Abstract
Chemotherapeutic options to treat tuberculosis are severely restricted by the intrinsic resistance of Mycobacterium tuberculosis to the majority of clinically applied antibiotics. Such resistance is partially provided by the low permeability of their unique cell envelope. Here we describe a complementary system that coordinates resistance to drugs that have penetrated the envelope, allowing mycobacteria to tolerate diverse classes of antibiotics that inhibit cytoplasmic targets. This system depends on whiB7, a gene that pathogenic Mycobacterium shares with Streptomyces, a phylogenetically related genus known as the source of diverse antibiotics. In M. tuberculosis, whiB7 is induced by subinhibitory concentrations of antibiotics (erythromycin, tetracycline, and streptomycin) and whiB7 null mutants (Streptomyces and Mycobacterium) are hypersusceptible to antibiotics in vitro. M. tuberculosis is also antibiotic sensitive within a monocyte model system. In addition to antibiotics, whiB7 is induced by exposure to fatty acids that pathogenic Mycobacterium species may accumulate internally or encounter within eukaryotic hosts during infection. Gene expression profiling analyses demonstrate that whiB7 transcription determines drug resistance by activating expression of a regulon including genes involved in ribosomal protection and antibiotic efflux. Components of the whiB7 system may serve as attractive targets for the identification of inhibitors that render M. tuberculosis or multidrug-resistant derivatives more antibiotic-sensitive.
Keywords: multidrug resistance, Streptomyces, WhiB, microarray, gene expression
The World Health Organization has estimated that between 2000 and 2020, nearly one billion people will be newly infected, 200 million people will get sick, and 35 million will die from tuberculosis (TB) (1). It is the remarkable antibiotic tolerance of the infectious agent Mycobacterium tuberculosis to many commonly used broad-spectrum antibiotics that limits chemotherapeutic options and is the root cause of all treatment failure (2). Tolerance may reflect physiological adaptations that occur within the host, perhaps including an undefined developmental or physiological state that underlies persistent infection (3). As a result, patients must be treated with multiple antibiotics for 6-12 months. Patient noncompliance or inadequate drug dosage favors the sequential acquisition of mutations providing resistance and the emergence of multidrug-resistant M. tuberculosis strains. In contrast to acquired resistance, intrinsic resistance in Mycobacterium has largely been attributed to its impermeable mycolic acid-containing cell envelope (4, 5) that is not found in many other Actinomycetes including Streptomyces. However, Jarlier and Nikaido (4) have also pointed out that this permeability barrier is insufficient to fully explain the high levels of drug resistance in Mycobacterium, suggesting that there must be synergistic systems effective against drugs that penetrate this barrier. Indeed, several mycobacterial genes not involved in outer envelope assembly confer resistance to specific, broad-spectrum antibiotics (6-8).
Although the best-known Mycobacterium species are pathogenic, most are ubiquitous environmental saprophytes belonging to the Actinomycete taxon (9). The taxon also include Streptomyces species, filamentous bacteria known for their extraordinary capacity to produce thousands of diverse antibiotics as a part of a developmental program leading to sporulation. Antibiotic biosynthetic genes are found in clusters that typically include the corresponding resistance genes to provide self-protection (10). However, as in other bacteria, genes scattered throughout the genome that may have alternative physiological roles can also confer antibiotic resistance (11). Intuitively, the protective activity of these resistance genes should be a prerequisite for the evolution of antibiotic biosynthetic pathways.
Indeed, at sublethal concentrations, antibiotics can induce a wide variety of genes, many not known to provide antibiotic resistance (12-14). The expression of some of these antibiotic-induced genes is under the control of stress inducible systems that respond to (13) or lead to (15) decreases in growth rate. The underlying control elements that affect antibiotic resistance include general stress-responsive sigma factors (16) and transcriptional activator proteins of the AraC family (MarA, SoxR, and Rob) (17), as well as genetic systems that provide for more specific adaptation to DNA damage (15) or oxidative stress (8). Such systems are commonly found in diverse bacterial groups and typically modulate antibiotic resistance within a rather narrow concentration range (8, 17). Here we describe a multidrug-resistance system that apparently evolved in the ancestors of antibiotic producing bacteria, which has been retained in saprophytes and pathogens belonging to the Actinomycete taxon.
Methods
Media and Strains. Streptomyces lividans 1326 was grown in the nutrient-rich liquid media YEME and cultivated on NE solid media (18). The slow growing mycobacteria Mycobacterium bovis bacillus Calmette-Guérin, M. tuberculosis H37Rv, and the clinical M. tuberculosis isolate 1254 were propagated in 7H9 media (19), supplemented with 10% ADS (5% BSA/2% dextrose/0.8% sodium chloride).
Plasmid Constructions and Mutant Analyses. Annotated whiB7 ORFs were deleted in the genomes of S. lividans and Streptomyces coelicolor (nucleotide coordinates 5,647,587-5,648,293; http://jic-bioinfo.bbsrc.ac.uk/streptomyces/ScoDB), M. tuberculosis H37Rv (nucleotide coordinates 3,568,405-3,568,801; http://genolist.pasteur.fr/TubercuList), and M. bovis bacillus Calmette-Guérin (nucleotide coordinates 3,523,346-3,522,950; http://genolist.pasteur.fr/BoviList) as described Supporting Text and Data Set, which are published as supporting information on the PNAS web site. Corresponding ORFs were expressed from vector promoters.
