Abstract
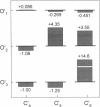
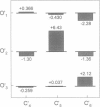
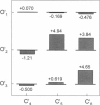
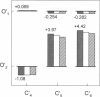
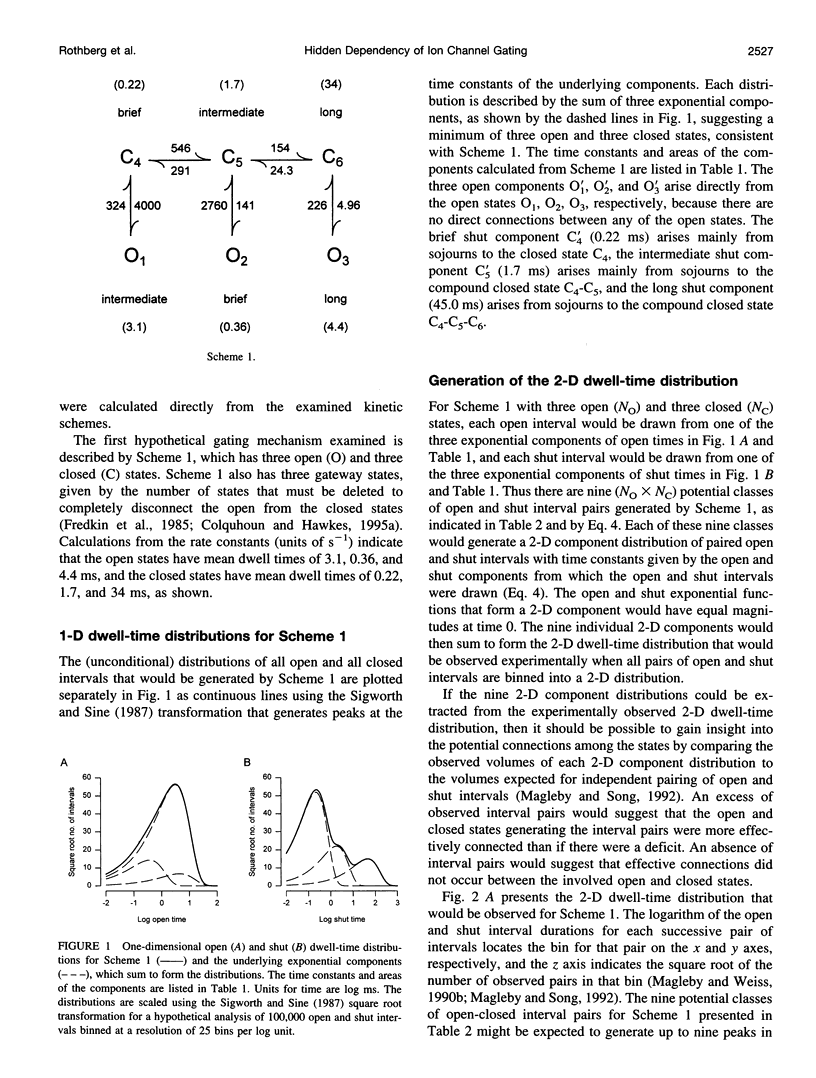
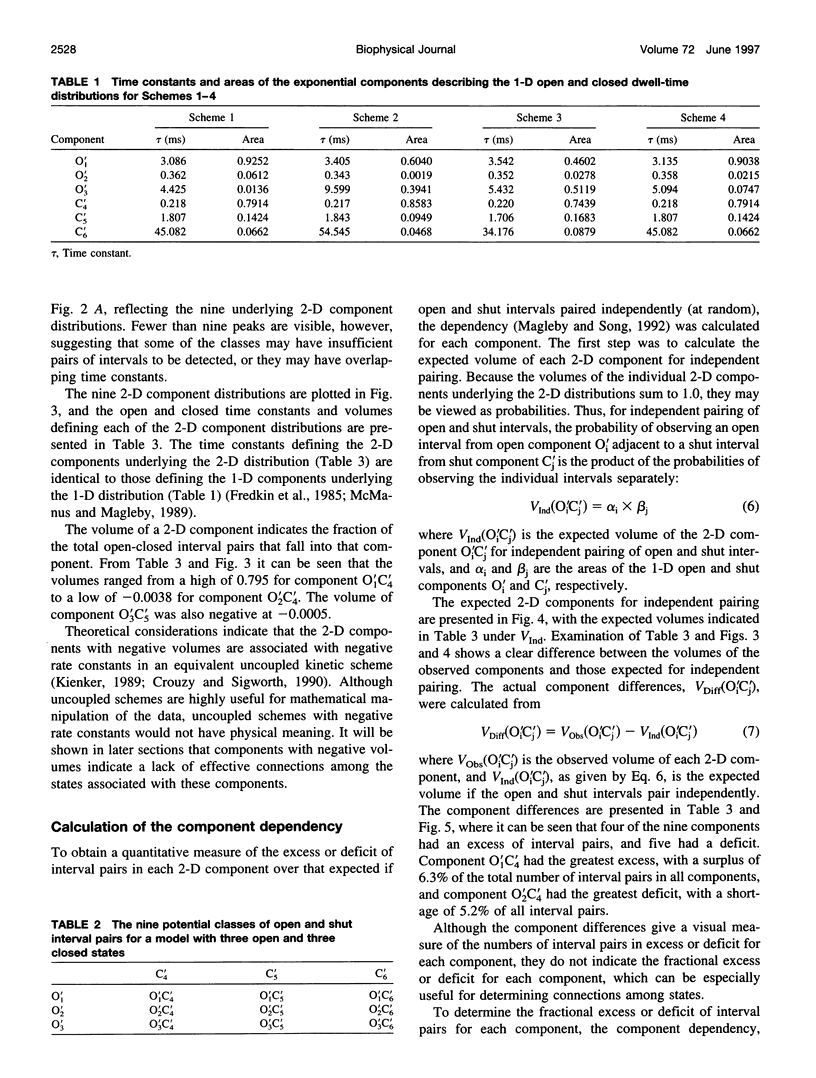
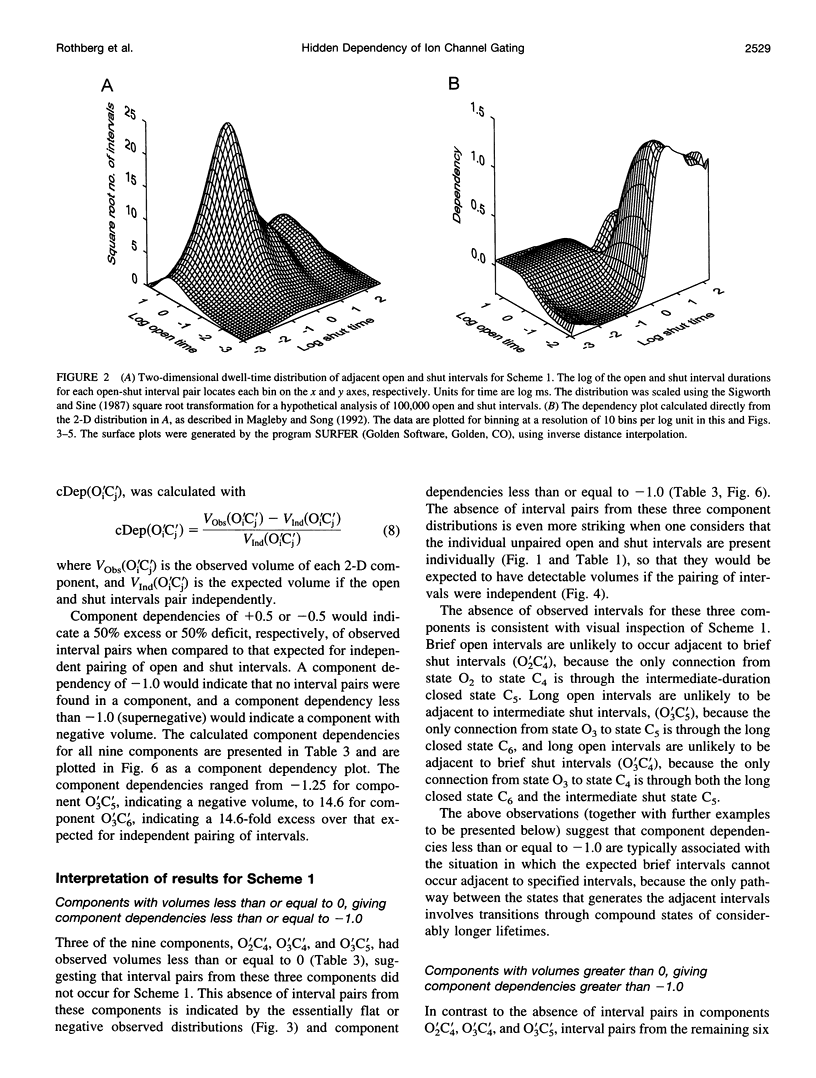
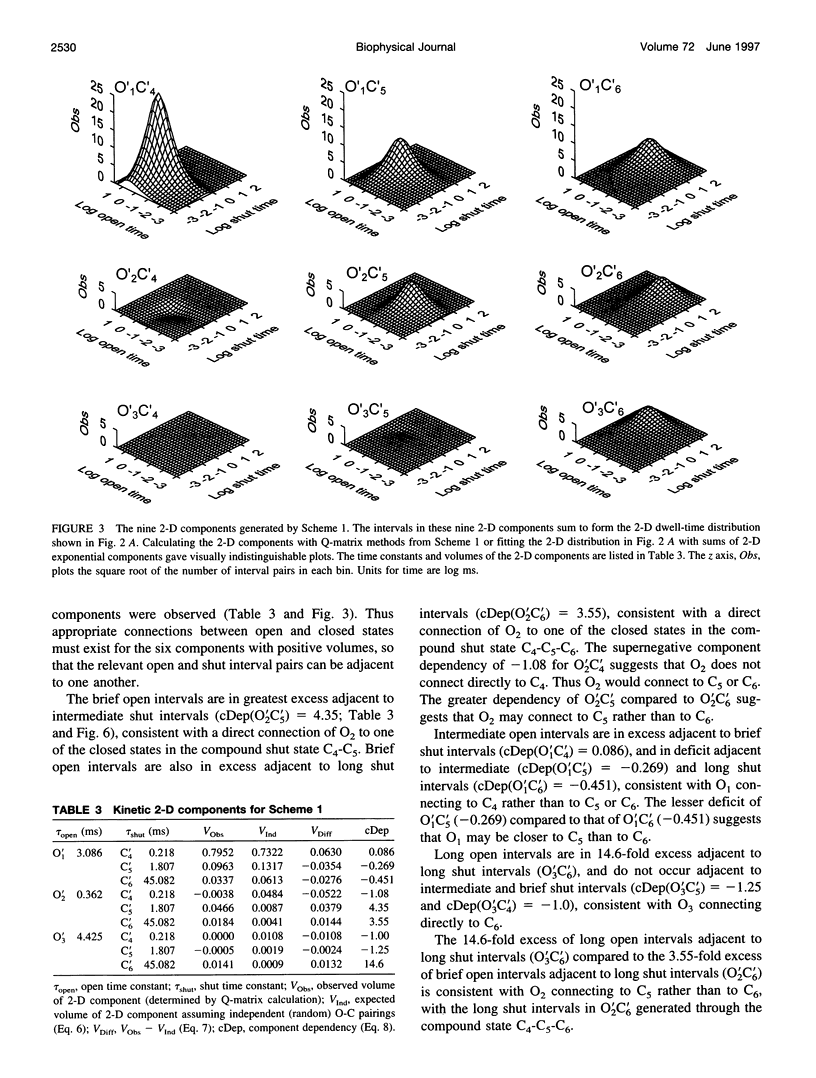
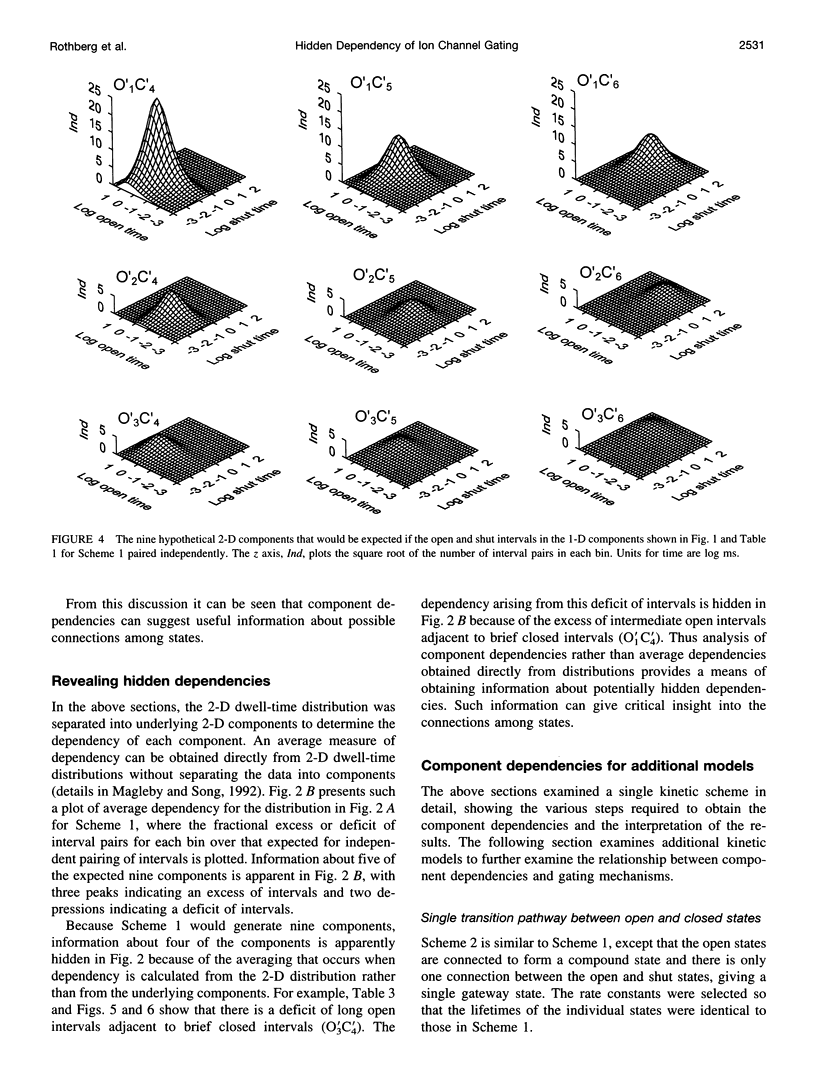
