from one to four. The predicted list of miRNAs is striking. miR-280 is predicted to interact with 14 of the 18 genes. This suggests that miR-280 is an important mediator of translation regulation at this stage of Drosophila development. Indeed, highly ranked target predictions for miR-280 include several genes that function in late oogenesis/early embryogenesis, including Toll, staufen, nudel, germ cell-less, oskar, and par-6 (13, 14, 17).
Table 3. Predicted miRNA-binding sites in 3′ UTRs of identified genes.
| Gene/CG number | 3′ UTR (bases) | Predicted miRNA sites (miR-) | S score, site number | Z score, binding energy |
|---|---|---|---|---|
| Hspc4 | 302 | 280, 275 | 3.1 | 2.1 |
| Hsp60 | 483 | 280, 1,* 5, 11, 31a | 6.1 | 5.3 |
| Hsp23 | 209 | 4, 305, 308 | 9.4 | 2.8 |
| CG7033 | 437 | 280(2),† 1, 305 | 11.6 | 8.4 |
| FKBP56 | 130 | 280(2)‡ | 1.9 | 2.5 |
| GDI | 166 | 280, 2b, 33, 287 | 8.3 | 10.5 |
| Stub | 96 | 280 | 13.9 | 2.4 |
| Me31B | 127 | 280‡ | 0.6 | 1.1 |
| Uba1 | 481 | 280, 1, 5, 14 | 5.0 | 5.6 |
| CG5384 | 69 | 280‡ | 0.9 | 3.6 |
| Pros35 | 107 | 12‡ | 0.9 | 2.8 |
| Mov34 | 254 | 280(2), 92b, 308 | 9.3 | 3.2 |
| CG10602 | 129 | 280‡ | 0.8 | 0.9 |
| TepII | 108 | None | ||
| CG6084 | 269 | 280† | 0.3 | 1.9 |
| Enolase | 370 | 280, 8,* 308 | 6.2 | 2.2 |
| Nurf38 | 137 | 4, 79 | 4.7 | 2.3 |
| Actin5C | 349 | 280, 1, 287, 288 | 9.9 | 9.9 |
We determined the significance of these sites by two methods. First, we found that the presence of putative binding sites correlated with genes that are down-regulated by Dcr-1. We scanned the dcr-1-depleted genes, reasoning that few of these genes should contain miRNA-binding sites in their 3′ UTRs. Indeed, we found an average of 0.2 binding sites per gene in the depleted class (data not shown), in contrast to the 2.4 sites per gene in the enriched class. A second method we applied to determine significance was a dual statistical test. We calculated the probability that the mfold ΔG value for each miRNA-binding interaction was greater than random average (Table 4). This Z score was considered statistically significant (P < 0.05) if it was >2.0, which indicates that it is at least 2-fold greater than the standard deviation for background measurements. We also calculated the probability of finding the seed sequences in each 3′ UTR by chance. The resulting S score was considered significant (P < 0.05) if it was >2.0, again indicating that it is at least 2-fold greater than the standard deviation for randomly finding those seeds. Using these criteria, we conclude that most of the genes that we identified by proteomic analysis are predicted to contain specific binding sites for miRNAs.
Many of the binding sites identified in our analysis were also predicted from published computational analyses of the Drosophila melanogaster genome (13, 14) (Table 3). Despite this apparent concordance, we did not detect a few binding sites predicted from those analyses. Moreover, several binding sites that our analysis identified were not found in the other analyses. These analyses considered only sites conserved in more than one Drosophila species. Failure to identify sites might have occurred because orthologous genes were not found, as happened with 35% of genes. Alternatively, sites in D. melanogaster genes might not be conserved in the other species.
Discussion
At the completion of Drosophila oocyte maturation, the number of genes negatively regulated by miRNAs appears to be limited. We detected 41 of 1,003 proteins that are down-regulated by miRNA production, which represents a maximum 4% of genes that might be directly inhibited. Although this value is close to the genome-wide 7% level predicted by sequence comparisons (13), we cannot be certain that 4% represents a typical fraction of genes regulated by miRNAs in a cell. First, this estimate reflects only the 1,000 most-abundant proteins at this stage of development. Many less-abundant proteins are not detected by DIGE, and so we do not know the fraction of those that are regulated. For example, dcr-1 mutant oocytes exhibit defective translation of Oskar and Gurken proteins, leading to a mild ventralization phenotype (data not shown). However, these two proteins are too rare to have been detected by our proteomic analysis. Second, we do not know whether the relative fraction of target genes varies during development. Possibly, fewer genes are regulated by miRNAs during oogenesis. It is known that RNAi is activated during oocyte maturation, and that the short interfering RNA pathway depends upon translation of target mRNAs (23). It is possible that there are fewer miRNA targets in mature oocytes due to dependence on different translation control mechanisms.
