Abstract
Successful implantation relies on precisely orchestrated and reciprocal signaling between the implanting blastocyst and the receptive uterus. We have examined the role of the Wnt/β-catenin signaling pathway during the process of implantation and demonstrate that this pathway is activated during two distinct stages. Wnt/β-catenin signaling is first transiently activated in circular smooth muscle forming a banding pattern of activity within the uterus on early day 4. Subsequently, activation is restricted to the luminal epithelium at the prospective site of implantation. Activation at both sites requires the presence of the blastocyst. Furthermore, inhibition of Wnt/β-catenin signaling interferes with the process of implantation. Our results demonstrate that the Wnt/β-catenin signaling pathway plays a central role in coordinating uterus–embryo interactions required for implantation.
Keywords: blastocyst, uterus
Acrucial event during mammalian embryonic development is the process of implantation, during which the free-living blastocyst attaches to the uterine endometrium. Successful implantation depends on precisely orchestrated and reciprocal signaling between the implanting blastocyst and the receptive uterus (1). For instance, blastocysts can only implant once they have been activated by uterine factor(s) that are regulated by ovarian steroid hormones (2). Conversely, expression of several uterine genes are regulated by a signal(s) emanating from activated blastocysts (3, 4). Multiple signaling pathways have been shown to participate in the implantation process, and several cytokines and growth factors have been shown to be expressed in the uterus at the time of implantation and to play important roles in this process (5). However, there is increasing evidence that members of other families of growth factors implicated in embryogenesis may also participate in the implantation process. It has been demonstrated that members of the hedgehog, bone morphogenetic, and Wnt proteins are expressed in the uterus at the time of implantation (6). However, the precise role of these different growth factor signaling pathways in the implantation process has not been determined. Furthermore, the cell types within the uterus that respond to these growth factors are not known. In this study, we set out to determine the role of the canonical Wnt/β-catenin signaling pathway in the implantation process. We demonstrate that this pathway is activated during two distinct stages before implantation. Signaling is transiently detected in circular smooth muscle forming a banding pattern of activity. Subsequently, activation is restricted to the luminal epithelium at the prospective site of implantation. Activation at both sites requires the presence of the blastocyst. Furthermore, we show that inhibition of Wnt/β-catenin signaling interferes with the process of implantation.
Materials and Methods
Mating and Experimental Manipulation of Transgenic Animals. The generation and characterization of the TCF/Lef-LacZ transgenic mice have been described in ref. 7. Transgenic females were either mated with fertile males or vasectomized males and the day of plug was considered to be day 1. The numbers of transgenic females tested for each experiment is shown in Table 3, which is published as supporting information on the PNAS web site. TCF/Lef-lacZ transgenic females used to determine whether the embryo was required to activate the Wnt pathway in the uterus were ovariectomized from one side only, or the junction between the oviduct and the uterus was tightly ligated with 6-0 silk thread (Ethicon, Somerville, NJ). Animals were left to recover for 2–3 weeks and then mated with fertile males. The uteri were recovered at the indicated time points and processed for β-galactosidase activity. TCF/Lef-lacZ females used for embryo transfer experiments were mated with vasectomized males, and then 5–10 blastocysts were transferred into each uterine horn on the morning of day 4. Uteri were recovered at the times indicated in the Results and Discussion and processed for β-galactosidase activity. TCF/Lef-lacZ females used for artificial decidualization were mated with vasectomized males, and 10 μl of sesame oil was injected into each horn in the morning of day 4. Uteri were recovered from these animals and processed for β-galactosidase activity. TCF/Lef-LacZ transgenic females used for the estrogen dependency study were ovariectomized on the morning of day 4 of pregnancy between 7:00 a.m. and 9:00 a.m. Immediately after surgery, mice received a s.c. injection of only progesterone (2 mg per mouse) or a s.c. injection of progesterone and β-estradiol (50 ng per mouse). Uteri were recovered and processed for β-galactosidase activity.
