Abstract
Background
Male factors leading to subfertility account for at least half of all cases of subfertility worldwide. Although some causes of male subfertility are treatable, treatment of idiopathic male factor subfertility remains empirical. Researchers have used gonadotrophins to improve sperm parameters in idiopathic male factor subfertility with the ultimate goal of increasing birth and pregnancy rates, but results have been conflicting.
Objectives
To determine the effect of systemic follicle‐stimulating hormone (FSH) on live birth and pregnancy rates when administered to men with idiopathic male factor subfertility .
Search methods
We searched the Cochrane Menstrual Disorders and Subfertility Group Specialised Register (14 January 2013), the Cochrane Central Register of Controlled Trials (CENTRAL; The Cochrane Library, Issue 12 of 12, 2012), Ovid MEDLINE In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE Daily and Ovid MEDLINE (1946 to 14 January 2013), Ovid EMBASE (1980 to week 2 of 2013), Ovid PsycINFO (1806 to week 2 of 2013), trial registers for ongoing and registered trials at ClinicalTrials.gov (19 January 2013), the World Health Organisation International Trials Registry Platform (19 January 2013), The Cochrane Library Database of Abstracts of Reviews of Effects (19 January 2013) and OpenGrey for grey literature from Europe (19 January 2013). Searches were not limited by language. Bibliographies of included and excluded trials and abstracts of major meetings were searched for additional trials.
Selection criteria
Randomised controlled trials (RCTs) in which gonadotrophins were compared with placebo or no treatment for participants with idiopathic male factor subfertility.
Data collection and analysis
Two review authors independently selected the trials, assessed risk of bias and extracted data on live birth, pregnancy and adverse effects. We included data on pregnancies that occurred during or after gonadotrophin therapy. Study authors and pharmaceutical companies were asked to provide missing and unpublished data and/or additional information.
Main results
Six RCTs with 456 participants and variable treatment and follow‐up periods were included. From the limited data, the live birth rate per couple randomly assigned (27% vs 0%; Peto odds ratio (OR) 9.31, 95% confidence interval (CI) 1.17 to 73.75, one study, 30 participants, very low‐quality evidence) and the spontaneous pregnancy rate per couple randomly assigned (16% vs 7%; Peto OR 4.94, 95% CI 2.13 to 11.44, five studies, 412 participants, I2 = 0%, moderate‐quality evidence) were significantly higher in men receiving gonadotrophin treatment than in men receiving placebo or no treatment. No significant difference between groups was noted when intracytoplasmic sperm injection (ICSI) or intrauterine insemination (IUI) was performed. None of the included studies reported miscarriage rates, and adverse events data were sparse.
Authors' conclusions
Encouraging preliminary data suggest a beneficial effect on live birth and pregnancy of gonadotrophin treatment for men with idiopathic male factor subfertility, but because the numbers of trials and participants are small, evidence is insufficient to allow final conclusions. Large multi‐centre trials with adequate numbers of participants are needed.
Keywords: Female; Humans; Male; Pregnancy; Birth Rate; Follicle Stimulating Hormone; Follicle Stimulating Hormone/therapeutic use; Gonadotropins; Gonadotropins/therapeutic use; Infertility, Male; Infertility, Male/drug therapy; Oligospermia; Oligospermia/drug therapy; Pregnancy Rate; Randomized Controlled Trials as Topic
Plain language summary
Gonadotrophins for idiopathic male factor subfertility
Background
Male subfertility contributes to at least 50% of subfertility in couples. Around 39% of subfertile men have idiopathic subfertility (male subfertility with an unknown cause or origin). Gonadotrophins (hormones that stimulate sperm production) have been used in the treatment of men with this condition, but results have been inconsistent. We reviewed the evidence.
Study characteristics
We found six randomised controlled trials, with 456 participants.
Key results
There was a trend towards an increase in live birth and pregnancy rates during and within three months of gonadotrophin treatment. The quality of the evidence was very low. We did not find enough studies to allow final conclusions about the use of gonadotrophins in the treatment of men with idiopathic male factor subfertility. The quality of the evidence was very low. More studies on this subject are needed. The evidence is current to January 2013.
Summary of findings
Summary of findings for the main comparison. Gonadotrophins versus placebo/no treatment for the treatment of idiopathic male subfertility for idiopathic male factor subfertility.
| Gonadotrophins versus placebo/no treatment for the treatment of idiopathic male subfertility | ||||||
| Population: Men with idiopathic male factor subfertility Setting: Assisted reproduction | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo/no treatment for the treatment of idiopathic male subfertility | Gonadotrophins | |||||
| Live birth rate per couple randomly assigned | 0 per 1000 | 0 per 1000 (0 to 0) | OR 9.31 (1.17 to 73.75) | 30 (1 study) | ⊕⊝⊝⊝ very low1,2 | |
| Spontaneous pregnancy rate per couple randomly assigned | 14 per 1000 | 67 per 1000 (30 to 142) | OR 4.94 (2.13 to 11.44) | 412 (5 studies) | ⊕⊕⊕⊝ moderate3 | |
| *The basis for the assumed risk is the median control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR: Odds ratio. | ||||||
| GRADE Working Group grades of evidence: High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Authors did not report on allocation concealment. 2Only one included trial (i.e. inconsistency cannot be assessed). 3All trials suffered from at least one potential risk of bias.
Background
Description of the condition
Around 15% of couples experience delay in conceiving. Although male factors alone are responsible for at least 30% of cases of subfertility, a combination of male and female factors accounts for another 20%. Thus, it is estimated that male factors contribute to causes of subfertility in at least 50% of subfertile couples (Mecham 1996) and that around 39% of subfertile men have idiopathic subfertility (WHO 1987).
Description of the intervention
Men given a diagnosis of idiopathic male factor subfertility are often treated with a variety of empirical treatments. One of the most commonly used treatments is human gonadotrophins.
Follicle‐stimulating hormone (FSH) and luteinizing hormone (LH) are naturally circulating gonadotrophins that play an important role in the process of spermatogenesis by maintaining the production of adequate numbers of good‐quality sperm. LH affects spermatogenesis via its effect on testosterone synthesis, but the mechanism by which FSH regulates spermatogenesis is poorly understood. In animal models (adult male rhesus and bonnet monkeys), blocking of FSH receptors by active immunisation or pituitary FSH desensitisation results in suppression of spermatogenesis, a drop in sperm count, poor sperm motility and deficiency of acrosomal enzymes, leading to subfertility (Wickings 1980; Moudgal 1992). Neutralisation of circulating endogenous FSH by ovine anti‐FSH antibodies in human volunteers was associated with a drop in the quality and quantity of sperm cells produced (Moudgal 1997).
How the intervention might work
Acosta and colleagues (Acosta 1991; Acosta 1992) reported that systemic administration of FSH to men with severe male factor subfertility improves fertilisation and pregnancy rates in in vitro fertilisation (IVF) cycles. More recently, the effect of FSH administration on sperm parameters in subfertile men has been considered in randomised controlled trials. One study reported that administration of recombinant human FSH (r‐hFSH) (recombinant means 'produced by genetic engineering') was not associated with a change in sperm parameters in men with idiopathic subfertility (Kamischke 1998), although other studies of men with idiopathic oligozoospermia (unexplained low sperm count) reported an increase in sperm concentration and spermatogonia population (mother cells of sperms) on fine‐needle aspiration (Foresta 1998; Foresta 2002). Furthermore, the administration of gonadotrophins was associated with a significant increase in fertilisation rate in IVF cycles in a subgroup of men with poor sperm motility, morphology and count (oligoasthenoteratozoospermia) (Ben‐Rafael 2000). Such an increased fertilisation rate was not observed in other studies in men with severe male factor subfertility or oligoasthenoteratozoospermia undergoing intracytoplasmic sperm injection (ICSI) cycles (Ashkenazi 1999).
Most of the research addressing this topic has focused on the effects of gonadotrophins on semen parameters such as motility, number and morphology. However, it is necessary to direct research toward patient‐oriented outcomes such as pregnancy rate or live birth rate rather than toward surrogate outcomes as semen parameters. Many studies have shown improved sperm parameters after FSH therapy in male subfertility (Iacono 1996 , Ashkenazi 1999 , Ben‐Rafael 2000 , Foresta 2002 , Caroppo 2003). Unfortunately, conventional semen analysis does not provide accurate information about the ability of the sperm to fertilise the ovum (Liu 2002), and the use of semen quality as a surrogate outcome measure is not strongly correlated with improved pregnancy rates.
Why it is important to do this review
Therapeutic modalities that use semen quality rather than pregnancy rate as an outcome measure might prove misleading. Semen quality as a surrogate outcome measure is not strongly correlated with improved pregnancy rates. Randomised controlled trials that report pregnancy rates after administration of gonadotrophin to subfertile men have been conducted, and these findings should be used in decision making. Use of r‐hFSH or purified FSH in men with idiopathic subfertility was not associated with a significant increase in pregnancy rates during intrauterine insemination (IUI) and intracytoplasmic sperm injection (ICSI) cycles in some studies (Ashkenazi 1999; Kamischke 1998), although it was associated with a significant increase in pregnancy rates in others (Baccetti 2004). Given these conflicting results, we decided to systematically review the evidence for gonadotrophin administration to men with idiopathic male factor subfertility.
Objectives
To determine the effect of systemic follicle‐stimulating hormone (FSH) on live birth and pregnancy rates when administered to men with idiopathic male factor subfertility.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials of fertility treatments in which gonadotrophins were administered for the treatment of idiopathic male factor subfertility compared with placebo or no treatment. Trials in which couples received one or more cycles of fertility treatment after randomisation were included. We excluded quasi‐randomised trials (e.g. alternate randomisation, reference to hospital number or to date of birth), cross‐over trials if data before the cross‐over were not available and trials that did not report on outcomes of importance to the review.