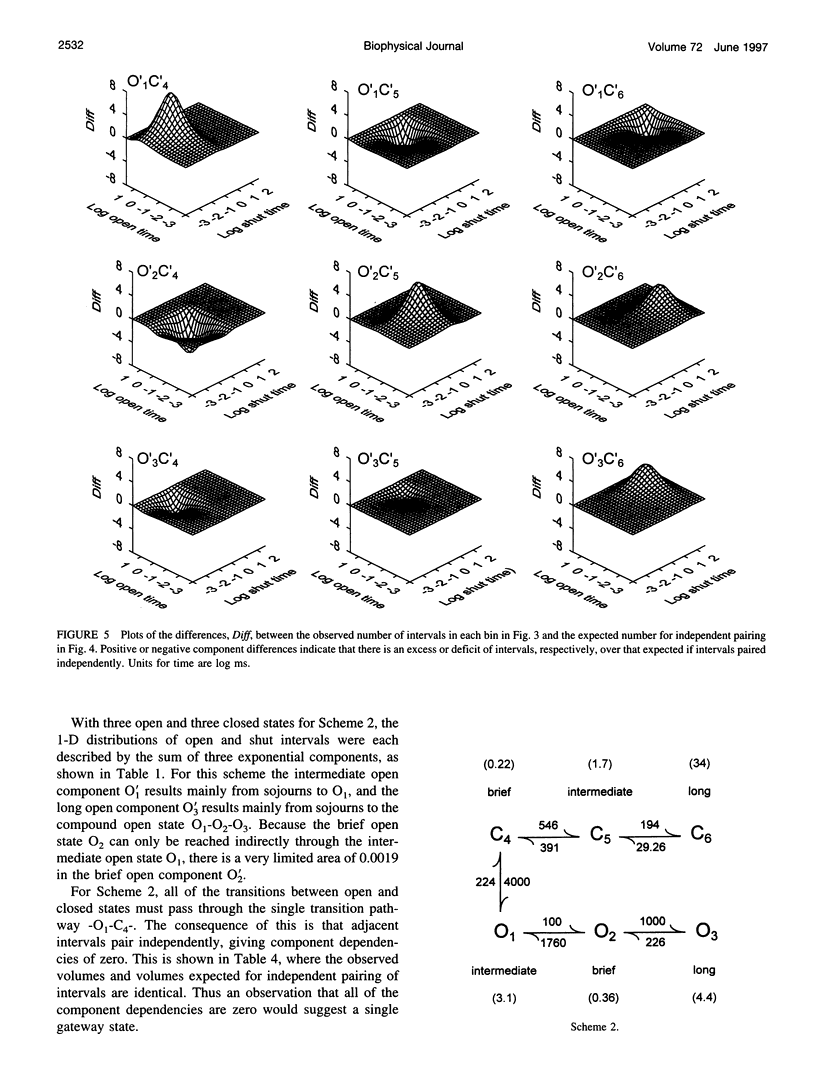
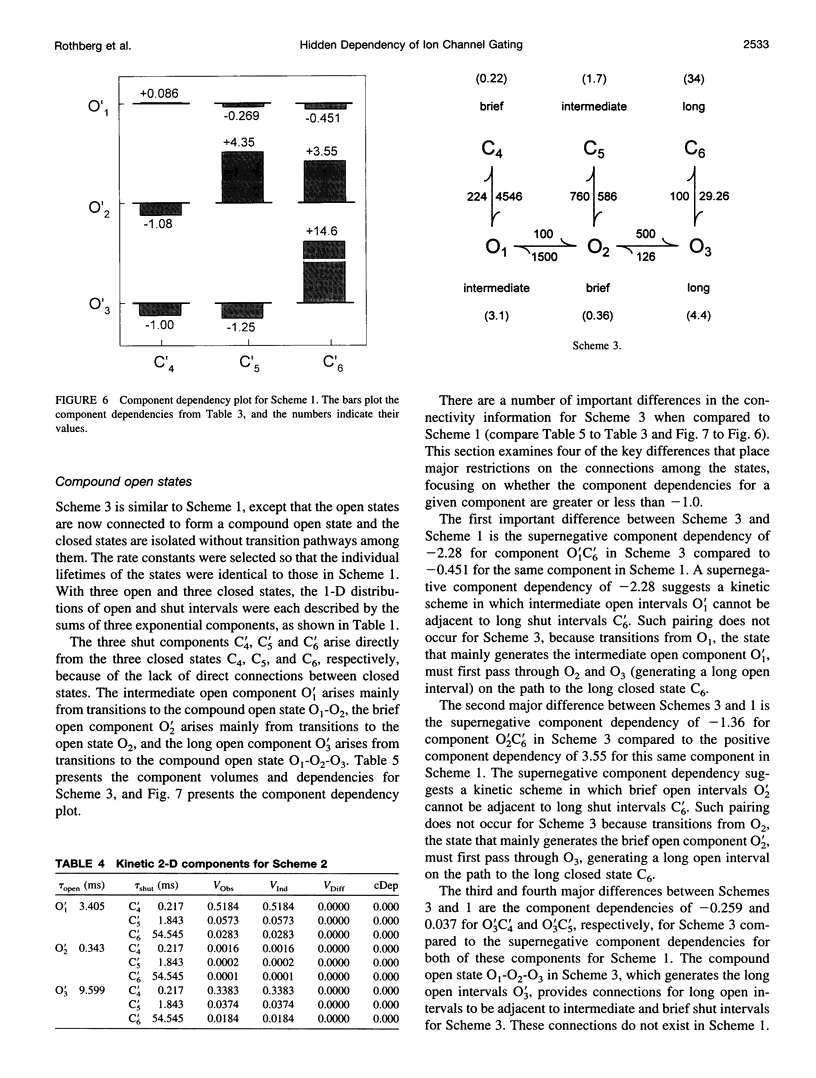
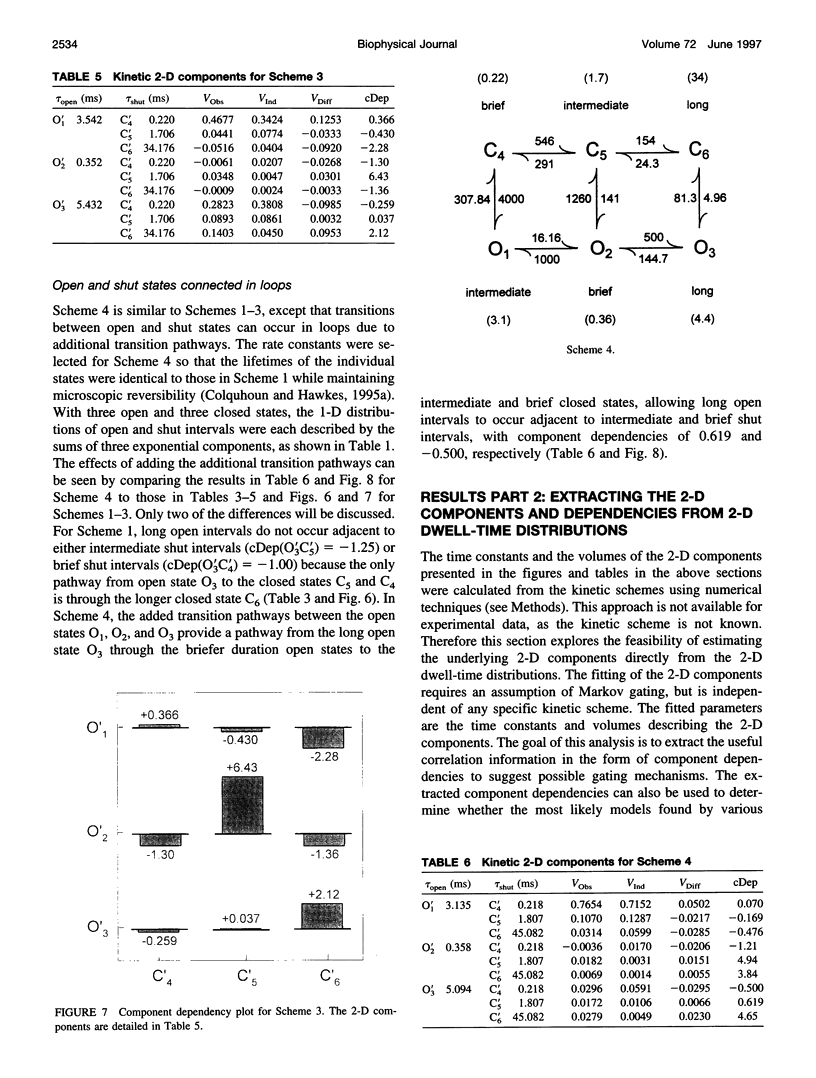
Correlations between the durations of adjacent open and shut intervals recorded from ion channels contain information about the underlying gating mechanism. This study presents an additional approach to extracting the correlation information. Detailed correlation information is obtained directly from single-channel data and quantified in a manner that can provide insight into the connections among the states underlying the gating. The information is obtained independently of any specific kinetic scheme, except for the general assumption of Markov gating. The durations of adjacent open and shut intervals are binned into two-dimensional (2-D) dwell-time distributions. The 2-D (joint) distributions are fitted with sums of 2-D exponential components to determine the number of 2-D components, their volumes, and their open and closed time constants. The dependency of each 2-D component is calculated by comparing its observed volume to the volume that would be expected if open and shut intervals paired independently. The estimated component dependencies are then used to suggest gating mechanisms and to provide a powerful means of examining whether proposed gating mechanisms have the correct connections among states. The sensitivity of the 2-D method can identify hidden components and dependencies that can go undetected by previous correlation methods.