Injection of Wnts and Secreted Frizzled-Related Proteins (sFRPs). Parental L cells, Wnt5a cells, and Wnt7a cells were generated as follows. The cDNA fragments of Wnt5a and Wnt7a were provided by Jan Kitajewski (University of North Carolina, Chapel Hill) (8). These cDNA fragments were subcloned into the retrovirus vector, pHAN(puro). To prepare ecotropic retrovirus, Phoenix-ECO packaging cells were transfected with retrovirus vectors by using Lipofectamine (Invitrogen). Viral supernatants were harvested 30 h after transfection and used to infect L cells (American Type Culture Collection CRL-2648) in the presence of 8 μg/ml polybrene (Sigma). Infected cells were selected 24 h after infection with 5 μg/ml puromycin (Sigma). Selected Wnt-expressing cells were grown in 100-mm dishes, washed twice with PBS, and lysed in 0.5 ml radioimmunoprecipitation assay buffer (50 mM Tris·HCl, pH 7.5/150 mM NaCl/0.5% Nonidet P-40/0.1% deoxycholate) containing protease inhibitor mixture (Roche). Cell extracts were collected and spun in a microcentrifuge at 15,000 × g for 5 min. Total proteins (5 μg) were separated by 10% SDS/PAGE and transferred to Immobilon-P (Millipore). The membranes were probed with primary antibodies followed by horseradish peroxidase-conjugated secondary antibodies at 1:5,000 (Bio-Rad) and developed by using ECL Plus (Amersham Pharmacia Biosciences). Membranes were exposed to BioMax film (Kodak). The primary antibody used in this work was anti-HA at a 1:5,000 ratio (HA7 clone, Sigma).
Parental L cells, Wnt5a cells, and Wnt7a cells were cultured to 80–90% confluency and the conditioned medium was harvested and concentrated 2- to 3-fold by using a centricon column. Five μl of parental and Wnt-conditioned medium was injected in each uterine horn in the morning of day 4. Uteri were isolated on day 5 and stained for β-galactosidase activity. To coat Cytodex-3 beads (Amersham Pharmacia Biotech) with parental or Wnt-expressing L cells, cells were cultured in the presence of beads according to the manufacturer's specifications. Fully coated beads were collected and washed in PBS, and three to five cell-coated beads were injected in one uterine horn as done for the conditioned medium.
Purified human sFRP1 and mouse sFRP2 were purchased from R & D Systems. Isolated blastocysts along with 500 ng of BSA, sFRP1, or sFRP2 were transferred into pseudopregnant females on day 4, and the uteri were isolated on day 6. Stably transfected sFRP2-expressing ES cells were generated and characterized in Austin Smith's laboratory (Institute for Stem Cell Research, University of Edinburgh, Edinburgh) (9). sFRP2-conditioned medium was collected in the same manner as Wnt-conditioned medium. Embryoid bodies were generated by culturing the ES cells in the absence of leukemia inhibitory factor according to standard procedures. Five to eight embryoid bodies of sizes similar to a blastocyst were transferred in each uterine horn along with 5 μl of conditioned medium.
Detection of β-Galactosidase Activity. Uteri were dissected in PBS, pH 7.3, rinsed in 100 mM sodium phosphate, pH 7.3, and then fixed in 0.2% glutaraldehyde/2 mM MgCl2/5 mM EGTA/100 mM sodium phosphate, pH 7.3, for 10 min at room temperature. Uteri were then washed three times in wash buffer (0.02% Nonidet P-40/0.01%deoxycholate/2 mM MgCl2/100 mM sodium phosphate, pH 7.3) for 15 min each at room temperature. To reveal β-galactosidase activity, uteri were incubated in 1 mg/ml X-gal/5 mM K3Fe(CN)6/5 mM K4Fe(CN)6/0.02% Nonidet P-40/0.01% deoxycholate/2 mM MgCl2/100 mM sodium phosphate, pH 7.3, overnight at 37°C. The uteri were then rinsed with wash buffer and PBS and postfixed overnight in 4% paraformaldehyde in PBS at 4°C.
Preparation of Progesterone and Estrogen. Progesterone (4-pregnen-3, 20-dione) was purchased from Steraloids (Newport, RI) (catalog no. Q2600-000) and was dissolved in sesame oil at a concentration of 20 mg/ml. Each mouse received a s.c. dose of 100 μl (2 mg per mouse). Estrogen [1,3,5(10)-estratriene-3, 17 β-diol] (β-estradiol) was purchased from Sigma (catalog no. E-8875) and was dissolved in sesame oil at a concentration of 500 ng/ml. Each mouse received a s.c. dose of 100 μl (50 ng per mouse).
Results and Discussion
To define the sites of active Wnt/β-catenin signaling in the uterus, we used a TCF/Lef-LacZ transgenic mouse reporter line that faithfully monitors activation of this pathway (7). In the uteri of nonpregnant transgenic females, Wnt/β-catenin signaling activity was found to be constitutively active in the oviducts at all stages of the estrous cycle, but no signaling was detectable in the uterus of nonpregnant females (Fig. 1A). This observation allowed us to determine whether this pathway was activated during the implantation period. Analysis of uteri isolated during the periimplantation period demonstrated a dynamic pattern of signaling activity. Activity was first detected at ≈1500 hours on day 4 in groups of cells that formed a series of bands visible in whole-mount β-galactosidase uterine preparations. The bands of Wnt/β-catenin signaling activity first appeared in the uterine region closest to the ovary (Fig. 1B), and, by late day 4, five to seven bands of activity were evenly distributed along each uterine horn (Fig. 1C). The number of bands present in each uterus was found not to correlate with the number of blastocysts because a greater number of bands were observed compared with the number of embryos present in each uterus. At this stage, activity was restricted to the circular smooth muscle cells of the myometrium on the antimesometrial side of the uterus, although weak activity was sometimes detected on the mesometrial side (Fig. 1 D and E). By the morning of day 5, activity was no longer detected in this region. Recently, the Wnt antagonist sFRP4 was shown to be expressed in this region at this time, which may account for silencing of this pathway (10).
