Abstract
The ability to generate antibodies that cross-neutralize diverse primary isolates is an important goal for human immunodeficiency virus type 1 (HIV-1) vaccine development. Most of the candidate HIV-1 vaccines tested in humans and nonhuman primates have failed in this regard. Past efforts have focused almost entirely on the envelope glycoproteins of a small number of T-cell line-adapted strains of the virus as immunogens. Here we assessed the immunogenicity of noninfectious virus-like particles (VLP) consisting of Gag, Pro (protease), and Env from R5 primary isolate HIV-1Bx08. Immunogens were delivered to rhesus macaques in the form of either purified VLP, recombinant DNA and canarypox (ALVAC) vectors engineered to express VLP, or a combination of these products. Seroconversion to Gag and Pro was detected in all of the immunized animals. Antibodies that could neutralize HIV-1Bx08 were detected in animals that received (i) coinoculations with DNABx08 and VLPBx08, (ii) DNABx08 followed by ALVACBx08 boosting, and (iii) VLPBx08 alone. The neutralizing antibodies were highly strain specific despite the fact that they did not appear to be directed to linear epitopes in the V3 loop. Virus-specific cellular immune responses also were generated, as judged by the presence of Gag-specific gamma interferon (IFN-γ)-producing cells. These cellular immune responses required the inclusion of DNABx08 in the immunization modality, since few or no IFN-γ-producing cells were detected in animals that received either VLPBx08 or ALVACBx08 alone. The results demonstrate the feasibility of generating neutralizing antibodies and cellular immune responses that target an R5 primary HIV-1 isolate by vaccination in primates.
Accumulating evidence supports a positive role for neutralizing antibodies and CD8+ cytotoxic T lymphocytes (CTL) in the antiviral immune response to human immunodeficiency virus type 1 (HIV-1) and simian immunodeficiency virus (SIV) (33, 44). The ability to generate antibodies that neutralize a broad spectrum of primary HIV-1 isolates has proven to be one of the more difficult challenges for vaccine development (44). Major targets for neutralizing antibodies are the surface gp120 and transmembrane gp41 envelope glycoproteins of the virus (12). Antibodies that bind these viral glycoproteins with adequate affinity and appropriate specificity can prevent HIV-1 from entering host cells (32, 41, 45, 71, 78, 83, 84) and have provided potent protection against infection in passive-antibody experiments in animal models (2, 20, 22, 35, 38, 76). Attempts at inducing cross-reactive neutralizing antibodies through vaccination have, however, had limited success. Most attempts have focused on the envelope glycoproteins of a small number of T-cell line-adapted (TCLA) strains of virus. Although the envelope glycoproteins of those strains generate high titers of neutralizing antibodies, those antibodies mostly target strain-specific epitopes in the third variable cysteine-cysteine loop (V3 loop) of gp120 (10, 45) and have been highly specific for TCLA strains of virus (23, 50). Importantly, the antibodies have failed to neutralize primary isolates (4, 5, 10, 37) and often possess little or no detectable neutralizing activity against heterologous TCLA strains (5, 10).
The B-cell response leading to HIV-1-specific neutralizing antibody production could be fundamentally different for primary isolates compared to TCLA strains. For example, whereas TCLA strains are highly sensitive to neutralization by V3 loop-specific antibodies (26, 68), the V3 loop on primary isolates is occluded by N-linked glycans and tertiary folds on the native gp120 molecule (30, 62, 73–75, 90, 91), making it a poor target for neutralizing antibodies (9, 77, 86). The envelope glycoproteins of TCLA strains and many, but not all, primary isolates also exhibit different coreceptor preferences for virus entry. Generally speaking, primary isolates utilize either CCR5 (R5), CXCR4 (X4), or both coreceptors (R5/X4) whereas all TCLA strains have the X4 phenotype (6, 7, 67). For reasons that are poorly understood, most transmitted strains of HIV-1 have an R5 phenotype (63, 87) and, although no association has been found between coreceptor preference and neutralization sensitivity (31, 43, 81), it seems prudent to target R5 strains when designing candidate HIV-1 vaccines.
Much of what is known about the neutralization epitopes on primary isolates comes from studies of three human monoclonal antibodies (MAbs), immunoglobulin G1b12 (IgG1b12), 2G12, and 2F5. Each of these MAbs can neutralize diverse primary isolates (12) and, when combined, exhibit synergistic neutralizing activity (36). A vaccine that generates antibodies equal to the combination of these specificities is highly desirable and deserving of intense investigation. Unfortunately, the corresponding epitopes have proven to be poorly immunogenic in natural infections and in experimentally immunized animals. Other neutralization epitopes are present on primary isolates that are less conserved. The latter epitopes account for the sporadic, low-level neutralization of heterologous isolates by sera from infected individuals (46, 58, 89). They might also account for the potent neutralization of primary isolates by autologous serum samples that are obtained months or years after the time of virus isolation (29, 48, 56, 58, 89), which would require the cognate epitopes to be immunogenic and well exposed on the virus surface. The presence of neutralization epitopes that are both immunogenic and antigenic on primary isolates affords opportunities for vaccine development that have received little attention.
Part of the reason why the latter epitopes have received little attention for vaccine development relates to their high degree of variability, which may not be suitable for cross-reactive neutralizing antibody induction. Given the paucity of information on the immunogenicity of primary isolate Env, however, it would be premature to conclude that no monovalent Env will generate cross-reactive neutralizing antibodies. Also, should monovalent primary isolate Env be capable of generating antibodies that neutralize the autologous primary isolate, it might ultimately make it feasible to formulate a composite (polyvalent) Env vaccine that will target a broad spectrum of primary isolates.
Few studies have examined the immunogenicity of primary-isolate envelope glycoproteins. VanCott et al. (85) showed that immunization with the gp120 protein from the R5 primary isolate HIV-1CM235 generated antibodies in baboons that neutralized TCLA strains, including the TCLA version of the vaccine strain, but not the autologous primary isolate. Similar results were obtained by Beddows et al. (4), who immunized healthy, HIV-1-seronegative volunteers with the gp120 protein from R5/X4 strain HIV-1W61D. Only Berman et al. (8) have succeeded at generating antibodies that neutralized an R5 primary isolate; this was done by immunizing rabbits with the gp120 protein from HIV-1CM244.
In addition to neutralizing antibodies, increasing evidence supports a critical role for virus-specific CD8+ T cells in protective immunity against HIV-1 (11, 15, 55, 61, 64, 69, 70, 92). CD8+ T-cell responses to HIV-1 can be both cytotoxic (11, 15, 55, 61, 69, 92) and virus suppressive (80, 88). Recent reports of the effect of CD8+ cell depletion on viremia and CD4+ cell counts in SIV- or SIV-HIV-infected macaques (27, 40, 72) has given additional in vivo relevance to the importance of the CD8+ antiviral response in the control of this viral infection. Even more recently, vaccine-induced, virus-specific CTL strongly correlated with viremia control and the preservation of CD4+ lymphocytes in the highly pathogenic SIV-HIV 89.6P–macaque model (3).
The immunogenicity of Gag, Pro, and Env from R5 primary isolate HIV-1Bx08 was investigated here in rhesus monkeys. Immunogens were delivered in the form of either purified noninfectious virus-like particles (VLP), recombinant DNA and canarypox vectors engineered to express VLP, or a combination of delivery modes. Our results reveal the priming of Gag-specific cellular responses by DNA-based immunization. We also demonstrate that envelope glycoprotein from an R5 primary isolate can be used to generate antibodies in nonhuman primates that are capable of neutralizing the autologous primary isolate.
MATERIALS AND METHODS
Cells and viruses.
MT-2 is a CD4+ human lymphoblastoid cell line that is highly permissive to cytopathic infection with TCLA strains of HIV-1 (24). Stocks of HIV-1 strains IIIB, MN, and SF2 were produced in H9 cells as previously described (47). MT-2 and H9 cells were maintained at 37°C in 5% CO2 in growth medium consisting of RPMI 1640 medium supplemented with 12% heat-inactivated fetal bovine serum (FBS) and 50 μg of gentamicin/ml. Peripheral blood mononuclear cells (PBMC) were purified from buffy coats of healthy HIV-1-seronegative individuals and stimulated for 1 day with phytohemagglutinin P as described previously (46). Growth medium for PBMC consisted of RPMI 1640 medium supplemented with 20% heat-inactivated FBS, 4% interleukin-2, and 50 μg of gentamicin/ml. Monkey kidney Vero cells used for Bx08 VLP production were grown and passaged biweekly in Dulbecco's modified Eagle's medium (Flow Laboratories, McLean, Va.) supplemented with 10% heat-inactivated FBS, glutamine (2 mM), penicillin (50 IU/ml), and streptomycin (50 μg/ml). Stably transfected cells secreting VLP were maintained in the presence of 0.5 mg of Geneticin (Gibco BRL, Grand Island, N.Y.) per ml.
Clade B primary isolate Bx08 was isolated from an HIV-1-infected French individual 8 months postseroconversion (48) and was kindly provided by H. J. A. Fleury (Bordeaux, France). The other clade B primary isolates used in this study were Bal, JR-FL, P15, and P27. Bal and JR-FL were obtained from the National Institutes of Health AIDS Research and Reference Reagent Program (Bal was contributed by Suzanne Gartner, Mikulas Popovic, and Robert Gallo; JR-FL was contributed by Irvin Chen). Bal was isolated from explanted human infant lung tissue (21), JR-FL was isolated from frontal lobe brain tissue of a patient with AIDS dementia (53), and P15 and P27 were isolated during early seroconversion by PBMC coculture (58). Bal, JR-FL, P15, and P27 all use the CCR5 coreceptor (10, 18). Stocks of primary isolates were produced in PBMC as previously described (46). All virus stocks were made cell free by 0.45-μm (pore size) filtration and stored in aliquots at −80°C until use. Coreceptor usage of Bx08 was assessed in MT-2, U87-CD4-CCR5, and U87-CD4-CXCR4 cells (25) by using p24 production as a measure of infection.
sCD4 and MAbs.
