Abstract
Stimulation of fibroblast growth factor receptor-1 (FGFR-1) is known to result in phosphorylation of tyrosine 766 and the recruitment and subsequent activation of phospholipase C-γ (PLC-γ). To assess the role of tyrosine 766 in endothelial cell function, we generated endothelial cells expressing a chimeric receptor, composed of the extracellular domain of the PDGF receptor-α and the intracellular domain of FGFR-1. Mutation of tyrosine 766 to phenylalanine prevented PLC-γ activation and resulted in a reduced phosphorylation of FRS2 and reduced activation of the Ras/MEK/MAPK pathway relative to the wild-type chimeric receptor. However, FGFR-1–mediated MAPK activation was not dependent on PKC activation or intracellular calcium, both downstream mediators of PLC-γ activation. We report that the adaptor protein Shb is also able to bind tyrosine 766 in the FGFR-1, via its SH2 domain, resulting in its subsequent phosphorylation. Overexpression of an SH2 domain mutant Shb caused a dramatic reduction in FGFR-1–mediated FRS2 phosphorylation with concomitant perturbment of the Ras/MEK/MAPK pathway. Expression of the chimeric receptor mutant and the Shb SH2 domain mutant resulted in a similar reduction in FGFR-1–mediated mitogenicity. We conclude, that Shb binds to tyrosine 766 in the FGFR-1 and regulates FGF-mediated mitogenicity via FRS2 phosphorylation and the subsequent activation of the Ras/MEK/MAPK pathway.
INTRODUCTION
Fibroblast growth factors (FGFs) constitute a family of heparin-binding polypeptides involved in the regulation of biological responses such as growth, differentiation and angiogenesis. The FGF family currently consists of 23 members, with FGF-1 (acidic FGF) and FGF-2 (basic FGF) the most extensively studied. The biological effects of FGFs are mediated by four structurally related receptor tyrosine kinases, denoted FGFR-1, FGFR-2, FGFR-3, and FGFR-4. The binding of FGF to its receptor, results in receptor dimerization and subsequent autophosphorylation on specific tyrosine residues, within the intracellular domain (Ullrich and Schlessinger, 1990). A number of autophosphorylation sites in the FGFR-1 have been identified (for a review, see Cross and Claesson-Welsh, 2001): Y463 in the juxtamembrane, which is responsible for binding Crk (Larsson et al., 1999), Y583/585 in the kinase insert, Y653/654 in the kinase domain, which are critical for kinase activity, Y730, and the C-terminal Y766, which is the binding site for phospholipase C-γ (PLC-γ) (Mohammadi et al., 1991).
Unlike other receptor tyrosine kinases, the FGF receptors do not directly bind Grb2. Instead, activation of the Ras/MEK/MAPK pathway is achieved via the phosphorylation of a 90-kDa protein denoted FRS2 (Klint et al., 1995; Kouhara et al., 1997). FRS2 lacks an SH2 domain but contains a phosphotyrosine binding (PTB) domain that mediates phosphotyrosine-independent binding to the juxtamembrane region of the FGFR-1 (Xu et al., 1998). Activation of FGFR-1 leads to the tyrosine phosphorylation of FRS2 on several sites, allowing the binding of Grb2 and the phosphotyrosine phosphatase Shp-2. Binding of both these molecules is required for maximal activation of MAPK (Hadari et al., 1998).
The binding of PLC-γ to tyrosine 766 in the FGFR-1 results in the phosphorylation and activation of this molecule. Active PLC-γ hydrolyzes the membrane phospholipid phosphatidylinositol 4,5-bisphosphate (PIP2), generating inositol 1,4,5-trisphosphate (IP3) and diacylglycerol (DAG). IP3 is responsible for releasing calcium from internal stores whereas DAG activates certain PKC isoforms (Divecha and Irvine, 1995). The physiological role of PLC-γ in FGFR-1 signaling has been investigated by mutation of tyrosine 766 to phenylalanine (Y766F), which prevents the binding and subsequent activation of PLC-γ. A number of studies have reported that FGFR-1–mediated PLC-γ activation is not required for FGFR-1–mediated mitogenesis (Mohammadi et al., 1992; Peters et al., 1992), chemotaxis (Clyman et al., 1994; Landgren et al., 1998), and neurite outgrowth (Spivak-Kroizman et al., 1994). However, recent data have shown that mutation of tyrosine 766 has a profound effect on FGFR-1–mediated cytoskeletal reorganization in endothelial cells (Cross et al., 2000). Biochemical analysis has demonstrated that mutation of tyrosine 766 results in a reduced activation of MAPK, which occurs at the level of Raf (Huang et al., 1995). However, the conclusions drawn from all these studies assume that PLC-γ is the sole protein binding to tyrosine 766 in the FGFR-1.
Shb is a ubiquitously expressed adaptor protein that was first identified as a serum-inducible gene in insulin-producing βTC-1 cells (Welsh et al., 1994). Shb contains a carboxy terminal SH2 domain, which interacts with the PDGF β-receptor (Welsh et al., 1994), the FGFR-1, and the T-cell receptor–associated ζ-chain (Karlsson et al., 1995; Welsh et al., 1998), a central PTB domain that interacts with phosphorylated p36/38 (LAT; Lindholm et al., 1999) and a number of N-terminal proline-rich motifs that mediate its interaction with the SH3 domains of Eps 8, p85α subunit of PI3-kinase, Grb2, and Src (Karlsson et al., 1995). Overexpression of Shb in PC12 cells has been shown to enhance NGF- and FGF-stimulated neurite outgrowth (Karlsson et al., 1998), suggesting that Shb may play a role in FGF signaling.
We have used an immortalized brain endothelial cell line (IBE; Kanda et al., 1996) and retroviral-mediated gene transfer to generate a number of clones expressing chimeric receptors, composed of the extracellular domain of the PDGFR-α and the intracellular domain of the FGFR-1. We sought to define the molecular mechanism by which tyrosine 766, in the FGFR-1, regulates the MAPK pathway and mitogenicity, in endothelial cells.
MATERIALS AND METHODS
Antibodies and Reagents
The goat polyclonal anti–PDGFR-α (extracellular domain) antibody was purchased from R&D (Abingdon, United Kingdom). The rabbit polyclonal anti-FGFR-1 (intracellular domain) antibody was generated against a synthetic peptide encompassing the final 16 C-terminal amino acid residues in the FGFR-1. The rabbit polyclonal anti-Shb antibody was generated as previously described (Karlsson and Welsh, 1996). The anti-FGFR mAb (McAb6) was a kind gift from Dr. P. Maher (The Scripps Institute, La Jolla, CA). The anti-FRS2 polyclonal antibody was a kind gift from Dr. I. Lax (New York University). The anti-Shp-2 antibody was purchased from Santa Cruz (Santa Cruz, CA). Phosphospecific antibodies against MEK (Ser 217/221), p42/44 MAPK (Thr 202/Tyr 204), and antibodies against MEK and p42/44 MAPK were purchased from New England Biolabs (Beverly, MA). The 4G10 antiphosphotyrosine antibody was purchased from Upstate Biotechnology (Lake Placid, NY). The monoclonal pan-Ras antibody was purchased from Oncogene Science (Manhasset, NY). Gö 6983, GF109203X, 8-Br-cAMP, forskolin, PD98059, U0126, and PP2, p13-Agarose were purchased from Calbiochem (La Jolla, CA). Phorbol 12-myristate 13-acetate (PMA), leupeptin, chloroquine, polybrene, puromycin, fibronectin (Human), fatty-acid–free bovine serum albumin (BSA), and gelatin (porcine) were purchased from Sigma (St. Louis, MO). FGF-2 and isopropyl-1-thio-β-d-galactopyranoside (IPTG) were purchased from Boehringer Mannheim (Indianapolis, IN). The BCA protein assay kit was purchased from Pierce (Rockford, IL). PDGF-AA and murine interferon-γ (IFN-γ) were purchased from Peprotec (Rocky Hill, NJ). AG-X8 anion exchange resin was from Bio-Rad. All cell culture media was purchased from Life Technologies (Rockville, MD). Glutathione sepharose, [3H]myo-inositol, [3H]thymidine, and [γ-32P]ATP were purchased from Amersham/Pharmacia Biotech (Piscataway, NJ). Protein-A sepharose was purchased from EC Diagnostics AB (Uppsala, Sweden).
Cell Culture and Retrovirus-mediated Gene Expression
IBE cells were routinely cultured on 1% gelatin-coated dishes in Ham's F-12 medium containing 15% fetal calf serum (FCS) and 20 U/ml IFN-γ at 33°C (Kanda et al., 1996). For experimental purposes, cells were trypsinized and plated at the required density on plastic dishes coated with fibronectin (20 μg/ml) and 0.1% gelatin in Ham's F-12 medium containing 15% FCS in the absence of IFN-γ and cultured at 33°C. The chimeric PDGFR-α/FGFR-1 (denoted α/wt) cDNA and PDGFR- α/FGFR-1:Y766F mutant denoted (α/Y766F) cDNA were constructed as previously described (Landgren et al., 1998). The chimeric receptor cDNAs were inserted into the retroviral vector pBABE Puro and all constructs verified by sequencing. The wild-type Shb cDNA and R522K Shb mutant cDNA (point mutation resulting in the inactivation of SH2 domain binding; (Welsh et al., 1998) were inserted into the retroviral vector pBABE Puro. The packaging cell line Bosc-23 (Pear et al., 1993) was incubated in 25 μM chloroquine before transient transfection with the relevant pBABE Puro cDNA construct. Supernatant containing viruses was collected 48 h later. This supernatant was then added to IBE cells in the presence of polybrene (4 μg/ml), and cells were incubated for 5 h. Fresh growth medium was then added, and the cells grown for a further 48 h before selection in puromycin (5 μg/ml). Selection medium was changed every other day. Clones were selected after 7 d. IBE clones expressing either wild-type or mutant chimeric receptors were identified by Western blotting followed by in vitro kinase assay. IBE clones overexpressing either wild-type Shb or the Shb mutant were identified by Western blotting.
