Abstract
The nonstructural human immunodeficiency virus type 1 Vpr protein is packaged into progeny virions at significant levels (∼200 copies/virion). Genetic analyses have demonstrated that efficient Vpr packaging is dependent upon a leucine-X-X-leucine-phenylalanine (LXXLF) motif located in the p6Gag domain of the structural Gag polyprotein. Recombinant proteins spanning full-length Vpr (Vpr1–97) or the amino-terminal 71 amino acids (Vpr1–71) formed specific complexes with recombinant p6 proteins in vitro. Complex formation required an intact LXXLF motif and exhibited an intrinsic dissociation constant of ∼75 μM. Gel filtration and cross-linking analyses further revealed that Vpr1–71 self-associated in solution. Our experiments demonstrate that Vpr can bind directly and specifically to p6 and suggest that oligomerization of both Vpr and Gag may serve to increase the avidity and longevity of Vpr-Gag complexes, thereby ensuring efficient Vpr packaging.
Human immunodeficiency virus type 1 (HIV-1) encodes a 97-amino-acid accessory protein called Vpr (an initialism for viral protein R). Although Vpr is dispensable for HIV-1 growth in culture, the protein presumably plays an important role(s) in replication in vivo since simian immunodeficiency virus derived from rhesus macaques lacking the two vpr homologs (vpr and vpx) replicates at reduced levels and displays attenuated pathogenicity in infected animals (12, 15). Although significant quantities of Vpr are packaged into budding HIV-1 particles (5, 23, 25, 26), the biological significance of this remains to be fully elucidated. For instance, the stimulation of postentry viral nucleoprotein complex nuclear import (11, 13), the capacity of virion-associated Vpr to induce target cell apoptosis or G2/M arrest (16, 27), and the Vpr-mediated recruitment of the DNA repair enzyme uracil DNA glycosylase into virions (24) have each been reported. From a practical standpoint, efficient virion incorporation has enabled researchers to target heterologous proteins into assembling virions as Vpr fusion proteins (39); this approach has been used to study the early steps of virus infection (4, 8, 36, 37), to deliver inhibitory factors to virions (38), and to tag virions fluorescently for direct visualization during infection (30, 33).
The structural p55Gag protein creates the viral particle and is either directly or indirectly responsible for packaging of all of the essential components of the virion (10). Gag is translated as a multidomain polyprotein that assembles into an immature viral particle and then buds from the cell. Coincident with budding, Gag is proteolytically processed into a series of new, discrete proteins—termed matrix, capsid, nucleocapsid, and p6. These Gag-derived proteins rearrange to form the mature, infectious virus, which is characterized by the presence of a central conical core structure. In addition to the Gag proteins, HIV-1 cores also contain the essential enzymes reverse transcriptase, integrase, and protease and the viral RNA genome, as well as the virus-encoded accessory proteins Vif, Nef, and Vpr (1, 19, 21, 35). Of these latter proteins, Vpr is packaged with substantially greater efficiency, with a recent estimate of the Vpr/Gag ratio being ∼1:7 (25).
The mechanism of Vpr incorporation into virus particles, as well as its retention in uncoated (postentry) cores, is not fully understood. The carboxy-terminal p6Gag region of p55Gag appears to play an important role in Vpr packaging because deletions and mutations in p6Gag can block Vpr incorporation, and transfer of the p6 coding sequence to a heterologous retroviral Gag protein endows the chimeric virus particles with the capacity to package Vpr (18, 22). Genetic mapping experiments have established that a conserved LXXLF motif at positions 41 to 45 in p6Gag is required for efficient Vpr packaging and that an alanine substitution at any of the three conserved hydrophobic residues abolishes Vpr packaging (17).
Consistent with these packaging experiments, yeast two-hybrid analyses have shown that Vpr interacts with p55Gag in a p6Gag-dependent manner (2, 32). Similarly, in vitro binding studies have also indicated that Vpr interacts with p55Gag, although the dependence of this interaction on the p6Gag region was not demonstrated (2). It is noteworthy that neither of these experimental methods can eliminate the possibility that another molecule, e.g., RNA, might serve as a bridge between Vpr and p6Gag. Moreover, and somewhat unexpectedly, both two-hybrid and biochemical analyses have indicated that Vpr either interacts weakly or is unable to interact with p6 when the rest of p55Gag is removed (6, 32). Instead, it has been reported that Vpr can interact with the upstream nucleocapsid or matrix protein (6, 20, 28, 32). Nevertheless, in spite of the lack of an unequivocal demonstration of direct Vpr-p6 binding, it is generally assumed that such interactions do occur in infected cells as a prerequisite to Vpr packaging. In an effort to validate this model further, we have characterized the direct binding of Vpr to p6 in vitro by using purified recombinant proteins. By adopting this approach, we have been able to define kinetic and thermodynamic details of the interaction.