Why are these particular gene products repressed by miRNAs during oocyte maturation? In most animal species, translation serves as the main mechanism to regulate gene expression during oocyte maturation and early embryogenesis (36). Indeed, oocyte maturation and early embryogenesis proceed without transcription of nuclear RNA, including rRNA. In parallel, no rps are synthesized de novo, and consequently no new ribosomes are produced. In Drosophila, rp-mRNA levels are constant throughout oogenesis and embryogenesis, but their translation drops as oogenesis ends and embryogenesis begins (37, 38). Translation of rp-mRNAs then rises in conjunction with the onset of rRNA transcription in the embryo. We have found that the accumulation of rp-S2 protein is inhibited by miRNAs during oocyte maturation, suggesting that the translation block exerted on Drosophila rp proteins is partially mediated by the miRNA pathway. We do not know the status of other r-proteins, because most are basic in charge and would not be detected in the 2D gels used in the DIGE analysis.
A lack of new ribosome production during oocyte maturation and early embryogenesis would also eliminate the need to synthesize other factors involved in protein biogenesis, reflecting a coordinated effort to balance various steps along the biogenesis pathway. Our study indicates that the synthesis of several chaperones and other biogenic factors is specifically inhibited during oocyte maturation, possibly for those reasons. Another cellular process that appears to be attenuated by miRNAs at this developmental stage is protein turnover. Why is proteolysis dampened? Possibly, reduced proteolysis allows preexisting ribosomes to remain abundant during the period when they cannot be replenished. Another possibility is that reduced proteolysis links the rate of protein biogenesis to the rate of protein turnover, thereby maintaining steady-state protein levels. Finally, lowered protein turnover during this highly dynamic stage of development would allow for rapid and global accumulation of new proteins necessary for early embryogenesis.
Supplementary Material
Acknowledgments
We thank D. Marks and C. Sander for helpful input, members of the J.S.M. laboratory for help and advice, and E. Sontheimer and Y. S. Lee for greatly improving the manuscript. This work was supported by Grant GM07345 from the National Institutes of Health awarded to R.W.C. The MALDI-TOF MS in the Center for Molecular Analysis at Carnegie Mellon University is supported by National Science Foundation Grant CHE-9808188.
Author contributions: K.N., K.K., J.S.M., and R.W.C. designed research; K.N., K.K., C.S., and S.R.D. performed research; J.S.M. contributed new reagents/analytic tools; K.N., K.K., S.R.D., and R.W.C. analyzed data; and K.N., J.S.M., and R.W.C. wrote the paper.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: miRNA, microRNA; DIGE, difference gel electrophoresis; dcr-1, dicer-1; rp, ribosomal protein.
References
- 1.Nakahara, K. & Carthew, R. W. (2004) Curr. Opin. Cell Biol. 16, 127–133. [DOI] [PubMed] [Google Scholar]
- 2.Novina, C. D. & Sharp, P. A. (2004) Nature 430, 161–164. [DOI] [PubMed] [Google Scholar]
- 3.Pasquinelli, A. E. & Ruvkun, G. (2002) Annu. Rev. Cell Dev. Biol. 18, 495–513. [DOI] [PubMed] [Google Scholar]
- 4.Ambros, V., Lee, R. C., Lavanway, A., Williams, P. T. & Jewell, D. (2003) Curr. Biol. 13, 807–818. [DOI] [PubMed] [Google Scholar]
- 5.Bartel, D. P. (2004) Cell 116, 281–297. [DOI] [PubMed] [Google Scholar]
- 6.Lee, R. C., Feinbaum, R. L. & Ambros, V. (1993) Cell 75, 843–854. [DOI] [PubMed] [Google Scholar]
- 7.Wightman, B., Ha, I. & Ruvkun, G. (1993) Cell 75, 855–862. [DOI] [PubMed] [Google Scholar]
- 8.Reinhart, B. J., Slack, F. J., Basson, M., Pasquinelli, A. E., Bettinger, J. C., Rougvie, A. E., Horvitz, H. R. & Ruvkun, G. (2000) Nature 403, 901–906. [DOI] [PubMed] [Google Scholar]
