Abstract
Mounting evidence suggests that human immunodeficiency virus type 1 (HIV-1) Gag-specific T helper cells contribute to effective antiviral control, but their functional characteristics and the precise epitopes targeted by this response remain to be defined. In this study, we generated CD4+ T-cell clones specific for Gag from HIV-1-infected persons with vigorous Gag-specific responses detectable in peripheral blood mononuclear cells. Multiple peptides containing T helper epitopes were identified, including a minimal peptide, VHAGPIAG (amino acids 218 to 226), in the cyclophilin binding domain of Gag. Peptide recognition by all clones examined induced cell proliferation, gamma interferon (IFN-γ) secretion, and cytolytic activity. Cytolysis was abrogated by concanamycin A and EGTA but not brefeldin A or anti-Fas antibody, implying a perforin-mediated mechanism of cell lysis. Additionally, serine esterase release into the extracellular medium, a marker for cytolytic granules, was demonstrated in an antigen-specific, dose-dependent fashion. These data indicate that T helper cells can target multiple regions of the p24 Gag protein and suggest that cytolytic activity may be a component of the antiviral effect of these cells.
Increasing evidence indicates that virus-specific T helper cells may play an important role in host immune responses against human immunodeficiency virus type 1 (HIV-1) infection (4, 17, 30, 43, 44). An inverse association between HIV-1 plasma RNA virus load and Gag-specific T helper cell responses is observed in untreated, chronic infection, suggesting a role in the control of viral replication (22, 44). In treated acute HIV-1 infection, preserved HIV-specific T helper cell responses are associated with an enhanced ability to contain viremia when antiretroviral therapy is discontinued (43). Studies involving early treatment of simian immunodeficiency virus (SIV) or DNA vaccination with or without interleukin-2 (IL-2) therapy prior to SIV infection demonstrated improved control of viremia, along with vigorous CD4+ T-cell responses (2, 4, 17, 30). The central role of T helper cells in maintaining control of viremia is consistent with findings from murine systems. CD4+ T-cell-depleted mice are unable to clear lymphocytic choriomeningitis virus, gammaherpesvirus 68, and Rauscher murine leukemia virus infections (5, 9, 18, 56).
While HIV-1-specific T helper cell responses appear to be associated with virologic control, the functional characteristics of these cells and the precise epitopes targeted remain to be defined. It is hypothesized that lack of appropriate HIV-1-specific T helper cell responses seen in the majority of HIV-1-infected individuals contributes to the waning of virus-specific cytotoxic T cells (CTL) and eventually results in disease progression (5, 13, 23, 35, 40). Another possibility is that CD4+ T cells play a direct role in the suppression of viral replication. CD4+ cytotoxic T cells have been described in a number of viral infections, including herpes simplex virus (53), hepatitis B virus (3), measles virus (20), human herpesvirus 6 (52), and Epstein-Barr virus (6). CD4+ T cells with gp120-specific cytolytic activity were first described in the cerebrospinal fluid of patients with AIDS (46). However, they have been most extensively observed in HIV-1-seronegative individuals vaccinated with recombinant gp160 (15, 37, 39, 48, 49).
Few data exist at a clonal level on the functional characteristics of HIV-1-specific T helper cells (31, 34). To further characterize HIV-1-specific T helper cells, we cloned these cells at limiting dilution. Our results reveal multiple discrete epitopes in the HIV-1 Gag protein, including an epitope in the cyclophilin binding domain known to be important for the viral life cycle prior to reverse transcription (RT), following membrane binding and fusion (8). Moreover, clones to this and other epitopes were shown to mediate cytotoxic activity as well as gamma interferon (IFN-γ) production.
MATERIALS AND METHODS
Study subjects.
Four persons with vigorous p24-specific T helper cell proliferative responses were selected for study. Subject CTS-01 is a 50-year-old African-American male infected with HIV-1 for at least 20 years. Without antiretroviral therapy his viral load has been always less than 1,000 RNA copies/ml and his CD4+ T-cell count above 500 cells/ml. Subjects AC-01, AC-25, and AC-36 were treated with antiretroviral therapy during acute HIV-1 infection (43), and clones were isolated 11 to 18 months after initiation of therapy. Clones from AC-01 and AC-36 were isolated before a supervised therapy interruption and from AC-25 after treatment interruption and reinstitution. Thirty-five HIV-1-seronegative individuals' peripheral blood mononuclear cells (PBMC) were used as controls for proliferative responses to p24 protein (43).
Peptides and antibodies.
Recombinant p24 protein (amino acids 133 to 373) derived from the NY-5 strain of HIV-1 was produced in a baculovirus expression system with 90 to 95% purity (Protein Science, Meriden, onn.T). Shorter p24 peptides were generated as free acids with an Advanced ChemTech 396Ω peptide synthesizer (44). Flow cytometry antibodies were obtained from Becton Dickinson (San Jose, Calif.).
T-cell clones.
Culture media (R+) consisted of RPMI 1640 (Sigma, St. Louis, Mo.) with penicillin-streptomycin (Mediatech, Herndon, Va.), HEPES (Mediatech), and l-glutamine (Mediatech). T-cell clones were maintained in R+ and 10% heat-inactivated human AB serum (R10H). Clones were generated by limiting dilution. Freshly isolated PBMC (107) were suspended in 10 ml of R10H in a T25 flask and stimulated with p24 (1 μg/ml) and IL-2 (100 U/ml; Hoffmann-La Roche). Indinavir (Merck, 0.4 μM), zidovudine (AZT; Glaxo Wellcome; 0.5 μM), and lamivudine (Glaxo Wellcome; 3 μM) were added to the media for the first 4 weeks of culture. After 2 weeks the PBMC were restimulated with p24 protein (1 μg/ml), IL-2 (100 U/ml), and 107 irradiated (30 Gy), autologous PBMC. Three days after restimulation the PBMC were plated at limiting dilution with 10, 3, or 1 cell per well. Clones were screened in a 2-day proliferation assay using autologous B-lymphoblastoid cell lines (B-LCL) as antigen-presenting cells and p24 protein as the stimulus. Clones that proliferated in response to p24 protein were restimulated every 2 to 3 weeks with an anti-CD3-specific antibody 12F6 (obtained from Johnson Wong, Massachusetts General Hospital), IL-2 (100 U/ml), and 107 irradiated allogeneic PBMC.
TCR Vβ chain analysis.
In order to determine if the T-cell lines were monoclonal, T-cell receptor (TCR) Vβ expression was determined by an established method (12). Twenty-four sets of primers specific for TCR Vβ RNA were used. PBMC (105) were used as positive controls, and distilled water was used for negative controls.
Proliferation assays.
To assay T helper cell responses in fresh PBMC, 105 cells were incubated with p24 protein at 1 μg/ml for 6 days and then pulsed with [3H]thymidine for 6 h before harvesting as previously described (44). To test T-cell clones, antigen was presented by autologous or partially HLA matched B-LCL. The B-LCL were irradiated (120 Gy) and incubated overnight in R10H with the appropriate antigen at 1 μg/ml. The following day B-LCL and T-cell clones were plated in triplicate wells at 50,000 cells/well each in 96-well plates in R10H. After 48 h, 1 μCi of [3H]thymidine (Dupont NEN, Boston, Mass.) in 50 μl of R10H was added per well. Plates were harvested onto glass fiber filters after 18 h. Results were expressed as stimulation index, the ratio of counts from wells with antigen divided by the counts obtained from wells without antigen. Alternately, results were shown as net counts per minute, the difference between the counts. Based on previous studies of HIV-1-specific proliferative responses, stimulation indexes greater than 5 were considered significant (44).