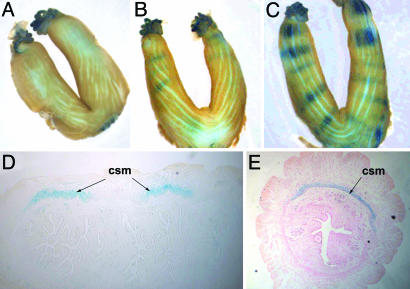
Fig. 1.
The Wnt/β-catenin signaling pathway is activated in the circular smooth muscle cells of the myometrium. (A) Whole-mount β-galactosidase staining of a uterus from a nonpregnant transgenic female. (B) Whole-mount β-galactosidase staining of a uterus from a TCF/Lef-lacZ transgenic female at mid-day 4. (C) Whole-mount β-galactosidase staining of a uterus from a TCF/Lef-lacZ transgenic female on the evening of day 4. (D and E) Sagittal (D) and transverse (E) sections of the uterus in C showing activity in the circular smooth muscle cells (csm).
At 1200 hours on day 5, Wnt/β-catenin signaling became active at specific sites within the uterus (Fig. 2A). At this stage, signaling activity was only detected on the antimesometrial side of the luminal epithelium directly apposed to the implanting blastocyst (Fig. 2 B and C). By 1600 hours, activity had intensified and spread to the mesometrial side (Fig. 2 D and E). Importantly, analysis of serial sections confirmed that endometrial Wnt/β-catenin activity was observed only where a blastocyst was present (Figure 6 A–C, which is published as supporting information on the PNAS web site). Wnt/β-catenin signaling was maintained in the uterine epithelium throughout day 5 but was no longer detected by day 6 (data not shown). Thus, Wnt/β-catenin signaling was transiently activated in the regions of the uterine endometrium directly apposed to the blastocyst at the time of implantation.
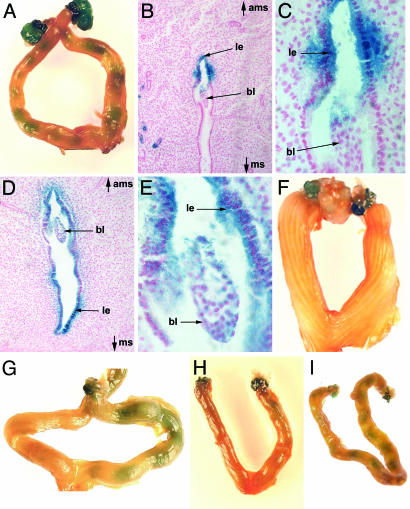
Fig. 2.
Activation of the Wnt/β-catenin signaling pathway in the luminal epithelium requires the presence of the embryo and is estrogen-dependent. (A) Whole-mount β-galactosidase staining of a TCF/Lef-lacZ transgenic uterus at 1200 hours on day 5 showing restricted activity within the uterus. (B) Transverse section from a uterus at 1200 hours on day 5 showing activation of the reporter gene in the luminal epithelium (le) adjacent to the blastocyst (bl) only on the antimesometrial side (ams) of the uterus and not on the mesometrial side (ms). (C) Higher magnification of B (×50). (D) Transverse section from a TCF/Lef-lacZ uterus at 1600 hours on day 5 demonstrating that activity has spread throughout the luminal epithelium by this stage. (E) Higher magnification of D (×200). (F) Whole-mount β-galactosidase staining of a uterus from a TCF/Lef-lacZ transgenic female mated with a vasectomized male. (G) Wholemount β-galactosidase staining of a uterus from a TCF/Lef-lacZ transgenic female where the junction between the oviduct and the uterus was tightly ligated on one side. Speckled staining in uterine horn corresponds to endometrial glands. (H and I) Uterus from a pregnant TCF/Lef-lacZ transgenic female in which both ovaries were removed on the morning of day 4, before the estrogen surge, and injected with progesterone alone (H) or progesterone and estrogen (I).
