Abstract
The peroxisome proliferator-activated receptor subtype γ (PPARγ) ligands, namely the synthetic insulin-sensitizing thiazolidinedione (TZD) compounds, have demonstrated great potential in the treatment of type II diabetes. However, their clinical applicability is limited by a common and serious side effect of edema. To address the mechanism of TZD-induced edema, we generated mice with collecting duct (CD)-specific disruption of the PPARγ gene. We found that mice with CD knockout of this receptor were resistant to the rosiglitazone- (RGZ) induced increases in body weight and plasma volume expansion found in control mice expressing PPARγ in the CD. RGZ reduced urinary sodium excretion in control and not in conditional knockout mice. Furthermore, RGZ stimulated sodium transport in primary cultures of CD cells expressing PPARγ and not in cells lacking this receptor. These findings demonstrate a PPARγ-dependent pathway in regulation of sodium transport in the CD that underlies TZD-induced fluid retention.
Keywords: roziglitazone, Cre recombinase, Evans blue technique
Thiazolidinediones (TZDs), synthetic insulin-sensitizing drugs that include troglitazone, pioglitazone, and rosiglitazone (RGZ), are highly effective in the treatment of type II diabetes. TZDs are believed to mediate their antidiabetic effect via activation of peroxisome proliferator-activated receptor γ (PPARγ) (1). In addition to lowering blood glucose, these drugs also benefit cardiovascular parameters, such as blood pressure and endothelial function (2, 3). However, fluid retention, presented as rapid weight gain, and peripheral and pulmonary edema have emerged as the most common and serious side effects of TZDs (4–6). Global awareness of this side effect has increased as a result of the growing number of reported cases. In a recent issue of Circulation (7), the American Heart Association and American Diabetes Association jointly issued a Consensus Statement commenting on the safety of TZD as related to edema. The mechanisms of fluid retention in patients treated with TZDs are poorly understood and may involve a number of factors, including reduction of urinary sodium excretion (8), alteration of endothelial permeability (9), increased sympathetic nervous system activity (10), or altered interstitial ion transport (11). To evaluate the relative contributions of these individual mechanisms, tissue- or cell-type-specific approaches are needed in carefully designed studies.
PPARs are a group of zinc finger-containing transcription factors, representing a family of the nuclear hormone receptor gene superfamily. To date, three subtypes of PPARs encoded by different genes have been described from several species: PPARα, -β/δ, and -γ (12, 13). They share a high degree of similarity in their overall amino acid sequences, particularly in the DNA-binding domain (14). The three isoforms of the PPARs heterodimerize with retinoid X receptor, bind to the same peroxisome proliferator-responsive element in the promoter regions of their target genes, and modulate gene transcription (13). Distinct functions for PPAR family members are suggested from their tissue-specific expression patterns: PPARα is mainly expressed in liver, kidney, and heart, where it controls fatty acid and lipid metabolism; PPARγ is highly expressed in fat tissues, where it controls adipocyte differentiation and lipid storage; and PPARδ is widely (15) expressed and its function largely remains unclear, although recent evidence suggests a role in fatty acid and lipid metabolism (16).
Within the kidney, PPARγ is predominantly expressed in the inner medulla and in the inner medullary collecting duct (CD) (17, 18), a critical site for the control of fluid metabolism. Therefore, we hypothesize that activation of PPARγ in the distal nephron may serve as the primary mechanism responsible for TZD-induced fluid retention. To examine this hypothesis, the present study uses the Cre-loxP system to generate mice with CD-specific deletion of PPARγ. The CD PPARγ knockout (KO) mice were generated by genetic cross between PPARγ floxed mice and transgenic mice expressing Cre recombinase under the control of the mouse AQP2 promoter. We report that TZD-induced fluid retention is remarkably blocked in CD PPARγ KO mice as compared with controls.
Methods
Transgenic Mice. We generated mice with CD-specific KO of the PPARγ gene by genetic cross between PPARγ floxed mice and AQP2-Cre mice. The PPARγ floxed mice contain two loxP sites inserted into introns 1 and 2 of the PPARγ gene flanking the critical exon 2 by homologous recombination in ES cells (19). AQP2-Cre mice contain a transgene, with 11 kb of the mouse AQP2 gene 5′ flanking region driving expression of the Cre recombinase (20). Both PPARγ floxed and AQP2-Cre mice were phenotypically normal. Homozygous PPARγ floxed (PPARγf/f) mice were mated with female AQP2-Cre mice to yield mice heterozygous for floxed PPARγ and heterozygous for AQP2-Cre. These mice were bred with mice homozygous for floxed PPARγ to obtain mice homozygous for floxed PPARγ (termed CD PPARγ KO).
All animal procedures were approved by the University of Utah Institutional Animal Care and Use Committee.
Genotyping. The genotype of the AQP2-Cre mice was performed as described (20). Genotyping the PPARγ gene involved the use of primers PPARγ F1 (5′-CTCCAATGTTCTCAAACTTAC-3′) and PPARγ R1 (5′-GAT GAGTCATGTAAGTTGACC-3′), which yielded a 225-bp band from the wild-type allele and a 275-bp band from the floxed allele. The DNA recombination was assessed in both kidney regions and microdissected nephron segments. Renal cortex and inner medulla from CD PPARγ KO and PPARγf/f mice were dissected and subjected to DNA extraction by using TRIzol reagent. The DNA was amplified by using primers PPARγ F2 (5′-GACAGCACAACA ATGTTCCCA-3′) and PPARγ R2 (5′-GTATTCTATGGCTTCCAGTGC-3′), which flanked the loxP sites and yielded a 2,423-bp band from the floxed allele and a 438-bp band from the recombined allele. To evaluate the CD-specific recombination event, CD PPARγ KO mice were anesthetized by isoflurance, and the left kidney was perfused with DMEM containing 2 mg/ml each collagenase Type 1 (Worthington) and hyaluronidase (Sigma). Kidney slices were incubated in the same solution for 45 min at 37°C. Under a stereomicroscope, 20–30 of each of the following nephron segments, including glomerulus, proximal convoluted tubule, cortical and medullary thick ascending limb, cortical and inner medullary CD, were dissected. The recombined allele from the microdissected nephron segments was detected as a 438-bp band by using primers PPARγ F2 and PPARγ R2 and the floxed allele as a 1,419-bp band by using primers PPARγ F3 (5′-CTCCAATGT TCTCAA ACTTAC-3′) and PPARγ R3 (5′-CATGAACTCCATAGTGGA AGCC-3′). Because PPARγ R3 was positioned within the loxP sites, no band was detected from recombined allele by using PPARγ F3 and PPARγ R3.
