Abstract
We have investigated the effects of Nef on infectivity in the context of various viral envelope proteins. These experiments were performed with a minimal vector system where Nef is the only accessory protein present. Our results support the hypothesis that the route of entry influences the ability of Nef to enhance human immunodeficiency virus (HIV) infectivity. We show that HIV particles pseudotyped with Ebola virus glycoprotein or vesicular stomatitis virus glycoprotein (VSV-G), which fuse at low pH, do not require Nef for optimal infectivity. In contrast, Nef significantly enhances the infectivity of virus particles that contain envelope proteins that fuse at neutral pH (CCR5-dependent HIV Env, CXCR4-dependent HIV Env, or amphotropic murine leukemia virus Env). In addition, our results demonstrate that virus particles containing mixed CXCR4-dependent HIV and VSV-G envelope proteins show a conditional requirement for Nef for optimal infectivity, depending on which protein is allowed to facilitate entry.
Human immunodeficiency virus (HIV) particles produced in the presence of Nef are more infectious than particles produced in the absence of this protein (9, 23, 24). While this infectivity enhancement effect is well documented, the details of the mechanism underlying this effect remain obscure. However, it is believed that Nef influences the early stages of the infection process since, compared to wild-type virus, virus lacking Nef is inefficient at synthesizing proviral DNA (1, 2, 8, 30).
In single-round infection assays, it has also been shown that providing Nef in trans to cells producing virus restores optimal infectivity, while providing Nef in trans to target cells has no effect (2, 25, 27). These findings suggest either that Nef enhances particle infectivity by altering the virus particle during production or that Nef is incorporated into the assembling virus particle, enabling it to act during the early stages of infection. Intriguingly, Nef has been found in HIV particles, where it appears to be associated with the viral core, but there is little evidence directly correlating the presence of Nef in virus particles with any phenotypic effects in target cells (5, 16, 27, 34).
In addition, it has been reported that HIV particles containing CXCR4-dependent (X4) HIV or amphotropic murine leukemia virus (ampho MLV) envelope proteins require Nef for optimal infectivity, while HIV particles pseudotyped with vesicular stomatitis virus (VSV) glycoprotein (VSV-G) do not (1, 19). These findings correlate with the facts that virus particles containing X4 HIV and ampho MLV envelope proteins fuse at the cell surface at neutral pH (3, 22, 32), while particles pseudotyped with VSV-G use the endocytic low pH route (37). Based on this information, it has been suggested that the route of viral entry may influence the ability of Nef to enhance infectivity (1, 19). Alternatively, VSV-G may induce some modification in the particle that substitutes for Nef function.
To distinguish between these possibilities, we wanted to test the effect that Nef had on the infectivity of HIV vectors pseudotyped with envelope proteins that had not been previously tested. To do this, we used the previously described HIV-based packaging cell line B4.14, along with the vector pTR167. B4.14 packaging cells are CMT3 COS cells (13) that stably express Gag, Gag-Pol, and Rev in the absence of the other viral accessory proteins (Vif, Vpr, Vpu, and Nef) (31). pTR167 is a derivative of pNL4–3 (HIV type 1 [HIV-1] strain NL43 [GenBank accession number M19921]) and is described in detail elsewhere (29). Its primary feature is a hygromycin resistance gene that is under the control of an internal simian virus 40 early promoter and that is capable of conferring hygromycin resistance to target cells. In addition, this vector expresses the HIV-1 Nef protein from a spliced mRNA initiated from the viral long terminal repeat. For these studies, we also produced a Nef− version of this vector (pTR167Nef−) by creating a frameshift in the Nef coding region at the internal XhoI site.
