Abstract
A limited number of tissues can spontaneously regenerate following injury, and even fewer can regenerate to a state comparable to mature, healthy adult tissue. Mesenchymal stem cells (MSCs) were first described in the 1960s-1970s by Friedenstein et al as a small population of bone marrow cells with osteogenic potential and abilities to differentiate into chondrocytes. In 1991, Arnold Caplan coined the term “mesenchymal cells” after identifying these cells as a theoretical precursor to bone, cartilage, tendon, ligament, marrow stroma, adipocyte, dermis, muscle, and connective tissues. MSCs are derived from periosteum, fat, and muscle. Another attractive property of MSCs is their immunoregulatory and regenerative properties, which result from crosstalk with their microenvironment and components of the innate immune system. Collectively, these properties make MSCs potentially attractive for various therapeutic purposes. MSCs offer potential in sports medicine, aiding in muscle recovery, meniscal tears, and tendon and ligament injuries. In joint disease, MSCs have the potential for chondrogenesis and reversing the effects of osteoarthritis. MSCs have also demonstrated potential application to the treatment of degenerative disc disease of the cervical, thoracic, and lumbar spine.
Keywords: mesenchymal stem cells, musculoskeletal diseases, regenerative medicine, tissue engineering, orthopedic surgery, immunomodulation
Graphical Abstract
Graphical Abstract.
Significance Statement.
Stem cells have regenerative potential in a variety of tissues. Specifically, mesenchymal stem cells (MSCs) have shown promise for bone and cartilage repair due to their unique immune-modulatory and anti-inflammatory properties. This article reviews the clinical evidence supporting the use of MSCs in orthopedics, outlining their potential for muscle injury and repair, tendon injuries, ligament tears, and mitigating the effects of osteoarthritis. The therapeutic use of stem cells has the potential to revolutionize regenerative medicine.
Introduction
The past 2 decades have witnessed growing insights into innate regenerative capacity of mammalian adult tissues and the underlying biology of stem cell niches that govern endogenous repair and rejuvenation. The regenerative capacity of tissues and organs ranges substantially from continuous renewal (eg, hair follicles and gut) to extremely limited repair capacity (eg, heart and CNS). As limited tissues have the capacity to regenerate to a completely healthy tissue following injury, there is intense interest in using exogenous cells and cell-derived products to stimulate endogenous repair pathways. Among these strategies, the use of culture-expanded mesenchymal stem/stromal cells (MSCs) has occupied the attention of many scientists and Orthopedic clinicians resulting in extensive research aimed at defining the potential of these cells and related products for cartilage and bone repair.1
MSCs are a heterogenous subset of multipotent regenerative cells that can be harvested from various sources including bone marrow (BM), amniotic fluid, placenta, adipose tissue, joint synovium, synovial fluid, dental pulp, endosteum, and periosteum.2 MSCs were first described by Friedenstein in the late 1960s to early 1970s as a small population of BM cells characterized by osteogenic potential, adherence to plastic, and the ability to form new, fibroblast-like progeny over many culture passages.3,4Friedenstein found that these cells could differentiate in vitro not only into osteocytes but also into chondrocytes and adipocytes. Subsequently, Caplan et al in 1991 termed these cells “mesenchymal stem cells” after discovering their ability to differentiate into more than one type of cell that formed connective tissue in many organs and had many different phenotypic endpoints of differentiation. Caplan pioneered the differentiation of the MSCs into bone-forming osteoblasts and cartilage-forming chondrocytes and described the ideal culture medium and conditions for each.5
Growing interest in MSCs led to controversy regarding the nomenclature and criteria for defining populations of cells as MSCs. To address this, the International Society for Cellular Therapy (ISCT) published its position specifying the criteria defining the population of MSCs and recommending the use of the name multipotent mesenchymal stromal cells; however, mesenchymal stem cells remain the most popular name. The ISCT’s published criteria described 3 major requirements: (1) cells must be plastic adherent when maintained under standard culture conditions; (2) cells must express cluster of differentiation (CD)73, CD90, and CD 105 markers and should not express CD34, CD45, CD14, human leukocyte antigen complex (HLA)-DR, CD11b or CD19; (3) cells must possess the ability to differentiate into osteoblasts, chondroblasts, and adipocytes in vitro.6,7
One potential conflict with these criteria is the relationship between MSCs and vascular pericytes. Pericytes are contractile cells that line the outer walls of capillaries and other vascular structures. They have similar cell surface markers and characteristics of MSCs and exhibit many similar properties to MSCs when grown ex vivo.8-11 For these reasons, some investigators propose that MSCs are derived from pericytes.12 However, lineage tracing techniques in transgenic mice reveal that pericytes are not present as local progenitor cells in vivo and are not equivalent to MSCs.13 Additional clarification derives from the findings of Khosravi et al14,15 using intra-vital microscopy supporting the idea that tissue-resident mesenchymal progenitor cells are localized between capillaries and represent a distinct population from pericytes. Their work suggests that within bony compartments tissue-resident mesenchymal cells are of perivascular origin and can differentiate into functional pericytes in addition to fibroblasts and osteogenic cells.
Also worth noting is that within the bony compartments are different types of endothelial cell (EC)-lined vessels that are selectively surrounded by a perivascular population of cells that are Runx2 and SP7 positive committed osteoprogenitor cells. Endosteal ECs that strongly express CD31/platelet endothelial cell adhesion molecule-1 and endomucin are termed H-type ECs, which are abundantly surrounded by osteoprogenitor cells. These findings confirm the earlier observations of Aubin et al16 who described populations of committed and inducible mesenchymal cells in BM. These H-type ECs mediate local growth of vasculature and provide niche signals for perivascular osteoprogenitors, which is important for compromised fracture healing and bone density loss.17 The concept of perivascular progenitor cells and their H-type EC microenvironment provides an interesting explanation for loss of bone mass density and a possible therapeutic target to enhance osteogenesis and fracture healing.
To conduct this review, we searched for studies using Pubmed and Data.gov including keywords and headings such as “MSCs, stem cells, muscle, articular cartilage, osteoarthritis, meniscus tear, tendon tear, ligament tear, intervertebral disc, fracture, and trauma.” Preference was given to RTCs conducted since 2017. Animal and preclinical studies were included to demonstrate the biological rational and potential mechanisms underlying the use of MSCs.
MSC administration
The administration of MSCs is specific to the type of condition being treated along with the intended effect. For most immunological or systemic disorders, intravenous administration has been widely applied whereas for certain types of soft-tissue injuries, MSCs have been directly injected locally into wounds and specific sites. MSCs were originally believed to migrate to sites of injury where they engraft and differentiate into functional tissue cells. However, cell tracking technology as well as long-term follow-up studies have illustrated that this is likely not the case. A high portion of intravenous-infused cells are trapped within organs with large capillary beds such as the lungs and a majority do not migrate to other sites. In addition, the majority of infused cells do not survive more than 24 hours.18 As a result, the mechanism of action of MSCs has evolved to the concept that these cells act as trophic mediators to modulate the function of immune cells and resident progenitor cells rather than differentiate into various cell lines.19
For the purpose of bone repair, MSCs are usually seeded on transplantable scaffolds such as bioceramics, polymers, and composite biomaterials. Bioceramics such as calcium phosphate and calcium silica demonstrate high bioactivity and biocompatibility but have relatively low toughness and insufficient strength. Polymers such as poly-lactic acid, poly-glycolic acid, and poly-lactic-co-glycolic acid demonstrate good plasticity with the ability to support osteoprogenitor cell adhesion and growth. However, they pose disadvantages of weak mechanical properties with risk of plastic deformity and lack of boney integration with provocation of giant cell response. Composite biomaterials typically consist of a composite of ceramics combined with polymers to provide advantages of both scaffolding types and can be tailored for an appropriate balance of strength and toughness.20 However, clinical administration and utilization of scaffolding constructs requires further illustration of MSCs’ mechanism of action, standardization of both MSC sourcing, culturing, osteogenic signaling factors as well as scaffold design and composition before further progress can be made toward elucidating the optimal therapeutic approach.
There have been discrepancies between positive results obtained from preclinical animal models and their translation into clinical efficacy in human trials.21 Galipeau et al suggested that such differences could arise from the preferential use of fresh MSC preparations in preclinical models compared to cryopreserved in clinical trials. To address this issue, Hoogduijin and Lombardo22 performed a concise review of publications using MSCs in experimental animal models of inflammatory diseases and identified methodological details regarding the origin, immunological matching, and status of cells prior to administrations and therapeutic outcomes. Their review suggested xenogeneic MSCs to be equally efficacious for both autologous and allogeneic MSCs in animal models. They cautioned, however, that in other animal models, these results could be opposing such as the case seen in a rat corneal transplantation model in which human MSCs in contrast to rat MSCs failed to prolong allograft survival. Interestingly, freshly cultured allogeneic rat MSCs showed equal efficacy as the same cells after cryopreservation.23 Accordingly, Hoogduijin and Lombardo22 concluded that MSC administered immediately following thawing retained therapeutic potential equivalent to cultured MSCs in animal models, thereby challenging the disparity between preclinical and clinical therapeutic effects associated with cryopreserved MSCs, fresh MSCs, autologous MSCs, and allogeneic MSCs. These data should be interpreted with some caution as additional studies comparing cultured and thawed MSCs are needed to make definitive conclusions.
With respect to dosing regimens, MSCs are usually administered in rodents at a dose of 50 million cells/kg, while clinical trials in humans range between 1 and 10 million cells/kg. There has been no clear transition made between effective dosing in rodents and humans and data continues to remain limited. In addition, comprehensive studies comparing the effects of single versus repeat dosing at different time points even in animal models are limited.22
Cell sourcing of MSCs
One of the most interesting aspects of MSCs is that their differentiation potential varies based on their tissue of origin. Depending on the specific tissue-derived MSC, these cells display different transcriptomes, proteomes, immunophenotypes, and immunomodulatory activities. As a result, depending on the tissue-specific derived MSC there is a tendency to differentiate into different end-stage lineage cells including osteoblast and chondrocytes.24 Bone marrow-derived MSCs (BMD-MSCs) exhibit a superior capacity for osteogenesis and chondrogenesis while; synovial MSCs (S-MSCs) demonstrate greater chondrogenic and proliferation potential than adipose-derived MSCS (AD-MSCs), BMD-MSCs, and MSCs derived from periosteum, fat, and muscle.25-27 Umbilical cord blood-derived MSCs (UCB-MSCs) were found to have the highest rate of cell proliferation and clonality and significantly lower expression of markers for senescence compared to BMD-MSCs and AD-MSCs.28
The frequency of successful collection and primary frequency of cells harvested from cord blood (CB) are typically low as described by Kögler et al.29 However, controversy exists regarding the superiority of certain extraction protocols and harvest compartments for CB. Typically, MSCs are sampled from CB via needle passage inserted into Wharton’s jelly, which does represent a rich population of MSCs.30 Still controversial, it is believed that the low frequency of cells harvested from CB is a result of harvesting technique and not a lack of abundance of cells present. Although there is low primary frequency in CB, these cells can be expanded to at least 1015 cells while maintaining a normal karyotype. Schugar et al31 suggest that the most abundant source of MSCs arises from the perivascular zones of human umbilical cord (UC) and propose a technique of digesting the UC with collagenase so as to select against hematopoietic and endothelial cells while maximizing yields of cells expressing CD146 and MSC markers. Sarugaser et al32 using a colony forming unit frequency (CFU-F) assay as a surrogate MSC measure demonstrated a CFU-F of 1:300 at harvest from an isolated UC perivascular population. In contrast, the CFU-F is estimated to be 1:10 000 to <1:100 000 reported for BMD-MSCs. Also, the population doubling (PD) time of UC-MSCs has been reported to be 24 hours as compared to 40 hours for BMD-MSCs.33,34 These differences in proliferative capacities, extraction methods, and differentiation proclivities are significant because the location of MSC extraction has an impact on differentiation potential in recipient tissues and may necessitate the addition of different cytokines or paracrine factors to obtain the desired potential as will be discussed below. In addition, the difference in PD times between specific tissue-derived MSC affects ex vivo culture expansion times and viability of cell banking. Therefore, researchers and clinicians must be mindful of the different tissue-derived MSCs and tailor their choices according to their desired purpose. These differences also highlight how the yield of MSCs can depend on a multitude of tissue, biologic, and manufacturing factors and a growing need for standardization both in research and clinical practice to further advance the field.