In Vitro Kinase Assay
Cells were grown in six-well plates until confluent. The medium was changed to serum-free Ham's F-12 containing 0.2% BSA, and the cells incubated for a further 24 h. Cells were stimulated with either medium or PDGF-AA (30 ng/ml) for 8 min at 37°C. After stimulation, cells were lysed in 20 mM Tris/HCl, pH 7.5, 150 mM NaCl, 1% Triton X-100, 1 mM dithiothreitol (DTT), 500 μM Na3VO4, 10 μg/ml aprotinin, 10 μg/ml leupeptin, and 1 mM phenylmethylsulfonyl fluoride (PMSF). Lysates were transferred to Eppendorf tubes and centrifuged at 14,000 × g for 20 min at 4°C. The supernatants were then transferred to fresh Eppendorf tubes and incubated with antisera for 1 h at 4°C followed by incubation for 30 min with Protein A Sepharose (EC Diagnostics AB). Immune complexes were collected by centrifugation and washed twice with lysis buffer and twice with phosphate-buffered saline (PBS). Kinase assays were performed for 15 min at 37°C in 40 μl of kinase buffer (20 mM HEPES, pH 7.5, 10 mM MnCl2, and 0.05% Triton X-100, containing 5 μCi [γ-32P]ATP). Reactions were terminated by addition of 40 μl of Laemmli buffer (8% SDS, 0.5 M Tris/HCl, pH 8.8, 1 M sucrose, 10 mM EDTA, 0.02% Bromophenol Blue, and 4% β-mercaptoethanol), and samples were boiled for 5 min. Proteins were resolved by SDS-PAGE on 8% polyacrylamide gels. After fixation in 10% glutaraldehyde the gel was treated with 1 M KOH at 55°C for 45 min to remove serine phosphorylation. The gel was dried and analyzed using a Bio-Imager BAS-1800II (Fuji).
PLC Assay
PLC activity was determined by measuring the accumulation of [3H]inositol phosphates in the presence of 20 mM LiCl from [3H]myo-inositol–labeled cells as previously described (Plevin et al., 1990).
Expression and Purification of GST-fusion Proteins
The bacteria Escherichia coli (BL-21) harboring the plasmid pGEX-KG containing the Raf1-Ras binding domain (Raf1-RBD; amino acids 1–149) fused to glutathione S-transferase (GST) was kindly provided by Dr. Bengt Hallberg (University of Umeå, Sweden). The culture was grown overnight at 37°C with agitation. The next day the bacterial culture was split 1:10 and grown for 1 h at 37°C with shaking. Expression was induced by the addition of 1 mM IPTG for 3 h at 37°C with shaking. The bacteria were lysed by sonication in 20 mM Tris, pH 7.5, containing 1% Triton X-100, 1 mM EDTA, 1 mM EGTA, 10 μg/ml aprotinin, 10 μg/ml leupeptin, 1 mM PMSF, and 1 mM DTT. The lysate was clarified by centrifugation and incubated with glutathione sepharose for 1 h at 4°C with end-over-end mixing. The sepharose beads were washed four times in lysis buffer and finally resuspended as a 1:1 slurry in lysis buffer containing 50% glycerol and stored at −20°C. The Shb-SH2-GST fusion protein was prepared as previously described (Welsh et al., 1994).
Active Ras Pulldown Assay
The activation status of Ras was determined by using a GST-fusion protein of the Ras-binding domain (RBD) of Raf-1 (1–149), which has been shown to have a high affinity for active GTP-Ras (Taylor and Shalloway, 1996). Cells were plated at 6 × 105 cells per 10-cm dish in Ham's F-12 containing 15% FCS and cultured for 48 h at 33°C. The medium was changed to Ham's F-12 containing 0.2% BSA, and the cells were grown for a further 24 h. Cells were stimulated for various periods with either 30 ng/ml PDGF-AA, 10 ng/ml FGF-2, or buffer. Cells were washed in ice-cold PBS and lysed in lysis buffer containing 20 mM Tris, pH 7.5, containing 1% Triton X-100, 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 10 mM MgCl2, 10 μg/ml aprotinin, 10 μg/ml leupeptin, 1 mM PMSF, and 1 mM DTT. Lysates were clarified by centrifugation, and 500 μg of total cellular protein incubated with ∼50 μg of GST-Raf1-(RBD) fusion protein for 1 h at 4°C with end-over-end mixing. The beads were collected by centrifugation and washed four times in lysis buffer. The beads were then resuspended in SDS-sample buffer, and samples were boiled for 5 min. Proteins were resolved by SDS-PAGE using a 15% gel, transferred electrophoretically, and visualized using enhanced chemiluminescence (ECL).
Precipitation and Immunoblotting
Cells were seeded at 2 × 105 per well of six-well plates in Hams F-12 containing 15% FCS and grown at 33°C for 48 h. The medium was changed to Ham's F-12 containing 0.2% BSA and cells incubated at 33°C for 24 h. Cells were then stimulated with either medium, FGF-2 (10 ng/ml), or PDGF-AA (30 ng/ml) for various times at 37°C. After stimulation, cells were rinsed in ice-cold PBS and lysed in 20 mM Tris/HCl pH 7.5, 150 mM NaCl, 1% Triton X-100, 10% glycerol, 500 μM Na3VO4, 10 μg/ml aprotinin, 10 μg/ml leupeptin, and 1 mM PMSF. Lysates were transferred to Eppendorf tubes and centrifuged at 14,000 × g for 20 min at 4°C. The supernatants were transferred to fresh Eppendorf tubes, and protein was determined by BCA protein assay. Laemmli sample buffer was added to 50 μg of total protein. Samples were boiled for 5 min, and proteins were resolved by SDS-PAGE on 10% polyacrylamide gels. Proteins were transferred to nitrocellulose membranes (Hybond-C extra; Amersham Pharmacia Biotech). Membranes were blocked for 2 h at 4°C in Tris-buffered saline (TBS) containing 0.1% Tween 20 and 5% BSA, before incubation overnight with the relevant antisera at 4°C. The membranes were then washed in TBS/Tween and incubated for 1 h with the relevant secondary antibody coupled to peroxidase (Amersham Pharmacia Biotech). Bound antibodies were visualized using ECL detection (Amersham Pharmacia Biotech). The nitrocellulose membranes were stripped in 62.5 mM Tris/HCl, pH 6.7, 2% SDS, and 100 mM β-mercaptoethanol for 30 min at 55°C. The membranes were then reprobed with the relevant antibodies. For detection of FRS2 phosphorylation, 500 μg of cell lysates were incubated with 10 μl of p13-Agarose for 1 h at 4°C with end-over-end mixing. Lysates were washed four times in lysis buffer and finally resuspended in Laemmli sample buffer. Proteins were separated by SDS-PAGE and transferred to nitrocellulose. Phosphorylated proteins were detected using the 4G10 antiphosphotyrosine antibody and visualized by ECL.
Thymidine Assay
Cells were plated at 2.5 × 104 cells/well of a 24-well plate in 1 ml of Ham's F-12 containing 15% FCS and grown for 24 h at 33°C. Cells were then washed in Ham's F-12 containing 0.2% BSA and incubated for a further 48 h at 33°C. Cells were stimulated with agonist and incubated for 16 h at 33°C before the addition of [3H]thymidine (1 μCi/ml), followed by incubation for a further 4 h at 33°C. Cells were placed on ice and washed in ice-cold PBS followed by ice-cold 10% TCA for 20 min, before washing in ice-cold ethanol and solubilized in 0.2 M NaOH. Well contents were transferred to scintillation vials, and [3H]thymidine incorporation quantified by liquid scintillation counting.
RESULTS
Characterization of Transfected Cells
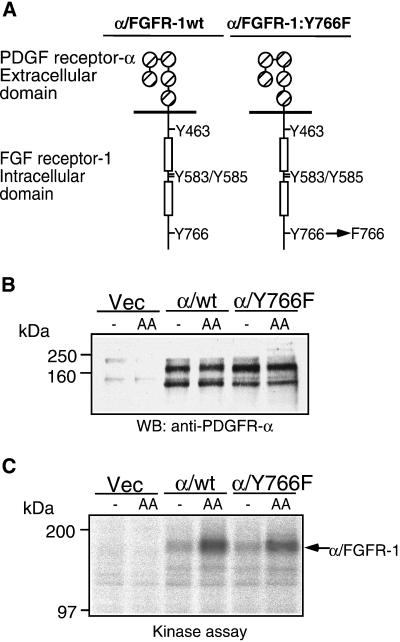
The immortomouse brain endothelial (IBE) cells display a number of markers characteristic of primary endothelial cells (Kanda et al., 1996) and represent a useful in vitro cell model to study FGF-mediated signal transduction in endothelial cell proliferation. We decided to study the role of tyrosine 766, the PLC-γ binding site, in FGFR-1–mediated signal transduction. The IBE cells express an endogenous FGFR-1 but show no expression of PDGFR-α (Kanda et al., 1996); therefore, we chose to transfect them with a chimeric receptor cDNA composed of the extracellular part of the PDGFR-α fused to the intracellular part of the FGFR-1 (Figure 1A). Such a receptor can be specifically stimulated with the ligand PDGF-AA and transduce FGFR-1–dependent effects (Landgren et al., 1998). Stable IBE cell clones expressing the chimeric receptors were generated by use of retroviral-mediated gene delivery. Clones expressing similar levels of the chimeric receptor were identified by Western blotting (Figure 1B). The α/wt and α/Y766F receptors showed similar levels of ligand-inducible kinase activity, indicating that the Y766F mutation did not affect receptor kinase activity (Figure 1C). At least two separate wild-type and Y766F receptor-expressing clones were used for all subsequent experiments with identical results obtained to those presented.