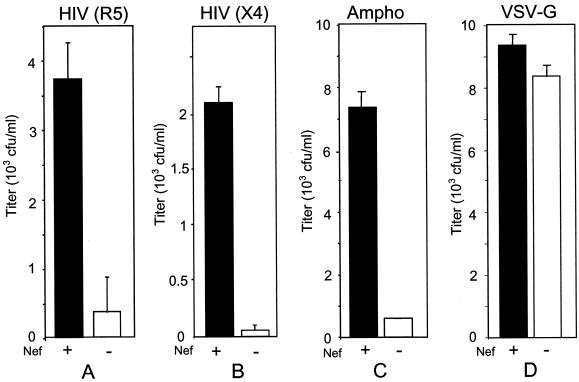
The first envelope protein to be tested in this system was a CCR5-dependent (R5) envelope protein from HIV-1JR-CSF (GenBank accession number M38429). The ability of Nef to enhance the infectivity of particles pseudotyped with this envelope protein was compared to that observed with X4 HIV Env, ampho MLV Env, and VSV-G. Although several reports have demonstrated that Nef enhances the infectivity of HIV particles that contain an envelope protein that utilizes CXCR4 as a coreceptor (12), there have been no reports to show that this is also true when entry is mediated by an envelope protein that uses CCR5 (10). To investigate this notion, Nef+ and Nef− pTR167 vectors were transfected into packaging cells together with plasmids expressing the various envelope proteins, and the infectivity of the different viral stocks was determined with HeLa-CD4 cells that had been stably transfected with CCR5. Nef-mediated enhancement of infectivity was clearly observed with R5 HIV Env (Fig. 1A), and this enhancement was similar to that observed with X4 HIV Env (Fig. 1B) or ampho MLV envelope protein (Fig. 1C). In contrast, this enhancement was not seen when the vectors were pseudotyped with VSV-G (Fig. 1D).
FIG. 1.
Effect of Nef on infectivity determined with viruses pseudotyped with various envelope proteins. Nef+ and Nef− viruses were produced by transfecting B4.14 packaging cells (31) with an envelope construct, pCMVtat, and either pTR167 (black columns) or pTR167Nef− (white columns). HIVJR-CSF and HIVHXB2 envelope proteins were expressed from plasmids derived from pCMV (pHR1374 and pHR1904) (18, 31); ampho MLV Env was expressed from pSV-A-MLV (pHR1794) (17); and VSV-G was expressed from pMD-G (pHR2004) (26). Virus containing supernatant was collected 3 days after transfection and cleared of cellular debris. The presence or absence of Nef had no effect on p24 production, as measured by a p24 enzyme-linked immunosorbent assay. One milliliter of supernatant was used to infect either HeLa-CD4-CCR5 (A) or HeLa-CD4 (B, C, and D) cells. Titers were determined by counting the number of hygromycin-resistant colonies after 2 weeks of selection. Particles were pseudotyped with HIVJR-CSF (A), HIVHXB2 (B), ampho MLV (C), or VSV-G (D) envelope proteins. Each experiment was performed in duplicate; error bars indicate standard deviations. HeLa-CD4-CCR5 cells were derived from HeLa-CD4 cells (6) by cotransfection of a pCDM8-derived plasmid (Invitrogen) expressing CCR5 (pHR1911) and a plasmid expressing puromycin resistance, pPuro (Clontech) (pHR1375).
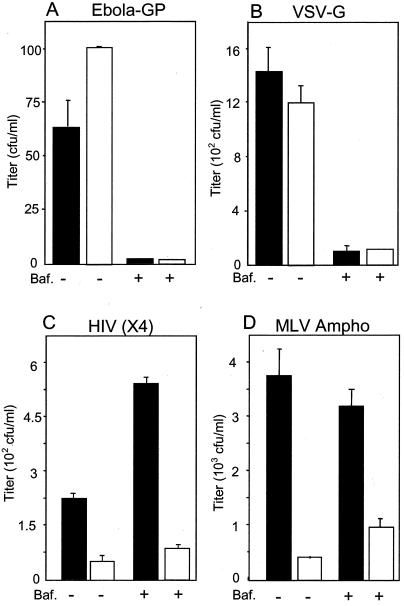
A common feature of the three different envelope proteins that were shown to require Nef for optimal infectivity is that they all fuse at the cell surface at neutral pH. In contrast, Nef did not increase the infectivity of virus particles pseudotyped with VSV-G, confirming previous results. Fusion facilitated by VSV-G requires the low pH of the endosome (21). To further test the hypothesis that fusion at low pH abrogates Nef enhancement, we produced Nef+ and Nef− HIV vectors that were pseudotyped with Ebola virus glycoprotein (Ebola-GP), which has recently been suggested to facilitate fusion through a low-pH-dependent mechanism (7, 35). When the infectivity of these samples was determined, no Nef enhancement phenotype was observed (Fig. 2A).