MSC interaction with host cells
The current prevailing theory regarding MSC mechanism of action is that these cells produce a variety of trophic mediators including growth factors, angiogenic factors, and immune regulatory factors that modulate the function of immune, vascular, and progenitor cells.35-39 The secretomes of MSCs include extracellular vesicles (EVs) containing microRNA, organelles, and proteins that control cell function. An elegant example is the release of osteoclastic factor receptor activator of nuclear factor kappa-B ligand (RANKL) by MSCs delivered on biomimetic scaffolds. The secretomes containing RANKL serve as a supplement of the cytokine to enhance the formation of tartrate-resistant acid phosphatase positive (TRAP+) cells in bone, yielding a potential treatment for osteopetrosis.40 Additional studies demonstrate the potential of cell-free therapies utilizing MSC secretomes. For example, Joseph et al41 used immunohistochemistry and Western blot to demonstrate that MSC conditioned culture media contained important growth factors and cytokines including vascular endothelial growth factor (VEGF), transforming growth factor-beta 1 (TGF-β 1), fibroblast growth factor 2, insulin like growth factor 1, stem cell factor, and interleukin-6 (IL-6). This study further demonstrated that allogeneic and xenogeneic application of MSC conditioned culture media significantly improved wound healing with minimal scar formation. As a result, MSC secretomes may be effective cell-free therapies and also provide support for the idea that MSCs exert important bioactivity through paracrine signaling. It is important to note that studies evaluating the introduction of secretome-deficient MSCs evoked similar responses as MSC infusion with secretome-sufficient MSCs. The spike in serum cytokines and chemokines observed from these studies from host cells rather than MSCs implies that the effects attributed to MSC secretomes may have other origins as well or may require heterocellular coupling35-37,42 Another possible mechanism is that specific endogenous cells such as H-Type ECs play in coupling angiogenesis and osteoprogenitors in fracture healing. The work of Zhang et al43 proposes that type H ECs mediate local angiogenesis and provide niche signals for perivascular osteoprogenitors by particular molecular pathways, which can be targeted for osteogenesis. Type H vascular ECs have been found to secrete Noggin and VEGF, which stimulate proliferation and differentiation of osteoprogenitors to regulate osteogenesis. Their work has also shown that the administration of exogenous platelet-derived growth factor type BB (PDGF-BB) and activation of different signaling pathways in bony defects can promote formation of type H vessels and restoration of bone.42,43
Immunomodulatory effects of MSCs
Another attractive property of MSCs is their immune-modulatory and anti-inflammatory effects. Through their production of soluble factors, MSCs can suppress both the innate and adaptive immune systems by attenuating maturation and capacity for antigen presentation of dendritic cells, inhibiting activation and proliferation of T and B lymphocytes, and reducing cytotoxicity of natural killer (NK) and natural killer T (NKT) cells.44 In addition, MSCs lack HLA class II/major histocompatibility complex (MHC) and associated cell surface costimulatory molecules allowing MSCs to evade recognition by the immune system.45,46 MSC expression of MHC I allows these cells to evade NK cell-mediated cytotoxicity, and lack of expression of MHC II prevents identification by CD4+ T cells, respectively.3,12 However despite previous literature suggesting solely inhibitory effects on immune cells, more recent observational studies emphasize the dynamic role of MSCs through crosstalk with the microenvironment. This is exemplified by MSC communication with the innate immune system to act as immunostimulatory cells partly by upregulating MHC class II expression when there are lower levels of interferon gamma.47 Therefore, MSCs balance immunosuppression and immunoactivation effects depending on their differentiation state and the host inflammatory microenvironment. This is apparent during fracture healing as MSCs crosstalk with macrophages resulting in downregulation of proinflammatory M1 macrophages and promotion of anti-inflammatory M2 macrophages to support bone formation.47
A concern with MSC therapies is the controversial risk of tumor formation.48 In this regard, several studies, mostly conducted in rodents or in vitro, demonstrate the capacity for MSCs to contribute to the progression of certain tumor cells. MSCs migrate toward inflammatory sites and incorporate into tumors.46,48 Importantly, this concern has not been borne out in clinical evaluation. For example, Hernigou et al48 examined 1873 patients treated with BM-derived concentrated cells and monitored for cancer incidence both at the treatment site and systemic locations over a 12.5-year period. Results demonstrated a cancer incidence below that expected in the general population leading to the conclusion that there is no increase in cancer risk in patients after the application of autologous cell-based therapy using BM-derived stromal progenitor cells.22 In addition, Lalu et al49 performed a systematic review and meta-analysis of prospective clinical trials of intravascular delivery of MSCs in adult and mixed adult/pediatric populations to further explore the safety of MSCs. Outcome measures included acute infusion toxicity, fever, organ system complications, infection, and longer-term adverse events including death and malignancy. A total of 8 RCTs with 321 participants were included. There was no difference in incidence of acute infusion toxicity, organ system complications, infection, death, or malignancy between controls and those that received MSC infusions. The results support the safety of MSCs not only with regards to tumor formation but also for other adverse events. More recently, a phase 1 study assessed safety and cancer-homing ability of allogenic BMD-MSCs in men with localized prostate cancer to determine an ideal cell-based therapeutic vector for delivery of cytotoxic agents. Seven patients received allogenic BMD-MSCs intravenous infusions 4-6 days prior to radical prostatectomy. Pathologic assessment of the prostates demonstrated no detectable MSCs; however, the high rate of lung sequestration of infused MSCs led to early termination of the trial due to perceived futility.50 Importantly, the study demonstrated that infusion of MSCs was safe in males with prostate cancer and there was no evidence for MSC homing to the primary malignancy. Although there remains controversy regarding the increased risk of cancer with MSC therapies, and there are preclinical studies to suggest homing of MSCs to sites of primary and metastatic tumor site, clinical data suggest that MSC application in human subjects is safe and poses a limited risk of tumor formation as described above.
The remainder of this review will discuss current and potential applications of MSCs in the treatment of musculoskeletal (MSK) diseases including fracture management, muscle injury and repair, articular cartilage, meniscus pathology, tendon injury, ligament repair, and intervertebral disc applications.
MSCs application in fracture healing
Fracture healing occurs as a result of a complex sequence of events that often results in complete bone repair to a pre-injury condition. A key feature of fracture healing proceeds through a combination of endochondral and intramembranous ossification.12 Intramembranous ossification occurs when bone formation ensues without a cartilaginous phase and the majority of cells that contribute are present in the inner osteogenic layer of the periosteum and endosteum. Ossification begins with the condensation of MSCs producing a collagen-proteoglycan matrix that can bind calcium and become mineralized. A BM cavity is formed and calcified bone spicules become surrounded by compact MSCs that form the periosteum and endosteum. Osteogenesis is made possible due to the presence of MSCs that form the inner sheet of the periosteum and the endosteum.51,52
Endochondral ossification occurs when an initial synthesis of cartilaginous scaffolding is followed by its calcification.53 This calcified scaffold is then resorbed by osteoclasts, which permits vascular invasion and osteogenesis to occur. This process of angiogenesis after a fracture is stimulated by hematoma formation and a subsequent series of intercellular connections and release of growth factors, cytokines, and interleukins that cause chemotaxis to the fracture site. Angiogenesis is crucial for trafficking of MSCs from the systemic circulation and also from previously described perivascular osteoprogenitor cells to the fracture site.51,54 In addition, H-type ECs mediate local growth of the vasculature and provide a niche signal for perivascular osteoprogenitors.16,17
Capitalizing on these events, BM extracts have been used for treating nonunion fractures with interest in specific molecular pathways to boost H-type vessel formation and osteogenesis associated with compromised fracture healing or bone mass loss.55 Two factors that have been found to act synergistically to promote MSCs migration to fracture sites and fracture site repair are stromal cell-derived factor (SDF)-1 and monocyte chemotactic protein-3.56 Others have noted that the expression of bone morphogenic protein-2 and (SDF)-1 increase MSC migration and differentiation to stimulate fracture healing. SDF-1 promotes the autocrine release of VEGF and basic fibroblast growth factor (bFGF) paracrine factor which are both associated with inhibiting cellular apoptosis.56 It is important to note that VEGF also plays an essential role in coupling vascular and skeletal morphogenesis. VEGF stimulates the vascular tip as well as bone-forming progenitors along with helping to incite mobilization of nutrition, oxygen, and minerals necessary for osteogenesis. Release of VEGF from MSCs not only plays a role in necessary angiogenesis before osteogenesis but also influences lineage fate between osteoblasts, chondrocytes, or adipocytes.57 Also of note, platelets release TGF-β in the inflammatory phase of fracture healing which in turn participates in callus formation and effective chemotaxis of BMD-MSCs.58
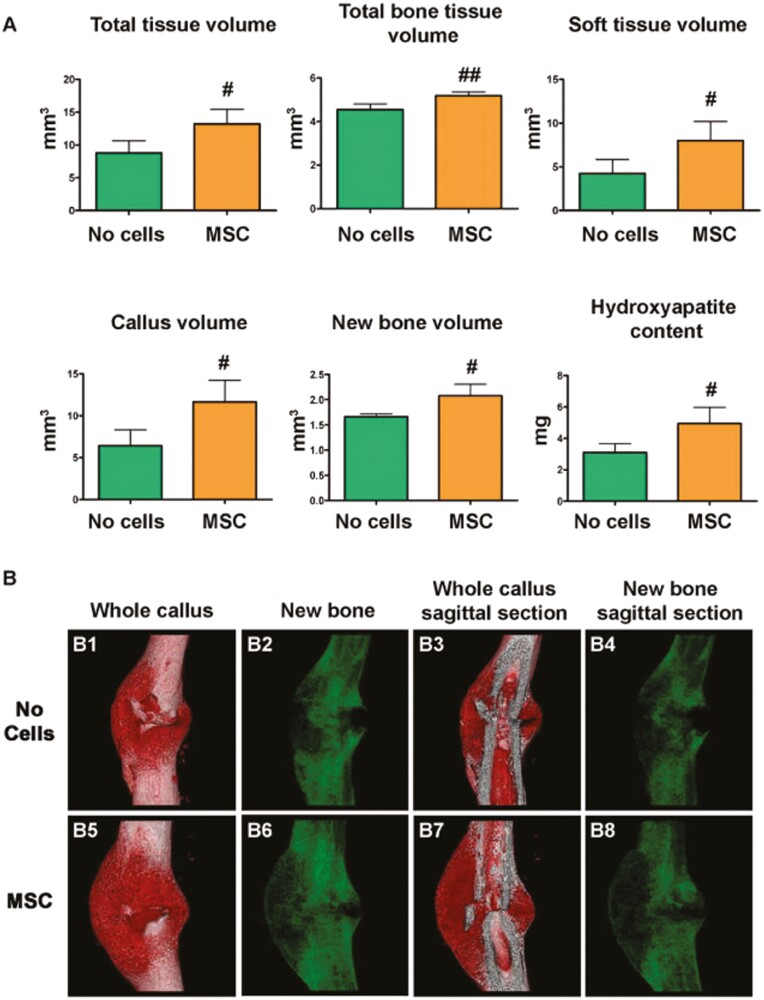
Understanding the multiple pathways involved in MSC regenerative abilities in bone osteogenesis is important in allowing the design of novel MSC-based therapies to treat fractures. For example, Granero-Moltó et al51 used a stabilized tibia fracture mouse model to determine the dynamic migration of transplanted MSCs to the fracture site and engraftment within the callus endosteum. In addition, they clarified MSCs’ contribution to the repair process and their role in modulating the injury-related inflammatory response (Figure 1). Building on these concepts, Wang et al59 used a C57 murine unilateral, transverse femur fracture model to determine the optimal time to inject BMD-MSCs for fracture healing. Their study had multiple implications: (1) injection of BMD-SCs improved bone healing, (2) injection of BMD-SCs 7 days postfracture promoted accelerated fracture healing and callus/bone quality, (3) the SDF-1-chemokine receptor 4 (CXCR) pathway played a key role in the fracture healing process, and (4) MSCs contributed therapeutically to delayed fracture healing and nonunions. Moreover, Emadedin et al60 demonstrated the safety of percutaneous autologous BMD-MSC implantation for the reconstruction of human lower limb long bone atrophic nonunion. However, it was noted that further double-blind, controlled clinical trials are necessary to assess the efficacy of this treatment. Recently, Mott et al61 preformed a systematic review of 94 studies assessing the evidence for the use of stem cells in fracture healing. The authors found that there was insufficient high-quality evidence to determine the efficacy of stem cells for fracture healing with lack of technique standardization cited as their major limitation. Also, the heterogeneity of assessment of fracture consolidations along with the lack of uniformity in outcome measurements creates significant obstacles to formulating control subjects and measuring outcomes.62 To date, there are no registered clinical trials with published data on MSCs and fracture or bone healing. However, in the last 10 years, there are at least 8 clinical trials registered with respect to fracture healing or treatment of nonunion with mesenchymal stem cells. Most trials focus on targeting BMD-MSCs as the primary mesenchymal stem cell treatment for fracture healing (Table 1).63–70 One trial is randomizing 32 patients, aged 70-85, to treatment with either endomedullary nailing + BMD-MSC treatment or isolated endomedullary nailing. These patients will be followed for 1 year, primarily assessing safety, CT and X-ray, clinical status, and quality of life.66 Improvement in clinical status and quality of life for these patients could have a major clinical impact due to the high levels of morbidity and mortality associated with hip fractures in older individuals (Table 1).71
Figure 1.
MSC transplant increases callus size and changes callus morphology in fracture healing sites. (A) Depicts a μCT analysis performed 14 days after fracture calluses dissected from mice that received MSC transplant compared to controls with no transplant. Callous and new bone volume was calculated after subtracting the cortical bone volume from the total volume and the total bone tissue volume. #P < .05 versus no cells; ##P < .01 versus no cells by Student’s t-test. No cells, n = 3; MSC, n = 6. (B) Three-dimensional reconstruction of whole calluses (B1, B2, B5, and B6) and sagittal sections (B3, B4, B7, and B8) were obtained 14 days after tibial fracture in calluses from mice that were transplanted with MSC or control untransplanted (no cells).51 Figure 1 and caption reprinted from Granero-Moltó et al.51 Copyright 2009 by Oxford University Press. Reprinted with permission.
Table 1.
Recent clinical trials on MSCs in fracture healing.
| Title | Cell type | Year initiated/reported | CT.gov ID |
|---|---|---|---|
| Autologous bone marrow concentration for femoral shaft fracture union | Auto-BMD-MSC | 2019 | NCT03794622 |
| A comparative study of 2 doses of BM autologous H-MSC+biomaterial vs iliac crest autograft for bone healing in non-union (ORTHOUNION) | Auto-BMD-MSC | 2017 | NCT03325504 |
| Autologous BM-MSC transplantation in combination with platelet lysate (PL) for nonunion treatment | Auto-BMD-MSC | 2015 | NCT02448849 |
| Allogeneic mesenchymal stromal cells in elderly patients with hip fracture | Allo-BMD-MSC | 2015 | NCT02630836 |
| Mesenchymal stromal cells for the treatment of non-union fractures of long bones | Auto-BMD-MSC | 2014 | NCT02230514 |
| Allogenic mesenchymal stem cell for bone defect or non-union fracture (AMSC) | Allo-UCB-MSC, BMD-MSC, AD-MSC | 2014 | NCT02307435 |
| Allogeneic mesenchymal stem cell transplantation in tibial closed diaphyseal fractures | Allo-AD-MSC | 2014 | NCT02140528 |
| Evaluation the treatment of nonunion of long bone fracture of lower extremities (femur and tibia) using mononuclear stem cells from the iliac wing within a 3-D tissue engineered scaffold | Auto-BMD-MSC | 2013 | NCT01958502 |
MSCs application in muscle injury and repair
Following muscle fiber injury, the nuclei of damaged fibers undergo apoptosis. Failure of the replacement of these nuclei partially explains the muscle atrophy and weakness that patients experience after moderate or severe skeletal muscle damage.72 The endogenous repair mechanism for this injury derives from skeletal muscle stem cells called satellite cells (also called myosatellite cells), which are activated by injury, migrate to the site of injury, and fuse with injured fibers to repopulate the lost nuclei.72 Deploying satellite cells as a therapy represents a possible strategy for the treatment of muscle injuries. However, in vitro expansion of these cells has proven difficult and they exhibit limited post-transplant survival.12
As an alternative, BMD-MSCs have been explored as a possible therapeutic strategy. In multiple studies conducted in mice, BMD-MSCs were incorporated into myofibrils of injured muscles and gave rise to Pax7+ satellite cells, a marker for muscle regeneration.73,74 Furthermore, engrafted MSCs survived repeated cycles of damage without additional transplant with 89% efficiency.74 Stromal cell-derived factor 1 SDF-1/CXCR4 signaling and chemokine receptor (CCR2) are implicated in BM cell homing to muscle injury sites.73 A study examining the role of CCR2 on muscle regeneration and BM-derived cells found that mice lacking CCR2 demonstrated fewer BM-derived cells and macrophages along with decreased muscle regeneration.75 Klimczak et al76 hypothesized that the delivery of MSCs into dystrophic muscle will create a microenvironment/ niche supporting homing of myogenic precursor and enhance tropism of stem cells of myogenic origin with CXCR4+ expression to injured muscle expressing SDF-1. These investigators found that MSCs were viable in injured muscle and contributed to regeneration supporting the importance of microenvironment/niche in MSCs muscle therapy. Iyer et al77 also demonstrated that exosomes derived from MSCs facilitate recovery of muscle strain injury in small animal models via reduced expression of TGF-β. They concluded that this resulted from modulation of inflammation, fibrosis, and myogenesis. Large animal model studies demonstrated that MSCs can be used to prevent fatty atrophy that can occur with muscle injury. Flück et al78 demonstrated molecular, microscopic, and macroscopic evidence of the prevention of fatty atrophy of detached rotator cuff muscle after tendon repair in an ovine model. They concluded that injection of micro-tissues from MSCs result in a paracrine cascade of events results in expansion of extracellular ground substance, increased tissue water content, and introduction of tenascin C-associated myogenic reactions. Coupled with downregulation of differentiation marker peroxisome proliferator-activated receptor gamma ultimately leads to prevention of fatty atrophy in repaired infraspinatus detachment in sheep models.