Mycobacterial Survival in Monocytes. Resting or activated J774 monocytes were grown in DMEM/FCS. Monocytes were exposed to Mycobacterium bovis bacillus Calmette-Guérin and the corresponding whiB7 mutant at a multiplicity of one. Activation was achieved by 16-h exposure to 500 units/ml IFN-γ followed by 4-h exposure to both IFN-γ and 1 μg/ml LPS. The monocytes were washed twice with PBS and incubated for 45 min at 37°C/5% CO2 with amikacin (200 μg/ml). Cells were again washed twice in PBS and incubated in DMEM/FCS. Survival was determined at the indicated incubation times by bacterial incorporation of tritiated uracil followed by liquid scintillation counting (20). For antibiotic susceptibility testing, the infected monocytes were incubated in the presence of indicated spectinomycin concentrations for 48 h before permeabilization and mycobacterial labeling (see Supporting Text for details).
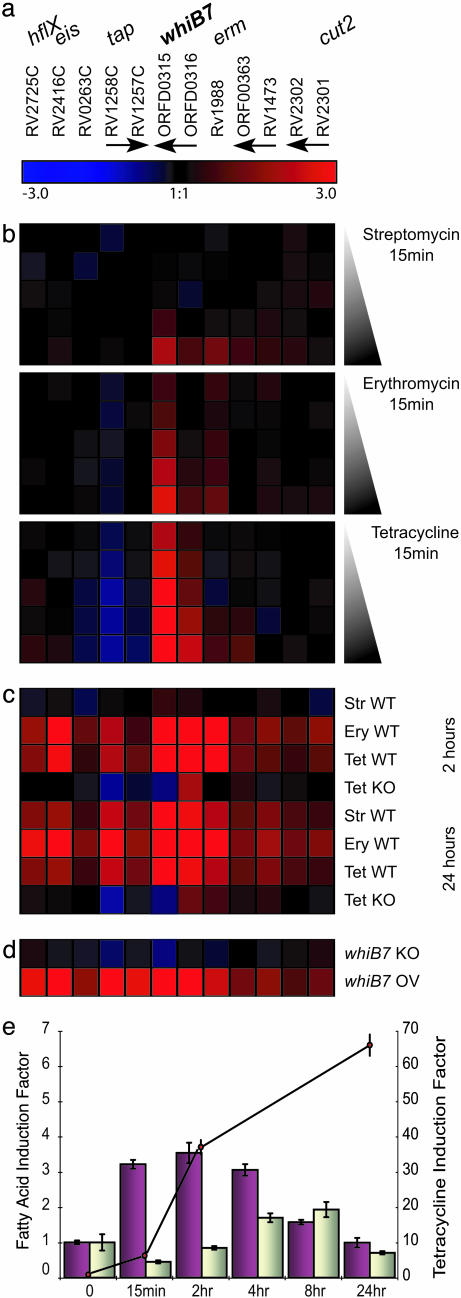
Microarray Expression Profiling and Analysis. Labeling of RNA and hybridizations to 70-mer oligonucleotide-based microarrays (Operon) was performed as described (21). Microarrays were scanned by using GenePix 4000A (Axon Instruments). Fluorescence intensities of the two channels at each spot were quantified by using the scanalyze software (http://rana.lbl.gov/EisenSoftware.htm). After data for each array were normalized (21), expression ratios were averaged from two biological replicates for antibiotic-induced cultures or from three cultures for the mid-log comparison, and with two microarrays for each of the biological replicates. Data from each experimental condition was analyzed separately by using significance analysis of microarrays (22) with a false discovery ratio ≤0.3%. Significantly regulated genes for all experimental conditions were combined to generate a data set containing 2,879 genes. Within this list, gene expression data could be present for one experimental condition and absent from another. To aid hierarchical clustering, these genes were then filtered to include those that were present across 95% of the 25 experimental conditions (total number of rows in Fig. 3 b-d) and a differential expression >2-fold under at least three of these conditions. The resulting 880 filtered genes were organized according to their expression profiles by average linkage clustering using genesis software (http://genome.tugraz.at/Software/GenesisCenter.html).
Fig. 3.
Identification of antibiotic resistance genes as parts of the M. tuberculosis whiB7 regulon. Significantly altered gene expression ratios from all experiments were averaged, log2 transformed, and clustered according to the displayed color code. Red and blue indicate higher and lower gene expression, respectively, in experimental samples, in comparison to the reference. Black represents no difference. (a) Genes of the whiB7 regulon. Rv and ORF numbers designate annotated M. tuberculosis genes, and arrows indicate contiguous genes. (b) Treatment (15 min) of M. tuberculosis 1254 with 0.5, 1.0, 5, 25 and 100 μg/ml of erythromycin, streptomycin, and tetracycline. (c) Extended antibiotic treatment for 2 and 24 h (1 μg/ml). (d) M. tuberculosis H37Rv was used as reference and compared to a whiB7 null mutant (WhiB7 KO) and a H37Rv strain engineered to overexpress whiB7 (WhiB7 OV). Cells were assayed at an optical density of 0.4 at 600 nm. (e) whiB7 induction by fatty acids. Quantitative RT-PCR-determined induction factors of whiB7 expression. Primary y axis: M. tuberculosis grown in MDG fed 50 μM palmitic acid (purple) or its unsaturated form, oleic acid (gray). Secondary y axis: induction to prolonged exposure of tetracycline (connected dots) (≈2 μM, 1 μg/ml).