Figure 1.
Characterization of transfected cells. (A) IBE cells expressing a chimeric receptor composed of the PDGFR-α extracellular domain and the FGFR-1 intracellular domain were generated by using retroviral-mediated gene transfer. (B) Clones expressing the chimeric receptor were identified by Western blotting. (C) The ability of the chimeric receptor to induce ligand-stimulated kinase activity was analyzed by in vitro kinase assay after stimulation with PDGF-AA (30 ng/ml) for 10 min.
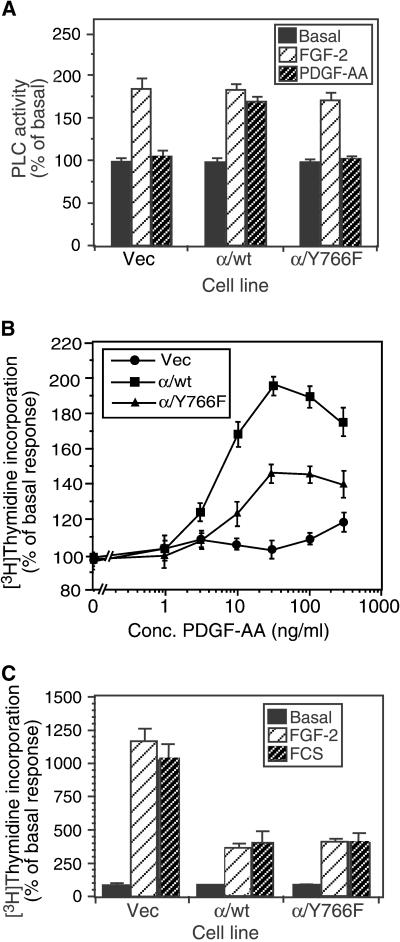
Analysis of PLC activity, showed that although all cell types responded to FGF-2 stimulation, cells expressing the α/Y766F receptor failed to stimulate PLC activity in response to PDGF-AA (Figure 2A). This is consistent with tyrosine 766 being the binding site for PLC-γ and required for activation of this enzyme. Previous reports have shown that tyrosine 766 is not required for a mitogenic response to FGF. However, in the chimeric receptor-expressing IBE cells, loss of tyrosine 766 resulted in a 50% decrease in thymidine incorporation in response to PDGF-AA treatment, compared with the wild-type chimeric receptor, indicating that tyrosine 766 was required for a full mitogenic response (Figure 2B). Analysis of mitogenicity in the Vec, α/wt, and α/Y766F cells revealed that FGF-2 showed a similar response to FCS, indicating that FGF-2 could evoke a full mitogenic response in all cells (Figure 2C). However, we noticed that in the α/wt and α/Y766F cells, both FGF and FCS could not stimulate a mitogenic responses to the same degree as in the Vec cells. We believe that this was due to the elevated basal mitogenic activity, which seemed to be a feature of the chimeric receptor-expressing cells.
Figure 2.
Effect of Y766F mutation on PLC activity and mitogenicity. (A) PLC-γ activity was analyzed by the generation of [3H]inositol phosphates after stimulation with either FGF-2 (10 ng/ml) or PDGF-AA (30 ng/ml) for 30 min. (B) Mitogenicity was analyzed by the incorporation of [3H]thymidine after stimulation with a range of concentrations of PDGF-AA for 24 h. (C) Mitogenicity in response to FGF-2 (10 ng/ml) and FCS (10%). The data shown are representative of three independent experiments. Results are expressed as the percentage of basal response (mean ± SD, n = 3) from a single experiment representative of three. The relevant basal d.p.m. were as follows: (A) Vec, 2110 ± 57; α/wt, 5064 ± 163; α/Y766F, 5014 ± 122; (B and C) Vec, 979 ± 51; α/wt, 3894 ± 81; α/Y766F, 3068 ± 125.
Tyrosine 766 Is Required for Sustained Activation of FRS2 and the Ras/MEK/MAPK Pathway
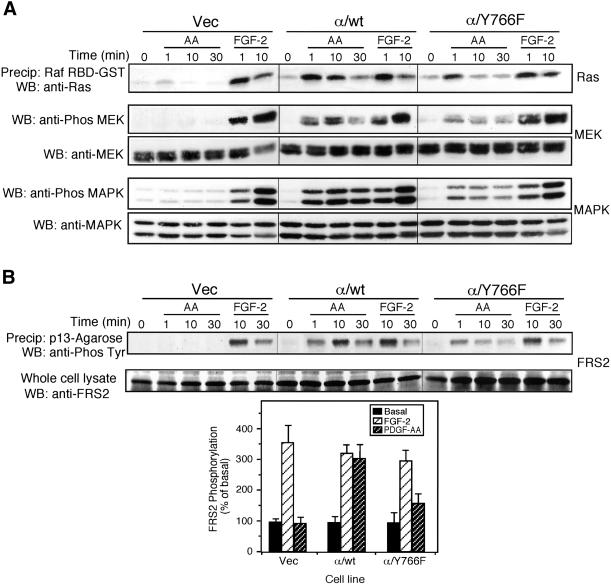
Tyrosine 766 has been previously shown to be required for maximal activation of p42/44 MAPK (Huang et al., 1995). Activation of the Ras/MEK/MAPK cascade was studied in cells expressing the chimeric receptors. PDGF-AA stimulation of cells expressing the α/Y766F chimeric receptor resulted in a weaker and more transient activation of Ras compared with cells expressing the wild-type chimeric receptor (Figure 3A). Analysis of MEK and MAPK phosphorylation also showed a weaker and more transient response in cells expressing the α/Y766F receptor compared with cells expressing the wild-type chimeric receptor. Activation of the endogenous FGFR-1, with FGF-2, evoked a similar robust response in all transfected cells.
Figure 3.
Tyrosine 766 regulates the Ras/MEK/MAPK cascade at the level of FRS2. Vec, α/wt, and α/Y766F cells were stimulated with either PDGF-AA (30 ng/ml) or FGF-2 (10 ng/ml) for the time periods shown. (A) Analysis of the Ras/MEK/MAPK signaling cascade. Cells were lysed, and active GTP-Ras was precipitated using a Raf1 RBD-GST fusion protein. Samples were resolved by SDS-PAGE, transferred eletrophoretically, and analyzed by Western blotting using a pan-Ras antibody. For the analysis of MEK and MAPK activity, a sample of the cell lysate was mixed with Laemmli buffer and resolved by SDS-PAGE. Proteins were transferred electrophoretically and blotted for active MEK using a phospho-specific MEK antibody or for active MAPK using a phospho-specific (p42/44) MAPK antibody. Blots were stripped and reprobed with either an anti-MEK antibody or an anti-(p42/44) MAPK antibody. (B) Analysis of FRS2 phosphorylation. Cells were lysed and FRS2 precipitated using p13-Agarose. Samples were resolved by SDS-PAGE, transferred electrophoretically, and blotted with an antiphosphotyrosine antibody (4G10). The degree of FRS2 phosphorylation at 10 min was quantified by NIH Image software. The results are expressed as the percentage of basal response (mean ± SE, n = 3) from three independent experiments.
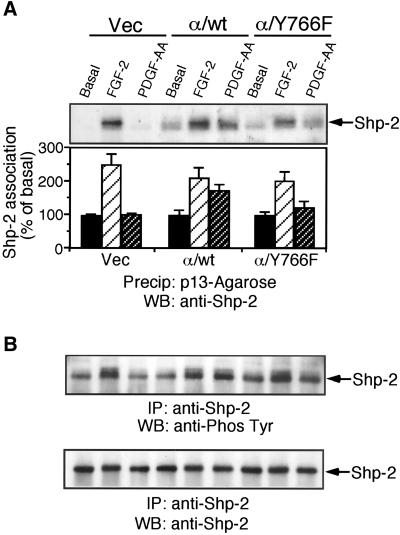
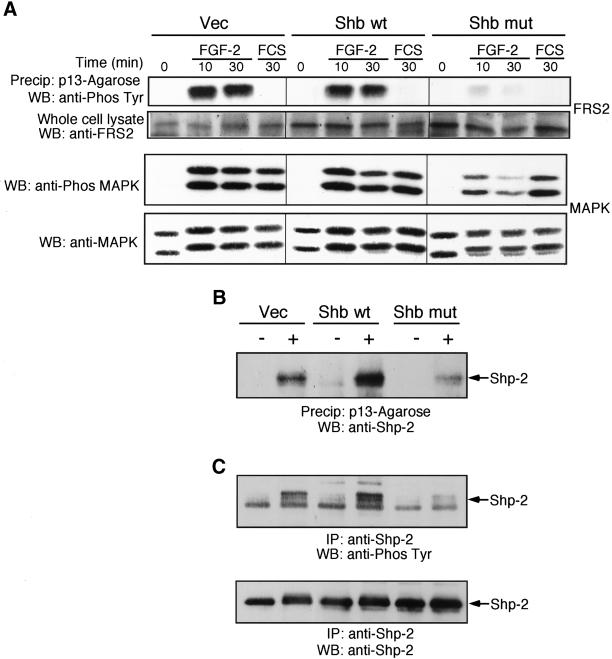
Tyrosine phosphorylation of FRS2 has been shown to be required for FGF-stimulation of Ras activity. Analysis of FRS2 phosphorylation in the chimeric receptor cells showed that PDGF-AA stimulation of cells expressing the α/Y766F chimeric receptor resulted in diminished FRS2 phosphorylation after 10 and 30 min, compared with cells expressing the wild-type chimeric receptor (Figure 3B). This suggested that loss of tyrosine 766 perturbed the Ras/MEK/MAPK pathway at the level of FRS2. We next looked at the phosphorylation and association of the tyrosine phosphatase, Shp-2 with FRS2. Precipitation of FRS2 using p13-Agarose revealed that in cells expressing the α/Y766F chimeric receptor, substantially less Shp-2 was associated with FRS2 after receptor activation, compared with cells expressing the wild-type chimeric receptor (Figure 4A). Furthermore, Shp-2 tyrosine phosphorylation was also reduced in cells expressing the α/Y766F chimeric receptor, compared with cells expressing the wild-type chimeric receptor (Figure 4B).