At present, there are no clinical trials registered to investigate the effect of MSCs on muscle healing, as all trials focus on the myotendinous junction or the muscle-tendon unit, rather than isolation of the muscle itself. Current studies are also exploring MSC use in the treatment of various dystrophic diseases or spastic palsies.79–82 However, there is ongoing research investigating the potential role for MSCs in frailty patients to help mitigate sarcopenia, dynapenia and loss of strength with aging. Clinical trials in this area have demonstrated some potential in the CRATUS phase 1 and 2 trials exploring the concept of rejuvenating aged niches and recapitulating their bioactivity using allogenic BMD-MSC in patients with mild frailty. The results of these trials suggest evidence of bioactivity and effectiveness of BMD-MSCs to combat sarcopenia and age-related muscle wasting with improved hand grip strength, increased 6-minute walk distance and reduced circulating TNF-α (inflammatory cytokine linked to frailty related sarcopenia and loss of strength with aging).83–87 In turn, based upon these promising findings, future clinical trials dedicated to orthopedic muscle injury are warranted to identify treatment targets for muscle strains, traumatic lacerations, and other injuries to muscle belly tissue.
MSCs application in articular cartilage disease and osteoarthritis
An estimated 30.8 million adults in the United States and in excess of 300 million worldwide suffer from osteoarthritis (OA).88 Articular cartilage is the hyaline cartilage at the end of long bones and comprises a thin layer bonded to the perforated cortical bone adjacent to intervertebral discs that provides a low friction surface for articulation and transmission of weight between joints.88,89 Although the half-life of collagen within cartilage is long, articular cartilage heals very slowly and lesions are difficult to manage because of tissue biochemical properties, acellularity, and limited self-renewal potential.12,88 Moreover, OA is not solely a wear and tear disease but represents a complex process that includes inflammatory and metabolic factors such as metalloproteases and inflammatory cytokines.88 In addition, OA affects not just the articular cartilage but the entire joint and surrounding soft tissues particularly the synovium, infrapatellar fat pad, and subchondral bone.88
Subchondral bone is the layer of bone beneath the hyaline cartilage and cement line, divided anatomically into the subchondral bone plate and subchondral bone trabeculae. It provides mechanical and nutritional support for cartilage and changes in its microenvironment can impact cartilage metabolism.90 The synovium is a specialized tissue lining diarthrodial joints, responsible for maintaining synovial fluid volume and composition to aid in chondrocyte nutrition, and has an outer layer rich in collagen and a relatively acellular inner layer dominated by fibroblasts as well as macrophages. It is important to note that synovial fluid facing macrophages are master regulators of joint homeostasis and play an essential role in the changes that lead to osteoarthritis and other joint inflammation.91 In osteoarthritis, the synovium undergoes histological changes, characterized by synovial lining hyperplasia, fibrosis, and stromal vascularization, which leads to macrophage infiltration and subsequent cytokine production, angiogenesis, and cartilage degradation.92 The secretome of MSCs, which includes growth factors, cytokines, chemokines, and lipids, among others, plays a crucial role in their therapeutic properties. These paracrine factors act on the microenvironment to influence the phenotype of target cells such as promoting anti-inflammatory M2 macrophage polarization.93,94 Extracellular vesicles (EVs), such as microvesicles or exosomes, are a key part of the secretome and facilitate communication between different cell types and prior in vitro studies suggest immunomodulatory, regenerative, anti-catabolic, and chondroprotective properties.95 EVs are an attractive cell-free therapy because they provide the same clinical benefits without the potential risks of live cell infusions. In addition, EVs show promise in the treatment of OA by reactivating aging MSCs; however, the main clinical challenge is the low production of exosomes.93
Given the multitude of tissues affected and the involvement of different inflammatory pathways, the proposed versatility of MSCs have emerged as a potential therapeutic option in the treatment of OA. Several clinical studies have examined the possible benefits of MSCs for OA. A variety of delivery methods and cell processing protocols have been deployed, with encouraging results along with at least one approved product for clinical use.96–118
MSCs have shown promising clinical benefits in patients with osteochondral defects. Bashir et al96 reviewed 4 clinical trials, testing MSCs injection, that demonstrated improved visual analog scale (VAS) pain scores, reduced cartilage defect as seen on magnetic resonance imaging (MRI), and improved functional measures such as functional rating index and ability to climb stairs. A systematic review by Migliorini et al97 of MSC injections for knee OA including 18 studies comprising 1069 treated knees tested the impact of treatment on a variety of clinical outcomes, including the VAS, Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) score, mean walking distance, Lequesne scale, and Knee Injury and Osteoarthritis Outcome (KOOS) score at 6- and 12-month follow-up. This analysis supported the conclusion that MSC injections for knee OA represent a feasible therapy producing a meaningful improvement of all clinical and functional outcomes, regardless of cell source. Most notably, mean walking distance improved from 71.90 to 152.22 and 316.72 m at 6- and 12-month follow-up, respectively. In addition, mean VAS improved from 18.37 to 30.98 and 36.91 at 6- and 12-month follow-up, respectively. However, these results should be taken with caution as many studies do not provide convincing evidence of long-term benefits after 3-5 years.98 For example, the meta-analysis performed by Kim et al99 examined clinical outcomes of intra-articular injections of MSCs for cartilage repair in knee OA. In their study, they looked at a total of 5 RCTs and found a significant improvement in Lysholm score and WOMAC when MSCs were used in recommended concentrations but no significant difference in cartilage repair when assessed with MRI. They found that despite favorable clinical outcomes there is limited evidence of pain relief and functional improvement in knee OA and that further rigorously conducted, well-powered RCTs with long-term follow-up are required before MSCs can be supported for use for cartilage repair in OA.
In an attempt to provide more clarification on the potential role of MSCs in the treatment of knee OA, Di Zhao et al100 performed a systematic review and network meta-analysis evaluating the clinical efficacy of intra-articular injection of MSCs. The study evaluated intra-articular injections of hyaluronic acid (HA), leukocyte-poor platelet-rich plasma (LP-PRP), leukocyte-rich PRP (LR-PRP), BMD-MSCs, and AD-MSCs against a saline placebo with 6- and 12-month follow-up. The analysis included 43 studies and looked at VAS scores and WOMAC pain subscores. The results were discussed in comparison to HA injections and demonstrated that at 6 months AD-MSCs provided the most pain relief and that LP-PRP was the most effective for functional improvement. During the 12-month follow-up, both AD-MSCs and LP-PRP showed potential clinical pain relief effects; however, the most effective improvements of functional improvement was achieved with LP-PRP.
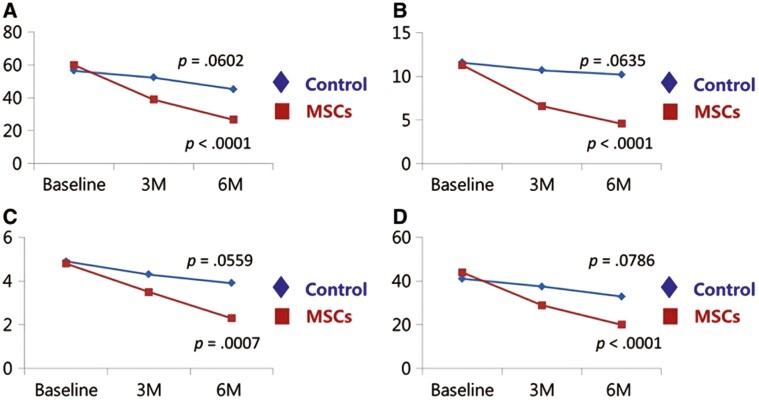
Importantly, RCTs continue to emerge supporting the clinical application of MSCs in the treatment of knee OA. A randomized phase 2B placebo-controlled clinical trial conducted that tested the efficacy, safety, and outcome scores of intra-articular injections of autologous AD-MSCs for the treatment of knee OA. AD-MSCs were administered to 12 patients and the group was compared with 12 knees injected with normal saline as a control and were followed for 6 months. The WOMAC score was the primary outcome and with secondary outcomes including radiographic examination and MRI results. The study concluded that a single injection of AD-MSCs led to a significant improvement in the WOMAC score at 6 months compared to the control group. No serious adverse events were observed. MRI evaluation showed no significant changes in cartilage defects at 6 months in the MSC group whereas the defect in the cartilage defects in the control group increased (Figure 2).101
Figure 2.
Changes in the WOMAC score during the 6‐month period after intra‐articular injection in the MSC group and control group of OA patients. Patients with injection of AD‐MSC showed significant improvement in the WOMAC score. Patients in the control group did not significantly change in the WOMAC score. (A) The WOMAC total score. (B) The pain subscore of the WOMAC. (C) The stiffness subscore of the WOMAC. (D) The physical function subscore of the WOMAC. Abbreviations: MSC, mesenchymal stem cell; WOMAC, Western Ontario and McMaster Universities Osteoarthritis index.101 Figure 2 and caption was reprinted from Lee et al.101 Copyright 2009 by Oxford University Press. Reprinted with permission.
There are multiple registered phase 2/3 clinical trials of note showing promising application of MSC in knee OA (Table 2).102–115 Of note, 2 clinical trials show promise for improvement in outcomes of pain and/or functional ability after therapy. In addition to the clinical improvements, radiographic assessments of articular cartilage improved with the use of BM-MSCs in the knee.116,117 One trial followed 32 patients with knee OA over a span of 12 months assessing pain and patient-reported quality of life after treatment with BMD-MSCs, compared to patients randomized to hyaluronate gel treatment. They found the most improvement in the BM-MSC group at 12 months, consistent with other studies.12,88,100,106 Additionally, the 5-year follow-up of a multicenter clinical trial with 114 patients with symptomatic, large, full-thickness defects showed superiority of the combination treatment of allogeneic UCB-MSCs and 4% hyaluronate (UCB-MSC-HA) when compared to microfracture with respect to cartilage grading on second look arthroscopy.118
Table 2.
Recent phase 2 and 3 clinical trials on MSCs in articular cartilage disease and osteoarthritis.
| Title | Cell type | Last update posted | Results | Number of patients | CT.gov ID |
|---|---|---|---|---|---|
| Allogenic adipose tissue-derived mesenchymal progenitor cells for the treatment of knee osteoarthritis | Allo-AD-MSC | 2023 | No results available | 104 | NCT04208646 |
| Effect of mesenchymal stem cells in primary knee osteoarthritis | Auto-AD-MSC | 2023 | No results available | 84 | NCT05783154 |
| Multicenter trial of stem cell therapy for osteoarthritis (MILES) | Auto-BMD-MSC, AD-MSC, and UCB-MSC | 2023 | BM-MSCs improved VAS scores at all timepoints from 1 to 12 points more significantly than AD-MSCs and hUCMSCs. BM-MSCs had comparable VAS scores to cortisteroid injections all timepoints from 1 to 12 months | 475 | NCT03818737 |
| A phase 3 study to evaluate the efficacy and safety of JointStem in treatment of osteoarthritis | Auto-AD-MSC | 2022 | Intra-articular injection of autologous culture-expanded AD-MSCs provided significant pain relief and functional improvements in patients with K-L grade 3 osteoarthritis | 261 | NCT03990805 |
| A phase 2 study to evaluate the efficacy and safety of JointStem in treatment of osteoarthritis | Auto-AD-MSC | 2021 | Decreased pain, impairment in physical functioning, emotional limitation at 9 and 12 months | 28 | NCT02674399 |
| Efficacy of allogeneic UCMSCs for treating large defects knee injury | Allo-UCB-MSC | 2021 | No results available | 50 | NCT05016011 |
| Chondrochymal for subjects with knee osteoarthritis (knee OA) | Allo-BMD-MSC | 2021 | No results available | 70 | NCT05027581 |
| Evaluation of safety and exploratory efficacy of CARTISTEM, a cell therapy product for articular cartilage defects | Allo-UCB-MSC | 2021 | Higher IKDC score at 12 months | 12 | NCT01733186 |
| Phase 2B clinical study of chondrogen for treatment of knee osteoarthritis | Allo-UCB-MSC | 2020 | No results available | 100 | NCT04520945 |
| Bone marrow versus adipose autologous mesenchymal stem cells for the treatment of knee osteoarthritis | Auto-BMD-MSC and AD-MSC | 2020 | No results available | 54 | NCT04351932 |
| Investigation of mesenchymal stem cell therapy for the treatment of osteoarthritis of the knee | Auto-BMD-MSC | 2019 | Both treatment groups experienced clinically and statistically significant improvement across the KOOS subscales | 32 | NCT02958267 |
| Clinical trial to evaluate efficacy and safety of JOINTSTEM in patients with degenerative arthritis | Auto-AD-MSC | 2019 | No results available | 24 | NCT02658344 |
| Autologous adipose tissue derived mesenchymal stem cells transplantation in patient with degenerative arthritis | Auto-AD-MSC | 2019 | No results available | 18 | NCT01300598 |
| Clinical trial to compare ReJoinTM to sodium hyaluronate injection for knee osteoarthritis cartilage defects | Auto-AD-MSC | 2018 | No results available | 28 | NCT02855073 |
There are currently 2 commercially available products available for use outside of the United States that are evaluated in phase 3 trials. JointStem represents an autologous stem cell-based medication injected into the joint cavity of patients with degenerative arthritis for renewal of cartilage along with alleviation of pain and improved joint function at 9- and 12-month time points. The product is currently approved by the Ministry of Health, Labor and Welfare of Japan and is used in a phase 2b/3a double-blinded multicenter trial scheduled for completion on December 30, 2023.105 CARTISTEM represents an allogenic UCB-MSC source injected in knee joint cavities and marketed as a treatment of cartilage defects in patients with degrative or repetitive traumatic osteoarthritis. Phase 1/2a clinical trials focusing on the treatment of grade 3-4 articular cartilage defects in knees demonstrated promising IKDC scores at 12 months. The product is currently market-approved for commercial sale by the Ministry of Food & Drug Safety and is currently undergoing phase 3 clinical trial in Japan.109
In summary, the totality of evidence demonstrates that MSCs, regardless of source, are safe for clinical use to treat knee OA. There have been a number of RCTs demonstrating the potential promising benefits of MSCs with regards to outcome measures such as the WOMAC, VAS, and mean walking distance. However, some studies still demonstrate some inconsistences with regards to clinical pain relief compared to current PRP injections/standards of practice. For example, a recent phase 2/3 four-arm parallel, multicenter, single-blind, RCT with 480 patients demonstrated non-superiority of autologous BM aspirate concentrate, UC tissue-derived MSCs, PRP, and cortical steroid injections in patients with knee OA after 1 year.119 A significant amount of heterogeneity still exists in the literature in regards to outcome, dosing protocols, and dose-dependent effects. In large part, results are short-term demonstrating some improvement over 12 months with few studies showing 5-year follow-up. Important to note that our review preferred more recent studies, for a more comprehensive discussion, Carneiro et al98 provides an in-depth review.
