Abstract
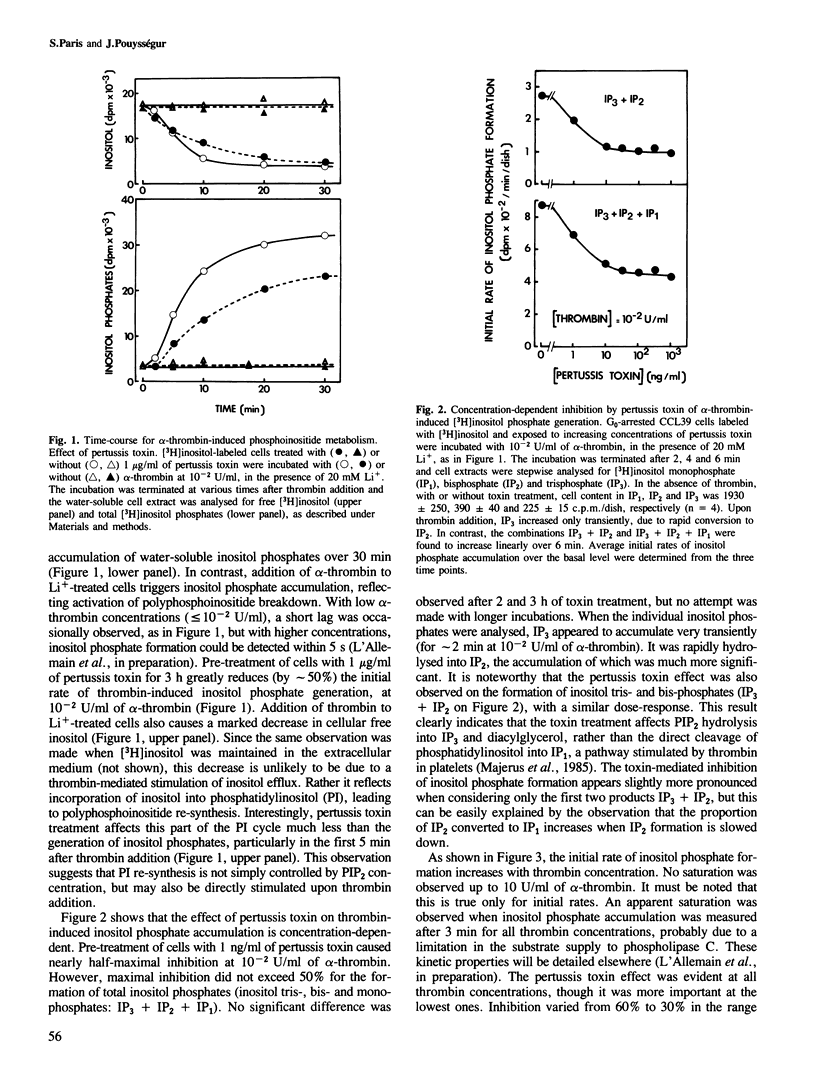
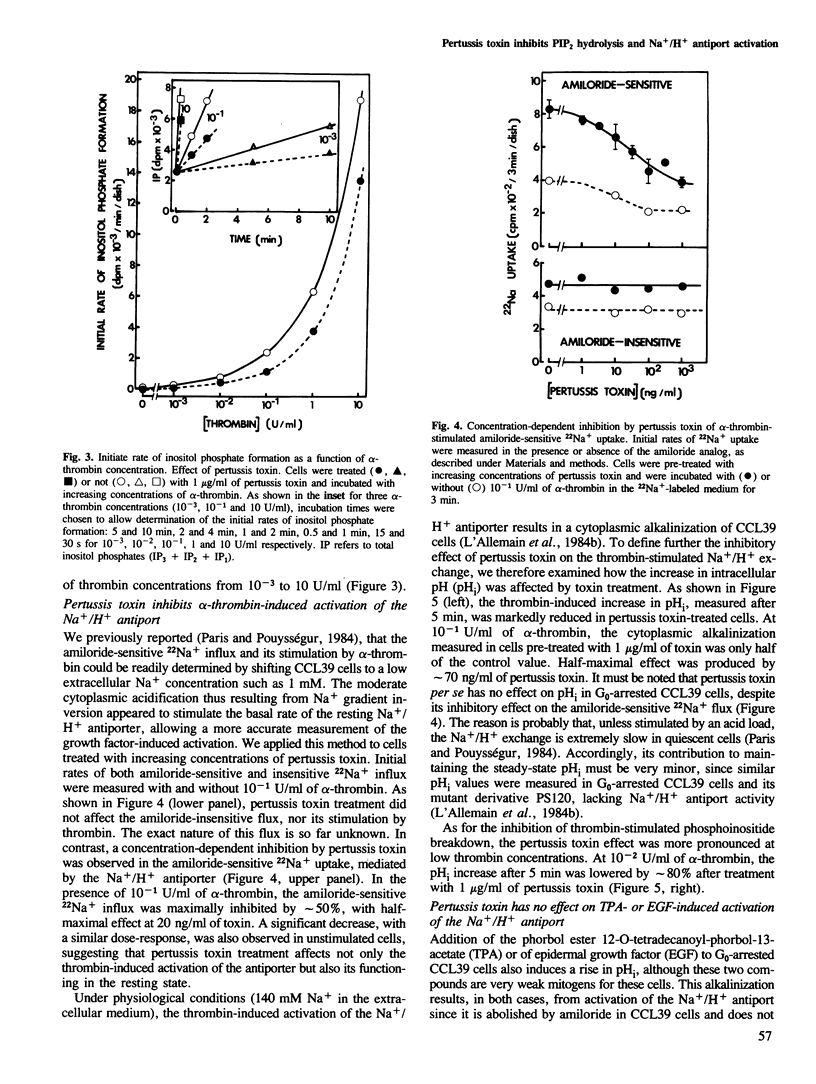
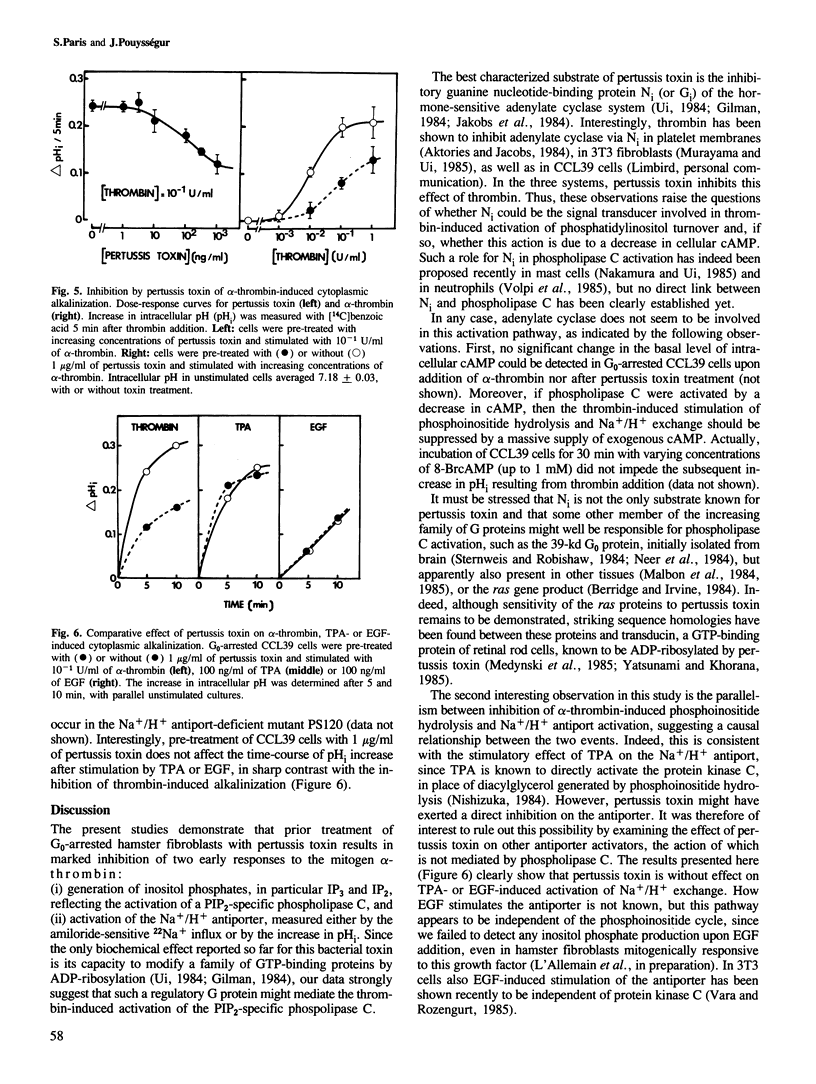
Prior treatment with pertussis toxin of G0-arrested hamster fibroblasts (CCL39) results in a dose-dependent inhibition of two early events of the mitogenic response elicited by alpha-thrombin: accumulation of inositol phosphates in Li+-treated cells, and activation of the Na+/H+ antiport, measured either by the amiloride-sensitive 22Na+ influx or by the increase in intracellular pH. At 10(-1) U/ml of alpha-thrombin, the maximal inhibition was approximately 50% for these two early cellular responses, but the pertussis toxin effect was more pronounced at lower thrombin concentrations. In contrast, pertussis toxin does not affect the Na+/H+ antiport activation induced by phorbol esters or EGF, the action of which is not mediated by the phosphoinositide-metabolizing pathway in CCL39 cells. Therefore, our data suggest the following. A GTP-binding regulatory protein is probably involved in signal transduction between thrombin receptors and the phosphatidylinositol 4,5-bisphosphate-specific phospholipase C. This regulation does not seem to be exerted via modulations of cyclic AMP levels. The thrombin-induced activation of Na+/H+ antiport is, at least in part, mediated by the protein kinase C, as a consequence of stimulation of phosphatidylinositol turnover.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aktories K., Jakobs K. H. Ni-mediated inhibition of human platelet adenylate cyclase by thrombin. Eur J Biochem. 1984 Dec 3;145(2):333–338. doi: 10.1111/j.1432-1033.1984.tb08558.x. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Downes C. P., Hanley M. R. Lithium amplifies agonist-dependent phosphatidylinositol responses in brain and salivary glands. Biochem J. 1982 Sep 15;206(3):587–595. doi: 10.1042/bj2060587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and diacylglycerol as second messengers. Biochem J. 1984 Jun 1;220(2):345–360. doi: 10.1042/bj2200345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984 Nov 22;312(5992):315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Besterman J. M., Cuatrecasas P. Phorbol esters rapidly stimulate amiloride-sensitive Na+/H+ exchange in a human leukemic cell line. J Cell Biol. 1984 Jul;99(1 Pt 1):340–343. doi: 10.1083/jcb.99.1.