Types of participants
Men with idiopathic male factor subfertility diagnosed by subnormal semen parameters as defined by the World Health Organisation (WHO) criteria, including oligospermia, teratospermia, asthenospermia and non‐obstructive azoospermia, or as defined by the study author.
Subnormal sperm parameters defined by WHO 1999 include (WHO 1999):
sperm concentration ≤ 20 million sperm/mL;
motility ≤ 50% motile sperm; and
normal morphology ≤ 30%.
Types of interventions
Systemic administration of any type of FSH (urinary, purified or highly purified or recombinant) compared with placebo or no treatment.
Types of outcome measures
Primary outcomes
Live birth rate per couple randomly assigned
Secondary outcomes
Pregnancy rate per couple randomly assigned confirmed by ultrasound and/or pregnancy test
Miscarriage rate per couple randomly assigned
Adverse effects of treatment
Search methods for identification of studies
Electronic searches
We searched the following sources, without restriction by language or publication status and in consultation with the Cochrane Menstrual Disorders and Subfertility Group Trials Search Co‐ordinator:
-
The Menstrual Disorders & Subfertility Group's Specialised Register of controlled trials (14 January 2013) for any trials with FSH or LH administration to subfertile men in the title, abstract or keywords section. See the Review Group for more details on the make‐up of the Specialised Register.
See Appendix 1.
-
The Cochrane Central Register of Controlled Trials (CENTRAL; The Cochrane Library, Issue 12 of 12, 2012).
See Appendix 2.
The following electronic databases, using Ovid software:
-
MEDLINE In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE(R) Daily and Ovid MEDLINE (1946 to 14 January 2013).
See Appendix 3.
-
EMBASE (1980 to week 2 2013).
See Appendix 4.
-
PsycINFO (1806 to January week 2 2013).
See Appendix 5.
-
The MEDLINE search was combined with the Cochrane highly sensitive search strategy for identifying randomised trials, which appears in the Cochrane Handbook for Systematic Reviews of Interventions (version 5.0.2; Chapter 6, 6.4.11).
The EMBASE search was combined with trial filters developed by the Scottish Intercollegiate Guidelines Network (SIGN) http://www.sign.ac.uk/mehodology/filters.html#random.
ClinicalTrials.gov (19 January 2013) for federally and privately supported ongoing and registered trials and clinical trials including studies sponsored by the National Institutes of Health, other US federal agencies and private industry.
The World Health Organisation International Trials Registry Platform (19 January 2013) at http://www.who.int/trialsearch/Default.aspx.
The metaRegister of Controlled Trials, a major international searchable database of ongoing randomised controlled trials in all areas of healthcare, built by combining registers held by public, charitable and commercial sponsors of trials (http://controlled‐trials.com/mrct/). This database contains the National Research Register (NRR), entries from the Medical Research Council's Clinical Trials Register and details on reviews in progress collected by the NHS Centre for Reviews and Dissemination, as well as others.
OpenSigle and OpenGrey (http://www.opengrey.eu/) for Grey literature from Europe (19 January 2013).
The Database of Abstracts of Reviews of Effects (19 January 2013).
Searching other resources
The citation lists of relevant publications, review articles and abstracts of major scientific meetings and the bibliographies of included and excluded trials were also searched for additional trials.
Data collection and analysis
Selection of studies
The search strategy described above was used to obtain titles and, where possible, abstracts of studies potentially relevant to the review. The titles and abstracts were screened by one review author (AMA), who discarded studies that were clearly ineligible but aimed to be overly inclusive rather than risk losing relevant studies. Two review authors (AMA, HGAl) independently assessed studies for inclusion in accordance with the mentioned criteria. Disagreements were resolved by consensus or through arbitration by the third review author (AMAS). Further information was sought from the authors when papers contained insufficient information to allow a decision to be made regarding eligibility.
Data extraction and management
All data extraction was performed independently by two of the three review authors (AMA, HGAI), using forms designed in accordance with Cochrane guidelines. The third review author (AMAS) resolved discrepancies. Extracted trial data included the following (Characteristics of included studies).
Method of randomisation: randomly assigned by computer, random number tables or drawing of lots, or method not clear (e.g. stated but not further described). Quasi‐randomised trials were excluded from the review (e.g. allocation by hospital number or date of birth).
Concealment of allocation: details recorded.
Presence or absence of blinding to treatment allocation.
Duration and type of follow‐up.
Number of participants recruited, randomly assigned, excluded, analysed or lost to follow‐up.
Location of trial: single‐centre or multi‐centre.
Timing of trial.
Whether an intention‐to‐treat analysis was done.
Source of funding.
Criteria for including participants and assessing outcomes.
Additional information on trial methodology and/or original trial data were sought from the authors of trials that appeared to meet the eligibility criteria but had aspects of methodology that were unclear, or for which the data were provided in a form unsuitable for meta‐analysis.
Assessment of risk of bias in included studies
All assessments of the risk of bias of trials were performed independently by two of the three review authors (AMA, AMAS), using the Cochrane risk of bias assessment tool (www.cochrane‐handbook.org). A third review author resolved discrepancies (HGAI).
Measures of treatment effect
Statistical analysis was performed in accordance with the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We used the number of events in the control and intervention groups of each study to calculate Peto odds ratios (ORs).
Live birth and pregnancy are considered positive consequences of treatment; therefore a higher proportion of women achieving these outcomes is considered a benefit. However, the outcome of adverse effects is a negative consequence; therefore higher numbers are considered detrimental. This needs to be taken into consideration when the summary graphs are viewed.
Studies varied in length of follow‐up after completion of gonadotrophin therapy. Because FSH therapy has a "short‐term effect" on fertility, we restricted our analysis to events that occurred spontaneously during FSH treatment and within 3 months after completion of FSH therapy, and in case of treatment of the female partner with IUI/IVF/ICSI, we restricted our analysis to the first cycle only.
Unit of analysis issues
To prevent unit of analysis errors when the outcome was presented for several time periods in the same trial (multiple treatment cycles), we included data only from the first time period.
Dealing with missing data
Data were extracted and analysed to allow an intention‐to‐treat analysis, defined as including in the denominator all originally randomly assigned participants. In addition, all participants were analysed according to their allocated groups, regardless of treatment eligibility, compliance or treatment given.
Assessment of heterogeneity
Homogeneity of the data from included trials was assessed by visual inspection of the outcomes tables and by use of the Chi2 test (✗2 test) for heterogeneity with a 10% level of statistical significance; a P value of 0.1 was selected as the cut‐off point for rejection of the null hypothesis of study homogeneity to limit type II errors. In addition, heterogeneity was quantified using the I2 statistic, which describes the percentage of variability in effect estimates that is due to heterogeneity rather than sampling error (chance). An I2 value > 50% may be considered to represent substantial heterogeneity.
Assessment of reporting biases
In the event that at least 10 included studies provided data for any given analysis, we planned to investigate publication bias by means of a funnel plot. If publication bias was suspected or detected by the aforementioned method, it would be confirmed or rejected using Egger's regression test.
Data synthesis
Although all included trials might be statistically homogeneous, differences in clinical parameters may be considerable (clinical heterogeneity). These differences were taken into account when the pooled results were analysed and interpreted. Clinical heterogeneity in infertility cannot be avoided because most centres use their own "materials and methods", which can vary along several parameters. If trials met the inclusion criteria and provided the same intervention for male factor subfertility, we considered it appropriate to pool their results.
We combined the data using a fixed effect model to calculate pooled Peto odds ratios (ORs) and 95% confidence intervals (CIs).
Subgroup analysis and investigation of heterogeneity
Subgroup analysis was performed according to treatment modality, whereby pregnancies were divided into spontaneous pregnancies resulting from natural intercourse and pregnancies that followed IUI or assisted reproduction (IVF/ ICSI) Figure 1. We also performed a subgroup analysis that was restricted to trials that enrolled 'normal' female partners (Figure 2). We planned to explore clinical and methodological differences between the studies if substantial statistical heterogeneity was noted.
1.
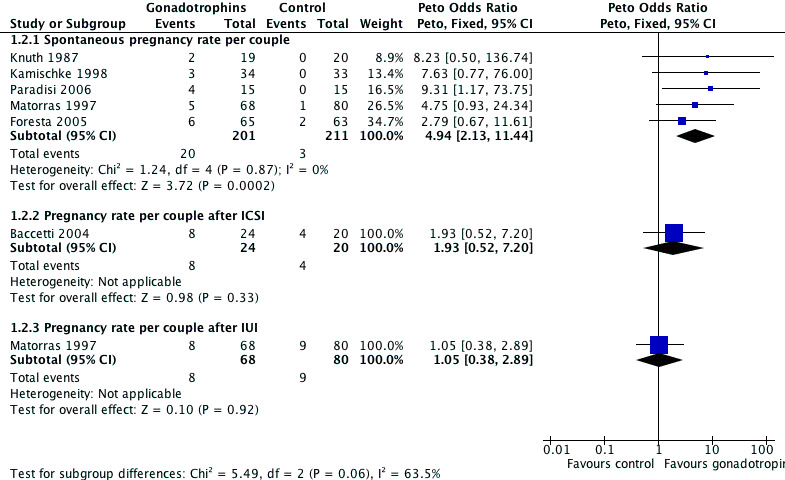
Forest plot of comparison: 1 Gonadotrophins versus placebo/no treatment for the treatment of idiopathic male subfertility, outcome: 1.2 Pregnancy rate per couple randomly assigned.