RGZ Treatment and Metabolic Studies. RGZ was incorporated into a chow-based diet (LabDiet Rodent Chow 5001; Purina) at a level of 320 mg/kg diet. RGZ was made by GlaxoSmithKline and purchased from the University of Utah Hospital. The gelled diets were made by melting agar (1% by weight) in water (65%), cooling, and adding the drug (0.1%), ground chow (33.9%), and NaCl (0.5%). The final content of NaCl became 0.8%. The same gelled diets without the drug served as controls. Adult PPARγf/f and CD PPARγ KO mice (3∼4-month-old) were acclimatized to metabolic cages (Hatteras Instruments, Cary, NC) and the control diet for 7 days. The numbers of males and females were roughly even in each group. After the 7-day acclimation period, PPARγf/f and CD PPARγ KO mice were placed on the gelled diet with or without RGZ for 9 days. Measurements of body weight and collection of 24-hour urine were performed. Hematocrit (Hct) was measured before and after RGZ treatment; at the end of experiments, plasma volume and blood pressure were determined as described in the following.
Measurement of Hct. The sphenous vein was punctured by using a #23-gauge needle, and one drop of blood (≈5–10 μl) was collected by using a 10-μl capillary glass (Idaho Technology, Salt Lake City). One side of the tube was sealed with Hemato-Seal and then centrifuged for 4 min in a Thermo IEC (Boston) microcentrifuge machine.
Measurements of Plasma Volume and Blood Pressure. Under general anesthetization with isoflurane (2 ml/min), catheters were placed in the carotid artery for direct measurement of systolic blood pressure and in the jugular vein for infusion of Evans blue. Blood pressure was recorded by using a pressure transducer (Abbott Critical Care System) and a data acquisition system (Dataq Instruments, Akron, OH). After recording of blood pressure for 5 min, 25 μl of 2 mg/ml Evans blue was injected via jugular vein catheterization. Seven minutes later, ≈700–800 μl of blood was withdrawn from the vena cava by using a heparin-coated 1-cc syringe with bent #23-gauge needle. Plasma was separated by centrifugation of the blood at 5,000 × g for 4 min. Absorbance was read at 620 nm, and plasma Evans blue concentrations were calculated according to a standard curve generated by a serial dilution of the 2 mg/ml Evans blue-saline solution. Plasma volume was calculated by using the dilution factors of Evans blue.
Aldosterone RIA. Plasma aldosterone concentrations were determined by using Coat-A-Count RIA kit (Diagnostic Products, Los Angeles). Twenty microliters of plasma was diluted with 100 μl of diluent before the assay.
Primary Cultures of CD Cells and Measurements of Sodium Transport. The primary cultures of CD cells derived from the whole kidneys of PPARγf/f and CD PPARγ KO mice were performed by using lectin-coated Dynabeads, as described (21).
For measurements of sodium transport, the CD cells were subcultured onto permeable filter supports (0.4-μm pore size, 1.13-cm2 surface area; Transwell, Corning Costar). Cells were kept on filters for at least 10 days until a confluent transporting cell monolayer had developed. This was evaluated by measurement of the [14C]-inulin leak, as described (22). Transepithelial resistance was determined by using “chopstick” electrodes (EVOM, World Precision Instruments, Sarasota, FL). Transepithelial transport of 22Na was determined by adding 10 μCi (1 Ci = 37 GBq) of 22Na to the apical compartment followed by measurement of the radioactivity in the basal compartment. All studies described in this report were performed on cells between the third and fifth passages.
Statistical Analysis. Values shown represent means ±SE. Statistical analysis was performed by unpaired t tests or ANOVA and Bonferroni tests, with a P value of <0.05 being considered statistically significant.