Figure 4.
Tyrosine 766 is required for FGFR-1–mediated association of Shp-2 and FRS2 and Shp-2 phosphorylation. Vec, α/wt, and α/Y766F cells were stimulated with either PDGF-AA (30 ng/ml) or FGF-2 (10 ng/ml) for 10 min. (A) Cells were lysed and FRS2 precipitated using p13-Agarose. Samples were resolved by SDS-PAGE, transferred electrophoretically, and blotted with an anti-Shp-2 antibody. The degree of FRS2 association was quantified by NIH Image software. The results are expressed as the percentage of basal response (mean ± SE, n = 3) from three independent experiments. (B) Cells were lysed and Shp-2 immunoprecipitated. Samples were resolved by SDS-PAGE, transferred electrophoretically, and blotted with an antiphosphotyrosine antibody (4G10). The blot was stripped and reprobed with an anti–Shp-2 antibody.
FGFR-1–mediated Activation of MAPK Is Independent of PKC, PKA, and Src in IBE Cells
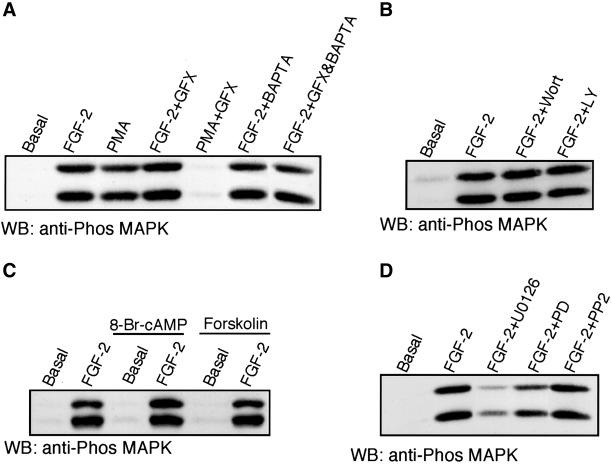
Tyrosine 766 has been shown to bind PLC-γ, which results in the generation of DAG and IP3. The resulting rise in intracellular calcium and generation of DAG leads to the activation of a number of PKC isoforms. Because PKC has been shown to activate MAPK in a variety of cells (Chao et al., 1992; Schonwasser et al., 1998), we were interested in the potential regulation of FGFR-1–mediated MAPK activation by PKC; such a mechanism may explain the reduced MAPK activation in cells expressing the α/Y766F mutant receptor. Pretreatment of IBE cells with the PKC inhibitor GF109203X (GFX), which inhibits the classical and atypical PKC isoforms (Toullec et al., 1991), failed to inhibit FGF-2–stimulated MAPK activity (Figure 5A). The phorbol ester, PMA, also stimulated MAPK activity, which was inhibited by GFX, indicating that the drug was able to block PKC activation in these cells. Pretreatment with the calcium chelator BAPTA-AM, either alone or in conjunction with GFX, also failed to inhibit FGF-2–mediated MAPK activation (Figure 5A). Pretreatment with another bisindolymaleimide PKC inhibitor, Gö 6983, also failed to inhibit FGF-2–stimulated PKC activity in the IBE cells. Recently it has been shown that certain PKC isoforms can also be activated via PI3-kinase–mediated activation of PDK1 (Le Good et al., 1998). Pretreatment with the PI3-kinase inhibitors wortmannin and LY294002, had no effect on FGF-2–stimulated MAPK activation in the IBE cells (Figure 5B), suggesting that PI3-kinase activity was not required for FGFR-1–mediated MAPK activation. Identical results using PKC inhibitors and PI3-kinase inhibitors were obtained using cells expressing the α/wt chimeric receptor stimulated with PDGF-AA. Taken together, this suggests that FGFR-1–mediated MAPK activation is not dependent on DAG and Ca2+-mediated activation of the classical PKC isoforms or DAG-mediated activation of the novel PKC isoforms. Therefore, the reduction in MAPK activity, seen in cells expressing the α/Y766F chimeric receptor, cannot be explained by a loss of PLC-γ–mediated PKC activity.
Figure 5.
FGFR-1–mediated MAPK activation is not dependent on PKC, PKA, and Src activity. IBE cells were preincubated with a number of signal transduction modulators and then stimulated with FGF-2 (5 ng/ml) or PMA (100 nM) for 10 min. Cells were lysed in Laemmli buffer, and equal amounts resolved by electrophoresis, followed by electrophoretic transfer to nitrocellulose. The membrane was blotted with antiphospho (p42/44) MAPK. (A) Cells were preincubated with the PKC inhibitor GF109203X (GFX; 5 μM for 30 min), the calcium chelator BAPTA-AM (BAPTA; 20 μM for 1 h) ,or a combination of both GFX and BAPTA. (B) Cells were preincubated with the PI 3-kinase inhibitors wortmannin (Wort; 100 nM for 30 min or LY294002 (LY; 30 μM for 30 min). (C) Cells were preincubated with the cell permeable cAMP analogue 8-Br-cAMP (1 mM for 15 min) or the adenylate cyclase activator forskolin (50 μM for 15 min). (D) Cells were preincubated with the MEK inhibitors U0126 (10 μM for 30 min), PD 98059 (PD; 30 μM for 1 h), or the Src-family kinase inhibitor PP2 (5 μM for 30 min).
Another pathway that has been implicated in FGF-2–stimulated MAPK activation is protein kinase A (PKA; D'Angelo et al., 1997), which is known to inhibit MAPK activation via phosphorylation of Raf (Cook and McCormick, 1993). Preincubation of IBE cells with the cell permeable cAMP analogue, 8-bromo-cAMP and the PKA activator forskolin had no effect on FGF-2–stimulated MAPK activity (Figure 5C). The Src-family inhibitor PP2 also showed no effect on FGF-2–stimulated MAPK activity (Figure 5D). Taken together, this suggests that PKA and Src activity are not critical for FGFR-1–mediated MAPK activity.
To confirm that FGFR-1–mediated activation of MAPK was downstream of the Ras/Raf/MEK cascade, we used the well-characterized MEK inhibitor PD98059 (Alessi et al., 1995) and a recent, more potent MEK inhibitor, U0126 (Favata et al., 1998). Preincubation with PD98059 showed only a partial inhibition of FGF-2–stimulated MAPK activation (Figure 5D). In contrast, U0126 was able to inhibit FGF-2–stimulated MAPK activation (Figure 5D). Interestingly, a similar inhibition profile of FGF-2–stimulated MAPK phosphorylation with PD98059 and U0126 has also been observed in Swiss 3T3 cells (Maher, 1999).
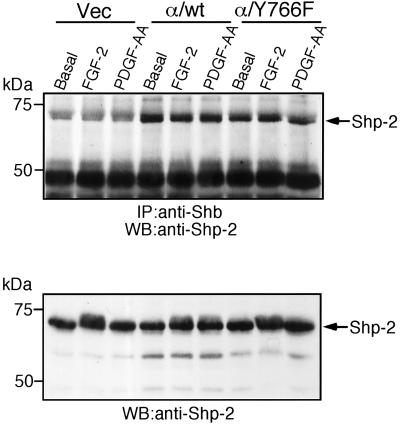
The Shb Adaptor Protein Binds to Tyrosine 766 and Regulates the Ras/MEK/MAPK Pathway
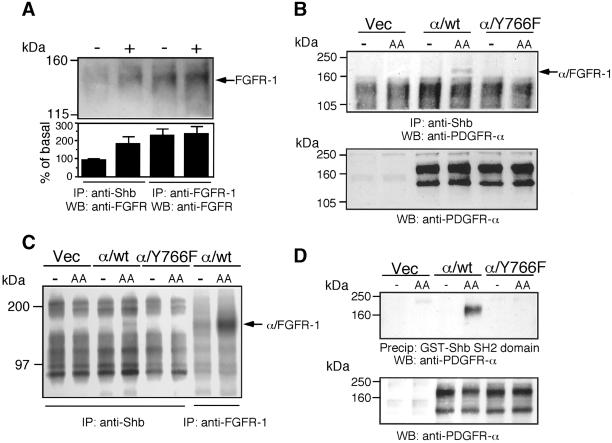
To explain the loss of regulation of FRS2 and the Ras/MEK/MAPK pathway in the Y766F cells, we were interested in other candidate proteins that may bind to Y766, in addition to PLC-γ. The Shb adaptor protein has been previously shown to bind to the consensus motif pY-T/V/I-X-L (Karlsson et al., 1995). The closest match to this site in the FGFR-1 is tyrosine 766. To investigate if Shb could bind directly to the FGF receptor, untransfected IBE cells were stimulated with FGF-2. Shb was immunoprecipitated and analyzed for coimmunoprecipitation of the FGF receptor. As shown in Figure 6A, Shb was found to complex with the FGF receptor upon agonist stimulation. To determine if Shb bound to tyrosine 766 in the FGFR-1, we immunoprecipitated Shb from cells expressing the chimeric receptors, after stimulation with the ligand PDGF-AA. We then Western-blotted with an antibody that specifically detected the extracellular domain of the PDGFR-α. Figure 6B shows that we detected an association between the chimeric receptor and Shb only in the stimulated α/wt cells and not in the α/Y766F cells. To certify further the interaction between Shb and the chimeric receptor, we also performed a kinase assay on Shb immunoprecipitates. Figure 6C shows that we detected a phosphorylated band of ∼180 kDa only with cells expressing the α/wt receptor. This band migrated at the same molecular mass as the α/FGFR-1 chimeric receptor. Taken together, the data in Figure 6, B and C, suggests that tyrosine 766, in the FGFR-1, was required for receptor interaction with Shb. To further clarify the role of tyrosine 766 in binding Shb, we used a GST-Shb-SH2 domain fusion protein. This fusion protein was able to precipitate the wild-type but not the α/Y766F chimeric receptor from stimulated cells (Figure 6D), suggesting that the SH2 domain of Shb binds to tyrosine 766 in the FGFR-1.