On the morning of day 5, weak Wnt/β-catenin signaling was also detected in the endometrial glands (Fig. 2B and Fig. 6, speckled staining). However, unlike Wnt/β-catenin signaling activity in the luminal epithelium, which was restricted to the site where the embryo was present, activity in the endometrial glands was detected throughout the uterus. Furthermore, in uteri that contained embryos in only one of the uterine horns, Wnt/β-catenin signaling activity was also present in the endometrial glands of the uterine horn that did not contain embryos (Fig. 2G). However, in the uteri of mice mated with vasectomized males, which contained no embryos, no Wnt/β-catenin signaling activity was detected in the endometrial glands (Fig. 2F). These results demonstrate that activation of Wnt/β-catenin signaling activity in the endometrial glands is not directly activated by the blastocysts but is rather a global response of the uterus because of the presence of embryos.
Because activation of uterine Wnt/β-catenin signaling is only observed where blastocysts were present, we wanted to determine whether activation of this pathway depended on the presence of the embryo. We first mated transgenic females with vasectomized males and examined the uteri of these pseudopregnant females. No activity was detected on either day 4 or day 5 (Fig. 2F and data not shown). Next, we prevented the entry of embryos into one uterine horn by either removing one ovary while keeping the other intact or by tightly ligating the junction between the oviduct and the uterus on one side, and, 2 weeks after surgery, we mated those females with fertile males. Active Wnt/β-catenin signaling was detected only on the side in which the ovary was kept intact or the uterotubal junction were kept intact (Table 3 and Fig. 2G). These results clearly demonstrate that activation of this pathway in the uterus requires the presence of the embryo. To determine whether activation of this pathway in the uterus was a result of a decidualization response, we induced artificial decidualization in pseudopregnant transgenic females by injecting sesame oil into each uterine horn on the morning of day 4. No Wnt/β-catenin signaling activity was detected on the evening of day 4 or throughout day 5, although deciduas were visible by day 6 (Table 3 and data not shown). Thus, activation of the Wnt/β-catenin signaling pathway in the uterus is likely triggered by signals emanating from the embryo before implantation.
In mice, implantation depends on an ovarian estrogen surge that occurs during the morning of day 4 (11, 12). To evaluate whether activation of Wnt/β-catenin signaling was regulated by estrogen, we removed both ovaries from pregnant transgenic females before the onset of the estrogen surge and administered animals with either progesterone alone or a mix of progesterone and estrogen. After this treatment, activation of this pathway was not detected in the uteri of females that received progesterone alone, whereas normal activation was detected in females that received progesterone and estrogen (Table 3 and Fig. 2 H and I). Thus, estrogen injection, which is required to reactivate dormant blastocysts in utero and the resumption of implantation (11, 12), is also required for blastocysts to trigger Wnt/β-catenin signaling in the uterine endometrium.
We next determined whether, independently of the presence of the blastocyst, Wnt proteins could activate this pathway in the uterus. Two members of the Wnt family of proteins were selected for this study, Wnt5a and Wnt7a. Genes coding for both Wnt molecules are expressed in the blastocyst at the time of implantation (13). Wnt5a is known not to signal through the canonical Wnt/β-catenin pathway, whereas Wnt 7a does signal through this pathway (14–16). Freshly collected conditioned medium from parental L cells or L cells expressing Wnt5a or Wnt7a was injected in the uteri of pseudopregnant transgenic females on the morning of day 4. On day 5, the uteri were isolated and stained for β-galactosidase activity. No Wnt/β-catenin signaling activity was detected in uteri injected with conditioned medium obtained from the parental cells or from the cells expressing Wnt5a (Fig. 3A and data not shown). These results demonstrate that the parental cells do not secrete any molecules capable of activating the Wnt/β-catenin signaling pathway in the uterus. Furthermore, Wnt5a, which has been shown to activate the noncanonical Wnt pathway (17–19) and antagonizes the canonical Wnt/β-catenin activity in Xenopus embryos and mammalian cells (17–19), was incapable of activating the Wnt/β-catenin signaling pathway in the uterus (Fig. 3A). In contrast, robust activation of the Wnt/β-catenin signaling pathway was observed when Wnt7a-conditioned medium was injected in one or both uterine horns (Fig. 3 B and C). Furthermore, activation of Wnt/β-catenin signaling was detected throughout the uterine horns, demonstrating that the uterus is capable of activating this pathway along its entire length and not just in spatially restricted domains. These results demonstrate that Wnt proteins known to activate the canonical Wnt pathway can selectively activate Wnt/β-catenin signaling in the uterus.
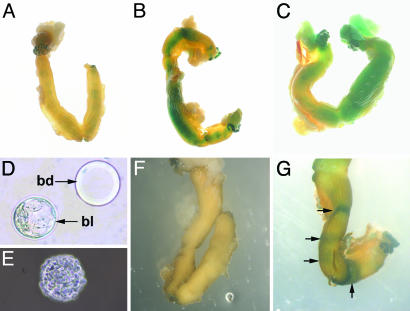
Fig. 3.