Results
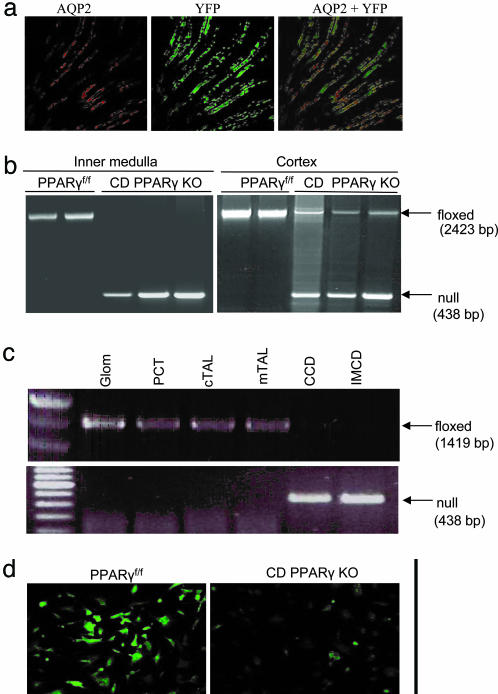
Generation of Mice with CD-Specific KO of PPARγ. The PPARγ floxed mice, containing two loxP sites inserted into introns 1 and 2 of the PPARγ gene flanking the critical exon 2, were generated by homologous recombination in ES cells (19). AQP2-Cre transgenic mice expressing Cre under the control of mouse AQP2 promoter (20) were used to produce a CD-specific disruption of the PPARγ gene. To assess CreTag activity in vivo, AQP2-Cre mice were bred with reporter mice (ROSA26-YFP). YFP expression was restricted to kidney and testes but not other tissues examined, including lung, brain, heart, muscle, intestine, stomach, spleen, and liver (data not shown). In the kidney, YFP expression was found in AQP2-expressing CD cells (Fig. 1a). Homozygous PPARγ floxed (PPARγf/f) mice were mated with female AQP2-Cre mice to yield mice heterozygous for the floxed PPARγ allele and heterozygous for the AQP2-Cre transgene. These mice were bred with mice homozygous for the floxed PPARγ to obtain mice homozygous for the floxed PPARγ (termed CD PPARγ KO). CD PPARγ KO mice had no gross morphological abnormalities until at least 6 months of age.
Fig. 1.
Validation of CD-specific KO of PPARγ. (a) Colocalization of YFP expression and AQP2 immunofluorescence in mice doubly heterozygous for ROSA26-YFP and AQP2-Cre. A representative photomicrograph is shown from three separate animals (×600). (b) PCR analysis of AQP2-Cre mediated recombination of the PPARγ gene in the inner medulla and cortex. Null band (438 bp) is the recombination product after deletion of exon 2 of the PPARγ. Exon 2 was nearly completely deleted in the inner medulla and partially deleted in the cortex of CD PPARγ KO mice (n = 3) as compared with the floxed controls (n = 2). (c) PCR analysis of AQP2-Cre-mediated recombination of the PPARγ gene in microdissected nephron segments from PPARγ KO mice. (d) Immunocytochemistry analysis of PPARγ protein expression in CD cells derived from PPARγ KO mice. CD cells were isolated by using lectin-coated dynabeads and grown in a chamber slide. Immunocytochemistry was performed by using a polyclonal antibody against PPARγ. Shown is a representative from three separate experiments.
The 438-bp products derived from the recombined allele were detected in both the inner medulla and cortex of CD PPARγ KO mice but not in those of PPARf/f mice (Fig. 1b). The 2,423-bp products derived from the floxed allele were almost undetectable in the inner medulla and were substantially reduced in the renal cortex of CD PPARγ KO mice, compared with the floxed controls. To confirm the CD-specific recombination, microdissected nephron segments from CD PPARγ KO mice were examined for the existence of the floxed and recombined alleles. The recombined allele, detected as a 438-bp band, was found only in the CD (cortical and inner medullary CDs) but not in other segments; the floxed allele, detected as 1,419-bp band, was present in Glom, PCT, cTAL, and mTAL, but was almost undetectable in the CD (Fig. 1c). Using lectin-coated Dynabeads, we isolated CD cells from PPARγf/f and CD PPARγ KO mice. The PPARγf/f cells expressed abundant PPARγ protein mostly in the nucleus, as assessed by immunocytochemistry. In contrast, PPARγ protein expression in the PPARγ KO cells was markedly reduced (Fig. 1d).
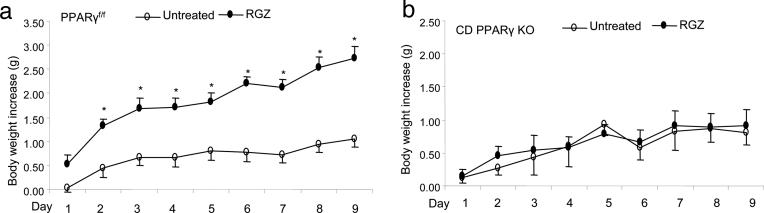
Comparison of Body Weights. TZDs induce body weight gain in both humans and rats as a result of fluid retention. Therefore, we monitored changes in body weights in PPARγf/f and CD PPARγ KO mice after a 9-day RGZ treatment. We found that RGZ induced a gradual and significant increase in body weights in the floxed mice as compared with the untreated floxed animals (2.74 ± 0.25 vs. 1.05 + 0.16 g on day 9, P < 0.05). In contrast, body weight gains between RGZ-treated and untreated CD PPARγ KO mice were not significantly different (0.90 ± 0.25 vs. 0.81 ± 0.19 g on day 9, P > 0.05) (Fig. 2).
Fig. 2.
Body-weight gains in untreated and RGZ-treated PPARγf/f mice (a) and CD PPARγ KO mice (b). PPARγf/f per vehicle, n = 11; PPARγf/f per RGZ, n = 9; CD PPARγ KO per vehicle, n = 8; CD PPARγ KO group, n = 9. *, P < 0.05 vs. vehicle at the corresponding time point.
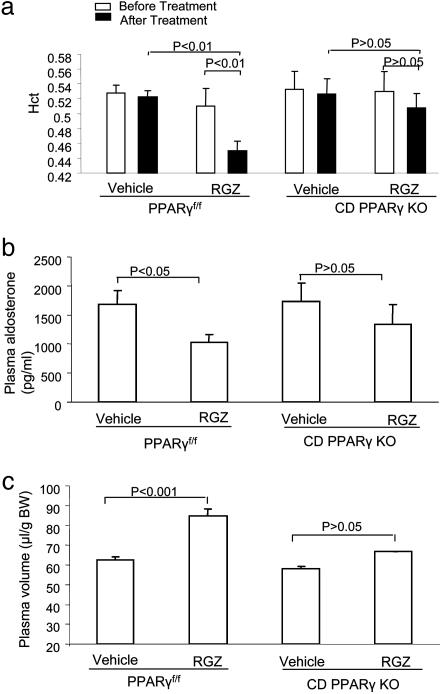
Comparison of Plasma Volumes. We monitored Hct changes in PPARγf/f and CD PPARγ KO mice before and after RGZ treatment. Blood samples were collected from the sphenous vein in awake animals for determination of Hct. RGZ treatment consistently induced a fall of Hct from 51.0 ± 2.3% to 45.0 ± 1.2% (P < 0.01) in PPARγf/f mice (Fig. 3a). A trend for a reduction of Hct was seen in CD PPARγ KO mice but did not reach statistical significance.