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bone E. A., Fretten P., Palmer S., Kirk C. J., Michell R. H. Rapid accumulation of inositol phosphates in isolated rat superior cervical sympathetic ganglia exposed to V1-vasopressin and muscarinic cholinergic stimuli. Biochem J. 1984 Aug 1;221(3):803–811. doi: 10.1042/bj2210803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford P. G., Rubin R. P. Pertussis toxin inhibits chemotactic factor-induced phospholipase C stimulation and lysosomal enzyme secretion in rabbit neutrophils. FEBS Lett. 1985 Apr 22;183(2):317–320. doi: 10.1016/0014-5793(85)80801-2. [DOI] [PubMed] [Google Scholar]
- Brandt S. J., Dougherty R. W., Lapetina E. G., Niedel J. E. Pertussis toxin inhibits chemotactic peptide-stimulated generation of inositol phosphates and lysosomal enzyme secretion in human leukemic (HL-60) cells. Proc Natl Acad Sci U S A. 1985 May;82(10):3277–3280. doi: 10.1073/pnas.82.10.3277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassel D., Whiteley B., Zhuang Y. X., Glaser L. Mitogen-independent activation of Na+/H+ exchange in human epidermoid carcinoma A431 cells: regulation by medium osmolarity. J Cell Physiol. 1985 Feb;122(2):178–186. doi: 10.1002/jcp.1041220203. [DOI] [PubMed] [Google Scholar]
- Castagna M., Takai Y., Kaibuchi K., Sano K., Kikkawa U., Nishizuka Y. Direct activation of calcium-activated, phospholipid-dependent protein kinase by tumor-promoting phorbol esters. J Biol Chem. 1982 Jul 10;257(13):7847–7851. [PubMed] [Google Scholar]
- Cockcroft S., Gomperts B. D. Role of guanine nucleotide binding protein in the activation of polyphosphoinositide phosphodiesterase. Nature. 1985 Apr 11;314(6011):534–536. doi: 10.1038/314534a0. [DOI] [PubMed] [Google Scholar]
- Dicker P., Rozengurt E. Phorbol ester stimulation of Na influx and Na-K pump activity in Swiss 3T3 cells. Biochem Biophys Res Commun. 1981 May 15;100(1):433–441. doi: 10.1016/s0006-291x(81)80115-5. [DOI] [PubMed] [Google Scholar]
- Gilman A. G. G proteins and dual control of adenylate cyclase. Cell. 1984 Mar;36(3):577–579. doi: 10.1016/0092-8674(84)90336-2. [DOI] [PubMed] [Google Scholar]
- Grinstein S., Cohen S., Goetz J. D., Rothstein A., Gelfand E. W. Characterization of the activation of Na+/H+ exchange in lymphocytes by phorbol esters: change in cytoplasmic pH dependence of the antiport. Proc Natl Acad Sci U S A. 1985 Mar;82(5):1429–1433. doi: 10.1073/pnas.82.5.1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesketh T. R., Moore J. P., Morris J. D., Taylor M. V., Rogers J., Smith G. A., Metcalfe J. C. A common sequence of calcium and pH signals in the mitogenic stimulation of eukaryotic cells. Nature. 1985 Feb 7;313(6002):481–484. doi: 10.1038/313481a0. [DOI] [PubMed] [Google Scholar]