Full text
PDF


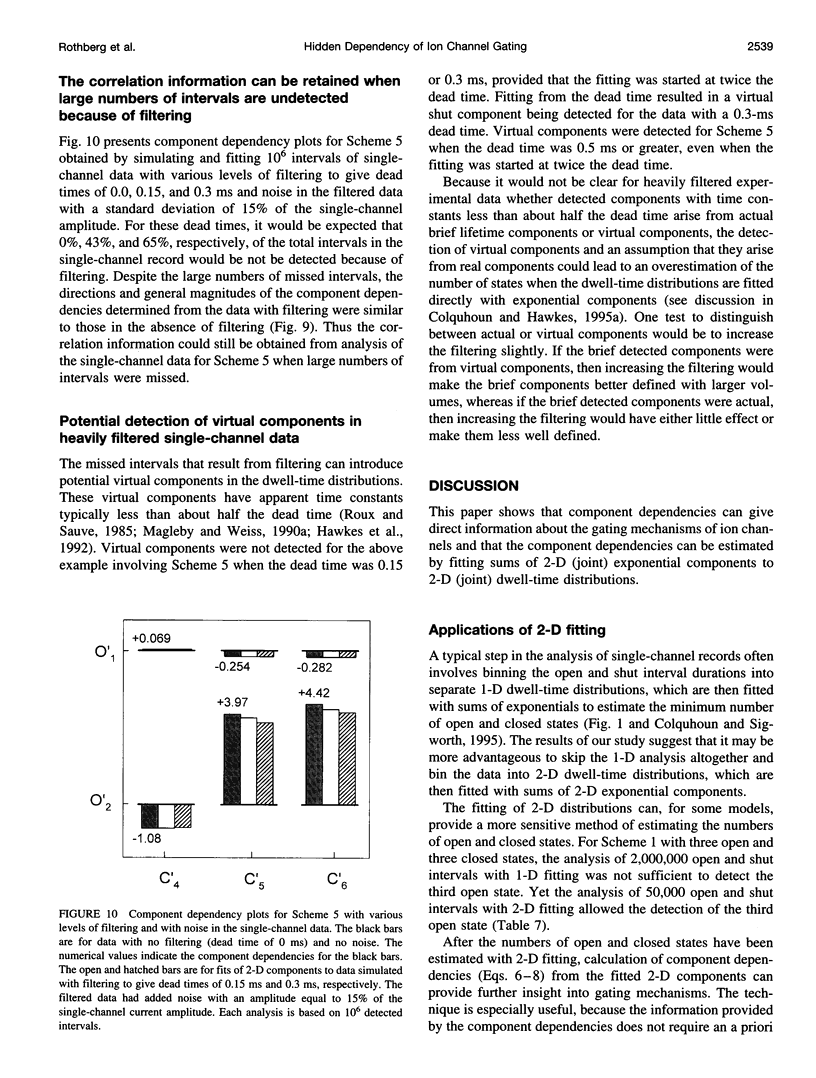




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aldrich R. W., Corey D. P., Stevens C. F. A reinterpretation of mammalian sodium channel gating based on single channel recording. Nature. 1983 Dec 1;306(5942):436–441. doi: 10.1038/306436a0. [DOI] [PubMed] [Google Scholar]
- Auerbach A. A statistical analysis of acetylcholine receptor activation in Xenopus myocytes: stepwise versus concerted models of gating. J Physiol. 1993 Feb;461:339–378. doi: 10.1113/jphysiol.1993.sp019517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball F. G., Kerry C. J., Ramsey R. L., Sansom M. S., Usherwood P. N. The use of dwell time cross-correlation functions to study single-ion channel gating kinetics. Biophys J. 1988 Aug;54(2):309–320. doi: 10.1016/S0006-3495(88)82961-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball F. G., Sansom M. S. Ion-channel gating mechanisms: model identification and parameter estimation from single channel recordings. Proc R Soc Lond B Biol Sci. 1989 May 22;236(1285):385–416. doi: 10.1098/rspb.1989.0029. [DOI] [PubMed] [Google Scholar]
- Bauer R. J., Bowman B. F., Kenyon J. L. Theory of the kinetic analysis of patch-clamp data. Biophys J. 1987 Dec;52(6):961–978. doi: 10.1016/S0006-3495(87)83289-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezanilla F., Perozo E., Stefani E. Gating of Shaker K+ channels: II. The components of gating currents and a model of channel activation. Biophys J. 1994 Apr;66(4):1011–1021. doi: 10.1016/S0006-3495(94)80882-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatz A. L., Magleby K. L. Adjacent interval analysis distinguishes among gating mechanisms for the fast chloride channel from rat skeletal muscle. J Physiol. 1989 Mar;410:561–585. doi: 10.1113/jphysiol.1989.sp017549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatz A. L., Magleby K. L. Correcting single channel data for missed events. Biophys J. 1986 May;49(5):967–980. doi: 10.1016/S0006-3495(86)83725-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung S. H., Krishnamurthy V., Moore J. B. Adaptive processing techniques based on hidden Markov models for characterizing very small channel currents buried in noise and deterministic interferences. Philos Trans R Soc Lond B Biol Sci. 1991 Dec 30;334(1271):357–384. doi: 10.1098/rstb.1991.0122. [DOI] [PubMed] [Google Scholar]
- Colquhoun D., Hawkes A. G. A note on correlations in single ion channel records. Proc R Soc Lond B Biol Sci. 1987 Feb 23;230(1258):15–52. doi: 10.1098/rspb.1987.0008. [DOI] [PubMed] [Google Scholar]
- Colquhoun D., Hawkes A. G. On the stochastic properties of single ion channels. Proc R Soc Lond B Biol Sci. 1981 Mar 6;211(1183):205–235. doi: 10.1098/rspb.1981.0003. [DOI] [PubMed] [Google Scholar]
- Colquhoun D., Sakmann B. Fast events in single-channel currents activated by acetylcholine and its analogues at the frog muscle end-plate. J Physiol. 1985 Dec;369:501–557. doi: 10.1113/jphysiol.1985.sp015912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crouzy S. C., Sigworth F. J. Yet another approach to the dwell-time omission problem of single-channel analysis. Biophys J. 1990 Sep;58(3):731–743. doi: 10.1016/S0006-3495(90)82416-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredkin D. R., Rice J. A. Maximum likelihood estimation and identification directly from single-channel recordings. Proc Biol Sci. 1992 Aug 22;249(1325):125–132. doi: 10.1098/rspb.1992.0094. [DOI] [PubMed] [Google Scholar]