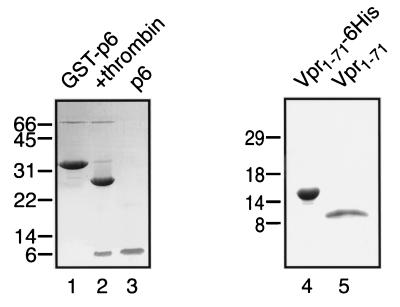
Studies of HIV-1 Vpr in vitro have frequently been hampered by difficulties in obtaining and/or maintaining preparations of soluble proteins. In the course of testing different subfragments of Vpr in a variety of Escherichia coli expression systems, we found that the amino-terminal 71 residues of Vpr from the YU-2 isolate (hereafter called Vpr1–71) can be produced at high levels by using a pET27b-based vector (Novagen). This fragment corresponds closely to a protease-resistant fragment of Vpr identified by others (41). Vpr1–71-His6 was purified under nondenaturing conditions by using nickel chelate affinity chromatography, and the hexahistidine tag was then removed by enterokinase cleavage (this left a nonnative five-amino-acid [aspartic acid4-lysine] extension at the protein's carboxy terminus). The resulting Vpr1–71 protein was purified to near homogeneity by anion-exchange chromatography on Q Sepharose (Fig. 1, lane 5). Preparations of Vpr1–71 typically displayed good solubility at concentrations of up to ∼15 mg/ml in detergent-free buffers of low ionic strength (e.g., 20 mM HEPES-NaOH [pH 7.3]). HIV-1 p6 was expressed as a glutathione S-transferase (GST) fusion protein and was isolated by glutathione affinity chromatography, released from the GST moiety by thrombin cleavage, and purified to near homogeneity by cation-exchange chromatography on S Sepharose (Fig. 1, lane 3).
FIG. 1.
Purification of recombinant p6 and Vpr1–71. Shown are SDS-PAGE and Coomassie blue staining of GST-p6 following glutathione-Sepharose affinity chromatography (lane 1), GST-p6 after cleavage with thrombin (lane 2), p6 after purification over S Sepharose (lane 3), Vpr1–71-His6 following Ni1+ chelate affinity chromatography (lane 4), and Vpr1–71 after cleavage with enterokinase and purification over Q Sepharose (lane 5). In both cases, mass spectrometry confirmed that the final products had the expected molecular masses (data not shown). The values on the left are molecular masses in kilodaltons.
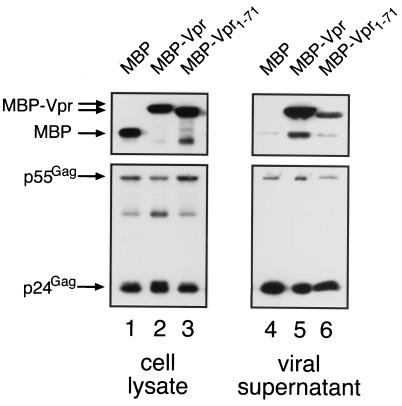
In addition to its solubility and purity, Vpr1–71 was judged to be a useful reagent for studying the in vitro binding properties of Vpr based on the following functional criteria. One, the fusion of either Vpr1–71 or full-length Vpr to heterologous proteins results in their nuclear accumulation (9). Consistent with this demonstration of nuclear import function, cytoplasmic microinjection of our recombinant Vpr1–71 into HeLa cells, followed by an indirect immunofluorescence assay, resulted in localization to the nucleus (data not shown). Two, and most pertinent for the experiments discussed here, Vpr1–71, like wild-type Vpr, can recruit appended proteins into progeny virions (Fig. 2). This was shown by cotransfection of 293T cells with the vpr-deficient HIV-1 provirus vector pYU-2/Δvpr and expression vectors for maltose-binding protein (MBP) (pCMV/MBP) or MBP-Vpr fusions (pCMV/MBP-Vpr and pCMV/MBP-Vpr1–71) (9). Whole-cell lysates and pelleted virions were then analyzed by Western blotting by using an MBP-specific primary antibody (New England Biolabs). Even though the full-length MBP-Vpr chimera was packaged at a higher level, both Vpr fusions were specifically incorporated into virions whereas MBP alone was not (compare lanes 5 and 6 with lane 4). These results are consistent with previous reports that the C-terminal end of Vpr is not required for packaging but that truncation of Vpr may diminish protein stability (7).
FIG. 2.
Virion packaging of MBP-Vpr fusion proteins. 293T cells (107) were cotransfected with pYU-2/Δvpr and a vector encoding MBP, MBP-Vpr, or MBP-Vpr1–71, as indicated. At 24 h, culture supernatants were clarified by centrifugation and filtered through 0.45-μm-pore-size membranes, and virions were pelleted through a 20% sucrose cushion by ultracentrifugation at 100,000 × g for 90 min. The transfected cells (lanes 1 to 3) and pelleted virions (lanes 4 to 6) were resuspended in loading buffer and resolved in parallel by SDS-PAGE. Gag and MBP-containing proteins were detected by Western blotting by using a p24Gag-specific monoclonal antibody or an MBP-specific rabbit antiserum, respectively, and an enhanced chemiluminescence assay.
