Abstract
We have used defined subcellular fractions to reconstitute in a cell-free system vesicle fusions occurring in the endocytic pathway. The endosomal fractions were prepared by immuno-isolation using as antigen an epitope located on a foreign protein, the transmembrane glycoprotein G (G-protein) of vesicular stomatitis virus. The G-protein was first implanted in the cell plasma membrane and subsequently endocytosed for 15 to 30 min at 37 degrees C. The endosomal fractions were immuno-isolated on a solid support using as antigen the cytoplasmic domain of the G-protein in combination with a specific monoclonal antibody. For comparative studies the plasma membrane was immuno-isolated from cells in the absence of G internalization with a monoclonal antibody against the exoplasmic domain of the G-protein. The immuno-isolated endosomal vesicles contained 70% of horseradish peroxidase internalized in the endosome fluid phase, exhibited an acidic luminal pH as shown by acridine orange fluorescence and differed in their protein composition from the immuno-isolated plasma membrane fraction. The fusion of endocytic vesicles originating from different stages of the pathway was studied in a cell-free assay using both a bio-chemical and a morphological detection system. These well defined endosomal vesicles were immuno-isolated with the G-protein on the solid support and provided the recipient compartment of the fusion (acceptor). They were mixed with a post-nuclear supernatant containing endosomes loaded with exogenous lactoperoxidase (donor) at 37 degrees C. Fusion delivered the donor peroxidase to the lumen of acceptor vesicles permitting fusion-specific iodination of the G-protein itself. The fusion of vesicles required ATP and was detected only with an endosomal fraction prepared after internalization of the G-protein for 15 min at 37 degrees C but not with a plasma membrane or with an endosomal fraction prepared after 30 min G-protein internalization.
Full text
PDF

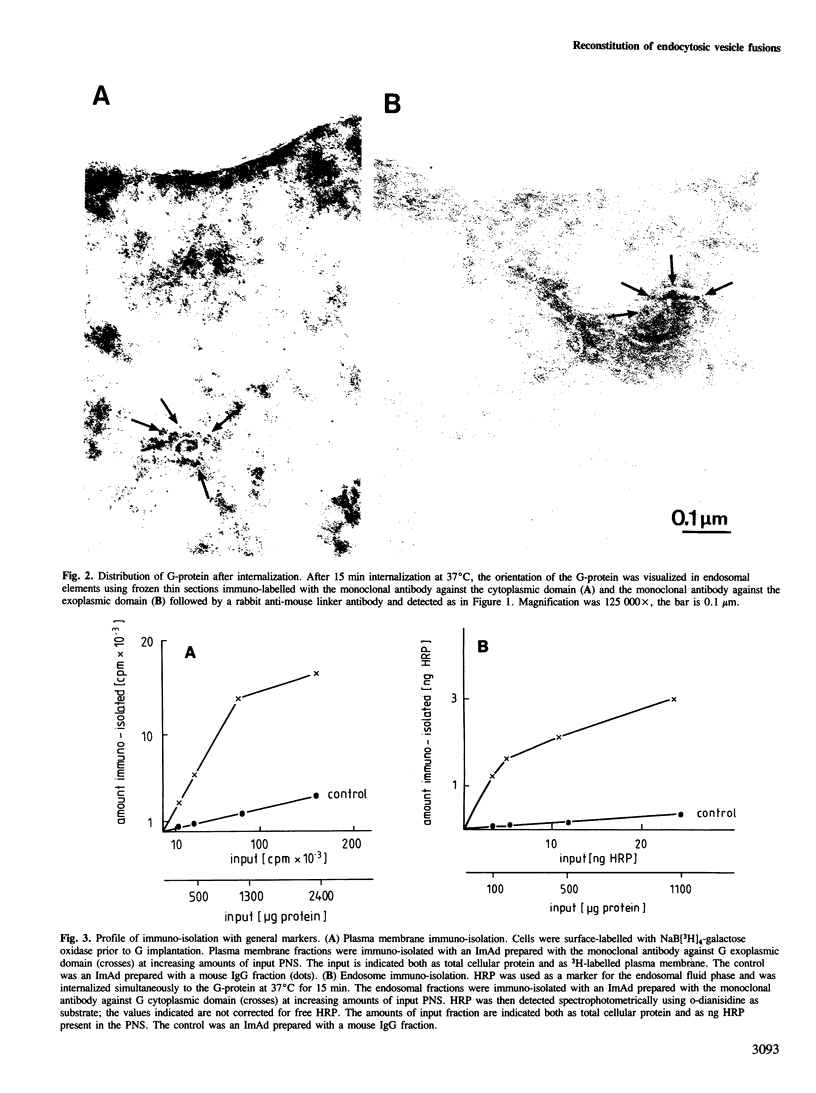








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson R. G., Goldstein J. L., Brown M. S. A mutation that impairs the ability of lipoprotein receptors to localise in coated pits on the cell surface of human fibroblasts. Nature. 1977 Dec 22;270(5639):695–699. doi: 10.1038/270695a0. [DOI] [PubMed] [Google Scholar]
- Balch W. E., Dunphy W. G., Braell W. A., Rothman J. E. Reconstitution of the transport of protein between successive compartments of the Golgi measured by the coupled incorporation of N-acetylglucosamine. Cell. 1984 Dec;39(2 Pt 1):405–416. doi: 10.1016/0092-8674(84)90019-9. [DOI] [PubMed] [Google Scholar]
- Balch W. E., Glick B. S., Rothman J. E. Sequential intermediates in the pathway of intercompartmental transport in a cell-free system. Cell. 1984 Dec;39(3 Pt 2):525–536. doi: 10.1016/0092-8674(84)90459-8. [DOI] [PubMed] [Google Scholar]