MSCs application in meniscus injuries
The knee meniscus functions to transmit joint reactive forces, lubricate, provide nutrition to the cartilage, and act as a shock absorber.120 It is a complex tissue comprised of both cells, specialized extracellular matrix molecules, and region-specific innervation and vascularization. In a healthy adult, there are 3 distinct regions of the meniscus: outer vasculature/neural region (red-red zone), inner completely avascular/aneural region (white-white zone), and a region that separates the two called a red-white region which has attributes of both. The vascularization of this tissue is highly relevant to the regenerative potential of the meniscus with the red-red zone having the greatest potential and the white-white zone having the least.121 Current repair techniques for arthroscopic meniscus repair, namely the inside-out, outside-in, and all-inside techniques are effective in treating lesions located in the peripheral vascularized zone; however, satisfactory treatment of the inner avascular region remains a clinical challenge.121–123 As a result, there has been a shift toward the utilization of biomaterials and bioengineering for meniscus repair incorporating stem cells and synthetic scaffolds. For example, a study examining the application of nanofiber-based scaffolds seeded with MSCs for the healing of avascular meniscus tears reported that the nanofiber-based scaffolds released collagenase which, enhanced meniscus healing in both in vitro and in vivo settings.123
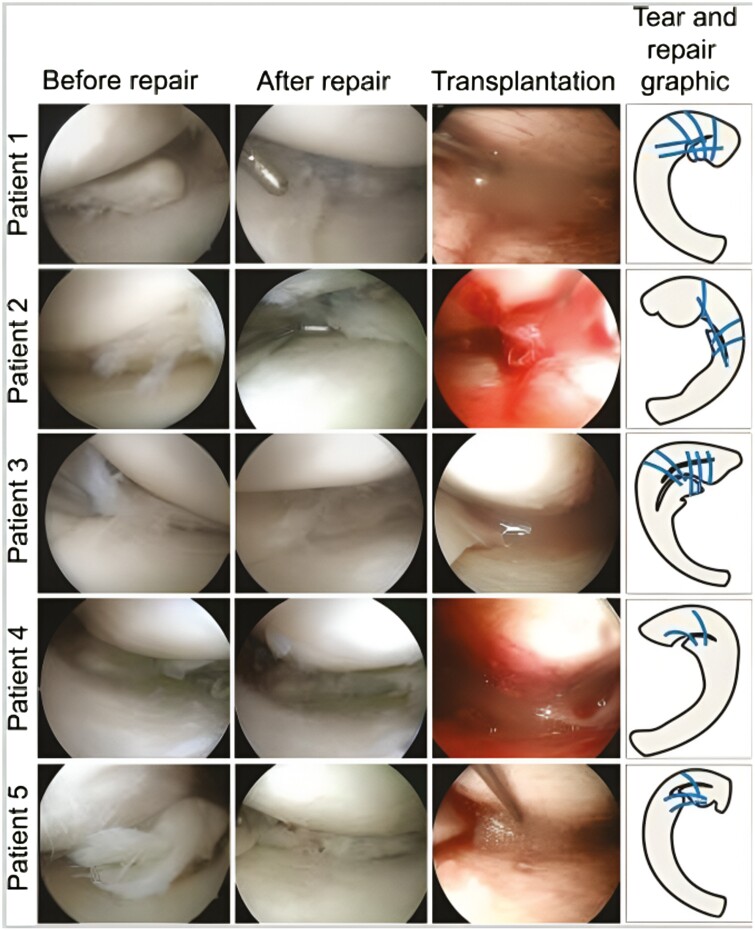
Recently, several clinical series employing MSCs have been conducted, including 2 by Sekiya et al124. The first combined surgical repair and S-MSCs transplantation. This series studied 5 patients with complex degenerative tears of the medial meniscus. They received surgical repair by standard surgical procedure with all-inside and inside-out repairs and a suspension of in vitro cultured autologous S-MSCs were transplanted into the repair utilizing an 18-gauge needle 2 weeks after surgery (Figure 3). Two years following cell transplantation there was an improvement in scores for “pain,” “daily living,” and “sports activities.”125 The second study combined meniscus repair with S-MSC transplantation in 6 patients with degenerative flaps and radial tears of the medial meniscus using the same procedure described above. The investigators performed arthroscopy and Lysholm scores along with the appearance of the repair site. The 4 flap tear patients showed tear zones that were completely stable and smooth in 2 patients and partial restoration in 2 patients at 52 weeks. The radial tear patients were reported to have completely healed at the final follow-up as well. The arthroscopy and Lysholm scores at 1 year were found to be higher compared to preoperative levels in all patients. Although the results of these series are encouraging, it is important to note that these studies lacked control subjects.126
Figure 3.
Torn meniscus before and after repair and transplantation of synovial MSCs. Arthroscopic images before and after repair and during transplantation of synovial MSCs, and tear & repair with suture threads (indicated by blue lines) graphics are shown.125 Figure 3 and caption reproduced from Sekiya et al.125 Copyright by Sage. Reprinted with permission.
There is only one randomized double-blind controlled trial studying the effects of MSC injection in knees post partial medial meniscectomy.127 The study consisted of 55 subjects in 3 groups receiving percutaneous injections of allogeneic MSCs: 50 × 106 cells, 150 × 106, and a control group only receiving HA. At 12 months, MRI scans demonstrated a significant increase in meniscal volume in 24% of the patients receiving 50 × 106 cells and 6% receiving 150 × 106 cells. None of the control group patients demonstrated increased meniscal volume. However, the study is limited because MRI was the only outcome measure used and clinical outcomes were not assessed.127–129 Whitehouse et al130 published a case series describing 5 patients who received BMD-MSCs injected onto a collagen scaffold and sutured into the white-white zone meniscus tears. At 12 months, 3 of the 5 patients demonstrated significant clinical improvement scores and MRI scans demonstrated in situ repair along with reduced abnormal scaffold signals; however, the other 2 patients had repeat tears and failed treatment at the 15-month follow-up.131 Despite optimistic results of current human studies, MSC studies in humans with meniscal pathology are still in the early stages and require more studies before a reliable assessment can be made.
With respect to the treatment of meniscal injuries/damage with MSCs, there are currently 3 registered clinical trials in the last 5 years (Table 3).132–134 The most recent trial investigates the efficacy of SMCS for degenerative meniscal tears.132 Another study from 2019 is evaluating the safety of Human Embryonic Stem Cell (hESC)-derived MSC-like cells for a treatment target in meniscus injury.133 The final study is incorporating BMD-MSCs for degenerative meniscal tears. Currently, degenerative disease is the predominant focus of meniscal MSC use, which may translate to traumatic sports injury of the meniscus in the future.134
Table 3.
Recent clinical trials on MSCs in meniscus injuries.
| Title | Cell type | Year initiated/reported | CT.gov ID |
|---|---|---|---|
| Clinical efficacy of exosome in degenerative meniscal injury (KNEEXO) | S-MSC (exosomes) | 2022 | NCT05261360 |
| Safety observation on hESC derived MSC like cell for the meniscus injury | Allo-hESC derived MSC like cell | 2019 | NCT03839238 |
| Mesenchymal stromal cells for degenerative meniscus injury | Auto-BMD-MSC | 2018 | NCT02033525 |
MSCs application in tendon and ligament injuries
Ligament and tendon injuries are common orthopedic problems affecting approximately 17 million patients a year in the United States. These injuries often result in slow healing that requires lengthy rehabilitation and the formation of scar tissue that requires years of remodeling to achieve functional tissue.135–137 More serious injuries with complete ligament and tendon disruption of require operative repair or tissue augmentation. Multiple approaches have been used in reconstructive surgeries for ligaments and tendons such as allografts, autografts, and synthetic biomaterials, but all approaches present challenges including donor-site morbidity, tissue rejection, graft failure, and transmission of infectious diseases.138 As a result, alternative methods such as cell-based therapies with MSC or MSC-like cells are being explored as adjuncts to surgical approaches.
The majority of studies regarding tendon and ligament healing have used BMD-MSCs, AD-MSCs, endogenous ligament-derived stem cells, and tendon-derived stem cells (TD-SCs). These studies also investigate a variety of delivery approaches including direct injection with or without collagen gel or tissue-engineered scaffolds seeded with MSCs to provide immediate mechanical support to injuries.135 Roth et al139 recently described that the therapeutic effects of locally applied MSCs are influenced by the cellular and molecular microenvironment at the injury site, in a bidirectional fashion. Modulating macrophages and promoting M1/M2 macrophage phenotype switch with MSCs plays a crucial role in reducing inflammation in injured tendons and joints. Based on this, it is reasonable to pursue future investigations relating to priming regimes of MSCs and tailoring to specific cell types or ECM components. Animal models investigating application of MSCs for ligament healing have focused on the anterior cruciate ligament (ACL) and generally show promise. Kanaya et al140 performed intra-articular injection of BMD-MSCs in Sprague-Dawley rats following surgical partial transection of the ACL. At 4 week follow-up, there was healing tissue within the transection gap compared to the contralateral side that received only ACL transection. Oe et al141 demonstrated similar positive outcomes when they demonstrated improved biomechanics and histology after administering MSCs in rats with partial ACL transection compared to saline control. In addition, the equine superficial digital flexor tendon and the human Achilles tendon have demonstrated numerous similarities in structure, function, and injury pathophysiology. Specifically, it has been shown that intralesional injection of MSCs in equine models has produced favorable effects, likely enabled by granulation tissue and the enclosed nature of core lesions that may have provided an appropriate scaffolding opportunity. It is hypothesized that a delivery vehicle may allow for various types of scaffolding or 3D tendon-like tissue constructs may improve stem cell retention at the site of injury.142 One RCT demonstrated that AD-MSCs treatment might lead to increased collagen cross-linking of remodeling scar tissue in the equine population.143 Another smaller study also showed that AD-MSCs and PRP can be considered a safe and effective strategy for the treatment of superficial digital flexor tendonitis in horses.144
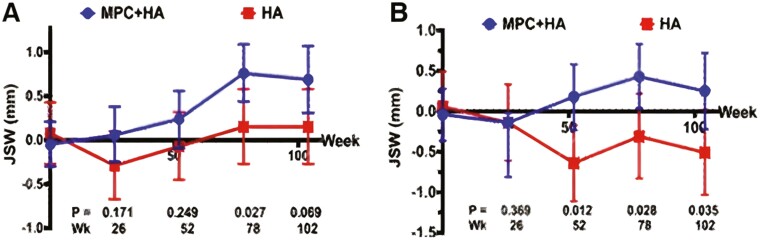
In comparison to the number of animal studies, there is a relative lack of translational human studies involving MSCs and ligament repair. One investigation performed by Wang et al145 provides promising evidence evaluating the efficacy of MSC augmented ligament reconstruction. A double-blinded RCT examined 17 patients that received a single intra-articular injection of 75 × 106 allogeneic immature mesenchymal precursor cells (MPCs) in a HA medium coupled with ACL reconstruction. Patients that had associated meniscal injury requiring one-third resection or reconstruction that would alter usual ACL rehabilitation were excluded. At 24-month follow-up, patients who received MSCs demonstrated significantly higher improvements in KOOS and pain scores. In addition, these patients were found to have reduced lateral tibiofemoral joint space narrowing at 24 months (Figure 4). The study did not, however, incorporate measurements of ACL healing such as clinical graft laxity grading or MRI signs demonstrating graft incorporation.146
Figure 4.
Change from baseline in tibiofemoral joint space width over 24 months (medical joint space width) following ACL reconstruction. (A) Change from baseline in tibiofemoral joint space width over 24 months (medial joint space width. (B) Mean and 95% CI shown. Abbreviations: HA, hyaluronan; MPC, mesenchymal precursor cells.145 Figure 4 reproduced from Wang et al.145 Copyright by BMC. Reproduced under Creative Commons Attribution (CC-BY) license.
Another study was performed by Moon et al147 examining the outcomes of UCB-MSCs in enhancing tendon-graft healing following ACL reconstruction (ACLR). The study enrolled 27 after the exclusion of 3 patients. In the experimental group, UCB-MSCs were suspended in a HA mixture and applied to tendon-bone interfaces of femoral tunnels during ACL reconstruction. A HA only group served as the control. There were no significant differences in the groups when measuring KT-2000 Knee Arthrometer (KT-2000) measurement, pivot shift, or International Knee Documentation Committee (IKDC) scores.