Analysis of Mycobacterial RNA with Quantitative Real-Time RT-PCR. Real time PCR to confirm microarray analysis of in vitro grown cultures was performed by using SYBR green (Applied Biosystems). A standard curve was generated for the relative quantification of all genes, and a control reaction lacking reverse transcriptase was performed for every RNA sample. The major housekeeping sigma factor gene sigA was used to normalize mRNA levels. Gene induction values were calibrated by comparison with the reference RNA isolated for each experiment.
Results
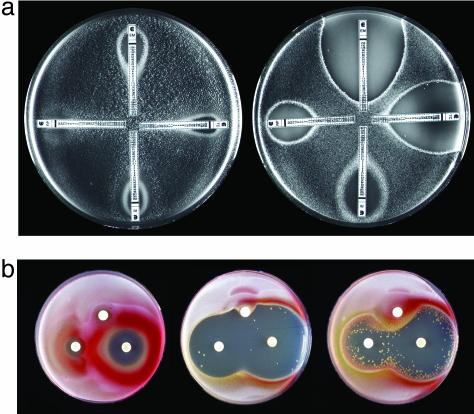
Identification of a Multidrug Resistance Regulator in Streptomyces. We isolated a spontaneous mutant of S. lividans, RM1, that was hypersensitive to a diverse array of chemically and functionally unrelated clinical antibiotics that it does not synthesize, including chloramphenicol, fusidic acid, imipenem, lincosamides (clindamycin and lincomycin), macrolides (erythromycin, oleandomycin, and spiramycin), rifampicin, streptogramins (pristinamycin and virginiamycin), and tetracycline (see Fig. 5, which is published as supporting information on the PNAS web site). Sensitivities of the mutant and wild-type parent were quantified by using Etest diffusion strips to compare their minimal inhibition concentrations to four structurally and functionally distinct classes of antibiotics. RM1 was 600-, 400-, 25-, and 40-fold more sensitive to erythromycin, tetracycline, rifampicin, and the pristinamycin derivatives quinupristin/dalfopristin, respectively (Fig. 1a). The mutant displayed large decreases in intrinsic antibiotic resistance, but its susceptibility to a variety of other toxic, nonantibiotic stresses, including detergents, antiseptics, and oxidative stress inducers, was unchanged (Fig. 6, which is published as supporting information on the PNAS web site). Standard cloning, sequencing, and site-directed mutagenesis experiments (described in Supporting Text) identified the gene responsible for this multidrug resistance as whiB7 in both S. lividans (GenBank accession no. AF205848) and S. coelicolor genomes (Fig. 2) (23). Sequence comparison of the wild-type locus to that of RM1 revealed a frame shift in the whiB7 gene resulting from the insertion of a cytosine at nucleotide position 239. This locus was not linked to any recognizable antibiotic biosynthetic cluster. whiB7 encodes a 122-aa protein related to whiB, a putative S. coelicolor transcriptional regulatory gene (24). In M. tuberculosis, a WhiB-like protein (WhiB3) may act as a transcriptional regulator by binding and modulating the activity of RpoV (SigA), the principle sigma factor in M. tuberculosis (25). To further demonstrate the correlation of whiB7 transcriptional activity with antibiotic resistance, the S. lividans mutant RM1 was engineered to allow inducible expression (26) of a wild-type copy of whiB7. Fig. 1b shows that the circular zones of inhibition caused by diffusion of tetracycline (an aromatic polyketide) or oleandomycin (a macrolide) were distorted and reduced by a radial gradient of the inducer (thiostrepton), indicating higher levels of resistance associated with increased whiB7 transcription.
Fig. 1.
Identification of whiB7, a gene providing intrinsic antibiotic resistance in Streptomyces. (a) Antibiotic susceptibility of the wild-type S. lividans strain (Left) and the spontaneous whiB7 mutant RM1 (Right). Etest strips were applied to seeded spores, and minimal inhibition concentration values were read from the scale (μg/ml) at the point of intersection between inhibition ellipse edge and the strip. Upper, erythromycin; right, tetracycline; lower, rifampicin; left, quinupristin/dalfopristin. (b) S. lividans RM1 was engineered to allow thiostrepton-inducible expression of whiB7 by using the expression plasmid pIJ8600. Seeded spores of S. lividans wild-type/pIJ8600 (Left), the whiB7 mutant RM1/pIJ8600 (Center), or RM1/pIJ8600::whiB7 (Right) were exposed to radial gradients by discs containing 100 μg of oleandomycin (left), chlorotetracycline (right), or thiostrepton (top). The high frequency of suppressor colonies in the RM1/pIJ8600::whiB7 culture are presumed to be promoter-up mutants that allow unregulated whiB7 expression. Tetracycline induced synthesis of a red pigment (likely to be the antibiotic undecylprodigiosin) in both wild-type S. lividans and the whiB7 mutant.
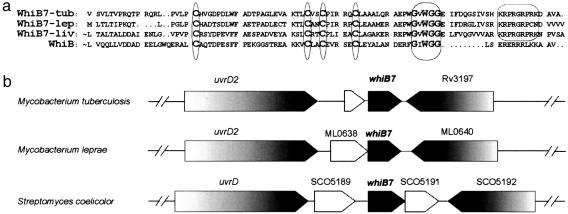
Fig. 2.