- Bordier C. Phase separation of integral membrane proteins in Triton X-114 solution. J Biol Chem. 1981 Feb 25;256(4):1604–1607. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bretscher M. S., Thomson J. N., Pearse B. M. Coated pits act as molecular filters. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4156–4159. doi: 10.1073/pnas.77.7.4156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Davey J., Hurtley S. M., Warren G. Reconstitution of an endocytic fusion event in a cell-free system. Cell. 1985 Dec;43(3 Pt 2):643–652. doi: 10.1016/0092-8674(85)90236-3. [DOI] [PubMed] [Google Scholar]
- Devaney E., Howell K. E. Immuno-isolation of a plasma membrane fraction from the Fao cell. EMBO J. 1985 Dec 1;4(12):3123–3130. doi: 10.1002/j.1460-2075.1985.tb04054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson R. B., Beguinot L., Hanover J. A., Richert N. D., Willingham M. C., Pastan I. Isolation and characterization of a highly enriched preparation of receptosomes (endosomes) from a human cell line. Proc Natl Acad Sci U S A. 1983 Sep;80(17):5335–5339. doi: 10.1073/pnas.80.17.5335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gahmberg C. G., Andersson L. C. Identification and characterization of normal and malignant human blood leukocytes by surface glycoprotein patterns. Ann N Y Acad Sci. 1978 Jun 20;312:240–255. doi: 10.1111/j.1749-6632.1978.tb16806.x. [DOI] [PubMed] [Google Scholar]
- Geuze H. J., Slot J. W., Strous G. J., Lodish H. F., Schwartz A. L. Intracellular site of asialoglycoprotein receptor-ligand uncoupling: double-label immunoelectron microscopy during receptor-mediated endocytosis. Cell. 1983 Jan;32(1):277–287. doi: 10.1016/0092-8674(83)90518-4. [DOI] [PubMed] [Google Scholar]
- Gluck S., Kelly S., Al-Awqati Q. The proton translocating ATPase responsible for urinary acidification. J Biol Chem. 1982 Aug 25;257(16):9230–9233. [PubMed] [Google Scholar]
- Goldstein J. L., Brown M. S., Anderson R. G., Russell D. W., Schneider W. J. Receptor-mediated endocytosis: concepts emerging from the LDL receptor system. Annu Rev Cell Biol. 1985;1:1–39. doi: 10.1146/annurev.cb.01.110185.000245. [DOI] [PubMed] [Google Scholar]
- Griffiths G., Pfeiffer S., Simons K., Matlin K. Exit of newly synthesized membrane proteins from the trans cisterna of the Golgi complex to the plasma membrane. J Cell Biol. 1985 Sep;101(3):949–964. doi: 10.1083/jcb.101.3.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths G., Simons K., Warren G., Tokuyasu K. T. Immunoelectron microscopy using thin, frozen sections: application to studies of the intracellular transport of Semliki Forest virus spike glycoproteins. Methods Enzymol. 1983;96:466–485. doi: 10.1016/s0076-6879(83)96041-x. [DOI] [PubMed] [Google Scholar]
- Gruenberg J., Howell K. E. Immuno-isolation of vesicles using antigenic sites either located on the cytoplasmic or the exoplasmic domain of an implanted viral protein. A quantitative analysis. Eur J Cell Biol. 1985 Sep;38(2):312–321. [PubMed] [Google Scholar]