- Gibb A. J., Colquhoun D. Activation of N-methyl-D-aspartate receptors by L-glutamate in cells dissociated from adult rat hippocampus. J Physiol. 1992 Oct;456:143–179. doi: 10.1113/jphysiol.1992.sp019331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahin R. Removal of inactivation causes time-invariant sodium current decays. J Gen Physiol. 1988 Sep;92(3):331–350. doi: 10.1085/jgp.92.3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkes A. G., Jalali A., Colquhoun D. Asymptotic distributions of apparent open times and shut times in a single channel record allowing for the omission of brief events. Philos Trans R Soc Lond B Biol Sci. 1992 Sep 29;337(1282):383–404. doi: 10.1098/rstb.1992.0116. [DOI] [PubMed] [Google Scholar]
- Horn R., Lange K. Estimating kinetic constants from single channel data. Biophys J. 1983 Aug;43(2):207–223. doi: 10.1016/S0006-3495(83)84341-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn R. Statistical methods for model discrimination. Applications to gating kinetics and permeation of the acetylcholine receptor channel. Biophys J. 1987 Feb;51(2):255–263. doi: 10.1016/S0006-3495(87)83331-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson M. B., Wong B. S., Morris C. E., Lecar H., Christian C. N. Successive openings of the same acetylcholine receptor channel are correlated in open time. Biophys J. 1983 Apr;42(1):109–114. doi: 10.1016/S0006-3495(83)84375-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kienker P. Equivalence of aggregated Markov models of ion-channel gating. Proc R Soc Lond B Biol Sci. 1989 Apr 22;236(1284):269–309. doi: 10.1098/rspb.1989.0024. [DOI] [PubMed] [Google Scholar]
- Magleby K. L., Pallotta B. S. Calcium dependence of open and shut interval distributions from calcium-activated potassium channels in cultured rat muscle. J Physiol. 1983 Nov;344:585–604. doi: 10.1113/jphysiol.1983.sp014957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magleby K. L., Song L. Dependency plots suggest the kinetic structure of ion channels. Proc Biol Sci. 1992 Aug 22;249(1325):133–142. doi: 10.1098/rspb.1992.0095. [DOI] [PubMed] [Google Scholar]
- Magleby K. L., Weiss D. S. Estimating kinetic parameters for single channels with simulation. A general method that resolves the missed event problem and accounts for noise. Biophys J. 1990 Dec;58(6):1411–1426. doi: 10.1016/S0006-3495(90)82487-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magleby K. L., Weiss D. S. Identifying kinetic gating mechanisms for ion channels by using two-dimensional distributions of simulated dwell times. Proc Biol Sci. 1990 Sep 22;241(1302):220–228. doi: 10.1098/rspb.1990.0089. [DOI] [PubMed] [Google Scholar]
- McManus O. B., Blatz A. L., Magleby K. L. Inverse relationship of the durations of adjacent open and shut intervals for C1 and K channels. Nature. 1985 Oct 17;317(6038):625–627. doi: 10.1038/317625a0. [DOI] [PubMed] [Google Scholar]
- McManus O. B., Blatz A. L., Magleby K. L. Sampling, log binning, fitting, and plotting durations of open and shut intervals from single channels and the effects of noise. Pflugers Arch. 1987 Nov;410(4-5):530–553. doi: 10.1007/BF00586537. [DOI] [PubMed] [Google Scholar]