Recombinant soluble human CD4 (sCD4) containing the full-length extracellular domain of human CD4 was obtained from Progenics Pharmaceuticals (Tarrytown, N.Y.). Human MAbs IgG1b12, 2G12, and 2F5 have been shown to neutralize diverse primary isolates (14, 19, 28, 59, 82). IgG1b12 recognizes an epitope in the CD4-binding domain of gp120 that is sensitive to mutations in V2 and C3 (42). 2G12 recognizes an epitope comprising residues within C2-V4 of gp120 that involved sites of N glycosylation (82). 2F5 recognizes a linear epitope in the ectodomain of gp41 having the amino acid sequence ELDKWA (51).
DNA vectors.
The nucleotide and amino acid numbering used throughout for HIV was that of Myers et al. (52). All pseudovirion-expressing vectors were constructed from p83-19 (57, 65), which was, in turn, derived from plasmid pMTHIVd25 (66). Plasmid p83-19 was engineered to incorporate several mutations to enhance safety, such as deletion of a 25-bp DNA fragment (nucleotides 753 to 777; LAI sequence) containing viral RNA packaging sequences (1, 34) and deletion of a 1.9-kbp DNA fragment containing most of the pol gene, effectively eliminating the coding sequences for reverse transcriptase and integrase. However, the deletion left intact the gene encoding the viral protease (pro), resulting in the expression of particles with processed Gag antigens. In p83-19, transcription of the HIV-1 coding sequences is regulated by the inducible human metallothionein IIa (MT) promoter and a simian virus 40 polyadenylation sequence. In order to produce pseudovirions with the Env glycoprotein from the Bx08 primary isolate, the HIV-1LAI env gene was replaced with that of HIV-1Bx08. To this end, a 2,440-bp fragment containing the entire gp160-encoding gene of HIV-1Bx08 was amplified by PCR from cells infected with this isolate. The PCR product was then used to replace the corresponding region in p83-19, giving rise to plasmid p133B1.
In order to produce a plasmid DNA vector for immunization studies, a construct was prepared incorporating the env gene from HIV-1Bx08 under the control of the cytomegalovirus promoter. The construct, pCMV3Bx08, is derived from plasmid pCMVgDtat−vpr−Bx08, which was, in turn, engineered from p83-19 after several modifications. These included (i) replacement of the MT promoter with a 1.6-kbp DNA fragment containing the human cytomegalovirus immediate-early gene promoter, enhancer, and intron A sequences; (ii) deletion of the coding sequences for HIV-1 Tat and Vpr; (iii) replacement of the HIV-1 gp120Bx08 signal peptide sequence with the human herpes simplex virus glycoprotein D signal peptide sequence; (iv) replacement of the Ampr-encoding gene with the Kanr-encoding gene; and (v) replacement of the simian virus 40 polyadenylation sequence with that of the bovine growth hormone-encoding gene. The nucleotide sequences of all constructs were confirmed by DNA sequencing.
Production of noninfectious VLP for immunogenicity studies.
Vero cells were transfected at passage 141 with plasmid p133B1 by the calcium phosphate method as specified previously (57), and stable transfectants expressing noninfectious HIV-1-like particles were established. VLP were isolated from the supernatants of a stable clone (DD19) after induction of the Vero cells with heavy metals (57). Expression of Gag and Env was verified by Western blot assay using, respectively, an anti-HIV-1 p24 mouse MAb (NEA-9306; Dupont Canada, Inc., Markham, Ontario, Canada) and MAb K3A, which recognizes gp120 envelope proteins from different clades (kindly supplied by Ronald Kennedy). Fully assembled, Env-containing particles were isolated from the supernatants of the stably engineered Vero DD19 cell clone by ultracentrifugation through a glycerol cushion and purified by sucrose gradient fractionation (66). The p24 contents of the various particle species were determined by a p24-specific enzyme immunoassay (Coulter Immunology, Hialeah, Fla.). The cell line secreted approximately 800 to 950 μg of p24 per liter.
Construction of recombinant canary poxvirus vCP1579.
Recombinant vCP1579 was engineered to express HIV-1LAI Gag and Pro, together with HIV-1Bx08 gp120 fused to a 28-amino-acid fragment of the transmembrane domain of HIV-1LAI gp41, and a synthetic polypeptide containing Pol and Nef CTL epitopes. This recombinant is similar to vCP1452 (10, 79), except that the gene fragment encoding gp120LAI was replaced with its cognate sequences from HIV-1Bx08. Due to the absence of the gp41 ectodomain, the expressed envelope glycoprotein would be expected to consist of membrane-anchored gp120. The construct was generated in several steps. Briefly, the vector-modifying sequences from the pMPC6H6K3E3 insertion vector, encoding E3L and K3L, were inserted into the C6 locus of recombinant vCP1566. Recombinant vCP1566 was, in turn, generated by insertion of an expression cassette encoding a synthetic polypeptide containing Pol and Nef CTL epitopes into the C5 locus of vCP1453. Recombinant vCP1453 was obtained after coinsertion of the genes encoding the HIV-1Bx08 Env and HIV-1LAI Gag-Pro products into the ALVAC genome at the C3 locus.
Macaque immunizations.
Rhesus macaques were divided into six groups with four animals in each group, as summarized in Table 1. Briefly, animals in groups 1 and 2 were inoculated with either a high dose (3 mg) or a low dose (600 μg) of DNABx08, respectively, together with 50 μg of VLPBx08 in QS21 (100 μg) at weeks 0, 4, 24, and 44. Animals in groups 3 and 4 were inoculated with either the high dose or the low dose of DNABx08, respectively, at weeks 0 and 4 and were boosted with ALVACBx08 at weeks 24 and 44 (4 × 108 PFU at week 24 and 4 × 108 PFU at week 44). Animals in group 5 were inoculated with 50 μg of VLPBx08 in QS21 adjuvant at weeks 0, 4, 24, and 44. Animals in group 6 received 3 mg of control DNA at weeks 0 and 4 and were boosted with ALVACBx08 at weeks 24 and 44 (same doses as above). All immunogens were administered in a volume of 2 ml intramuscularly. The QS21 adjuvant was prepared and filter sterilized before filling in of single-dose vials and stored at −20°C.
TABLE 1.
Vaccination groups and schedules
| Macaque group no. | Inoculation(s) at wk 0 and wk 4 | Inoculation(s) at wk 24 and wk 44 |
|---|---|---|
| 1 | 3 mg of DNABx08, 50 μg of VLPBx08, 100 μg of QS21 | Same as wk 0 and wk 4 |
| 2 | 600 μg of DNABx08, 50 μg of VLPBx08, 100 μg of QS21 | Same as wk 0 and wk 4 |
| 3 | 3 mg of DNABx08 | ALVACBx08 |
| 4 | 600 μg of DNABx08, | ALVACBx08 |
| 5 | 50 μg of VLPBx08, 100 μg of QS21 | Same as wk 0 and wk 4 |
| 6 | Control DNA | ALVACBx08 |
Western blot.
Antibodies specific for HIV-1 antigens were assessed with a commercial HIV-1 Western blot kit (Genetic Systems, Sanofi Diagnostics Pasteur, Inc.) that is based on the LAI strain of HIV-1.
Enzyme-linked immunosorbent assay.
Bx08-V3 peptide-specific binding antibodies were assessed in Nunc (Roskilde, Denmark) Immunoplates (MaxiSorb F96) using alkaline phosphatase-conjugated goat anti-monkey IgG as described previously (16). Plasma samples were assayed in duplicate at a 1:50 dilution, and values are given as the average A405. The Bx08-V3 peptide had the amino acid sequence TRPNNNTRKSIHIGPGRAFYTTGDIIGDIR and was made by SynPep Corporation (Dublin, Calif.). The peptide was judged to be 91.5% pure by high-pressure liquid chromatography analysis. This same peptide was used in neutralization competition assays as described below.
Neutralizing antibody assays.
Neutralization of HIV-1Bx08 and other primary isolates was assessed in human PBMC by using a reduction in p24 Gag antigen synthesis as described previously (46, 58). Briefly, 500 50% tissue culture-infective dose of virus were incubated with various dilutions of serum samples for 1 h at 37°C before the addition of PBMC. Cells were washed three times with 200 μl of growth medium and resuspended in 200 μl of fresh interleukin-2 growth medium 1 day later. Culture supernatants were assessed for p24 content at a time when p24 synthesis in virus control wells (no test sample) was in a linear phase of increase, which is when optimum sensitivity is achieved in this assay (93). Neutralization titers were defined as either the dilution of serum or concentration of sCD4 and MAbs at which p24 synthesis was reduced by 80% relative to that of a negative control. These titers refer to conditions under which serum samples were incubated with virus before the addition of cells. All serum samples were heat inactivated for 1 h at 56°C prior to assay. V3 peptide neutralization competition assays were performed by preincubating serum samples with either phosphate-buffered saline (PBS) or Bx08-V3 peptide (50 μg/ml [final concentration]) for 1 h at 37°C. The serum samples were then assayed at a 1:3 dilution on the virus as described above.