The orthologous whiB7 loci of Streptomyces and Mycobacterium. (a) Alignment of the WhiB7 proteins from both Mycobacterium tuberculosis (WhiB7-tub) and leprae (WhiB7-lep) with the Streptomyces WhiB7 and the prototypic family member WhiB from Streptomyces coelicolor. Four absolutely conserved cysteine residues and a tryptophan-containing/glycine-rich motif are conserved throughout the WhiB family (circled). An A/T-Hook DNA binding consensus sequence is found only in WhiB7 paralogs. ∼, N-terminal sequence not shown. (b) Gene organization of the whiB7 genomic region. Shaded block arrows represent conserved ORFs.
Members of the whiB gene lineage, including whiB7 of S. lividans (alternatively named wblC), are restricted to the Actinomycetes (27); blast searches did not identify orthologs in any other published bacterial genome sequences. The prototype of this gene family, whiB, was identified as a developmental gene in Streptomyces species that is essential for the differentiation of mycelium into pigmented spores (white) (28). The family signature is defined by four absolutely conserved cysteines that form an oxygen sensitive iron sulfur cluster (ref. 29 and L.N., P. Jensen, R.P.M., M. Folcher, S. Durr, S. Grzesiek, and C.J.T., unpublished data), and a tryptophan within a glycine-rich sequence (Fig. 2a). In addition, whiB7 paralogs also encode a C-terminal “A/T Hook” domain that is known to bind AT-rich DNA sequences. Although the M. tuberculosis genome encodes seven whiB-like genes (whiB1-7), both homology and synteny predict a minimal core of five orthologous whiB-like genes common to M. tuberculosis, Mycobacterium leprae, and Streptomyces species (whiB1-4 and -7). tblastn searches of the 201 completed bacterial genomes identified whiB7 orthologs in all species of Streptomyces (S. coelicolor and Streptomyces averimidilus), Mycobacterium (tuberculosis H37Rv, tuberculosis CDC1551, bovis AF2122/97, leprae TN, avium subsp. paratuberculosis), and Nocardia (farcinica IFN10152). The role of the streptomycete whiB7 gene in determining broad spectrum drug resistance, predicted that it might play a similar role in pathogenic M. tuberculosis.
A whiB7 Ortholog Controls Multidrug Resistance in M. tuberculosis. Intrinsic resistance in M. tuberculosis could be partially due to a whiB7 ortholog that is able to provide resistance to antibiotics that have penetrated the cell envelope and entered the cytoplasm. To test this hypothesis, we constructed a gene replacement mutant in M. tuberculosis, strain H37Rv. The mutant grew normally, but was defective in its resistance (Table 1) to a variety of antibiotics including macrolides, a lincosamide, and an aminoglycoside. The whiB7 gene was cloned into the integrative vector pMV361 to provide expression from a strong constitutive promoter (hsp60). Integration of this plasmid (pRPM251) into the chromosome of the M. tuberculosis whiB7 mutant restored normal, or slightly elevated levels of antibiotic resistance (Table 1). Multidrug sensitivity also resulted from disruption of the whiB7 gene of the fast growing saprophytic Mycobacterium smegmatis (R.P.M. and C.J.T., unpublished results).
Table 1. Multidrug resistance in M. tuberculosis determined by whiB7.
| Antibiotic | whiB7 KO | WT | whiB7 OV |
|---|---|---|---|
| Chloramphenicol | 4.0 | 8.0 | 8.0 |
| Clarithromycin | 0.4 | 6.4 | 6.4 |
| Erythromycin | 40.0 | 80.0 | 160.0 |
| Lincomycin | 20.0 | 640.0 | 1,280.0 |
| Spectinomycin | 16.0 | 128.0 | 128.0 |
| Streptomycin | <0.5 | 2.0 | 2.0 |
| Tetracycline | 20.0 | 20.0 | 20.0 |
M. tuberculosis H37Rv antibiotic sensitivities (minimal inhibitory concentrations in μg/ml) were determined by conventional BA CTEC assays. whiB7KO, whiB7 null mutant; WT, wild type; whiB7OV, the wild-type strain carrying a plasmid (pR PM251) engineered to provide for overexpression of whiB7.
Induction of the M. tuberculosis whiB7 Gene by Multiple Antibiotics and Fatty Acids. Antibiotic resistance genes typically confer resistance to one class of antibiotic and are specifically activated by the corresponding drugs. Experiments were carried out to determine whether the broad spectrum of resistance conferred by whiB7 might be controlled by regulatory systems that are responsive to dissimilar drugs (30). Microarray transcript profiling of all annotated M. tuberculosis genes was used to monitor expression in response to three chemically distinct classes of common antibiotics: the frontline antimycobacterial drug streptomycin, as well as erythromycin and tetracycline (Fig. 3b). M. tuberculosis 1254 cultures were treated with five concentrations of each antibiotic, spanning three orders of magnitude (0.5-100 μg/ml) including the minimal inhibition concentration. Expression was assayed 15 min after exposure to maximize detection of genes whose regulation most directly reflected whiB7 activity, rather than downstream pleiotropic effects. whiB7 expression was significantly induced by subinhibitory concentrations of both erythromycin (1.0 μg/ml) and tetracycline (0.5 μg/ml) and also higher levels of streptomycin (25 μg/ml). After longer exposure (24 h), concentrations of streptomycin as low as 1 μg/ml induced whiB7 (Fig. 3c). The levels of induction were dose dependent for all three antibiotics (Fig. 3b). Activation of whiB7 transcription by tetracycline (1 μg/ml) was confirmed by quantitative RT-PCR showing that whiB7 RNA levels progressively increased ≈70-fold during 24 h of exposure (Fig. 3e).
