Abstract
Herpes simplex virus type 1 (HSV-1) establishes a latent infection in neurons of the peripheral nervous system. During latent HSV-1 infection, viral gene expression is limited to latency-associated transcripts (LAT). HSV-1 remains latent until an unknown mechanism induces reactivation. The ability of the latent virus to periodically reactivate and be shed is essential to the transmission of disease. In vivo, the stimuli that induce reactivation of latent HSV-1 include stress, fever, and UV damage to the skin at the site of initial infection. In vitro, in primary neurons harboring latent HSV-1, nerve growth factor (NGF) deprivation or forskolin treatment induces reactivation. However, the mechanism involved in the induction of reactivation remains poorly understood. An in vitro neuronal model of HSV-1 latency was used to investigate potential mechanisms involved in the induction of reactivation of latent HSV-1. In situ hybridization analysis of neuronal cultures harboring latent HSV-1 showed a marked, rapid decrease in the percentage of LAT-positive neurons following induction of reactivation by NGF deprivation or forskolin treatment. Western blot analysis showed a corresponding increase in expression of the cellular transcription factor inducible cyclic AMP early repressor (ICER) during reactivation. In transient-transfection assays, ICER downregulated LAT promoter activity. Expression of ICER from a recombinant adenoviral vector induced reactivation and decreased the percentage of LAT-positive neurons in neuronal cultures harboring latent HSV-1. These results indicate that ICER represses LAT expression and induces reactivation of latent HSV-1.
During latent herpes simplex virus type 1 (HSV-1) infection in sensory neurons, the viral genome is maintained in a nonreplicating state and viral gene expression is silenced, with the exception of the viral gene that encodes the latency-associated transcripts (LAT) (34). Reactivation of latent HSV-1 is induced by many different stimuli, including fever, stress, and UV irradiation or abrasion to the skin. Studies using LAT mutants indicate that LAT enhances the establishment of latency as well as the reactivation of latent HSV-1 (3, 6, 11, 22, 23, 33). The signaling mechanisms controlling the induction of reactivation of latent HSV-1 are not yet understood.
Cyclic AMP (cAMP) and nerve growth factor (NGF)-mediated pathways are involved in the induction of reactivation. Forskolin, chlorophenylthio-cAMP, or NGF deprivation results in reactivation of latent HSV-1 in primary neuronal cultures (29). Activation of these pathways is shown to result in phosphorylation and activation of the CRE-binding protein (CREB) (8, 9). Functional CREB response elements (CREs) have been identified within the LAT promoter at positions −85 and −43 from the site of transcription initiation (4, 15, 24). The CRE at −43 has been shown to be cAMP responsive in transient-transfection assays, and mutagenesis of this CRE results in reduced reactivation in rabbits latently infected with the recombinant virus (4). Characterization of the CRE at −85 is primarily limited to the observation that members of the CREB/ATF family can interact with the promoter in electrophoretic mobility shift assays (13, 17). Based on this evidence, it is possible that CREs in the LAT promoter may have a role in signaling that results in the reactivation of latent HSV-1.
Previous studies have focused on activation of LAT transcription by signaling pathways (15, 24). Based on the presence of elements in the promoter of LAT, the role of the inducible cAMP early repressors (ICER) in the induction of reactivation of latent HSV-1 was examined. The CRE modulator (CREM) gene family encodes transcriptional activators and repressors that are structurally related to the CREB/ATF family (26). The best-characterized CREM repressors are the ICER isoforms (18). ICER is a member of the basic-leucine zipper family and represses by virtue of its ability to heterodimerize with members of the CREB/ATF family of transcription factors. These inactive complexes form on CREs and block transcription because ICER lacks an activation domain (12, 14). The CREM P2 intronic promoter that drives ICER expression contains multiple CREs, which convey cAMP responsiveness, thus making ICER the only known CREB that is itself inducible by cAMP. ICER activity is regulated by protein abundance rather than by posttranslational modification (7).
Signaling pathways that result in ICER expression may be involved in reactivation of latent HSV-1. The roles of ICER expression and LAT regulation during HSV-1 reactivation from latency in an in vitro neuronal model were examined.
MATERIALS AND METHODS
Cell culture.
Vero cells (from the American Type Culture Collection) were maintained in Dulbecco's modified Eagle's medium supplemented with 5% fetal bovine serum (Life Technologies). Human T-cell Jurkat cells were maintained in Iscove's modified Eagle's medium supplemented with glutamine and fetal bovine serum (Life Technologies). Sensory neuron cultures were prepared from dorsal root ganglia of embryonic day 15 Sprague-Dawley rats as described previously (31, 37). Dulbecco's Eagle's medium supplemented with 10% newborn bovine serum and 100-ng/ml 2.5 S nerve growth factor (Harlan Bioproducts) was used to maintain neuronal cultures (neuronal maintenance medium). Cultures were treated with fluorodeoxyuridine (20 μM) for 7 to 10 days after plating to reduce the nonneuronal cell population. Cultures were plated to provide 1 × 103 to 5 × 103 neurons per culture.
Establishment and reactivation of latent HSV-1 infections.
Latent HSV-1 infections in neuronal cultures were established as previously described (31, 37). After neuronal cultures had been established for 2 weeks, 50 μM acyclovir was added to the cultures 24 h prior to inoculation with virus and for the following 7 days after inoculation. Neuronal cultures were infected with approximately 10 PFU of HSV-1 per neuron. NGF deprivation-induced reactivation was performed by adding media containing 1% anti-NGF serum to the cultures as previously described (31, 37). Forskolin-induced reactivation was performed by adding 0.1 mM forskolin (Sigma) in dimethyl sulfoxide to the neuronal maintenance medium as previously described (29). For reactivation studies using adenoviral vectors, latently infected cultures were coinfected with recombinant adenovirus (described below) at a multiplicity of infection of approximately 100 PFU per neuron in neuronal maintenance medium. Cells were harvested 4 days after adenoviral vector infection, and plaque formation assays were performed using Vero cells.