2.
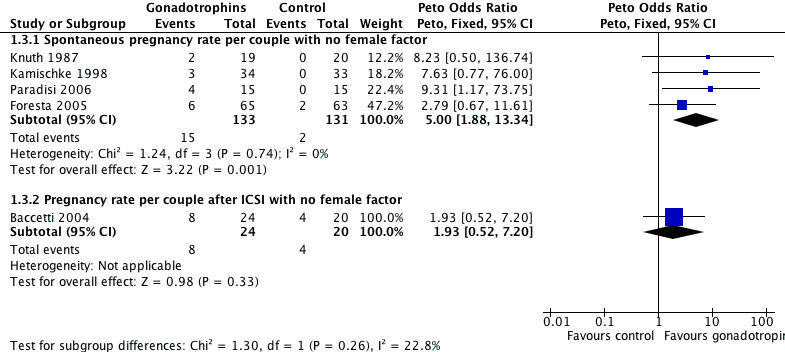
Forest plot of comparison: 1 Gonadotrophins versus placebo/no treatment for the treatment of idiopathic male subfertility, outcome: 1.3 Subgroup analysis: pregnancy rate per couple randomly assigned with no female factor.
Sensitivity analysis
We did not plan any sensitivity analyses.
Results
Description of studies
Design and setting
All studies were parallel‐group randomised controlled trials.
Duration of follow‐up: The duration of follow‐up varied between studies. Couples were followed up only during the treatment period (12 weeks) in one study (Baccetti 2004). In another study, the follow‐up period was extended by another 12 weeks after completion of therapy (Knuth 1987). In the Kamischke 1998 trial, participants were followed‐up during the treatment period, then for another 12 weeks, during which assessment examinations were done, and then for another 3 months, during which pregnancies were recorded. In the Foresta 2005 trial, couples were followed up during treatment and for 3 months after completion of treatment; then, depending on their sperm count, participants were either allocated directly or re‐randomly assigned into groups to receive either three cycles of IUI or one cycle of IVF/ICSI. One trial followed up with participants through a maximum of six IUI cycles (Matorras 1997). In the remaining trial, the duration of follow‐up was not reported (Paradisi 2006).
Sources of funding: Two trials did not report sources of funding (Foresta 2005; Matorras 1997). The trial of Baccetti 2004 was funded by a grant from the Italian Ministry for Universities and Technological Research (2000); that of Kamischke 1998 was supported in part by the Federal Health Ministry (Bonn), the Deutsche Forschungsgemeinschaft (DFG, Bonn) and Ares‐Serono (Unterschleissheim, Germany); and in the study of Knuth 1987, human menopausal gonadotrophin (HMG)/human chorionic gonadotrophin (HCG) and placebo preparations were offered by Serono Co. The remaining trial was funded by Serono Co (supplied r‐hFSH and placebo injections) (Paradisi 2006).
Participants
Male partners
All trials included male partners with idiopathic subfertility based on subfertile semen parameters. Male subfertility was diagnosed in a different way in each trial: diagnostic criteria according to WHO 1987 criteria (Matorras 1997), WHO 1992 criteria (Kamischke 1998) or WHO 1999 criteria (Baccetti 2004); sperm count between 0.1 and 10 Mil/mL (Knuth 1987) and sperm count < 10 million/mL on at least three separate occasions (Foresta 2005); or poor semen quality in the form of moderate to severe oligoasthenozoospermia (range 1 to 15 106/mL sperm concentration) (Paradisi 2006). The age of participants was comparable in the four trials (details in Characteristics of included studies).
Female partners
Female partners had normal fertility (as reported by authors) in five trials (Baccetti 2004; Foresta 2005; Kamischke 1998; Knuth 1987; Paradisi 2006). One trial reported female causes of subfertility in 50% of couples (14.9% tubal factor, 12.2% endometriosis, 14.9% ovulatory disorders, 2.0% hyperprolactinaemia and 6.0% mixed causes) (Matorras 1997).
Interventions
Male partners
HMG/HCG treatment was used in one study (Knuth 1987), purified or highly purified FSH was used in two studies (Baccetti 2004; Matorras 1997) and recombinant FSH was administered in three studies (Foresta 2005; Kamischke 1998; Paradisi 2006). HMG/HCG was given at a dose of 150 IU HMG three times a week and 2500 IU HCG twice a week for 13 weeks (Knuth 1987). Purified FSH was given at a dose of 150 IU/day for 12 weeks (Baccetti 2004; Matorras 1997). Recombinant FSH was given at a dose of 150 IU daily for 12 weeks (Kamischke 1998), 100 IU r‐hFSH IM on alternate days for 3 months (Foresta 2005) or 300 IU r‐hFSH SC daily for at least 4 months (Paradisi 2006). FSH therapy was compared with placebo in three trials (Kamischke 1998; Knuth 1987; Paradisi 2006) and with no treatment in the control group in the other three trials (Baccetti 2004; Foresta 2005; Matorras 1997).
Female partners
Female partners received no treatment in one trial (Knuth 1987), and ICSI was performed after ovarian stimulation, follicular monitoring and aspiration in another trial (Baccetti 2004). The trial of Kamischke 1998 did not state the exact numbers of participants who underwent treatment with assisted reproductive technologies (ART), although pregnancies were reported to occur, besides spontaneously, after IUI, IVF or ICSI. In the trial of Foresta 2005, no intervention was applied with female partners in the first 6 months (3 months of therapy and 12 weeks of follow‐up) of the trial; then participants were re‐randomly assigned to IVF, ICSI or IUI, depending on the sperm count. The re‐randomisation did not influence the data included in this review, but it invalidated the results of the last 3‐month period of the study because it interfered with the first randomisation and rendered the chances of pregnancy due to the treatment studied unequal between the two groups. Moreover, ART was used for longer than 3 months after completion of FSH, which we considered the limit of attributing pregnancies to treatment in our analysis. The trial by Matorras et al. (Matorras 1997) reported using ovarian stimulation, follicular monitoring and HCG administration before participants underwent IUI. The remaining trial did not report whether female partners received any fertility treatment or were monitored during the course of treatment of their male partners (Paradisi 2006).
Outcomes
Live birth rate per couple randomly assigned.
Only one of the six included trials reported live birth rates (Paradisi 2006).
Pregnancy rate per couple randomly assigned
The pregnancy rate was reported in all included trials. Pregnancy was diagnosed by ultrasound (US) 6 weeks after embryo transfer (Baccetti 2004; Matorras 1997), by US and beta‐HCG concentration increase (Kamischke 1998) or by beta‐HCG plasma level (Foresta 2005); one study did not mention the method of diagnosing pregnancy (Knuth 1987), and Paradisi 2006 reported only that all pregnancies went to term successfully.
Miscarriage rate per pregnancy
None of the six trials reported on the miscarriage rate.
Adverse events
Only one trial clearly addressed the side effects of the drugs used (Knuth 1987), another trial reported on adverse events in general (Matorras 1997) and a third trial reported that no adverse events occurred (Paradisi 2006).
Other outcomes
Other surrogate outcomes such as fertilisation rates and effects on sperm count, motility and morphology were not included in the analysis, as we excluded studies that reported only such surrogate outcomes without reporting pregnancy rates, and because such outcomes are intermediate ones that are of secondary importance compared with the participant‐oriented outcome (e.g. live birth or pregnancy rate).
Results of the search
A total of fifteen potentially eligible controlled trials were identified. Electronic searches identified 14 controlled trials that used gonadotrophin treatment for idiopathic male subfertility, and another trial was obtained via personal communication with the author (Foresta 2005) that was published later. All trials were thoroughly appraised for their eligibility to be included in the review, and their risk of bias was assessed.
Included studies
Six trials with 456 participants met the inclusion criteria (Baccetti 2004 with 44 participants, Foresta 2005 with 128 participants, Kamischke 1998 with 67 participants, Knuth 1987 with 39 participants, Matorras 1997 with 148 participants, Paradisi 2006 with 30 participants). Details of each study are provided in the Characteristics of included studies.
Excluded studies
Nine trials were excluded because they did not fulfil our inclusion criteria. One trial was excluded because of quasi‐randomisation (Ashkenazi 1999). One RCT was excluded because the authors compared two gonadotrophin regimens in hypogonadotrophic men (Bouloux 2003). Two RCTs were excluded because outcomes of the study did not include pregnancy rates (Foresta 1998; Foresta 2002). We contacted the authors of these trials to ask for data on pregnancy rates that were not published but received confirmation on the absence of such data. Another RCT was excluded because it was a partial cross‐over study, and because pregnancy rates were not reported (Ben‐Rafael 2000). The other four trials were excluded because all were non‐randomised (Caroppo 2003; Dirnfeld 2000; Iacono 1996; Thomalla‐Sauter 2001).
Risk of bias in included studies
3.
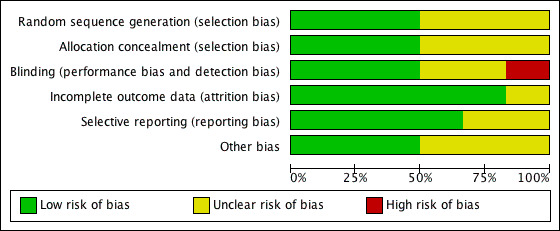
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
4.