Figure 6.
The Shb adaptor protein binds to tyrosine 766 in the FGFR-1. (A) Untransfected IBE cells were stimulated with FGF-2 (10 ng/ml) for 10 min. Cells were lysed, and Shb was immunoprecipitated. As a control, a portion of cell lysate was used for immunoprecipitation of the FGFR-1. Samples were resolved by electrophoresis, transferred elctrophoretically, and blotted with an anti-FGFR mAb. The intensity of each band was quantified by NIH Image software. The results are expressed as the percentage of basal response (mean ± SE, n = 3) from three independent experiments. (B) Vec, α/wt, and α/Y766F cells were stimulated with PDGF-AA (30 ng/ml) for 10 min. Cells were lysed, and Shb was immunoprecipitated. Immunoprecipitates were Western blotted with an anti–PDGFR-α antibody. (C) Vec, α/wt, and α/Y766F cells were stimulated with PDGF-AA (30 ng/ml) for 10 min. Cells were lysed, and Shb was immunoprecipitated. As a control, a portion of cell lysate from α/wt cells was used to immunoprecipitate the FGFR-1. Samples were then subjected to an in vitro kinase assay. (D) Vec, α/wt, and α/Y766F cells were stimulated with PDGF-AA (30 ng/ml) for 10 min. Cells were lysed and incubated with Sepharose coupled GST-Shb SH2. Samples were resolved by electrophoresis, transferred electrophoretically, and blotted with anti–PDGFR-α antibody. Aliquots of total cell lysate were also analyzed by blotting with the anti–PDGFR-α antibody.
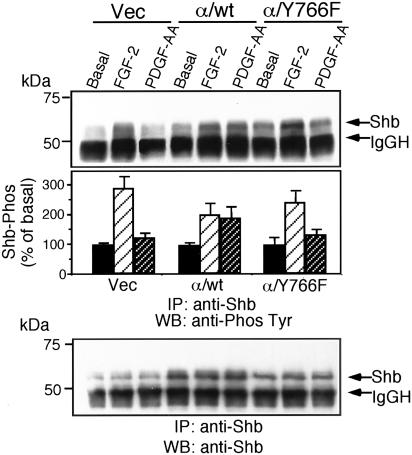
Analysis of Shb phosphorylation after stimulation with PDGF-AA showed that cells expressing the wild-type chimeric receptor were able to stimulate the tyrosine phosphorylation of Shb, a response that was lost in cells expressing the α/Y766F chimeric receptor (Figure 7). FGF-2 stimulation resulted in Shb phosphorylation in all transfected cells.
Figure 7.
The Shb adaptor protein is phosphorylated in response to receptor binding. Vec, α/wt, and α/Y766F cells were stimulated with FGF-2 (10 ng/ml) for 10 min or PDGF-AA (30 ng/ml) for 10 min. Cells were lysed, and samples were immunoprecipitated with an anti-Shb antibody. Samples were resolved by SDS-PAGE, transferred electrophoretically, and blotted with antiphosphotyrosine antibody (4G10). The intensity of each band was quantified by NIH Image software. The results are expressed as the percentage of basal response (mean ± SE, n = 3) from three independent experiments.
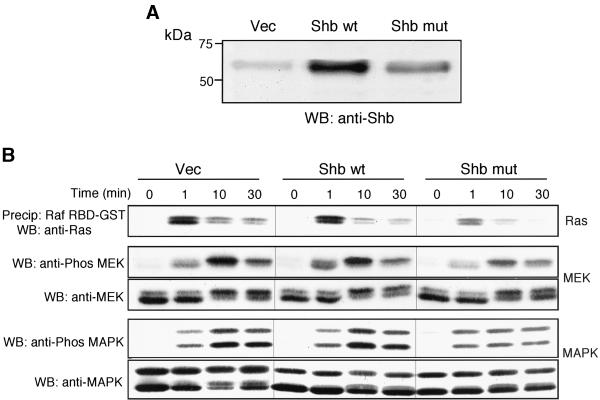
To determine the effect of Shb on FGFR-1 signaling, we used retrovirus-mediated gene transfer to generate IBE cells overexpressing either wild-type Shb or a Shb SH2 domain mutant incapable of phosphotyrosine binding (Waksman et al., 1992). A number of stable clones expressing the respective forms of Shb were identified by Western blotting (Figure 8A). At least two separate clones for wild-type and mutant Shb were used for all subsequent experiments with identical results obtained to those presented. FGF-2 stimulation of cells expressing the mutant Shb showed a reduced activation of the Ras/MEK/MAPK pathway, compared with cells expressing the wild-type Shb and Vec (Figure 8B). This would suggest that Shb plays a role in regulating the Ras/MEK/MAPK pathway in response to FGF-2.
Figure 8.
The Shb adaptor protein regulates FGFR-1–mediated Ras/MEK/MAPK activity. (A) IBE cells overexpressing Shb and a Shb SH2 domain mutant (R522K) were generated using retroviral-mediated gene transfer. Cells were lysed, and the expression level of the relevant Shb protein was analyzed by Western blotting. (B) Cells were stimulated with FGF-2 (10 ng/ml) for the times indicated. Cells were lysed, and active GTP-Ras was precipitated using Raf1 RBD-GST fusion protein. Samples were resolved by SDS-PAGE, transferred electrophoretically, and blotted for Ras using a pan-Ras antibody. For the analysis of MEK and MAPK activity, a sample of the cell lysate was mixed with Laemmli buffer and resolved by SDS-PAGE (10% polyacrylamide). Proteins were transferred electrophoretically and blotted for active MEK using a phospho-specific MEK antibody or for active MAPK using a phospho-specific (p42/44) MAPK antibody. Blots were stripped and reprobed with either an anti-MEK antibody or an anti-(p42/44) MAPK antibody.
Shb Binds Shp-2 and Regulates FRS2 Phosphorylation
To identify the point upstream of Ras, regulated by Shb, we next analyzed FRS2 phosphorylation. FGF-2 stimulation resulted in tyrosine phosphorylation of FRS2 in the Vec cells and Shb wild-type cells, whereas this response was markedly reduced in the Shb mutant cells (Figure 9A). Interestingly, FCS stimulation of the cells, which did not induce tyrosine phosphorylation of FRS2, resulted in a similar activation of MAPK in all cells. This indicates that the Shb mutant did not block activation of MAPK per se. Analysis of Shp-2 association with FRS2 revealed that in the cells overexpressing wild-type Shb enhanced association of FRS2, and Shp-2 was observed upon FGF-2 stimulation (Figure 9B); an increase in FGF-2–stimulated Shp-2 phosphorylation was also observed (Figure 9C). In contrast, cells expressing the mutant Shb displayed a reduced FGF-2–stimulated FRS2 and Shp-2 association and reduced Shp-2 phosphorylation. This experiment demonstrates that Shb has the potential to regulate the association of FRS2 and Shp-2.
Figure 9.
Shb regulates FGFR-1–mediated FRS2 phosphorylation, Shp-2 phosphorylation, and the association of FRS2 and Shp-2. (A) Vec, Shb wt, and Shb mut cells were stimulated either with FGF-2 (10 ng/ml) for the times shown or with FCS (10%) for 30 min. Cells were lysed, and FRS2 was precipitated using p13-Agarose. Samples were resolved by SDS-PAGE (10% polyacrylamide), transferred electrophoretically, and blotted with an antiphosphotyrosine antibody (4G10). For the analysis of MAPK activity, a sample of the cell lysate was mixed with Laemmli buffer and resolved by SDS-PAGE. Proteins were transferred electrophoretically and blotted for active MAPK using a phospho-specific (p42/44) MAPK antibody. Blots were stripped and reprobed with an anti-(p42/44) MAPK antibody. (B) Vec, Shb wt, and Shb mut cells were stimulated with FGF-2 (10 ng/ml) for 10 min. Cells were lysed, and FRS2 was precipitated using p13-Agarose. Samples were resolved by SDS-PAGE, transferred electrophoretically, and blotted with an anti-Shp-2 antibody. (C) Cells were lysed, and Shp-2 was immunoprecipitated. Samples were resolved by SDS-PAGE, transferred electrophoretically, and blotted with an antiphosphotyrosine antibody (4G10). The blot was stripped and reprobed with an anti–Shp-2 antibody.