Neutralization of HIV-1 strains IIIB, MN, and SF2 was measured in an MT-2 cell-killing assay by using neutral red to quantify the fraction of cells that survived virus-induced cytopathic effects (47). Briefly, 500 50% tissue culture-infective doses of virus were incubated with multiple dilutions of serum samples for 1 h at 37°C before the addition of cells. The incubation was continued until extensive syncytium formation had just occurred in wells that contained no serum sample (usually 4 to 6 days). Neutralizing antibody titers are defined as the serum dilution (before the addition of cells) at which 50% of the cells were protected from virus-induced killing. A 50% reduction in cell-killing corresponds to an approximately 90% reduction in viral Gag antigen synthesis in this assay (10, 54). Each set of assays included a positive control serum that had been assayed multiple times and had a known average titer.
ELISpot assay for IFN-γ release from antigen-specific PBMC.
PBMC were separated from whole blood by Lymphocyte Separation Medium (Cellgro), washed twice, counted, and viably frozen in freezing medium for time course studies. Cells were thawed, washed twice, resuspended at 4 × 106/ml, and stimulated for 48 h with 2 μg of HIV-1HXB2 Gag peptide pools (National Institutes of Health AIDS Research and Reference Reagent Program) per ml. The peptides were 20-mers overlapping by 10 amino acids and were divided into two pools of peptides with 25 peptides per pool. Pool 1 represented amino acids 1 to 260, while pool 2 represented amino acids 251 to 500. Ovalbumin peptide was used as a negative control, and 50 ng of phorbol myristate acetate (Sigma) per ml plus 1 μg of ionomycin (Sigma) per ml served as a positive control. Ninety-six-well mixed cellulose ester filtration plates (Millipore) were coated with 50 μl of a 5-μg/ml concentration of mouse anti-human IFN-γ MAb 1-D1K (Biosource International) overnight at 4°C. Plates were then washed four times with PBS and blocked with RPMI (Cellgro) medium supplemented with l-glutamine, penicillin, and 10% heat-inactivated FBS (HyClone) for 1 h at 37°C. Prestimulated cells were then added to the coated plate in 100 μl of R10. Duplicate wells containing either 5 × 105 or 2.5 × 105 stimulated PBMC were plated for each sample and incubated for 16 to 20 h at 37°C. Plates were washed four times with PBS (Cellgro) and then four times with PBS containing 0.05% Tween 20 (Sigma) to remove all cells and debris from the plates. Next, 100 μl of 1-μg/ml biotinylated anti-IFN-γ MAb 1-D1K (Mabtech) diluted in PBS was added. After 2 h, the plates were washed four times with PBS containing 0.1% Tween 20 and incubated with 100 μl of Vectastain ABC (Vector Laboratories) per well for 1 h. Following four washes with PBS plus 0.1% Tween 20, Stable DAB (Research Genetics) was added to the wells and monitored for color development (approximately 5 min). The Stable DAB reaction was stopped by washing the wells three times with H2O. Wells were counted visually under a dissecting microscope (Olympus) after the plates had dried.
RESULTS
Biologic and immunologic properties of HIV-1Bx08.
Coreceptor usage was determined by infection of cells that coexpressed CD4 and either CCR5 or CXCR4. HIV-1Bx08 was able to infect U87-CD4-CCR5 cells but not U87-CD4-CXCR4 cells or MT-2 cells (data not shown). This outcome is consistent with an R5 phenotype for this virus. As a control for the integrity of the cells used for X4 phenotype classification, HIV-1IIIB was able to infect U87-CD4-CXCR4 and MT-2 cells but not U87-CD4-CCR5 cells.
The neutralization sensitivity of HIV-1Bx08 was assessed with sCD4 and human MAbs in the PBMC assay. The virus was sensitive to all four reagents. The minimum concentration of sCD4 required to inhibit HIV-1Bx08 was 9.9 μg/ml. This concentration of sCD4 is approximately 500 to 5,000 times higher than that required to inhibit TCLA strains (10, 17, 49) but was moderately low compared to other primary isolates, most of which resist inhibition by this same preparation of sCD4 at concentrations of up to 50 μg/ml (10). The virus was also neutralized by the MAbs, where the 80% inhibition doses were 11.5 (IgG1b12), 1.2 (2G12), and 27.9 (2F5) μg/ml.
HIV-1 seroconversion.
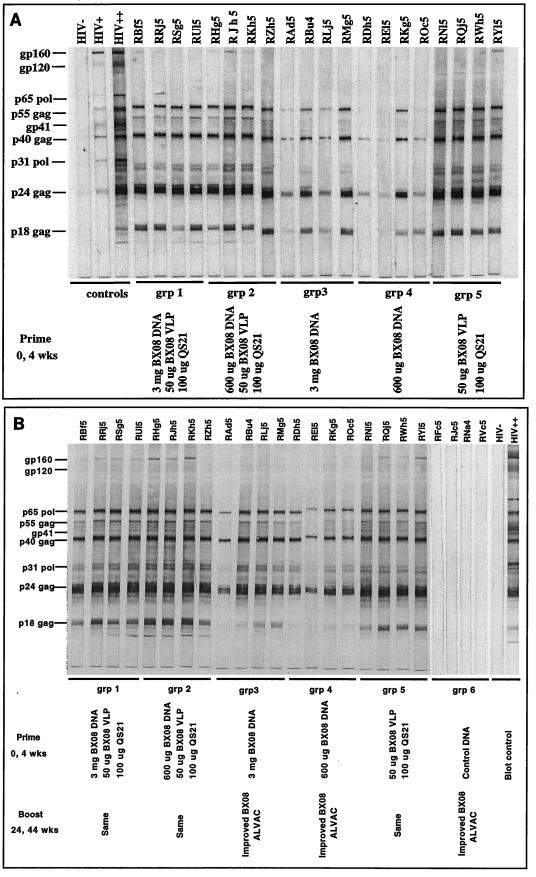
HIV-1-specific IgG was assessed by Western blot assay at multiple time points throughout the immunization schedule. Figure 1A shows that all of the immunized animals possessed Gag-specific antibodies by week 6 (2 weeks after the second inoculation). Gag-specific seroconversion was strongest in animals that received VLPBx08 either alone (group 5) or in combination with the high and low doses of DNABx08 (groups 1 and 2, respectively). Gag-specific seroconversion was variable and less potent at this time in animals that received the high and low doses of DNABx08 (groups 3 and 4, respectively). A similar pattern was noted for Pro-specific antibodies (p31), with the exception of very low levels of anti-Pro antibodies in animals inoculated with the low dose of DNABx08 (group 4). Little or no Env-specific antibody was detected at this time.
FIG. 1.
Western blot analysis of HIV-1 antigen-specific antibodies. Serum samples were assayed at a 1:100 dilution. Panels: A, sera obtained at week 6; B, sera obtained at week 46. wks, weeks; HIV−, HIV negative; HIV+, weakly HIV positive; HIV++, strongly HIV positive.
Western blot reactivities had intensified by 2 weeks following the final immunization (week 46) where, in addition to anti-Gag antibodies, all immunized animals showed evidence of Pro-specific seroconversion (Fig. 1B). We noted that the anti-Gag and anti-Pro reactivity was relatively weak in animals RAd5 (group 3) and REl5 (group 4). Env-specific antibodies (for gp160 and gp41 but not for gp120) were detected at this time in animals in groups 1, 2, and 5 only. The lack of detection of gp120-specific antibodies in these animals could be due to genetic and antigenic dissimilarities in the gp120 of Bx08 compared to the IIIB gp120 antigen used in the Western blot assay strips. All of the animals immunized with control DNA and boosted with ALVACBx08 were seronegative for Env at week 46 (Fig. 1B). A boosting effect at weeks 26 and 46 (2 weeks after the third and fourth inoculations, respectively) was seen in each animal, where evidence of seroconversion remained present 12 weeks after the final inoculation (data not shown).
Neutralization of HIV-1Bx08 with sera from immunized macaques.
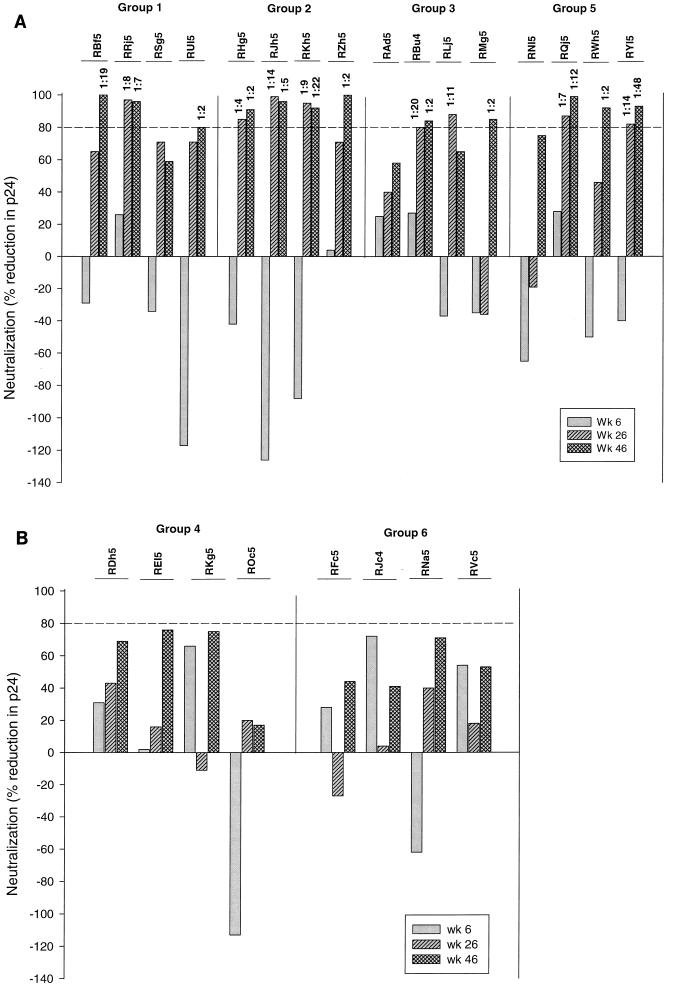
Neutralization of HIV-1Bx08 was assessed in human PBMC with serum collected preimmunization and after the second, third, and fourth (final) immunizations. These assays were performed without knowledge of the animal group assignments. Figure 2A shows that neutralizing antibodies were detected in three of the four animals in group 1 (high-dose DNABx08 plus VLPBx08), four of the four animals in group 2 (low-dose DNABx08 plus VLPBx08), three of the four animals in group 3 (high-dose DNABx08 plus ALVACBx08 boosting), and three of the four animals in group 5 (VLPBx08). These neutralization titers ranged from 1:2 to 1:48. The highest titer was achieved in an animal (RYl5) immunized four times with VLPBx08. Notably, a 1:2 dilution of sera from several animals in groups 1, 2, and 5 achieved >90% neutralization and serum from at least one animal in each of the latter groups achieved 99 to 100% neutralization. The potency of neutralization at a 1:2 serum dilution did not correlate with the 80% neutralization titer.