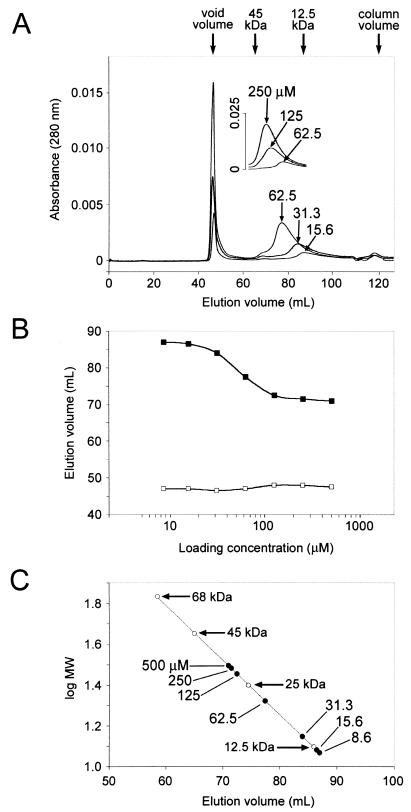
Several groups have reported that different fragments of Vpr can self-associate (3, 14, 29, 31, 41). To test whether the Vpr1–71 protein could also self-associate, we carried out both gel filtration (Fig. 3) and covalent cross-linking (Fig. 4) analyses. Vpr1–71 samples ranging in concentration from 8.6 to 500 μM were applied to a Superdex 75 column (Pharmacia), and the column was developed in 130 ml of buffer (100 mM NaCl, 20 mM sodium phosphate, pH 7.2). A subset of these elution profiles is shown in Fig. 3A. In each case, Vpr1–71 eluted in two separate peaks, with the first always appearing at the void volume (∼47 ml). The fraction of Vpr1–71 in this peak slowly increased as the protein was stored in solution for extended periods, and upon collection and rechromatography, this fraction eluted exclusively with the void volume (data not shown). Based on their large Stokes radii and invariant behavior, we have assumed that these complexes represent large, inert aggregates of Vpr1–71.
FIG. 3.
Size exclusion chromatography of Vpr1–71. (A) Elution profiles of pure Vpr1–71 on Superdex 75 at the indicated initial concentrations. (B) Concentration dependence of the elution volume for the aggregate (□) and oligomeric (▪) fractions. (C) Standard curve showing elution volumes of protein markers (○) and Vpr1–71 (●) at the indicated concentrations. The molecular mass (MW) standards are as follows: bovine serum albumin, 68 kDa; ovalbumin, 45 kDa; chymotrypsinogen A, 25 kDa; cytochrome c, 12.5 kDa.
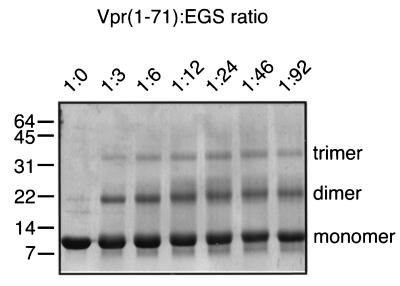
FIG. 4.
Cross-linking of Vpr1–71. Vpr1–71 at 50 μM was incubated with the indicated molar ratios of EGS for 60 min at 37°C and visualized by SDS-PAGE and Coomassie staining. Cross-linking intermediates representing monomer, dimer, and trimer forms of Vpr1–71 are indicated. The values on the left are molecular masses in kilodaltons.
In contrast, the retention time of the second peak increased with decreasing protein concentrations (Fig. 3A and B). The smaller size and concentration-dependent mobility of this peak suggested that it was composed of discrete Vpr1–71 oligomers that could rapidly and reversibly reequilibrate. The masses of Vpr1–71 complexes in the second peak were estimated by comparing the concentration-dependent retention times to a standard curve of retention times of globular proteins whose masses were known (Fig. 3C). These experiments indicated that the maximal and minimal hydrodynamic masses of Vpr1–71 were ∼11.7 kDa (at limiting low concentrations) and ∼31.3 kDa (at limiting high concentrations). We assume that the smaller species represents monomeric Vpr1–71 (actual mass, 9.051 kDa), and our data are therefore most consistent with the formation of trimeric Vpr1–71 complexes at high protein concentrations.
A limitation of these experiments is that gel filtration measurements reflect the Stokes radius (rather than true mass) of the protein or complex under study. We attempted to circumvent this problem by using equilibrium sedimentation experiments to quantitate Vpr oligomerization. Although these data were again most consistent with the trimerization of Vpr1–71, we were unable to obtain satisfactory global fits of our data over a large range of protein concentrations, presumably owing to difficulties with fitting of the fraction of Vpr1–71 that aggregated irreversibly.