In situ hybridization.
In situ hybridization was performed as described previously (27, 36). Neuronal cultures, prepared on coverslips, were latently infected with either HSV-1(F) or HSV-1(17+). Cultures were fixed with 4% paraformaldehyde and dehydrated. The digoxigenin-labeled riboprobe for detection of LAT was prepared from the bacterial plasmid pLAT as previously described (28). The digoxigenin-labeled riboprobe for detection of ICP0 was prepared from the bacterial plasmid pBL-2 as previously described (27). Representative fields were photographed on TMax 100 film using a Nikon Optiphot-2 equipped with Hoffman optics. The slides were scanned with a Nikon LS-1000 film scanner, and the images were prepared using Photoshop 5.0 software (Adobe Systems).
Western blot analysis.
Western blot analysis was performed on samples from neuronal cultures harboring latent HSV-1 at various times after forskolin treatment. Neuronal cultures were pooled (approximately 7 × 104 neurons per sample) in 500 μl of radioimmunoprecipitation assay buffer and frozen at −20°C until analysis by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Proteins were quantified with the bicinchoninic acid protein assay kit (Pierce Immunochemicals, Rockford, Ill.). Proteins were separated on an SDS–15% PAGE denaturing gel and transferred to nitrocellulose (Pro-bind; Amersham-Pharmacia) for Western blot analysis. The nitrocellulose blots were blocked with phosphate buffered saline (pH 7.4) plus 0.1% Tween-20 and 1.0× Uniblock (Analytical Genetic Testing Center, Inc., Denver, Colo.) overnight at 4°C. The primary antibody, anti-CREB (C-21; Santa Cruz Biotechnology, Inc., Santa Cruz, Calif.), was diluted 1:100 in blocking solution and incubated with the blots for 1 h. After washing, an anti-rabbit immunoglobulin G secondary antibody conjugated to horseradish peroxidase (Vector Labs) was diluted 1:750 in blocking solution and incubated with the blot for 1 h. Blots were developed using an NEN chemiluminescent detection kit, and the signal was recorded on Kodak Biomax film. The films were scanned, and the resulting images were analyzed using NIH Image to compare the relative levels of ICER expression.
Recombinant plasmids and viruses.
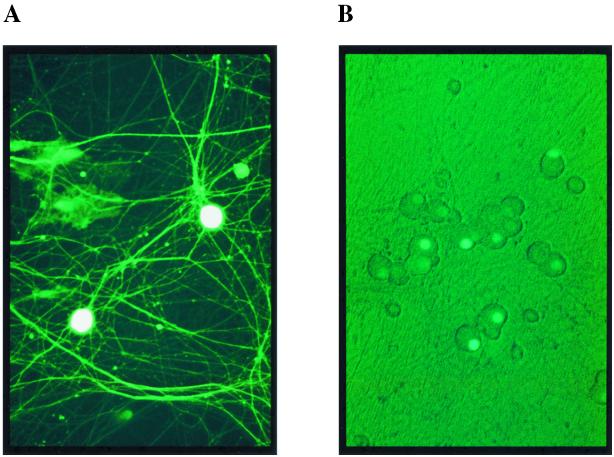
Human ICER II cDNA was cloned by RT-PCR from RNA extracted from Jurkat cells as previously described (5). The entire ICERII coding region was placed under the control of the cytomegalovirus (CMV) promoter in a mammalian expression plasmid and used for transient-transfection experiments. The full LAT promoter-luciferase reporter construct was made by inserting a 981-bp PCR product consisting of the LAT promoter from HSV-1(F) into the luciferase reporter plasmid pGL2-basic (Promega). The PCR fragment comprises positions −866 to +115 with respect to the LAT promoter 1 transcriptional start site. The minimal LAT promoter consists of the PstI fragment (−137 to +63) from HSV-1(17+) cloned into pGL2-basic. Recombinant adenoviruses Ad-ICER, Ad-EGFP, and Ad-EGFP-ICER were constructed using methods previously described (10, 16). EGFP is the humanized form of the green fluorescent protein (Clontech). EGFP-ICER is a fusion between ICER and EGFP such that EGFP is fused to the amino terminus of ICER. The expression of proteins in the recombinant adenoviruses was under the control of the human CMV immediate early promoter. Expression of EGFP and EGFP-ICER was monitored by fluorescence microscopy. Images of representative fields were captured using an inverted fluorescence microscopy (Nikon) with a Cool Snap charge-coupled device camera (RS Photometrics) and Image Pro Software (Media Cybernetics).
Transient-transfection assays.
Lipofectamine (Life Technologies) was used for transfections. DNA for transfection was prepared using Qiagen Plasmid Maxi kit. Vero cells were plated for transfection at a density of 2.5 × 105 cells per 33-mm well and harvested according to Promega's luciferase assay protocols.
RESULTS
LAT rapidly decreases during reactivation of latent HSV-1 induced by either NGF deprivation or forskolin treatment.