- Jakobs K. H., Aktories K., Schultz G. Mechanism of pertussis toxin action on the adenylate cyclase system. Inhibition of the turn-on reaction of the inhibitory regulatory site. Eur J Biochem. 1984 Apr 2;140(1):177–181. doi: 10.1111/j.1432-1033.1984.tb08083.x. [DOI] [PubMed] [Google Scholar]
- L'Allemain G., Franchi A., Cragoe E., Jr, Pouysségur J. Blockade of the Na+/H+ antiport abolishes growth factor-induced DNA synthesis in fibroblasts. Structure-activity relationships in the amiloride series. J Biol Chem. 1984 Apr 10;259(7):4313–4319. [PubMed] [Google Scholar]
- L'Allemain G., Paris S., Pouysségur J. Growth factor action and intracellular pH regulation in fibroblasts. Evidence for a major role of the Na+/H+ antiport. J Biol Chem. 1984 May 10;259(9):5809–5815. [PubMed] [Google Scholar]
- Litosch I., Wallis C., Fain J. N. 5-Hydroxytryptamine stimulates inositol phosphate production in a cell-free system from blowfly salivary glands. Evidence for a role of GTP in coupling receptor activation to phosphoinositide breakdown. J Biol Chem. 1985 May 10;260(9):5464–5471. [PubMed] [Google Scholar]
- Malbon C. C., Mangano T. J., Watkins D. C. Heart contains two substrates (Mr = 40,000 and 41,000) for pertussis toxin-catalyzed ADP-ribosylation that co-purify with Ns. Biochem Biophys Res Commun. 1985 Apr 30;128(2):809–815. doi: 10.1016/0006-291x(85)90119-6. [DOI] [PubMed] [Google Scholar]
- Malbon C. C., Rapiejko P. J., Garciá-Sáinz J. A. Pertussis toxin catalyzes the ADP-ribosylation of two distinct peptides, 40 and 41 kDa, in rat fat cell membranes. FEBS Lett. 1984 Oct 29;176(2):301–306. doi: 10.1016/0014-5793(84)81184-9. [DOI] [PubMed] [Google Scholar]
- Manning D. R., Fraser B. A., Kahn R. A., Gilman A. G. ADP-ribosylation of transducin by islet-activation protein. Identification of asparagine as the site of ADP-ribosylation. J Biol Chem. 1984 Jan 25;259(2):749–756. [PubMed] [Google Scholar]
- Medynski D. C., Sullivan K., Smith D., Van Dop C., Chang F. H., Fung B. K., Seeburg P. H., Bourne H. R. Amino acid sequence of the alpha subunit of transducin deduced from the cDNA sequence. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4311–4315. doi: 10.1073/pnas.82.13.4311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moolenaar W. H., Tertoolen L. G., de Laat S. W. Phorbol ester and diacylglycerol mimic growth factors in raising cytoplasmic pH. Nature. 1984 Nov 22;312(5992):371–374. doi: 10.1038/312371a0. [DOI] [PubMed] [Google Scholar]