To evaluate the oligomerization of Vpr1–71 by covalent cross-linking, 50 μM Vpr1–71 was incubated with increasing concentrations of the bifunctional agent ethylene glycol bis-(succinimidylsuccinate) (EGS; Pierce) for 60 min at 37°C. Cross-linked adducts were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and visualized following staining with Coomassie blue (Fig. 4). As above, unmodified Vpr1–71 migrated as a single species with an apparent relative molecular mass of ∼9 kDa (lane 1). At all of the concentrations of EGS added, two additional bands of Vpr1–71 were observed (lanes 2 to 7). Based on their relative electrophoretic mobililties, these species appear to correspond to dimers and trimers. Although very small quantities of higher-order oligomers were sometimes observed at high cross-linker concentrations (∼5 mM), these species never formed efficiently. Overall, these data again support the conclusion that Vpr1–71 can trimerize in solution.
Having defined some of the properties of the Vpr1–71, reagent, we next turned our attention to the analysis of Vpr-p6 interactions. As an initial approach to the examination of direct binding, standard pull-down experiments were conducted by using purified Vpr1–71 and GST-p6; despite testing a variety of assay conditions, we consistently failed to detect an interaction between the two proteins (data not shown). Although the absence of observable binding might have reflected a lack of complex formation, we also considered the alternative possibility that the p6-Vpr1–71 complex dissociates rapidly and is therefore undetectable under the nonequilibrium binding conditions required in this experimental configuration (i.e., one that includes extensive washing steps).
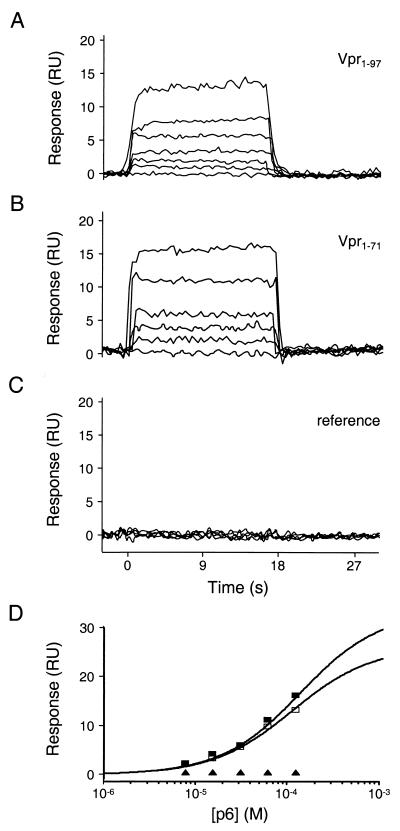
The Vpr-p6 interaction was therefore examined by using optical biosensor experiments that measure binding in real time and have the potential to detect transient complexes. All surface plasmon resonance measurements were performed with a BIACORE 2000 or 3000 instrument (Biacore AB, Uppsala, Sweden) equipped with research grade CM5 sensor chips. Initially, the binding of pure, soluble, monomeric p6 to hexahistidine-tagged Vpr proteins was examined. Vpr-His6 proteins were captured directly from crude bacterial expression lysates by using an immobilized anti-His4 monoclonal antibody (Qiagen). Importantly, this strategy circumvented the aforementioned difficulties associated with purifying aggregation-prone Vpr proteins and made it possible to study binding to both Vpr1–71 and full-length Vpr (Vpr1–97). Approximately 1,000 response units (RU) of Vpr1–97-His6 was captured in flow cell 2, and approximately 600 RU of Vpr1–71-His6 was captured in flow cell 3. Flow cells 1 and 4 served as reference surfaces. Replicates of the analyte, p6, in 20 mM Tris–100 mM NaCl–0.005% polyoxyethylene 20-sorbitan monolaurate (P20) (pH 8.0) were injected over the four flow cells at concentrations of 7.5, 15.5, 31.2, 62.5, 125, and 250 μM at a flow rate of 50 μl/min. The analyses were performed at 20°C, and no regeneration of the flow cell surfaces was required; data were collected at a rate of 2 Hz.
As illustrated in Fig. 5A to C, p6 bound each Vpr protein in a concentration- and Vpr-dependent fashion. Both Vpr-p6 complexes dissociated (and associated) very rapidly, with the half-lives of both complexes being less than 1 s. These rapid rates of dissociation are consistent with our inability to detect Vpr-p6 binding by using pull-down assays. Dissociation constants were estimated by fitting the equilibrium phases of the p6 binding isotherms by using simple 1:1 binding models (40), and both complexes bound with dissociation constants of ∼75 μM (Table 1). To test the specificity of the interaction, we also examined the binding of p6 to a surface derivatized with Vpr1–71-His6 harboring a leucine-to-alanine substitution at residue 30 (A30L). This mutation, which inhibits Vpr packaging in vivo (7), prevented detectable p6 binding (Table 1) and thereby confirmed the validity of studying Vpr-p6 interactions by surface plasmon resonance.
FIG. 5.
Biosensor analysis of p6 binding to Vpr1–97 and Vpr1–71. Representative sensorgrams of p6 injected at concentrations of 0, 7.5, 15.5, 31.2, 62.5, and 125 μM over Vpr1–97, (A) Vpr1–71 (B), and a reference surface (C). (D) Responses at equilibrium (t = 15 s) were plotted versus the injected p6 concentrations. Responses were fitted to simple 1:1 binding models to determine dissociation constants (Kds) of 71 ± 8 and 84 ± 6 μM for Vpr1–97 and Vpr1–71, respectively. Symbols: ▪, Vpr1–97; □, Vpr1–71; ▴, reference surface.