Wnt proteins known to activate the canonical Wnt pathway can selectively activate Wnt/β-catenin signaling in the uterus. (A) Whole-mount β-galactosidase staining of a TCF/Lef-lacZ transgenic uterus injected with Wnt5a-conditioned media in both uterine horns. (B) Whole-mount β-galactosidase staining of a TCF/Lef-lacZ transgenic uterus injected with Wnt7a-conditioned media in both uterine horns. (C) Whole-mount β-galactosidase staining of a TCF/Lef-lacZ transgenic uterus injected with Wnt7a-conditioned media only in the right uterine horn. (D) A Cytodex-3 bead (bd) is approximately the same size as a wild-type blastocyst (bl). (E) A Cytodex-3 bead coated with Wnt7a-expressing cells. (F) Whole-mount β-galactosidase staining of a TCF/Lef-lacZ transgenic uterus injected with Wnt5a-coated Cytodex-3 beads. (G) Whole-mount β-galactosidase staining of a TCF/Lef-lacZ transgenic uterus injected with Wnt7a-coated Cytodex-3 beads.
We have previously demonstrated that the blastocyst expresses multiple Wnt genes (13), suggesting that Wnt proteins secreted by the blastocyst may be responsible for activating Wnt/β-catenin signaling in the uterus. We therefore tested whether a small group of Wnt-expressing cells of the size of a blastocyst were capable of activating Wnt/β-catenin signaling in the uterus. To achieve this, Cytodex-3 beads of sizes similar to the blastocyst (Fig. 3D) were coated with either Wnt5a- or Wnt7a-expressing cells (Fig. 3E), and three to five cell-coated beads were injected in each uterine horn of pseudopregnant transgenic females on the morning of day 4. On day 5, the uteri were isolated and stained for β-galactosidase activity. No Wnt/β-catenin signaling activity was detected in uteri injected with beads coated with Wnt5a-expressing cells (Fig. 3F). In contrast, in the uteri injected with beads coated with Wnt7a-expressing cells, Wnt/β-catenin signaling activity was detected in discrete bands along the uterine horn (Fig. 3G), which mimics the banding pattern observed in naturally mated females (Fig. 2 A). These results demonstrate that a small group of cells expressing Wnt7a is sufficient to activate Wnt/β-catenin signaling activity in discrete bands along the uterus, supporting the possibility that Wnts secreted by the blastocyst activate Wnt/β-catenin signaling in the endometrium.
Having demonstrated that Wnts were sufficient to activate signaling in the uterus, we next determined whether activation of the Wnt/β-catenin signaling pathway before implantation was required for successful implantation. To achieve this, we took advantage of the ability of sFRP proteins to physically interact with Wnt proteins and thereby prevent the activation of the Wnt/β-catenin signaling pathway (20, 21). Eight to 10 blastocysts were transferred along with BSA, purified sFRP1 protein, or purified sFRP2 protein in each uterine horn of wild-type pseudopregnant females. Embryonic development was allowed to proceed until day 6, when recipient females were killed and the ability of the transferred blastocysts to implant was assessed (Table 1). Transfer of blastocysts with BSA or sFRP1 had no significant effect on embryo implantation (Table 1 and Fig. 4 A and B). However, transfer of blastocysts along with purified sFRP2 resulted in a significant decrease in the frequency of implantation (35%) (Table 1 and Fig. 4C). Furthermore, although embryos did implant and formed deciduas, sites of implantation were always located close to the junction of the two uterine horns and no implantation occurred in the more proximal region of the uterus as observed with blastocysts incubated with either BSA or sFRP1. Because the embryos and sFRP2 were transferred in the proximal region of the uterine horn and that implantation occurred only in the distal region raises the possibility that these embryos may have implanted in regions where sFRP2 protein concentrations were low.
Table 1. sFRP2 prevents implantation of blastocytes transferred to pseudopregnant females.
| Sample injected | Embryos transfered, n | Embryos implanted, n | Implantation, % |
|---|---|---|---|
| BSA + embryos | 40 | 32 | 80 |
| sFRP1 + embryos | 40 | 27 | 67.5 |
| sFRP2 + embryos | 40 | 14 | 35* |
Fisher's exact test, P = 0.015
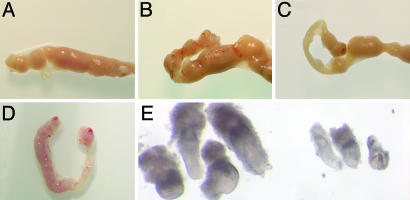
Fig. 4.