IFN-γ Elispot assays.
Elispot plates were coated with anti-IFN-γ antibody (Endogen, Woburn, Mass.) and incubated overnight at 4°C. The following day plates were washed six times with phosphate-buffered saline (PBS; Mediatech). B-LCL (5 × 104) and antigen (1 μg/ml) were added in 100 μl of R10H. T-cell clones were added at 100 cells/well in 100 μl of R10H. After overnight incubation the cells were discarded and plates were washed six times with PBS, and biotinylated anti- IFN-γ (Endogen) was added for 1.5 h at 25°C. The plates were then washed six times with PBS. Streptavidin (Bio-Rad, Hercules, Calif.) (100 μl/well) was added, and plates were incubated 45 min at 25°C. After six more PBS washes 100 μl/well of coloring reagent (nitro blue tetrazolium–5-bromo-4-chloro-3-indolylphosphate [NBT/BCIP]; Bio-Rad) was added. Once dots appeared, the reaction was stopped with three water washes. Background responses to wells with no antigen or irrelevant antigen ranged from 0 to 1.5 spots per well, or 0 to 15,000 spot-forming cells (SFC) per 106 cells. Responses greater than 50,000 SFC per 106 cells and 5 times maximum background were considered significant.
Cytotoxicity assays.
Target cells (B-LCL) were incubated overnight with whole p24 or the cognate 22-amino-acid peptide at 1 μg/ml and Na251CrO4 (60 μCi/ml; Dupont NEN). The following day the targets were washed twice with cold R10H before effectors were added. Effectors and targets were incubated together in triplicate wells for 4 h at 37°C, then the plates were centrifuged at 1,000 rpm for 5 min at 4°C. A 25-μl aliquot of reaction supernatant was assayed for 51Cr release. Maximal release was obtained by mixing the targets with 100 μl of 1% Triton X-100. The percent specific 51Cr release was calculated as 100 × [(cpm experimental release − cpm spontaneous release)/(cpm maximal release − cpm spontaneous release)]. Some lysis inhibition experiments were performed in the presence of 2 mM EGTA and MgCl2. These reagents chelate extracellular calcium and were added at the time clones and targets were combined. We also performed cytolytic assays in the presence of inhibitors with concentrations varying from 0 to 10 μg/ml of concanamycin A (Sigma), brefeldin A (Sigma), or anti-Fas antibody (Coulter Immunotech, Miami, Fla.). The effector cells were incubated with inhibitors for 2 h prior to plating with B-LCL, and the inhibitors remained present throughout the assay. Spontaneous lysis was less than 30% unless otherwise noted. For the purpose of interpretation, specific lysis of greater than 10% was considered significant.
Lytic granule release assay.
To quantify lytic granule release, autologous B-LCL were pulsed with various concentrations of cognate peptide for 1 h at 37oC and combined with CD4+ T cells at an effector-to-target cell (E:T) ratio of 1:1 in round-bottomed 96-well plates. The density of the CTL in the mixture was 7 × 105 per ml (1.5 × 105 per well). After 4 h at 37oC the culture supernatant was collected, and activity of serine esterase, an essential component of the secreted granules, was measured using the BLT (N-α-benzyloxycarboxyl-l-lysinethiobensyl ester) substrate in the presence of DTNB [5,5′-dithio-bis(2-nitrobenzoic acid)] by reading the optical density (OD) at 405 nm (method communicated by Pierre Henkart). In our experiments DTNB was added prior to the addition of BLT to measure the background OD at 405 nm. The background was then subtracted from the final reading for each sample.
Intracellular staining for IFN-γ production.
Quantitation for intracellular production of IFN-γ upon antigen stimulation was performed as described elsewhere (51). Briefly, 5 × 105 clone cells were incubated with B cells pulsed with peptide at 5 μg/ml and anti-CD28 and anti-CD49 antibodies at 1 μg/ml each for 2 h at 37C at a 5° slant in 1 ml of R10H in a fluorescence-activated cell sorting (FACS) tube. At 2 h brefeldin A was added at 10 μg/ml, and incubation was continued as above for 4 more h. The samples were then refrigerated overnight. The next day the samples were washed in PBS plus 1% fetal calf serum, then stained with surface antibodies for 20 min. After washing in PBS the cells were fixed and permeabilized with two 15-min incubations with Caltag reagents according to the manufacturer's instructions. The cells were then stained with intracellular IFN-γ–fluorescein isothiocyanate (FITC), washed twice in PBS, and analyzed on the Becton Dickinson FACS machine. For the concanamycin A inhibition experiments, the cloned cells were first incubated with concanamycin A for 1 h prior to the addition of B-LCL and antigen. At least 50,000 total events were counted for each condition.
RESULTS
Proliferative responses of PBMC to p24 Gag protein.
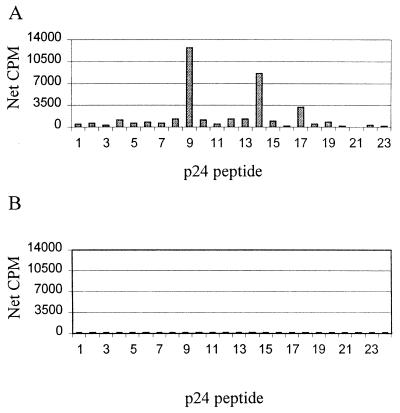
Previous studies have shown dominant HIV-1-specific T helper cell responses directed against the p24 Gag protein and that these responses are inversely correlated to HIV-1 viral load (22, 44). To determine the breadth of this response, overlapping peptides were used to map discrete targeted regions. An example is shown for subject CTS-01, an individual with long-term nonprogressive HIV-1 infection and a robust p24-specific T helper cell response. The dominant epitopes targeted were defined using synthetic 22-amino-acid peptides overlapping by 12 amino acids and spanning the p24 protein. Significant peptide-specific proliferative responses were detected to 3 of the 23 peptides tested (Fig. 1A), indicating a polyclonal response. The dominant response was directed against peptide 9, sequence DRVHPVHAGPIAPGQMREPRGS (44). Furthermore, responses of 10 HIV-1-seronegative controls to the panel of proteins were negligible (Fig. 1B).
FIG. 1.
(A) PBMC from subject CTS-01 were tested in a lymphocyte proliferation assay for responses to synthetic 22-amino-acid peptides spanning HIV-1 p24 protein sequence (peptides 1 to 23). These PBMC recognized several epitopes within p24, with the dominant response directed against peptide 9, sequence DRVHPVHAGPIAPGQMREPRGS. (B) The average proliferative response (net cpm) of PBMC from 10 HIV-1-seronegative control subjects to each of peptides 1 to 23 is shown.
Generation of CD4+ T-cell clones and mapping.