Fig. 3.
Changes in plasma volume in PPARγf/f and CD PPARγ KO mice after RGZ treatment. (a) Hct in PPARγf/f and CD PPARγ KO mice before and after RGZ treatment. PPARγf/f per vehicle, n = 4; PPARγf/f per RGZ, n = 5; CD PPARγ KO per vehicle, n = 4; CD PPARγ KO group, n = 4. (b) Plasma aldosterone levels in PPARγf/f and CD PPARγ KO mice after RGZ treatment. n = 4 in each group. (c) Determination of plasma volume PPARγf/f and CD PPARγ KO mice by the Evans blue technique. PPARγf/f per vehicle, n = 5; PPARγf/f per RGZ, n = 6; CD PPARγ KO per vehicle, n = 4; CD PPARγ KO group, n = 4.
Plasma aldosterone levels are widely used as a reliable index of plasma volume. Thus we compared RGZ-induced changes in plasma aldosterone levels between the two strains of mice. We observed a significant fall of plasma aldosterone levels in PPARf/f mice after RGZ treatment, in contrast to the insignificant changes in CD PPARγ KO mice (Fig. 3b). The pattern of changes in plasma aldosterone levels was similar to those in Hct levels.
To confirm the results obtained with indirect measurements, we used the Evans blue technique to accurately measure plasma volume. The Evans blue technique is widely used to determine plasma volume in humans (23, 24), dog (25), and rat (26). Plasma volume in untreated PPARf/f mice was 62.5 ± 1.6 μl per gram, which approximates the value of 54 ± 7 ml/kg in dog (25). A 9-day RGZ treatment induced a 32.2% increase in plasma volume in PPARγf/f mice. In the basal state, CD PPARγ KO mice had a normal plasma volume that was not significantly different from the floxed controls. However, the KO mice had a significantly reduced plasma volume expansion induced by RGZ (Fig. 3c).
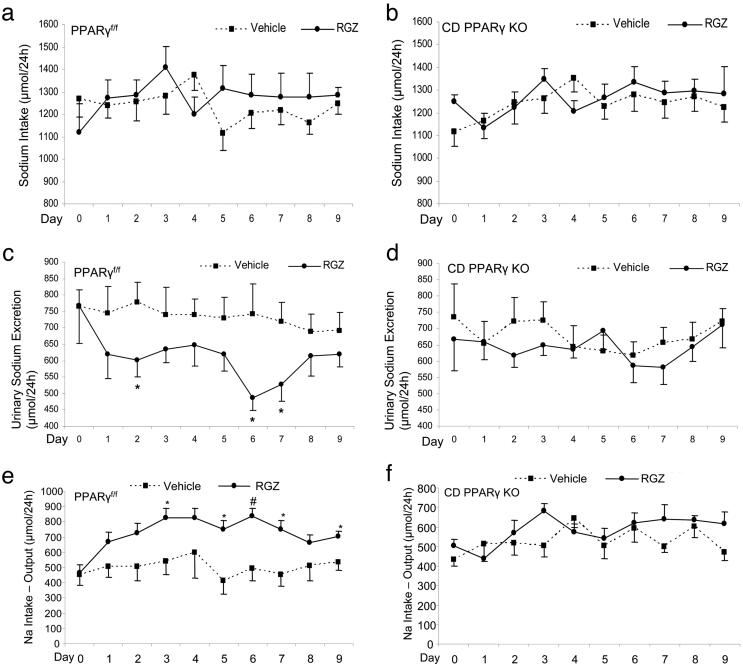
Comparison of Sodium Balance. We performed metabolic studies to determine sodium intake and excretion in PPARγf/f and CD PPARγ KO mice before and after RGZ treatment. After RGZ treatment, PPARf/f mice had unchanged sodium intake but a significant reduction of urinary sodium excretion that peaked at day 6 and returned to normal at day 8; CD PPARγ KO mice did not exhibit significant changes in either sodium intake or urinary sodium excretion (Fig. 4 a–d). Sodium balance was further determined by subtracting output from intake. RGZ treatment induced a positive sodium balance in the control mice but not in CD PPARγ KO mice (Fig. 4 e and f).
Fig. 4.
Comparison of urinary sodium excretion and sodium intake between PPRAγf/f and CD PPARγ KO mice. After a 7-day acclimation period, PPRAγf/f and CD PPARγ KO mice were treated for 9 days with the gelled diet incorporated with or without RGZ. Shown are daily sodium intake (a and b), urinary sodium excretion (c and d), and sodium balance (intake–output) (e and f). n = 9 in each group. *, P < 0.05 vs. vehicle; #, P < 0.001 vs. vehicle at the corresponding time point.