Activation of the Wnt/β-catenin signaling pathway in the uterus is required for proper blastocyst implantation. (A) Uterine horn on day 6 in which blastocysts along with BSA had been transferred on day 4. (B) Uterine horn on day 6 in which blastocysts along with sFRP1 had been transferred on day 4. (C) Uterine horn on day 6 in which blastocysts along with sFRP2 had been transferred on day 4. (D) Uterus on day 6 in which sFRP2-conditioned media along with embryoid bodies expressing sFRP2 had been injected in the right uterine horn on the morning of day 4. The left horn was not injected and served as a control. (E) Embryos dissected from a uterine horn that had been injected with sFRP2-conditioned media along with embryoid bodies expressing sFRP2 (Right) and embryos from the same uterus from the control uterine horn (Left).
We next tested whether sFRP2 could inhibit normal implantation of blastocysts in naturally mated wild-type females. Conditioned media from wild-type ES cells or ES cells overexpressing sFRP2 were collected. In addition, to favor continued expression of sFRP2 in the uterus, embryoid bodies were generated from both ES cell lines. Conditioned medium and embryoid bodies were injected together into one uterine horn of naturally mated wild-type females on the morning of day 4. The second uterine horn was left intact as a control. Injection of four to five wild-type embryoid bodies along with conditioned medium from wild-type ES cells had no effect on implantation, because a similar number of implanted embryos were observed as compared with control (Table 2). In contrast, injection of four to five embryoid bodies overexpressing sFRP2 along with sFRP2-conditioned medium resulted in a 57% decrease in the implantation rate (Table 2). Although embryos were found in each decidua, the embryos that had implanted on the treated side consistently showed a developmental delay of ≈1 day as compared with embryos implanted on the control untreated side (Fig. 4E). Interestingly, injection of sFRP2 on the evening of day 4, when activation of this pathway had already occurred, had no effect on blastocyst implantation and embryonic development (data not shown). This result suggests that the growth retardation observed in embryos treated with sFRP2 on the morning of day 4 is likely due to delayed implantation of these embryos rather than a growth retardation effect caused by sFRP2.
Table 2. Addition of sFRP2 in the uteri of wild-type pregnant females inhibits implantation.
| Embryos, n
|
||||
|---|---|---|---|---|
| Sample injected | Uteri, n | Control | Exp. | Implantation, % |
| Conditioned media | ||||
| Parental cell + WT EB | 4 | 27 | 24 | 89 |
| sFRP2-conditioned media + sFRP2 | 4 | 28 | 12 | 43* |
EB, embyroid bodies; Exp., experimental.
Fisher's exact test, P = 0.04
We have demonstrated that the Wnt/β-catenin signaling pathway is active in two distinct regions of the uterus during the periimplantation period. Wnt/β-catenin signaling is first transiently activated in circular smooth muscle forming a banding pattern of activity within the uterus on early day 4. Subsequently, activation is restricted to the luminal epithelium at the prospective site of implantation. We have shown that the embryo is required for activation of Wnt/β-catenin signaling in the luminal epithelium, demonstrating that this pathway is active in embryo–uterus communication. Finally, we have shown that inhibiting Wnt signaling at the time of implantation severely reduces the rate of embryo implantation, demonstrating an essential role of the Wnt pathway in the process of implantation.
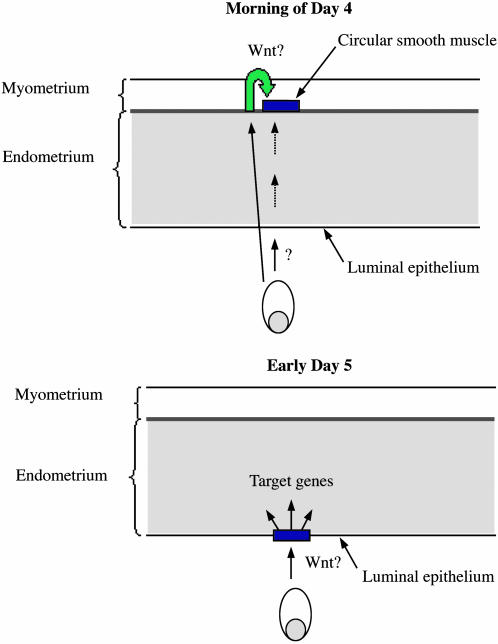
Our results are consistent with the model shown in Fig. 5. On the morning of day 4, shortly after the estrogen surge, the blastocyst emits a signal that results in the activation of the Wnt/β-catenin signaling pathway in discrete groups of cells within the circular smooth muscle that form bands along the uterus. Because of the substantial distance between the location of the embryo and the domain of activation of the pathway, it is unlikely that this activation is mediated by Wnts secreted by the blastocyst. A more likely explanation would be that the blastocyst emits a signal(s) that directly or indirectly activates the Wnt/β-catenin signaling pathway in circular smooth muscle cells. These results demonstrate that the uterus exhibits regionalization along it proximodistal axis before implantation and, more intriguingly, that activation of the Wnt/β-catenin pathway occurs at regularly spaced intervals along the uterus. The function of Wnt/β-catenin signaling in these cells is currently not known. Because circular smooth muscle cells have been suggested to play a role in proper spacing of the embryos (22) and because activity occurs at regularly spaced intervals, it is possible that Wnt/β-catenin signaling in these cells may be involved in regulating embryo spacing.