Many antibiotics, including erythromycin and tetracycline, are based on polyketides, fatty acid-like molecules with carbon backbones synthesized by enzyme complexes similar to fatty acid synthase. Like antibiotics, many fatty acids are known to suppress growth of diverse bacteria (31), including Mycobacterium spp. (32). Palmitic acid, as well as an unsaturated derivative, oleic acid, were likewise tested for their abilities to induce whiB7 transcription by quantitative RT-PCR. Although both fatty acids activated whiB7 transcription, the palmitic acid response was more rapid and achieved higher levels of induction. Although induction kinetics were concentration dependent, at least for the antibiotics tested, higher concentrations of externally applied palmitic acid were needed and lower levels of maximal induction were achieved (3- to 4-fold compared to 70-fold for tetracycline).
In conclusion, whiB7 expression was progressively induced at the transcriptional level by sublethal concentrations of antibiotics and fatty acids. Up-regulation of whiB7 expression may be required for the induction of other genes that could plausibly provide antibiotic resistance. These observations suggested that whiB7 encoded a regulator whose transcriptional induction activated a regulon providing intrinsic antibiotic resistance.
Identification of Genes in the whiB7 Regulon by Microarray Analyses. To determine whether the induction of whiB7 was correlated with the expression of genes associated with antibiotic resistance, microarray expression profiles of mid-log phase cultures of the whiB7 deletion mutant and a strain overexpressing whiB7 were compared to parental strain M. tuberculosis H37Rv (Fig. 3d). These global analyses (details not presented) showed that whiB7 was the only gene induced initially, after exposure to minimal concentrations of antibiotic (0.5 mg/ml tetracycline for 15 min, for example). Thus, whiB7 represented a primary regulatory gene whose expression was followed by transcription of other genes in its regulon. Average distance hierarchical clustering identified 12 significantly regulated genes (sam false discovery rate ≤ 0.3%) whose expression profile appeared to be influenced by antibiotic exposure and the activity of whiB7 (Fig. 3). The whiB7-dependent set of eight transcripts includes three genes that may provide intrinsic antibiotic resistance: tap (Rv1258c), encoding an efflux pump that confers low-level resistance to aminoglycosides and tetracycline (33); an unstudied ORF encoding a putative macrolide transporter (Rv1473) with an ATP-binding cassette; and erm (Rv1988), homologous to ribosomal methyltransferases and conferring MLS (macrolide, lincosamide, and streptogramin) resistance by modification of 23S rRNA (7, 34). Although the whiB7 regulon may include unrecognized antibiotic resistance determinants, other functions were also suggested. These include eis (Rv2416C), a putative acetyl-transferase providing enhanced survival within macrophages, Rv0263C, a putative carboxylase catalyzing urea degradation, and cut2, a putative cutinase/lipase that is reported to be exposed on the outside of the cell membrane and potentially able to release fatty acids from external lipids (35). These possible functions of other genes in the putative whiB7 regulon, not known to be antibiotic resistance determinants, require further investigation. Some may play roles in bacterial physiology, a recognized but not well understood determinant of antibiotic resistance (36).
Quantitative RT-PCR was used to independently confirm the induction data for genes within the whiB7 regulon, including Rv1258, Rv1473, and Rv1988. Furthermore, primers targeting an intergenic sequence upstream of whiB7 showed that whiB7 was transcriptionally coupled to the small upstream unannotated ORF, ORFD0316 (Fig. 7, which is published as supporting information on the PNAS web site). Strains engineered to constitutively express whiB7 in trans (whiB7 OV) were associated with elevated levels of ORFD0316 transcription, and ORFD0316 was down-regulated in the whiB7 mutant, suggesting that whiB7 positively autoregulates its own transcription.
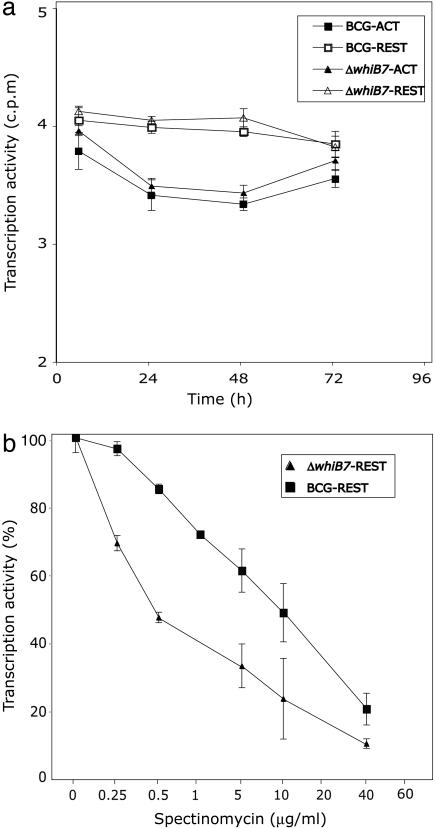
Multidrug Resistance in a Monocyte Model System. The physiology of Mycobacterium growing in laboratory cultures is much different from their natural state during host cell infection (36). To investigate whether whiB7 controls survival or antibiotic resistance in a eukaryotic cellular environment, we monitored the intracellular survival of M. bovis bacillus Calmette-Guérin and a constructed isogenic whiB7 mutant harbored within untreated or spectinomycin-treated J774, a monocyte-like cell line most commonly used for antibiotic sensitivity testing. In the absence of antibiotic, the wild type and whiB7 mutant had similar survival curves in resting or IFN-γ-activated J774 during the first 72 h of infection (Fig. 4a). Compared to liquid cultures, both bacillus Calmette-Guérin wild type and the whiB7 mutant were more sensitive to spectinomycin in J774. However, more importantly, in resting J774, the whiB7 mutant was >10-fold more sensitive to spectinomycin (as reflected by the concentration of antibiotic needed to reduce transcription by 50%; Fig. 4b).