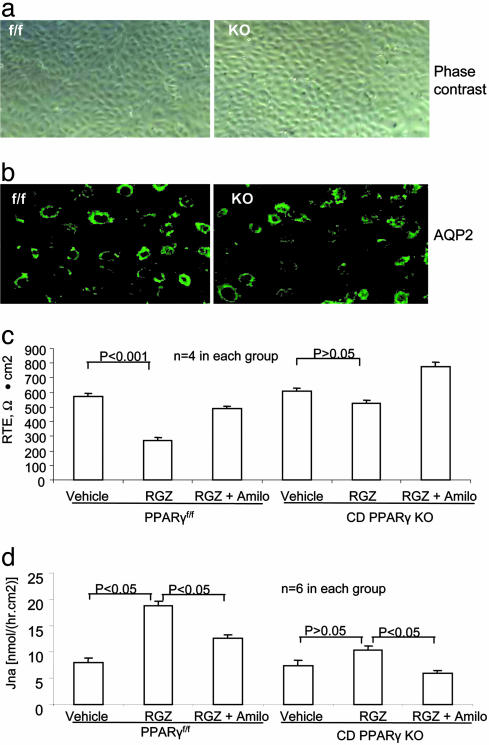
Comparison of Sodium Transport in the Primary Culture of CD Cells. We attempted to address the cellular mechanism of differences in the RGZ-induced plasma volume expansion between PPARf/f and CD PPARγ KO mice. Therefore, we established primary cultures of CD cells derived from the two strains of mice for a parallel examination of sodium transport in response to RGZ treatment. We used biotinylated Dolichos biflorus agglutinin-coated Dynabeads to isolated CD cells. These cells exhibited epithelial cell like morphology (Fig. 5a). In the basal state, they expressed abundant AQP2 mostly in cytoplasm as assessed by immunocytochemistry (Fig. 5b). Cell morphology between the control and CD PPARγ KO cells was not obviously different nor was the expression of AQP2. For measurements of sodium transport, the CD cells were subcultured onto permeable filter supports. Measurement of 14C-inulin leak across the monolayers was performed to determine whether confluent cells were a tight monolayer. The leak of 14C-inulin from the apical to basal was 0.46 ± 0.12% and 0.87 ± 0.24% in the period of 10 min and 1 h, respectively. There were no differences detected between the control and PPAR KO cells (0.58 ± 0.21% vs. 0.35 ± 0.01% at 10 min, and 0.90 ± 0.35% vs. 0.83 ± 0.37% at 1 h, respectively, P > 0.05). Exposure of the control cells to 1 μM RGZ for 24 h significantly reduced transepithelial resistance in an amiloride-sensitive manner, suggesting activation of epithelial sodium channel (ENaC)-mediated sodium transport (Fig. 5c). In contrast, the same RGZ treatment had no obvious effect on transepithelial resistance in the CD PPARγ KO cells. Similarly, the RGZ treatment induced a significant increase in transepithelial 22Na flux in the control cells in an amiloride-sensitive manner. The RGZ-induced changes in 22Na flux were significantly blocked in the PPARγ KO cells.
Fig. 5.
Comparison of sodium transport between the cultured CD cells derived from PPARγf/f and CD PPARγ KO mice. (a) Phase-contrast micrograph of confluent CD cells derived from PPARγf/f and CD PPARγ KO mice. (b) AQP2 immunocytochemistry showing AQP2 expression in the unstimulated control and CD PPARγ KO cells. (c) Changes of transepithelial resistance (RTE) in the control and CD PPARγ KO cells after RGZ treatment. (d) Changes in 22Na flux in the control and PPARγ KO cells after RGZ treatment.
Discussion
The PPARγ activators, TZDs, are insulin-sensitizing agents that improve insulin sensitivity as well as parameters of blood pressure and endothelial function. Currently, two TZDs, RGZ and pioglitazone, are being widely used for the treatment of type II diabetes as efficient insulin sensitizers alone or in combination with other antidiabetic agents such as metformin, sulfonylureas, or insulin. However, these drugs are associated with significant fluid retention as the most common and serious side effect. The current study describes a Cre-Loxp approach to testing the role of CD PPARγ in TZD-induced fluid retention. To achieve CD-specific deletion of PPARγ, we used the mouse AQP2 promoter to drive Cre expression specifically in the CD. Specific recombination in the CD was validated by PCR on microdissected nephron segments. A 9-day RGZ treatment consistently increased body weight in PPARγf/f but not in CD PPARγ KO mice. After RGZ treatment, PPARγf/f mice exhibited severe plasma volume expansion, as reflected by significant decreases in Hct and plasma aldosterone levels, and increases in plasma volume, as measured by the Evans blue technique. In contrast, the RGZ-induced plasma volume expansion was remarkably blunted in CD PPARγ KO mice.
It is well documented that TZD-induced edema is caused by a positive sodium balance (intake > excretion). In this regard, Song et al. (27) reported that chronic 3-day administration of RGZ to Sprague–Dawley rats significantly reduced urine volume (by 22%) and sodium excretion (by 44%). Because electrolyte and water metabolism are largely maintained at the renal level, it is reasonable to speculate that renal mechanisms will play a major role in TZD-induced fluid retention. Within the kidney, PPARγ is highly expressed in the renal medullary CD, with lower expression levels in glomeruli, proximal tubules, and microvasculature, as demonstrated by both RT-PCR and micro-dissection and by in situ hybridization techniques (17, 18, 28). This distribution pattern suggests the possibility that local activation of PPARγ in the CD may stimulate sodium reabsorption and account for the fluid retention. This notion is supported by several lines of direct and indirect evidence from previous studies. First, in a cultured human cortical CD cell line, PPARγ agonists increase levels of cell surface ENaCα, paralleled by stimulation of gene expression of serum and glucocorticoid regulated kinase 1 (SGK1), a key mediator of aldosterone activation of ENaC (29). Second, a PPARγ ligand, GI262570, increased expressions of ENaCα, SGK1, and Na-K-ATPaseα in the renal medulla (26). Finally, GI262570 caused sodium retention but did not affect glomerular filtration rate, renal plasma flow, and renal filtration fraction (30, 31), indirectly supporting the local action of TZDs. In line with these observations, we report that RGZ-induced plasma volume expansion was significantly blunted in PPARγ KO mice. Taken together, the observations from these previous along with our current studies provide solid evidence supporting a role for the distal nephon in fluid retention associated with TZDs.