FIG. 2.
Neutralization of HIV-1Bx08 by sera from immunized animals. Bar height represents the percent reduction in p24 relative to the amount of p24 produced in the presence of the corresponding preimmunization serum from each animal as assayed at a 1:2 dilution. Values above the bars are the highest serum dilutions at which p24 production was reduced by 80%. Panels: A, animal groups with detectable neutralizing antibodies; B, animal groups with no detectable neutralizing antibodies.
As shown in Fig. 2B, no HIV-1Bx08-specific neutralization was detected with sera from animals immunized with the low dose of DNABx08 plus ALVACBx08 (group 4) or control DNA plus ALVACBx08 (group 6). Sera from these animals reduced p24 synthesis by −113% to +76% at the lowest dilution tested (1:2), which is within the normal variation range of this assay. Although an 80% reduction in p24 Gag antigen synthesis (fivefold decrease in infectivity) has proven to be the most reliable minimum cutoff for a true positive (10), two animals in the group that received the low dose of DNABx08 plus ALVACBx08 (REl5 and RHg5) had neutralization values of 76 and 75%, respectively, after final boosting.
Absence of cross-reactive neutralizing antibodies.
Cross-neutralizing activity was assessed with three TCLA strains (IIIB, MN, and SF2) and four heterologous R5, clade B primary isolates. In only two cases were neutralizing antibodies detected with a TCLA strain as measured in the MT-2 cell assay (data not shown). These cases occurred 2 weeks after final boosting and included sera from animal RLj5 in group 3 and animal REl5 in group 4, both having weak neutralizing activity against HIV-1MN (titers of 1:29 and 1:50, respectively). Nine serum samples that neutralized HIV-1Bx08 were further tested for the ability to neutralize heterologous R5 primary isolates in the PBMC assay. The results of the latter assays were all negative (Table 2).
TABLE 2.
Neutralization of heterologous R5 primary HIV-1 isolates
| Animala | Titer of NAb to HIV-1Bx08b | % Reduction in p24c with HIV-1 isolate:
|
|||
|---|---|---|---|---|---|
| Bal | JR-FL | P15 | P27 | ||
| RY15 | 1:48 | 47 | 72 | 47 | 45 |
| RKh5 | 1:22 | 64 | 39 | 3 | 50 |
| RBf5 | 1:19 | 52 | 47 | 28 | 16 |
| RQj5 | 1:12 | 66 | 44 | 30 | 61 |
| RJh5 | 1:5 | 70 | 47 | 15 | 53 |
| RZh5 | 1:2 | 69 | 44 | 37 | 0 |
| RWh5 | 1:2 | 38 | 28 | 7 | 37 |
| RHg5 | 1:2 | 67 | 36 | 17 | 24 |
| RBu4 | 1:2 | 3 | 3 | 7 | 3 |
Serum samples were obtained at week 46.
Titers are reproduced from Fig. 2A and represent the serum dilutions at which p24 production was reduced by 80% relative to that obtained with preimmunization serum. NAb, neutralizing antibody.
Serum samples were assayed at a 1:4 dilution with virus in the PBMC blast assay. Percent reduction in p24 was calculated relative to the amount of p24 produced in the presence of pooled preimmunization serum samples from animals RBf5, RSg5, RHg5, RRj5, and RU15. Amounts of p24 produced in the presence of pooled negative serum sample: Bal, 64 ng/ml; JR-FL, 36 ng/ml; P15, 60 ng/ml; P27, 38 ng/ml.
Antibody specificity for the V3 loop.
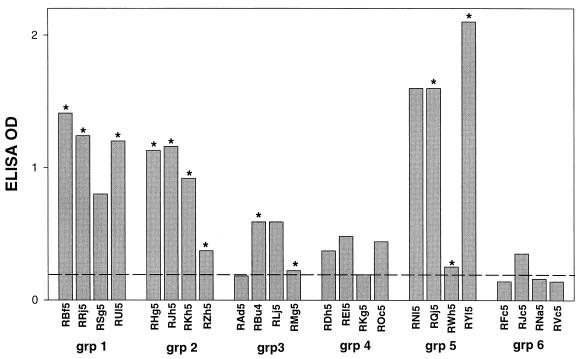
Serum samples obtained 2 weeks after final boosting (week 46) were tested for the presence antibodies that could bind a Bx08-V3 peptide. Sera from animals in groups 1, 2, and 5 and, to a lesser extent, groups 3 and 4 tested positive by enzyme-linked immunosorbent assay (Fig. 3). The detection of V3-specific antibodies did not always predict the ability to neutralize HIV-1Bx08. For example, at least three serum samples that neutralized HIV-1Bx08 had no detectable V3-specific antibodies (RZh5, RMg5, and RWh5). In addition, two samples that were positive for V3-specific antibodies (RSg5 and RNl5) failed to neutralize HIV-1Bx08. The frequent detection of antibodies to the V3 loop of Bx08 is strong evidence of the presence of gp120-specific antibodies that were not detected by the Western blot assay (Fig. 1).
FIG. 3.
Bx08-V3 peptide-binding antibodies. Serum samples obtained 2 weeks after the fourth immunization (week 46) were tested at a 1:50 dilution for antibodies reactive with a peptide corresponding to the V3 loop of HIV-1Bx08. Bar height represents the average A450 of duplicate tests. All duplicate values agreed within 5% of the average. The value obtained with serum from an HIV-1-naive macaque is shown by a dashed line. Values greater than twice that of the negative control were considered to be positive. Serum samples that neutralized HIV-1Bx08 are indicated by asterisks above the bars. OD, optical density; grp, group.
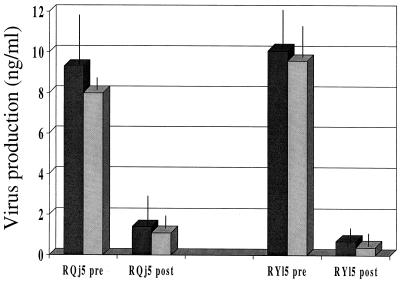
Two serum samples that contained antibodies reactive with the Bx08 V3 peptide and neutralized HIV-1Bx08 were tested for neutralizing activity in the presence and absence of Bx08 V3 peptide. Similar experiments have shown this to be a very effective means by which to determine whether a portion of the neutralizing antibodies are V3 specific (10, 16). The results of these assays showed that the Bx08 V3 peptide (50 μg/ml) was not able to outcompete the neutralizing activity of these serum samples (Fig. 4).
FIG. 4.
Inability to detect neutralizing antibodies specific for the HIV-1Bx08 V3 loop. Two serum samples that contained antibodies reactive with a Bx08 V3 peptide and that neutralized HIV-1Bx08 were tested for neutralizing activity in the presence and absence of the Bx08 V3 peptide. Samples were preincubated with either peptide (50 μg/ml) or an equal volume of sterile PBS for 1 h at 37°C and assayed at a 1:3 dilution. Both postimmunization serum samples were obtained at week 46 (2 weeks after final boosting). Dark-shaded bars, no V3 peptide; light-shaded bars, with V3 peptide; pre, preimmunization serum; post, postimmunization serum. Each error bar represents the standard deviation of the average of triplicate values.
Analysis of IFN-γ secretion.
PBMC from the vaccinated and control animals were tested for the ability to secrete IFN-γ upon stimulation with two pools of overlapping peptides representing the Gag sequence of HIV-1HXB2. Peptide pool 1 spanned amino acids 1 to 260, while peptide pool 2 spanned amino acids 251 to 500. The number of IFN-γ spots per 106 PBMC for each animal at each time point is shown in Fig. 5, and the average value for each group is shown in Fig. 6. We measured Gag-specific responses 2 weeks after the second immunization, a time when near-peak cellular responses would be expected, and then 3 months later (16 weeks), when memory responses from the first two immunizations would be in effect. The PBMC were then screened 2 weeks after each of the next two immunizations (weeks 26 and 46) to determine boosting effects and finally at week 56 (3 months following the final immunization) for immunologic memory for the HIV-1 antigen. The presence of ≥20 ELISpots in an assay was considered a positive test.
FIG. 5.
Number of HIV-1 Gag-specific IFN-γ-producing cells detected by ELISpot assay with PBMC from individual animals. Solid bars represent cells stimulated with peptide pool 1, covering the amino-terminal half of Gag. Hatched bars represent cells stimulated with peptide pool 2, covering the carboxyl-terminal half of Gag. Values on the y axis are numbers of IFN-γ-producing cells per million cells. ND, not done.