Fig. 4.
The phenotype of the whiB7 mutation in J774, a monocyte-derived cell line. Mycobacterial RNA transcription, an indicator of survival, was assayed by incorporation of tritiated uracil. Shown are mean values (±SD) of six to eight determinations. Resting (REST) or activated (ACT) J774 monocytes growing in microtiter plates were exposed to M. bovis bacillus Calmette-Guérin and the corresponding whiB7 mutant. (a) RNA synthesis of internalized WT and whiB7 mutant cells decreased at the same rate in resting or activated monocytes, presumably because of bactericidal activity of the monocytes. In contrast, growth did occur in the control experiment where the bacteria were cultured in the same medium without monocytes (data not shown). (b) For antibiotic susceptibility testing, infected monocytes were incubated with medium containing the indicated concentrations of spectinomycin; % transcriptional activity is the percentage of incorporation rates in spectinomycin-treated vs. untreated bacteria. The same results were obtained in infected monocytes treated with amikacin after infection to confirm that incorporation rates reflected internalized mycobacteria (data not shown).
Discussion
The fact that the whiB7 gene and its multidrug resistance phenotype have been retained in most Actinomycetes, including those that have not been continuously exposed to antibiotics in the environment, provides important insights into the evolution and biological function of “antibiotic resistance” genes and their regulatory systems. There are 14 genes belonging to the whiB family in the sequenced genome of S. coelicolor (23). Site-directed mutagenesis of this set of genes (B. Gust and K. Chater, personal communication) has shown that, like whiB7, many do not have obvious sporulation (white) defects under standard growth conditions and that the multidrug sensitive phenotype is a unique characteristic of whiB7 mutants (L.N. and C.J.T., unpublished results). Studies of whiB paralogs in Mycobacterium species have shown that the M. smegmatis whiB2 gene (also called whmD) is essential (37) and that another, whiB3, plays a role in virulence in some model systems (25). Here we focus on the ability of whiB7 to determine multiple antibiotic resistances in Actinomycetes and suggest that, in mycobacterial species, it acts synergistically with a rather impermeable cell envelope to provide high levels of intrinsic resistance.
whiB7 is notably different from systems reported in other bacteria that allow adaptation to a variety of different nonspecific stress conditions and may incidentally provide multiantibiotic resistance. whiB7 does not confer resistance to antiseptics, but rather to antibiotics having specific targets (see Figs. 5 and 6). whiB7 function is also unique in that it confers relatively high levels of resistance: in the S. lividans mutant, antibiotic sensitivity increased by orders of magnitude; this is distinct from the general stress adaptive system, which confers much lower levels of multidrug resistance (mar) in enteric bacteria (17, 38) by using any one of three transcriptional activators having highly redundant functions.
whiB7 is a putative transcriptional activator that is induced by antibiotics and controls the expression of at least two documented antibiotic resistance genes. The presence of these structural genes and corresponding regulatory systems in Mycobacterium suggests that this system provides selective advantage. The retention of the multiple antibiotic-responsive regulatory system controlling M. tuberculosis whiB7 provides circumstantial evidence that toxic metabolites of various structures may have played a key role in directing the early evolution of the regulon to provide antibiotic resistance. The whiB7 gene, as well as 5 of its 10 M. tuberculosis target genes (Fig. 3a), are present in M. leprae (Rv1473, Rv1257c, Rv1258c, Rv0263, and Rv2725), whose genome has undergone dramatic reduction during evolution within metazoan (presumably mammalian) hosts. The presence of the functionally conserved whiB7 locus in all Streptomyces and Mycobacterium spp. (also including saprophytic M. smegmatis) genomes now sequenced records its origin in their presumed soil dwelling ancestor. Although it is not clear why this capacity should be retained by M. tuberculosis and M. leprae, long after their progenitor left the antibiotic containing soil, some of these genes may have been adapted to protect the microbe against compounds of the mammalian immune system. The whiB7 system was active in a monocyte model system; mutant was more sensitive to spectinomycin in J774 (Fig. 4b).
This evolutionary retention of whiB7, along with the observation that antibiotics with different structures activate it, implies a common endogenous inducer made by actinomycetes in response to antibiotics. Indeed, sublethal concentrations of some antibiotics induce synthesis of other secondary metabolites as demonstrated in Streptomyces (Figs. 1B and 5) that may also be autotoxic. Although Mycobacterium species are not recognized as antibiotic producers, they do have a remarkably large repertoire of polyketide biosynthesis gene clusters (39), some of which may encode biosynthetic pathways for autotoxic compounds.