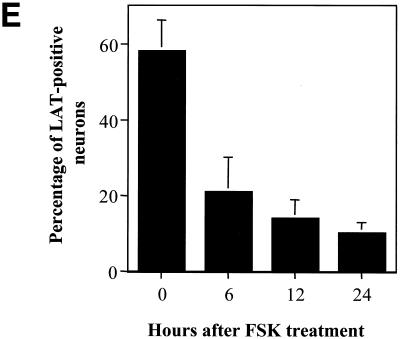
The presence of LAT was determined during reactivation of latently infected neuronal cultures. In situ hybridization was performed to identify and quantify LAT-positive neurons during latency and reactivation. As shown in Fig. 1, the percentage of LAT-positive neurons decreased significantly by 12 h after induction of reactivation by NGF deprivation. Similar results were obtained using HSV-1(17+) (data not shown).
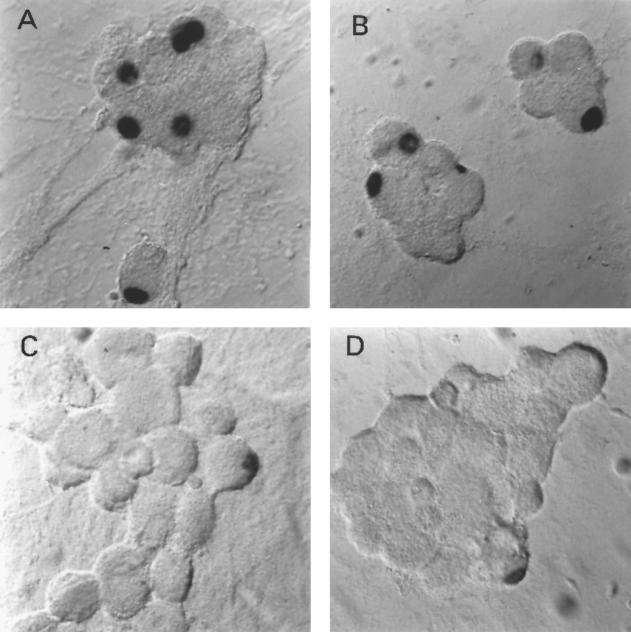
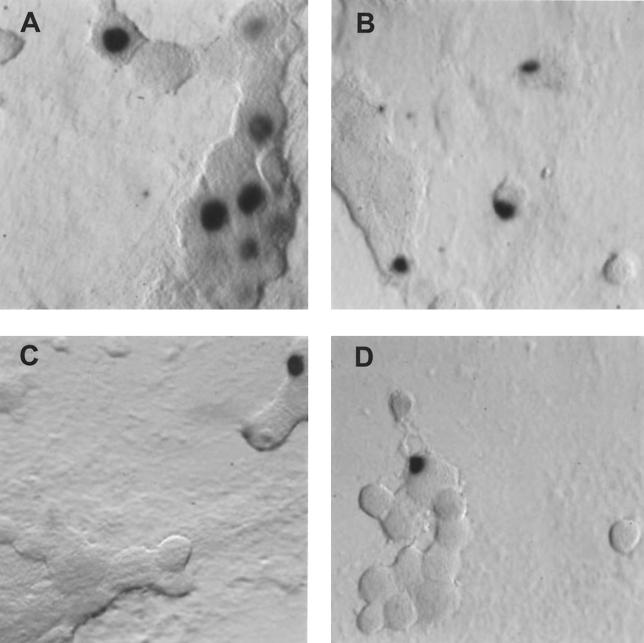
FIG. 1.
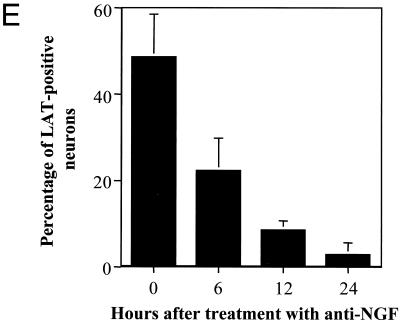
In situ hybridization using a riboprobe to detect LAT in neuronal cultures latently infected with HSV-1 following the induction of reactivation by NGF deprivation. A digoxigenin-labeled riboprobe was used to detect LAT in neuronal cultures 2 weeks after the establishment of latency with HSV-1(F) following NGF deprivation. Representative fields are shown for cultures after induction of reactivation using NGF deprivation at 0 (A), 6 (B), 12 (C), and 24 (D) h after induction of reactivation. (E) Percentage of neurons per culture expressing LAT at the indicated time points. Values are means plus standard errors of the means (n = 4).
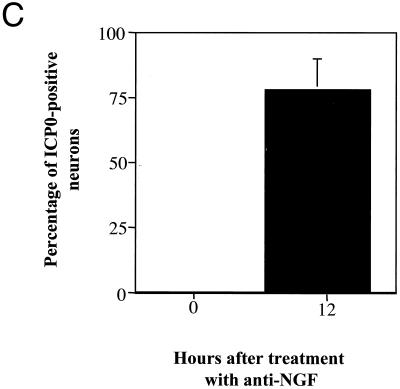
ICP0 transcripts were undetectable during latency but were detected 12 h after induction of reactivation (Fig. 2). Twelve hours after anti-NGF treatment, less than 10% of the neurons were LAT positive, whereas more than 75% of neurons were positive for ICP0 transcripts.
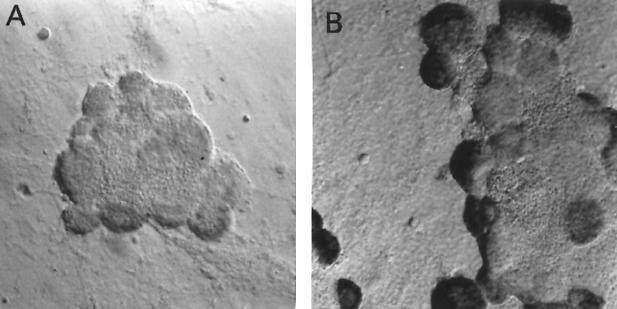
FIG. 2.