- McManus O. B., Magleby K. L. Accounting for the Ca(2+)-dependent kinetics of single large-conductance Ca(2+)-activated K+ channels in rat skeletal muscle. J Physiol. 1991 Nov;443:739–777. doi: 10.1113/jphysiol.1991.sp018861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McManus O. B., Magleby K. L. Kinetic states and modes of single large-conductance calcium-activated potassium channels in cultured rat skeletal muscle. J Physiol. 1988 Aug;402:79–120. doi: 10.1113/jphysiol.1988.sp017195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McManus O. B., Magleby K. L. Kinetic time constants independent of previous single-channel activity suggest Markov gating for a large conductance Ca-activated K channel. J Gen Physiol. 1989 Dec;94(6):1037–1070. doi: 10.1085/jgp.94.6.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E., Stevens C. F. Conductance fluctuations and ionic pores in membranes. Annu Rev Biophys Bioeng. 1977;6:345–381. doi: 10.1146/annurev.bb.06.060177.002021. [DOI] [PubMed] [Google Scholar]
- Petracchi D., Barbi M., Pellegrini M., Pellegrino M., Simoni A. Use of conditioned distributions in the analysis of ion channel recordings. Eur Biophys J. 1991;20(1):31–39. doi: 10.1007/BF00183277. [DOI] [PubMed] [Google Scholar]
- Qin F., Auerbach A., Sachs F. Estimating single-channel kinetic parameters from idealized patch-clamp data containing missed events. Biophys J. 1996 Jan;70(1):264–280. doi: 10.1016/S0006-3495(96)79568-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux B., Sauvé R. A general solution to the time interval omission problem applied to single channel analysis. Biophys J. 1985 Jul;48(1):149–158. doi: 10.1016/S0006-3495(85)83768-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigworth F. J., Sine S. M. Data transformations for improved display and fitting of single-channel dwell time histograms. Biophys J. 1987 Dec;52(6):1047–1054. doi: 10.1016/S0006-3495(87)83298-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sine S. M., Claudio T., Sigworth F. J. Activation of Torpedo acetylcholine receptors expressed in mouse fibroblasts. Single channel current kinetics reveal distinct agonist binding affinities. J Gen Physiol. 1990 Aug;96(2):395–437. doi: 10.1085/jgp.96.2.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song L., Magleby K. L. Testing for microscopic reversibility in the gating of maxi K+ channels using two-dimensional dwell-time distributions. Biophys J. 1994 Jul;67(1):91–104. doi: 10.1016/S0006-3495(94)80458-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg I. Z. Frequencies of paired open-closed durations of ion channels. Method of evaluation from single-channel recordings. Biophys J. 1987 Jul;52(1):47–55. doi: 10.1016/S0006-3495(87)83187-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg I. Z. Relationship between statistical properties of single ionic channel recordings and the thermodynamic state of the channels. J Theor Biol. 1987 Jan 7;124(1):71–87. doi: 10.1016/s0022-5193(87)80253-9. [DOI] [PubMed] [Google Scholar]
- Weiss D. S., Magleby K. L. Gating scheme for single GABA-activated Cl- channels determined from stability plots, dwell-time distributions, and adjacent-interval durations. J Neurosci. 1989 Apr;9(4):1314–1324. doi: 10.1523/JNEUROSCI.09-04-01314.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagotta W. N., Hoshi T., Aldrich R. W. Shaker potassium channel gating. III: Evaluation of kinetic models for activation. J Gen Physiol. 1994 Feb;103(2):321–362. doi: 10.1085/jgp.103.2.321. [DOI] [PMC free article] [PubMed] [Google Scholar]