With regards to MSCs and tendon healing, the majority of studies have focused on rotator cuff repair. A study by Gulotta et al148 used BMD-MSCs to augment supraspinatus repair compared to supraspinatus repair alone in a rat model. There was no difference in load-to-failure testing at 30 days but a significantly increased strength at the insertion site in animals receiving BMD-MSCs at 45 days. Ina separate study, Hernigou149 demonstrated improved healing rates and a lower subsequent tear rate in a case-controlled study that involved applying 51,000 ± 25,000 autogenic BMD-MSCs during arthroscopic full-thickness supraspinatus repair.
Two clinical trials have been completed in the last 5 years employing MSCs in the treatment of tendinous injuries.150,151 Another 5 clinical trials are either active, recruiting, or planning to recruit participants to investigate healing using MSCs (Table 4).152–156 At this stage, while minimal adverse events have been reported, the literature is not convincing for the use of MSCs in the treatment of tendon or ligamentous injuries.150,151
Table 4.
Recent clinical trials on MSCs in tendon and ligament injuries.
| Title | Cell type | Year initiated/reported | Results | Number of patients | CT.gov ID |
|---|---|---|---|---|---|
| Treatment of tendon injury using allogenic adipose-derived mesenchymal stem cells (rotator cuff tear) | Allo-AD-MSC | 2021 | No difference between MSC, control group and placebo in pain, function, or appearance on MR at time points from 6 weeks up to 2 years | 24 | NCT02298023 |
| Treatment of tendon injury using mesenchymal stem cells (ALLO-ASC) | Allo-AD-MSC | 2021 | No difference in pain, performance, or appearance on ultrasound | 12 | NCT01856140 |
| Regeneration of posterior cruciate ligament injury using hypoxic conditioned allogenic adipose mesenchymal stem cell and condition medium | Allo-AD-MSC | 2021 | No results posted | N/A | NCT04889963 |
| Augmentation of anterior cruciate ligament reconstruction using mesenchymal stem cells and collagen matrix carrier (BioACL) | Auto-MSC (ACL stump) | 2020 | No results posted | N/A | NCT05582226 |
| Mesenchymal stem cells and amniotic membrane composite for supraspinatus tendon repair augmentation | Allo-AD-MSC | 2020 | No results posted | N/A | NCT04670302 |
| Treatment of tendon disease using autologous adipose-derived mesenchymal stem cells | Auto-AD-MSC | 2018 | No results posted | N/A | NCT03279796 |
| Treatment of intractable common extensor tendon injury using mesenchymal stem cells (Allo-ASC) | Allo-AD-MSC | 2018 | No results posted | N/A | NCT03449082 |
Applications of MSCs in treatment of intervertebral disc disease
Degenerative disc disease (DDD) is an umbrella term for intervertebral disc diseases, generally referring to pathologies of the lumbar spine from lumbar disc herniation or other discogenic low back pain. This collection of common diseases that increase with age and are growing in prevalence, has also been a target for treatment with MSCs.157 A 2021 meta-analysis of RCTs demonstrated that MSC therapy could decrease VAS scores and Oswestry Disability Index scores, suggesting potential for MSCs as a novel therapeutic intervention for DDD.158 The pathological processes of DDD are complex and multifactorial, but are comprised of immunological components involving various IL and tumor necrosis factor-alpha (TNF-α), both of which are implicated in the repair of discogenic disease by stem cells cocultured in an IDD-like environment.159
Specifically, at least 5 types of cells have been identified as potential treatment vectors for IDD: BMD-MSCs, AD-MSCs, UCB-MSCs, intervertebral-derived stem cells IDVSCs, and pluripotent stem cells (PSCs).157 BMSCs possess the capability to repair degenerated IVD tissues through the promotion of cell proliferation and enhancement of ECM components, including COL I and proteoglycans, while exosomes from BMD-MSCs can increase ECM production and encourage the expression of NP cell-related genes, ultimately achieving the desired effect of IVD self-repair.160 AD-MSCs, like BMD-MSCs, have the capacity to differentiate into NP cells, generate distinct growth factors, and contribute to IVD restoration at various levels.25 Several studies indicate that AD-MSCs are better suited than BMD-MSCs for the treatment of DDDs due to their superior differentiation potential, but they may also exhibit inferior endochondral osteogenic ability when compared to BMD-MSCs.161–163 Like other MSCs, UCB-MSCs are relatively new and have strong proliferation and differentiation abilities, low immunogenicity, and no tumorigenicity.164–166 Beeravolu et al167 demonstrated that differentiation of UCB-MSCs into chondrogenic progenitor cells in vitro resulted in significant improvements in cell structure, ECM, and glycosaminoglycan content compared to original UCB-MSCs. Furthermore, other studies have indicated that UCB-MSCs promote ECM formation by significantly increasing proteoglycan and COL II expression in the NP.168
The applications of IVDSCs face challenges, including low efficiency in separating cartilage endplate stem cells (CESCs) from cartilage endplates (CEPs), difficulty inducing CESCs to differentiate into hyaline cartilage tissue, and a harsh microenvironment of the IVD that poses a challenge to the viability of implanted stem cells.157 PSCs are stem cells with high self-renewal, proliferation, and differentiation abilities, and include IPSCs and ESCs. IPSCs are generated by inducing the expression of recombinant transcription factors, while ESCs are derived from early embryos and can differentiate into all 3 germ layers.169,170 However carcinogenicity, along with legal and ethical issues have limited the use of PSCs thus far. Presently, the greatest promise for stem cell therapy seems to be with BMD-SCs, AD-MSCs, and UCB-MSCs, with the question of optimal dose, frequency, time, and route of MSC transplantation at different stages of DDD needing to be explored in various clinical trials.158
In the last 10 years, there have been 3 completed clinical trials and 3 additional clinical trials currently registered to investigate the treatment of IDD with MSCs. One of which reported results showing the use of BMD-MSCs directly injected into the nucleus pulposus decreased lumbar pain VAS scores at all time points, decreased sciatic pain VAS scores at 6 and 12 months, and increased water content on MRI in lumbar discs at 12 months.171 Two other studies are exploring the use of various umbilical MSC types, which could theoretically be superior in restoring disc height in comparison to other cell types due to their actions on the ECM and prior benefits in cell structure.167,172–176 Lastly, there is one trial assessing safety of MPCs, which have not been explored as much as other cell types and may provide a new avenue for the stem cell treatment of IDD (Table 5).174
Table 5.
Recent clinical trials on MSCs in intervertebral disc disease.
| Title | Cell type | Year initiated/reported | Results | CT.gov ID |
|---|---|---|---|---|
| Effectiveness and safety of mesenchymal stem cell (MSC) implantation on degenerative discus disease patients (MSC) | Allo-UC-MSC | 2020 | No results posted | NCT04499105 |
| Human umbilical cord mesenchymal stem cells for the treatment of lumbar disc degeneration disease | Allo-UCB-MSC | 2020 | No results posted | NCT04414592 |
| Safety and preliminary efficacy study of mesenchymal precursor cells (MPCs) in subjects with lumbar back pain | Allo-MPC | 2020 | No results posted | NCT01290367 |
| Clinical trial based on the use of mesenchymal stem cells from autologous bone marrow in patients with lumbar intervertebral degenerative disc disease | Auto-BMD-MSC | 2017 | No results posted | NCT01513694 |
| Human autograft mesenchymal stem cell mediated stabilization of the degenerative lumbar spine | Auto vs allo-“MSCs” | 2017 | No results posted | NCT02529566 |
| Treatment of degenerative disc disease with allogenic mesenchymal stem cells (MSV) (Disc_allo) | Allo-BMD-MSC | 2017 | Decreased lumbar pain VAS scores at all time points, decreased sciatic pain VAS scores at 6 and 12 months, and increased water content on MRI in lumbar discs at 12 months | NCT01860417 |
Conclusion
The application of cell-based therapies with MSCs in musculoskeletal diseases is a burgeoning and promising area of medicine. MSCs have the potential to provide a diverse and versatile means of treating musculoskeletal diseases. Multiple animal and human models have demonstrated promising results in treating orthopedic injuries/diseases driven largely by the promotion of regeneration of tissues; however, studies to-date have not demonstrated consistent efficacy and further research is required to confirm their safe clinical applications. MSCs can be extracted from a variety of tissues, and biochemical experiments are unraveling the benefits and possible specific roles of specific tissue harvestings. One of the most attractive properties of MSCs is their anti-inflammatory and immunomodulatory effects. However, there are ongoing obstacles to their usage. The majority of studies to-date lack standardization of cell extraction, expansion, preparation, and delivery, which makes a comparison of studies and homogeneous results difficult. In addition, there is ongoing controversy and concern regarding MSCs’ potential risks of tumor formation; however, human trials using MSCs have established their safety in trials so far. Another issue is the relative paucity of human trials, which requires further research to evaluate the safety, efficacy, and applicability of MSCs in orthopedic surgery and the treatment of musculoskeletal diseases. Despite these challenges, progress is being made, and the potential for MSCs to enter the therapeutic armamentarium for musculoskeletal disease remains promising. The application of MSCs has the potential to revolutionize the way we approach and manage musculoskeletal diseases.
Contributor Information
Justin Trapana, Department of Orthopaedics, University of Miami Miller School of Medicine, Miami, United States; Interdisciplinary Stem Cell Institute, University of Miami Miller School of Medicine, Miami, United States.
Jonathan Weinerman, Department of Orthopaedics, University of Miami Miller School of Medicine, Miami, United States.
Danny Lee, Department of Orthopaedics, University of Miami Miller School of Medicine, Miami, United States.
Anil Sedani, Department of Orthopaedics, University of Miami Miller School of Medicine, Miami, United States.
David Constantinescu, Department of Orthopaedics, University of Miami Miller School of Medicine, Miami, United States.
Thomas M Best, Department of Orthopaedics, University of Miami Miller School of Medicine, Miami, United States; Interdisciplinary Stem Cell Institute, University of Miami Miller School of Medicine, Miami, United States.
Francis J Hornicek, Jr, Department of Orthopaedics, University of Miami Miller School of Medicine, Miami, United States; Interdisciplinary Stem Cell Institute, University of Miami Miller School of Medicine, Miami, United States.
Joshua M Hare, Interdisciplinary Stem Cell Institute, University of Miami Miller School of Medicine, Miami, United States.
Author contributions
Justin Trapana: Conception and design, Administrative support, Collection and/or assembly of data, Data analysis and interpretation, Manuscript writing, Final approval of manuscript. Jonathan Weinerman: Conception and design, Administrative support, Manuscript writing, Final approval of manuscript. Danny Lee, Anil Sedani, David Constantinescu: Conception and design, Administrative support, Final approval of manuscript. Thomas M. Best, Francis J. Hornicek, Jr.: Conception and design, Administrative support, Data analysis and interpretation, Final approval of manuscript. Joshua M. Hare, MD: Conception and design, Administrative support, Data analysis and interpretation, Manuscript writing, Final approval of manuscript
Conflicts of interest
J.M.H. reports having a patent for cardiac cell-based therapy and holds equity in Vestion Inc. and maintains a professional relationship with Vestion Inc. as a consultant and member of the Board of Directors and Scientific Advisory Board. Vestion Inc. did not play a role in the design, conduct, or funding of the study. J.M.H. is the Chief Scientific Officer, a compensated consultant and board member for Longeveron Inc. and holds equity in Longeveron. J.M.H. is also the co-inventor of intellectual property licensed to Longeveron. Longeveron did not play a role in the design, conduct, or funding of the study. The University of Miami is an equity owner in Longeveron Inc., which has licensed intellectual property from the University of Miami.