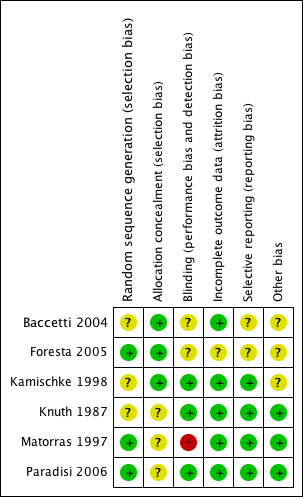
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
Allocation
Half the trials reported using a proper method of randomisation (Foresta 2005; Matorras 1997; Paradisi 2006). Two trials reported "true randomisation" by a third party without reporting any further details of the method (Baccetti 2004; Kamischke 1998). One trial was reported to be a randomised trial but did not report the method of randomisation used (Knuth 1987).
Allocation concealment was considered adequate in the three trials that used third party randomisation (Baccetti 2004; Foresta 2005; Kamischke 1998), but it was not reported in the remaining trials (Knuth 1987; Matorras 1997; Paradisi 2006).
Blinding
Half the trials were considered to be properly blinded (Kamischke 1998; Knuth 1987; Paradisi 2006), and one trial was open label (Matorras 1997). It was not clear from the description whether blinding was used in the remaining two trials (Baccetti 2004; Foresta 2005).
Incomplete outcome data
Three studies reported no withdrawals or dropouts in assessment of pregnancy rates (Baccetti 2004; Knuth 1987; Paradisi 2006). In the remaining studies, dropouts were variable (see Characteristics of included studies for further details).
We analysed the results on an intention‐to‐treat basis, adding the 16 excluded and dropout cases in the trial of Foresta 2005, the four dropout cases along with their corresponding groups in the Kamischke 1998 trial and the 12 excluded cases from the trial by Matorras 1997. The one excluded pregnant case in the Kamischke 1998 trial has been added to the treatment group (upon contacting the author, we were informed that the case belonged in the treatment group).
Selective reporting
None of the included trials had protocols available for review, but no differences were noted between the methods and results sections of the respective trial reports.
Other potential sources of bias
Only three of the included trials (Knuth 1987; Matorras 1997; Paradisi 2006) clearly reported homogeneity among baseline characteristics of included populations.
Effects of interventions
See: Table 1
Gonadotrophins versus placebo/no treatment for participants with idiopathic male subfertility
Primary outcome
Live birth rate per couple randomly assigned
Only one included trial, with a sample size of 30, reported live birth rate per couple (Paradisi 2006). A statistically significant increase in live births was noted following the use of FSH (4/15; 27%) as compared with placebo (0/15; 0%) (Peto OR 9.31, 95% CI 1.17 to 73.75) (Analysis 1.1; Figure 5).
1.1. Analysis.
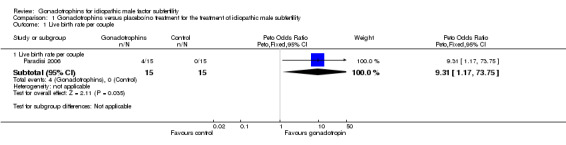
Comparison 1 Gonadotrophins versus placebo/no treatment for the treatment of idiopathic male subfertility, Outcome 1 Live birth rate per couple.
5.
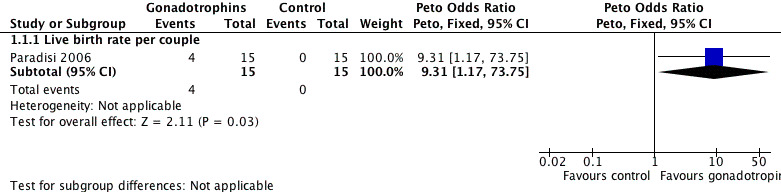
Forest plot of comparison: 1 Gonadotrophins versus placebo/no treatment for the treatment of idiopathic male subfertility, outcome: 1.1 live‐birth rate per couple randomly assigned.
Secondary outcomes
Pregnancy rate per couple randomly assigned
Spontaneous pregnancy rate per couple randomly assigned
We analysed pregnancies that occurred spontaneously within the gonadotrophin treatment period or within 3 months after completion of gonadotrophin treatment. Five trials reported spontaneous pregnancies after gonadotrophin treatment (Foresta 2005, Kamischke 1998, Knuth 1987; Matorras 1997; Paradisi 2006). Pooling of results from the five trials revealed a significant difference in the overall pregnancy rate per couple randomly assigned in favour of the gonadotrophin treatment group (Peto OR 4.94, 95% CI 2.13 to 11.44) with a 16% pregnancy rate (20/201) in the gonadotrophin group and 7% (3/211) in the control group (Analysis 1.2; Figure 1).
1.2. Analysis.
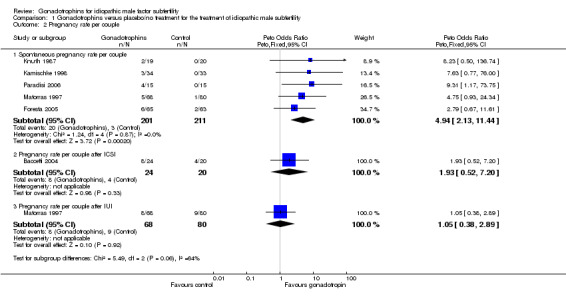
Comparison 1 Gonadotrophins versus placebo/no treatment for the treatment of idiopathic male subfertility, Outcome 2 Pregnancy rate per couple.
The results of a subgroup analysis in trials enrolling 'normal' female partners (excluding Matorras 1997) showed a statistically significant result in favour of FSH treatment (Peto OR 5, 95% CI 1.88 to 13.34) with an 11.3% pregnancy rate (15/133) in the gonadotrophin group and 1.5% (2/131) in the control group (Analysis 1.3; Figure 2).
1.3. Analysis.
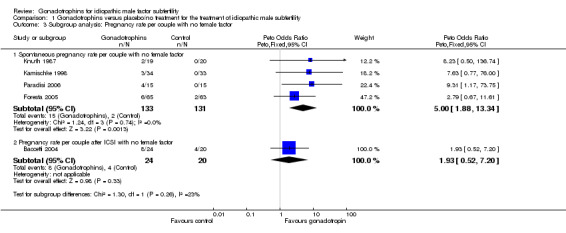
Comparison 1 Gonadotrophins versus placebo/no treatment for the treatment of idiopathic male subfertility, Outcome 3 Subgroup analysis: Pregnancy rate per couple with no female factor.
Pregnancy rate after ICSI per couple randomly assigned
Only one study reported pregnancies that occurred after the ICSI cycle within the gonadotrophin treatment period or within 3 months after FSH treatment (Baccetti 2004). Results of the study showed a favourable, but non‐significant, pregnancy rate after ICSI cycles in the FSH group compared with controls (OR 1.93, 95% CI 0.52 to 7.2), with a 33.3% pregnancy rate (8/24) in the FSH group and 20% (4/20) in the control group.
Pregnancy rate after IUI per couple randomly assigned
Only one trial (Matorras 1997) was performed in couples undergoing IUI. The results were equivocal with a non‐significant difference in pregnancy rates between the two groups (Peto OR 1.05; 95% CI 0.38 to 2.89) (pregnancy rate of 11.8% in the treatment group and 11.3% in the control group).
Miscarriage rate per pregnancy
None of the trials reported this outcome.
Adverse effects
Three studies did not report adverse effects as an outcome (Baccetti 2004, Foresta 2005, Kamischke 1998). Three studies reported adverse effects as an outcome (Knuth 1987; Matorras 1997; Paradisi 2006): One participant in the treatment group developed a temporary side effect in the form of breast tenderness and mild gynaecomastia that resolved spontaneously during the treatment period (Knuth 1987); another participant in the control group suffered an intracranial haemorrhage (Matorras 1997); and the third trial reported that no adverse events occurred during the treatment and follow‐up periods (Paradisi 2006).
Discussion
Summary of main results
Only one included trial, with a sample size of 30, reported live birth rate per couple; it reported a statistically significant increase in live births following the use of FSH. Five trials reported spontaneous pregnancies after gonadotrophin treatment as well as a significant difference in the overall pregnancy rate per couple, favouring gonadotrophins.
Use of gonadotrophins before IUI (one study) and ART (one study) did not lead to a significant increase in pregnancy rates. This could be attributed to the impact of the technology of IUI and ART in overcoming barriers to natural conception such as reduced sperm count, motility, or fertilisability (effect‐modifying factor).
Overall completeness and applicability of evidence
Unfortunately, the present systematic review identified only six valid RCTs that addressed the pregnancy rate after gonadotrophin therapy for idiopathic male subfertility (including 456 participants), but none of them was of adequate sample size. Besides, although pooling of different included studies showed positive outcomes in terms of spontaneous pregnancy rate after gonadotrophin administration, the collective sample size was not sufficient to achieve adequate power.
The included studies used different types of gonadotrophins (hCG, HMG, purified and highly purified FSH and recombinant hFSH) in different regimens and doses. Follow‐up periods also varied between the studies. Some studies reported spontaneous pregnancy rates and others reported pregnancies after IUI and after ARTs. This represents an obvious clinical heterogeneity. Furthermore, we assumed that any benefit derived from FSH therapy is not expected to last longer than 3 months after completion of treatment, as spermatogenesis, the maturation of germ cells to mature spermatozoa, requires on average 72 to 75 days. Thus, we restricted our analysis to the treatment period and 3 months' follow‐up and performed a stratified analysis according to whether pregnancy was achieved spontaneously, by IUI, or by ART. In view of this clinical heterogeneity, the conclusions may be less generalisable, although all trials demonstrated a treatment effect in the same direction.