FIG. 2.
Effect of Nef and bafilomycin A1 on infectivity of HIV vectors pseudotyped with various envelope proteins. Nef+ and Nef− viruses were produced by transfecting B4.14 packaging cells with an envelope construct, pCMVtat, and either pTR167 (black columns) or pTR167Nef− (white columns). Ebola-GP was expressed from pVR1012-GP(Z) (pHR2186) (36, 38). Other envelope proteins were expressed from the vectors described in the legend to Fig. 1. Ebola-GP (A), VSV-G (B), HIVHXB2 (C), and ampho MLV(D) pseudotyped viruses were used to infect HeLa-CD4 cells as described in the legend to Fig. 1, with or without the addition of 10 mM bafilomycin A1 (Baf.). After 4 h of incubation, the cells were washed twice with phosphate-buffered saline and 5 ml of medium (Iscove's medium containing 10% bovine calf serum) was added. Hygromycin selection was started 24 h later and continued for 2 weeks, at which time the number of resistant colonies was determined. Each experiment was performed in duplicate; error bars show standard deviations.
It is also interesting to note that while pseudotyping with VSV-G increased the absolute infectivity (more CFU per milliliter) of the vector particles relative to particles with X4 HIV Env, Ebola-GP actually decreased it. Thus, the data clearly rule out the possibility that the loss of Nef-mediated infectivity enhancement with these pseudotyped viruses is simply a consequence of a general increase in infectivity that overwhelms any potential role of Nef.
To ensure that the Ebola-GP and VSV-G pseudotyped viruses entered through a low-pH-dependent mechanism in these assays and that the particles containing HIV X4 Env and ampho MLV Env utilized a neutral-pH fusion mechanism, we tested the infectivity of these viruses in the presence of the drug bafilomycin A1 (Fig. 2). Bafilomycin A1 prevents the acidification of endosomes by blocking the function of the vacuolar H+ ATPase (4, 33, 39), allowing fusion only through a pH-independent mechanism. Figure 2 shows that both Ebola-GP and VSV-G pseudotyped viral stocks demonstrated a dramatic loss in infectivity (Fig. 2A and B) when bafilomycin A1 was present during the early stages of infection. In contrast, the virus containing HIV X4 Env or ampho MLV Env showed no decrease in infectivity in the presence of the drug (Fig. 2C and D). This was true for both Nef+ and Nef− vectors. Thus, these results showed a perfect correlation between bafilomycin A1 sensitivity (low pH fusion) and the abrogation of Nef enhancement.
It is interesting that bafilomycin A1 treatment consistently caused an approximately twofold increase in the infectivity of particles containing HIV Env. One explanation for this result could be that a fraction of the HIV Env-containing particles normally start to enter cells by the endocytic route but fail to survive the subsequent acidification. Treatment with bafilomycin A1 would prevent acidification and therefore could cause this usually nonproductive route of entry to become productive. Consistent with this notion are reports in the literature describing endocytic entry for HIV in specific instances (11, 14, 28).
It seems reasonable to hypothesize that entry through the endocytic route somehow compensates for Nef function, since Nef enhancement of infectivity is lost when viruses are pseudotyped with either VSV-G or Ebola-GP. However, a second possibility is that pseudotyping with VSV-G or Ebola-GP modifies the particle differently than when the other envelope proteins are used. This modification could somehow prevent or replace Nef function. To address this issue, we created particles that contained both VSV-G and HIV envelope proteins and determined if there was a Nef effect when VSV-G-dependent entry was inhibited.