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
References
- 1. Barry F. MSC therapy for osteoarthritis: an unfinished story. J Orthop Res. 2019;37(6):1229-1235. 10.1002/jor.24343 [DOI] [PubMed] [Google Scholar]
- 2. Balkan W, Banerjee M, Trapana J, Hare JM.. Marrow-Derived Stroma Cells for Cardiac Regeneration. Vol 1. Emerging Technologies for Heart Diseases. Elsevier; 2020. [Google Scholar]
- 3. Fridenshteĭn A, Piatetskiĭ S II, Petrakova KV.. Kosteobrazovanie v transplantatakh kostnomozgovykh kletok. [Osteogenesis in transplants of bone marrow cells]. Arkh Anat Gistol Embriol. 1969;56(3):3-11. [PubMed] [Google Scholar]
- 4. Andrzejewska A, Lukomska B, Janowski M.. Concise review: mesenchymal stem cells: from roots to boost. Stem Cells. 2019;37(7):855-864. 10.1002/stem.3016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Caplan AI. Mesenchymal stem cells. J Orthop Res. 1991;9(5):641-650. 10.1002/jor.1100090504 [DOI] [PubMed] [Google Scholar]
- 6. Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315-317. 10.1080/14653240600855905 [DOI] [PubMed] [Google Scholar]
- 7. Hardy WR, Moldovan NI, Moldovan L, et al. Transcriptional networks in single perivascular cells sorted from human adipose tissue reveal a hierarchy of mesenchymal stem cells. Stem Cells. 2017;35(5):1273-1289. 10.1002/stem.2599 [DOI] [PubMed] [Google Scholar]
- 8. Covas DT, Panepucci RA, Fontes AM, et al. Multipotent mesenchymal stromal cells obtained from diverse human tissues share functional properties and gene-expression profile with CD146+ perivascular cells and fibroblasts. Exp Hematol. 2008;36(5):642-654. 10.1016/j.exphem.2007.12.015 [DOI] [PubMed] [Google Scholar]
- 9. Wagner W, Wein F, Seckinger A, et al. Comparative characteristics of mesenchymal stem cells from human bone marrow, adipose tissue, and umbilical cord blood. Exp Hematol. 2005;33(11):1402-1416. 10.1016/j.exphem.2005.07.003 [DOI] [PubMed] [Google Scholar]
- 10. Crisan M, Yap S, Casteilla L, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3(3):301-313. 10.1016/j.stem.2008.07.003 [DOI] [PubMed] [Google Scholar]
- 11. Guimarães-Camboa N, Cattaneo P, Sun Y, et al. Pericytes of multiple organs do not behave as mesenchymal stem cells in vivo. Cell Stem Cell. 2017;20(3):345-359.e5. 10.1016/j.stem.2016.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Volarevic V, Gazdic M, Simovic Markovic B, et al. Mesenchymal stem cell-derived factors: immuno-modulatory effects and therapeutic potential. Biofactors. 2017;43(5):633-644. 10.1002/biof.1374 [DOI] [PubMed] [Google Scholar]
- 13. Berebichez-Fridman R, Gómez-García R, Granados-Montiel J, et al. The holy grail of orthopedic surgery: mesenchymal stem cells-their current uses and potential applications. Stem Cells Int. 2017;2017:2638305. 10.1155/2017/2638305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Khosravi N, DaCosta RS, Davies JE.. New insights into spatio-temporal dynamics of mesenchymal progenitor cell ingress during peri-implant wound healing: provided by intravital imaging. Biomaterials. 2021;273:120837. 10.1016/j.biomaterials.2021.120837 [DOI] [PubMed] [Google Scholar]
- 15. Khosravi N, Maeda A, DaCosta RS, Davies JE. Nanosurfaces modulate the mechanism of peri-implant endosseous healing by regulating neovascular morphogenesis. Commun Biol. 2018;1(1):72. 10.1038/s42003-018-0074-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Aubin JE. Osteoprogenitor cell frequency in rat bone marrow stromal populations: role for heterotypic cell-cell interactions in osteoblast differentiation. J Cell Biochem. 1999;72(3):396-410. [PubMed] [Google Scholar]
- 17. Kusumbe AP, Ramasamy SK, Adams RH.. Coupling of angiogenesis and osteogenesis by a specific vessel subtype in bone. Nature. 2014;507(7492):323-328. 10.1038/nature13145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Musiał-Wysocka A, Kot M, Majka M.. The pros and cons of mesenchymal stem cell-based therapies. Cell Transplant. 2019;28(7):801-812. 10.1177/0963689719837897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Keating A. Mesenchymal stromal cells. Curr Opin Hematol. 2006;13(6):419-425. 10.1097/01.moh.0000245697.54887.6f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Stamnitz S, Klimczak A.. Mesenchymal stem cells, bioactive factors, and scaffolds in bone repair: from research perspectives to clinical practice. Cells. 2021;10(8):1925. 10.3390/cells10081925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Galipeau J, Sensébé L.. Mesenchymal stromal cells: clinical challenges and therapeutic opportunities. Cell Stem Cell. 2018;22(6):824-833. 10.1016/j.stem.2018.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hoogduijn MJ, Lombardo E.. Mesenchymal stromal cells anno 2019: dawn of the therapeutic era? Concise review. Stem Cells Transl. Med. 2019;8(11):1126-1134. 10.1002/sctm.19-0073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lohan P, Murphy N, Treacy O, et al. Third-party allogeneic mesenchymal stromal cells prevent rejection in a pre-sensitized high-risk model of corneal transplantation. Front Immunol. 2018;9:2666. 10.3389/fimmu.2018.02666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cao Y, Gomes SA, Rangel EB, et al. S-Nitrosoglutathione reductase-dependent PPARγ denitrosylation participates in MSC-derived adipogenesis and osteogenesis. J Clin Invest. 2015;125(4):1679-1691. 10.1172/JCI73780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mochizuki T, Muneta T, Sakaguchi Y, et al. Higher chondrogenic potential of fibrous synovium- and adipose synovium-derived cells compared with subcutaneous fat-derived cells: distinguishing properties of mesenchymal stem cells in humans. Arthritis Rheum. 2006;54(3):843-853. 10.1002/art.21651 [DOI] [PubMed] [Google Scholar]
- 26. Shariatzadeh M, Song J, Wilson SL.. Correction to: the efficacy of different sources of mesenchymal stem cells for the treatment of knee osteoarthritis. Cell Tissue Res. 2019;378(3):559. 10.1007/s00441-019-03086-8 [DOI] [PubMed] [Google Scholar]
- 27. Futami I, Ishijima M, Kaneko H, et al. Isolation and characterization of multipotential mesenchymal cells from the mouse synovium. PLoS One. 2012;7(9):e45517. 10.1371/journal.pone.0045517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jin HJ, Bae YK, Kim M, et al. Comparative analysis of human mesenchymal stem cells from bone marrow, adipose tissue, and umbilical cord blood as sources of cell therapy. Int J Mol Sci. 2013;14(9):17986-18001. 10.3390/ijms140917986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kögler G, Sensken S, Airey JA, et al. A new human somatic stem cell from placental cord blood with intrinsic pluripotent differentiation potential. J Exp Med. 2004;200(2):123-135. 10.1084/jem.20040440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Palsson BO, Palsson H, Lightfoot EN.. Mathematical modelling of dynamics and control in metabolic networks. III. Linear reaction sequences. J Theor Biol. 1985;113(2):231-259. 10.1016/s0022-5193(85)80226-5 [DOI] [PubMed] [Google Scholar]
- 31. Schugar RC, Chirieleison SM, Wescoe KE, et al. High harvest yield, high expansion, and phenotype stability of CD146 mesenchymal stromal cells from whole primitive human umbilical cord tissue. J Biomed Biotechnol. 2009;2009:789526. 10.1155/2009/789526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sarugaser R, Lickorish D, Baksh D, Hosseini MM, Davies JE.. Human umbilical cord perivascular (HUCPV) cells: a source of mesenchymal progenitors. Stem Cells. 2005;23(2):220-229. 10.1634/stemcells.2004-0166 [DOI] [PubMed] [Google Scholar]
- 33. Lu LL, Liu YJ, Yang SG, et al. Isolation and characterization of human umbilical cord mesenchymal stem cells with hematopoiesis-supportive function and other potentials. Haematologica. 2006;91(8):1017-1026. [PubMed] [Google Scholar]
- 34. Davies JE, Walker JT, Keating A.. Concise review: wharton’s jelly: the rich, but enigmatic, source of mesenchymal stromal cells. Stem Cells Transl. Med. 2017;6(7):1620-1630. 10.1002/sctm.16-0492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. de Witte SFH, Luk F, Sierra Parraga JM, et al. Immunomodulation by therapeutic mesenchymal stromal cells (MSC) is triggered through phagocytosis of MSC by monocytic cells. Stem Cells. 2018;36(4):602-615. 10.1002/stem.2779 [DOI] [PubMed] [Google Scholar]
- 36. Mayourian J, Cashman TJ, Ceholski DK, et al. Experimental and computational insight into human mesenchymal stem cell paracrine signaling and heterocellular coupling effects on cardiac contractility and arrhythmogenicity. Circ Res. 2017;121(4):411-423. 10.1161/CIRCRESAHA.117.310796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mayourian J, Ceholski DK, Gorski PA, et al. Exosomal microRNA-21-5p mediates mesenchymal stem cell paracrine effects on human cardiac tissue contractility. Circ Res. 2018;122(7):933-944. 10.1161/CIRCRESAHA.118.312420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Banerjee MN, Bolli R, Hare JM.. Clinical studies of cell therapy in cardiovascular medicine: recent developments and future directions. Circ Res. 2018;123(2):266-287. 10.1161/CIRCRESAHA.118.311217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pittenger MF, Discher DE, Peault BM, et al. Mesenchymal stem cell perspective: cell biology to clinical progress. NPJ Regen Med. 2019;4(22):1-15. 10.1038/s41536-019-0083-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Menale C, Campodoni E, Palagano E, et al. Mesenchymal stromal cell-seeded biomimetic scaffolds as a factory of soluble RANKL in rankl-deficient osteopetrosis. Stem Cells Transl. Med. 2019;8(1):22-34. 10.1002/sctm.18-0085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Joseph A, Baiju I, Bhat IA, et al. Mesenchymal stem cell-conditioned media: a novel alternative of stem cell therapy for quality wound healing. J Cell Physiol. 2020;235(7-8):5555-5569. 10.1002/jcp.29486 [DOI] [PubMed] [Google Scholar]
- 42. Luk F, de Witte SF, Korevaar SS, et al. Inactivated mesenchymal stem cells maintain immunomodulatory capacity. Stem Cells Dev. 2016;25(18):1342-1354. 10.1089/scd.2016.0068 [DOI] [PubMed] [Google Scholar]
- 43. Zhang J, Pan J, Jing W.. Motivating role of type H vessels in bone regeneration. Cell Prolif. 2020;53(9):e12874. 10.1111/cpr.12874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Jacobs SA, Roobrouck VD, Verfaillie CM, Van Gool SW.. Immunological characteristics of human mesenchymal stem cells and multipotent adult progenitor cells. Immunol Cell Biol. 2013;91(1):32-39. 10.1038/icb.2012.64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ryan JM, Barry FP, Murphy JM, Mahon BP. Mesenchymal stem cells avoid allogeneic rejection. J Inflamm (Lond). 2005;2(8):1-11. 10.1186/1476-9255-2-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ridge SM, Sullivan FJ, Glynn SA.. Mesenchymal stem cells: key players in cancer progression. Mol Cancer. 2017;16(1):31. 10.1186/s12943-017-0597-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Oryan A, Monazzah S, Bigham-Sadegh A.. Bone injury and fracture healing biology. Biomed Environ Sci. 2015;28(1):57-71. 10.3967/bes2015.006 [DOI] [PubMed] [Google Scholar]
- 48. Hernigou P, Homma Y, Flouzat-Lachaniette CH, et al. Cancer risk is not increased in patients treated for orthopaedic diseases with autologous bone marrow cell concentrate. J Bone Joint Surg Am. 2013;95(24):2215-2221. 10.2106/JBJS.M.00261 [DOI] [PubMed] [Google Scholar]
- 49. Lalu MM, McIntyre L, Pugliese C, et al. ; Canadian Critical Care Trials Group. Safety of cell therapy with mesenchymal stromal cells (SafeCell): a systematic review and meta-analysis of clinical trials. PLoS One. 2012;7(10):e47559. 10.1371/journal.pone.0047559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Schweizer MT, Wang H, Bivalacqua TJ, et al. A phase I study to assess the safety and cancer-homing ability of allogeneic bone marrow-derived mesenchymal stem cells in men with localized prostate cancer. Stem Cells Transl. Med. 2019;8(5):441-449. 10.1002/sctm.18-0230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Granero-Moltó F, Weis JA, Miga MI, et al. Regenerative effects of transplanted mesenchymal stem cells in fracture healing. Stem Cells. 2009;27(8):1887-1898. 10.1002/stem.103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Colnot C. Skeletal cell fate decisions within periosteum and bone marrow during bone regeneration. J Bone Miner Res. 2009;24(2):274-282. 10.1359/jbmr.081003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Neagu TP, Ţigliş M, Cocoloş I, Jecan CR.. The relationship between periosteum and fracture healing. Rom J Morphol Embryol. 2016;57(4):1215-1220. [PubMed] [Google Scholar]
- 54. Han Y, Li X, Zhang Y, et al. Mesenchymal stem cells for regenerative medicine. Cells. 2019;8(8):886. 10.3390/cells8080886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wang X, Wang Y, Gou W, et al. Role of mesenchymal stem cells in bone regeneration and fracture repair: a review. Int Orthop. 2013;37(12):2491-2498. 10.1007/s00264-013-2059-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Giannelli M, Chellini F, Sassoli C, et al. Photoactivation of bone marrow mesenchymal stromal cells with diode laser: effects and mechanisms of action. J Cell Physiol. 2013;228(1):172-181. 10.1002/jcp.24119 [DOI] [PubMed] [Google Scholar]
- 57. Hu K, Olsen BR.. The roles of vascular endothelial growth factor in bone repair and regeneration. Bone. 2016;91:30-38. 10.1016/j.bone.2016.06.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Mendelson A, Frank E, Allred C, et al. Chondrogenesis by chemotactic homing of synovium, bone marrow, and adipose stem cells in vitro. FASEB J. 2011;25(10):3496-3504. 10.1096/fj.10-176305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Wang X, Wang C, Gou W, et al. The optimal time to inject bone mesenchymal stem cells for fracture healing in a murine model. Stem Cell Res Ther. 2018;9(1):272. 10.1186/s13287-018-1034-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Emadedin M, Labibzadeh N, Fazeli R, et al. Percutaneous autologous bone marrow-derived mesenchymal stromal cell implantation is safe for reconstruction of human lower limb long bone atrophic nonunion. Cell J. 2017;19(1):159-165. 10.22074/cellj.2016.4866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Mott A, Mitchell A, McDaid C, et al. Systematic review assessing the evidence for the use of stem cells in fracture healing. Bone Jt Open. 2020;1(10):628-638. 10.1302/2633-1462.110.BJO-2020-0129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Khatkar H, See A.. Stem cell therapy in the management of fracture non-union - evaluating cellular mechanisms and clinical progress. Cureus. 2021;13(3):e13869. 10.7759/cureus.13869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Lee PY. Autologous bone marrow concentration for femoral shaft fracture union. ClinicalTrials.gov Identifier: NCT03794622; 2019. Retrieved from: https://clinicaltrials.gov/study/NCT03794622 [Google Scholar]
