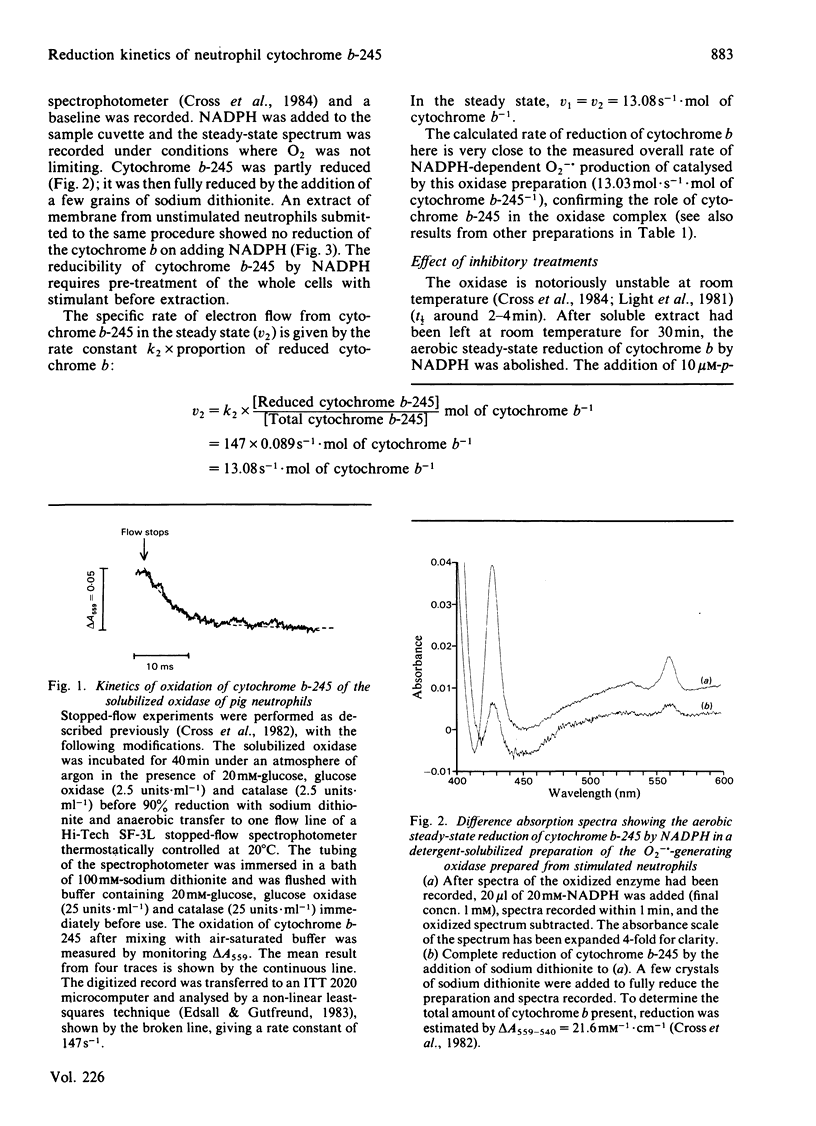
Abstract
A soluble oxidase from phorbol-stimulated pig neutrophils contained FAD and cytochrome b-245. A typical preparation produced 13.03 mol of superoxide (O2-.) X S-1 X mol of cytochrome b-1 (348 nmol X min-1 X mg of protein-1). In the aerobic steady state, cytochrome b was 8.9% reduced. Steady-state cytochrome b reduction was absent from extracts of unstimulated cells; Km values for NADPH, for O2-. production and cytochrome b reduction were similar. The calculated aerobic rate of cytochrome b reduction was equal to the measured rate of O2-. production in a variety of preparations and in the presence of a range of inhibitors. Under anaerobic conditions the rate was slow: O2 is apparently required for rapid electron flow into the oxidase complex. Cytochrome b is shown to be kinetically competent to act as part of the O2-.-generating complex.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andréasson L. -E., Malmström B. G., Strömberg C., Vänngård T. The reaction of ferrocytochrome c with cytochrome oxidase: A new look. FEBS Lett. 1972 Dec 15;28(3):297–301. doi: 10.1016/0014-5793(72)80735-x. [DOI] [PubMed] [Google Scholar]
- Antonini E., Brunori M., Colosimo A., Greenwood C., Wilson M. T. Oxygen "pulsed" cytochrome c oxidase: functional properties and catalytic relevance. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3128–3132. doi: 10.1073/pnas.74.8.3128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babior B. M., Curnutte J. T., McMurrich B. J. The particulate superoxide-forming system from human neutrophils. Properties of the system and further evidence supporting its participation in the respiratory burst. J Clin Invest. 1976 Oct;58(4):989–996. doi: 10.1172/JCI108553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babior B. M. Oxygen-dependent microbial killing by phagocytes (first of two parts). N Engl J Med. 1978 Mar 23;298(12):659–668. doi: 10.1056/NEJM197803232981205. [DOI] [PubMed] [Google Scholar]