To investigate the molecular mechanism by which Shb may regulate both FRS2 and Shp-2 phosphorylation, we analyzed the potential interaction of Shb and Shp-2. Immunoprecipitation of Shb revealed that Shp-2 was constitutively associated with Shb, and this was not increased upon FGF-2 stimulation (Figure 10). Furthermore, mutation of tyrosine 766 in the FGFR-1 did not affect the association between Shb and Shp-2.
Figure 10.
Shb is constitutively associated with Shp-2. Vec, α/wt, and α/Y766F cells were stimulated with either FGF-2 (10 ng/ml) or PDGF-AA (30 ng/ml) for 10 min. Cells were lysed, and Shb was immunoprecipitated. Samples were resolved by SDS-PAGE, transferred electrophoretically, and blotted with an anti–Shp-2 antibody. A portion of total cell lysate was analyzed by Western blotting with an anti–Shp-2 antibody.
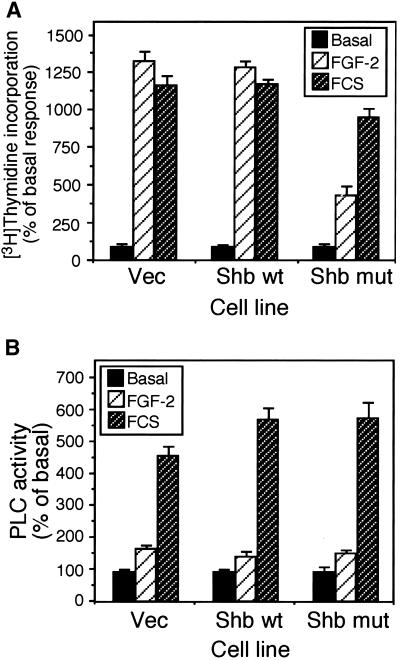
Analysis of thymidine incorporation showed that in cells expressing the Shb mutant, FGF-2–stimulated mitogenicity was reduced by ∼60% (Figure 11A). In contrast, FCS-stimulated mitogenicity was only reduced by ∼18% in cells expressing the Shb mutant. Because both PLC-γ and Shb appear to bind to tyrosine 766, we decided to examine if overexpression of Shb affected the activity of PLC-γ. Stimulation of Vec cells and cells overexpressing wild-type Shb and mutant Shb with either FGF-2 or FCS resulted in a similar increase in PLC activity (Figure 11B), indicating that overexpression of Shb or mutant Shb did not affect FGF-2–stimulated PLC activity. This would also suggest that Shb and PLC-γ do not compete for binding to tyrosine 766 in the FGFR-1.
Figure 11.
Shb regulates FGFR-1–mediated mitogenicity but not PLC-γ activation. (A) Mitogenicity was analyzed by the incorporation of [3H] thymidine after stimulation with either FGF-2 (10 ng/ml) or FCS (10%) for 24 h. Results are expressed as the percentage of basal response (mean ± SD, n = 3) from a single experiment representative of three. The relevant basal d.p.m. were as follows: Vec, 1323 ± 120; Shb wt, 1143 ± 57; Shb mut, 5812 ± 272. (B) PLC activity was determined by the generation of [3H]inositol phosphates after stimulation of [3H]inositol-labeled cells with FGF-2 (10 ng/ml) for 30 min. Results are expressed as the percentage of basal response (mean ± SD, n = 3) from a single experiment representative of three. The relevant basal d.p.m. were Vec, 2983 ± 129; Shb wt, 2289 ± 63; Shb mut, 1395 ± 165.
DISCUSSION
PLC-γ was the first protein identified to bind directly to the FGFR-1, and subsequently, its role in FGF-mediated biological responses has been extensively investigated. Mutation of tyrosine 766, the PLC-γ binding site, has revealed that PLC-γ activity is not required for FGFR-1–mediated mitogenicity (Mohammadi et al., 1992; Peters et al., 1992). In contrast, other studies have shown that mutation of tyrosine 766 can affect FGFR-1–mediated proliferation of L6 myoblasts (Klint et al., 1995) and to a lesser degree of Ba/F3 cells (Huang et al., 1995). However, all these studies have used transformed cell lines that do not express the endogenous FGF receptor. The IBE cells expressing a chimeric receptor allow studies of FGFR-1 signaling in a more biologically relevant model, where there is endogenous expression of the FGFR-1.
In this article, we describe a novel pathway involving binding of the adaptor protein Shb to tyrosine 766 in the FGFR-1. This interaction is required for maximal FGF-mediated mitogenicity via activation of the Ras/MEK/MAPK pathway. We further identify Shb binding as critical for FGFR-1–mediated FRS2 phosphorylation via interaction with Shp-2.
Previous work on signaling cascades downstream of tyrosine 766, the PLC-γ binding site in the FGFR-1, has shown that mutation of tyrosine 766 leads to a reduction in FGFR-1–mediated MAPK activity, an effect attributed to loss of PKC-mediated Raf phosphorylation (Huang et al., 1995). A number of PKC isoforms have been shown to directly phosphorylate Raf-1 (Cai et al., 1997; Schonwasser et al., 1998) and regulate MEK and MAPK phosphorylation in vivo. In the IBE cells, the broad-spectrum PKC inhibitors GF109203X and Gö 6983 had no effect on FGF-mediated MAPK activation (Figure 5). Therefore, although we indeed noticed a reduction in MAPK activity with the α/Y766F mutation (Figure 3), FGFR-1–mediated MAPK activation was not dependent on PKC activation. The ability of FGF to stimulate MAPK, independent of PKC, has also been observed in L6 myoblasts (van Dijk and van Blitterswijk, 1998), suggesting that it is not confined to IBE cells.
The ability of tyrosine 766 to regulate MAPK activity was dependent on FRS2 phosphorylation. Phosphorylation of FRS2 is known to be critical for FGF-stimulated MAPK activation (Kouhara et al., 1997), because FGFR-1–mediated tyrosine phosphorylation of FRS2 allows the binding of Grb2, thus linking FGFR-1 to the Ras/MEK/MAPK pathway. The inability of calcium chelation or PKC inhibition to affect FGFR-1–mediated MAPK activation suggests, that although PLC-γ was activated by binding to tyrosine 766, its downstream effectors, IP3 and DAG, play no role in the regulation of the FRS2/Ras/MEK/MAPK pathway in these cells. Furthermore, other pathways such as PI3-kinase, PKA and Src, which have been implicated in FGFR-1–mediated MAPK activation, do not appear to be regulating MAPK activity in the IBE cells.
A search for other proteins that may bind to tyrosine 766 in the FGFR-1 led us to examine Shb, an adaptor protein that has been previously reported to affect FGF-stimulated MAPK activation in PC12 cells (Karlsson et al., 1998). The preferred binding sequence for the Shb SH2 domain is pY-T/V/I-X-L with Leu in position +3 as the main determinant (Karlsson et al., 1995). In the FGFR-1, the closest match is Y766, which has the sequence pY-L-D-L. Shb bound to the endogenous FGF receptors on IBE cells, which was mediated through tyrosine 766 in the FGFR-1 (Figure 6, B and C). Use of a GST-Shb-SH2 domain fusion protein indicated that the SH2 domain of Shb interacts directly with phosphorylated tyrosine 766 in the FGFR-1. Phosphorylation of Shb was inhibited in cells expressing the Y766F chimeric receptor, suggesting that receptor association is required for phosphorylation. FGF-2 stimulation of IBE cells overexpressing a Shb SH2 domain mutant (R522K) revealed a perturbment in the Ras/MEK/MAPK pathway (Figure 9A), similar to that seen in cells expressing the α/Y766F chimeric receptor mutant (Figure 3). In both instances, there was a severe reduction of FRS2 phosphorylation in response to FGFR-1 activation.
In exploring the molecular mechanism whereby Shb modulates FRS2 phosphorylation, we discovered that both the Y766F mutation (Figure 4, A and B) and expression of a Shb mutant (Figure 9, B and C) perturbed the phosphorylation of the tyrosine phosphatase Shp-2 and its association with FRS-2. Previous work has shown that association of FRS-2 and Shp-2 is required for efficient FGFR-1–mediated FRS2 phosphorylation and activation of the MAPK cascade (Hadari et al., 1998). Overexpression of Shb potentiated the FGF-mediated phosphorylation of Shp-2 and its association with FRS-2, further suggesting that Shb could directly regulate Shp-2 phosphorylation and its association with FRS-2. However, we observed no concomitant increase in the phosphorylation of FRS2 upon overexpression of wild-type Shb, suggesting that in the IBE cells, the endogenous association of Shp-2 and FRS2 was sufficient to cause maximal phosphorylation of FRS2. The mechanism whereby Shp-2 regulates FRS2 phosphorylation and MAPK activation remains obscure, although it has been demonstrated that the catalytic activity of Shp-2 is required for sustained MAPK activation in response to FGF (Hadari et al., 1998).