In situ hybridization using a riboprobe to detect ICP0 in neuronal cultures latently infected with HSV-1 following the induction of reactivation by NGF deprivation. A digoxigenin-labeled riboprobe was used to detect ICP0 in neuronal cultures 2 weeks after the establishment of latency with HSV-1(F) following NGF deprivation. Representative fields are shown for cultures after induction of reactivation using NGF deprivation at 0 (A) and 12 (B) h after induction of reactivation. (C) Percentage of neurons per culture expressing ICP0 at the indicated time points. Values are means plus standard errors of the means (n = 4).
Similar results showing a rapid decrease in the percentage of LAT-positive neurons were obtained using forskolin treatment to induce reactivation (Fig. 3). The decrease in the percentage of LAT-positive neurons followed a pattern essentially identical to that observed during reactivation induced by NGF deprivation. These results show that LAT expression rapidly and significantly decreased as the virus reactivated from latency in response to NGF deprivation or forskolin treatment.
FIG. 3.
In situ hybridization using a riboprobe to detect LAT in neuronal cultures latently infected with HSV-1 following treatment with forskolin (FSK). A digoxigenin-labeled riboprobe was used to detect LAT in neuronal cultures 2 weeks after establishment of latency with HSV-1(17+). Representative fields are shown for cultures after induction of reactivation using 0.5 mM forskolin at 0 (A), 6 (B), 12 (C), and 24 (D) h after induction of reactivation. (E) Percentage of neurons expressing LAT from the indicated time points. Values are means plus standard errors of the means (n = 4).
Forskolin treatment induces ICER expression in neurons in culture.
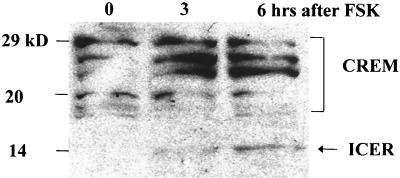
Since LAT expression decreased upon induction of reactivation, it was possible that the LAT promoter was repressed during the induction of reactivation. ICER expression is shown to be upregulated by phospho-CREB in the neuronal cell line PC-12 (19). Since ICER is a factor that could potentially repress the LAT promoter, ICER expression in neurons in vitro was examined during reactivation. Figure 4 shows the results of Western blot analysis on samples from neuronal cultures harboring latent HSV-1 and following treatment with forskolin. The relative level of ICER expression was upregulated threefold by 3 h after forskolin treatment and eightfold by 6 h after forskolin treatment. A similar pattern, showing the induction of ICER RNA, was observed following NGF deprivation (data not shown).
FIG. 4.
Western blot analysis shows increased ICER in neuronal cultures latently infected with HSV-1 following treatment with forskolin (FSK). Neuronal cultures harboring latent HSV-1(17+) were treated with 0.1 mM forskolin. The anti-CREB primary antibody recognizes ICER as well as other CREM isoforms, producing a pattern of detected products similar to published results (21). The arrow indicates the predicted size of ICER.
ICER represses LAT promoter activity.
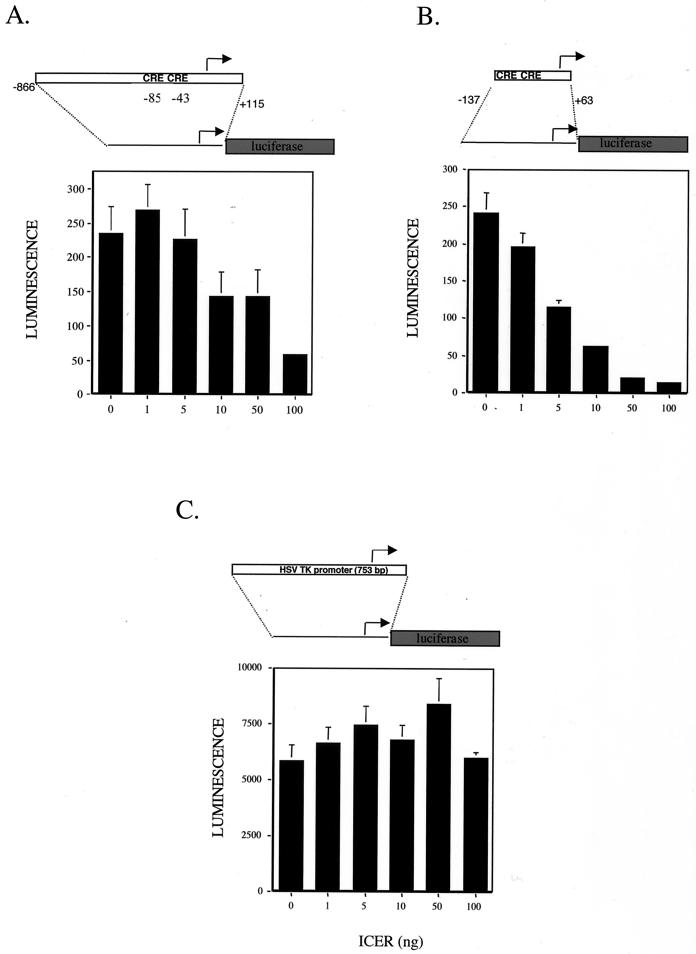
The LAT promoter contains several CREs, which are potential sites for repression by ICER. To examine the effects of ICER on LAT promoter activity, transient-transfection experiments were performed. An expression plasmid containing human ICERII cDNA was constructed as previously described (5). The entire ICERII coding region was placed under the control of the CMV promoter in a mammalian expression plasmid and used for transient-transfection experiments. Vero cells were cotransfected with a reporter plasmid containing the LAT promoter driving luciferase with increasing amounts of the ICER expression plasmid (Fig. 5A). As predicted, increasing amounts of ICER expression plasmid resulted in decreasing luminescence. This suggests that ICER can downregulate LAT expression by repressing through the CREs in the LAT promoter.
