Abstract
Avian influenza A virus subtype H5N1 can infect humans to cause a severe viral pneumonia with mortality rates of more than 30%. The biological basis for this unusual disease severity is not fully understood. We previously demonstrated that in contrast to human influenza A virus subtypes including H1N1 or H3N2, the H5N1 virus associated with the “bird flu” outbreak in Hong Kong in 1997 (H5N1/97) hyperinduces proinflammatory cytokines, including tumor necrosis factor alpha (TNF-α), in primary human macrophages in vitro. To delineate the molecular mechanisms involved, we analyzed the role of transcription factor NF-κB and cellular kinases in TNF-α dysregulation. H5N1 and H1N1 viruses did not differ in the activation of NF-κB or degradation of IκB-α in human macrophages. However, we demonstrated that unlike H1N1 virus, H5N1/97 strongly activates mitogen-activated protein kinase (MAPK), including p38 MAPK and extracellular signal-regulated kinases 1 and 2. Specific inhibitors of p38 MAPK significantly reduced the H5N1/97-induced TNF-α expression in macrophages. Taken together, our findings suggest that H5N1/97-mediated hyperinduction of cytokines involves the p38 MAPK signaling pathway. These results may provide insights into the pathogenesis of H5N1 disease and rationales for the development of novel therapeutic strategies.
The “bird flu” outbreak in Hong Kong in 1997 caused by influenza A virus subtype H5N1 (H5N1/97) was the first documented instance of a purely avian influenza virus causing respiratory disease and death in humans. It was associated with an overall mortality rate of 33% (6 of 18 patients; 4, 30, 36). The clinical features were those of a viral pneumonia progressing to acute respiratory distress and multiple organ dysfunction syndromes associated with lymphopenia and hemophagocytosis (36). These clinical features have been associated with cytokine dysregulation (8, 13, 33). From 2001 onward, the precursor H5N1 viruses continued to reassort and gave rise to novel virus genotypes. One of these H5N1 genotypes was associated with the re-emergence of human disease in 2003 (12, 27) and led to a widespread outbreak in Asia with transmission to humans in Vietnam and Thailand in 2004 (34; World Health Organization avian influenza information at http://www.who.int/csr/disease/avian_influenza/en/). This situation poses a significant threat to human health and the potential for the emergence of a pandemic influenza virus.
We previously showed that in contrast to human influenza virus subtypes H3N2 and H1N1, the H5N1 viruses causing human disease in 1997 (H5N1/97) and 2003 (H5N1/03) induce high levels of tumor necrosis factor alpha (TNF-α) and other proinflammatory cytokines and chemokines in monocyte-derived macrophages in vitro (3, 12). Viral factors associated with the H5N1-induced hyperinduction of TNF-α and related cytokines remain to be fully defined. Using reverse genetics, we demonstrated that the nonstructural (NS) gene of the H5N1/97 influenza virus contributes, in part, to this effect. However, the human H5N1 virus isolates of 1997 and 2003 do not share common NS genes (12) and therefore other viral genes may play an important role in TNF-α induction. The cellular transcription factors and signaling pathways associated with H5N1-associated cytokine hyperinduction also remain to be investigated. Putative targets for investigation include the pathways associated with transcription factor NF-κB and the mitogen-activated protein kinases (MAPK) together with their downstream signaling cascades.
Transcription factor NF-κB plays a critical role in gene activation in response to infection, inflammation, and stress signals. Overexpression of influenza virus hemagglutinin, nucleoprotein, or matrix protein in infected cells triggers the NF-κB signaling pathway through activation of the inhibitor of NF-κB (IκB) kinase (IKK) (9). NF-κB belongs to the Rel family of dimeric transcription factors including p65 (RelA), RelB, c-Rel, p100, and p105 (16). Activation of NF-κB requires sequential phosphorylation, ubiquitination, and ultimately degradation of IκB. IκB degradation is triggered by activation of a complex of multiple kinases that is composed of IKKα and IKKβ and a structural regulatory subunit, IKKγ or NEMO. Phosphorylation of IKKα and IKKβ is activated by their upstream kinases including NF-κB-inducing kinase (16).
MAPK and their downstream signaling cascades also play crucial roles in immunological and cellular responses to external stimuli. Three well-characterized members of the MAPK superfamily are p38 MAPK, extracellular signal-regulated kinases 1 and 2 (ERK1/2), and c-jun NH2-terminal kinases (JNK). These kinases regulate gene expression at both the transcription and posttranscription levels by different mechanisms (7). Recent studies by using specific kinase inhibitors and/or dominant-negative mutant forms of kinases have demonstrated the functions of MAPK signaling pathways in influenza A virus infection. For example, it has been shown that p38 MAPK and JNK, but not ERK1/2, regulate RANTES expression in influenza virus-infected bronchial epithelial cells (20). Viral RNA induction of activator protein 1-dependent gene expression is mediated by MAPK kinase 4/JNK and MAPK kinase 7/JNK signaling cascades (21). Moreover, MAPK/ERK kinase-specific inhibitors have been used to inhibit the nuclear export of viral ribonucleoprotein complexes and impair the activity of nuclear-export protein, NEP/NS2, in the virus replication cycle (28).
To investigate the underlying mechanisms of avian influenza virus H5N1-induced cytokine dysregulation, we compared the activation of transcription factor NF-κB and MAPK signaling pathways in H5N1/97- and H1N1-infected primary human monocyte-derived macrophages in vitro. Our results showed that both H5N1/97 and H1N1 viruses activate NF-κB signaling pathways in human macrophages to comparable extents. On the other hand, within 1 h of infection, H5N1/97 differentially hyperinduces p38 MAPK and ERK1/2 phosphorylation compared with H1N1 virus. These findings are supported by the use of specific inhibitors of p38 MAPK (SB203580) and ERK1/2 (PD98059) to show that the TNF-α expression in H5N1-infected cells is significantly suppressed by SB203580 but not by PD98059. Taken together, our findings suggest that the p38 MAPK signaling pathway plays a regulatory role in H5N1-induced cytokine dysregulation.
MATERIALS AND METHODS
Reagents and antibodies.