- Kreis T. E. Microinjected antibodies against the cytoplasmic domain of vesicular stomatitis virus glycoprotein block its transport to the cell surface. EMBO J. 1986 May;5(5):931–941. doi: 10.1002/j.1460-2075.1986.tb04306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrman M. A., Goldstein J. L., Brown M. S., Russell D. W., Schneider W. J. Internalization-defective LDL receptors produced by genes with nonsense and frameshift mutations that truncate the cytoplasmic domain. Cell. 1985 Jul;41(3):735–743. doi: 10.1016/s0092-8674(85)80054-4. [DOI] [PubMed] [Google Scholar]
- Luzio J. P., Newby A. C., Hales C. N. A rapid immunological procedure for the isolation of hormonally sensitive rat fat-cell plasma membrane. Biochem J. 1976 Jan 15;154(1):11–21. doi: 10.1042/bj1540011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh M., Bolzau E., Helenius A. Penetration of Semliki Forest virus from acidic prelysosomal vacuoles. Cell. 1983 Mar;32(3):931–940. doi: 10.1016/0092-8674(83)90078-8. [DOI] [PubMed] [Google Scholar]
- Matlin K. S., Reggio H., Helenius A., Simons K. Infectious entry pathway of influenza virus in a canine kidney cell line. J Cell Biol. 1981 Dec;91(3 Pt 1):601–613. doi: 10.1083/jcb.91.3.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matlin K., Bainton D. F., Pesonen M., Louvard D., Genty N., Simons K. Transepithelial transport of a viral membrane glycoprotein implanted into the apical plasma membrane of Madin-Darby canine kidney cells. I. Morphological evidence. J Cell Biol. 1983 Sep;97(3):627–637. doi: 10.1083/jcb.97.3.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellman I. S., Steinman R. M., Unkeless J. C., Cohn Z. A. Selective iodination and polypeptide composition of pinocytic vesicles. J Cell Biol. 1980 Sep;86(3):712–722. doi: 10.1083/jcb.86.3.712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellman I., Fuchs R., Helenius A. Acidification of the endocytic and exocytic pathways. Annu Rev Biochem. 1986;55:663–700. doi: 10.1146/annurev.bi.55.070186.003311. [DOI] [PubMed] [Google Scholar]
- Slot J. W., Geuze H. J. Sizing of protein A-colloidal gold probes for immunoelectron microscopy. J Cell Biol. 1981 Aug;90(2):533–536. doi: 10.1083/jcb.90.2.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall D. A., Hubbard A. L. Receptor-mediated endocytosis of asialoglycoproteins by rat liver hepatocytes: biochemical characterization of the endosomal compartments. J Cell Biol. 1985 Dec;101(6):2104–2112. doi: 10.1083/jcb.101.6.2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts C. In situ 125I-labelling of endosome proteins with lactoperoxidase conjugates. EMBO J. 1984 Sep;3(9):1965–1970. doi: 10.1002/j.1460-2075.1984.tb02077.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White J., Kartenbeck J., Helenius A. Fusion of Semliki forest virus with the plasma membrane can be induced by low pH. J Cell Biol. 1980 Oct;87(1):264–272. doi: 10.1083/jcb.87.1.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wileman T., Harding C., Stahl P. Receptor-mediated endocytosis. Biochem J. 1985 Nov 15;232(1):1–14. doi: 10.1042/bj2320001. [DOI] [PMC free article] [PubMed] [Google Scholar]