FIG. 5.
Transient-transfection assays show that the ICER represses LAT promoter activity. Luciferase assays were performed to measure the ability of ICER to negatively regulate the LAT promoter activity. Vero cells were transiently transfected with 1 μg of reporter plasmid. Values are in luminescence units and are means plus standard deviations (n = 4). Cells were cotransfected with the indicated amount of ICER expression plasmid and the luciferase reporter containing plasmid. Luciferase expression from the full LAT promoter (A), the minimal LAT promoter (B), and the HSV thymidine kinase (TK) promoter (C) is shown.
To demonstrate that the region of the LAT promoter necessary for repression contains the CREs, a minimal LAT promoter-luciferase plasmid was cotransfected with increasing amounts of the ICER expression plasmid. As indicated by the diagram in Fig. 4, the minimal LAT promoter construct (−137 to +63) was significantly shorter than the full promoter (−866 to +115). This minimal region is reported to be required for reactivation of latent HSV-1 (4). As shown in Fig. 5B, increasing amounts of ICER expression plasmid corresponded to decreasing luminescence values in a dose-dependent manner. Since luminescence was drastically reduced by transfection with the ICER expression plasmid, it was necessary to confirm that ICER specifically repressed the LAT promoter and was not toxic. The same amounts of ICER expression plasmid were cotransfected with an internal control plasmid, pRLTK. As shown in Fig. 5C, this reporter has a region of the HSV thymidine kinase promoter driving luciferase. This control promoter contains no CREs. The results show that ICER did not affect transcription in a nonspecific manner. ICER appeared to repress LAT promoter activity specifically through the CREs. To corroborate that overexpression of ICER does not cause toxic effects, DAPI (4′,6′-diamidino-2-phenylindole) staining of ICER-transfected and mock-transfected Vero cells was carried out, and it revealed no apparent differences in cell viability (data not shown). These data demonstrate that overexpression of ICER resulted in decreased LAT promoter activity.
Expression of ICER from an adenoviral vector results in reactivation of latent HSV-1.
The effect of expression of ICER was examined in neuronal cultures harboring latent HSV-1. Since introduction of DNA by transfection into neurons is very inefficient, recombinant adenoviruses were constructed to express EGFP (Ad-EGFP) or the EGFP-ICER fusion (Ad-EGFP-ICER). Adenoviral vectors are shown to efficiently infect sensory neurons in vitro without cytotoxicity (30). Immunoblotting, performed using antibodies against EGFP or CREB, showed the expression of the predicted proteins in Vero cells infected with the recombinant adenoviral vector (data not shown). Neuronal cultures infected with recombinant EGFP-expressing adenoviruses confirmed expression of the transgenes (Fig. 6A and B). Following infection with Ad-EGFP, EGFP was uniformly distributed in the cytoplasm, nucleus, and neuronal processes, whereas with Ad-EGFP-ICER, EGFP-ICER was detected almost exclusively in the nucleus, as predicted.
FIG. 6.
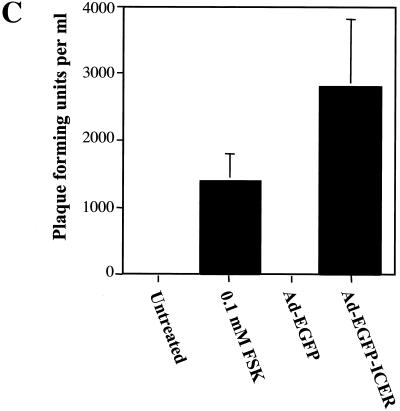
Expression and induction of reactivation of latent HSV-1 in neurons using an adenoviral vector to express ICER fused to EGFP. Neurons in culture following infection with adenoviral vectors Ad-EGFP (A) and Ad-EGFP-ICER (B) showed expression of EGFP and EGFP-ICER, respectively. (C) Neuronal cultures, 2 weeks after establishment of latent HSV-1(17+) infections, were infected with the indicated adenoviral vector or treated with forskolin (FSK). Cultures were assayed for infectious virus 4 days posttreatment in plaque formation assays. Values are means plus standard errors of the means (n = 6).
Neuronal cultures harboring latent HSV-1 were infected with either Ad-EGFP or Ad-EGFP-ICER. Plaque formation assays were performed and showed that the adenoviral vector expressing ICER induced reactivation of latent HSV-1 (Fig. 6C). The Ad-EGFP control showed that neither adenovirus infection nor EGFP expression resulted in reactivation of latent HSV-1. These data indicate that ICER expression induced reactivation of latent HSV-1 in neuronal cultures.
LAT expression decreases during reactivation of latent HSV-1 after coinfection with adenoviral vectors expressing ICER.