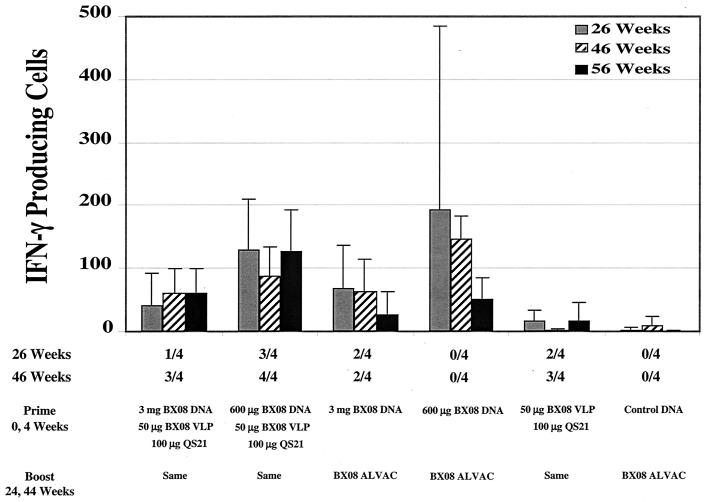
FIG. 6.
Average number of IFN-γ-producing cells in each group of animals. The values below the x axis are the fractions of animals that were positive for Bx08-specific neutralizing antibodies at weeks 26 and 46.
All groups of animals primed with DNABx08 showed clear positive Gag-specific ELISpot responses following the third immunization, while the groups of animals receiving only VLPBx08 or ALVACBx08 failed to score positive. Following the first two inoculations with DNABx08, the groups receiving the low dose of DNABx08 had more animals with >20 ELISpots (five of eight animals) than did the groups receiving the high dose of DNABx08 (none of eight animals). Following the first boost with either VLPBx08 or ALVACBx08, all DNABx08-primed groups contained animals that were positive, with 12 of 16 animals scoring >20 ELISpots. Animals that scored positive typically did so with both peptide pools (28 of 42 positive samples). The second boost did not markedly increase the magnitude or frequency of the ELISpot responses. At the end of the experiment, the frequency of Gag-specific ELISpot-positive animals was only slightly lower (11 of 16 animals) than at 2 weeks after the first boost (12 of 16 animals). Boosting with ALVACBx08 was no more effective than boosting with DNABx08 combined with VLPBx08. By comparison, a typical response to corresponding SIV Gag peptide pools using cells from SIV-infected rhesus macaques is 50 to 150 ELISpots, with most animals responding to both peptide pools (data not shown).
DISCUSSION
Despite a growing desire to generate antibodies that are capable of neutralizing primary isolates, very few primary-isolate Envs have been investigated as potential immunogens for inclusion in an HIV-1 vaccine. Here we show that noninfectious VLP containing the surface envelope glycoprotein of an R5 primary isolate were capable of generating antibodies that neutralized the vaccine strain of virus. The antibodies could be generated by (i) coinoculation with DNABx08 and VLPBx08, (ii) priming with DNABx08 and boosting with ALVACBx08, and (iii) inoculation with VLPBx08 alone. Most notable was the fact that, in certain cases, neutralizing antibodies were induced by recombinant vectors without the need for protein boosting—a milestone that has not been reported previously with respect to primary-isolate neutralization by macaque serum.
Unlike the neutralizing antibodies generated by most of the candidate HIV-1 Env vaccines tested to date, the neutralizing antibodies generated here did not appear to be directed to linear epitopes in the V3 loop of gp120. Two lines of evidence support this conclusion. First, some serum samples with neutralizing activity had little or no antibody reactive with a Bx08 V3 loop peptide. Second, the neutralizing activity of two serum samples with strong reactivity to the Bx08 V3 loop peptide was not reduced in the presence of a high concentration (50 μg/ml) of the V3 loop peptide. These results suggest that the neutralizing antibodies targeted one or more epitopes outside the V3 loop. We note, however, that our experiments with linear peptides do not eliminate the possibility that the epitope(s) consisted of a conformational structure within V3 that was not represented by the linear peptide.
A disadvantage of the neutralizing antibodies seen here was their minimal cross-reactivity. Specifically, the antibodies had little or no neutralizing activity against TCLA strains and heterologous R5 primary isolates. The neutralizing antibody response generated by these candidate vaccines needs to be improved to target a broad spectrum of HIV-1 antigenic variants. We also noted that the Bx08-specific neutralizing antibodies were low in titer (1:2 to 1:48); however, the small magnitude of this response may be less of a concern than the limited strain specificity. For example, results of recent passive-immunization experiments with nonhuman primates indicate that preexisting neutralization titers of 1:10 or less may provide complete or partial protection against intravenous and mucosal challenge with simian-human immunodeficiency virus (35, 38, 76). Given that natural HIV-1 transmission appears to be virus dose dependent (60), where not all exposures result in infection (39), it is conceivable that a 5- or 10-fold reduction in virus infectivity by minimally diluted serum in vitro will correspond to an undiluted potency in vivo that is effective prophylactically. A number of recombinant vaccine vectors are also known to prime B cells for a rapid secondary neutralizing antibody response generated by HIV-1 Env protein boosting (23, 79), and at least one example exists of a dramatic secondary neutralizing antibody response after a virus challenge of macaques that had been primed with recombinant modified vaccinia virus strain Ankara-SIV vaccines (54). It may be expected that DNA vectors expressing VLP will prime for similar secondary neutralizing antibody production in response to HIV-1 infection.
We also detected HIV-1 Gag-specific cellular immune responses in the groups of animals that were inoculated with DNABx08. All groups of animals receiving this immunogen scored positive for IFN-γ-producing cells, and with the exception of a weak positive response to Gag pool 1 in one animal (RWh5, week 56), no IFN-γ-producing cells were detected in animals inoculated with either VLPBx08 or ALVACBx08 alone. Thus, as judged by the best overall immune responses (i.e., IFN-γ-producing cells and HIV-1Bx08 neutralizing antibody), coinoculation with a low dose of DNABx08 plus VLPBx08 was the superior immunization modality investigated (Fig. 6). The Gag peptides used for PBMC stimulation were not completely matched to the vaccine strain, making it possible that some strain-specific reactivities went undetected in our assay. Despite this, it is noteworthy that IFN-γ production could be stimulated in many cases with two overlapping peptide pools covering the amino-terminal and carboxyl-terminal halves of Gag as evidence that at least two specificities of the cellular response were induced by vaccination.
While we consider the combined humoral and cellular immune responses elicited by these vaccine candidates to be encouraging, we also readily acknowledge the limited benefit that can be expected from neutralizing antibodies that are so narrow in specificity. Nonetheless, our results point to several vaccine strategies that may be useful for delivering a more appropriate immunogen for cross-reactive neutralizing antibody induction once that immunogen has been identified. We also did not determine whether the structure of Bx08 Env incorporated into the VLP is optimal for neutralizing antibody induction. For example, it has been argued that the native oligomeric (trimeric) structure of gp120-gp41 heterodimers must be preserved for optimal immunogenicity (13). Although it is possible that the Bx08 VLP expressed by our DNA vector contained native oligomeric envelope glycoproteins, we have not confirmed this. Furthermore, the envelope glycoproteins expressed by ALVACBx08 consisted of membrane-anchored gp120 lacking the gp41 ectodomain, which may not represent the most suitable structure for optimal immunogenicity. Additional studies are required to determine the full potential of VLP as an immunogen for neutralizing antibody induction.
ACKNOWLEDGMENTS
We thank H. J. A. Fleury for HIV-1Bx08 and Dennis Burton, Herman Katinger, and John Mascola for MAbs. We also thank Ginger James, Lauren Rodrigues, Heike Marshall, and Lisa Murdin for excellent technical assistance.
This work was supported by funding from Aventis Pasteur and the National Institutes of Health (AI-85343).