Quality of the evidence
The quality of the evidence was variable. The randomisation method was not adequately described in three trials (Baccetti 2004; Kamischke 1998; Knuth 1987), data about allocation concealment were unclear in one study (Knuth 1987) and follow‐up periods varied. Only one study reported live birth, and the quality of the evidence for this outcome was rated as very low. Five studies reported spontaneous pregnancy: Their findings were consistent, but all suffered from at least one potential risk of bias, and the quality of the evidence for this outcome was rated as moderate.
Potential biases in the review process
No potential biases were identified during the review process.
Agreements and disagreements with other studies or reviews
Upon searching the literature, we did not find any similar systematic reviews studying gonadotrophin administration to males for the treatment of idiopathic male subfertility.
Authors' conclusions
Implications for practice.
Encouraging preliminary data suggest a beneficial effect on live birth and pregnancy of gonadotrophin treatment for men with idiopathic male subfertility, but the numbers of trials and participants are small; therefore evidence is insufficient to permit final conclusions.
Implications for research.
Large multi‐centre trials with adequate numbers of participants are needed to provide conclusive evidence on the effects of gonadotrophin treatment for men with idiopathic male subfertility. Investigators should report live birth and pregnancy as outcomes.
What's new
| Date | Event | Description |
|---|---|---|
| 25 July 2013 | New search has been performed | Review updated. AM Attia updated the review and reran the search to January 2013. Two new studies were included. Authorship edited and Ahmed M Abou‐Setta added to the review team. Risk of bias assessments added for all included studies. Strength of evidence graded and 'Summary of findings' table added. |
| 25 July 2013 | New citation required and conclusions have changed | Conclusions amended to reflect new evidence. |
History
Protocol first published: Issue 1, 2005 Review first published: Issue 1, 2006
| Date | Event | Description |
|---|---|---|
| 24 October 2008 | Amended | Minor formatting completed Authorship edited |
| 20 May 2008 | Amended | Converted to new review format. |
| 22 June 2007 | New citation required and conclusions have changed | Substantive amendment |
| 30 May 2007 | New search has been performed | AM Attia revised and rerun the search in 2007. One study was identified and excluded (Paradisi 2006). |
Acknowledgements
We would like to acknowledge the work of:
Michelle L Proctor, a former Review Group Co‐ordinator of the Cochrane Menstrual Disorders and Subfertility Group. She shared in authoring of the first version of this review by commenting on drafts of the text of the protocol, in particular the search strategy and methods sections. For the first version of the review, she was responsible for resolving disagreements in data extraction and quality assessment; and
Marian Showell, the Trials Search Co‐ordinator of the Cochrane Menstrual Disorders and Subfertility Group, who provided us with the main search in major databases in February 2012 and January 2013.
Appendices
Appendix 1. MDSG
Keywords CONTAINS "male factor subfertility" or "male factor" or "male fertility" or "male immune subfertility" or "male infertility" or "male subfertility" or "Sperm" or "oligo‐spermatozoa" or "oligoasthenoteratozoospermia"or "oligospermia"or "oligozoospermia"or "asthenospermia"or "asthenozoospermia"or "azoospermia"or "subfertility‐male " or Title CONTAINS "male factor subfertility" or "male factor" or "male fertility" or "male immune subfertility" or "male infertility" or "male subfertility" or "Sperm" or "oligo‐spermatazoa" or "oligoasthenoteratozoospermia"or "oligospermia"or "oligozoospermia"or "asthenospermia"or "asthenozoospermia"or "azoospermia"or "subfertility‐male "
AND
Keywords CONTAINS "gonadotrophins"or"gonadotropin"or"FSH"or"FSH HMG"or"lh"or"follitropin"or"Follitropin A"or"follitropin alfa"or"follicle stimulating hormone"or"urinary FSH"or"u‐FSH "or"u‐hMG"or"u‐LH "or"uFSH"or"uHCG"or"luteinizing hormone"or"Luteinising hormone releasing hormone"or"recombinant FSH"or"recombinant hFSH"or"recombinant HCG"or"recombinant LH"or"human recombinant follitropin‐alpha"or"human menopausal gonadotrophin"or"HMG"or "human menopausal gonadotrophin"or"human chorionic gonadotrophin"or"human chorionic gonadotropin"or"HCG"or"menotropin"or"menotrophin" or Title CONTAINS"gonadotrophins"or"gonadotropin"or"FSH"or"FSH HMG"or"lh"or"follitropin"or"Follitropin A"or"follitropin alfa"or"follicle stimulating hormone"or"urinary FSH"or"u‐FSH "or"u‐hMG"or"u‐LH "or"uFSH"or"uHCG"or"luteinizing hormone"or"Luteinising hormone releasing hormone"or"recombinant FSH"or"recombinant hFSH"or"recombinant HCG"or"recombinant LH"or"human recombinant follitropin‐alpha"
Appendix 2. CENTRAL
The Cochrane Central Register of Controlled Trials (CENTRAL) on Issue 12, 2012 of The Cochrane Library was searched in all fields using the following words: 1 gonadotropins/ or exp gonadotropins, pituitary/ or exp follicle stimulating hormone/ or exp luteinizing hormone/ (3536) 2 gonadotrop$.tw. (2412) 3 follicle stimulating hormone$.tw. (1045) 4 (luteinizing hormone$ or luteinising hormone$).tw. (1193) 5 (LH or FSH).tw. (2778) 6 (ufsh or ulh).tw. (21) 7 (rfsh or rlh).tw. (168) 8 or/1‐7 (6449) 9 exp infertility, male/ or exp aspermia/ or exp asthenozoospermia/ or exp azoospermia/ or exp oligospermia/ (473) 10 (male$ adj2 infertil$).tw. (293) 11 (male$ adj2 subfertil$).tw. (54) 12 (men adj2 infertil$).tw. (103) 13 (men adj2 subfertil$).tw. (19) 14 oligospermia.tw. (43) 15 asthenospermia.tw. (26) 16 azoospermia.tw. (122) 17 asthenozoospermia.tw. (35) 18 oligoasthenoteratozoospermia.tw. (7) 19 (sperm or semen).tw. (1668) 20 or/9‐19 (1906) 21 8 and 20 (492) 22 limit 21 to yr="2007 ‐Current" (86)
Appendix 3. MEDLINE
The MEDLINE search was combined with the Cochrane highly sensitive search strategy for identifying randomized trials which appears in the Cochrane Handbook of Systematic Reviews of Interventions (Version 5.0.2 chapter 6, 6.4.11)
MEDLINE database was searched using the following subject headings and keywords: 1 gonadotropins/ or exp gonadotropins, pituitary/ or exp follicle stimulating hormone/ or exp luteinizing hormone/ (92423) 2 gonadotrop$.tw. (54032) 3 follicle stimulating hormone$.tw. (14034) 4 (luteinizing hormone$ or luteinising hormone$).tw. (24704) 5 (LH or FSH).tw. (52061) 6 (ufsh or ulh).tw. (75) 7 (rfsh or rlh).tw. (533) 8 or/1‐7 (139500) 9 exp infertility, male/ or exp aspermia/ or exp asthenozoospermia/ or exp azoospermia/ or exp oligospermia/ (20736) 10 (male$ adj2 infertil$).tw. (6771) 11 (male$ adj2 subfertil$).tw. (538) 12 (men adj2 infertil$).tw. (2860) 13 (men adj2 subfertil$).tw. (387) 14 oligospermia.tw. (1043) 15 asthenospermia.tw. (240) 16 azoospermia.tw. (4131) 17 asthenozoospermia.tw. (439) 18 oligoasthenoteratozoospermia.tw. (192) 19 (sperm or semen).tw. (61703) 20 or/9‐19 (74403) 21 8 and 20 (6570) 22 randomized controlled trial.pt. (318727) 23 controlled clinical trial.pt. (83412) 24 randomized.ab. (234709) 25 placebo.tw. (136295) 26 clinical trials as topic.sh. (157393) 27 randomly.ab. (172722) 28 trial.ti. (100443) 29 (crossover or cross‐over or cross over).tw. (52145) 30 or/22‐29 (781229) 31 (animals not (humans and animals)).sh. (3557624) 32 30 not 31 (721295) 33 32 and 21 (580) 34 (2007$ or 2008$ or 2009$ or 2010$ or 2011$ or 2012$).ed. (4353905) 35 33 and 34 (134)
Appendix 4. EMBASE
The EMBASE search is combined with trial filters developed by the Scottish Intercollegiate Guidelines Network (SIGN) http://www.sign.ac.uk/mehodology/filters.html#random
The EMBASE database was searched using the following subject headings and keywords: 1 exp gonadotropin/ (19928) 2 exp follitropin/ (38779) 3 exp luteinizing hormone/ (44768) 4 gonadotrop$.tw. (54764) 5 follitropin.tw. (578) 6 luteinizing hormone$.tw. (22001) 7 follicle stimulating hormone$.tw. (13564) 8 luteinising hormone$.tw. (1169) 9 (LH or FSH).tw. (54401) 10 (ufsh or ulh).tw. (93) 11 (rfsh or rlh).tw. (776) 12 or/1‐11 (120267) 13 exp male infertility/ or exp asthenospermia/ or exp azoospermia/ or exp male sterility/ or exp oligospermia/ (27195) 14 (male$ adj2 infertil$).tw. (8279) 15 (male$ adj2 subfertil$).tw. (641) 16 (men adj2 infertil$).tw. (3292) 17 (men adj2 subfertil$).tw. (412) 18 oligospermia.tw. (1072) 19 asthenospermia.tw. (286) 20 azoospermia.tw. (4550) 21 asthenozoospermia.tw. (488) 22 oligoasthenoteratozoospermia.tw. (239) 23 (sperm or semen).tw. (65277) 24 or/13‐23 (82036) 25 24 and 12 (7630) 26 Clinical Trial/ (823603) 27 Randomized Controlled Trial/ (296357) 28 exp randomization/ (55579) 29 Single Blind Procedure/ (14735) 30 Double Blind Procedure/ (102763) 31 Crossover Procedure/ (31733) 32 Placebo/ (191694) 33 Randomi?ed controlled trial$.tw. (68283) 34 Rct.tw. (8403) 35 random allocation.tw. (1087) 36 randomly allocated.tw. (16142) 37 allocated randomly.tw. (1728) 38 (allocated adj2 random).tw. (691) 39 Single blind$.tw. (11480) 40 Double blind$.tw. (120977) 41 ((treble or triple) adj blind$).tw. (256) 42 placebo$.tw. (164540) 43 prospective study/ (181244) 44 or/26‐43 (1171442) 45 case study/ (14547) 46 case report.tw. (213228) 47 abstract report/ or letter/ (806112) 48 or/45‐47 (1029684) 49 44 not 48 (1137623) 50 25 and 49 (1089) 51 (2010$ or 2011$ or 2012$).em. (2585757) 52 50 and 51 (211)