HIV-1 particles bearing both HIV-1 Env and VSV-G were produced in 293T cells (106, 6-cm dishes) by cotransfection of wild-type (R9) or Nef-defective (R9ΔNef) proviruses (10 μg) and the VSV-G expression plasmid pHCMV-G (40 ng) in a biosafety level 3 laboratory using stringent precautions (1, 2). Two days after transfection, supernatants were harvested, passed through 0.45-μm-pore-size cellulose acetate filters to remove cellular debris, assayed for p24 content, and frozen at −80°C. After thawing, HIV-1 infectious titers were determined by titration on HeLa/LTR-lacZ or HeLa-CD4/LTR-lacZ(P4–2) cells as previously described (15). For comparison, Nef+ and Nef− viral stocks prepared without the addition of VSV-G were also produced and the titers were determined.
When measured in cells containing CD4, the presence of Nef resulted in only a 2-fold higher titer for the particles with mixed envelope proteins, compared to a 13-fold enhancement for the particles made without VSV-G (Table 1). In cells lacking CD4, virtually no Nef enhancement was seen in the particles with mixed envelope proteins and, as expected, the particles made without VSV-G did not infect these cells at all (Table 1). These results indicated that the presence of VSV-G in particles abrogated the effect of Nef on infectivity, even when HIV-1 Env was also present. This result may have occurred because the VSV-G entry pathway was dominant over the HIV-1 entry pathway or because VSV-G modified the particle in some way to make it no longer responsive to Nef.
TABLE 1.
Effect of VSV neutralization on infectivity of HIV-1 particles bearing both HIV-1 and VSV envelope glycoproteins
| Virus | No. of infectious units per ng of p24 inoculum for the following cellsa:
|
|||
|---|---|---|---|---|
| CD4+, no antiserum | CD4−, no antiserum | CD4+, antiserum | CD4−, antiserum | |
| HIV-1 Nef+ plus VSV | 1,754 ± 105 | 241 ± 6 | 213 ± 12 | 1 ± 1 |
| HIV-1 Nef− plus VSV | 870 ± 85 | 181 ± 17 | 9 ± 2 | 1 ± 1 |
| HIV-1 Nef+ | 132 ± 8 | 0 | 135 ± 9 | ND |
| HIV-1 Nef− | 10 ± 3 | 0 | 11 ± 3 | ND |
Antiserum, pretreatment of virus with VSV-neutralizing antiserum; no antiserum, lack of such treatment. Values are the mean and one standard deviation from triplicate infections. ND, not determined.
To distinguish between these possibilities, we reasoned that particles containing mixed envelope proteins should not be able to utilize the VSV-G entry pathway if VSV-G function were completely blocked with an anti-VSV antibody. However, there should be some residual infectivity after this treatment, due to infectivity mediated exclusively by the HIV envelope protein. If the entry pathway and not particle modification affects Nef enhancement, there should be a more significant difference in titer between virus made in the presence and virus made in the absence of Nef after treatment with the anti-VSV antibody.
For antibody inhibition studies, viral stocks were preincubated with a 1:50 dilution of rabbit anti-VSV neutralizing serum (purchased from Lee Biomedical Research Laboratories) for 30 min at room temperature prior to infection of the reporter cells. As expected, treatment of the mixed particle stocks with anti-VSV neutralizing antibody reduced the titer to near zero on cells lacking CD4 (Table 1), while a small fraction of infectivity remained resistant to neutralization when tested on CD4+ cells (Table 1). In this fraction, there was now a dramatic difference in titer between the particles produced in the presence of Nef and those produced in the absence of Nef. A 24-fold difference was observed. These results exclude the possibility that VSV-G modifies the particle in a way that abrogates or substitutes for the Nef enhancement function. Furthermore, these observations support the conclusion that the lack of Nef enhancement in HIV-1 (VSV) particles is due to the specific interactions that take place between VSV-G and its receptor on target cells.
These results, together with our observation that there is a loss of Nef enhancement function with Ebola-GP pseudotypes, strengthen the hypothesis that fusion at the cell surface is required for Nef enhancement of infectivity. In addition, these results suggest that the infection process is different when fusion occurs at the cell surface (neutral pH) than when it occurs within an endosome (low pH).