REFERENCES
- 1.Aldovini A, Young R. Mutations of RNA and protein sequences involved in human immunodeficiency virus type 1 packaging result in production of noninfectious virus. J Virol. 1990;64:1920–1926. doi: 10.1128/jvi.64.5.1920-1926.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baba T W, Liska V, Hofmann-Lehmann R, Vlasak J, Xu W, Ayehunie S, Cavacini L A, Posner M R, Katinger H, Stiegler G, Bernacky B J, Rizvi T A, Schmidt R, Hill L R, Keeling M E, Lu Y, Wright J E, Chou T-C, Ruprecht R M. Human neutralizing monoclonal antibodies of the IgG1 subtype protect against mucosal simian-human immunodeficiency virus infection. Nat Med. 2000;6:200–206. doi: 10.1038/72309. [DOI] [PubMed] [Google Scholar]
- 3.Barouch D H, Santra S, Schmitz J E, Kuroda M J, Fu T-M, Wagner W, Bilska M, Craiu A, Zheng X X, Krivulka G R, Beaudry K, Lifton M A, Nickerson C E, Trigona W L, Punt K, Freed D C, Guan L, Dubey S, Casimiro D, Simon A, Davies M-E, Chastain M, Strom T B, Gelman R S, Montefiori D C, Lewis M G, Emini E A, Shiver J W, Letvin N L. Control of viremia and prevention of clinical AIDS in rhesus monkeys by cytokine-augmented DNA vaccination. Science. 2000;290:486–492. doi: 10.1126/science.290.5491.486. [DOI] [PubMed] [Google Scholar]
- 4.Beddows S, Lister S, Cheingsong R, Bruck C, Weber J. Comparison of the antibody repertoire generated in healthy volunteers following immunization with a monomeric recombinant gp120 construct derived from a CCR5/CXCR4-using human immunodeficiency virus type 1 isolate with sera from naturally infected individuals. J Virol. 1999;73:1740–1745. doi: 10.1128/jvi.73.2.1740-1745.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Belshe R B, Gorse G J, Mulligan M J, Evans T G, Keefer M C, Excler J-L, Duliege A-M, Tartaglia J, Cox W I, McNamara J, Hwang K-L, Bradney A, Montefiori D, Weinhold K J. Induction of immune responses to HIV-1 canarypox virus (ALVAC) HIV-1 and gp120 SF-2 recombinant vaccines in uninfected volunteers. AIDS. 1998;12:2407–2415. doi: 10.1097/00002030-199818000-00009. [DOI] [PubMed] [Google Scholar]
- 6.Berger E A. HIV entry and tropism: the chemokine receptor connection. AIDS. 1997;11(Suppl. A):S3–S16. [PubMed] [Google Scholar]
- 7.Berger E A, Doms R W, Fenyö E M, Korber B T M, Littman D R, Moore J P, Sattentau Q J, Schuitemaker H, Sodrsoki J, Weiss R A. HIV-1 phenotypes classified by co-receptor usage. Nature. 1998;391:240. doi: 10.1038/34571. [DOI] [PubMed] [Google Scholar]
- 8.Berman P W, Huang W, Riddle L, Gray A M, Wrin T, Vennari J, Johnson A, Klaussen M, Prashad H, Köhne C, deWit C, Gregory T J. Development of bivalent (B/E) vaccines able to neutralize CCR5-dependent viruses from the United States and Thailand. Virology. 1999;265:1–9. doi: 10.1006/viro.1999.0031. [DOI] [PubMed] [Google Scholar]
- 9.Bou-Habib D C, Roderiquez G, Oravecz T, Berman P W, Lusso P, Norcross M A. Cryptic nature of envelope V3 region epitopes protects primary monocytotropic human immunodeficiency virus type 1 from antibody neutralization. J Virol. 1994;68:6006–6013. doi: 10.1128/jvi.68.9.6006-6013.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bures R, Gaitan A, Zhu T, Graziosi C, McGrath K M, Tartaglia J, Caudrelier P, El Habib R, Klein M, Lazzarin A, Stablein D M, Deers M, Corey L, Greenberg M L, Schwartz D H, Montefiori D C. Immunization with recombinant canarypox vectors expressing membrane-anchored gp120 followed by gp160 protein boosting fails to generate antibodies that neutralize R5 primary isolates of human immunodeficiency virus type 1. AIDS Res Hum Retrovir. 2000;16:2019–2035. doi: 10.1089/088922200750054756. [DOI] [PubMed] [Google Scholar]
- 11.Burrow P, Lewicki H, Hahn B H, Shaw G M, Oldstone M B A. Virus-specific CD8+ cytotoxic T-lymphocyte activity associated with control of viremia in primary human immunodeficiency virus type 1 infection. J Virol. 1994;68:6103–6110. doi: 10.1128/jvi.68.9.6103-6110.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burton D R, Montefiori D C. The antibody response in HIV-1 infection. AIDS. 1997;11(Suppl. A):S87–S98. [PubMed] [Google Scholar]
- 13.Burton D R. A vaccine for HIV type 1: the antibody perspective. Proc Natl Acad Sci USA. 1997;94:10018–10023. doi: 10.1073/pnas.94.19.10018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burton D R, Pyati J, Koduri R, Sharp S J, Thornton G B, Parren P W H I, Sawyer L S W, Hendry R M, Dunlop N, Nara P L, Lamacchia M, Garratty E, Stiehm E R, Bryson Y J, Cao Y, Moore J P, Ho D D, Barbas C F., III Efficient neutralization of primary isolates of HIV-1 by a recombinant human monoclonal antibody. Science. 1994;266:1024–1027. doi: 10.1126/science.7973652. [DOI] [PubMed] [Google Scholar]
- 15.Cao Y, Qin L, Zhang L, Safrit J, Ho D D. Virologic and immunologic characterization of long-term survivors of HIV-1 infection. N Engl J Med. 1994;332:201–208. doi: 10.1056/NEJM199501263320401. [DOI] [PubMed] [Google Scholar]
- 16.Crawford J M, Earl P L, Moss B, Reimann K A, Wyand M S, Manson K H, Bilska M, Zhou J T, Pauza C D, Parren P W H I, Burton D R, Sodroski J G, Letvin N L, Montefiori D C. Characterization of primary isolate-like variants of simian-human immunodeficiency virus. J Virol. 1999;73:10199–10207. doi: 10.1128/jvi.73.12.10199-10207.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Daar E S, Li X L, Moudgil T, Ho D D. High concentrations of recombinant soluble CD4 are required to neutralize primary human immunodeficiency type 1 isolates. Proc Natl Acad Sci USA. 1990;87:6574–6578. doi: 10.1073/pnas.87.17.6574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deng H, Unutmaz D, KewalRamani V N, Littman D R. Expression cloning of new receptors used by simian and human immunodeficiency viruses. Nature. 1997;388:296–300. doi: 10.1038/40894. [DOI] [PubMed] [Google Scholar]
- 19.D'Souza M P, Livnat D, Bradac J A, Bridges S H. Evaluation of monoclonal antibodies to human immunodeficiency virus type 1 primary isolates by neutralization assays: performance criteria for selecting candidate antibodies for clinical trials. J Infect Dis. 1997;175:1056–1062. doi: 10.1086/516443. [DOI] [PubMed] [Google Scholar]
- 20.Foresman L, Jia F, Li Z, Wang C, Stephens E B, Sahni M, Narayan O, Joag S V. Neutralizing antibodies administered before, but not after, virulent SHIV prevent infection in macaques. AIDS Res Hum Retrovir. 1998;14:1035–1043. doi: 10.1089/aid.1998.14.1035. [DOI] [PubMed] [Google Scholar]
- 21.Gartner S, Markovits P, Markovitz D M, Kaplan M H, Gallo R C, Popovic M. The role of mononuclear phagocytes in HTLV/LAV infection. Science. 1986;233:215–219. doi: 10.1126/science.3014648. [DOI] [PubMed] [Google Scholar]
- 22.Gaudin M-C, Parren P W H I, Weir R, Barbas C F, Burton D R, Koup R A. Passive immunization with a human monoclonal antibody protects hu-PBL-SCID mice against challenge by primary isolates of HIV-1. Nat Med. 1997;3:1389–1393. doi: 10.1038/nm1297-1389. [DOI] [PubMed] [Google Scholar]
- 23.Graham B S. Serologic responses to candidate AIDS vaccines. AIDS Res Hum Retrovir. 1994;10(Suppl. 2):S145–S148. [PubMed] [Google Scholar]
- 24.Harada S, Koyanagi Y, Yamamoto N. Infection of HTLV-III/LAV in HTLV-I-carrying cells MT-2 and MT-4 and application in a plaque assay. Science. 1985;229:563–566. doi: 10.1126/science.2992081. [DOI] [PubMed] [Google Scholar]
- 25.Hill C M, Deng H, Unutmaz D, Kewalramani V N, Bastiani L, Gorny M K, Zolla-Pazner S, Littman D R. Envelope glycoproteins from human immunodeficiency virus types 1 and 2 and simian immunodeficiency virus can use human CCR5 as a coreceptor for viral entry and make direct CD4-dependent interactions with this chemokine receptor. J Virol. 1997;71:6296–6304. doi: 10.1128/jvi.71.9.6296-6304.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Javaherian K, Langlois A J, McDanal C, Ross K L, Eckler L I, Jellis C L, Profy A T, Rusche J R, Bolognesi D P, Putney S D, Matthews T J. Principal neutralizing domain of the human immunodeficiency virus type 1 envelope protein. Proc Natl Acad Sci USA. 1989;86:6768–6772. doi: 10.1073/pnas.86.17.6768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jin X, Bauer D E, Tuttleton S E, Lewin S, Gettie A, Blanchard J, Irwin C E, Safrit J T, Mittler J, Weinberger L, Kostrikis L G, Zhang L, Perelson A S, Ho D D. Dramatic rise in plasma viremia after CD8+ T cell depletion in simian immunodeficiency virus-infected macaques. J Exp Med. 1999;189:991–998. doi: 10.1084/jem.189.6.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kessler J A, II, McKenna P M, Emini E A, Chan C P, Patel M D, Gupta S K, Mark III G E, Barbas III C F, Burton D R, Conley A J. The recombinant human monoclonal antibody IgG1b12 neutralizes diverse human immunodeficiency virus type 1 primary isolates. AIDS Res Hum Retrovir. 1997;13:575–582. doi: 10.1089/aid.1997.13.575. [DOI] [PubMed] [Google Scholar]