Compared with control (PPARγf/f) mice, CD PPARγ KO mice exhibited a significantly blunted response to RGZ, as revealed by the extent of fluid retention; however, they still showed trends in changes in Hct, aldosterone, and plasma volume. The reason for the residual responses remaining in PPAR KO mice is unclear. One possibility is that this residual response may be due to incomplete PPARγ deletion in the CD. However, the nearly complete absence of PCR products derived from the unrecombinant allele from the inner medulla and the CD of CD PPARγ KO mice does not support this possibility. Apart from the distal nephron, other sites of action of TZDs may also contribute to fluid retention. PPARγ agonists decrease lithium clearance in humans, suggesting some stimulation of reabsorption in the proximal tubules (32). Several proximal tubule transporters including sodium hydrogen exchanger-3 undergo changes in gene expression in response to RGZ treatment (27). However, these observations are not supported by the finding that PPARγ mRNA is not detected in proximal tubules (18). PPARγ is expressed in the vascular system (33), such as in endothelial cells (34, 35), vascular smooth muscle cells (36), and monocyte/macrophages (37, 38), where TZDs may affect vascular function, leading to edema. In line with this notion, PPARγ activators RGZ and pioglitazone prevent hypertension as well as the vascular changes induced by angiotensin II infusion (39). RGZ also lowers blood pressure in normotensive rats (27). A recent study by Ryan et al. (40) showed that TZDs exerted a direct vasodilatory effect in isolated carotid artery but through PPARγ-independent mechanisms. Overall, other sites than the distal nephron, such as the vasculature, may still contribute to the TZD-induced fluid retention; however, any contribution from these sites is unlikely to be significant.
It is conceivable that the TZD-induced fluid retention is mediated by a PPARγ-dependent activation of sodium transport in the distal nephron. Therefore, primary cultures of CD cells derived from PPARγf/f and CD PPARγ KO mice were established for parallel examination of sodium transport in response to RGZ treatment. In control cells, RGZ treatment had a direct stimulatory effect on sodium transport, as assessed by both transepithelial resistance and transepithelial 22Na flux. In sharp contrast, the RGZ effect was almost completely blocked in the CD PPARγ KO cells. These observations strongly suggest that the stimulatory effect of RGZ on sodium transport is mediated by PPARγ, ruling out nonspecific mechanisms. It is evident that PPARγ functions as a positive regulator of sodium transport process in the distal nephron, which likely underlies TZD-induced fluid retention.
Besides addressing the mechanism of TZD-induced fluid retention, the data presented in the present study strongly support the notion that PPARγ may function as a physiological regulator of sodium transport process in the distal nephron. In cases of modest changes in sodium intake, CD PPARγ KO mice do not exhibit signs of altered balance of electrolytes and water. These findings suggest that PPARγ in the distal nephron is not required for the maintenance of fluid homeostasis in the normal physiological state. However, this does not rule out the possibility that PPARγ may play a role under more stressful conditions with significant changes in salt or water intake. If PPARγ serves as a physiological regulator of the CD function, a question arises as to what is the endogenous ligand for the nuclear receptor. 15-deoxy-Delta(12,14)-prostaglandin J2 (15d-PGJ2), a product of prostaglandin D2, is an effective activator of PPARγ in several in vitro systems (41, 42). Despite some evidence for endogenous biosynthesis of 15d-PGJ2 in a number of cell types (43, 44), it remains uncertain whether 15d-PGJ2 is produced in the kidney in a sufficient amount to act as an effective endogenous ligand for PPARγ in vivo.
Conclusion
The present study generated mice with CD-specific deletion of PPARγ by genetic cross between PPARγ floxed mice with transgenic mice expressing Cre under the control of the mouse AQP2 promoter. After chronic treatment with the PPARγ ligand, control mice developed severe volume expansion in sharp contrast to the significantly blunted response in CD PPARγ KO mice. Overall, our study provides insight into TZD-induced edema and also suggests an area of research concerning PPARγ-dependent mechanisms for the control of fluid homeostasis.
Table 1. Routine physiological data.
| PPARγf/f
|
CD PPARγ KO
|
|||
|---|---|---|---|---|
| Vehicle (n = 4) | RGZ (n = 5) | Vehicle (n = 4) | RGZ (n = 4) | |
| Plasma Na, mmol/l | 157 ± 0.95 | 162 ± 3.15 | 158 ± 1.65 | 162 ± 1.33 |
| Plasma K, mmol/l | 4.08 ± 0.1 | 4.55 ± 0.36 | 3.9 ± 0.17 | 4.2 ± 0.03 |
| Plasma Cl, mmol/l | 109 ± 0.91 | 112 ± 2.84 | 109 ± 2.40 | 113 ± 0.67 |
| Plasma creatinine, mg/dl | 1.02 ± 0.06 | 0.9 ± 0.04 | 0.95 ± 0.028 | 1 ± 0.00 |
| Plasma BUN, mg/dl | 27 ± 3.24 | 29 ± 2.86 | 26 ± 1.87 | 26 ± 3.21 |
| Urine volume, μl/24 hr | 1625 ± 188.68 | 1,749 ± 257.11 | 1,904 ± 199.27 | 1,762 ± 152.46 |
| Urine creatinine, mg/dl | 20.33 ± 3.60 | 25.5 ± 4.69 | 21.75 ± 1.15 | 19.6 ± 1.63 |
| ClCr, ml/min | 0.22 ± 0.02 | 0.30 ± 0.02 | 0.30 ± 0.05 | 0.25 ± 0.05 |
| SBP, mmHg | 112.67 ± 4.67 | 107.50 ± 2.75 | 123.50 ± 7.77 | 117.25 ± 6.85 |
ClCr, creatinine clearance; SBP, systolic blood pressure. No statistical significance was found between any groups.