Appendix 5. PSYCINFO
PSYCINFO was searched using the following words:
1 exp Gonadotropic Hormones/ (3427) 2 exp Luteinizing Hormone/ or exp Follicle Stimulating Hormone/ (665) 3 gonadotrop$.tw. (1195) 4 follicle stimulating hormone$.tw. (414) 5 (luteinizing hormone$ or luteinising hormone$).tw. (1128) 6 (LH or FSH).tw. (2253) 7 (ufsh or ulh).tw. (1) 8 (rfsh or rlh).tw. (9) 9 or/1‐8 (5830) 10 exp Infertility/ (1489) 11 (male$ adj2 infertil$).tw. (135) 12 (male$ adj2 subfertil$).tw. (6) 13 (men adj2 infertil$).tw. (57) 14 (men adj2 subfertil$).tw. (0) 15 oligospermia.tw. (15) 16 asthenospermia.tw. (2) 17 azoospermia.tw. (15) 18 asthenozoospermia.tw. (0) 19 oligoasthenoteratozoospermia.tw. (1) 20 (sperm or semen).tw. (1703) 21 or/10‐20 (3130) 22 9 and 21 (64) 23 random.tw. (34342) 24 control.tw. (267676) 25 double‐blind.tw. (15544) 26 clinical trials/ (5727) 27 placebo/ (3095) 28 exp Treatment/ (506101) 29 or/23‐28 (765513) 30 22 and 29 (28)
Data and analyses
Comparison 1. Gonadotrophins versus placebo/no treatment for the treatment of idiopathic male subfertility.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Live birth rate per couple | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 1.1 Live birth rate per couple | 1 | 30 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 9.31 [1.17, 73.75] |
| 2 Pregnancy rate per couple | 6 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 2.1 Spontaneous pregnancy rate per couple | 5 | 412 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 4.94 [2.13, 11.44] |
| 2.2 Pregnancy rate per couple after ICSI | 1 | 44 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.93 [0.52, 7.20] |
| 2.3 Pregnancy rate per couple after IUI | 1 | 148 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.05 [0.38, 2.89] |
| 3 Subgroup analysis: Pregnancy rate per couple with no female factor | 5 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 3.1 Spontaneous pregnancy rate per couple with no female factor | 4 | 264 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 5.00 [1.88, 13.34] |
| 3.2 Pregnancy rate per couple after ICSI with no female factor | 1 | 44 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.93 [0.52, 7.20] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Baccetti 2004.
| Methods | Country of study: Germany Number of centres: single centre Consent: reported Ethical approval: not reported Timing of trial: not reported Source of funding: a grant from the Italian Ministry for Universities and Technological Research, 2000 Numbers of participants
|
|
| Participants | Pre‐allocation examinations and studies: Andrological and gynaecological examinations for males and their partners. Semen analysis and transmission electron microscopy. Female partners underwent a complete infertility work‐up Inclusion criteria
Exclusion criteria
Age
Duration of follow‐up: 12 weeks of therapy |
|
| Interventions | Treatment group: 150 IU/day s.c.Highly purified FSH (Fertinorm HP, Serono) Control group: no treatment Female partners underwent hormonal stimulation with HMG and HCG, sonographic monitoring of follicular growth, oocyte aspiration, ICSI; up to 3 embryos were transferred to the uterine cavity two days after oocyte retrieval Duration of intervention: 12 weeks Follow‐up examinations: semen analysis by optical microscopy before and after FSH treatment. Sperm count and motility were assessed using a Makler counting chamber. Aliquots of each semen sample were examined by transmission electron microscopy (TEM) | |
| Outcomes | Principal and secondary: sperm parameters and pregnancy rate Methods of assessing outcome measures: Clinical pregnancy was determined by ultrasound evidence of a foetal sac 6 weeks after embryo transfer Adverse events: not reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Authors reported using "true randomisation" with no further details |
| Allocation concealment (selection bias) | Low risk | Authors reported using third party randomisation |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | The authors did not report blinding of participants or outcome assessors. From the study design (treatment vs no treatment), the most probable scenario was an open‐label study design |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Authors did not report any dropouts and analysed outcomes using the intention‐to‐treat principle |
| Selective reporting (reporting bias) | Unclear risk | Protocol was not reviewed, but outcomes in the methods and results sections are similar |
| Other bias | Unclear risk | Baseline characteristics for both groups reported with no mention of similarity between groups nor of results of any statistical comparison of homogeneity |
Foresta 2005.
| Methods | Country of study: Italy Number of centres: single centre Consent: reported Ethical approval: reported Timing of trial: not reported Source of funding: not reported Numbers of participants
|
|
| Participants | Pre‐allocation examinations and studies: Exclusion of common conditions such as history of cryptorchidism, post‐mumps orchitis, testicular torsion or trauma, varicocele; seminal tract infections, anti‐sperm antibodies and Y chromosome microdeletion, karyotypic abnormalities and CFTR gene mutations. Ultrasound scanning of the testis to evaluate testicular size and morphology, followed by testicular aspiration Testicular structure was analysed in all participants by means of bilateral fine‐needle aspiration cytology (FNAC). Semen evaluations were performed in a blinded fashion by the same operator. FSH, LH and testosterone plasma concentrations were measured by RIA using standard methods. Inhibin B plasma concentrations were measured by a solid phase sandwich enzyme‐linked immunosorbent assay (ELISA) specific for the dimeric inhibin‐B Inclusion criteria
Exclusion criteria: none stated Age
Duration of follow‐up: 3 months' treatment, 3 months' follow‐up, then re‐randomisation and ART trials (3 months) |
|
| Interventions | Treatment group: 100 IU r‐hFSH IM on alternate days for 3 months Control group: no treatment Duration of intervention: 3 months Follow‐up examinations: The study was divided into 3 periods
|
|
| Outcomes | Principal and secondary: sperm parameters and pregnancy rate Methods of assessing outcome measures: measuring B‐hCG plasma levels Adverse events: not reported | |
| Notes | Changes in trial protocol: after the initial 6‐month period (3 months' treatment and 3 months' follow‐up) a major change in the initial protocol necessitated exclusion of this period from the analysis in the SR Contact with author: The study was first sent to us upon personal communication (unpublished data), but later, it was published (September 2005) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Authors reported that participants were randomly allocated to treatment or no treatment groups with a random number generator |
| Allocation concealment (selection bias) | Low risk | Authors reported using third party randomisation |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Authors did not report blinding of participants or outcome assessors, except for semen analysis. From the study design (treatment vs no treatment), the most probable scenario was an open‐label study design |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Sixty‐five men were allocated to the treatment group and 63 to the no treatment group. Of these, 6 couples (2 from the treatment group and 4 from the non‐treatment group) were subsequently excluded from the study because of concurrent illnesses. Ten participants (1 from the treatment group and 9 from the no treatment group) dropped out before completing the study: 2 in the no treatment group were lost, 7 in the no treatment group dropped out by participant request and 1 in the treatment group discontinued intervention. Therefore, 112 men (87.5%) affected by idiopathic oligozoospermia completed the study (62 in the treatment group and 50 in the no treatment group), were analysed and are described |
| Selective reporting (reporting bias) | Unclear risk | Protocol was not reviewed, but outcomes in the methods and results sections are similar |
| Other bias | Unclear risk | Baseline characteristics for both groups are reported, but no mention is made of similarity between groups. Statistical comparison of homogeneity between baseline characteristics of the groups shows statistically significant differences in sperm concentration and forward motility in the control group |
Kamischke 1998.