- 29.Koup R A, Safrit J T, Cao Y, Andrews C A, McLeod G, Borkowsky W, Farthing C, Ho D D. Temporal association of cellular immune responses with initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J Virol. 1994;68:4650–4655. doi: 10.1128/jvi.68.7.4650-4655.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kwon P D, Wyatt R, Robinson J, Sweet R W, Sodroski J, Hendrickson W A. Structure of an HIV gp120 envelope glycoprotein in complex with the CD4 receptor and a neutralizing human antibody. Nature. 1998;393:648–659. doi: 10.1038/31405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.LaCasse R A, Follis K E, Moudgil T, Trahey M, Binley J M, Planelles V, Zolla-Pazner S, Nunberg J H. Coreceptor utilization by human immunodeficiency virus type 1 is not a primary determinant of neutralization sensitivity. J Virol. 1998;72:2491–2495. doi: 10.1128/jvi.72.3.2491-2495.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.LaCasse R A, Follis K E, Trahey M, Scarborough J D, Littman D R, Nunberg J H. Fusion-competent vaccines: broad neutralization of primary isolates of HIV. Science. 1999;283:357–362. doi: 10.1126/science.283.5400.357. [DOI] [PubMed] [Google Scholar]
- 33.Letvin N L. Progress in the development of an HIV-1 vaccine. Science. 1998;280:1875–1880. doi: 10.1126/science.280.5371.1875. [DOI] [PubMed] [Google Scholar]
- 34.Lever A, Gottlinger H, Haseltine W, Sodroski J. Identification of a sequence required for efficient packaging of human immunodeficiency virus type 1 RNA into virions. J Virol. 1989;63:4085–4087. doi: 10.1128/jvi.63.9.4085-4087.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mascola J R, Lewis M G, Stiegler G, Harris D, VanCott T C, Hayes D, Louder M K, Brown C R, Sapan C V, Frankel S S, Lu Y, Robb M L, Katinger H, Birx D L. Protection of macaques against pathogenic simian/human immunodeficiency virus 89.6PD by passive transfer of neutralizing antibodies. J Virol. 1999;73:4009–4018. doi: 10.1128/jvi.73.5.4009-4018.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mascola J R, Louder M K, VanCott T C, Sapan C V, Lambert J S, Muenz L R, Bunow B, Birx D L, Robb M L. Potent and synergistic neutralization of human immunodeficiency virus (HIV) type 1 primary isolates by hyperimmune anti-HIV immunoglobulin combined with monoclonal antibodies 2F5 and 2G12. J Virol. 1997;71:7198–7206. doi: 10.1128/jvi.71.10.7198-7206.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mascola J R, Snyder S W, Weislow O S, Belay S M, Belshe R B, Schwartz D H, Clements M L, Dolin R, Graham B S, Gorse G J, Keefer M C, McElrath M J, Walker M C, Wagner K F, McNeil J G, McCutchan F E, Burke D S. Immunization with envelope subunit vaccine products elicits neutralizing antibodies against laboratory-adapted but not primary isolates of human immunodeficiency virus type 1. J Infect Dis. 1996;173:340–348. doi: 10.1093/infdis/173.2.340. [DOI] [PubMed] [Google Scholar]
- 38.Mascola J R, Stiegler G, VanCott T C, Katinger H, Carpenter C B, Hanson C E, Beary H, Hayes D, Frankel S S, Birx D L, Lewis M G. Protection of macaques against vaginal transmission of a pathogenic HIV-1/SIV chimeric virus by passive infusion of neutralizing antibodies. Nat Med. 2000;6:207–210. doi: 10.1038/72318. [DOI] [PubMed] [Google Scholar]
- 39.Mastro T D, Kitayaporn D. HIV type 1 transmission probabilities: estimates from epidemiologic studies. AIDS Res Hum Retrovir. 1998;14(Suppl. 3):S223–S227. [PubMed] [Google Scholar]
- 40.Matano T, Shibata R, Siemon C, Connors M, Lane H C, Martin M A. Administration of an anti-CD8 monoclonal antibody interferes with the clearance of chimeric simian/human immunodeficiency virus during primary infections of rhesus macaques. J Virol. 1998;72:164–169. doi: 10.1128/jvi.72.1.164-169.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McDougal J S, Kennedy M S, Orloff S L, Nicholson J K A, Spira T J. Mechanism of human immunodeficiency virus type 1 (HIV-1) neutralization: irreversible inactivation of infectivity by anti-HIV-1 antibody. J Virol. 1996;70:5236–5245. doi: 10.1128/jvi.70.8.5236-5245.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mo H, Stamatatos L, Ip J E, Barbas C F, Parren P W H I, Burton D R, Moore J P, Ho D D. Human immunodeficiency virus type 1 mutants that escape neutralization by human monoclonal antibody IgG1b12. J Virol. 1997;71:6869–6874. doi: 10.1128/jvi.71.9.6869-6874.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Montefiori D C, Collman R G, Fouts T R, Zhou J Y, Bilska M, Hoxie J A, Moore J P, Bolognesi D P. Evidence that antibody-mediated neutralization of human immunodeficiency virus type 1 is independent of coreceptor usage. J Virol. 1998;72:1886–1893. doi: 10.1128/jvi.72.3.1886-1893.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Montefiori D C, Evans T G. Toward an HIV-1 vaccine that generates potent, broadly cross-reactive neutralizing antibodies. AIDS Res Hum Retrovir. 1999;15:689–698. doi: 10.1089/088922299310773. [DOI] [PubMed] [Google Scholar]
- 45.Montefiori D C, Graham B S, Zhou J T, Zhou J Y, Bucco R, Cavacini L A, Posner M R The NIH AIDS Vaccine Clinical Trials Network. V3-specific neutralizing antibodies in sera from HIV-1 gp160-immunized volunteers block virus fusion and act synergistically with human monoclonal antibody to the conformation-dependent CD4 binding region of gp120. J Clin Investig. 1993;92:840–847. doi: 10.1172/JCI116658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Montefiori D C, Pantaleo G, Fink L M, Zhou J T, Zhou J Y, Bilska M, Miralles G D, Fauci A S. Neutralizing and infection-enhancing antibody responses to human immunodeficiency virus type 1 in long-term nonprogressors. J Infect Dis. 1996;173:60–67. doi: 10.1093/infdis/173.1.60. [DOI] [PubMed] [Google Scholar]
- 47.Montefiori D C, Robinson W E, Jr, Schuffman S S, Mitchell W M. Evaluation of antiviral drugs and neutralizing antibodies to human immunodeficiency virus by a rapid and sensitive microtiter infection assay. J Clin Microbiol. 1988;26:231–235. doi: 10.1128/jcm.26.2.231-235.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moog C, Fleury H J A, Pellegrin I, Kirn A, Aubertin A M. Autologous and heterologous neutralizing antibody responses following initial seroconversion in human immunodeficiency virus type 1-infected individuals. J Virol. 1997;71:3734–3741. doi: 10.1128/jvi.71.5.3734-3741.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moore J P, McKeating J A, Huang Y X, Ashkenazi A, Ho D D. Virions of primary human immunodeficiency virus type 1 isolates resistant to soluble CD4 (sCD4) neutralization differ in sCD4 binding and glycoprotein gp120 retention from sCD4-sensitive isolates. J Virol. 1992;66:235–243. doi: 10.1128/jvi.66.1.235-243.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mulligan M J, Weber J. Human trials of HIV-1 vaccines. AIDS. 1999;13(Suppl. A):S105–S112. [PubMed] [Google Scholar]
- 51.Muster T, Steindl F, Purtscher M, Trkola A, Klima A, Himmler G, Ruker F, Katinger H. A conserved neutralization epitope on gp41 of human immunodeficiency virus type 1. J Virol. 1993;67:6642–6647. doi: 10.1128/jvi.67.11.6642-6647.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Myers G, Berzofsky J A, Rabson A B, Smith T F, Wong-Staal F, editors. Human retroviruses and AIDS. Theoretical Biology and Biophysics Group T-10. Los Alamos, N.Mex: Los Alamos National Laboratory; 1990. [Google Scholar]
- 53.O'Brien W A, Koyanagi Y, Namazie A, Zhao J Q, Diagne A, Idler K, Zack J A, Chen I S. HIV-1 tropism for mononuclear phagocytes can be determined by regions of gp120 outside the CD4-binding domain. Nature. 1990;348:69–73. doi: 10.1038/348069a0. [DOI] [PubMed] [Google Scholar]
- 54.Ourmanov I, Bilska M, Hirsch V H, Montefiori D C. Recombinant modified vaccinia virus Ankara expressing the surface gp120 of simian immunodeficiency virus (SIV) primes for a rapid neutralizing antibody response to SIV infection in macaques. J Virol. 2000;74:2960–2965. doi: 10.1128/jvi.74.6.2960-2965.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pantaleo G, Menzo S, Vaccarezza M, Graziosi C, Cohen O J, Demarest J F, Biddison W E, Montefiori D, Orenstein J M, Fox C, Schrager L K, Margolick J B, Detels R, Buchbinder S, Giorgi J V, Rinaldo C R, Phair J P, Fauci A S. Studies in subjects with long-term nonprogressive human immunodeficiency virus infection. N Engl J Med. 1995;332:209–216. doi: 10.1056/NEJM199501263320402. [DOI] [PubMed] [Google Scholar]
- 56.Pellegrin I, Legrand E, Neau D, Bonot P, Masquelier B, Pellegrin J-L, Ragneud J-M, Bernard N, Fleury H J A. Kinetics of appearance of neutralizing antibodies in 12 patients with primary or recent HIV-1 infection and relationship with plasma and cellular viral loads. J Acquir Immun Defic Syndr Hum Retrovirol. 1996;11:438–447. doi: 10.1097/00042560-199604150-00003. [DOI] [PubMed] [Google Scholar]
- 57.Persson R H, Cao S X, Cates G, Yao F L, Klein M H, Rovinski B. Modifications of HIV-1 retrovirus-like particles to enhance safety and immunogenicity. Biologicals. 1998;26:255–265. doi: 10.1006/biol.1998.0142. [DOI] [PubMed] [Google Scholar]