One possible reason for these differences is that obstacles to infection may be present when infection occurs via fusion at the cell surface that are not present when it occurs in the endosome. The mechanism by which Nef functions to enhance infectivity may involve overcoming such obstacles. For example, following fusion at the cell surface, the viral core may not be in a location that is conducive to the early stages of infection unless it was produced in the presence of Nef. In contrast, fusion with the endosome may release the viral core into a cellular compartment that facilitates these events. This idea is supported by the fact that other viruses (Semliki Forest virus and VSV) are unable to infect CHO cells when forced to enter at the cell surface but retain the ability to infect these cells when allowed to enter through an endocytic route (20).
Further analysis of the mechanism by which Nef enhances infectivity will likely require a thorough investigation of the infection steps that immediately follow fusion, prior to proviral DNA synthesis. Because little is known about these early stages, Nef may provide an important tool for better understanding the HIV infection process.
Acknowledgments
We thank Didier Trono (University of Geneva), Ned Landau (Salk Institute), and Anthony Sanchez (Centers for Disease Control and Prevention, Atlanta, Ga.) for gifts of plasmids; David Camerini (University of Virginia) and Warren Pear (University of Pennsylvania) for cell lines; and Susan Prasad and Joy Niesen-Morgenegg for expert technical assistance.
This work was supported by grants AI38186 and AI44349 to D.R. and AI 40364 to C.A. from the National Institutes of Health. Salary support for D.R. and M.-L.H. was provided by the Myles H. Thaler and Charles Ross, Jr., endowments at the University of Virginia.
REFERENCES
- 1.Aiken C. Pseudotyping human immunodeficiency virus type 1 (HIV-1) by the glycoprotein of vesicular stomatitis virus targets HIV-1 entry to an endocytic pathway and suppresses both the requirement for Nef and the sensitivity to cyclosporin A. J Virol. 1997;71:5871–5877. doi: 10.1128/jvi.71.8.5871-5877.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aiken C, Trono D. Nef stimulates human immunodeficiency virus type 1 proviral DNA synthesis. J Virol. 1995;69:5048–5056. doi: 10.1128/jvi.69.8.5048-5056.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bates P. Chemokine receptors and HIV-1: an attractive pair? Cell. 1996;86:1–3. doi: 10.1016/s0092-8674(00)80070-7. [DOI] [PubMed] [Google Scholar]
- 4.Bowman E J, Siebers A, Altendorf K. Bafilomycins: a class of inhibitors of membrane ATPases from microorganisms, animal cells, and plant cells. Proc Natl Acad Sci USA. 1988;85:7972–7976. doi: 10.1073/pnas.85.21.7972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bukovsky A A, Dorfman T, Weimann A, Gottlinger H G. Nef association with human immunodeficiency virus type 1 virions and cleavage by the viral protease. J Virol. 1997;71:1013–1018. doi: 10.1128/jvi.71.2.1013-1018.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Camerini D, Seed B. A CD4 domain important for HIV-mediated syncytium formation lies outside the virus binding site. Cell. 1990;60:747–754. doi: 10.1016/0092-8674(90)90089-w. [DOI] [PubMed] [Google Scholar]
- 7.Chan S Y, Speck R F, Ma M C, Goldsmith M A. Distinct mechanisms of entry by envelope glycoproteins of Marburg and Ebola (Zaire) viruses. J Virol. 2000;74:4933–4937. doi: 10.1128/jvi.74.10.4933-4937.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chowers Y M, Pandori M W, Spina C A, Richman D D, Guatelli J C. The growth advantage conferred by HIV-1 nef is determined at the level of viral DNA formation and is independent of CD4 downregulation. Virology. 1995;212:451–457. doi: 10.1006/viro.1995.1502. [DOI] [PubMed] [Google Scholar]