TABLE 1.
Estimated dissociation constants for HIV-1 Vpr-p6 complexes
| Protein-protein interaction | Kd (μM) |
|---|---|
| Vpr1–97-His6–p6 | 71 (8),a 66 (6) |
| Vpr1–71-His6–p6 | 84 (4), 77 (3) |
| Vpr1–71A30L-His6–p6 | —b |
| GST-p6–Vpr1–71 | 18 ± 11c |
| GST-p6L41A–Vpr1–71 | — |
| GST-p6R42A,S43A–Vpr1–71 | 71 (3) |
| GST-p6L44A–Vpr1–71 | — |
| GST-p6F45A–Vpr1–71 | — |
The values in parentheses represent estimated errors of 1 standard deviation in the final digit and were derived from the fits of a single binding experiment. Data from multiple independent binding experiments are listed sequentially.
—, binding was too weak to quantify (>200 μM).
This value is the average of four independent measurements made with different protein preparations. The error is the standard deviation in the repeated experiments.
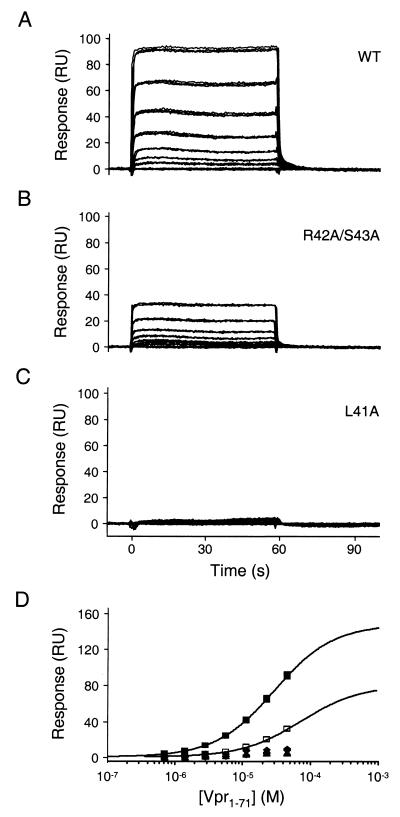
Biosensor analysis was also used to quantitate the Vpr-p6 binding interaction in the opposite orientation. In this format, GST fusion proteins were captured by using an immobilized GST-specific antibody as follows: flow cell 1, 1,200 RU of GST-p6 (wild type); flow cell 2, 1,200 RU of GST-p6R42A,S43A (contains alanine substitutions at the second and third positions of the LXXLF motif); flow cell 3, 700 RU of recombinant GST (reference sample); flow cell 4, 1,000 RU of GST-p6L44A (contains an inactivating mutation in the LXXLF motif) (17). To collect equilibrium binding data, triplicate injections of Vpr1–71 were allowed to flow over the four flow cells at concentrations of 0.7, 1.4, 2.9, 5.7, 11.5, 22.9, and 45.8 μM and a flow rate of 50 μl/min. Analogous binding studies were performed for two additional packaging-defective mutant forms of p6, L41A (∼1,200 RU) and F45A (∼1,300 RU) (17).
Consistent with our previous results, Vpr1–71 bound to p6 in a dose-dependent fashion, with complex dissociation again being very rapid (Fig. 6A). Within a single experiment, the binding measurements were highly reproducible (compare the overlaid triplicates in Fig. 6A), although absolute binding levels did vary somewhat between independent repetitions. The apparent dissociation constant derived from four such repetitions was 18 ± 11 μM. Both the variability and apparently tighter Vpr1–71-p6 complexation observed in this orientation likely reflect avidity effects arising from Vpr oligomerization and aggregation; as a result, the data do not provide a true measure of the intrinsic equilibrium dissociation constant of the complex.
FIG. 6.
Biosensor analysis of Vpr1–71 binding to p6. Representative sensorgrams of Vpr1–71 injected in triplicate at concentrations of 0, 0.72, 1.4, 2.9, 5.7, 11.5, 22.9, and 45.8 μM over wild-type (WT; A), R42A/S43A (B), and L41A (C) GST-p6. (D) Responses at equilibrium (t = 50 s) were plotted versus the injected Vpr1–71 concentrations. Responses were fitted to simple 1:1 binding models for the wild-type and R42A/S43A p6 proteins. Binding of the other mutant p6 proteins was too weak to quantify. Symbols: ▪, wild-type GST-p6; □, R42A/S43A; ⧫, F45A; ▴, L44A; ●, L41A.