Fig. 5.
Model for blastocyst-induced Wnt/β-catenin signaling in the uterus. On the morning of day 4, the blastocyst emits a signal that results either directly or indirectly in the activation of the Wnt/β-catenin signaling pathway in discrete groups of cells within the circular smooth muscle, which form bands along the uterus. On late day 4 to early day 5, the blastocyst activates the Wnt/β-catenin signaling pathway in the luminal epithelium of the uterus. Activation of the Wnt/β-catenin signaling pathway results in the modulation of target genes in the uterus, which facilitates implantation.
On late day 4 to early day 5, the blastocyst activates the Wnt/β-catenin signaling pathway in the luminal epithelium of the uterus. Activation first occurs on the antimesometrial side of the uterus, where the embryo will implant, and is later detected throughout the luminal epithelium. By late day 5, Wnt/β-catenin signaling is no longer active in the uterus. Whether Wnt proteins secreted by the blastocyst or other blastocyst-secreted factors are responsible for this activation is not known. However, several lines of evidence suggest that blastocyst-secreted Wnt proteins may be involved. We have previously shown that the blastocyst expresses multiple Wnt genes before implantation (13). Furthermore, beads coated with Wnt-expressing cells are sufficient to activate Wnt/β-catenin signaling in a fashion similar to the blastocyst, and injection of sFRP2 inhibits activation of this pathway in the uterus. Taken together, these findings strongly suggest that Wnt secreted by the blastocyst activates the Wnt/β-catenin signaling pathway in the luminal epithelium of the uterus. Interestingly, none of the individual Wnt gene mutations have resulted in preimplantation lethality because of implantation failure. Because the blastocyst has been shown to express multiple Wnt genes at the time of embryo implantation (13), it is possible that several of these Wnt genes may have redundant functions and are able to activate the Wnt/β-catenin signaling pathway in the luminal epithelium of the uterus. Thus, the inactivation of multiple Wnt genes expressed by the blastocyst would be required to affect implantation. Although our results support the idea that the blastocyst-secreted Wnt proteins directly activate Wnt/β-catenin signaling, we cannot exclude the possibility that the blastocyst secretes a factor that activates the expression of Wnt genes in the luminal epithelium, which, in an autocrine fashion, activates signaling.
Inhibiting Wnt/β-catenin signaling in the uterus by the addition of sFRP2 significantly decreases implantation, demonstrating the importance of this signaling pathway during this process. That Wnt/β-catenin signaling is only active on days 4 and 5 and is not active during the decidualization process suggests that it plays a role early during implantation. Activation of the Wnt/β-catenin signaling pathway in the luminal epithelium most probably results in modulating the expression of target genes, which allows successful implantation to take place. Indeed, the expression of several genes, such as HB-EGF and COX-2, have been shown to be activated in the endometrium by the presence of the blastocyst (5). The signaling pathway as well as the mechanism of activation of these genes are still unknown. The Wnt/β-catenin signaling is an excellent candidate pathway to be involved in the activation of some of these genes. Supporting this view, it has been demonstrated that Wnt/β-catenin is involved in the activation of the COX-2 promoter (8).
Although embryo–uterus cross-talk is known to play an essential role in facilitating implantation, few signaling pathways specifically activated in the uterus in response to signals secreted by the blastocyst have previously been identified. Our results demonstrate that the blastocyst activates Wnt-dependent β-catenin signaling in the uterine endometrium and that activation of this pathway is required for implantation, strongly suggesting that this paracrine signaling mechanism plays a central role in coordinating uterus–embryo interactions required for implantation.
Supplementary Material
Acknowledgments
We thank Drs. Tom Kennedy and Makoto Nagano for critical reading of the manuscript, Dr. Riaz Farookhi for helpful suggestions with experiments, and Dr. Austin Smith for providing sFRP2-expressing ES cells. O.A.M. was supported by a scholarship from the Libyan Ministry of Higher Education. This work was supported by the Canadian Institut of Health Research. D.D. is a Chercheur Boursier du Fonds de la Recherche en Sante du Quebec.