Specific antibodies against phosphorylated and unphosphorylated forms of p38 MAPK (catalog no. 9211 and 9212), ERK1/2 (catalog no. 9101 and 9102), and JNK (catalog no. 9255 and 9252) were purchased from Cell Signaling Technology, New England Biolabs, Beverly, MA, for Western blot analysis. The specificity of the antibodies had been confirmed by previous reports (20). Anti-IκB-α (sc-203) and anti-actin (sc1616) antibodies were purchased from Santa Cruz Biotechnology, Santa Cruz, CA. Horseradish peroxidase-conjugated secondary antibodies used were anti-rabbit immunoglobulin G (IgG) and anti-mouse IgG (Transduction Lab, BD Biosciences Pharmingen, San Diego, CA) and anti-goat IgG (DAKO). Kinase inhibitors for p38 MAPK (SB203580), ERK1/2 (PD98059), and JNK (SP600159) were purchased from Calbiochem, La Jolla, CA.
Cells and viruses.
Blood mononuclear cells from healthy donors (Hong Kong Red Cross Blood Transfusion Service) were separated by Ficoll-Paque centrifugation and purified by the adherence method as previously described (3). The purity of harvested monocytes was determined by staining with fluorescein isothiocyanate-conjugated anti-CD14 antibody (Beckman Coulter, Fullerton, CA) and analyzed by using flow cytometry. Monocytes were seeded in 24-well (0.5 × 106 cells/well) or 6-well (2 × 106 cells/well) tissue culture plates for studies of RNA and protein, respectively. Monocytes were differentiated in RPMI 1640 (Gibco, Invitrogen, Grand Island, NY) supplemented with 5% heat-inactivated autologous plasma. Differentiated macrophages were obtained after 14 days of culture as previously described (3). Influenza viruses A/HK/483/97 (H5N1), isolated from a patient with H5N1 infection in 1997, and A/HK/54/98 (H1N1) were used for the experiments. The viruses were grown in MDCK cells and purified by preadsorption to and elution from turkey red blood cells (3). Virus infectivity was determined by titration on MDCK cells.
Virus infection.
The procedure of influenza virus infection was described in our previous study (3). Macrophages were infected with virus at a multiplicity of infection of 2 for 30 min at 37°C. The supernatant containing the virus inoculum was then removed, and the cells were incubated in macrophage serum-free medium (Gibco, Invitrogen, Grand Island, NY) supplemented with 0.6 μg/ml penicillin, 60 μg/ml streptomycin, and 2 μg/ml l-1-tosylamide-2-phenylethyl chloromethyl ketone-treated trypsin (Sigma, St. Louis, MO). The mock-treated control was incubated with the buffer under parallel conditions.
Real-time reverse transcription (RT)-PCR analysis of mRNA in virus-infected macrophages.
Total RNA was DNase treated and then reverse transcribed by using a TaqMan reverse transcription reagent kit (Applied Biosystems, Foster City, CA) according to the manufacturer's protocol. Analysis of real-time RT-PCR was performed with reference to the comparative CT, cycle number to threshold, as described in user bulletin no. 2 of the ABI Prism 7700 Sequence Detection System. Briefly, the levels of TNF-α mRNA and 18S rRNA (18S), the reference gene, were assayed by TaqMan gene-specific ASSAYS-ON-DEMAND reagent kits (Applied Biosystems, Foster City, CA). The CT of TNF-α was normalized to that of 18S in each sample (i.e., ΔCT). The mRNA level of virus-infected cells was relative to the uninfected cells (i.e., ΔΔCT), and the amount was given by 2−ΔΔCT. The quantity of the mRNA was statistically analyzed by two-tailed, paired t test.
The mRNA levels of H5N1 matrix and β-actin genes were assayed by SYBR Green PCR reagent kit according to the manufacturer's protocol. (Applied Biosystems, Foster City, CA). In brief, the cDNA was amplified with gene-specific primers and their sequences were as follows: H5N1 matrix protein forward, 5′-TTACTCAACTGGTGCGCTTG-3′; H5N1 matrix protein reverse, 5′-GTTGGTGGTAGTCGCCATCT-3′; human β-actin forward, 5′-TCACCCACACTGTGCCCATCT-3′; human β-actin reverse, 5′-GAACCGCTCATTGCCAATGG-3′. At the end of the PCR, dissociation curve analysis was performed to examine the specificity of the PCR product. The SYBR Green PCR data were statistically analyzed by the CT method as described above. The CT of the matrix gene was normalized to that of β-actin in each sample. The mRNA levels of the matrix gene in H5N1-infected cells with kinase inhibitors were relative to that of virus-infected cells without the inhibitors.
Preparation of cytoplasmic and nuclear proteins.
The nuclear extract preparation method used was a modified form of that of Schreiber et al. (29). In brief, macrophages (2 × 106 cells/well) were harvested at different times after infection and washed with phosphate-buffered saline. The cells were treated with cold buffer A (10 mM HEPES [pH 7.9], 10 mM KCl, 0.1 mM EDTA, 0.1 mM EGTA, 1 mM dithiothreitol [DTT], protease inhibitor cocktail) on ice for 15 min. Then, NP-40 was added to a final concentration of 0.6% to the cell lysate and removed with a cell scraper. The cytoplasmic portion of the lysate was harvested by centrifugation for 1 min at 4°C. The nuclear pellet was resuspended in 50 μl of ice-cold buffer C (20 mM HEPES [pH 7.9], 0.4 M NaCl, 1 mM EDTA, 1 mM EGTA, 1 mM DTT, protease inhibitor cocktail), and the nuclei were disrupted on ice with agitation for 15 min. The nuclear protein lysate was harvested by centrifugation for 5 min at 4°C, and all protein samples were frozen in aliquots at −70°C. The harvested proteins were quantified with a Coomassie blue staining kit (Pierce, Rockford, IL).
Electrophoretic mobility shift assay (EMSA).