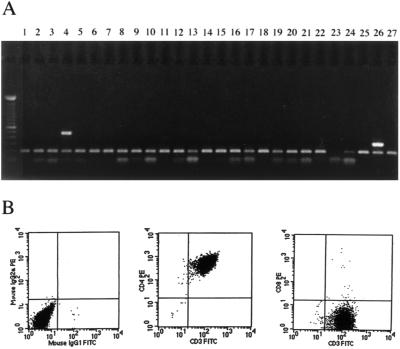
In order to define functional characteristics of T helper cell responses and fine map the epitopes targeted, clones were established. Limiting-dilution cloning of PBMC from subject CTS-01 was performed to derive 10 T-cell lines that proliferated to p24 protein. The fine specificity of the proliferative response was mapped using a set of overlapping p24 peptides. All T-cell lines but one were found to proliferate exclusively in response to peptide 9, DRVHPVHAGPIAPGQMREPRGS, with stimulation indices ranging from 25 to 230, net cpm 1,675 to 9,530 (data not shown). Clonality was determined by RT-PCR for Vβ region T-cell receptor usage. Clone 16 was selected for in-depth analysis because it expressed T-cell receptor Vβ4 exclusively and was presumed to be clonal (Fig. 2A). The remaining T-cell lines all expressed Vβ4 but also expressed one to three additional Vβ regions and are likely not clonal. T-cell lines were all functionally clonal and are referred to as clones in the remainder of the article. Each of the T-cell clones was >99.2% CD4+, and the majority were less than 0.5% CD8+ by FACS analysis (Fig. 2B).
FIG. 2.
(A) TCR Vβ repertoire analysis: cDNA was derived from clone 16 from subject CTS-01 by RT-PCR as previously described (12). The cDNA was amplified using primer sets specific for different variable segments of the TCR Vβ chain (Vβ1 to Vβ24). Only the Vβ4-specific primer pair yielded a PCR product (lane 4). Each reaction also contained T-cell receptor α chain constant region 3′ and 5′ primers that produced a 100-bp fragment, included as a positive PCR control in each lane. Lanes 25 and 27 contained no Vβ-specific primers (negative control), whereas lane 26 contained a primer pair specific for the Vβ constant region (positive control). The DNA ladder shown at left contained 100-bp incremental fragments. (B) Flow cytometric analysis of clone 16 showed that the clone was 99.57% CD4+ and 0.34% CD8+.
p24-specific cytotoxic responses mediated by CD4+ CD8− T-cell clones.
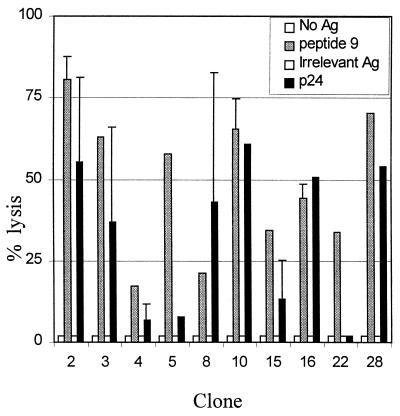
CD4+ T cells are classically thought to assist in antiviral control by providing help to both CD8+ CTL and B cells. CD4+ T cells have also been shown to secrete a number of antiviral chemokines, such as IFN-γ, RANTES, MIP-1α, and MIP-1β. We queried whether or not these CD4+ T-cell clones would also possess direct cytolytic function. The 10 CD4+ T cell clones derived from subject CTS-01 were assessed for their ability to lyse B-LCL incubated with peptide 9. All 10 clones assayed lysed autologous B-LCL incubated with peptide 9 at an E:T ratio of 100:1 (17 to 68% lysis), but not B-LCL incubated without peptide or with an irrelevant control peptide (Fig. 3). Seven of 10 clones also exhibited specific lysis in response to whole p24 protein at an E:T ratio of 100:1 (13 to 61% lysis; Fig. 3).
FIG. 3.
Ten p24-specific lines from donor CTS-01 were assayed for their ability to lyse B-LCL pulsed with p24 protein or peptide 9. A standard chromium release assay was performed, with results expressed as percent specific lysis. Negative controls included B cells pulsed with no antigen or with a control 22-amino-acid peptide derived from a portion of p24 not recognized by the subject. Lines were assayed at an E:T ratio of 100:1. Error bars were calculated by determining the standard deviation of assay results performed on at least two separate occasions.
Fine epitope mapping of proliferative, cytotoxic, and IFN-γ-secreting responses.
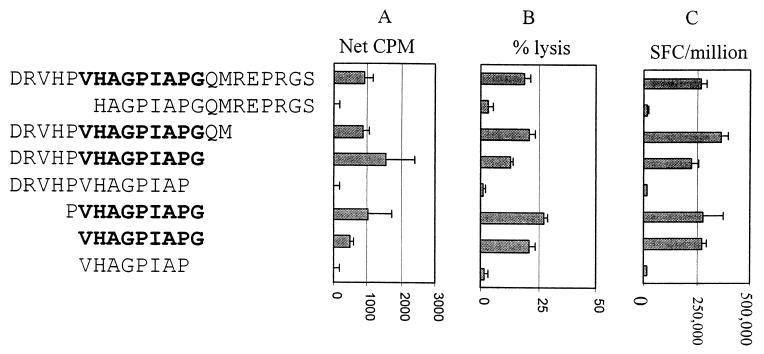
T helper cell responses are typically targeted at exogenously processed proteins 10 to 17 amino acids in length. Truncations of peptide 9 were synthesized to define the minimal epitope recognized by the clones from subject CTS-01. Three clones were examined, and data for one representative clone are shown. All three clones recognized the same minimal 9-amino-acid peptide, VHAGPIAPG (Fig. 4A). Deletion of either the N-terminal valine or C-terminal glycine resulted in complete abrogation of the proliferative response. However, longer peptides containing these residues were all recognized, and some were associated with stronger proliferative responses.
FIG. 4.
Fine mapping of the peptide-specific proliferative, cytotoxic, and Elispot responses of clone 16 from subject CTS-01. The peptide sequences are given on the left, followed by the graphs of (A) proliferation, (B) cytotoxicity, and (C) Elispot responses. Peptides that contained the minimal epitope are in bold. Elispot results were expressed as SFC/106 clone cells added. Antigen concentration was 1 μg/ml for the proliferation and Elispot assays and 10 μg/ml for the cytotoxicity assays. In each assay the minimal epitope recognized was VHAGPIAPG.
We next compared proliferation to cytolytic activity and IFN-γ secretion using these truncated peptides. Fine mapping of the cytolytic response revealed the same minimal epitope (Fig. 4B). The clone was found to secrete IFN-γ in response to stimulation by B-LCL pulsed with cognate peptide (2.8 × 105 SFC/106; Fig. 4C), but not by B-LCL incubated with an irrelevant peptide (0 to 1.5 × 104 SFC/106; data not shown). The minimal epitope for stimulation of IFN-γ secretion was identical to the minimal epitope required to induce proliferation and cytolysis, VHAGPIAPG.
Clonal responses were DQ7 restricted.
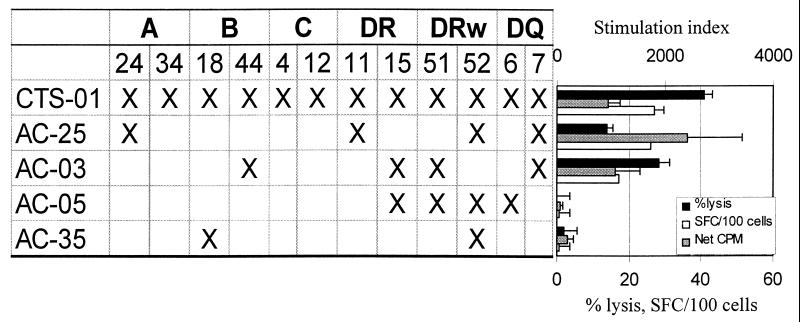
The HLA restriction of clones targeting the minimal peptide VHAGPIAPG was determined using partially matched B-LCL as antigen-presenting cells in proliferation, cytolytic, and IFN-γ Elispot assays. B-LCL that could present antigen effectively in the three assays all had allele DQ7, while those lacking DQ7 could not present antigen effectively (Fig. 5, left panel). Multiple other B-LCL were assayed, and the results consistently identified DQ7 as the only allele associated with effective antigen presentation (Fig. 5 and data not shown). These data thus confirm that the effector functions mediated by these HIV-1-specific T helper cell clones are HLA class II restricted.