- 64. Gomez-Barrena E. A comparative study of 2 doses of BM autologous H-MSC+biomaterial vs iliac crest autograft for bone healing in non-union (ORTHOUNION). ClinicalTrials.gov Identifier: NCT03325504; 2017. Retrieved from: https://clinicaltrials.gov/study/NCT03325504 [Google Scholar]
- 65. Aghdami N. Autologous BM-MSC transplantation in combination with platelet lysate (PL) for nonunion treatment. ClinicalTrials.gov Identifier: NCT02448849; 2015. Retrieved from: https://clinicaltrials.gov/study/NCT02448849. [Google Scholar]
- 66. Segur JM. Allogeneic mesenchymal stromal cells in elderly patients with hip fracture. ClinicalTrials.gov Identifier: NCT02630836; 2015. Retrieved form: https://clinicaltrials.gov/study/NTC02630836. [Google Scholar]
- 67. Granell F. Mesenchymal stromal cells for the treatment of non-union fractures of long bones. ClinicalTrials.gov Identifier: NCT02230514; 2014. Retrieved from: https://clinicaltrials.gov/study/NTC02230514 [Google Scholar]
- 68. Dilogo IH. Allogenic mesenchymal stem cell for bone defect or non union fracture (AMSC). ClinicalTrials.gov Identifier: NCT02307435; 2014. Retrieved from: https://clinicaltrials.gov/study/NTC02307435. [Google Scholar]
- 69. Ghorbani M. Allogeneic mesenchymal stem cell transplantation in tibial closed diaphyseal fractures. ClinicalTrials.gov Identifier: NCT02140528; 2014. Retrieved from: https://clinicaltrials.gov/study/NTC02140528. [Google Scholar]
- 70. Peivandi MT. Evaluation the treatment of nonunion of long bone fracture of lower extremities (femur and tibia) using mononuclear stem cells from the iliac wing within a 3-D tissue engineered scaffold. ClinicalTrials.gov Identifier: NCT01958502; 2013. Retrieved from: https://clinicaltrials.gov/study/NTC01958502. [Google Scholar]
- 71. Raichandani K, Agarwal S, Jain H, Bharwani N.. Mortality profile after 2 years of hip fractures in elderly patients treated with early surgery. J Clin Orthop Trauma. 2021;18:1-5. 10.1016/j.jcot.2021.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Dueweke JJ, Awan TM, Mendias CL.. Regeneration of skeletal muscle after eccentric injury. J Sport Rehabil. 2017;26(2):171-179. 10.1123/jsr.2016-0107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Yin H, Price F, Rudnicki MA.. Satellite cells and the muscle stem cell niche. Physiol Rev. 2013;93(1):23-67. 10.1152/physrev.00043.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Dezawa M, Ishikawa H, Itokazu Y, et al. Bone marrow stromal cells generate muscle cells and repair muscle degeneration. Science. 2005;309(5732):314-317. 10.1126/science.1110364 [DOI] [PubMed] [Google Scholar]
- 75. Sun D, Martinez CO, Ochoa O, et al. Bone marrow-derived cell regulation of skeletal muscle regeneration. FASEB J. 2009;23(2):382-395. 10.1096/fj.07-095901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Klimczak A, Kozlowska U, Kurpisz M.. Muscle stem/progenitor cells and mesenchymal stem cells of bone marrow origin for skeletal muscle regeneration in muscular dystrophies. Arch Immunol Ther Exp (Warsz). 2018;66(5):341-354. 10.1007/s00005-018-0509-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Iyer SR, Scheiber AL, Yarowsky P, et al. Exosomes isolated from platelet-rich plasma and mesenchymal stem cells promote recovery of function after muscle injury. Am J Sports Med. 2020;48(9):2277-2286. 10.1177/0363546520926462 [DOI] [PubMed] [Google Scholar]
- 78. Flück M, Kasper S, Benn MC, et al. Transplant of autologous mesenchymal stem cells halts fatty atrophy of detached rotator cuff muscle after tendon repair: molecular, microscopic, and macroscopic results from an ovine model. Am J Sports Med. 2021;49(14):3970-3980. 10.1177/03635465211052566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Strickland M. Allogeneic human umbilical cord mesenchymal stem cells for a single male patient with duchenne muscular dystrophy (DMD). ClinicalTrials.gov Identifier: NCT02235844; 2014. Retrieved from: https://clinicaltrials.gov/study/NCT02235844. [Google Scholar]
- 80. Arab L. Intramuscular transplantation of muscle derived stem cell and adipose derived mesenchymal stem cells in patients with facioscapulohumeral dystrophy (FSHD). ClinicalTrials.gov Identifier: NCT02208713; 2014. Retrieved from: https://clinicaltrials.gov/study/NCT02208713. [Google Scholar]
- 81. Ashrafi M. Efficacy of allogeneic umbilical cord derived hematopoietic and mesenchymal stem cells in cerebral palsy. ClinicalTrials.gov Identifier: NCT03795974; 2019. Retrieved form: https://clinicaltrials.gov/study/NCT03795974. [Google Scholar]
- 82. Smid P. Clinical study on mesenchymal stem cells used in the reconstruction surgery of the supraspinatus muscle lesions. ClinicalTrials.gov Identifier: NCT03068988; 2017. Retrieved from: https://clinicaltrials.gov/study/NCT03068988. [Google Scholar]
- 83. Florea V, Bagno L, Rieger AC, Hare JM.. Attenuation of frailty in older adults with mesenchymal stem cells. Mech Ageing Dev. 2019;181:47-58. 10.1016/j.mad.2019.111120 [DOI] [PubMed] [Google Scholar]
- 84. Schulman IH, Balkan W, Hare JM.. Mesenchymal stem cell therapy for aging frailty. Front Nutr. 2018;5(108):1-11. 10.3389/fnut.2018.00108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Hare JM, Beerman I.. Potential of stem cell-based therapy to restore function in aging systems: are we there yet? J Gerontol A Biol Sci Med Sci. 2022;77(7):1292-1294. 10.1093/gerona/glac003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Golpanian S, DiFede DL, Khan A, et al. Allogeneic human mesenchymal stem cell infusions for aging frailty. J Gerontol A Biol Sci Med Sci. 2017;72(11):1505-1512. 10.1093/gerona/glx056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Tompkins BA, DiFede DL, Khan A, et al. Allogeneic mesenchymal stem cells ameliorate aging frailty: a phase II randomized, double-blind, placebo-controlled clinical trial. J Gerontol A Biol Sci Med Sci. 2017;72(11):1513-1522. 10.1093/gerona/glx137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Abramoff B, Caldera FE.. Osteoarthritis: pathology, diagnosis, and treatment options. Med Clin North Am. 2020;104(2):293-311. 10.1016/j.mcna.2019.10.007 [DOI] [PubMed] [Google Scholar]
- 89. Balkovec C, Adams MA, Dolan P, McGill SM.. Annulus fibrosus can strip hyaline cartilage end plate from subchondral bone: a study of the intervertebral disk in tension. Global Spine J. 2015;5(5):360-365. 10.1055/s-0035-1546956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Hu Y, Chen X, Wang S, Jing Y, Su J.. Subchondral bone microenvironment in osteoarthritis and pain. Bone Res. 2021;9(1):20. 10.1038/s41413-021-00147-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Kemble S, Croft AP.. Critical role of synovial tissue-resident macrophage and fibroblast subsets in the persistence of joint inflammation. Front Immunol. 2021;12:715894. 10.3389/fimmu.2021.715894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Mathiessen A, Conaghan PG.. Synovitis in osteoarthritis: current understanding with therapeutic implications. Arthritis Res Ther. 2017;19(1):18. 10.1186/s13075-017-1229-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Luo P, Yuan Q, Wan X, Yang M, Xu P. Effects of immune cells and cytokines on different cells in OA. J Inflamm Res. 2023;2023(16):2329-2343. 10.2147/JIR.S413578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Rizzo MG, Best TM, Huard J, et al. Therapeutic perspectives for inflammation and senescence in osteoarthritis using mesenchymal stem cells, mesenchymal stem cell-derived extracellular vesicles and senolytic agents. Cells. 2023;12(10):1421. 10.3390/cells12101421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. D’Arrigo D, Roffi A, Cucchiarini M, et al. Secretome and extracellular vesicles as new biological therapies for knee osteoarthritis: a systematic review. J Clin Med. 2019;8(11):1867. 10.3390/jcm8111867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Bashir J, Sherman A, Lee H, Kaplan L, Hare JM.. Mesenchymal stem cell therapies in the treatment of musculoskeletal diseases. Pm r. 2014;6(1):61-69. 10.1016/j.pmrj.2013.05.007 [DOI] [PubMed] [Google Scholar]
- 97. Migliorini F, Rath B, Colarossi G, et al. Improved outcomes after mesenchymal stem cells injections for knee osteoarthritis: results at 12-months follow-up: a systematic review of the literature. Arch Orthop Trauma Surg. 2020;140(7):853-868. 10.1007/s00402-019-03267-8 [DOI] [PubMed] [Google Scholar]
- 98. Carneiro DC, Araujo LT, Santos GC, et al. Clinical trials with mesenchymal stem cell therapies for osteoarthritis: challenges in the regeneration of articular cartilage. Int J Mol Sci. 2023;24(12):9939. 10.3390/ijms24129939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Kim SH, Ha CW, Park YB, et al. Intra-articular injection of mesenchymal stem cells for clinical outcomes and cartilage repair in osteoarthritis of the knee: a meta-analysis of randomized controlled trials. Arch Orthop Trauma Surg. 2019;139(7):971-980. 10.1007/s00402-019-03140-8 [DOI] [PubMed] [Google Scholar]
- 100. Zhao D, Pan JK, Yang WY, et al. Intra-articular injections of platelet-rich plasma, adipose mesenchymal stem cells, and bone marrow mesenchymal stem cells associated with better outcomes than hyaluronic acid and saline in knee osteoarthritis: a systematic review and network meta-analysis. Arthroscopy. 2021;37(7):2298-2314.e10. 10.1016/j.arthro.2021.02.045 [DOI] [PubMed] [Google Scholar]
- 101. Lee WS, Kim HJ, Kim KI, Kim GB, Jin W.. Intra-articular injection of autologous adipose tissue-derived mesenchymal stem cells for the treatment of knee osteoarthritis: a phase IIb, randomized, placebo-controlled clinical trial. Stem Cells Transl Med. 2019;8(6):504-511. 10.1002/sctm.18-0122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Bao C, Zhang C, Wang G, et al. Allogenic adipose tissue-derived mesenchymal progenitor cells for the treatment of knee osteoarthritis. ClinicalTrials.gov Identifier: NCT04208646; 2019. Retrieved from: https://clinicaltrials.gov/study/04208646. [Google Scholar]
- 103. Khasru MR. Effect of mesenchymal stem cells in primary knee osteoarthritis. ClinicalTrials.gov Identifier: NCT05783154; 2023. Retrieved from: https://clinicaltrials.gov/study/NCT05783154.
- 104. Boden SD. Multicenter trial of stem cell therapy for osteoarthritis (MILES). ClinicalTrials.gov Identifier: NCT03818737; 2019. Retrieved from: https://clinicaltrials.gov/study/NCT03818737. [Google Scholar]
- 105. Kim K, Lee W, Hwang S, et al. A phase 3 study to evaluate the efficacy and safety of JointStem in treatment of osteoarthritis. ClinicalTrials.gov Identifier: NCT03990805; 2019. Retrieved from: https://clinicaltrials.gov/study/NCT03990805. [Google Scholar]
- 106. Nature Cell Co. A phase 2 study to evaluate the efficacy and safety of JointStem in treatment of osteoarthritis. ClinicalTrials.gov Identifier: NCT02674399; 2016. Retrieved from: https://clinicaltrials.gov/study/NCT02674399. [Google Scholar]
- 107. Hisham BA. Efficacy of allogeneic UCMSCs for treating large defects knee injury. ClinicalTrials.gov Identifier: NCT05016011; 2021. Retrieved from: https://clinicaltrials.gov/study/NCT05016011. [Google Scholar]
- 108. Taiwan Bio Therapuetics Co. Chondrochymal® for subjects with knee osteoarthritis (knee OA). ClinicalTrials.gov Identifier: NCT05027581; 2021. Retrieved from: https://clinicaltrails.gov/study/NCT05027581. [Google Scholar]
- 109. Cole BJ, Gomoll AH. Evaluation of safety and exploratory efficacy of CARTISTEM®, a cell therapy product for articular cartilage defects. ClinicalTrials.gov Identifier: NCT01733186; 2012. Retrieved from: https://clinicaltrials.gov/study/NCT01733186. [Google Scholar]
- 110. Hisham BA. Phase 2B clinical study of chondrogen for treatment of knee osteoarthritis. ClinicalTrials.gov Identifier: NCT04520945; 2020. Retrieved from: https://clinicaltrials.gov/study/NCT04520945. [Google Scholar]
- 111. Chiriboga CA. Bone marrow versus adipose autologous mesenchymal stem cells for the treatment of knee osteoarthritis. ClinicalTrials.gov Identifier: NCT04351932; 2020. Retrieved from: https://clinicaltrails.gov/study/NCT04351932. [Google Scholar]
- 112. Ruane JJ. Investigation of mesenchymal stem cell therapy for the treatment of osteoarthritis of the knee. ClinicalTrials.gov Identifier: NCT02958267; 2016. Retrieved from: https://clinicaltrials.gov/study/NCT02958267. [Google Scholar]
- 113. Kim K. Clinical trial to evaluate efficacy and safety of JOINTSTEM in patients with degenerative arthritis. ClinicalTrials.gov Identifier: NCT02658344; 2016. Retrieved from: https://clinicaltrials.gov/study/NCT02658344. [Google Scholar]
- 114. Yoon SK. Autologous adipose tissue derived mesenchymal stem cells transplantation in patient with degenerative arthritis. ClinicalTrials.gov Identifier: NCT01300598; 2008. Retrieved from: https://clinicaltrial.gov/study/NCT01300598. [Google Scholar]
- 115. Wang Y. Clinical trial to compare ReJoinTM to sodium hyaluronate injection for knee osteoarthritis cartilage defects. ClinicalTrials.gov Identifier: NCT02855073; 2016. Retrieved from: https://clinicaltrails.gov/study/NCT02855073. [Google Scholar]
- 116. Garcia-Sancho J, Vega A, Orozco L, Sanchez A, Moraleda JM. Treatment of knee osteoarthritis with allogenic mesenchymal stem cells (MSV_allo). ClinicalTrials.gov Identifier: NCT01586312; 2012. Retrieved from: https//clinicaltrials.gov/study/NCT01586312. [Google Scholar]
- 117. Garcia-Sancho J. Treatment of degenerative disc disease with allogenic mesenchymal stem cells (MSV) (Disc_allo). ClinicalTrials.gov Identifier: NCT01860417; 2013. Retrieved from: https//clinicaltrials.gov/study/NCT01860417. [Google Scholar]
- 118. Lim HC, Park YB, Ha CW, et al. ; Cartistem Research Group. Allogeneic umbilical cord blood-derived mesenchymal stem cell implantation versus microfracture for large, full-thickness cartilage defects in older patients: a multicenter randomized clinical trial and extended 5-year clinical follow-up. Orthop J Sports Med. 2021;9(1):2325967120973052. 10.1177/2325967120973052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Mautner K, Gottschalk M, Boden SD, et al. Cell-based versus corticosteroid injections for knee pain in osteoarthritis: a randomized phase 3 trial. Nat Med. 2023;29(12):3120-3126. 10.1038/s41591-023-02632-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Cameron HU, Macnab I.. The structure of the meniscus of the human knee joint. Clin Orthop Relat Res. 1972;89:215-219. [PubMed] [Google Scholar]