Nuclear protein (2 μg) was incubated with 20 fmol of [γ-32P]ATP-labeled, double-stranded κB enhancer oligodeoxynucleotides, with sequences obtained from the region of the human immunodeficiency virus type 1 long terminal repeat (5′-GATCAGGGACTTTCCGCTGGGGACTTTCC-3′ and 5′-GGAAAGTCCCCAGCGGAAAGTCCCTGATC-3′) in binding buffer (25 mM HEPES [pH 7.9], 0.5 mM EDTA, 0.5 mM DTT, 1% NP-40, 12% glycerol, 50 mM NaCl) and 0.5 μg of poly(dI-dC) for 30 min at 37°C. A pair of oligodeoxynucleotides encoding the mutated κB binding sites (5′-GATCACTCACTTTCCGCTGCTCACTTTCC-3′ and 5′-GGAAAGTGAGCAGCGGAAAGTGAGTGATC-3′) was included in parallel experiments to test for specific binding of the DNA-protein complexes. After incubation, the reaction complex was separated from the free labeled probe in a 5% nondenaturing polyacrylamide gel using Tris-glycine buffer (50 mM Tris, 200 mM glycine, 1 mM EDTA, pH 8.5). The gel was dried for 2 h in a gel dryer (Bio-Rad) and exposed to Fuji X-ray film.
Western blot analysis.
For Western blot analysis, 30 μg of cytoplasmic protein was heat denatured in sample buffer (125 mM Tris [pH 6.8], 4% sodium dodecyl sulfate, 20% glycerol, 5% β-mercaptoethanol, 0.01% bromophenol blue), separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and transferred to a nitrocellulose membrane for assaying phosphorylated kinases by using ECL solution (Amersham Biosciences, Buckinghamshire, United Kingdom). Blots were stripped and reprobed using different antibodies against the respective nonphosphorylated kinase to determine the total amount of the indicated kinase.
Quantitative analysis of TNF-α by enzyme-linked immunosorbent assay (ELISA).
The supernatant samples of macrophage cultures were collected at 6 h after infection and irradiated with UV light before the levels of TNF-α were measured by a specific TNF-α assay kit (R&D Systems, Minneapolis, MN) as previously described (3).
RESULTS
H5N1 and H1N1 influenza viruses induce NF-κB activation in human macrophages.
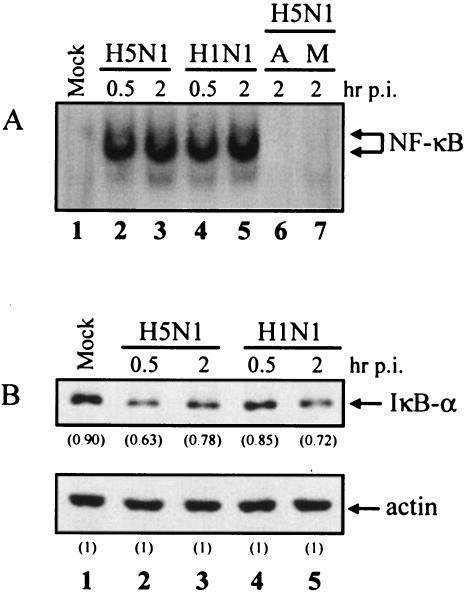
To investigate whether NF-κB is involved in H5N1-induced cytokine expression, we first measured the level of NF-κB activation by EMSA in the nuclear extract of H5N1- and H1N1-infected cells at 0.5 and 2 h after infection. Over the time course, there was a strong activation of NF-κB to comparable levels by both H5N1 and H1N1 viruses (Fig. 1A, lanes 2 to 5). This result was reproduced in macrophages from three healthy donors, suggesting that the finding was not donor specific (data not shown). The specificity of NF-κB binding was shown by incubating the nuclear extracts with 5 pmol of unlabeled κB oligodeoxynucleotide probe or with 20 fmol of the radioactively labeled, mutated κB probe (Fig. 1A, lanes 6 and 7). Our results indicate that H5N1 did not induce higher levels of NF-κB activation in primary blood macrophages compared to H1N1 virus.
FIG. 1.
(A) H5N1 and H1N1 influenza viruses activate NF-κB in human macrophages. Primary blood macrophages were mock treated (lane 1) or infected with H5N1 (lanes 2 to 3) or H1N1 (lanes 4 to 5) for 30 min (multiplicity of infection = 2). Nuclear extracts were harvested at 0.5 and 2 h after infection. The amounts of activated NF-κB were assayed by EMSA as described in Materials and Methods. Lane A, competition control (lane 6); lane M, radioactively labeled, mutated κB probe (lane 7). (B) Degradation of IκB-α in the cytoplasm of mock-treated cells (lane 1) or virus-infected cells (lanes 2 to 5) was detected by Western blotting with anti-IκB-α antibody as indicated at the top. Equal loading of the blot was shown by reprobing the blot with an anti-actin antibody (bottom). The density of the protein band was determined by using Bio-Rad Quantity One imaging software. The values in parentheses are density values of IκB-α relative to actin. Mock, uninfected cells; p.i., postinfection.
To further investigate the activation of the NF-κB signaling pathway in cytokine dysregulation, we examined the IκB-α degradation in the cytoplasmic portion of virus-infected cells by Western blot analysis. The protein levels of IκB-α in the H5N1- or H1N1-infected cells at 0 h were the same as in mock-treated cells (Fig. 1B, lane 1). IκB-α expression was reduced by 15 to 20% at 0.5 and 2 h after infection in both H5N1- and H1N1-infected cells (Fig. 1B, top, lanes 2 to 5). Similar results were found in three different donors (data not shown). Equal loading of protein samples was shown by reprobing the blot with an anti-actin antibody (Fig. 1B, bottom). Together with the EMSA, our results clearly demonstrated that H5N1 and H1N1 induce similar levels of NF-κB activation and IκB-α degradation.
H5N1 activates p38 MAPK and ERK1/2 phosphorylation but not JNK in human macrophages.
To investigate the role of MAPK in mediating H5N1-induced cytokine expression, we next measured the phosphorylation levels of p38 MAPK, ERK1/2, and JNK in the cytoplasmic protein lysate of virus-infected cells by Western analysis at different times.