FIG. 5.
HLA restriction of CD4+ T-cell clone 16 from subject CTS-01. Antigen was presented to the clone by partially HLA matched B-LCL pulsed overnight with peptide 9. Negative controls included no antigen or peptide 23, a 22-amino-acid peptide from p24 not recognized by the subject's PBMC (data not shown). Only B-LCL which expressed the DQ7 allele elicited antigen-specific responses in proliferation, cytotoxicity, and IFN-γ Elispot assays. The top scale is for net cpm, and the bottom scale is for percent lysis and SFC/106 cells. An E:T ratio of 10:1 was used for cytotoxicity assays.
CD4+ T-cell mediated cytolysis was seen in multiple individuals.
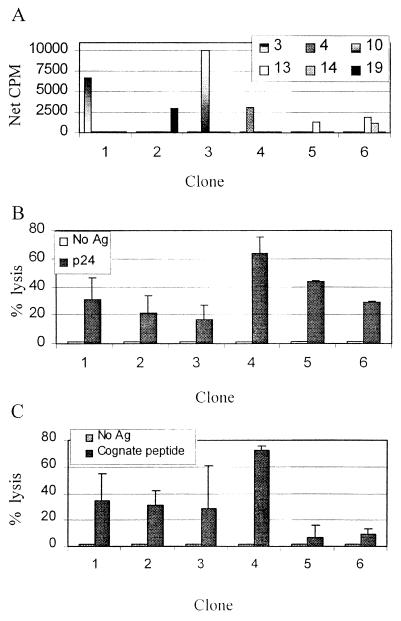
We explored whether CD4+ T-cell clones from other HIV-1-infected individuals would also exhibit antigen-specific cytolytic activity. We derived clones from three individuals who were diagnosed and treated with highly active antiretroviral therapy during acute HIV-1 infection who generated vigorous T helper cell responses to p24 antigen (43, 44). Three clones were derived from one individual, two from a second, and one from a third. Each clone recognized a different epitope within p24 as mapped by overlapping 22-amino-acid peptides (Fig. 6A). Clonality was determined by RT-PCR for Vβ T-cell receptor usage as described previously. Clones 1 to 3 and 6 each expressed a unique T-cell receptor Vβ region, clone 4 expressed two Vβ T-cell receptor products, and none of the tested Vβ T-cell receptors was identified for clone 5. The six clones were tested for p24-specific cytolytic activity at an E:T ratio of 100:1 (Fig. 6B). All six clones exhibited cytolytic activity, confirming that the observation of cytolytic CD4+ T-cell clones was not limited to one individual. The six clones were also tested for cytolytic activity directed against the 22-amino-acid peptide each was found to recognize in a proliferation assay. Four of the six clones maintained levels of specific lysis greater than 25% at an E:T ratio of 10:1 (Fig. 6C). These results provide evidence at a clonal level that HIV-1-specific T helper cells possess multiple effector functions, including proliferation, IFN-γ secretion, and cytolysis.
FIG. 6.
(A) Proliferative responses of each of six CD4+ T-cell clones derived from three individuals treated during acute HIV-1 infection are shown. The clones are labeled 1 to 6, with the first three derived from subject AC-01, the next two from subject AC-25, and the last from subject AC-36. Peptides 3 (amino acids 153 to 174), 4 (amino acids 163 to 184), 10 (amino acids 223 to 244), 13 (amino acids 253 to 274), 14 (amino acids 263 to 284), and 19 (amino acids 313 to 344) were recognized by one or more clones. (B) The six clones were tested for cytolytic ability. Autologous B-LCL were incubated with 51Cr and soluble p24 and used as targets in a 4-h CTL assay at an E:T ratio of 100:1. Spontaneous lysis ranged from 10 to 36%. Negative controls included B-LCL without added antigen. (C) The same six clones were assayed for their ability to kill B-LCL targets pulsed with the 22-amino-acid peptide within p24 recognized by each clone (see panel A). The assays were performed at an E:T ratio of 10:1, and negative controls included B-LCL without added antigen.
Cytolytic activity occurred at doses of peptide similar to those required to induce proliferation and IFN-γ production.
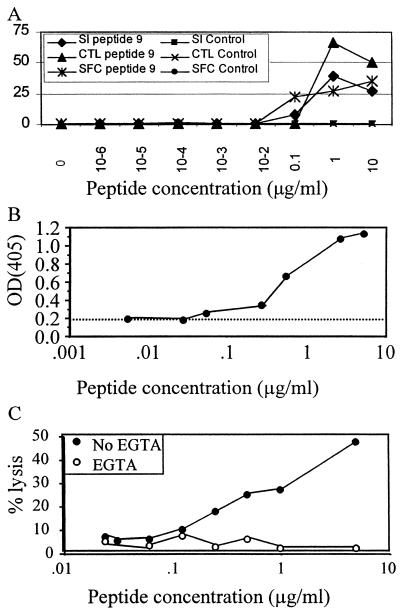
The in vivo significance of these cytolytic T helper cell clones is unknown. In order to determine if the cytolytic function of these clones was artificially induced by concentrations of antigen much higher than those required for proliferation and IFN-γ production, we performed a peptide titration. Figure 7A demonstrates that a peptide concentration of 1 μg/ml was able to elicit proliferation, IFN-γ production, and cytolysis in the clone from subject CTS-01, implying that the cytolysis observed was not merely an artifact due to unusually high antigen concentrations. Identical results were obtained with a clone from subject AC-25 (data not shown).
FIG. 7.
(A) Dependence of proliferative, cytolytic, and IFN-γ secretion activity on peptide concentration. Autologous B cells were incubated with peptide 9 from p24 or with the control peptide 23 from p24 (not recognized) from 0 to 10 μg/ml. CD4+ T-cell clone responses from subject CTS-01 were measured in each of the three assays and expressed as stimulation index (SI), percent lysis (CTL), and SFC per 100. (B) Elaboration of serine esterase upon antigen-specific stimulation of clone 4 from subject AC-25. Autologous B cells were incubated with peptide 4 from 0 to 10 μg/ml prior to use as antigen-presenting cells. Serine esterase release was quantified by modification of the substrate BLT, and results were expressed as OD405, with higher OD corresponding to greater granule secretion. The dotted line indicates the background release in response to the target cells without the peptide. (C) Abrogation of lysis mediated by clone 4 from subject AC-25 in the presence (○) and absence (●) of 2 mM Mg2+ and EGTA. Chelation of extracellular calcium caused abrogation of lysis over the range of peptide concentrations.
Lysis was mediated by the perforin pathway.