Fatty acids, serving as precursors for diverse lipids and for the assembly of biological membranes, are nevertheless toxic to a wide variety of bacteria. Unlike most other bacteria, and for reasons that are not well understood, Actinomycetes commonly synthesize and accumulate extremely large amounts (20-80% of their biomass) of triacyl glycerols (40). This includes the primary precursor of complex lipids, palmitic acid, along with several unsaturated fatty acid derivatives, oleic, linoleic (unpublished data) and arachidonic (unpublished data) acids. All induced whiB7, with palmitic acid being the most active (Fig. 3e). The fact that the whiB7 regulon, including antibiotic resistance genes, can be activated by palmitic acid has important implications for mycobacterial chemotherapy. Palmitic acid has been found in mycobacterial cytosol, and is considered to be a major source of carbon used by M. tuberculosis in the mammalian macrophage (41). It is also the principle fatty acid found in animal tissues and serum. Therefore, the whiB7 regulon may be induced when M. tuberculosis enters macrophages or other lipid rich cells, organs, or tissues, thereby allowing mycobacteria to more effectively resist some chemotherapeutic strategies, sheltered in specific areas of the body.
The intrinsic resistance of M. tuberculosis to antibiotics during in vivo growth and persistence underlies the need for protracted therapy for tuberculosis (2). Knowledge of such inducible intrinsic mycobacterial systems could generate derivatives of antibiotics that might circumvent detection by whiB7 regulators or perhaps WhiB7 inhibitors that augment conventional therapies by inactivating groups of genes that confer intrinsic resistance. Such developments could not only open up a powerful repertoire of currently redundant clinical antibiotics in the treatment of tuberculosis but also reduce the problematic duration of chemotherapy.
Supplementary Material
Acknowledgments
We thank Keith Chater and Berthold Gust (John Innes Centre, Norwich, U.K.) for providing us with a collection of S. coelicolor mutants having mutations in genes belonging to the whiB family. This work was supported by the Swiss National Science Foundation Grants 4049-69384 (to C.J.T. and J.P.), a World Health Organization grant (to J.P.), and National Institutes of Health Grant AI44826 (to G.S.).
Author contributions: R.P.M., L.N., G.S., and C.J.T. designed research; R.P.M., L.N., J.G., K.N., D.S., S.E., and Y.L. performed research; R.P.M., L.N., J.G., K.V., L.H., J.P., G.S., and C.J.T. contributed new reagents/analytic tools; R.P.M., L.N., J.G., K.V., J.P., G.S., and C.J.T. analyzed data; and R.P.M., L.N., G.S., and C.J.T. wrote the paper.
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (accession no. AF205848).
References
- 1.Dye, C., Scheele, S., Dolin, P., Pathania, V. & Raviglione, M. C. (1999) J. Am. Med. Assoc. 282, 677-686. [DOI] [PubMed] [Google Scholar]
- 2.McKinney, J. D. (2000) Nat. Med. 6, 1330-1333. [DOI] [PubMed] [Google Scholar]
- 3.Parrish, N. M., Dick, J. D. & Bishai, W. R. (1998) Trends Microbiol. 6, 107-112. [DOI] [PubMed] [Google Scholar]
- 4.Jarlier, V. & Nikaido, H. (1994) FEMS Microbiol. Lett. 123, 11-18. [DOI] [PubMed] [Google Scholar]
- 5.Nguyen, L., Chinnapapagari, S. & Thompson, C. J. (2005) J. Bacteriol., in press. [DOI] [PMC free article] [PubMed]
- 6.Ainsa, J. A., Blokpoel, M. C., Otal, I., Young, D. B., De Smet, K. A. & Martin, C. (1998) J. Bacteriol. 180, 5836-5843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Doucet-Populaire, F., Buriankova, K., Weiser, J. & Pernodet, J. L. (2002) Curr. Drug Targets Infect Disord. 2, 355-370. [DOI] [PubMed] [Google Scholar]
- 8.Rawat, M., Newton, G. L., Ko, M., Martinez, G. J., Fahey, R. C. & Av-Gay, Y. (2002) Antimicrob. Agents Chemother. 46, 3348-3355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cosma, C. L., Sherman, D. R. & Ramakrishnan, L. (2003) Annu. Rev. Microbiol. 57, 641-676. [DOI] [PubMed] [Google Scholar]
- 10.Davies, J. (1994) Science 264, 375-382. [DOI] [PubMed] [Google Scholar]
- 11.Folcher, M., Morris, R. P., Dale, G., Salah-Bey-Hocini, K., Viollier, P. H. & Thompson, C. J. (2001) J. Biol. Chem. 276, 1479-1485. [DOI] [PubMed] [Google Scholar]
- 12.Goh, E. B., Yim, G., Tsui, W., McClure, J., Surette, M. G. & Davies, J. (2002) Proc. Natl. Acad. Sci. USA 99, 17025-17030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Novotna, J., Vohradsky, J., Berndt, P., Gramajo, H., Langen, H., Li, X. M., Minas, W., Orsaria, L., Roeder, D. & Thompson, C. J. (2003) Mol. Microbiol. 48, 1289-1303. [DOI] [PubMed] [Google Scholar]
- 14.Murakami, T., Holt, T. G. & Thompson, C. J. (1989) J. Bacteriol. 171, 1459-1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miller, C., Thomsen, L. E., Gaggero, C., Mosseri, R., Ingmer, H. & Cohen, S. N. (2004) Science 305, 1629-1631. [DOI] [PubMed] [Google Scholar]
- 16.Wu, S., de Lencastre, H. & Tomasz, A. (1996) J. Bacteriol. 178, 6036-6042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barbosa, T. M. & Levy, S. B. (2000) J. Bacteriol. 182, 3467-3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kieser, T., Bibb, M. B., Buttner, M. J., Chater, K. F. & Hopwood, D. A. (2000) Practical Streptomyces Genetics (The John Innes Foundation, Norwich, U.K.).