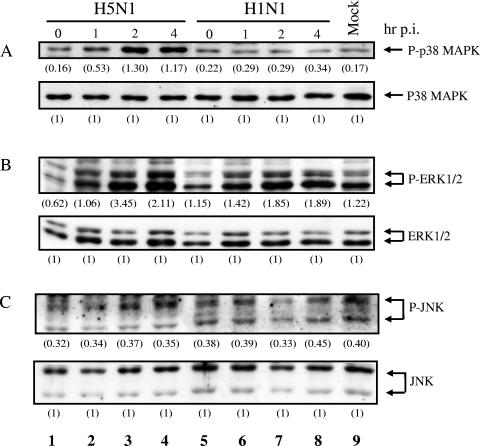
Phosphorylation of p38 MAPK was rapidly induced by the H5N1 virus at 1 h postinfection, compared with the H1N1 counterpart, in human macrophages (Fig. 2A). The maximal level of p38 MAPK activation in macrophages was reached at 2 h after H5N1 infection, and the level of activation was sustained at 4 h postinfection (Fig. 2A, lanes 1 to 4). There was a 7.6-fold induction of p38 MAPK phosphorylation in macrophages infected with H5N1 compared with mock-infected cells at 2 h postinfection (Fig. 2A, lanes 3 and 9). In contrast, there was no significant activation of p38 MAPK in H1N1-infected cells throughout the time course (Fig. 2A, lanes 5 to 8). In addition, the activation of ERK1/2 in H5N1-infected macrophages was higher than that in H1N1-infected cells at 2 and 4 h after infection (Fig. 2B, lanes 3 to 4 and 7 to 8). In contrast, there was no significant change in the levels of phosphorylation of JNK in both H5N1- and H1N1-infected cells (Fig. 2C, lanes 1 to 4 and 5 to 8). These experiments were performed in duplicate with macrophages obtained from five different blood donors with similar results. Thus, our results showed that H5N1 significantly induces the phosphorylation of p38 MAPK and moderately activates ERK1/2 but not JNK in primary human macrophages.
FIG. 2.
Activation of p38 MAPK and ERK1/2 in human macrophages infected with H5N1. Primary blood macrophages were infected with H5N1 (lanes 1 to 4) or H1N1 (lanes 5 to 8) or mock treated (lane 9) as described in Materials and Methods. Protein samples were harvested at 0, 1, 2, and 4 h after infection. The phosphorylation levels of p38 MAPK (A), ERK1/2 (B), and JNK (C) in mock-treated or virus-infected cells were assayed by Western analysis using specific antibodies. Steady-state expression levels of MAPK were found in all samples as indicated. The density of the protein band was determined by using Bio-Rad Quantity One imaging software. The values in parentheses are density values of phosphorylated MAPK relative to the total MAPK. Mock, uninfected cells; p.i., postinfection.
Inhibition of MAPK suppresses H5N1 induction of TNF-α in human macrophages.
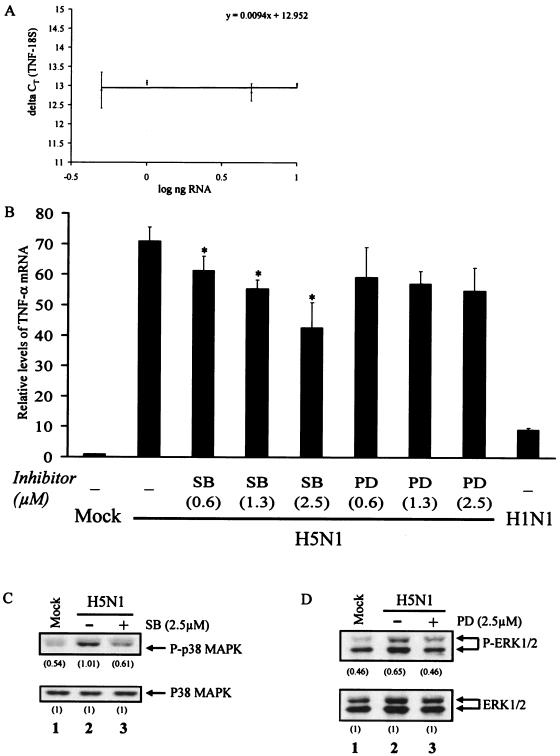
In light of the H5N1-induced activation of p38 MAPK and ERK1/2, we examined whether these kinase activities are required for cytokine dysregulation by real-time RT-PCR. We first determined the dose-dependent effects of MAPK kinase inhibitors on H5N1-induced TNF-α at 3 h postinfection, i.e., at the peak of TNF-α induction by H5N1, as previously described (3). Our assay system for TNF-α mRNA was validated by the linear relationship of the ΔCT of TNF-α and 18S to the log input of RNA (Fig. 3A). The slope of the validation plot was 0.0094 (i.e., <0.1), indicating equal amplification efficiencies of these two genes in our detection system.
FIG. 3.
Inhibition of p38 MAPK and ERK1/2 suppresses H5N1 induction of TNF-α transcription in human macrophages. The effects of p38 MAPK and ERK1/2 on H5N1-induced TNF-α transcription were investigated by kinase inhibitors SB203580 (SB) and PD98059 (PD), respectively. (A) Validation of real-time RT-PCR assay. Graded amounts of RNA from H1N1-infected cells were analyzed as described in Materials and Methods. The ΔCT results of TNF-α and 18S rRNAs were plotted against the log scale of input RNA. (B) Real-time RT-PCR analysis of the TNF-α mRNA in mock-treated and H5N1- or H1N1-infected cells. Primary blood macrophages were left untreated or treated with SB203580 (SB) or PD98059 (PD) at the indicated concentrations for 1 h, followed by infection with H5N1 for 3 h. The relative levels of TNF-α mRNA were analyzed by TaqMan real-time RT-PCR. Values represent the average ± the standard deviation of four different donors and were statistically analyzed by two-tailed, paired t test. *, P < 0.05. (C) Western analysis was performed to examine the activation of p38 MAPK and ERK1/2 in H5N1-infected cells with or without SB or PD treatment after 1 h of infection. The density of the protein band was determined by using Bio-Rad Quantity One imaging software. The values in parentheses are density values of the phosphorylated MAPK relative to the total MAPK. Mock, uninfected cells.