The finding that these Gag-specific T helper cell clones mediated cytolytic activity prompted us to examine the mechanism of cell lysis. We focused our attention on clone 16 from subject CTS-01 and clone 4 from subject AC-25. The two main mechanisms of CD4+ T-cell cytotoxicity that have been reported include perforin-mediated and Fas-mediated lysis (21, 32). Additionally, CD4+ CTL have been shown to secrete IFN-γ, granzyme A, and tumor necrosis factor (TNF)-α and -β (11, 29, 47, 54). Serine esterase granule secretion was measured after specific stimulation in the clone from subject AC-25 (Fig 7B). Peptide 4 from p24 was able to elicit serine esterase release as measured by conversion of the substrate BLT, implying that the clones contained and could release lytic granules. Furthermore, chelation of extracellular calcium with EGTA has been used as a method of selectively blocking perforin-mediated lysis. We examined lysis mediated by the AC-25 clone in the presence of EGTA and found it to be abrogated, implying that the clone utilized the perforin pathway of lysis (Fig. 7C). Perforin staining of the clone from AC-25 was performed and was 6 to 8 times the background staining of B-LCL or staining with control immunoglobulin (data not shown).
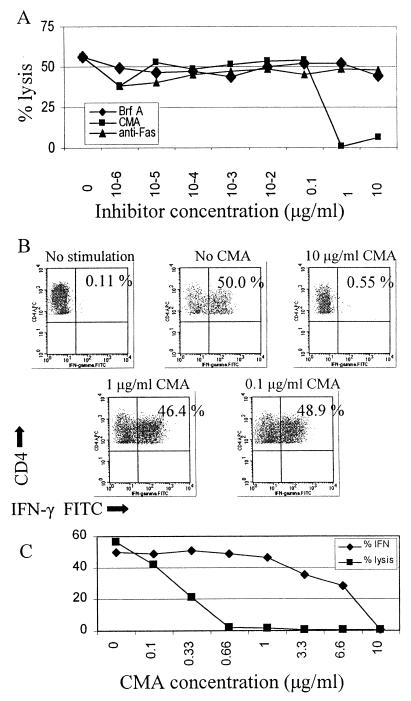
Concanamycin A and brefeldin A have been used as inhibitors of perforin-mediated and Fas-mediated cytotoxicity, respectively, to differentiate between these two mechanisms of lysis (24). Concanamycin A, an inhibitor of vacuolar-type H+-ATPase, accelerates degradation of perforin by increasing the pH of lytic granules. Brefeldin A inhibits intracellular glycoprotein transport and was shown to selectively block Fas-mediated killing. Anti-Fas antibody can also block Fas-mediated killing (55). We found that concanamycin A could abrogate lysis in a dose-dependent fashion in the clone from subject CTS-01 (Fig. 8A). Brefeldin A and anti-Fas antibody over the same concentration range showed no effect on cytolytic activity. To demonstrate that the clones retained functions other than perforin secretion in the presence of concanamycin A, we quantitated IFN-γ production in the presence of concanamycin A in the clone from subject AC-25 (Fig. 8B). At the highest concentration of concanamycin A, IFN-γ production was abrogated. However, IFN-γ production was unaffected over a range of concentrations of concanamycin A that resulted in complete abrogation of lysis (Fig. 8C), supporting the notion that perforin secretion was selectively inhibited by concanamycin A. These results imply that the Gag-specific T helper cell responses utilized a perforin-dependent pathway in killing targets.
FIG. 8.
(A) Effects of concanamycin A, brefeldin A, and anti-Fas antibody on cytolytic activity of the clone from subject CTS-01. T cells were incubated with various concentrations of concanamycin A, brefeldin A, or anti-Fas antibody for 2 h preceding a standard 4-h chromium release assay. The E:T ratio was 10:1, and B-cell targets were pulsed with 10 μg/ml of peptide 9 overnight prior to the assay. Similar results were obtained with clone 4 from subject AC-25. Spontaneous lysis ranged from 25 to 35%. (B) IFN-γ secretion after stimulation with peptide 4 was measured in clone 4 from subject AC-25 in the presence of various concentrations of concanamycin A (CMA). B-LCL were pulsed with peptide 4 and added at an E:T ratio of 100:1. The percentage of CD4+ T cells secreting IFN-γ is listed in the upper right of each dot plot. (C) Cytolysis was measured in clone 4 from subject AC-25 in the presence of various concentrations of concanamycin A and compared to IFN-γ production (from panel B). B-LCL were pulsed with peptide 4 and added at an E:T ratio of 10:1 for the CTL assay. While abrogation of IFN-γ secretion was seen at the highest concentration of concanamycin A, lysis was completely inhibited at concentrations of concanamycin A that had no effect on IFN-γ secretion. The left axis gives percent IFN-γ production and percent lysis.
DISCUSSION
Although HIV-specific T helper cells are a central component in antiviral control, an in-depth characterization of these immune responses has not been performed. We report that a CD4+ T-cell clone specific for a discrete 9-amino-acid peptide in the HIV-1 Gag protein responds to antigen by proliferation, IFN-γ secretion, and perforin-mediated lysis of p24-pulsed target cells. Moreover, we extend these studies to show that cytolytic activity is a frequently observed in vitro functional activity of HIV-1-specific T helper cell clones.
The extent to which cytolytic activity of T helper cells plays a role in vivo in controlling infections is not clear and remains controversial. In an experimental system with herpes simplex virus type 1-specific T-cell lines, cytolytic activity was demonstrated to be mediated solely by natural killer and CD4+ CD8− T cells (45). No herpes simplex virus type 1-specific CD8+ T cells were found, indicating that under certain circumstances CD4+ CTL may exert immune control of viral infections. Given that a number of cells infected by HIV-1 also express HLA class II molecules, one could speculate that CD4+ CTL could play a role in control of HIV-1 infection. Alternatively, deletion of HIV-1-specific antigen-presenting cells could have a downregulatory effect on the immune response and interfere with the delivery of help to CD8+ CTL.
Few data exist showing a direct correlation between CD4+ cytolytic T cells and disease progression. In a murine model of influenza virus infection, protection from a lethal challenge and over 100-fold reduction in lung virus titers was observed with adoptive transfer of a Th1 influenza virus-specific CD4+ T-cell clone with cytolytic activity (14, 33). Th2-type CD4+ T-cell clones were found to have neither cytolytic ability nor the ability to protect from lethal influenza virus challenge in this system. In a Friend retrovirus infection model, CD4+ T-cell clones were shown to possess cytolytic activity and to inhibit the production of virus in vitro (19). The clones that we isolated secreted IFN-γ and possessed cytolytic activity on specific stimulation, consistent with the Th1-type clones identified in murine studies. HIV-1-specific CD4+ CTL have primarily been described in the setting of gp160-vaccinated, seronegative volunteers (15, 37, 39, 48) and were found to be both Th0 and Th1 phenotypes (49). Few reports have demonstrated the existence of HIV-1-specific CD4+ CTL in natural infection (31, 46), and in some reports HIV-1-specific CD4+ T helper cell clones possessed no cytolytic activity (34), while the majority of the clones that we isolated possessed HIV-1-specific cytolytic activity. Efforts to demonstrate HIV-1-specific cytolytic activity from PBMC have met with mixed results (16, 28), and therefore it is unclear whether the clones that we isolated are a result of several rounds of in vitro stimulation or whether they exist in vivo.