- 19.Braunstein, M., Bardarov, S. & Jacobs, W. J. (2002) Methods Enzymol. 358, 67-99. [DOI] [PubMed] [Google Scholar]
- 20.Walburger, A., Koul, A., Ferrari, G., Nguyen, L., Prescianotto-Baschong, C., Huygen, K., Klebl, B., Thompson, C., Bacher, G. & Pieters, J. (2004) Science 304, 1800-1804. [DOI] [PubMed] [Google Scholar]
- 21.Schnappinger, D., Ehrt, S., Voskuil, M. I., Liu, Y., Mangan, J. A., Monahan, I. M., Dolganov, G., Efron, B., Butcher, P. D., Nathan, C. & Schoolnik, G. K. (2003) J. Exp. Med. 198, 693-704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tusher, V. G., Tibshirani, R. & Chu, G. (2001) Proc. Natl. Acad. Sci. USA 98, 5116-5121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bentley, S. D., Chater, K. F., Cerdeno-Tarraga, A. M., Challis, G. L., Thomson, N. R., James, K. D., Harris, D. E., Quail, M. A., Kieser, H., Harper, D., et al. (2002) Nature 417, 141-147. [DOI] [PubMed] [Google Scholar]
- 24.Davis, N. K. & Chater, K. F. (1992) Mol. Gen. Genet 232, 351-358. [DOI] [PubMed] [Google Scholar]
- 25.Steyn, A. J., Collins, D. M., Hondalus, M. K., Jacobs, W. R., Jr., Kawakami, R. P. & Bloom, B. R. (2002) Proc. Natl. Acad. Sci. USA 99, 3147-3152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sun, J., Kelemen, G. H., Fernandez-Abalos, J. M. & Bibb, M. J. (1999) Microbiology 145, 2221-2227. [DOI] [PubMed] [Google Scholar]
- 27.Soliveri, J. A., Gomez, J., Bishai, W. R. & Chater, K. F. (2000) Microbiology 146, 333-343. [DOI] [PubMed] [Google Scholar]
- 28.Chater, K. F. (2001) Curr. Opin. Microbiol. 4, 667-673. [DOI] [PubMed] [Google Scholar]
- 29.Jakimowicz, P., Cheesman, M. R., Bishai, W. R., Chater, K. F., Thomson, A. J. & Buttner, M. J. (2004) J. Biol. Chem. 280, 8309-8315. [DOI] [PubMed] [Google Scholar]
- 30.Salah-Bey, K., Blanc, V. & Thompson, C. J. (1995) Mol. Microbiol. 17, 1001-1012. [DOI] [PubMed] [Google Scholar]
- 31.Saito, H., Tomioka, H. & Yoneyama, T. (1984) Antimicrob. Agents Chemother. 26, 164-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kanetsuna, F. (1985) Microbiol. Immunol. 29, 127-141. [DOI] [PubMed] [Google Scholar]
- 33.De Rossi, E., Arrigo, P., Bellinzoni, M., Silva, P. A., Martin, C., Ainsa, J. A., Guglierame, P. & Riccardi, G. (2002) Mol. Med. 8, 714-724. [PMC free article] [PubMed] [Google Scholar]
- 34.Buriankova, K., Doucet-Populaire, F., Dorson, O., Gondran, A., Ghnassia, J. C., Weiser, J. & Pernodet, J. L. (2004) Antimicrob. Agents Chemother. 48, 143-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martinez, C., Nicolas, A., van Tilbeurgh, H., Egloff, M. P., Cudrey, C., Verger, R. & Cambillau, C. (1994) Biochemistry 33, 83-89. [DOI] [PubMed] [Google Scholar]
- 36.Nathan, C. (2004) Nature 431, 899-902. [DOI] [PubMed] [Google Scholar]
- 37.Gomez, J. E. & Bishai, W. R. (2000) Proc. Natl. Acad. Sci. USA 97, 8554-8559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hèachler, H., Cohen, S. P. & Levy, S. B. (1991) J. Bacteriol. 173, 5532-5538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cole, S. T., Brosch, R., Parkhill, J., Garnier, T., Churcher, C., Harris, D., Gordon, S. V., Eiglmeier, K., Gas, S., Barry, C. E., III, et al. (1998) Nature 393, 537-544. [DOI] [PubMed] [Google Scholar]
- 40.Alvarez, H. M. & Steinbuchel, A. (2002) Appl. Microbiol. Biotechnol. 60, 367-376. [DOI] [PubMed] [Google Scholar]
- 41.Wheeler, P. R. (1994) in Tuberculosis: Pathogenesis, Protection and Control, ed. Bloom, B. R. (Am. Soc. Microbiol. Press, Washington, DC), pp. 353-385.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.