Primary blood macrophages were mock treated or treated with increasing concentrations of pharmacological inhibitors for p38 MAPK (SB203580) or ERK1/2 (PD98059) for 1 h before virus infection. We did not find any cellular changes including morphology and viability at the indicated concentrations at the time of harvesting. Total RNA was obtained for RT-PCR and analyzed by TaqMan TNF-α assay kit (Fig. 3B). At 3 h postinfection, the H5N1-induced TNF-α mRNA expression decreased significantly with increasing concentrations of SB203580. Specifically, the TNF-α mRNA level in cells treated with 2.5 μM SB203580 was suppressed by 41% (P < 0.05), compared to the H5N1-infected cells without treatment with the kinase inhibitor. In contrast, PD98059 did not show any dose-dependent effect on the H5N1-induced TNF-α level. For example, the TNF-α mRNA level in cells treated with 2.5 μM PD98059 was not significantly suppressed by PD98059 (P > 0.05). The real-time RT-PCR results were reproduced in five separate macrophage cultures obtained from different donors.
We also performed Western analysis to examine the inhibitory effects of kinase inhibitors on the activities of p38 MAPK or ERK1/2 after H5N1 infection. The macrophages were left untreated or treated with SB203580 or PD98059 at 2.5 μM for 1 h. The cytoplasmic proteins of H5N1-infected cells were collected at 1 h after infection. The phosphorylation levels of p38 MAPK or ERK1/2 in macrophages infected with H5N1 were reduced significantly to the basal levels, compared to cells with mock treatment (Fig. 3C and D, lanes 1 and 3).
p38 MAPK regulates TNF-α production in H5N1-infected cells.
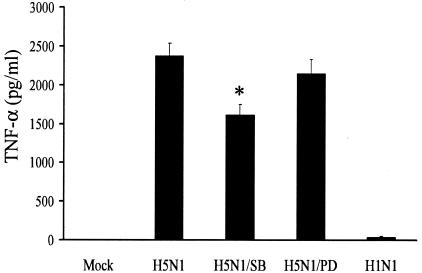
To further examine whether activation of p38 MAPK or ERK1/2 in H5N1 infection results in TNF-α production, we examined the quantities of TNF-α protein in the supernatants of kinase inhibitor-treated cells at 6 h after infection. Consistent with the real-time RT-PCR results, SB203580 reduced the TNF-α production by 32% (P < 0.05) whereas PD98059 did not have significant suppressive effects, as shown in Fig. 4. Taken together, our RT-PCR and ELISA results strongly indicated that p38 MAPK plays a statistically significant role in H5N1-induced cytokine dysregulation.
FIG. 4.
TNF-α expression in H5N1-infected cells is regulated by p38 MAPK. Primary blood macrophages were treated as described in the legend to Fig. 3. At 6 h post virus infection, the culture supernatants were harvested for ELISA. Values represent the average ± the standard deviation of three samples and were statistically analyzed by two-tailed, paired t test. *, P < 0.05; Mock, uninfected cells.
Infectivity of H5N1 is not affected by MAPK inhibitors.
To study the effects of the p38 MAPK and ERK1/2 inhibitors on viral infectivity, we measured the levels of the H5N1 matrix mRNA in virus-infected cells with or without kinase inhibitor treatment at 3 h postinfection by real-time RT-PCR assay. We did not find any significant change in the levels of matrix mRNA in cells with or without inhibitor treatment (P > 0.5; Fig. 5). The specificity of the PCR product was confirmed by using the dissociation curve software and agarose gel electrophoresis (data not shown). The results were derived from triplicate macrophage cultures, and each set of macrophages was obtained from three separate donors.
FIG. 5.
MAPK inhibitor did not show an inhibitory effect on H5N1 infectivity. Primary blood macrophages were treated with SB203580 (SB) or PD98059 (PD) at 2.5 μM for 1 h, followed by infection with H5N1 for 3 h. The relative (Rel.) levels of H5N1 matrix mRNA were analyzed by real-time RT-PCR. Cells without treatment with inhibitors were used as controls. Values represent the average ± the standard deviation of three different donors and were statistically analyzed by two-tailed, paired t test. #, P > 0.5.
DISCUSSION
We have previously shown that avian H5N1 influenza viruses associated with severe human disease in 1997 and 2003 were more potent inducers of proinflammatory cytokines in human macrophages than common human influenza virus subtype H1N1 or H3N2. Such hyperinduction of cytokines may well explain the unusual disease severity associated with these avian H5N1 viruses (3, 12, 27). In this study, we investigated the intracellular mechanisms by which H5N1/97 virus hyperinduces TNF-α production in primary human macrophages. We examined the activation of the NF-κB pathway, as well as the induction of MAPK, in H5N1/97 and H1N1 influenza virus-infected human macrophages.
It has been demonstrated that viral infection triggers the NF-κB pathway, resulting in transcriptional activation of a large number of proinflammatory genes, including cytokines and chemokines (16). To delineate the mechanisms of H5N1-induced cytokine production, we first measured NF-κB activation by EMSA in H5N1-infected macrophages at different times after infection. We chose the early time course of 0.5 and 2 h after infection in order to investigate the direct virus-induced effects and to minimize possible effects by autocrine-regulated events. The H1N1 virus was used as a control. Throughout the time period under investigation, both H5N1/97 and H1N1 viruses induced similar levels of NF-κB activation (Fig. 1A). In addition, there are no differential virus induction effects on activator protein 1 activation (data not shown). To further investigate the involvement of the NF-κB signaling pathways in H5N1-induced cytokine dysregulation, we examined IκB-α degradation in H5N1- or H1N1-infected cells by Western analysis. Consistent with our EMSA results, the level of IκB-α in macrophages infected with H5N1 or H1N1 was reduced at 2 h after infection (Fig. 1B). This lack of differential NF-κB activation indicates that the hyperinduction of TNF-α in H5N1-infected cells may be mediated by pathways independent of NF-κB.