The observation that a 9-amino-acid peptide is sufficient for activation of CD4+ T cells is in keeping with recent studies based on crystallization of murine major histocompatibility complex (MHC) class II molecule I-Ak with peptide and the T-cell receptor (42). Larger peptides are accommodated in the MHC class II binding pocket, but only 9 amino acids were shown to directly contact the T-cell receptor and MHC class II protein. HLA-DQ7 has been described to bind molecules lacking a P1 anchor residue, binding simple alanine octamers and even hexamers (41). More recent work modeling putative peptide-binding motifs predicted four essential pockets that could interact with peptides at positions P1, P4, P6, and P9, with P1 interacting with the N-terminal portion of the peptide (26). P1 was predicted to interact with aromatic or small hydrophobic residues, P4 with small residues, P6 with small hydrophobic or polar residues, and P9 with large amide, polar, or small residues. The peptide that we delineated supported the above model, as it matched the motif at all predicted pocket-binding positions: P1 (valine), P4 (glycine), P6 (isoleucine), and P9 (glycine). HLA-DQ7-restricted clones have been described in a number of other diseases, such as melanoma (38), human papillomavirus type 1 (50), and hepatitis C virus (10). Epidemiological studies have linked HLA-DQ7 with clearance of infection with hepatitis C virus (1, 36) and persistence of hepatitis B virus. While DR13 and DQ6 have been associated with delayed progression to AIDS (25), DQ7 has not been correlated with disease progression in HIV-1.
The epitope recognized by the clones derived from subject CTS-01, VHAGPIAPG, is contained within the binding sequence of Gag p24 to cyclophilin A, thought to be crucial to an early step in the HIV-1 viral life cycle (7). The epitope recognized by these clones is from a relatively conserved region of Gag (27). The critical interaction of Gag and cyclophilin A could provide pressure against viral mutation in this region of the genome, making immune escape more difficult at this epitope. Further studies will be needed to address sequence variation of autologous viral isolates from subject CTS-01 and other persons who target this epitope. Likewise, fine characterization of other epitopes may define additional targeted epitopes within functional HIV-1 domains.
The CD4+ T-cell clones we studied here exhibited antiviral activity via proliferation, IFN-γ secretion, and p24-specific cytolysis that appears to be perforin mediated. Further studies will be required to determine if CD4+ T cells can play a direct role in antiviral control. The demonstration of effector activity in CD4+ T-cell clones provides further impetus to include CD4+ T-cell epitopes in future vaccines. Fine epitope mapping and HLA restriction of responses provide an important first step in identifying areas to target in immunotherapeutic interventions.
ACKNOWLEDGMENTS
This work has been supported by NIH grants AI01698–01, AI01541, and AI40873. P.J.N. is grateful for funding from Cable Positive. P.J.N., E.S.R., and B.D.W. are supported by the Doris Duke Charitable Foundation. B.D.W. is a Doris Duke Distinguished Clinical Science Professor.
REFERENCES
- 1.Alric L, Fort M, Izopet J, Vinel J P, Charlet J P, Selves J, Puel J, Pascal J P, Duffaut M, Abbal M. Genes of the major histocompatibility complex class II influence the outcome of hepatitis C virus infection. Gastroenterology. 1997;113:1675–1681. doi: 10.1053/gast.1997.v113.pm9352872. [DOI] [PubMed] [Google Scholar]
- 2.Amara R R, Villinger F, Altman J D, Lydy S L, O'Neil S P, Staprans S I, Montefiori D C, Xu Y, Herndon J G, Wyatt L S, Candido M A, Kozyr N L, Earl P L, Smith J M, Ma H L, Grimm B D, Hulsey M L, Miller J, McClure H M, McNicholl J M, Moss B, Robinson H L. Control of a mucosal challenge and prevention of AIDS by a multiprotein DNA/MVA vaccine. Science. 2001;292:69–74. doi: 10.1126/science.1058915. [DOI] [PubMed] [Google Scholar]
- 3.Barnaba V, Franco A, Paroli M, Benvenuto R, De Petrillo G, Burgio V L, Santilio I, Balsano C, Bonavita M S, Cappelli G. Selective expansion of cytotoxic T lymphocytes with a CD4+CD56+ surface phenotype and a T helper type 1 profile of cytokine secretion in the liver of patients chronically infected with Hepatitis B virus. J Immunol. 1994;152:3074–3087. [PubMed] [Google Scholar]
- 4.Barouch D, Santra S, Schmitz J, Kuroda M, Fu T, Wagner W, Bilska M, Craiu A, Zheng X, Krivulka G, Beaudry K, Lifton M, Nickerson C, Trigona W, Punt K, Freed D, Guan L, Dubey S, Casimiro D, Simon A, Davies M, Chastain M, Strom T, Gelman R, Montefiori D, Lewis M, Emini E, Shiver J, Letvin N. Control of viremia and prevention of clinical AIDS in Rhesus monkeys by cytokine-augmented DNA vaccination. Science. 2000;290:486–492. doi: 10.1126/science.290.5491.486. [DOI] [PubMed] [Google Scholar]
- 5.Battegay M, Moskophidis D, Rahemtulla A, Hengartner H, Mak T W, Zinkernagel R M. Enhanced establishment of a virus carrier state in adult CD4+ T-cell-deficient mice. J Virol. 1994;68:4700–4704. doi: 10.1128/jvi.68.7.4700-4704.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bickham K, Munz C, Tsang M L, Larsson M, Fonteneau J F, Bhardwaj N, Steinman R. EBNA1-specific CD4(+) T cells in healthy carriers of Epstein-Barr virus are primarily Th1 in function. J Clin Investig. 2001;107:121–130. doi: 10.1172/JCI10209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Braaten D, Aberham C, Franke E K, Yin L, Phares W, Luban J. Cyclosporine A-resistant human immunodeficiency virus type 1 mutants demonstrate that Gag encodes the functional target of cyclophilin A. J Virol. 1996;70:5170–5176. doi: 10.1128/jvi.70.8.5170-5176.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Braaten D, Franke E K, Luban J. Cyclophilin A is required for an early step in the life cycle of human immunodeficiency virus type 1 before the initiation of reverse transcription. J Virol. 1996;70:3551–3560. doi: 10.1128/jvi.70.6.3551-3560.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cardin R D, Brooks J W, Sarawar S R, Doherty P C. Progressive loss of CD8+ T cell-mediated control of a gamma-herpesvirus in the absence of CD4+ T cells. J Exp Med. 1996;184:863–871. doi: 10.1084/jem.184.3.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cramp M, Rossol S, Carucci P, Williams R, Naumov N, Donaldson P. The influence of HLA class II allele DQB1*0301 on HCV-specific T helper responses. J Hepatol. 1998;28:94. [Google Scholar]