Specificity of binding was evaluated by using GST-p6 fusions that carried alanine substitutions in the LXXLF motif. As shown in Fig. 6B and C (and Table 1), each of the mutations known to block the packaging of Vpr into virions failed to bind Vpr1–71 at detectable levels. In contrast, noninterfering substitutions of residues 42 and 43 (17) reduced the apparent binding affinity only modestly (Kd = 71 μM). Finally, we also tested the binding of Vpr1–71 to a seven-residue minimal peptide spanning p6 residues 39 to 45 (immobilized as a GST fusion). Consistent with the ability of this motif to mediate efficient Vpr packaging when appended to a heterologous Gag protein (17), the apparent binding affinity was reduced only ∼1.7-fold relative to that of full-length p6 (Table 1). In sum, our binding data demonstrate that pure recombinant p6 and Vpr proteins form a direct, specific complex in vitro. Moreover, the sequence requirements for binding correlate very well with the proven requirements for Vpr packaging in vivo. The most parsimonious interpretation of these experiments is that the virion incorporation of Vpr involves a direct interaction with the LXXLF motif of p6Gag.
It is noteworthy that both HIV-1 Gag and Vpr oligomerize and form higher-order structures in solution (3, 10, 14, 29, 31, 41). It is most likely that such interactions will significantly increase the avidities and decrease the dissociation rates of Vpr-Gag complexes that form during virion assembly. Thus, although we cannot rule out the possibilities that Vpr might contact additional domains within p55Gag or that p6Gag might adopt a different structure in the context of the Gag precursor, we believe that efficient Vpr packaging need not require any additional sites of contact beyond the LXXLF motif. Interestingly, analysis of virus cores purified from mature HIV-1 virions has revealed that Vpr remains associated with the core but that the p6 protein is lost (1, 35). We suggest that this observation is also in accordance with our avidity model; specifically, because the fully processed p6 protein is a monomer (34), its removal from the rest of Gag would lower the affinity of the Vpr-p6 complex and facilitate the rapid dissociation of p6 away from the core. Indeed, we speculate that the use of avidity to amplify weak but specific binding interactions may provide a general mechanism for packaging of viral components. By subsequently exploiting proteolysis to separate packaging signals from sites of oligomerization, it may then be possible to free virion components and enable them to execute important functions in newly infected cells.
Acknowledgments
Y.J. and O.P. contributed equally to this work.
We thank Laurie Zimmerman for secretarial support.
This work was supported by NIH research grants AI46942 (M.H.M.), AI09996 (Y.J.), and AI45405 (W.I.S.).
REFERENCES
- 1.Accola M A, Öhagen A, Göttlinger H G. Isolation of human immunodeficiency virus type 1 cores: retention of Vpr in the absence of p6(gag) J Virol. 2000;74:6198–6202. doi: 10.1128/jvi.74.13.6198-6202.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bachand F, Yao X J, Hrimech M, Rougeau N, Cohen E A. Incorporation of Vpr into human immunodeficiency virus type 1 requires a direct interaction with the p6 domain of the p55 gag precursor. J Biol Chem. 1999;274:9083–9091. doi: 10.1074/jbc.274.13.9083. [DOI] [PubMed] [Google Scholar]
- 3.Bogerd H P, Fridell R A, Blair W S, Cullen B R. Genetic evidence that the Tat proteins of human immunodeficiency virus types 1 and 2 can multimerize in the eukaryotic cell nucleus. J Virol. 1993;67:5030–5034. doi: 10.1128/jvi.67.8.5030-5034.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bouyac-Bertoia M, Dvorin J D, Fouchier R A M, Jenkins Y, Meyer B E, Wu L I, Emerman M, Malim M H. HIV-1 infection requires a functional integrase NLS. Mol Cell. 2001;7:1025–1035. doi: 10.1016/s1097-2765(01)00240-4. [DOI] [PubMed] [Google Scholar]
- 5.Cohen E A, Terwilliger E F, Jalinoos Y, Proulx J, Sodroski J G, Haseltine W A. Identification of HIV-1 vpr product and function. J Acquir Immune Defic Syndr. 1990;3:11–18. [PubMed] [Google Scholar]
- 6.de Rocquigny H, Petitjean P, Tanchou V, Decimo D, Drouot L, Delaunay T, Darlix J L, Roques B P. The zinc fingers of HIV nucleocapsid protein NCp7 direct interactions with the viral regulatory protein Vpr. J Biol Chem. 1997;272:30753–30759. doi: 10.1074/jbc.272.49.30753. [DOI] [PubMed] [Google Scholar]