In contrast to the lack of preferential NF-κB activation, H5N1 induced strong activation of p38 MAPK and ERK1/2 in human macrophages at 1 h postinfection compared to that of the effects of H1N1 (Fig. 2A and B). In addition, the result of the kinetic study of MAPK activation after H5N1 infection was consistent with our previous report that the maximal induction of TNF-α was detected at 3 h postinfection (3). This indicates that H5N1-stimulated MAPK activation contributed to TNF-α hyperinduction. Interestingly, we did not find any delay in the activation of p38 MAPK in macrophages after H1N1 infection. The basal activity of ERK1/2 and JNK in mock-treated cells may be due to the presence of trypsin in macrophage SFM medium during virus infection (1, 3). Addition of trypsin to the culture medium may enhance the infectivity of human influenza virus (3).
H5N1-induced TNF-α expression, as measured by TNF-α-specific mRNA and protein levels, was significantly reduced by the inhibitor of p38 MAPK but not by inhibition of ERK1/2 (Fig. 3B and 4). Interestingly, there was no synergistic effect of p38 MAPK and ERK1/2 inhibitors on H5N1-induced TNF-α mRNA and protein expression (data not shown). Our results are consistent with the previous reports that the induction of inflammatory cytokines by respiratory viruses, including respiratory syncytial virus, reovirus, and rhinovirus, in human bronchial epithelial cells is regulated by p38 MAPK activity (11, 25). The increased ERK1/2 activity in H5N1-infected cells may involve other virus-induced intracellular events, including virus propagation and viral protein export (28).
Previous studies have shown that MAPK inhibitors, including those for p38 MAPK, may suppress replication of encephalomyocarditis virus and human immunodeficiency virus type 1 by different mechanisms (15, 26). However, whether the effects of kinase inhibitors occur at the early stage of viral infection is not clear. Here we showed that the levels of H5N1 matrix mRNA did not have any significant effect on cells with or without inhibitor treatment at 3 h postinfection (Fig. 5). This indicates that the infectivity of H5N1 was not changed by the kinase inhibitor at the indicated concentrations of inhibitors.
The contribution of p38 MAPK to TNF-α up-regulation appears to be at both the levels of transcriptional activation and posttranscriptional modifications, such as mRNA stability (18, 37). Analysis of the 3′ untranslated region of H5N1-induced genes indicates that it encodes a region of pentameric tandem repeats of the AUUUA sequence or AU-rich element (ARE) commonly found in cytokine genes, including that for TNF-α. The common characteristics of the ARE-containing genes are the transient nature of their expression and instability of the transcripts with early degradation of their mRNA (32). In general, ARE plays a critical role in posttranscriptional regulation of cytokine and growth factor genes.
Recent studies have shown that the mRNA stability of TNF-α is regulated by the interaction of cis-acting ARE and p38 MAPK-mediated trans-acting RNA binding factors. These factors include HuR, the mammalian homologs of embryonic lethal abnormal vision proteins, and the zinc finger protein tristetraprolin (5, 23). In fact, we are currently investigating the involvement of p38 MAPK in posttranscriptional regulation of TNF-α in cells infected by influenza viruses. Taken together, our findings indicate that the mechanism of H5N1-induced cytokine dysregulation is mediated by the p38 MAPK signaling pathway.
In H5N1 infections, other critical pathways for the recognition of pathogens and for the initiation of antiviral response may also involve activation of the Toll-like receptor (TLR) family of proteins leading to TNF-α hyperinduction. TLRs, a major class of molecular pattern recognition receptors, are activated by direct interaction of the extracellular domain of the receptor with a pathogen-associated molecular pattern, and this results in the activation of NF-κB, IRF3, and MAPK signaling pathways (2). Recent studies have shown that TLR3 activated by double-stranded RNA and TLR7- and -8-recognized single-stranded RNA are involved in the influenza virus-triggered host immune response (6, 14, 24). Since these TLR family members can be detected in the intracellular endosomes of immune cells, the TLR signaling pathways were probably activated by TLR3 during virus replication or by TLR7 and -8 through the receptor uptake of viral particles or by fusion of the budding virus (6, 14, 22). However, the precise roles of TLRs in virus-induced immune response are still being investigated.
Differential activation of host factors, including TNF-α induction by viral factors, remains to be investigated. We previously showed that the NS gene of the H5N1/97 virus contributes to the hyperinduction of TNF-α (3). Other reports have shown that the NS1 protein of influenza A plays a critical role in counteracting virus-stimulated immune responses by binding and sequestering the double-stranded RNA generated by virus replication, thereby inhibiting nuclear export of poly(A)-containing mRNA and splicing of pre-mRNA (19, 31, 35). However, the effects of NS expression on the p38 MAPK signaling pathway remain to be defined. Recently, Kobasa et al. reported that the hemagglutinin of the 1918 influenza virus is essential for virulent infection, as well as induction of cytokines and chemokines, in mice (17). Surprisingly, this highly virulent recombinant virus did not induce high levels of TNF-α in the lungs of infected animals. Hence, a better understanding of the mechanisms of H5N1-activated kinase signaling would provide insights into the pathogenesis of highly pathogenic avian and human influenza virus infections.
The geographical extent of recent avian H5N1 outbreaks in Asia (World Health Organization avian influenza information at http://www.who.int/csr/disease/avian_influenza/en/) and associated transmission to humans highlight the pandemic threat posed by this emerging virus. The continued opportunity for interspecies transmission events from the avian reservoir to humans increases the possibility of this virus adapting to the host environment, resulting in efficient human-to-human transmission, with potentially disastrous consequences. Severe human disease has also been reported in association with another avian influenza virus, viz., subtype H7N7 virus in Holland (10). This highlights the urgency for a better understanding of the pathogenesis underlying the severity of human diseases associated with these infections and the molecular mechanisms underlying the pathogenesis. Such understanding will underpin novel strategies for the treatment of human diseases associated with H5N1/97-like infections and enhance our investigation of the pathogenesis of zoonotic infections in general.
Acknowledgments
This work was supported by research grants to Allan S. Y. Lau from the Research Grants Council of Hong Kong (HKU 7430/03 M) and the Edward S. K. Hotung Paediatrics Education and Research Fund and to Malik Peiris from the Research Grants Council of Hong Kong (HKU 7459/03), as well as the 2003 Vice Chancellors Development Fund from The University of Hong Kong.