Acknowledgments
We thank Mark A. Knepper (National Heart, Lung, and Blood Institute, National Institutes of Health, Bethesda, MD) for providing the AQP2 antibody. This work was supported by National Institutes of Health Grants RO-1 HL079453, RO-1 DK066592, and K01 DK064981 (to T.Y.).
Author contributions: T.Y. designed research; H.Z., A.Z., D.E.K., R.D.N., F.J.G., and T.Y. performed research; H.Z., A.Z., and T.Y. analyzed data; and H.Z. and T.Y. wrote the paper.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: TZD, thiazolidinedione; PPARγ, peroxisome proliferator-activated receptor γ; CD, collecting duct; KO, knockout; Hct, hematocrit; RGZ, rosiglitazone; ENaC, epithelial sodium channel.
References
- 1.Cheng-Lai, A. & Levine, A. (2000) Heart Dis. 2, 326-333. [PubMed] [Google Scholar]
- 2.Parulkar, A. A., Pendergrass, M. L., Granda-Ayala, R., Lee, T. R. & Fonseca, V. A. (2001) Ann. Intern. Med. 134, 61-71. [DOI] [PubMed] [Google Scholar]
- 3.Haffner, S. M., Greenberg, A. S., Weston, W. M., Chen, H., Williams, K. & Freed, M. I. (2002) Circulation 106, 679-684. [DOI] [PubMed] [Google Scholar]
- 4.Hirsch, I. B., Kelly, J. & Cooper, S. (1999) Arch. Intern. Med. 159, 1811. [DOI] [PubMed] [Google Scholar]
- 5.Fuchtenbusch, M., Standl, E. & Schatz, H. (2000) Exp. Clin. Endocrinol. Diabetes 108, 151-163. [DOI] [PubMed] [Google Scholar]
- 6.Thomas, M. L. & Lloyd, S. J. (2001) Ann. Pharmacother. 35, 123-124. [DOI] [PubMed] [Google Scholar]
- 7.Nesto, R. W., Bell, D., Bonow, R. O., Fonseca, V., Grundy, S. M., Horton, E. S., Le Winter, M., Porte, D., Semenkovich, C. F., Smith, S., et al. (2003) Circulation 108, 2941-2948. [DOI] [PubMed] [Google Scholar]
- 8.Bando, Y., Ushiogi, Y., Okafuji, K., Toya, D., Tanaka, N. & Fujisawa, M. (1999) J. Int. Med. Res. 27, 53-64. [DOI] [PubMed] [Google Scholar]
- 9.Walker, A. B., Naderali, E. K., Chattington, P. D., Buckingham, R. E. & Williams, G. (1998) Diabetes 47, 810-814. [DOI] [PubMed] [Google Scholar]
- 10.Yoshimoto, T., Naruse, M., Nishikawa, M., Naruse, K., Tanabe, A., Seki, T., Imaki, T., Demura, R., Aikawa, E. & Demura, H. (1997) Am. J. Physiol. 272, E989-E996. [DOI] [PubMed] [Google Scholar]
- 11.Hosokawa, M., Tsukada, H., Fukuda, K., Oya, M., Onomura, M., Nakamura, H., Kodama, M., Yamada, Y. & Seino, Y. (1999) J. Pharmacol. Exp. Ther. 290, 1080-1084. [PubMed] [Google Scholar]
- 12.Keller, H., Mahfoudi, A., Dreyer, C., Hihi, A. K., Medin, J., Ozato, K. & Wahli, W. (1993) Ann. N.Y. Acad. Sci. 684, 157-173. [DOI] [PubMed] [Google Scholar]
- 13.Schoonjans, K., Staels, B. & Auwerx, J. (1996) Biochim. Biophys. Acta 1302, 93-109. [DOI] [PubMed] [Google Scholar]
- 14.Dreyer, C., Krey, G., Keller, H., Givel, F., Helftenbein, G. & Wahli, W. (1992) Cell 68, 879-887. [DOI] [PubMed] [Google Scholar]
- 15.Braissant, O., Foufelle, F., Scotto, C., Dauca, M. & Wahli, W. (1996) Endocrinology 137, 354-366. [DOI] [PubMed] [Google Scholar]
- 16.Gilde, A. J., van der Lee, K. A., Willemsen, P. H., Chinetti, G., van der Leij, F. R., van der Vusse, G. J., Staels, B. & van Bilsen, M. (2003) Circ. Res. 92, 518-524. [DOI] [PubMed] [Google Scholar]
- 17.Guan, Y., Zhang, Y., Davis, L. & Breyer, M. D. (1997) Am. J. Physiol. 273, F1013-F1022. [DOI] [PubMed] [Google Scholar]
- 18.Yang, T., Michele, D. E., Park, J., Smart, A. M., Lin, Z., Brosius, F. C., 3rd, Schnermann, J. B. & Briggs, J. P. (1999) Am. J. Physiol. 277, F966-F973. [DOI] [PubMed] [Google Scholar]
- 19.Akiyama, T. E., Sakai, S., Lambert, G., Nicol, C. J., Matsusue, K., Pimprale, S., Lee, Y. H., Ricote, M., Glass, C. K., Brewer, H. B., Jr., et al. (2002) Mol. Cell. Biol. 22, 2607-2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ge, Y., Ahn, D., Stricklett, P. K., Hughes, A. K., Yanagisawa, M., Verbalis, J. G. & Kohan, D. E. (2005) Am. J. Physiol., in press.