We could not detect any direct association between Shb and FRS2, suggesting that Shb affects FRS2 phosphorylation indirectly. However, we did find that Shb was constitutively associated with Shp-2 (Figure 10), providing a molecular link between Shb and FRS2. This interaction was surprising, because the Shb amino acid sequence (Welsh et al., 1994), does not contain the consensus pY-I/V-X-V/I/L/P binding region for the SH2 domains of Shp-2 (Songyang et al., 1993). It is possible that Shp-2 associates with Shb via another binding mechanism. Indeed, examination of the crystal structure of Shp-2, has revealed that the N-terminal SH2 domain of Shp-2 can bind intramolecularly to the phosphatase domain, in a phosphotyrosine-independent manner (Hof et al., 1998). However, it is also conceivable that Shp-2 interacts indirectly with Shb, possibly via Grb2, which is known to associate with the N-terminal proline-rich domain in Shb, via its SH3 domain (Karlsson et al., 1995), and with phosphorylated tyrosine residues in Shp-2, via its SH2 domain (Vogel and Ullrich, 1996). Therefore, it is possible that Shp-2 acts as a bridge to link Shb and FGFR-1 activation to FRS2 phosphorylation and the subsequent activation of MAPK.
Previous reports have shown that FRS2 interacts constitutively with the juxtamembrane region of the FGFR, via its PTB domain, in a phosphorylation-independent manner (Xu et al., 1998; Ong et al., 2000). However, these experiments have used overexpression in nonendothelial cell lines to show association between the FGFR-1 and FRS2. In the IBE cells, we could not detect association between either the endogenous FGFR or the α/wt and α/Y766F chimeric receptors and FRS2, suggesting that in our endothelial cells, any association between the FGFR-1 and FRS2 was possibly weak and transient in nature. Furthermore, our data show that the phosphorylation status of FRS2 is regulated via the tyrosine 766 site in the FGFR-1.
The ability of both the Y766F point mutation and the mutant Shb expression to affect FGFR-1–mediated mitogenicity further supports a role for Shb in the regulation of the MAPK cascade. Addition of the MEK inhibitors PD 98059 (30 μM) and U0126 (10 μM) resulted in 46.0 ± 7.4% and 69.6 ± 6.0% reduction in [3H]thymidine incorporation, respectively, in response to FGF-2 in untransfected IBE cells. When compared with the 60% reduction in [3H]thymidine incorporation observed in the Shb mutant cells (Figure 11A), this is in conformity with the view that Shb regulates FGFR-1–mediated mitogenicity via regulation of the Ras/MEK/MAPK cascade.
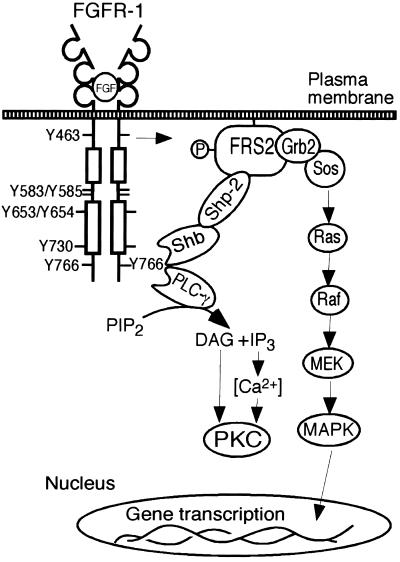
Therefore, the data presented here suggest that both Shb and PLC-γ bind to tyrosine 766 in the FGFR-1. A recent report has shown that in addition to tyrosine 766, tyrosines 677 and 701 are also required for FGFR-1–mediated PLC-γ activation (Foehr et al., 2001), suggesting that a number of additional residues also regulate PLC-γ binding; although tyrosine 766 appears to be the main binding site. The PLC-γ pathway is known to couple to PKC/Ca2+. The novel Shb pathway is able to regulate Shp-2 and FRS2 phosphorylation and subsequently the Ras/MEK/MAPK cascade leading to a mitogenic response (Figure 12). However, because both mutation of tyrosine 766 (Figure 3B) and expression of a Shb SH2 domain mutant (Figure 8B) did not completely inhibit all Ras/MEK/MAPK activity in the IBE cells, it is likely that the FGFR-1 activates the Ras/MEK/MAPK pathway by more than one mechanism. Indeed, we have previously reported that Y463, in the juxtamembrane domain of the FGFR-1, can also affect FRS2 phosphorylation and MAPK activity in endothelial cells (Larsson et al., 1999). The physiological importance of the MAPK cascade is highlighted by the fact that in the chicken chorioallantoic membrane (CAM) assay, addition of the MEK inhibitor PD 98059, prevents FGF-mediated angiogenesis (Eliceiri et al., 1998). The proliferation of endothelial cells is an important step in angiogenesis as the endothelial cells must migrate, proliferate and eventually differentiate to form a new, lumen containing vessel (Cross and Claesson-Welsh, 2001). By regulating the mitogenic response, our data suggest that Shb may play an important role in regulating FGF-mediated angiogenesis.
Figure 12.
Schematic model for the role of tyrosine 766 in the FGFR-1 regulating PLC-γ and Shb function. Both PLC-γ and Shb bind to phosphorylated tyrosine 766 after ligand stimulation of the receptor. PLC-γ generates IP3-mediated Ca2+ release and DAG, which results in the activation of PKC. Shb is constitutively associated with Shp-2 and regulates FRS2 phosphorylation and the Ras/MEK/MAPK pathway resulting in mitogenicity. Other structural motifs in the FGFR-1, such as Y463 and the juxtamembrane domain, may also contribute to regulation of the FRS2/Ras pathway in endothelial cells.
ACKNOWLEDGMENTS
This work was supported by grants from the Medical Research Foundation (31X-10822) to M.W., the Novo Nordisk Foundation, the Göran Gustafsson Foundation, the Association for International Cancer Research (AICR) and the Swedish Medical Research Foundation (project grant K2001–32X-12552–04B) to L.C.W.. M.J.C. was supported by a Marie Curie TMR fellowship.
Footnotes
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E02–02–0103. Article and publication date are at www.molbiolcell.org/cgi/doi/10.1091/mbc.E02–02–0103.
REFERENCES
- Alessi DR, Cuenda A, Cohen P, Dudley DT, Saltiel AR. PD 098059 is a specific inhibitor of the activation of mitogen-activated protein kinase kinase in vitro and in vivo. J Biol Chem. 1995;270:27489–27494. doi: 10.1074/jbc.270.46.27489. [DOI] [PubMed] [Google Scholar]
- Cai H, Smola U, Wixler V, Eisenmann-Tappe I, Diaz-Meco MT, Moscat J, Rapp U, Cooper GM. Role of diacylglycerol-regulated protein kinase C isotypes in growth factor activation of the Raf-1 protein kinase. Mol Cell Biol. 1997;17:732–741. doi: 10.1128/mcb.17.2.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao TS, Byron KL, Lee KM, Villereal M, Rosner MR. Activation of MAP kinases by calcium-dependent and calcium-independent pathways. Stimulation by thapsigargin and epidermal growth factor. J Biol Chem. 1992;267:19876–19883. [PubMed] [Google Scholar]
- Clyman RI, Peters KG, Chen YQ, Escobedo J, Williams LT, Ives HE, Wilson E. Phospholipase C gamma activation, phosphotidylinositol hydrolysis, and calcium mobilization are not required for FGF receptor-mediated chemotaxis. Cell Adhes Commun. 1994;1:333–342. doi: 10.3109/15419069409097264. [DOI] [PubMed] [Google Scholar]
- Cook SJ, McCormick F. Inhibition by cAMP of Ras-dependent activation of Raf. Science. 1993;262:1069–1072. doi: 10.1126/science.7694367. [DOI] [PubMed] [Google Scholar]
- Cross MJ, Claesson-Welsh L. FGF, and VEGF function in angiogenesis. signaling pathways, biological responses and therapeutic inhibition. Trends Pharmacol Sci. 2001;22:201–207. doi: 10.1016/s0165-6147(00)01676-x. [DOI] [PubMed] [Google Scholar]
- Cross MJ, Hodgkin MN, Roberts S, Landgren E, Wakelam MJ, Claesson-Welsh L. Tyrosine 766 in the fibroblast growth factor receptor-1 is required for FGF-stimulation of phospholipase C, phospholipase D, phospholipase A2, phosphoinositide 3-kinase, and cytoskeletal reorganisation in porcine aortic endothelial cells. J Cell Sci. 2000;113:643–651. doi: 10.1242/jcs.113.4.643. [DOI] [PubMed] [Google Scholar]
- D'Angelo G, Lee H, Weiner RI. cAMP-dependent protein kinase inhibits the mitogenic action of vascular endothelial growth factor and fibroblast growth factor in capillary endothelial cells by blocking Raf activation. J Cell Biochem. 1997;67:353–366. [PubMed] [Google Scholar]
- Divecha N, Irvine RF. Phospholipid signaling. Cell. 1995;80:269–278. doi: 10.1016/0092-8674(95)90409-3. [DOI] [PubMed] [Google Scholar]
- Eliceiri BP, Klemke R, Stromblad S, Cheresh DA. Integrin alphavbeta3 requirement for sustained mitogen-activated protein kinase activity during angiogenesis. J Cell Biol. 1998;140:1255–1263. doi: 10.1083/jcb.140.5.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favata MF, et al. Identification of a novel inhibitor of mitogen-activated protein kinase kinase. J Biol Chem. 1998;273:18623–18632. doi: 10.1074/jbc.273.29.18623. [DOI] [PubMed] [Google Scholar]