REFERENCES
- 1.Belham, C. M., R. J. Tate, P. H. Scott, A. D. Pemberton, H. R. Miller, R. M. Wadsworth, G. W. Gould, and R. Plevin. 1996. Trypsin stimulates proteinase-activated receptor-2-dependent and -independent activation of mitogen-activated protein kinases. Biochem. J. 15:939-946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boehme, K. W., and T. Compton. 2004. Innate sensing of viruses by Toll-like receptors. J. Virol. 78:7867-7873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheung, C. Y., L. L. Poon, A. S. Lau, W. Luk, Y. L. Lau, K. F. Shortridge, S. Gordon, Y. Guan, and J. S. Peiris. 2002. Induction of proinflammatory cytokines in human macrophages by influenza A (H5N1) viruses: a mechanism for the unusual severity of human disease? Lancet 360:1831-1837. [DOI] [PubMed] [Google Scholar]
- 4.Claas, E. C., A. D. Osterhaus, R. van Beek, J. C. De Jong, G. F. Rimmelzwaan, D. A. Senne, S. Krauss, K. F. Shortridge, and R. G. Webster. 1998. Human influenza A H5N1 virus related to a highly pathogenic avian influenza virus. Lancet 351:472-477. [DOI] [PubMed] [Google Scholar]
- 5.Dean, J. L., R. Wait, K. R. Mahtani, G. Sully, A. R. Clark, and J. Saklatvala. 2001. The 3′ untranslated region of tumor necrosis factor alpha mRNA is a target of the mRNA-stabilizing factor HuR. Mol. Cell. Biol. 21:721-730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Diebold, S. S., T. Kaisho, H. Hemmi, S. Akira, and C. Reis e Sousa. 2004. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science 303:1529-1531. [DOI] [PubMed] [Google Scholar]
- 7.Dong, C., R. J. Davis, and R. A. Flavell. 2002. MAP kinases in the immune response. Annu. Rev. Immunol. 20:55-72. [DOI] [PubMed] [Google Scholar]
- 8.Fishman, D. N. 2000. Hemophagocytic syndromes and infection. Emerg. Infect. Dis. 6:601-608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flory, E., M. Kunz, C. Scheller, C. Jassoy, R. Stauber, U. R. Rapp, and S. Ludwig. 2000. Influenza virus-induced NF-κB-dependent gene expression is mediated by overexpression of viral proteins and involves oxidative radicals and activation of IκB kinase. J. Biol. Chem. 275:8307-8314. [DOI] [PubMed] [Google Scholar]
- 10.Fouchier, R. A., P. M. Schneeberger, F. W. Rozendaal, J. M. Broekman, S. A. Kemink, V. Munster, T. Kuiken, G. F Rimmelzwaan, M. Schutten, G. J. Van Doornum, G. Koch, A. Bosman, M. Koopmans, and A. D. Osterhaus. 2004. Avian influenza A virus (H7N7) associated with human conjunctivitis and a fatal case of acute respiratory distress syndrome. Proc. Natl. Acad. Sci. USA 101:1356-1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Griego, S. D., C. B. Weston, J. L. Adams, R. Tal-Singer, and S. B. Dillon. 2000. Role of p38 mitogen-activated protein kinase in rhinovirus-induced cytokine production by bronchial epithelial cells. J. Immunol. 165:5211-5220. [DOI] [PubMed] [Google Scholar]
- 12.Guan, Y., L. L. M. Poon, C. Y. Cheung, T. M. Ellis, W. Lim, A. S. Lipatov, K. H. Chan, K. M. Strum-Ramirez, C. L. Cheung, Y. H. C. Leung, K. Y. Yuen, R. G. Webster, and J. S. Peiris. 2004. H5N1 influenza: a protean pandemic threat. Proc. Natl. Acad. Sci. USA 101:8156-8161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Headley, A. S., E. Tolley, and G. U. Meduri. 1997. Infections and the inflammatory response in acute respiratory distress syndrome. Chest 111:1306-1321. [DOI] [PubMed] [Google Scholar]
- 14.Heil, F., H. Hemmi, H. Hochrein, F. Ampenberger, C. Kirschning, S. Akira, G. Lipford, H. Wagner, and S. Bauer. 2004. Species-specific recognition of single-stranded RNA via Toll-like receptor 7 and 8. Science 303:1526-1529. [DOI] [PubMed] [Google Scholar]
- 15.Hirasawa, K., A. Kim, H. S. Han, J. Han, H. S. Jun, and J. W. Yoon. 2003. Effect of p38 mitogen-activated protein kinase on the replication of encephalomyocarditis virus. J. Virol. 77:5649-5656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karin, M., and B.-N. Yinon. 2000. Phosphorylation meets ubiquitination: the control of NF-κB activity. Annu. Rev. Immunol. 18:621-663. [DOI] [PubMed] [Google Scholar]
- 17.Kobasa, D., A. Takada, K. Shinya, M. Hatta, P. Halfmann, S. Theriault, H. Suzuki, H. Nishimura, K. Mitamura, N. Sugaya, T. Usui, T. Murata, Y. Maeda, S. Watanabe, M. Suresh, T. Suzuki, Y. Suzuki, H. Feldmann, and Y. Kawaoka. 2004. Enhanced virulence of influenza A viruses with the haemagglutinin of the 1918 pandemic virus. Nature 431:703-707. [DOI] [PubMed] [Google Scholar]
- 18.Kontoyiannis, D., A. Kotlyarov, E. Carballo, L. Alexopoulou, P. J. Blackshear, M. Gaestel, R. Davis, R. Flavell, and G. Kollias. 2001. Interleukin-10 targets p38 MAPK to modulate ARE-dependent TNF mRNA translation and limit intestinal pathology. EMBO J. 20:3760-3770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krug, R. M. 1998. Unique functions of the NS1 protein, p. 82-92. In K. G. Nicholson, R. G. Webster, and A. J. Hay (ed.), Textbook of influenza. Blackwell Science Ltd., Oxford, United Kingdom.