- Murayama T., Ui M. Receptor-mediated inhibition of adenylate cyclase and stimulation of arachidonic acid release in 3T3 fibroblasts. Selective susceptibility to islet-activating protein, pertussis toxin. J Biol Chem. 1985 Jun 25;260(12):7226–7233. [PubMed] [Google Scholar]
- Nakamura T., Ui M. Simultaneous inhibitions of inositol phospholipid breakdown, arachidonic acid release, and histamine secretion in mast cells by islet-activating protein, pertussis toxin. A possible involvement of the toxin-specific substrate in the Ca2+-mobilizing receptor-mediated biosignaling system. J Biol Chem. 1985 Mar 25;260(6):3584–3593. [PubMed] [Google Scholar]
- Neer E. J., Lok J. M., Wolf L. G. Purification and properties of the inhibitory guanine nucleotide regulatory unit of brain adenylate cyclase. J Biol Chem. 1984 Nov 25;259(22):14222–14229. [PubMed] [Google Scholar]
- Nishizuka Y. Turnover of inositol phospholipids and signal transduction. Science. 1984 Sep 21;225(4668):1365–1370. doi: 10.1126/science.6147898. [DOI] [PubMed] [Google Scholar]
- Paris S., Pouysségur J. Growth factors activate the Na+/H+ antiporter in quiescent fibroblasts by increasing its affinity for intracellular H+. J Biol Chem. 1984 Sep 10;259(17):10989–10994. [PubMed] [Google Scholar]
- Pouysségur J., Franchi A., L'Allemain G., Paris S. Cytoplasmic pH, a key determinant of growth factor-induced DNA synthesis in quiescent fibroblasts. FEBS Lett. 1985 Oct 7;190(1):115–119. doi: 10.1016/0014-5793(85)80439-7. [DOI] [PubMed] [Google Scholar]
- Rosoff P. M., Stein L. F., Cantley L. C. Phorbol esters induce differentiation in a pre-B-lymphocyte cell line by enhancing Na+/H+ exchange. J Biol Chem. 1984 Jun 10;259(11):7056–7060. [PubMed] [Google Scholar]
- Smith C. D., Lane B. C., Kusaka I., Verghese M. W., Snyderman R. Chemoattractant receptor-induced hydrolysis of phosphatidylinositol 4,5-bisphosphate in human polymorphonuclear leukocyte membranes. Requirement for a guanine nucleotide regulatory protein. J Biol Chem. 1985 May 25;260(10):5875–5878. [PubMed] [Google Scholar]
- Sternweis P. C., Robishaw J. D. Isolation of two proteins with high affinity for guanine nucleotides from membranes of bovine brain. J Biol Chem. 1984 Nov 25;259(22):13806–13813. [PubMed] [Google Scholar]