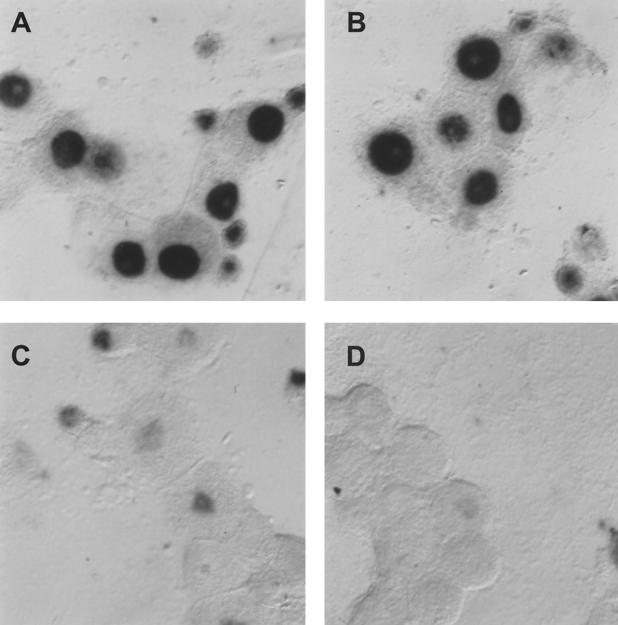
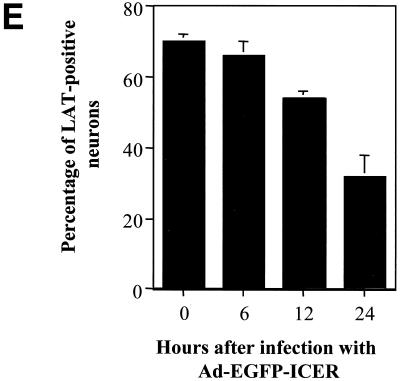
In situ hybridization was performed to quantify the LAT-positive neurons after infection with the adenoviral vectors. As shown in Fig. 7, the percentage of LAT-positive neurons decreased 50% by 24 h after coinfection with Ad-EGFP-ICER but was unaffected by coinfection with Ad-EGFP. The delay in the decrease of LAT-positive neurons compared to NGF deprivation or forskolin treatment may be the result of the time required for expression of ICER protein from the adenoviral vector. These results show that the percentage of LAT-positive neurons significantly decreased in response to infection with an adenoviral vector expressing ICER, similar to the results observed after NGF deprivation or forskolin treatment.
FIG. 7.
In situ hybridization using a riboprobe to detect LAT in neuronal cultures latently infected with HSV-1 following infection with Ad-EGFP or Ad-EGFP-ICER. A digoxigenin-labeled riboprobe was used to detect LAT in neuronal cultures 2 weeks after the establishment of latent HSV-1(17+) following infection with adenoviral vectors. Fields shown are representative of neuronal cultures after infection with an adenoviral vector expressing EGFP at 24 h after infection (A) or an adenoviral vector expressing ICER-EGFP at 6 (B), 12 (C), and 24 (D) h after infection. (E) Percentage of neurons expressing LAT at the indicated time points. Values are means plus standard errors of the means (n = 4).
DISCUSSION
It has previously been shown that activation of several signaling pathways results in reactivation of latent HSV-1 (29). ICER may have an important function in second-messenger-mediated HSV-1 reactivation, which appears to involve the downregulation of LAT expression. CREs in the LAT promoter appear to be important for reactivation (4, 13, 15, 17, 24). The results suggest that signals that induce phosphorylation of CREB have a role in the mechanism of reactivation, which may include repression of LAT promoter activity. However, it is possible that other targets are also involved.
To understand the relationship between cell signaling events and viral reactivation, we examined LAT expression during reactivation. A rapid decrease in LAT expression following forskolin or anti-NGF treatment was measured. A possible explanation for decreased LAT expression was that neurons died. However, neuronal cell counts do not indicate that significant cell death occurred. The detection of ICP0 transcripts also indicates that the neurons were viable. It is possible that there is a mechanism that results in decreased LAT abundance during reactivation by decreasing either LAT expression, stability, or both. Decreased expression of LAT is consistent with ICP4 repression of LAT transcription through the ICP4 binding element in the LAT promoter (2, 25). Similarly, it is also reported that the cellular protein Egr-1 negatively regulates LAT expression through a negative response element downstream from the TATA box (32). However, the correlation of the increase in expression of ICER with the decrease in LAT-positive neurons suggests a role for ICER in repression of the LAT promoter.
Forskolin has been shown to be effective in inducing reactivation in neuronal cultures harboring latent HSV-1 (29). Results presented here show that ICER expression was induced in neuronal cultures within 3 h of treatment with forskolin. Furthermore, ICER repressed LAT promoter activity in a dose-dependent manner in transient-transfection assays. Overexpression of ICER in neuronal cultures latently infected with HSV-1 resulted in robust reactivation. Although the correlation between ICER induction and LAT repression is strong, it is possible that ICER does not have a direct role in reactivation. ICER may act together with viral transcription factors, as it does with hepatitis B virus protein X, to transrepress viral and cellular gene expression (1). Similarly, in addition to repressing the LAT promoter, ICER may also inactivate cellular transcription factors or repress transcription of cellular factors necessary for maintenance of the latent HSV-1 infection.
LAT appears to be downregulated at the level of transcription during reactivation, suggesting the possibility that this repression is mediated through regulation of CREs of the LAT promoter. ICER is a likely candidate, since it binds CREs and it is inducible by stimuli that induce reactivation, including forskolin and NGF deprivation. In addition, ICER expression is shown to be induced in spinal cord neurons following peripheral thermal stimulation (20). This is intriguing, since in vivo reactivation of latent HSV-1 can be induced with peripheral noxious stimuli, including heat stress. These data indicate a role for ICER in the repression of LAT and the induction of reactivation of latent HSV-1.
ACKNOWLEDGMENTS
The minimal LAT promoter-luciferase plasmid was a gift from Kent Wilcox.
This work was supported by National Research Service Award postdoctoral fellowship F32NS10896 awarded to M.A.C. and Public Health Service grant NS29046 awarded to C.L.W.