| Methods | Country of study: Germany Number of centres: single centre Consent: reported Ethical approval: reported Timing of trial: March 1994 to November 1996 Source of funding: supported in part by the Federal Health Ministry (Bonn), the Deutsche Forschungsgemeinschaft (DFG, Bonn) and Ares‐Serono (Unterschleissheim, Germany) Numbers of participants
Complete physical, hormonal and semen examination. Detailed medical histories of the participant and female partner, physical examination, clinical chemistry, red blood cell count, clotting factors, hormones (luteinizing hormone (LH), FSH, prolactin, testosterone, oestradiol), semen analysis and flow cytometry of sperm DNA. In addition, scrotal content was examined by ultrasonography. at one pre‐examination and at cessation of medication. Electron microscopy (EM) was included in the analysis at the second pre‐examination and was performed in 31 participants
|
|
| Participants | Pre‐allocation examination and studies: At the first screening examination, a complete physical, hormonal and semen examination was performed. If the results of the first screening examination were in accordance with the inclusion criteria, a second pre‐examination was performed, including detailed medical histories of the participant and female partner, physical examination, clinical chemistry, red blood cell count, clotting factors, hormones (luteinizing hormone (LH), FSH, prolactin, testosterone, oestradiol), semen analysis and flow cytometry of sperm DNA. In addition, scrotal content was examined by ultrasonography at one pre‐examination and at cessation of medication. Electron microscopy (EM) was included in the analysis at the second pre‐examination and was performed in 31 participants Inclusion criteria
Exclusion criteria
Mean age 32.89 years. Mean BMI 25.63 kg/m2. Mean duration of infertility 4.6 years Duration of follow‐up 24 weeks (12 treatment + 12 follow‐up) + 3 months |
|
| Interventions | Treatment group: daily SC injections of 150 IU rhFSH (Gonal‐F, Serono) with 30 mg saccharose same time of day by participants themselves. Treatment started 1.2 ± 0.2 months after the last pre‐examination Control group: same method but with placebo containing saccharose 30 mg alone Duration of intervention: 12 weeks Follow‐up examinations: control examinations: 6 and 12 weeks after initiation of treatment and 6 and 12 weeks after cessation of treatment (medical histories of participant and female partner, detection of adverse events and side effects, physical evaluation and clinical chemistry, red blood cell count, clotting factors, hormone analysis (LH, FSH, inhibin B, testosterone, oestradiol), semen analysis and flow cytometry of sperm DNA). In participants for whom EM analysis had been performed at the pre‐examination, it was repeated 12 weeks after initiation and 12 weeks after cessation of treatment An additional ultrasound examination of scrotal content was performed 12 weeks after initiation of treatment Pregnancies in female partners were recorded a further 3 months after the last control examination | |
| Outcomes | Principal and secondary. Primary: sperm parameters. Secondary: pregnancy Methods of assessing outcome measures: US and HCG concentration increase Adverse events: not reported | |
| Notes | Excluded from the analysis by authors: After the 6‐month observation period after treatment, further pregnancies of female partners occurred with the aid of ICSI (treated n = 4, placebo n = 7), IVF (treated n = 1) or insemination (placebo n = 1), or spontaneously (treated n = 1) Contact with the author: contacted twice. At first, author informed us that the spontaneous pregnancy that occurred before treatment was reported in the treatment group. We then asked about the number of participants who underwent ART but received no answer until now | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Authors reported using "true randomisation" with no further details |
| Allocation concealment (selection bias) | Low risk | Authors reported "third party randomization" and that the code distinguishing the treatment groups was blinded for the examiners |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Authors reported that the code distinguishing the treatment groups was blinded for the examiners and that they used a placebo, but explicit description of blinding of participants was not reported and was assumed |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 67 participants were allocated to treated or placebo groups. One participant (placebo) dropped out for personal reasons before completing the study. Another participant (placebo) was excluded after completing the study because the mixed agglutination reaction (MAR) test at the last three examinations revealed IgG and IgA titres between 50% and 100% as a sign of immunological infertility. Intention‐to‐treat analyses were not performed |
| Selective reporting (reporting bias) | Low risk | Protocol was not reviewed, but outcomes in the methods and results sections are similar |
| Other bias | Unclear risk | Baseline characteristics for both groups are reported, but no mention is made of similarity between groups nor results of any statistical comparison of homogeneity |
Knuth 1987.
| Methods | Country of study: Germany Number of centres: single centre Consent: reported Ethical approval: not reported Timing of trial: not reported Source of funding: HMG/HCG and placebo preparations were offered by Serono Co Numbers of participants
|
|
| Participants | Pre‐allocation examinations and studies: assessment of endocrine parameters using 100 mcg GnRH and 10 mg metoclopramide monohydrochloride iv tests. Evaluation of Leydig cell responsiveness using 5000 IU hCG IM injection at the end of the GnRH test Inclusion criteria
Exclusion criteria
Age: in placebo group 33.2 years; in treatment group 31.1 years Duration of follow‐up: 6 months (13 weeks' treatment and 3 months' follow‐up) |
|
| Interventions | Treatment group: 150 IU hMG (Pregonal, Serono) three times per week in addition to 2500 IU hCG (Pregnesin, Serono) twice weekly or 13 weeks. Injections were given by the participant's general practitioner Control group: same schedule of injections with NaCl injections for 13 weeks (labelled ampoules in a double‐blind design) Duration of intervention: 13 weeks Follow‐up examinations: semen parameters and basal LH, FSH and testosterone levels were assessed 1 week after the last injection and were repeated three more times at monthly intervals | |
| Outcomes | Principal and secondary: semen parameters and pregnancy rate Methods of assessing outcome measures: not reported Adverse events: 1 in the treatment group developed side effects (temporary breast tenderness and gynaecomastia that resolved spontaneously within the treatment period) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Authors reported that the trial was a randomised trial and provided no further details |
| Allocation concealment (selection bias) | Unclear risk | Authors did report on allocation concealment |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Authors reported that the trial was a double‐blind trial that included the use of an appropriate placebo |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Two participants in the treatment group dropped out because they developed febrile illness. They were excluded from seminal parameters analysis but not from pregnancy analysis |
| Selective reporting (reporting bias) | Low risk | Protocol was not reviewed, but outcomes in the methods and results sections are similar |
| Other bias | Low risk | Baseline hormone characteristics for both groups were reported to be similar |
Matorras 1997.
| Methods | Country of study: Spain Number of centres: single centre Consent: reported Ethical approval: not reported Timing of trial: January 1991 to December 1994 Source of funding: not reported Numbers of participants
|
|
| Participants | Pre‐allocation examinations and studies:
Inclusion criteria
Exclusion criteria
Age: 34.06 years in FSH group versus 34.63 years in control group Duration of follow‐up: up to 6 cycles of IUI |
|
| Interventions | Treatment group: IM injections of 150 IU pure urinary FSH (Fertinorm; Serono in the first 2 years of study) and SC 150 IU highly purified FSH (Neo‐Fertinorm; Serono in the second 2 years of study) three times per week starting 3 months before the first IUI cycle and ending with the fifth IUI cycle Control group: no treatment for males and same treatment as treatment group for females Female partners: no IUI was performed in the first 3 months after randomisation in either group. Then all women underwent ovarian stimulation (with HMG or FSH) and were monitored by vaginal US and E2 levels. HCG administration and IUI with luteal phase supplementation with HCG or micronized progesterone were performed up to 5 cycles Duration of intervention: 3 months before IUI and up to 5 months (5 IUI cycles) during IUI Follow‐up examinations: A second sperm analysis was performed after 3 months of therapy in the FSH group and after 3 months of no treatment in the non‐FSH group | |
| Outcomes | Principal and secondary: clinical pregnancy rate and post‐FSH semen parameters Methods of assessing outcome measures: ultrasound identification of an embryonic sac at 6 to 7 weeks amenorrhoea and semen analysis Adverse events: none reported in the FSH group. One participant in the control group suffered an intracranial haemorrhage |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Authors reported randomly assigning participants using an "alleatory number table" |
| Allocation concealment (selection bias) | Unclear risk | Authors did report on allocation concealment |
| Blinding (performance bias and detection bias) All outcomes | High risk | Authors reported that the study was not double‐blind, nor was a placebo used in the control group (open‐label trial design) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Authors excluded 12/148 participants and provided reasons. Intention‐to‐treat analysis was not performed |
| Selective reporting (reporting bias) | Low risk | Protocol was not reviewed, but outcomes in the methods and results sections are similar |
| Other bias | Low risk | Baseline characteristics for both groups were reported to be similar |
Paradisi 2006.
| Methods | Country of study: Italy Number of centres: single centre Consent: reported Ethical approval: reported Timing of trial: not reported Source of funding: supported in part by grants from Ministero dell’Istruzione, dell’Università e della Ricerca, Rome, Italy, and from Serono Industries, Rome, Italy Numbers of participants
|
|
| Participants | Pre‐allocation examinations and studies Inclusion criteria
Exclusion criteria: testicular tumour, hypergonadotropic hypogonadism, hypogonadotropic hypogonadism, isolated gonadotropin deficiency, hyperprolactinaemia, severe scrotal varicocele, history of cryptorchidism, leucocytospermia, acute orchitis and other genital infections, positivity to seminal sperm antibodies, presence of Y chromosome microdeletions, obesity and other systemic severe chronic illness Age: not reported Duration of follow‐up: not reported |
|
| Interventions | Treatment group: 300 IU rhFSH SC every other day for >4 months Control group: placebo Duration of intervention: >4 months Follow‐up examinations: semen and hormone analyses | |
| Outcomes | Principal and secondary: semen and hormone profile, clinical pregnancy and live birth rates Methods of assessing outcome measures: semen and hormone analyses immediately after treatment; follow‐up of pregnancies Adverse events: none reported |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Authors reported using a "computer‐generated randomization list" |
| Allocation concealment (selection bias) | Unclear risk | Authors did report on allocation concealment |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Authors reported that the trial was double‐blind and used identical placebos |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts were reported. All 30 participants were analysed |
| Selective reporting (reporting bias) | Low risk | Protocol was not reviewed, but outcomes in the methods and results sections are similar |
| Other bias | Low risk | Baseline characteristics for both groups were reported to be similar |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ashkenazi 1999 | Quasi‐randomised trial |
| Ben‐Rafael 2000 | Partial cross‐over study with no data before cross‐over |
| Bouloux 2003 | No outcomes of importance to this review were reported |
| Caroppo 2003 | Prospective cohort study |
| Dirnfeld 2000 | Retrospective cohort study |
| Foresta 1998 | No outcomes of importance to this review were reported |
| Foresta 2002 | No outcomes of importance to this review were reported |
| Iacono 1996 | No outcomes of importance to this review were reported |
| Thomalla‐Sauter 2001 | Non‐randomised controlled trial |
Differences between protocol and review
Types of interventions: In the first protocol, we included only couples who were undergoing IUI/IVF/ICSI, but upon finding some studies that looked for spontaneous pregnancy and others that analysed both spontaneous pregnancies and those after ARTs, we removed this restriction. Outcome measures: We included pregnancies diagnosed by pregnancy test and removed semen parameters from secondary outcomes, as this is a surrogate outcome that provides no value when compared with pregnancy rate.