- Van Dop C., Yamanaka G., Steinberg F., Sekura R. D., Manclark C. R., Stryer L., Bourne H. R. ADP-ribosylation of transducin by pertussis toxin blocks the light-stimulated hydrolysis of GTP and cGMP in retinal photoreceptors. J Biol Chem. 1984 Jan 10;259(1):23–26. [PubMed] [Google Scholar]
- Van Obberghen-Schilling E., Chambard J. C., Paris S., L'Allemain G., Pouysségur J. alpha-Thrombin-induced early mitogenic signalling events and G0 to S-phase transition of fibroblasts require continual external stimulation. EMBO J. 1985 Nov;4(11):2927–2932. doi: 10.1002/j.1460-2075.1985.tb04025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Obberghen-Schilling E., Pérez-Rodriguez R., Pouysségur J. Hirudin, a probe to analyze the growth-promoting activity of thrombin in fibroblasts; reevaluation of the temporal action of competence factors. Biochem Biophys Res Commun. 1982 May 14;106(1):79–86. doi: 10.1016/0006-291x(82)92060-5. [DOI] [PubMed] [Google Scholar]
- Vara F., Rozengurt E. Stimulation of Na+/H+ antiport activity by epidermal growth factor and insulin occurs without activation of protein kinase C. Biochem Biophys Res Commun. 1985 Jul 31;130(2):646–653. doi: 10.1016/0006-291x(85)90466-8. [DOI] [PubMed] [Google Scholar]
- Vara F., Schneider J. A., Rozengurt E. Ionic responses rapidly elicited by activation of protein kinase C in quiescent Swiss 3T3 cells. Proc Natl Acad Sci U S A. 1985 Apr;82(8):2384–2388. doi: 10.1073/pnas.82.8.2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigne P., Frelin C., Lazdunski M. The Na+/H+ antiport is activated by serum and phorbol esters in proliferating myoblasts but not in differentiated myotubes. Properties of the activation process. J Biol Chem. 1985 Jul 5;260(13):8008–8013. [PubMed] [Google Scholar]
- Volpi M., Naccache P. H., Molski T. F., Shefcyk J., Huang C. K., Marsh M. L., Munoz J., Becker E. L., Sha'afi R. I. Pertussis toxin inhibits fMet-Leu-Phe- but not phorbol ester-stimulated changes in rabbit neutrophils: role of G proteins in excitation response coupling. Proc Natl Acad Sci U S A. 1985 May;82(9):2708–2712. doi: 10.1073/pnas.82.9.2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yatsunami K., Khorana H. G. GTPase of bovine rod outer segments: the amino acid sequence of the alpha subunit as derived from the cDNA sequence. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4316–4320. doi: 10.1073/pnas.82.13.4316. [DOI] [PMC free article] [PubMed] [Google Scholar]