- 121. Makris EA, Hadidi P, Athanasiou KA.. The knee meniscus: structure-function, pathophysiology, current repair techniques, and prospects for regeneration. Biomaterials. 2011;32(30):7411-7431. 10.1016/j.biomaterials.2011.06.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Kurzweil PR, Cannon WD, DeHaven KE.. Meniscus repair and replacement. Sports Med Arthrosc Rev. 2018;26(4):160-164. 10.1097/JSA.0000000000000224 [DOI] [PubMed] [Google Scholar]
- 123. Tarafder S, Gulko J, Sim KH, et al. Engineered healing of avascular meniscus tears by stem cell recruitment. Sci Rep. 2018;8(1):8150. 10.1038/s41598-018-26545-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Sekiya I, Koga H, Katano H, et al. Second-look arthroscopy after meniscus repair and synovial mesenchymal stem cell transplantation to treat degenerative flaps and radial tears of the medial meniscus: a case report. J Orthop Sci. 2022;27(4):821-834. 10.1016/j.jos.2021.04.015 [DOI] [PubMed] [Google Scholar]
- 125. Sekiya I, Koga H, Otabe K, et al. Additional use of synovial mesenchymal stem cell transplantation following surgical repair of a complex degenerative tear of the medial meniscus of the knee: a case report. Cell Transplant. 2019;28(11):1445-1454. 10.1177/0963689719863793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Bian Y, Wang H, Zhao X, Weng X.. Meniscus repair: up-to-date advances in stem cell-based therapy. Stem Cell Res Ther. 2022;13(1):207. 10.1186/s13287-022-02863-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Vangsness CT Jr, Farr J 2nd, Boyd J, et al. Adult human mesenchymal stem cells delivered via intra-articular injection to the knee following partial medial meniscectomy: a randomized, double-blind, controlled study. J Bone Joint Surg Am. 2014;96(2):90-98. 10.2106/JBJS.M.00058 [DOI] [PubMed] [Google Scholar]
- 128. Jacob G, Shimomura K, Krych AJ, Nakamura N.. The meniscus tear: a review of stem cell therapies. Cells. 2019;9(1):92. 10.3390/cells9010092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Chew E, Prakash R, Khan W.. Mesenchymal stem cells in human meniscal regeneration: a systematic review. Ann Med Surg (Lond). 2017;24:3-7. 10.1016/j.amsu.2017.09.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Whitehouse MR, Howells NR, Parry MC, et al. Repair of torn avascular meniscal cartilage using undifferentiated autologous mesenchymal stem cells: from in vitro optimization to a first-in-human study. Stem Cells Transl. Med.. 2017;6(4):1237-1248. 10.1002/sctm.16-0199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Dai TY, Pan ZY, Yin F.. In vivo studies of mesenchymal stem cells in the treatment of meniscus injury. Orthop Surg. 2021;13(8):2185-2195. 10.1111/os.13002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Sariboyaci AE, Uysal O, Inan U.. Clinical efficacy of exosome in degenerative meniscal injury (KNEEXO). ClinicalTrials.gov Identifier: NCT05261360; 2022. Retrieved from: https://clinicaltrials.gov/study/NCT05261360. [Google Scholar]
- 133. Chen H. Safety observation on hESC derived MSC like cell for the meniscus injury. ClinicalTrials.gov Identifier: NCT03839238; 2019. Retrieved from: https://clinicaltrials.gov/study/NCT03839238. [Google Scholar]
- 134. Monllau JC. Mesenchymal stromal cells for degenerative meniscus injury. ClinicalTrials.gov Identifier: NCT02033525; 2014. Retrieved from: https://clinicaltrials.gov/study/NCT02033525. [Google Scholar]
- 135. Leong NL, Kator JL, Clemens TL, et al. Tendon and ligament healing and current approaches to tendon and ligament regeneration. J Orthop Res. 2020;38(1):7-12. 10.1002/jor.24475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Snedeker JG, Foolen J.. Tendon injury and repair - a perspective on the basic mechanisms of tendon disease and future clinical therapy. Acta Biomater. 2017;63:18-36. 10.1016/j.actbio.2017.08.032 [DOI] [PubMed] [Google Scholar]
- 137. Wu F, Nerlich M, Docheva D.. Tendon injuries: basic science and new repair proposals. EFORT Open Rev. 2017;2(7):332-342. 10.1302/2058-5241.2.160075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Hogan MV, Bagayoko N, James R, et al. Tissue engineering solutions for tendon repair. J Am Acad Orthop Surg. 2011;19(3):134-142. 10.5435/00124635-201103000-00002 [DOI] [PubMed] [Google Scholar]
- 139. Roth SP, Burk J, Brehm W, Troillet A.. MSC in tendon and joint disease: the context-sensitive link between targets and therapeutic mechanisms. Front Bioeng Biotechnol. 2022;10:855095. 10.3389/fbioe.2022.855095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Kanaya A, Deie M, Adachi N, et al. Intra-articular injection of mesenchymal stromal cells in partially torn anterior cruciate ligaments in a rat model. Arthroscopy. 2007;23(6):610-617. 10.1016/j.arthro.2007.01.013 [DOI] [PubMed] [Google Scholar]
- 141. Oe K, Kushida T, Okamoto N, et al. New strategies for anterior cruciate ligament partial rupture using bone marrow transplantation in rats. Stem Cells Dev. 2011;20(4):671-679. 10.1089/scd.2010.0182 [DOI] [PubMed] [Google Scholar]
- 142. Shojaee A, Parham A.. Strategies of tenogenic differentiation of equine stem cells for tendon repair: current status and challenges. Stem Cell Res Ther. 2019;10(1):181. 10.1186/s13287-019-1291-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Geburek F, Roggel F, van Schie HTM, et al. Effect of single intralesional treatment of surgically induced equine superficial digital flexor tendon core lesions with adipose-derived mesenchymal stromal cells: a controlled experimental trial. Stem Cell Res Ther. 2017;8(1):129. 10.1186/s13287-017-0564-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Ricco S, Renzi S, Del Bue M, et al. Allogeneic adipose tissue-derived mesenchymal stem cells in combination with platelet rich plasma are safe and effective in the therapy of superficial digital flexor tendonitis in the horse. Int J Immunopathol Pharmacol. 2013;26(1 Suppl):61-68. 10.1177/03946320130260s108 [DOI] [PubMed] [Google Scholar]
- 145. Wang Y, Shimmin A, Ghosh P, et al. Safety, tolerability, clinical, and joint structural outcomes of a single intra-articular injection of allogeneic mesenchymal precursor cells in patients following anterior cruciate ligament reconstruction: a controlled double-blind randomised trial. Arthritis Res Ther. 2017;19(1):180. 10.1186/s13075-017-1391-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Hevesi M, LaPrade M, Saris DBF, Krych AJ.. Stem cell treatment for ligament repair and reconstruction. Curr Rev Musculoskelet Med. 2019;12(4):446-450. 10.1007/s12178-019-09580-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Moon SW, Park S, Oh M, Wang JH.. Outcomes of human umbilical cord blood-derived mesenchymal stem cells in enhancing tendon-graft healing in anterior cruciate ligament reconstruction: an exploratory study. Knee Surg Relat Res. 2021;33(1):32. 10.1186/s43019-021-00104-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Gulotta LV, Kovacevic D, Ehteshami JR, et al. Application of bone marrow-derived mesenchymal stem cells in a rotator cuff repair model. Am J Sports Med. 2009;37(11):2126-2133. 10.1177/0363546509339582 [DOI] [PubMed] [Google Scholar]
- 149. Hernigou P, Flouzat Lachaniette CH, Delambre J, et al. Biologic augmentation of rotator cuff repair with mesenchymal stem cells during arthroscopy improves healing and prevents further tears: a case-controlled study. Int Orthop. 2014;38(9):1811-1818. 10.1007/s00264-014-2391-1 [DOI] [PubMed] [Google Scholar]
- 150. Chung SG. Treatment of tendon injury using allogenic adipose-derived mesenchymal stem cells (rotator cuff tear). ClinicalTrials.gov Identifier: NCT02298023; 2014. Retrieved from: https://clinicaltrials.gov/study/NCT02298023. [Google Scholar]
- 151. Chung SG. Treatment of tendon injury using mesenchymal stem cells (ALLO-ASC). ClinicalTrials.gov Identifier: NCT01856140; 2013. Retrieved from: https://clinicaltrails.gov/study/NCT01856130. [Google Scholar]
- 152. Rhatomy S. Regeneration of posterior cruciate ligament injury using hypoxic conditioned allogenic adipose mesenchymal stem cell and condition medium. ClinicalTrials.gov Identifier: NCT04889963; 2021. Retrieved from: https://clinicaltrials.gov/study/NCT04889963. [Google Scholar]
- 153. Anz A. Augmentation of anterior cruciate ligament reconstruction using mesenchymal stem cells and collagen matrix carrier (BioACL). ClinicalTrials.gov Identifier: NCT05582226; 2022. Retrieved from: https://clinicaltrials.gov/study/NCT05582226. [Google Scholar]
- 154. Suroto H. Mesenchymal stem cells and amniotic membrane composite for supraspinatus tendon repair augmentation. ClinicalTrials.gov Identifier: NCT04670302; 2020. Retrieved from: https://clinicaltrials.gov/study/NCT04670302. [Google Scholar]
- 155. Second Affiliated, School of Medicine, Zhejiang University. Treatment of tendon disease using autologous adipose-derived mesenchymal stem cells. ClinicalTrials.gov Identifier: NCT03279796; 2017. Retrieved from: https://clinicaltrials.gov/study/NCT03279796. [Google Scholar]
- 156. Chung SG. Treatment of intractable common extensor tendon injury using mesenchymal stem cells (Allo-ASC). ClinicalTrials.gov Identifier: NCT03449082; 2018. Retrieved from: https://clinicaltrials.gov/study/NCT03449082. [Google Scholar]
- 157. Zhang W, Sun T, Li Y, et al. Application of stem cells in the repair of intervertebral disc degeneration. Stem Cell Res Ther. 2022;13(1):70. 10.1186/s13287-022-02745-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Xie B, Chen S, Xu Y, et al. Clinical efficacy and safety of human mesenchymal stem cell therapy for degenerative disc disease: a systematic review and meta-analysis of randomized controlled trials. Stem Cells Int. 2021;2021(1):9149315. 10.1155/2021/9149315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Vadala G, Ambrosio L, Russo F, Papalia R, Denaro V.. Stem cells and intervertebral disc regeneration overview-what they can and can’t do. Int J Spine Surg. 2021;15(s1):40-53. 10.14444/8054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Vadalà G, Ambrosio L, Russo F, Papalia R, Denaro V. Interaction between mesenchymal stem cells and intervertebral disc microenvironment: from cell therapy to tissue engineering. Stem Cells Int. 2019;2019(1):2376172. 10.1155/2019/2376172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Vasiliadis ES, Pneumaticos SG, Evangelopoulos DS, Papavassiliou AG.. Biologic treatment of mild and moderate intervertebral disc degeneration. Mol Med. 2014;20(1):400-409. 10.2119/molmed.2014.00145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Marfia G, Campanella R, Navone SE, et al. Potential use of human adipose mesenchymal stromal cells for intervertebral disc regeneration: a preliminary study on biglycan-deficient murine model of chronic disc degeneration. Arthritis Res Ther. 2014;16(5):457. 10.1186/s13075-014-0457-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Diekman BO, Rowland CR, Lennon DP, Caplan AI, Guilak F.. Chondrogenesis of adult stem cells from adipose tissue and bone marrow: induction by growth factors and cartilage-derived matrix. Tissue Eng Part A. 2010;16(2):523-533. 10.1089/ten.TEA.2009.0398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Xie L, Lin L, Tang Q, et al. Sertoli cell-mediated differentiation of male germ cell-like cells from human umbilical cord Wharton’s jelly-derived mesenchymal stem cells in an in vitro co-culture system. Eur J Med Res. 2015;20(1):9. 10.1186/s40001-014-0080-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Garzón I, Martín-Piedra MA, Alfonso-Rodríguez C, et al. Generation of a biomimetic human artificial cornea model using Wharton’s jelly mesenchymal stem cells. Invest Ophthalmol Vis Sci. 2014;55(7):4073-4083. 10.1167/iovs.14-14304 [DOI] [PubMed] [Google Scholar]
- 166. Chen H, Zhang Y, Yang Z, Zhang H.. Human umbilical cord Wharton’s jelly-derived oligodendrocyte precursor-like cells for axon and myelin sheath regeneration. Neural Regen Res. 2013;8(10):890-899. 10.3969/j.issn.1673-5374.2013.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Beeravolu N, McKee C, Alamri A, et al. Isolation and characterization of mesenchymal stromal cells from human umbilical cord and fetal placenta. J Vis Exp. 2017(122):55224. 10.3791/55224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Qi L, Wang R, Shi Q, et al. Umbilical cord mesenchymal stem cell conditioned medium restored the expression of collagen II and aggrecan in nucleus pulposus mesenchymal stem cells exposed to high glucose. J Bone Miner Metab. 2019;37(3):455-466. 10.1007/s00774-018-0953-9 [DOI] [PubMed] [Google Scholar]
- 169. Thomson JA, Itskovitz-Eldor J, Shapiro SS, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282(5391):1145-1147. 10.1126/science.282.5391.1145 [DOI] [PubMed] [Google Scholar]
- 170. Gou S, Oxentenko SC, Eldrige JS, et al. Stem cell therapy for intervertebral disk regeneration. Am J Phys Med Rehabil. 2014;93(11 Suppl 3):S122-S131. 10.1097/PHM.0000000000000152 [DOI] [PubMed] [Google Scholar]
- 171. Orozco L, Soler R, Morera C, et al. Intervertebral disc repair by autologous mesenchymal bone marrow cells: a pilot study. Transplantation. 2011;92(7):822-828. 10.1097/TP.0b013e3182298a15 [DOI] [PubMed] [Google Scholar]
- 172. Rahyussalim AJ. Effectiveness and safety of mesenchymal stem cell (MSC) implantation on degenerative discus disease patients (MSC). ClinicalTrials.gov Identifier: NCT04499105; 2020. Retrieved from: https://clinicaltrials.gov/study/NCT04499105. [Google Scholar]
- 173. Fu Q. Human umbilical cord mesenchymal stem cells for the treatment of lumbar disc degeneration disease. ClinicalTrials.gov Identifier: NCT04414592; 2020. Retrieved from: https://clinicaltrials.gov/study/NCT04414592. [Google Scholar]
- 174. Brown R. Safety and preliminary efficacy study of mesenchymal precursor cells (MPCs) in subjects with lumbar back pain. ClinicalTrials.gov Identifier: NCT01290367; 2011. Retrieved from: https://clinicaltrials.gov/study/NCT01290367. [Google Scholar]
- 175. Red de Terapia Celular. Clinical trial based on the use of mesenchymal stem cells from autologous bone marrow in patients with lumbar intervertebral degenerative disc disease. ClinicalTrials.gov Identifier: NCT01513694; 2012. Retrieved from: https://clinicaltrials.gov/study/NCT01513694. [Google Scholar]
- 176. Siddiqi FN. Human autograft mesenchymal stem cell mediated stabilization of the degenerative lumbar spine. ClinicalTrials.gov Identifier: NCT02529566; 2015. Retrieved from: https://clinicaltrials.gov/study/NCT02529566. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.