Contributions of authors
AMA registered the title and took the lead in writing the text of the protocol. For the review, he was responsible for performing searches of databases for trials, selecting trials for inclusion, independently extracting data and providing quality assessment, performing statistical analyses and interpreting the data.
AMA also had the search revised and rerun in 2007 and in 2013. One study was identified and excluded in 2007.
AMAS revised the review, provided methodological support and commented on the final draft.
HGAl initiated and conceptualised the review topic and shared writing of the protocol. For the review, he was responsible for selecting trials for inclusion, independently extracting data and providing quality assessment; he also commented on drafts of the review.
Sources of support
Internal sources
None, Not specified.
External sources
No sources of support supplied
Declarations of interest
None known.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Baccetti 2004 {published data only}
- Baccetti B, Piomboni P, Bruni E, Capitani S, Gambera L, Moretti E, et al. Effect of follicle‐stimulating hormone on sperm quality and pregnancy rate. Asian Journal of Andrology 2004;6:133‐7. [PubMed] [Google Scholar]
Foresta 2005 {published data only}
- Foresta C, Bettella A, Garolla A, Ambrosini G Ferlin A. Treatment of male idiopathic infertility with recombinant human follicle‐stimulating hormone: a prospective, controlled, randomized clinical study. Fertility and Sterility 2005;84:654–61. [DOI] [PubMed] [Google Scholar]
Kamischke 1998 {published data only}
- Kamischke A, Behre HM, Bergmann M, Simoni M, Shafer T, Nieschlag E. Recombinant follicle stimulating hormone for treatment of male idiopathic infertility: a randomised, double‐blind, placebo‐controlled, clinical trial. Human Reproduction 1998;13:596‐603. [DOI] [PubMed] [Google Scholar]
Knuth 1987 {published data only}
- Knuth UA, Honigl W, Bals‐Pratsch M, Schleicher G, Nieschlag E. Treatment of severe oligospermia with human chorionic gonadotropin/human menopausal gonadotropin: a placebo‐controlled, double blind trial. Journal of Clinical Endocrinology and Metabolism 1987;65(6):1081‐7. [DOI] [PubMed] [Google Scholar]
Matorras 1997 {published data only}
- Matorras R, Perez C, Corcostegui B, Pijoan JI, Ramon O, Delgado P, Rodriguez‐Escudero FJ. Treatment of the male with follicle‐stimulating hormone in intrauterine insemination with husband's spermatozoa: a randomized study. Human Reproduction 1997;12:24‐8. [DOI] [PubMed] [Google Scholar]
Paradisi 2006 {published data only}
- Paradisi R, Busacchi P, Seracchioli R, Porcu E, Venturoli S. Effects of high doses of recombinant human follicle‐stimulating hormone in the treatment of male factor infertility: results of a pilot study. Fertility & Sterility 2006;86(3):728‐31. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Ashkenazi 1999 {published data only}
- Ashkenazi J, Bar‐Hava I, Farhi J, Levy T, Feldberg D, Orvieto R, Ben‐Rafael Z. The role of purified follicle stimulating hormone therapy in the male partner before intracytoplasmic sperm injection. Fertility and Sterility 1999;72:670‐3. [DOI] [PubMed] [Google Scholar]
Ben‐Rafael 2000 {published data only}
- Ben‐Rafael Z, Farhi J, Feldberg D, Bartoov B, Kovo M, Eltes F, et al. Follicle‐stimulating hormone treatment for men with idiopathic oligoteratoasthenozoospermia before in vitro fertilization: its impact on sperm microstructure and fertilization potential. Fertility and Sterility 2000;73:24‐30. [DOI] [PubMed] [Google Scholar]
Bouloux 2003 {published data only}
- Bouloux PM, Nieschlag E, Burger HG, Skakkebaek NE, Wu FC, Handelsman DJ, et al. Induction of spermatogenesis by recombinant follicle‐stimulating hormone (puregon) in hypogonadotropic azoospermic men who failed to respond to human chorionic gonadotropin alone. Journal of Andrology 2003;4:604‐11. [DOI] [PubMed] [Google Scholar]
Caroppo 2003 {published data only}
- Caroppo E, Niederberger C, Vizziello GM, D'Amato G. Recombinant human follicle‐stimulating hormone as a pretreatment for idiopathic oligoasthenoteratozoospermic patients undergoing intracytoplasmic sperm injection. Fertility and Sterility 2003;80:1398‐403. [DOI] [PubMed] [Google Scholar]
Dirnfeld 2000 {published data only}
- Dirnfeld M, Katz G, Calderon I, Abramovici H, Bider D. Pure follicle‐stimulating hormone as an adjuvant therapy for selected cases in male infertility during in‐vitro fertilization is beneficial. European Journal of Obstetrics and Gynecology and Reproductive Biology 2000;93:105‐8. [DOI] [PubMed] [Google Scholar]
Foresta 1998 {published data only}
- Foresta C, Bettella A, Ferlin A, Garolla A, Rossato M. Evidence for a stimulatory role of follicle‐stimulating hormone on the spermatogonial population in adult males. Fertility and Sterility 1998;69:636‐42. [DOI] [PubMed] [Google Scholar]
Foresta 2002 {published data only}
- Foresta C, Bettella A, Merico M, Garolla A, Ferlin A, Rossato M. Use of recombinant human follicle‐stimulating hormone in the treatment of male factor infertility. Fertility and Sterility 2002;77:238‐44. [DOI] [PubMed] [Google Scholar]
Iacono 1996 {published data only}
- Iacono F, Barra S, Montano L, Lotti T. Value of high‐dose pure FSH in the treatment of idiopathic male infertility. Journal of Urology (Paris) 1996;102:81‐4. [PubMed] [Google Scholar]
Thomalla‐Sauter 2001 {published data only}
- Thomalla‐Sauter B, Denschlag D, Henze C, Bergmann M, Keck C. Recombinant follicle‐stimulating hormone for treatment of idiopathic male infertility [Behandlung der idiopathischen m?nnlichen Infertilit?t mit rekombinantem FSH: Ergebnisse einer kontrollierten Studie]. Geburtshilfe und Frauenheilkunde 2001;61:127‐32. [Google Scholar]
Additional references
Acosta 1991
- Acosta AA, Oehninger S, Ertunc H, Philput C. Possible role of pure human follicle‐stimulating hormone in the treatment of severe male factor infertility by assisted reproduction: preliminary report. Fertility and Sterility 1991;55:1150‐6. [DOI] [PubMed] [Google Scholar]
Acosta 1992
- Acosta AA, Khalifa E, Oehninger S. Pure human follicle stimulating hormone has a role in the treatment of severe male infertility by assisted reproduction: Norfolk's total experience. Human Reproduction 1992;7:1067‐72. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. www.cochrane‐handbook.org. The Cochrane Collaboration, 2011. [Google Scholar]
Liu 2002
- Liu DY, Baker HW. Evaluation and assessment of semen for IVF/ICSI. Asian Journal of Andrology 2002;4:281‐5. [PubMed] [Google Scholar]
Mecham 1996
- Meacham RB, Lipshultz LI, Howards SS. Male infertility. In: Gillenwater JY, Grayhack JT, Howards SS, Duckett JW editor(s). Adult and Pediatric Urology. St Louis: Mosby‐Year Book Inc, 1996:1747‐802. [Google Scholar]
Moudgal 1992
- Moudgal NR, Ravindranath N, Murthy GS, Dighe RR, Aravindan GR, Martin F. Long‐term contraceptive efficacy of vaccine of ovine follicle‐stimulating hormone in male bonnet monkeys (Macaca radiata). Journal of the Society of Reproduction and Fertility 1992;96:91‐102. [DOI] [PubMed] [Google Scholar]
Moudgal 1997
- Moudgal NR, Murthy GS, Prasanna Kumar KM, Martin F, Suresh R, Medhamurthy R, et al. Responsiveness of human male volunteers to immunization with ovine follicle stimulating hormone vaccine: results of a pilot study. Human Reproduction 1997;12:457‐63. [DOI] [PubMed] [Google Scholar]
WHO 1987
- World Health Organization. WHO: Towards more objectivity in diagnosis and management of male infertility. Results of a World Health Organization multi‐centre study. International Journal of Andrology 1987;(Suppl 7)10:1‐35. [Google Scholar]
WHO 1999
- World Health Organization. WHO Laboratory Manual for the Examination of Human Semen and Sperm‐Cervical Mucus Interaction. 4th Edition. Cambridge (UK): Cambridge University Press, 1999:p.62. [Google Scholar]
Wickings 1980
- Wickings EJ, Nieschlag E. Suppression of spermatognesis over two years in rhesus monkeys actively immunized with follicle stimulating hormone. Fertility and Sterility 1980;34:269‐74. [DOI] [PubMed] [Google Scholar]