- 11.Gagnon S J, Ennis F A, Rothman A L. Bystander target cell lysis and cytokine production by dengue virus-specific human CD4(+) cytotoxic T-lymphocyte clones. J Virology. 1999;73:3623–3629. doi: 10.1128/jvi.73.5.3623-3629.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Genevee C, Diu A, Nierat J, Caignard A, Dietrich P Y, Ferradini L, Roman-Roman S, Triebel F, Hercend T. An experimentally validated panel of subfamily-specific oligonucleotide primers (V alpha 1-w29/V beta 1-w24) for the study of human T cell receptor variable V gene segment usage by polymerase chain reaction. Eur J Immunol. 1992;22:1261–1269. doi: 10.1002/eji.1830220522. [DOI] [PubMed] [Google Scholar]
- 13.Gerlach J T, Diepolder H M, Jung M C, Gruener N H, Schraut W W, Zachoval R, Hoffmann R, Schirren C A, Santantonio T, Pape G R. Recurrence of hepatitis C virus after loss of virus-specific CD4(+) T-cell response in acute hepatitis C. Gastroenterology. 1999;117:933–941. doi: 10.1016/s0016-5085(99)70353-7. [DOI] [PubMed] [Google Scholar]
- 14.Graham M B, Braciale V L, Braciale T J. Influenza virus-specific CD4+ T helper type 2 T lymphocytes do not promote recovery from experimental virus infection. J Exp Med. 1994;180:1273–1282. doi: 10.1084/jem.180.4.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hammond S A, Bollinger R C, Stanhope P E, Quinn T C, Schwartz D, Clements M L, Siliciano R F. Comparative clonal analysis of human immunodeficiency virus type 1 (HIV-1)-specific CD4+ and CD8+ cytolytic T lymphocytes isolated from seronegative humans immunized with candidate HIV-1 vaccines. J Exp Med. 1992;176:1531–1542. doi: 10.1084/jem.176.6.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heinkelein M, Euler-Konig I, Klinker H, Ruckle-Lanz H, Jassoy C. Lysis of human immunodeficiency virus type 1 antigen-expressing cells by CD4 and CD8 T cells ex vivo. J Infect Dis. 1996;174:209–213. doi: 10.1093/infdis/174.1.209. [DOI] [PubMed] [Google Scholar]
- 17.Hel Z, Venzon D, Poudyal M, Tsai W P, Giuliani L, Woodward R, Chougnet C, Shearer G, Altman J D, Watkins D, Bischofberger N, Abimiku A, Markham P, Tartaglia J, Franchini G. Viremia control following antiretroviral treatment and therapeutic immunization during primary SIV251 infection of macaques. Nat Med. 2000;6:1140–1146. doi: 10.1038/80481. [DOI] [PubMed] [Google Scholar]
- 18.Hom R C, Finberg R W, Mullaney S, Ruprecht R M. Protective cellular retroviral immunity requires both CD4+ and CD8+ immune T cells. J Virol. 1991;65:220–224. doi: 10.1128/jvi.65.1.220-224.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iwashiro M, Peterson K, Messer R J, Stromnes I M, Hasenkrug K J. CD4(+) T cells and gamma interferon in the long-term control of persistent friend retrovirus infection. J Virol. 2001;75:52–60. doi: 10.1128/JVI.75.1.52-60.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jacobson S, Richert J R, Biddison W E, Satinsky A, Hartzman R J, McFarland H F. Measles virus-specific T4+ human cytotoxic T cell clones are restricted by class II HLA antigens. J Immunol. 1984;133:754–757. [PubMed] [Google Scholar]
- 21.Kagi D, Vignaux F, Ledermann B, Burki K, Depraetere V, Nagata S, Hengartner H, Golstein P. Fas and perforin pathways as major mechanisms of T cell-mediated cytotoxicity. Science. 1994;265:528–530. doi: 10.1126/science.7518614. [DOI] [PubMed] [Google Scholar]
- 22.Kalams S A, Buchbinder S P, Rosenberg E S, Billingsley J M, Colbert D S, Jones N G, Shea A K, Trocha A K, Walker B D. Association between virus-specific cytotoxic T-lymphocyte and helper responses in human immunodeficiency virus type 1 infection. J Virol. 1999;73:6715–6720. doi: 10.1128/jvi.73.8.6715-6720.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kalams S A, Goulder P J, Shea A K, Jones N G, Trocha A K, Ogg G S, Walker B D. Levels of human immunodeficiency virus type 1-specific cytotoxic T-lymphocyte effector and memory responses decline after suppression of viremia with highly active antiretroviral therapy. J Virol. 1999;73:6721–6728. doi: 10.1128/jvi.73.8.6721-6728.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kataoka T, Shinohara N, Takayama H, Takaku K, Kondo S, Yonehara S, Nagai K. Concanamycin A, a powerful tool for characterization and estimation of contribution of perforin- and Fas-based lytic pathways in cell-mediated cytotoxicity. J Immunol. 1996;156:3678–3686. [PubMed] [Google Scholar]
- 25.Keet I P, Tang J, Klein M R, LeBlanc S, Enger C, Rivers C, Apple R J, Mann D, Goedert J J, Miedema F, Kaslow R A. Consistent associations of HLA class I and II and transporter gene products with progression of human immunodeficiency virus type 1 infection in homosexual men. J Infect Dis. 1999;180:299–309. doi: 10.1086/314862. [DOI] [PubMed] [Google Scholar]
- 26.Khalil-Daher I, Boisgerault F, Feugeas J P, Tieng V, Toubert A, Charron D. Naturally processed peptides from HLA-DQ7 (alpha1*0501-beta1*0301): influence of both alpha and beta chain polymorphism in the HLA-DQ peptide binding specificity. Eur J Immunol. 1998;28:3840–3849. doi: 10.1002/(SICI)1521-4141(199811)28:11<3840::AID-IMMU3840>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 27.Korber B, Moore J, Brander C, Walker B, Haynes B, Koup R. HIV Molecular Immunology Database. Los Alamos, N. Mex: Los Alamos National Laboratory; 1999. [Google Scholar]
- 28.Kundu S K, Merigan T C. Equivalent recognition of HIV proteins, Env, Gag and Pol, by CD4+ and CD8+ cytotoxic T-lymphocytes. AIDS. 1992;6:643–649. . (Erratum, 6:1051.) [PubMed] [Google Scholar]
- 29.Lewinsohn D M, Bement T T, Xu J, Lynch D H, Grabstein K H, Reed S G, Alderson M R. Human purified protein derivative-specific CD4+ T cells use both CD95-dependent and CD95-independent cytolytic mechanisms. J Immunol. 1998;160:2374–2379. [PubMed] [Google Scholar]
- 30.Lifson J D, Rossio J L, Arnaout R, Li L, Parks T L, Schneider D K, Kiser R F, Coalter V J, Walsh G, Imming R J, Fisher B, Flynn B M, Bischofberger N, Piatak M, Hirsch V M, Bowak M A, Wodarz D. Containment of simian immunodeficiency virus infection: cellular immune responses and protection from rechallenge following transient postinoculation antiretroviral treatment. J Virol. 2000;74:2584–2593. doi: 10.1128/jvi.74.6.2584-2593.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Littaua R A, Oldstone M B, Takeda A, Ennis F A. A CD4+ cytotoxic T-lymphocyte clone to a conserved epitope on human immunodeficiency virus type 1 p24: cytotoxic activity and secretion of interleukin-2 and interleukin-6. J Virol. 1992;66:608–611. doi: 10.1128/jvi.66.1.608-611.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lowin B, Hahne M, Mattmann C, Tschopp J. Cytolytic T-cell cytotoxicity is mediated through perforin and Fas lytic pathways. Nature. 1994;370:650–652. doi: 10.1038/370650a0. [DOI] [PubMed] [Google Scholar]