- 7.Di Marzio P, Choe S, Ebright M, Knoblauch R, Landau N R. Mutational analysis of cell cycle arrest, nuclear localization, and virion packaging of human immunodeficiency virus type 1 Vpr. J Virol. 1995;69:7909–7916. doi: 10.1128/jvi.69.12.7909-7916.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fletcher T M, III, Soares M A, McPhearson S, Hui H, Wiskerchen M, Muesing M A, Shaw G M, Leavitt A D, Boeke J D, Hahn B H. Complementation of integrase function in HIV-1 virions. EMBO J. 1997;16:5123–5138. doi: 10.1093/emboj/16.16.5123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fouchier R A M, Meyer B E, Simon J H M, Fischer U, Albright A V, González-Scarano F, Malim M H. Interaction of the human immunodeficiency virus type 1 Vpr protein with the nuclear pore complex. J Virol. 1998;72:6004–6013. doi: 10.1128/jvi.72.7.6004-6013.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Freed E O. HIV-1 gag proteins: diverse functions in the virus life cycle. Virology. 1998;251:1–15. doi: 10.1006/viro.1998.9398. [DOI] [PubMed] [Google Scholar]
- 11.Gallay P, Stitt V, Mundy C, Oettinger M, Trono D. Role of the karyopherin pathway in human immunodeficiency virus type 1 nuclear import. J Virol. 1996;70:1027–1032. doi: 10.1128/jvi.70.2.1027-1032.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gibbs J S, Lackner A A, Lang S M, Simon M A, Sehgal P K, Daniel M D, Desrosiers R C. Progression to AIDS in the absence of a gene for vpr or vpx. J Virol. 1995;69:2378–2383. doi: 10.1128/jvi.69.4.2378-2383.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heinzinger N K, Bukrinsky M I, Haggerty S A, Ragland A M, Kewalramani V, Lee M-A, Gendelman H E, Ratner L, Stevenson M, Emerman M. The Vpr protein of human immunodeficiency virus type 1 influences nuclear localization of viral nucleic acids in nondividing host cells. Proc Natl Acad Sci USA. 1994;91:7311–7315. doi: 10.1073/pnas.91.15.7311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Henklein P, Bruns K, Sherman M P, Tessmer U, Licha K, Kopp J, de Noronha C M, Greene W C, Wray V, Schubert U. Functional and structural characterization of synthetic HIV-1 Vpr that transduces cells, localizes to the nucleus, and induces G2 cell cycle arrest. J Biol Chem. 2000;275:32016–32026. doi: 10.1074/jbc.M004044200. [DOI] [PubMed] [Google Scholar]
- 15.Hirsch V M, Sharkey M E, Brown C R, Brichacek B, Goldstein S, Wakefield J, Byrum R, Elkins W R, Hahn B H, Lifson J D, Stevenson M. Vpx is required for dissemination and pathogenesis of SIVSM PBj: evidence of macrophage-dependent viral amplification. Nat Med. 1998;4:1401–1408. doi: 10.1038/3992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hrimech M, Yao X J, Bachand F, Rougeau N, Cohen E A. Human immunodeficiency virus type 1 (HIV-1) Vpr functions as an immediate-early protein during HIV-1 infection. J Virol. 1999;73:4101–4109. doi: 10.1128/jvi.73.5.4101-4109.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kondo E, Göttlinger H G. A conserved LXXLF sequence is the major determinant in p6gag required for the incorporation of human immunodeficiency virus type 1 Vpr. J Virol. 1996;70:159–164. doi: 10.1128/jvi.70.1.159-164.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kondo E, Mammano F, Cohen E A, Göttlinger H G. The p6gag domain of human immunodeficiency virus type 1 is sufficient for the incorporation of Vpr into heterologous viral particles. J Virol. 1995;69:2759–2764. doi: 10.1128/jvi.69.5.2759-2764.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kotov A, Zhou J, Flicker P, Aiken C. Association of Nef with the human immunodeficiency virus type 1 core. J Virol. 1999;73:8824–8830. doi: 10.1128/jvi.73.10.8824-8830.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li M S, Garcia-Asua G, Bhattacharyya U, Mascagni P, Austen B M, Roberts M M. The Vpr protein of human immunodeficiency virus type 1 binds to nucleocapsid protein p7 in vitro. Biochem Biophys Res Commun. 1996;218:352–355. doi: 10.1006/bbrc.1996.0061. [DOI] [PubMed] [Google Scholar]
- 21.Liu H, Wu X, Newman M, Shaw G M, Hahn B H, Kappes J C. The Vif protein of human and simian immunodeficiency viruses is packaged into virions and associates with viral core structures. J Virol. 1995;69:7630–7638. doi: 10.1128/jvi.69.12.7630-7638.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lu Y-L, Bennett R P, Wills J W, Gorelick R, Ratner L. A leucine triplet repeat sequence (LXX)4 in p6gag is important for Vpr incorporation into human immunodeficiency virus type 1 particles. J Virol. 1995;69:6873–6879. doi: 10.1128/jvi.69.11.6873-6879.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lu Y-L, Spearman P, Ratner L. Human immunodeficiency virus type 1 viral protein R localization in infected cells and virions. J Virol. 1993;67:6542–6550. doi: 10.1128/jvi.67.11.6542-6550.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mansky L M, Preveral S, Selig L, Benarous R, Benichou S. The interaction of Vpr with uracil DNA glycosylase modulates the human immunodeficiency virus type 1 in vivo mutation rate. J Virol. 2000;74:7039–7047. doi: 10.1128/jvi.74.15.7039-7047.