- Foehr ED, Raffioni S, Murray-Rust J, Bradshaw RA. The role of tyrosine residues in fibroblast growth factor receptor 1 signaling in PC12 cells. Systematic site-directed mutagenesis in the endodomain. J Biol Chem. 2001;276:37529–37536. doi: 10.1074/jbc.M103234200. [DOI] [PubMed] [Google Scholar]
- Hadari YR, Kouhara H, Lax I, Schlessinger J. Binding of Shp2 tyrosine phosphatase to FRS2 is essential for fibroblast growth factor-induced PC12 cell differentiation. Mol Cell Biol. 1998;18:3966–3973. doi: 10.1128/mcb.18.7.3966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hof P, Pluskey S, Dhe-Paganon S, Eck MJ, Shoelson SE. Crystal structure of the tyrosine phosphatase SHP-2. Cell. 1998;92:441–450. doi: 10.1016/s0092-8674(00)80938-1. [DOI] [PubMed] [Google Scholar]
- Huang J, Mohammadi M, Rodrigues GA, Schlessinger J. Reduced activation of RAF-1 and MAP kinase by a fibroblast growth factor receptor mutant deficient in stimulation of phosphatidylinositol hydrolysis. J Biol Chem. 1995;270:5065–5072. doi: 10.1074/jbc.270.10.5065. [DOI] [PubMed] [Google Scholar]
- Kanda S, Landgren E, Ljungstrom M, Claesson-Welsh L. Fibroblast growth factor receptor 1-induced differentiation of endothelial cell line established from tsA58 large T transgenic mice. Cell Growth Differ. 1996;7:383–395. [PubMed] [Google Scholar]
- Karlsson T, Kullander K, Welsh M. The Src homology 2 domain protein Shb transmits basic fibroblast growth factor- and nerve growth factor-dependent differentiation signals in PC12 cells. Cell Growth Differ. 1998;9:757–766. [PubMed] [Google Scholar]
- Karlsson T, et al. Molecular interactions of the Src homology 2 domain protein Shb with phosphotyrosine residues, tyrosine kinase receptors and Src homology 3 domain proteins. Oncogene. 1995;10:1475–1483. [PubMed] [Google Scholar]
- Karlsson T, Welsh M. Apoptosis of NIH3T3 cells expressing the Src homology 2 domain protein Shb. Oncogene. 1996;13:955–961. [PubMed] [Google Scholar]
- Klint P, Kanda S, Claesson-Welsh L. Shc and a novel 89-kDa component couple to the Grb2-Sos complex in fibroblast growth factor-2-stimulated cells. J Biol Chem. 1995;270:23337–23344. doi: 10.1074/jbc.270.40.23337. [DOI] [PubMed] [Google Scholar]
- Kouhara H, Hadari YR, Spivak-Kroizman T, Schilling J, Bar-Sagi D, Lax I, Schlessinger J. A lipid-anchored Grb2-binding protein that links FGF-receptor activation to the Ras/MAPK signaling pathway. Cell. 1997;89:693–702. doi: 10.1016/s0092-8674(00)80252-4. [DOI] [PubMed] [Google Scholar]
- Landgren E, Klint P, Yokote K, Claesson-Welsh L. Fibroblast growth factor receptor-1 mediates chemotaxis independently of direct SH2-domain protein binding. Oncogene. 1998;17:283–291. doi: 10.1038/sj.onc.1201936. [DOI] [PubMed] [Google Scholar]
- Larsson H, Klint P, Landgren E, Claesson-Welsh L. Fibroblast growth factor receptor-1-mediated endothelial cell proliferation is dependent on the Src homology (SH) 2/SH3 domain-containing adaptor protein Crk. J Biol Chem. 1999;274:25726–25734. doi: 10.1074/jbc.274.36.25726. [DOI] [PubMed] [Google Scholar]
- Le Good JA, Ziegler WH, Parekh DB, Alessi DR, Cohen P, Parker PJ. Protein kinase C isotypes controlled by phosphoinositide 3-kinase through the protein kinase PDK1. Science. 1998;281:2042–2045. doi: 10.1126/science.281.5385.2042. [DOI] [PubMed] [Google Scholar]
- Lindholm CK, Gylfe E, Zhang W, Samelson LE, Welsh M. Requirement of the Src homology 2 domain protein Shb for T cell receptor-dependent activation of the interleukin-2 gene nuclear factor for activation of T cells element in Jurkat T cells. J Biol Chem. 1999;274:28050–28057. doi: 10.1074/jbc.274.39.28050. [DOI] [PubMed] [Google Scholar]
- Maher P. p38 mitogen-activated protein kinase activation is required for fibroblast growth factor-2-stimulated cell proliferation but not differentiation. J Biol Chem. 1999;274:17491–17498. doi: 10.1074/jbc.274.25.17491. [DOI] [PubMed] [Google Scholar]
- Mohammadi M, Dionne CA, Li W, Li N, Spivak T, Honegger AM, Jaye M, Schlessinger J. Point mutation in FGF receptor eliminates phosphatidylinositol hydrolysis without affecting mitogenesis. Nature. 1992;358:681–684. doi: 10.1038/358681a0. [DOI] [PubMed] [Google Scholar]
- Mohammadi M, et al. A. tyrosine-phosphorylated carboxy-terminal peptide of the fibroblast growth factor receptor (Flg) is a binding site for the S.H2 domain of phospholipase C-γ1. Mol Cell Biol. 1991;11:5068–5078. doi: 10.1128/mcb.11.10.5068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong SH, Guy GR, Hadari YR, Laks S, Gotoh N, Schlessinger J, Lax I. FRS2 proteins recruit intracellular signaling pathways by binding to diverse targets on fibroblast growth factor, and nerve growth factor receptors. Mol Cell Biol. 2000;20:979–989. doi: 10.1128/mcb.20.3.979-989.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pear WS, Nolan GP, Scott ML, Baltimore D. Production of high-titer helper-free retroviruses by transient transfection. Proc Natl Acad Sci USA. 1993;90:8392–8396. doi: 10.1073/pnas.90.18.8392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters KG, Marie J, Wilson E, Ives HE, Escobedo J, Del Rosario M, Mirda D, Williams LT. Point mutation of an FGF receptor abolishes phosphatidylinositol turnover and Ca2+ flux but not mitogenesis. Nature. 1992;358:678–681. doi: 10.1038/358678a0. [DOI] [PubMed] [Google Scholar]
- Plevin R, Palmer S, Gardner SD, Wakelam MJ. Regulation of bombesin-stimulated inositol 1,4,5-trisphosphate generation in Swiss 3T3 fibroblasts by a guanine-nucleotide-binding protein. Biochem J. 1990;268:605–610. doi: 10.1042/bj2680605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonwasser DC, Marais RM, Marshall CJ, Parker PJ. Activation of the mitogen-activated protein kinase/extracellular signal-regulated kinase pathway by conventional, novel, and atypical protein kinase C isotypes. Mol Cell Biol. 1998;18:790–798. doi: 10.1128/mcb.18.2.790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Songyang Z, et al. SH2 domains recognize specific phosphopeptide sequences. Cell. 1993;72:767–778. doi: 10.1016/0092-8674(93)90404-e. [DOI] [PubMed] [Google Scholar]
- Spivak-Kroizman T, Mohammadi M, Hu P, Jaye M, Schlessinger J, Lax I. Point mutation in the fibroblast growth factor receptor eliminates phosphatidylinositol hydrolysis without affecting neuronal differentiation of PC12 cells. J Biol Chem. 1994;269:14419–14423. [PubMed] [Google Scholar]
- Taylor SJ, Shalloway D. Cell cycle-dependent activation of Ras. Curr Biol. 1996;6:1621–1627. doi: 10.1016/s0960-9822(02)70785-9. [DOI] [PubMed] [Google Scholar]
- Toullec D, et al. The bisindolylmaleimide GF 109203X is a potent and selective inhibitor of protein kinase C. J Biol Chem. 1991;266:15771–15781. [PubMed] [Google Scholar]
- Ullrich A, Schlessinger J. Signal transduction by receptors with tyrosine kinase activity. Cell. 1990;61:203–212. doi: 10.1016/0092-8674(90)90801-k. [DOI] [PubMed] [Google Scholar]
- van Dijk MC, van Blitterswijk WJ. Lipid metabolism in fibroblast growth factor-stimulated L6 myoblasts: a receptor mutation (Y766F) abrogates phospholipase D and diacylglycerol kinase activities. Biochim Biophys Acta. 1998;1391:273–279. doi: 10.1016/s0005-2760(98)00016-2. [DOI] [PubMed] [Google Scholar]
- Vogel W, Ullrich A. Multiple in vivo phosphorylated tyrosine phosphatase SHP-2 engages binding to Grb2 via tyrosine 584. Cell Growth Differ. 1996;7:1589–1597. [PubMed] [Google Scholar]
- Waksman G, et al. Crystal structure of the phosphotyrosine recognition domain SH2 of v-src complexed with tyrosine-phosphorylated peptides. Nature. 1992;358:646–653. doi: 10.1038/358646a0. [DOI] [PubMed] [Google Scholar]
- Welsh M, Mares J, Karlsson T, Lavergne C, Breant B, Claesson-Welsh L. Shb is a ubiquitously expressed Src homology 2 protein. Oncogene. 1994;9:19–27. [PubMed] [Google Scholar]
- Welsh M, et al. Stimulation through the T cell receptor leads to interactions between SHB and several signaling proteins. Oncogene. 1998;16:891–901. doi: 10.1038/sj.onc.1201607. [DOI] [PubMed] [Google Scholar]
- Xu H, Lee KW, Goldfarb M. Novel recognition motif on fibroblast growth factor receptor mediates direct association and activation of SNT adapter proteins. J Biol Chem. 1998;273:17987–17990. doi: 10.1074/jbc.273.29.17987. [DOI] [PubMed] [Google Scholar]