Author contributions: D.D. designed research; O.A.M., M.J., and C.L.-D. performed research; K.K. contributed new reagents/analytic tools; H.J.C. and D.D. analyzed data; and D.D. wrote the paper.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviation: sFRP, secreted frizzled-related protein.
See Commentary on page 8397.
References
- 1.Paria, B. C., Reese, J., Das, S. K. & Dey, S. K. (2002) Science 296, 2185-2188. [DOI] [PubMed] [Google Scholar]
- 2.Ma, W. G., Song, H., Das, S. K., Paria, B. C. & Dey, S. K. (2003) Proc. Natl. Acad. Sci. USA 100, 2963-2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Das, S. K., Wang, X. N., Paria, B. C., Damm, D., Abraham, J. A., Klagsbrun, M., Andrews, G. K. & Dey, S. K. (1994) Development (Cambridge, U.K.) 120, 1071-1083. [DOI] [PubMed] [Google Scholar]
- 4.Das, S. K., Das, N., Wang, J., Lim, H., Schryver, B., Plowman, G. D. & Dey, S. K. (1997) Dev. Biol. 190, 178-190. [DOI] [PubMed] [Google Scholar]
- 5.Dey, S. K., Lim, H., Das, S. K., Reese, J., Paria, B. C., Daikoku, T. & Wang, H. (2004) Endocr. Rev. 25, 341-373. [DOI] [PubMed] [Google Scholar]
- 6.Paria, B. C., Ma, W., Tan, J., Raja, S., Das, S. K., Dey, S. K. & Hogan, B. L. (2001) Proc. Natl. Acad. Sci. USA 98, 1047-1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mohamed, O., Clarke, H. J. & Dufort, D. (2004) Dev. Dyn. 231, 416-424. [DOI] [PubMed] [Google Scholar]
- 8.Araki, Y., Okamura, S., Hussain, S. P., Nagashima, M., He, P., Shiseki, M., Miura, K. & Harris, C. C. (2003) Cancer Res. 63, 728-734. [PubMed] [Google Scholar]
- 9.Shimizu, H., Julius, M. A., Giarre, M., Zheng, Z., Brown, A. M. & Kitajewski, J. (1997) Cell Growth Differ. 12, 1349-1358. [PubMed] [Google Scholar]
- 10.Daikoku, T., Song, H., Guo, Y., Riesewijk, A., Mosselman, S., Das, S. K. & Dey, S. K. (2004) Mol. Endocrinol. 18, 1238-1250. [DOI] [PubMed] [Google Scholar]
- 11.McCormack J. T. & Greenwald, G. S. (1974) J. Reprod. Fertil. 41, 297-301. [DOI] [PubMed] [Google Scholar]
- 12.Yoshinaga, K. & Adams, C. E. (1966) J. Reprod. Fertil. 12, 593-595. [DOI] [PubMed] [Google Scholar]
- 13.Mohamed, O. A., Dufort, D. & Clarke, H. J. (2004) Biol. Reprod. 71, 417-424. [DOI] [PubMed] [Google Scholar]
- 14.Sheldahl, L. C., Park, M., Malbon, C. C. & Moon, R. T. (1999) Curr. Biol. 9, 695-698. [DOI] [PubMed] [Google Scholar]
- 15.Kuhl, M., Sheldahl, L. C., Park, M., Miller, J. R. & Moon, R. T. (2000) Trends Genet. 16, 279-283. [DOI] [PubMed] [Google Scholar]
- 16.Caricasole, A., Ferraro, T., Iacovelli, L., Barletta, E., Caruso, A., Melchiorri, D., Terstappen, G. C. & Nicoletti, F. (2003) J. Biol. Chem. 278, 37024-37031. [DOI] [PubMed] [Google Scholar]
- 17.Torres, M. A., Yang-Snyder, J. A., Purcell, S. M., DeMarais, A. A., McGrew, L. L. & Moon, R. T. (1996) J. Cell Biol. 133, 1123-1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ishitani, T., Kishida, S., Hyodo-Miura, J., Ueno, N., Yasuda, J., Waterman, M., Shibuya, H., Moon, R. T., Ninomiya-Tsuji, J. & Matsumoto, K. (2003) Mol. Cell. Biol. 23, 131-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Topol, L., Jiang, X., Choi, H., Garrett-Beal, L., Carolan, P. J. & Yang, Y. (2003) J. Cell Biol. 162, 899-908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jones, S. E. & Jomary, C. (2002) BioEssays 24, 811-820. [DOI] [PubMed] [Google Scholar]
- 21.Kawano, Y. & Kypta, R. (2003) J. Cell Sci. 116, 2627-2634. [DOI] [PubMed] [Google Scholar]
- 22.Crane, L. H. & Martin, L. (1991) Reprod. Fertil. Dev. 3, 233-244. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.