- 9.Chowers Y M, Spina C A, Kwoh T J, Fitch N J, Richman D D, Guatelli J C. Optimal infectivity in vitro of human immunodeficiency virus type 1 requires an intact nef gene. J Virol. 1994;68:2906–2914. doi: 10.1128/jvi.68.5.2906-2914.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deng H, Liu R, Ellmeier W, Choe S, Unutmaz D, Burkhart M, Di Marzio P, Marmon S, Sutton R E, Hill C M, Davis C B, Peiper S C, Schall T J, Littman D R, Landau N R. Identification of a major co-receptor for primary isolates of HIV-1. Nature. 1996;381:661–666. doi: 10.1038/381661a0. [DOI] [PubMed] [Google Scholar]
- 11.Fackler O T, Peterlin B M. Endocytic entry of HIV-1. Curr Biol. 2000;10:1005–1008. doi: 10.1016/s0960-9822(00)00654-0. [DOI] [PubMed] [Google Scholar]
- 12.Feng Y, Broder C C, Kennedy P E, Berger E A. HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor. Science. 1996;272:872–877. doi: 10.1126/science.272.5263.872. [DOI] [PubMed] [Google Scholar]
- 13.Gerard R D, Gluzman Y. New host cell system for regulated simian virus 40 DNA replication. Mol Cell Biol. 1985;5:3231–3240. doi: 10.1128/mcb.5.11.3231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grewe C, Beck A, Gelderblom H R. HIV: early virus-cell interactions. J Acquir Immune Defic Syndr. 1990;3:965–974. [PubMed] [Google Scholar]
- 15.Kimpton J, Emerman M. Detection of replication-competent and pseudotyped human immunodeficiency virus with a sensitive cell line on the basis of activation of an integrated beta-galactosidase gene. J Virol. 1992;66:2232–2239. doi: 10.1128/jvi.66.4.2232-2239.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kotov A, Zhou J, Flicker P, Aiken C. Association of Nef with the human immunodeficiency virus type 1 core. J Virol. 1999;73:8824–8830. doi: 10.1128/jvi.73.10.8824-8830.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Landau N R, Page K A, Littman D R. Pseudotyping with human T-cell leukemia virus type I broadens the human immunodeficiency virus host range. J Virol. 1991;65:162–169. doi: 10.1128/jvi.65.1.162-169.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lewis N, Williams J, Rekosh D, Hammarskjold M L. Identification of a cis-acting element in human immunodeficiency virus type 2 (HIV-2) that is responsive to the HIV-1 rev and human T-cell leukemia virus type I and II rex proteins. J Virol. 1990;64:1690–1697. doi: 10.1128/jvi.64.4.1690-1697.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luo T, Douglas J L, Livingston R L, Garcia J V. Infectivity enhancement by HIV-1 Nef is dependent on the pathway of virus entry: implications for HIV-based gene transfer systems. Virology. 1998;241:224–233. doi: 10.1006/viro.1997.8966. [DOI] [PubMed] [Google Scholar]
- 20.Marsh M, Bron R. SFV infection in CHO cells: cell-type specific restrictions to productive virus entry at the cell surface. J Cell Sci. 1997;110:95–103. doi: 10.1242/jcs.110.1.95. [DOI] [PubMed] [Google Scholar]
- 21.Matlin K S, Reggio H, Helenius A, Simons K. Pathway of vesicular stomatitis virus entry leading to infection. J Mol Biol. 1982;156:609–631. doi: 10.1016/0022-2836(82)90269-8. [DOI] [PubMed] [Google Scholar]
- 22.McClure M O, Sommerfelt M A, Marsh M, Weiss R A. The pH independence of mammalian retrovirus infection. J Gen Virol. 1990;71:767–773. doi: 10.1099/0022-1317-71-4-767. [DOI] [PubMed] [Google Scholar]
- 23.Miller M D, Feinberg M B, Greene W C. The HIV-1 nef gene acts as a positive viral infectivity factor. Trends Microbiol. 1994;2:294–298. doi: 10.1016/0966-842x(94)90007-8. [DOI] [PubMed] [Google Scholar]