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Müller B, Tessmer U, Schubert U, Kräusslich H-G. Human immunodeficiency virus type 1 Vpr protein is incorporated into the virion in significantly smaller amounts than Gag and is phosphorylated in infected cells. J Virol. 2000;74:9727–9731. doi: 10.1128/jvi.74.20.9727-9731.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paxton W, Connor R I, Landau N R. Incorporation of Vpr into human immunodeficiency virus type 1 virions: requirement for the p6 region of gag and mutational analysis. J Virol. 1993;67:7229–7237. doi: 10.1128/jvi.67.12.7229-7237.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Poon B, Grovit-Ferbas K, Stewart S A, Chen I S Y. Cell cycle arrest by Vpr in HIV-1 virions and insensitivity to antiretroviral agents. Science. 1998;281:266–269. doi: 10.1126/science.281.5374.266. [DOI] [PubMed] [Google Scholar]
- 28.Sato A, Yoshimoto J, Isaka Y, Miki S, Suyama A, Adachi A, Hayami M, Fujiwara T, Yoshie O. Evidence for direct association of Vpr and matrix protein p17 within the HIV-1 virion. Virology. 1996;220:208–212. doi: 10.1006/viro.1996.0302. [DOI] [PubMed] [Google Scholar]
- 29.Sawaya B E, Khalili K, Gordon J, Srinivasan A, Richardson M, Rappaport J, Amini S. Transdominant activity of human immunodeficiency virus type 1 Vpr with a mutation at residue R73. J Virol. 2000;74:4877–4881. doi: 10.1128/jvi.74.10.4877-4881.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schaeffer E, Geleziunas R, Greene W C. Human immunodeficiency virus type 1 Nef functions at the level of virus entry by enhancing cytoplasmic delivery of virions. J Virol. 2001;75:2993–3000. doi: 10.1128/JVI.75.6.2993-3000.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schuler W, Wecker K, de Rocquigny H, Baudat Y, Sire J, Roques B P. NMR structure of the (52–96) C-terminal domain of the HIV-1 regulatory protein Vpr: molecular insights into its biological functions. J Mol Biol. 1999;285:2105–2117. doi: 10.1006/jmbi.1998.2381. [DOI] [PubMed] [Google Scholar]
- 32.Selig L, Pages J C, Tanchou V, Prévéral S, Berlioz-Torrent C, Liu L X, Erdtmann L, Darlix J L, Benarous R, Benichou S. Interaction with the p6 domain of the Gag precursor mediates incorporation into virions of Vpr and Vpx proteins from primate lentiviruses. J Virol. 1999;73:592–600. doi: 10.1128/jvi.73.1.592-600.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stauber R H, Rulong S, Palm G, Tarasova N I. Direct visualization of HIV-1 entry: mechanisms and role of cell surface receptors. Biochem Biophys Res Commun. 1999;258:695–702. doi: 10.1006/bbrc.1999.0511. [DOI] [PubMed] [Google Scholar]
- 34.Stys D, Blaha I, Strop P. Structural and functional studies in vitro on the p6 protein from the HIV-1 gag open reading frame. Biochim Biophys Acta. 1993;1182:157–161. doi: 10.1016/0925-4439(93)90137-p. [DOI] [PubMed] [Google Scholar]
- 35.Welker R, Hohenberg H, Tessmer U, Huckhagel C, Kräusslich H-G. Biochemical and structural analysis of isolated mature cores of human immunodeficiency virus type 1. J Virol. 2000;74:1168–1177. doi: 10.1128/jvi.74.3.1168-1177.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu X, Liu H, Xiao H, Conway J A, Hehl E, Kalpana G V, Prasad V, Kappes J C. Human immunodeficiency virus type 1 integrase protein promotes reverse transcription through specific interactions with the nucleoprotein reverse transcription complex. J Virol. 1999;73:2126–2135. doi: 10.1128/jvi.73.3.2126-2135.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu X, Liu H, Xiao H, Conway J A, Hunter E, Kappes J C. Functional RT and IN incorporated into HIV-1 particles independently of the Gag/Pol precursor protein. EMBO J. 1997;16:5113–5122. doi: 10.1093/emboj/16.16.5113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu X, Liu H, Xiao H, Conway J A, Kappes J C. Inhibition of human and simian immunodeficiency virus protease function by targeting Vpx-protease-mutant fusion protein into viral particles. J Virol. 1996;70:3378–3384. doi: 10.1128/jvi.70.6.3378-3384.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu X, Liu H, Xiao H, Kim J, Seshaiah P, Natsoulis G, Boeke J D, Hahn B H, Kappes J C. Targeting foreign proteins to human immunodeficiency virus particles via fusion with Vpr and Vpx. J Virol. 1995;69:3389–3398. doi: 10.1128/jvi.69.6.3389-3398.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yoo S, Myszka D G, Yeh C, McMurray M, Hill C P, Sundquist W I. Molecular recognition in the HIV-1 capsid/cyclophilin A complex. J Mol Biol. 1997;269:780–795. doi: 10.1006/jmbi.1997.1051. [DOI] [PubMed] [Google Scholar]
- 41.Zhao L J, Wang L, Mukherjee S, Narayan O. Biochemical mechanism of HIV-1 Vpr function. Oligomerization mediated by the N-terminal domain. J Biol Chem. 1994;269:32131–32137. [PubMed] [Google Scholar]