- 21.Stokes, J. B., Grupp, C. & Kinne, R. K. (1987) Am. J. Physiol. 253, F251-F262. [DOI] [PubMed] [Google Scholar]
- 22.Wilson, P. D., Sherwood, A. C., Palla, K., Du, J., Watson, R. & Norman, J. T. (1991) Am. J. Physiol. 260, F420-F430. [DOI] [PubMed] [Google Scholar]
- 23.Ross, M. G. & Idah, R. (2004) J. Matern. Fetal Neonatal Med. 15, 104-108. [DOI] [PubMed] [Google Scholar]
- 24.Iwasaki, K., Zhang, R., Perhonen, M. A., Zuckerman, J. H. & Levine, B. D. (2004) Am. J. Physiol. 287, R1256-R1262. [DOI] [PubMed] [Google Scholar]
- 25.Merrill, D. C. & Cowley, A. W., Jr. (1987) Am. J. Physiol. 252, F26-F31. [DOI] [PubMed] [Google Scholar]
- 26.Chen, L., Yang, B., McNulty, J. A., Clifton, L. G., Binz, J. G., Grimes, A. M., Strum, J. C., Harrington, W. W., Chen, Z., Balon, T. W., et al. (2004) J. Pharmacol. Exp. Ther. 312, 718-725. [DOI] [PubMed] [Google Scholar]
- 27.Song, J., Knepper, M. A., Hu, X., Verbalis, J. G. & Ecelbarger, C. A. (2004) J. Pharmacol. Exp. Ther. 308, 426-433. [DOI] [PubMed] [Google Scholar]
- 28.Guan, Y., Zhang, Y., Schneider, A., Davis, L., Breyer, R. M. & Breyer, M. D. (2001) Am. J. Physiol. 281, F1036-F1046. [DOI] [PubMed] [Google Scholar]
- 29.Hong, G., Lockhart, A., Davis, B., Rahmoune, H., Baker, S., Ye, L., Thompson, P., Shou, Y., O'Shaughnessy, K., Ronco, P., et al. (2003) FASEB J. 17, 1966-1968. [DOI] [PubMed] [Google Scholar]
- 30.Yang, B., Clifton, L. G., McNulty, J. A., Chen, L., Brown, K. K. & Baer, P. G. (2003) J. Cardiovasc. Pharmacol. 42, 436-441. [DOI] [PubMed] [Google Scholar]
- 31.Gardiner, S. M., Nunez, D. J., Baer, P. G., Brown, K. K. & Bennett, T. (2004) J. Pharmacol. Exp. Ther. 310, 1226-1233. [DOI] [PubMed] [Google Scholar]
- 32.Zanchi, A., Chiolero, A., Maillard, M., Nussberger, J., Brunner, H. R. & Burnier, M. (2004) J. Clin. Endocrinol. Metab. 89, 1140-1145. [DOI] [PubMed] [Google Scholar]
- 33.Bishop-Bailey, D. (2000) Br. J. Pharmacol. 129, 823-834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Inoue, I., Shino, K., Noji, S., Awata, T. & Katayama, S. (1998) Biochem. Biophys. Res. Commun. 246, 370-374. [DOI] [PubMed] [Google Scholar]
- 35.Satoh, H., Tsukamoto, K., Hashimoto, Y., Hashimoto, N., Togo, M., Hara, M., Maekawa, H., Isoo, N., Kimura, S. & Watanabe, T. (1999) Biochem. Biophys. Res. Commun. 254, 757-763. [DOI] [PubMed] [Google Scholar]
- 36.Staels, B., Koenig, W., Habib, A., Merval, R., Lebret, M., Torra, I. P., Delerive, P., Fadel, A., Chinetti, G., Fruchart, J. C., et al. (1998) Nature 393, 790-793. [DOI] [PubMed] [Google Scholar]
- 37.Chinetti, G., Griglio, S., Antonucci, M., Torra, I. P., Delerive, P., Majd, Z., Fruchart, J. C., Chapman, J., Najib, J. & Staels, B. (1998) J. Biol. Chem. 273, 25573-25580. [DOI] [PubMed] [Google Scholar]
- 38.Ricote, M., Li, A. C., Willson, T. M., Kelly, C. J. & Glass, C. K. (1998) Nature 391, 79-82. [DOI] [PubMed] [Google Scholar]
- 39.Diep, Q. N., El Mabrouk, M., Cohn, J. S., Endemann, D., Amiri, F., Virdis, A., Neves, M. F. & Schiffrin, E. L. (2002) Circulation 105, 2296-2302. [DOI] [PubMed] [Google Scholar]
- 40.Ryan, M. J., Didion, S. P., Mathur, S., Faraci, F. M. & Sigmund, C. D. (2004) Hypertension 43, 661-666. [DOI] [PubMed] [Google Scholar]
- 41.Forman, B. M., Tontonoz, P., Chen, J., Brun, R. P., Spiegelman, B. M. & Evans, R. M. (1995) Cell 83, 803-812. [DOI] [PubMed] [Google Scholar]
- 42.Kliewer, S. A., Lenhard, J. M., Willson, T. M., Patel, I., Morris, D. C. & Lehmann, J. M. (1995) Cell 83, 813-819. [DOI] [PubMed] [Google Scholar]
- 43.Bell-Parikh, L. C., Ide, T., Lawson, J. A., McNamara, P., Reilly, M. & FitzGerald, G. A. (2003) J. Clin. Invest. 112, 945-955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gilroy, D. W., Colville-Nash, P. R., Willis, D., Chivers, J., Paul-Clark, M. J. & Willoughby, D. A. (1999) Nat. Med. 5, 698-701. [DOI] [PubMed] [Google Scholar]