REFERENCES
- 1.Barnabas S, Hai T, Andrisani O M. The hepatitis B virus X protein enhances the DNA binding potential and transcription efficacy of bZip transcription factors. J Biol Chem. 1997;272:20684–20690. doi: 10.1074/jbc.272.33.20684. [DOI] [PubMed] [Google Scholar]
- 2.Batchelor A H, O'Hare P. Regulation and cell-type-specific activity of a promoter located upstream of the latency-associated transcript of herpes simplex virus type 1. J Virol. 1990;64:3269–3279. doi: 10.1128/jvi.64.7.3269-3279.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Block T M, Deshmane S, Masonis J, Maggioncalda J, Valyi-Nagi T, Fraser N W. An HSV null mutant reactivates slowly from latent infection and makes small plaques on CV-1 monolayers. Virology. 1993;192:618–630. doi: 10.1006/viro.1993.1078. [DOI] [PubMed] [Google Scholar]
- 4.Bloom D C, Stevens J G, Hill J M, Tran R K. Mutagenesis of a cAMP response element within the latency-associated transcript promoter of HSV-1 reduces adrenergic reactivation. Virology. 1997;236:202–207. doi: 10.1006/viro.1997.8723. [DOI] [PubMed] [Google Scholar]
- 5.Bodor J, Spetz A L, Strominger J L, Habener J F. cAMP inducibility of transcriptional repressor ICER in developing and mature human T lymphocytes. Proc Natl Acad Sci USA. 1996;93:3536–3541. doi: 10.1073/pnas.93.8.3536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Drolet B S, Perng G C, Villosis R J, Slanina S M, Nesburn A B, Wechsler S L. Expression of the first 811 nucleotides of the herpes simplex virus type 1 latency-associated transcript (LAT) partially restores wild-type spontaneous reactivation to a LAT-null mutant. Virology. 1999;253:96–106. doi: 10.1006/viro.1998.9492. [DOI] [PubMed] [Google Scholar]
- 7.Folco E J, Koren G. Degradation of the inducible cAMP early repressor (ICER) by the ubiquitin-proteasome pathway. Biochem J. 1997;328:37–43. doi: 10.1042/bj3280037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ginty D D, Bonni A, Greenberg M E. Nerve growth factor activates a Ras-dependent protein kinase that stimulates c-fos transcription via phosphorylation of CREB. Cell. 1994;77:713–725. doi: 10.1016/0092-8674(94)90055-8. [DOI] [PubMed] [Google Scholar]
- 9.Gonzalez G A, Montminy M R. Cyclic AMP stimulates somatostatin gene transcription by phosphorylation of CREB at serine 133. Cell. 1989;59:675–680. doi: 10.1016/0092-8674(89)90013-5. [DOI] [PubMed] [Google Scholar]
- 10.Graham F L, Smiley J, Russell W C, Nairn R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J Gen Virol. 1977;36:59–74. doi: 10.1099/0022-1317-36-1-59. [DOI] [PubMed] [Google Scholar]
- 11.Hill J M, Sedarati F, Javier R T, Wagner E K, Stevens J G. Herpes simplex virus latent phase transcription facilitates in vivo reactivation. Virology. 1990;174:117–125. doi: 10.1016/0042-6822(90)90060-5. [DOI] [PubMed] [Google Scholar]
- 12.Inada A, Someya Y, Yamada Y, Ihara Y, Kubota A, Ban N, Watanabe R, Tsuda K, Seino Y. The cyclic AMP response element modulator family regulates the insulin gene transcription by interacting with transcription factor IID. J Biol Chem. 1999;274:21095–21103. doi: 10.1074/jbc.274.30.21095. [DOI] [PubMed] [Google Scholar]
- 13.Kenny J J, Millhouse S, Wotring M, Wigdahl B. Upstream stimulatory factor family binds to the herpes simplex virus type 1 latency-associated transcript promoter. Virology. 1997;230:381–391. doi: 10.1006/viro.1997.8501. [DOI] [PubMed] [Google Scholar]
- 14.Lamas M, Sassone-Corsi P. The dynamics of the transcriptional response to cyclic adenosine 3′,5′-monophosphate: recurrent inducibility and refractory phase. Mol Endocrinol. 1997;11:1415–1424. doi: 10.1210/mend.11.10.9988. [DOI] [PubMed] [Google Scholar]
- 15.Leib D A, Nadeau K C, Rundle S A, Schaffer P A. The promoter of the latency-associated transcripts of herpes simplex virus type 1 contains a functional cAMP-response element: role of the latency-associated transcripts and cAMP in reactivation of viral latency. Proc Natl Acad Sci USA. 1991;88:48–52. doi: 10.1073/pnas.88.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McGrory W J, Bautista D S, Graham F L. A simple technique for the rescue of early region I mutations into infectious human adenovirus type 5. Virology. 1988;163:614–617. doi: 10.1016/0042-6822(88)90302-9. [DOI] [PubMed] [Google Scholar]
- 17.Millhouse S, Kenny J J, Quinn P G, Lee V, Wigdahl B. ATF/CREB elements in the herpes simplex virus type 1 latency-associated transcript promoter interact with members of the ATF/CREB and AP-1 transcription factor families. J Biomed Sci. 1998;5:451–464. doi: 10.1007/BF02255935. [DOI] [PubMed] [Google Scholar]
- 18.Molina C A, Foulkes N S, Lalli E, Sassone-Corsi P. Inducibility and negative autoregulation of CREM: an alternative promoter directs the expression of ICER, an early response repressor. Cell. 1993;75:875–886. doi: 10.1016/0092-8674(93)90532-u. [DOI] [PubMed] [Google Scholar]
- 19.Monaco L, Sassone-Corsi P. Cross-talk in signal transduction: Ras-dependent induction of cAMP-responsive transcriptional repressor ICER by nerve growth factor. Oncogene. 1997;15:2493–2500. doi: 10.1038/sj.onc.1201636. [DOI] [PubMed] [Google Scholar]