- 9.Abrahante, J. E., Daul, A. L., Li, M., Volk, M. L., Tennessen, J. M., Miller, E. A. & Rougvie, A. E. (2003) Dev. Cell 4, 625–637. [DOI] [PubMed] [Google Scholar]
- 10.Lin, S. Y., Johnson, S. M., Abraham, M., Vella, M. C., Pasquinelli, A., Gamberi, C., Gottlieb, E. & Slack, F. J. (2003) Dev. Cell 4, 639–650. [DOI] [PubMed] [Google Scholar]
- 11.Chang, S., Johnston, R. J., Jr., Frokjaer-Jensen, C., Lockery, S. & Hobert, O. (2004) Nature 430, 785–789. [DOI] [PubMed] [Google Scholar]
- 12.Brennecke, J., Hipfner, D. R., Stark, A., Russell, R. B. & Cohen, S. M. (2003) Cell 113, 25–36. [DOI] [PubMed] [Google Scholar]
- 13.Enright, A. J., John, B., Gaul, U., Tuschl, T., Sander, S. & Marks, D. S. (2003) Genome Biol. 5, R1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stark, A., Brennecke, J., Russell, R. B. & Cohen, S. M. (2003) PLoS Biol. 1, E60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lewis, B. P., Shih, I. H., Jones-Rhoades, M. W., Bartel, D. P. & Burge, C. B. (2003) Cell 115, 787–798. [DOI] [PubMed] [Google Scholar]
- 16.John, B., Enright, A. J., Aravin, A., Tuschl, T., Sander, C. & Marks, D. S. (2004) PLoS Biol. 2, e363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rajewsky, N. & Socci, N. D. (2004) Dev. Biol. 267, 529–535. [DOI] [PubMed] [Google Scholar]
- 18.Lewis, B. P., Burge, C. B. & Bartel, D. P. (2005) Cell 120, 15–20. [DOI] [PubMed] [Google Scholar]
- 19.Chou, T. B. & Perrimon, N. (1996) Genetics 144, 1673–1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gong, L., Puri, M., Unlu, M., Young, M., Robertson, K., Viswanathan, S., Krishnaswamy, A., Dowd, S. R. & Minden, J. S. (2004) Development (Cambridge, U.K.) 131, 643–656. [DOI] [PubMed] [Google Scholar]
- 21.Perkins, D. N., Pappin, D. J., Creasy, D. M. & Cottrell, J. S. (1999) Electrophoresis 20, 3551–3567. [DOI] [PubMed] [Google Scholar]
- 22.Lee, Y. S., Nakahara, K., Pham, J. W., Kim, K., He, Z., Sontheimer, E. J. & Carthew, R. W. (2004) Cell 117, 83–94. [DOI] [PubMed] [Google Scholar]
- 23.Kennerdell, J. R., Yamaguchi, S. & Carthew, R. W. (2002) Genes Dev. 16, 1884–1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Unlu, M., Morgan, M. E. & Minden, J. S. (1997) Electrophoresis 18, 2071–2077. [DOI] [PubMed] [Google Scholar]
- 25.Mahowald, A. P., Goralski, T. J. & Caulton, J. H. (1983) Dev. Biol. 98, 437–445. [DOI] [PubMed] [Google Scholar]
- 26.Melnick, M. B., Noll, E. & Perrimon, N. (1993) Genetics 135, 553–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johnstone, O. & Lasko, P. (2001) Annu. Rev. Genet. 35, 365–406. [DOI] [PubMed] [Google Scholar]
- 28.Zaffran, S. (2000) Gene 246, 103–109. [DOI] [PubMed] [Google Scholar]
- 29.Ricard, C. S., Jakubowski, J. M., Verbsky, J. W., Barbieri, M. A., Lewis, W. M., Fernandez, G. E., Vogel, M., Tsou, C., Prasad, V., Stahl, P. D., et al. (2001) Genesis 31, 17–29. [DOI] [PubMed] [Google Scholar]
- 30.Holzl, H., Kapelari, B., Kellermann, J., Seemuller, E., Sumegi, M., Udvardy, A., Medalia, O., Sperling, J., Muller, S. A., Engel, A., et al. (2000) J. Cell Biol. 150, 119–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen, X., Overstreet, E., Wood, S. A. & Fischer, J. A. (2000) Dev. Genes Evol. 210, 603–610. [DOI] [PubMed] [Google Scholar]
- 32.Lagueux, M., Perrodou, E., Levashina, E. A., Capovilla, M. & Hoffmann, J. A. (2000) Proc. Natl. Acad. Sci. USA 97, 11427–11432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bownes, M. (1994) BioEssays 16, 745–752. [DOI] [PubMed] [Google Scholar]
- 34.Fujii, S. & Amrein, H. (2002) EMBO J. 21, 5353–5363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zuker, M. (2003) Nucleic Acids Res. 31, 3406–3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wormington, M. (1993) Curr. Opin. Cell Biol. 5, 950–954. [DOI] [PubMed] [Google Scholar]
- 37.Al-Atia, G. R., Fruscoloni, P. & Jacobs-Lorena, M. (1985) Biochemistry 24, 5798–5803. [DOI] [PubMed] [Google Scholar]
- 38.Kay, M. A. & Jacobs-Lorena, M. (1985) Mol. Cell. Biol. 5, 3583–3592. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.