- 33.Lukacher A E, Morrison L A, Braciale V L, Braciale T J. T lymphoscyte function in recovery from experimental viral infection: The influenza model. In: Steinman R M, North R J, editors. Mechanisms of host resistance to infectious agents, tumors, and allografts : a conference in recognition of the Trudeau Institute centennial. New York, N.Y: Rockefeller University Press; 1986. pp. 233–254. [Google Scholar]
- 34.Malhotra U, Holte S, Dutta S, Berrey M M, Delpit E, Koelle D M, Sette A, Corey L, McElrath M J. Role for HLA class II molecules in HIV-1 suppression and cellular immunity following antiretroviral treatment. J Clin Investig. 2001;107:505–517. doi: 10.1172/JCI11275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matloubian M, Concepcion R J, Ahmed R. CD4+ T cells are required to sustain CD8+ cytotoxic T-cell responses during chronic viral infection. J Virol. 1994;68:8056–8063. doi: 10.1128/jvi.68.12.8056-8063.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Minton E J, Smillie D, Neal K R, Irving W L, Underwood J C, James V. Association between MHC class II alleles and clearance of circulating hepatitis C virus. Members of the Trent Hepatitis C Virus Study Group. J Infect Dis. 1998;178:39–44. doi: 10.1086/515599. [DOI] [PubMed] [Google Scholar]
- 37.Miskovsky E P, Liu A Y, Pavlat W, Viveen R, Stanhope P E, Finzi D, Fox W M, 3rd, Hruban R H, Podack E R, Siliciano R F. Studies of the mechanism of cytolysis by HIV-1-specific CD4+ human CTL clones induced by candidate AIDS vaccines. J Immunol. 1994;153:2787–2799. [PubMed] [Google Scholar]
- 38.Olsen A C, Fossum B, Kirkin A F, Zeuthen J, Gaudernack G. A human melanoma cell line, recognized by both HLA class I and class II restricted T cells, is capable of initiating both primary and secondary immune responses. Scandinavian J Immunology. 1995;41:357–364. doi: 10.1111/j.1365-3083.1995.tb03579.x. [DOI] [PubMed] [Google Scholar]
- 39.Orentas R J, Hildreth J E, Obah E, Polydefkis M, Smith G E, Clements M L, Siliciano R F. Induction of CD4+ human cytolytic T cells specific for HIV-infected cells by a gp160 subunit vaccine. Science. 1990;248:1234–1237. doi: 10.1126/science.2190315. [DOI] [PubMed] [Google Scholar]
- 40.Ostrowski M A, Justement S J, Ehler L, Mizell S B, Lui S, Mican J, Walker B D, Thomas E K, Seder R, Fauci A S. The role of CD4(+) T cell help and CD40 ligand in the In vitro expansion of HIV-1-specific memory cytotoxic CD8(+) T cell responses. J Immunol. 2000;165:6133–6141. doi: 10.4049/jimmunol.165.11.6133. [DOI] [PubMed] [Google Scholar]
- 41.Raddrizzani L, Sturniolo T, Guenot J, Bono E, Gallazzi F, Nagy Z A, Sinigaglia F, Hammer J. Different modes of peptide interaction enable HLA-DQ and HLA-DR molecules to bind diverse peptide repertoires. J Immunol. 1997;159:703–711. [PubMed] [Google Scholar]
- 42.Reinherz E L, Tan K, Tang L, Kern P, Liu J, Xiong Y, Hussey R E, Smolyar A, Hare B, Zhang R, Joachimiak A, Chang H C, Wagner G, Wang J. The crystal structure of a T cell receptor in complex with peptide and MHC class II. Science. 1999;286:1913–1921. doi: 10.1126/science.286.5446.1913. [DOI] [PubMed] [Google Scholar]
- 43.Rosenberg E, Altfeld M, Poon S, Phillips M, Wilkes B, Eldridge R, Robbins G, D'Aquilla R, Goulder P, Walker B. Immune control of HIV-1 after early treatment of acute infection. Nature. 2000;407:523–526. doi: 10.1038/35035103. [DOI] [PubMed] [Google Scholar]
- 44.Rosenberg E S, Billingsley J M, Caliendo A M, Boswell S L, Sax P E, Kalams S A, Walker B D. Vigorous HIV-1-specific CD4+ T cell responses associated with control of viremia. Science. 1997;278:1447–1450. doi: 10.1126/science.278.5342.1447. [DOI] [PubMed] [Google Scholar]
- 45.Schmid D S. The human MHC-restricted cellular response to herpes simplex virus type 1 is mediated by CD4+, CD8- T cells and is restricted to the DR region of the MHC complex. J Immunol. 1988;140:3610–3616. [PubMed] [Google Scholar]
- 46.Sethi K K, Naher H, Stroehmann I. Phenotypic heterogeneity of cerebrospinal fluid-derived HIV-specific and HLA-restricted cytotoxic T-cell clones. Nature. 1988;335:178–181. doi: 10.1038/335178a0. [DOI] [PubMed] [Google Scholar]
- 47.Stalder T, Hahn S, Erb P. Fas antigen is the major target molecule for CD4+ T cell-mediated cytotoxicity. J Immunol. 1994;152:1127–1133. [PubMed] [Google Scholar]
- 48.Stanhope P E, Clements M L, Siliciano R F. Human CD4+ cytolytic T lymphocyte responses to a human immunodeficiency virus type 1 gp160 subunit vaccine. J Infect Dis. 1993;168:92–100. doi: 10.1093/infdis/168.1.92. [DOI] [PubMed] [Google Scholar]
- 49.Stanhope P E, Liu A Y, Pavlat W, Pitha P M, Clements M L, Siliciano R F. An HIV-1 envelope protein vaccine elicits a functionally complex human CD4+ T cell response that includes cytolytic T lymphocytes. J Immunol. 1993;150:4672–4686. [PubMed] [Google Scholar]
- 50.Steele J C, Stankovic T, Gallimore P H. Production and characterization of human proliferative T-cell clones specific for human papillomavirus type 1 E4 protein. J Virol. 1993;67:2799–2806. doi: 10.1128/jvi.67.5.2799-2806.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Waldrop S L, Pitcher C J, Peterson D M, Maino V C, Picker L J. Determination of antigen-specific memory/effector CD4+ T cell frequencies by flow cytometry: evidence for a novel, antigen-specific homeostatic mechanism in HIV-associated immunodeficiency. J Clin Investig. 1997;99:1739–1750. doi: 10.1172/JCI119338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yakushijin Y, Yasukawa M, Kobayashi Y. Establishment and functional characterization of human herpesvirus 6-specific CD4+ human T-cell clones. J Virol. 1992;66:2773–2779. doi: 10.1128/jvi.66.5.2773-2779.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yasukawa M, Inatsuki A, Kobayashi Y. Differential in vitro activation of CD4+CD8- and CD8+CD4- herpes simplex virus-specific human cytotoxic T cells. J Immunol. 1989;143:2051–2057. [PubMed] [Google Scholar]
- 54.Yasukawa M, Yakushijin Y, Hasegawa H, Miyake M, Hitsumoto Y, Kimura S, Takeuchi N, Fujita S. Expression of perforin and membrane-bound lymphotoxin (tumor necrosis factor-beta) in virus-specific CD4+ human cytotoxic T-cell clones. Blood. 1993;81:1527–1534. [PubMed] [Google Scholar]
- 55.Yonehara S, Nishimura Y, Kishil S, Yonehara M, Takazawa K, Tamatani T, Ishii A. Involvement of apoptosis antigen Fas in clonal deletion of human thymocytes. Int Immunol. 1994;6:1849–1856. doi: 10.1093/intimm/6.12.1849. [DOI] [PubMed] [Google Scholar]
- 56.Zajac A J, Blattman J N, Murali-Krishna K, Sourdive D J, Suresh M, Altman J D, Ahmed R. Viral immune evasion due to persistence of activated T cells without effector function. J Exp Med. 1998;188:2205–2213. doi: 10.1084/jem.188.12.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]