- 20.Naranjo J R, Mellstrom B, Carrion A M, Lucas J J, Foulkes N S, Sassone-Corsi P. Peripheral noxious stimulation induces CREM expression in dorsal horn: involvement of glutamate. Eur J Neurosci. 1997;9:2778–2783. doi: 10.1111/j.1460-9568.1997.tb01707.x. [DOI] [PubMed] [Google Scholar]
- 21.Pati D, Meistrich M L, Plon S E. Human Cdc34 and Rad6B ubiquitin-conjugating enzymes target repressors of cyclic AMP-induced transcription for proteolysis. Mol Cell Biol. 1999;19:5001–5013. doi: 10.1128/mcb.19.7.5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perng G C, Slanina S M, Yukht A, Drolet B S, Keleher W, Jr, Ghiasi H, Nesburn A B, Wechsler S L. A herpes simplex virus type 1 latency-associated transcript mutant with increased virulence and reduced spontaneous reactivation. J Virol. 1999;73:920–929. doi: 10.1128/jvi.73.2.920-929.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Perng G C, Slanina S M, Yukht A, Ghiasi H, Nesburn A B, Wechsler S L. The latency-associated transcript gene enhances establishment of herpes simplex virus type 1 latency in rabbits. J Virol. 2000;74:1885–1891. doi: 10.1128/jvi.74.4.1885-1891.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rader K A, Ackland-Berglund C E, Miller J K, Pepose J S, Leib D A. In vivo characterization of site-directed mutations in the promoter of the herpes simplex virus type 1 latency-associated transcripts. J Gen Virol. 1993;74:1859–1869. doi: 10.1099/0022-1317-74-9-1859. [DOI] [PubMed] [Google Scholar]
- 25.Rivera-Gonzalez R, Imbalzano A N, Gu B, Deluca N A. The role of ICP4 repressor activity in temporal expression of the IE-3 and latency-associated transcript promoters during HSV-1 infection. Virology. 1994;202:550–564. doi: 10.1006/viro.1994.1377. [DOI] [PubMed] [Google Scholar]
- 26.Sassone-Corsi P. Coupling gene expression to cAMP signalling: role of CREB and CREM. Int J Biochem Cell Biol. 1998;30:27–38. doi: 10.1016/s1357-2725(97)00093-9. [DOI] [PubMed] [Google Scholar]
- 27.Smith R L, Escudero J L, Wilcox C L. Regulation of the herpes simplex virus latency-associated transcript during establishment of latency in sensory neurons in vitro. Virology. 1994;202:49–60. doi: 10.1006/viro.1994.1321. [DOI] [PubMed] [Google Scholar]
- 28.Smith R L, Geller A I, Escudero K W, Wilcox C L. Long-term expression in sensory neurons in tissue culture from HSV-1 promoters in herpes-derived vectors. J Virol. 1995;69:4593–4599. doi: 10.1128/jvi.69.8.4593-4599.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith R L, Pizer L I, Johnson E M, Jr, Wilcox C L. Activation of second-messenger pathways reactivates latent herpes simplex virus in neuronal cultures. Virology. 1992;188:311–318. doi: 10.1016/0042-6822(92)90760-m. [DOI] [PubMed] [Google Scholar]
- 30.Smith R L, Traul D L, Schaack J, Clayton G H, Staley K J, Wilcox C L. Characterization of promoter function and cell-type-specific expression from viral vectors in the nervous system. J Virol. 2000;74:11254–11261. doi: 10.1128/jvi.74.23.11254-11261.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smith R L, Wilcox C L. Studies of herpes simplex virus 1 latency using primary neuronal cultures of dorsal root ganglion neurons. In: Lowenstein P, Enquist L, editors. Protocols for gene transfer in neuroscience: towards gene therapy of neurological disorders. Chichester, Sussex, United Kingdom: John Wiley & Sons, Ltd.; 1996. [Google Scholar]
- 32.Tatarowicz W A, Martin C E, Pekosz A S, Madden S L, Rauscher III F J, Chiang S Y, Beerman T A, Fraser N W. Repression of the HSV-1 latency-associated transcript (LAT) promoter by the early growth response (EGR) proteins: involvement of a binding site immediately downstream of the TATA box. J Neurovirol. 1997;3:212–224. doi: 10.3109/13550289709018296. [DOI] [PubMed] [Google Scholar]
- 33.Thompson R L, Sawtell N M. The herpes simplex virus type 1 latency-associated transcript gene regulates the establishment of latency. J Virol. 1997;71:5432–5440. doi: 10.1128/jvi.71.7.5432-5440.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wagner E K, Bloom D C. Experimental investigation of herpes simplex virus latency. Clin Microbiol Rev. 1997;10:419–443. doi: 10.1128/cmr.10.3.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wilcox C L, Johnson E M., Jr Characterization of nerve growth factor-dependent herpes simplex virus latency in neurons in vitro. J Virol. 1988;62:393–399. doi: 10.1128/jvi.62.2.393-399.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wilcox C L, Smith R L. HSV latency in vitro: in situ hybridization methods. In: Brown S M, MacLean A R, editors. Herpes simplex virus protocols. Totowa, N.J: Humana Press; 1998. pp. 317–326. [DOI] [PubMed] [Google Scholar]
- 37.Wilcox C L, Smith R L, Freed C R, Johnson E M., Jr Nerve growth factor-dependence of herpes simplex virus latency in peripheral sympathetic and sensory neurons in vitro. J Neurosci. 1990;10:1268–1275. doi: 10.1523/JNEUROSCI.10-04-01268.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]