- 24.Miller M D, Warmerdam M T, Gaston I, Greene W C, Feinberg M B. The human immunodeficiency virus-1 nef gene product: a positive factor for viral infection and replication in primary lymphocytes and macrophages. J Exp Med. 1994;179:101–113. doi: 10.1084/jem.179.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller M D, Warmerdam M T, Page K A, Feinberg M B, Greene W C. Expression of the human immunodeficiency virus type 1 (HIV-1) nef gene during HIV-1 production increases progeny particle infectivity independently of gp160 or viral entry. J Virol. 1995;69:579–584. doi: 10.1128/jvi.69.1.579-584.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Naldini L, Blomer U, Gallay P, Ory D, Mulligan R, Gage F H, Verma I M, Trono D. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science. 1996;272:263–267. doi: 10.1126/science.272.5259.263. [DOI] [PubMed] [Google Scholar]
- 27.Pandori W M, Fitch N J, Craig H M, Richman D D, Spina C A, Guatelli J C. Producer-cell modification of human immunodeficiency virus type 1: Nef is a virion protein. J Virol. 1996;70:4283–4290. doi: 10.1128/jvi.70.7.4283-4290.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pauza C D, Price T M. Human immunodeficiency virus infection of T cells and monocytes proceeds via receptor-mediated endocytosis. J Cell Biol. 1988;107:959–968. doi: 10.1083/jcb.107.3.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rizvi T A, Panganiban A T. Simian immunodeficiency virus RNA is efficiently encapsidated by human immunodeficiency virus type 1 particles. J Virol. 1993;67:2681–2688. doi: 10.1128/jvi.67.5.2681-2688.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schwartz O, Marechal V, Danos O, Heard J M. Human immunodeficiency virus type 1 Nef increases the efficiency of reverse transcription in the infected cell. J Virol. 1995;69:4053–4059. doi: 10.1128/jvi.69.7.4053-4059.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Srinivasakumar N, Chazal N, Helga-Maria C, Prasad S, Hammarskjold M L, Rekosh D. The effect of viral regulatory protein expression on gene delivery by human immunodeficiency virus type 1 vectors produced in stable packaging cell lines. J Virol. 1997;71:5841–5848. doi: 10.1128/jvi.71.8.5841-5848.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stein B S, Gowda S D, Lifson J D, Penhallow R C, Bensch K G, Engleman E G. pH-independent HIV entry into CD4-positive T cells via virus envelope fusion to the plasma membrane. Cell. 1987;49:659–668. doi: 10.1016/0092-8674(87)90542-3. [DOI] [PubMed] [Google Scholar]
- 33.Umata T, Moriyama Y, Futai M, Mekada E. The cytotoxic action of diphtheria toxin and its degradation in intact Vero cells are inhibited by bafilomycin A1, a specific inhibitor of vacuolar-type H(+)-ATPase. J Biol Chem. 1990;265:21940–21945. [PubMed] [Google Scholar]
- 34.Welker R, Kottler H, Kalbitzer H R, Krausslich H G. Human immunodeficiency virus type 1 Nef protein is incorporated into virus particles and specifically cleaved by the viral proteinase. Virology. 1996;219:228–236. doi: 10.1006/viro.1996.0240. [DOI] [PubMed] [Google Scholar]
- 35.Wool-Lewis R J, Bates P. Characterization of Ebola virus entry by using pseudotyped viruses: identification of receptor-deficient cell lines. J Virol. 1998;72:3155–3160. doi: 10.1128/jvi.72.4.3155-3160.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xu L, Sanchez A, Yang Z, Zaki S R, Nabel E G, Nichol S T, Nabel G J. Immunization for Ebola virus infection. Nat Med. 1998;4:37–42. doi: 10.1038/nm0198-037. [DOI] [PubMed] [Google Scholar]
- 37.Yamada S, Ohnishi S. Vesicular stomatitis virus binds and fuses with phospholipid domain in target cell membranes. Biochemistry. 1986;25:3703–3708. doi: 10.1021/bi00360a034. [DOI] [PubMed] [Google Scholar]
- 38.Yang Z, Delgado R, Xu L, Todd R F, Nabel E G, Sanchez A, Nabel G J. Distinct cellular interactions of secreted and transmembrane Ebola virus glycoproteins. Science. 1998;279:1034–1037. doi: 10.1126/science.279.5353.1034. [DOI] [PubMed] [Google Scholar]
- 39.Yoshimori T, Yamamoto A, Moriyama Y, Futai M, Tashiro Y. Bafilomycin A1, a specific inhibitor of vacuolar-type H(+)-ATPase, inhibits acidification and protein degradation in lysosomes of cultured cells. J Biol Chem. 1991;266:17707–17712. [PubMed] [Google Scholar]