- Cross A. R., Higson F. K., Jones O. T., Harper A. M., Segal A. W. The enzymic reduction and kinetics of oxidation of cytochrome b-245 of neutrophils. Biochem J. 1982 May 15;204(2):479–485. doi: 10.1042/bj2040479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross A. R., Jones O. T., Harper A. M., Segal A. W. Oxidation-reduction properties of the cytochrome b found in the plasma-membrane fraction of human neutrophils. A possible oxidase in the respiratory burst. Biochem J. 1981 Feb 15;194(2):599–606. doi: 10.1042/bj1940599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross A. R., Parkinson J. F., Jones O. T. The superoxide-generating oxidase of leucocytes. NADPH-dependent reduction of flavin and cytochrome b in solubilized preparations. Biochem J. 1984 Oct 15;223(2):337–344. doi: 10.1042/bj2230337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewald B., Baggiolini M., Curnutte J. T., Babior B. M. Subcellular localization of the superoxide-forming enzyme in human neutrophils. J Clin Invest. 1979 Jan;63(1):21–29. doi: 10.1172/JCI109273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieppe P. A., Doherty M. The role of particles in the pathogenesis of joint disease. Curr Top Pathol. 1982;71:199–233. doi: 10.1007/978-3-642-68382-4_7. [DOI] [PubMed] [Google Scholar]
- Gabig T. G., Schervish E. W., Santinga J. T. Functional relationship of the cytochrome b to the superoxide-generating oxidase of human neutrophils. J Biol Chem. 1982 Apr 25;257(8):4114–4119. [PubMed] [Google Scholar]
- Light D. R., Walsh C., O'Callaghan A. M., Goetzl E. J., Tauber A. I. Characteristics of the cofactor requirements for the superoxide-generating NADPH oxidase of human polymorphonuclear leukocytes. Biochemistry. 1981 Mar 17;20(6):1468–1476. doi: 10.1021/bi00509a010. [DOI] [PubMed] [Google Scholar]
- SBARRA A. J., KARNOVSKY M. L. The biochemical basis of phagocytosis. I. Metabolic changes during the ingestion of particles by polymorphonuclear leukocytes. J Biol Chem. 1959 Jun;234(6):1355–1362. [PubMed] [Google Scholar]
- Segal A. W., Cross A. R., Garcia R. C., Borregaard N., Valerius N. H., Soothill J. F., Jones O. T. Absence of cytochrome b-245 in chronic granulomatous disease. A multicenter European evaluation of its incidence and relevance. N Engl J Med. 1983 Feb 3;308(5):245–251. doi: 10.1056/NEJM198302033080503. [DOI] [PubMed] [Google Scholar]
- Wakeyama H., Takeshige K., Takayanagi R., Minakami S. Superoxide-forming NADPH oxidase preparation of pig polymorphonuclear leucocyte. Biochem J. 1982 Sep 1;205(3):593–601. doi: 10.1042/bj2050593. [DOI] [PMC free article] [PubMed] [Google Scholar]