- 20.Kujime, K., S. Hashimoto, Y. Gon, K. Shimizu, and T. Horie. 2000. p38 mitogen-activated protein kinase and c-jun-NH2-terminal kinase regulate RANTES production by influenza virus-infected human bronchial epithelial cells. J. Immunol. 164:3222-3228. [DOI] [PubMed] [Google Scholar]
- 21.Ludwig, S., C. Ehrhardt, E. R. Neumeier, M. Kracht, U. R. Rapp, and S. Pleschka. 2001. Influenza virus-induced AP-1-dependent gene expression requires activation of the JNK signaling pathway. J. Biol. Chem. 276:10990-10998. [PubMed] [Google Scholar]
- 22.Lund, J. M., L. Alexopoulou, A. Sato, M. Karow, N. C. Adams, N. W. Gale, A. Iwasaki, and R. A. Flavell. 2004. Recognition of single-stranded RNA viruses by Toll-like receptor 7. Proc. Natl. Acad. Sci. USA 101:5598-5603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mahtani, K. R., M. Brook, J. L. Dean, G. Sully, J. Saklatvala, and A. R. Clark. 2001. Mitogen-activated protein kinase p38 controls the expression and posttranslational modification of tristetraprolin, a regulator of tumor necrosis factor alpha mRNA stability. Mol. Cell. Biol. 21:6461-6469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matsumoto, M., K. Funami, H. Oshiumi, and T. Seya. 2004. Toll-like receptor 3: a link between Toll-like receptor, interferon and viruses. Microbiol. Immunol. 48:147-154. [DOI] [PubMed] [Google Scholar]
- 25.Meusel, T. R., and F. Imani. 2003. Viral induction of inflammatory cytokines in human epithelial cells follows a p38 mitogen-activated protein kinase-dependent but NF-κB-independent pathway. J. Immunol. 171:3768-3774. [DOI] [PubMed] [Google Scholar]
- 26.Muthumani, K., S. A. Wadsworth, N. S. Dayes, D. S. Hwang, A. Y. Choo, H. R. Abeysinghe, J. J. Siekierka, and D. B. Weiner. 2004. Suppression of HIV-1 viral replication and cellular pathogenesis by a novel p38/JNK kinase inhibitor. AIDS 18:739-748. [DOI] [PubMed] [Google Scholar]
- 27.Peiris, J. S. M., W. C. Yu, C. W. Leung, C. Y. Cheung, W. F. Ng, J. M. Nicholls, T. K. Ng, K. H. Chan, S. T. Lai, W. L. Lim, K. Y. Yuen, and Y. Guan. 2004. Re-emergence of fatal human influenza A subtype H5N1 disease. Lancet 363:617-619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pleschka, S., T. Wolff, C. Ehrhardt, G. Hobom, O. Planz, U. R. Rapp, and S. Ludwig. 2001. Influenza virus propagation is impaired by inhibition of the Raf/MEK/ERK signalling cascade. Nat. Cell Biol. 3:301-305. [DOI] [PubMed] [Google Scholar]
- 29.Schreiber, E., P. Matthias, M. M. Muller, and W. Schaffner. 1989. Rapid detection of octamer binding proteins with ‘mini-extracts’, prepared from a small number of cells. Nucleic Acids Res. 17:6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Subbarao, K., A. Klimov, J. Katz, H. Regnery, W. Lim, H. Hall, M. Perdue, D. Swayne, C. Bender, J. Huang, M. Hemphill, T. Rowe, M. Shaw, X. Xu, K. Fukuda, and N. Cox. 1998. Characterization of an avian influenza A (H5N1) virus isolated from a child with a fatal respiratory illness. Science 279:393-396. [DOI] [PubMed] [Google Scholar]
- 31.Talon, J., C. M. Horvath, R. Polley, C. F. Basler, T. Muster, P. Palese, and A. Garcia-Sastre. 2000. Activation of interferon regulatory factor 3 is inhibited by the influenza A virus NS1 protein. J. Virol. 74:7989-7996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tebo, J., S. Der, M. Frevel, K. S. Khabar, B. R. Williams, and T. A. Hamilton. 2003. Heterogeneity in control of mRNA stability by AU-rich elements. J. Biol. Chem. 278:12085-12093. [DOI] [PubMed] [Google Scholar]
- 33.To, K. F., P. K. Chan, K. F. Chan, W. K. Lee, W. Y. Lam, K. F. Wong, N. L. Tang, D. N. Tsang, R. Y. Sung, T. A. Buckley, J. S. Tam, and A. F. Cheng. 2001. Pathology of fatal human infection associated with avian influenza A H5N1 virus. J. Med. Virol. 63:242-246. [DOI] [PubMed] [Google Scholar]
- 34.Tran, T. H., T. L. Nguyen, T. D. Nguyen, T. S. Luong, P. M. Pham, V. C. Nguyen, T. S. Pham, C. D. Vo, T. Q. Le, T. T. Ngo, B. K. Dao, P. P. Le, T. T. Nguyen, T. L. Hoang, V. T. Cao, T. G. Le, D. T. Nguyen, H. N. Le, K. T. Nguyen, H. S. Le, V. T. Le, D. Christiane, T. T. Tran, J. de Menno, C. Schultsz, P. Cheng, W. Lim, P. Horby, J. Farrar, and the World Health Organization International Avian Influenza Investigative Team. 2004. Avian influenza A (H5N1) in 10 patients in Vietnam. N. Engl. J. Med. 350:1179-1188. [DOI] [PubMed] [Google Scholar]
- 35.Wang, X., M. Li, H. Zheng, T. Muster, P. Palese, A. A. Beg, and A. Garcia-Sastre. 2000. Influenza A virus NS1 protein prevents activation of NF-κB and induction of alpha/beta interferon. J. Virol. 74:11566-11573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yuen, K. Y., P. K. Chan, M. Peiris, D. N. Tsang, T. L. Que, K. F. Shortridge, P. T. Cheung, W. K. To, E. T. Ho, R. Sung, and A. F. Cheng. 1998. Clinical features and rapid viral diagnosis of human disease associated with avian influenza A H5N1 virus. Lancet 351:467-471. [DOI] [PubMed] [Google Scholar]
- 37.Zhu, W., J. S. Downey, J. Gu, F. Di Padova, H. Gram, and J. Han. 2000. Regulation of TNF expression by multiple mitogen-activated protein kinase pathways. J. Immunol. 164:6349-6358. [DOI] [PubMed] [Google Scholar]