- 58.Pilgrim A K, Pantaleo G, Cohen O J, Fink L M, Zhou J Y, Zhou J T, Bolognesi D P, Fauci A S, Montefiori D C. Neutralizing antibody responses to human immunodeficiency virus type 1 in primary infection and long-term nonprogressive infection. J Infect Dis. 1997;176:924–932. doi: 10.1086/516508. [DOI] [PubMed] [Google Scholar]
- 59.Purtscher M, Trkola A, Grassauer A, Schultz P M, Klima A, Döpper S, Gruber G, Buchacher A, Muster T, Katinger H. Restricted antigenic variability of the epitope recognized by the neutralizing gp41 antibody 2F5. AIDS. 1996;10:587–593. doi: 10.1097/00002030-199606000-00003. [DOI] [PubMed] [Google Scholar]
- 60.Quinn T C, Wawer M J, Sewankambo N, Serwadda D, Li C, Wabwire-Mangen F, Meehan M O, Lulato T, Gray R H. Viral load and heterosexual transmission of human immunodeficiency virus type 1. N Engl J Med. 2000;342:921–929. doi: 10.1056/NEJM200003303421303. [DOI] [PubMed] [Google Scholar]
- 61.Reimann K A, Tenner-Racz K, Racz P, Montefiori D C, Yasutomi Y, Lin W, Ransil B J, Letvin N. Immunopathogenic events in the acute infection of rhesus monkeys with simian immunodeficiency virus of macaques. J Virol. 1994;68:2362–2370. doi: 10.1128/jvi.68.4.2362-2370.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Reitter J N, Means R E, Desrosiers R C. A role for carbohydrates in immune evasion in AIDS. Nat Med. 1998;4:679–684. doi: 10.1038/nm0698-679. [DOI] [PubMed] [Google Scholar]
- 63.Richman D D, Bozzette S A. The impact of the syncytium-inducing phenotype of human immunodeficiency virus on disease progression. J Infect Dis. 1994;169:968–974. doi: 10.1093/infdis/169.5.968. [DOI] [PubMed] [Google Scholar]
- 64.Rosenberg E S, Altfeld M, Poon S H, Phillips M N, Wilkes B M, Eldridge R L, Robbins G K, D'Aquila R T, Goulder P J R, Walker B D. Immune control of HIV-1 following early treatment of acute infection. Nature. 2000;407:523–526. doi: 10.1038/35035103. [DOI] [PubMed] [Google Scholar]
- 65.Rovinski B, Dekaban G A, Cao S X, Tao F L, Persson R, Matthews T J, Klein M H. Engineering of noninfectious HIV-1-like particles containing mutant gp41 glycoproteins as vaccine candidates that allow vaccinees to be distinguished from HIV-1 infectees. Virology. 1999;257:438–448. doi: 10.1006/viro.1999.9667. [DOI] [PubMed] [Google Scholar]
- 66.Rovinski B, Haynes J R, Cao S X, James O, Sia C, Zolla-Pazner S, Matthews T J, Klein M H. Expression and characterization of genetically engineered human immunodeficiency virus-like particles containing modified envelope glycoproteins: implications for development of a cross-protective AIDS vaccine. J Virol. 1992;66:4003–4012. doi: 10.1128/jvi.66.7.4003-4012.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rucker J, Doms R W. Chemokine receptors as HIV coreceptors: implications and interactions. AIDS Res Hum Retrovir. 1998;14(Suppl. 3):S241–S246. [PubMed] [Google Scholar]
- 68.Rusche J R, Javaherian K, McDanal C, Petro J, Lynn D L, Grimaila R, Langlois A J, Gallo R C, Arthur L O, Fischinger P J, Bolognesi D P, Putney S D, Matthews T J. Antibodies that inhibit fusion of human immunodeficiency virus-infected cells bind a 24-amino acid sequence of the viral envelope gp120. Proc Natl Acad Sci USA. 1988;85:3198–3202. doi: 10.1073/pnas.85.9.3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Safrit J T, Andrews C A, Zhu T, Ho D D, Koup R A. Characterization of HIV-1-specific cytotoxic T lymphocyte clones isolated during acute seroconversion: recognition of autologous virus sequences within a conserved immunodominant epitope. J Exp Med. 1994;179:463–472. doi: 10.1084/jem.179.2.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Safrit J T, Lee A Y, Andrews C A, Koup R A. A region of the third variable loop of HIV-1 gp120 is recognized by HLA-B7-restricted cytotoxic T lymphocytes from two acute seroconversion patients. J Immunol. 1994;153:3822–3830. [PubMed] [Google Scholar]
- 71.Salzwedel K, Smith E D, Dey B, Berger E A. Sequential CD4-coreceptor interactions in human immunodeficiency virus type 1 Env function: soluble CD4 activates Env for coreceptor-dependent fusion and reveals blocking activities of antibodies against cryptic conserved epitopes on gp120. J Virol. 2000;74:326–333. doi: 10.1128/jvi.74.1.326-333.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schmitz J E, Kuroda M J, Santra S, Sasseville V G, Simon M A, Lifton M A, Racz P, Tenner-Racz K, Dalesandro M, Scallon B J, Ghrayeb J, Forman M A, Montefiori D C, Rieber E P, Letvin N L, Reimann K A. CD8+ lymphocytes control viremia in simian immunodeficiency virus infection. Science. 1999;283:857–860. doi: 10.1126/science.283.5403.857. [DOI] [PubMed] [Google Scholar]
- 73.Schønning K, Bolmstedt A, Novotny J, Søgaard Lund O, Olofsson S, Hansen J-E S. Induction of antibodies against epitopes inaccessible on the HIV type 1 envelope oligomer by immunization with recombinant monomeric glycoprotein 120. AIDS Res Hum Retrovir. 1998;16:1451–1456. doi: 10.1089/aid.1998.14.1451. [DOI] [PubMed] [Google Scholar]
- 74.Schønning K, Jansson B, Olofsson S, Hansen J-E S. Rapid selection for an N-linked oligosaccharide by monoclonal antibodies directed against the V3 loop of human immunodeficiency virus type 1. J Gen Virol. 1996;77:753–758. doi: 10.1099/0022-1317-77-4-753. [DOI] [PubMed] [Google Scholar]
- 75.Schønning K, Jansson B, Olofsson S, Neilsen J O, Hansen J-E S. Resistance to V3-directed neutralization caused by an N-linked oligosaccharide depends on the quaternary structure of the HIV-1 envelope oligomer. Virology. 1996;218:134–140. doi: 10.1006/viro.1996.0173. [DOI] [PubMed] [Google Scholar]
- 76.Shibata R, Igarashi T, Haigwood N, Buckler-White A, Ogert R, Ross W, Willey R, Cho M W, Martin M M. Neutralizing antibody directed against the HIV-1 envelope glycoprotein can completely block HIV-1/SIV chimeric virus infections of macaque monkeys. Nat Med. 1999;5:204–210. doi: 10.1038/5568. [DOI] [PubMed] [Google Scholar]
- 77.Spenlehauer C, Saragosti S, Fleury H J A, Kirn A, Aubertin A-M, Moog C. Study of the V3 loop as a target epitope for antibodies involved in the neutralization of primary isolates versus T-cell-line-adapted strains of human immunodeficiency virus type 1. J Virol. 1998;72:9855–9864. doi: 10.1128/jvi.72.12.9855-9864.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sullivan N, Sun Y, Sattentau Q, Thali M, Wu D, Denisova G, Gershoni J, Robinson J, Moore J, Sodroski J. CD4-induced conformational changes in the human immunodeficiency virus type 1 gp120 glycoprotein: consequences for virus entry and neutralization. J Virol. 1998;72:4694–4703. doi: 10.1128/jvi.72.6.4694-4703.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tartaglia J, Excler J L, El Habib R, Limbach K, Meignier B, Plotkin S, Klein M. Canarypox virus-based vaccines: prime-boost strategies to induce cell-mediated and humoral immunity against HIV. AIDS Res Hum Retrovir. 1998;14(Suppl. 3):S291–S298. [PubMed] [Google Scholar]
- 80.Tomaras G D, Lacey S F, McDanal C B, Ferrari G, Weinhold K J. CD8+ T cell-mediated suppressive activity inhibits HIV-1 after virus entry with kinetics indicating effects on virus gene expression. Proc Natl Acad Sci USA. 2000;97:3503–3508. doi: 10.1073/pnas.070521097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Trkola A, Ketas T, KewalRamani V N, Endorf F, Binley J M, Katinger H, Robinson J, Littman D R, Moore J P. Neutralization sensitivity of human immunodeficiency virus type 1 primary isolates to antibodies and CD4-based reagents is independent of their coreceptor usage. J Virol. 1998;72:1876–1885. doi: 10.1128/jvi.72.3.1876-1885.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Trkola A, Purtscher M, Muster T, Ballaun C, Buchacher A, Sullivan N, Srinivasa K, Sodroski J, Moore J P, Katinger H. Human monoclonal antibody 2G12 defines a distinctive neutralization epitope on the gp120 glycoprotein of human immunodeficiency virus type 1. J Virol. 1996;70:1100–1108. doi: 10.1128/jvi.70.2.1100-1108.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ugolini S, Mondor I, Parren P W H I, Burton D R, Tilley S A, Klasse P J, Sattentau Q J. Inhibition of virus attachment to CD4+ target cells is a major mechanism of T cell line-adapted HIV-1 neutralization. J Exp Med. 1997;186:1287–1298. doi: 10.1084/jem.186.8.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Valenzuela A, Blanco J, Krust B, Franco R, Hovanessian A G. Neutralizing antibodies against the V3 loop of human immunodeficiency virus type 1 gp120 block the CD4-dependent and -independent binding of virus to cells. J Virol. 1997;71:8289–8298. doi: 10.1128/jvi.71.11.8289-8298.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.VanCott T C, Mascola J R, Loomis-Price L D, Sinangil F, Zitomersky N, McNeil J, Robb M L, Birx D L, Barnett S. Cross-subtype neutralizing antibodies induced in baboons by a subtype E gp120 immunogen based on an R5 primary human immunodeficiency virus type 1 envelope. J Virol. 1999;73:4640–4650. doi: 10.1128/jvi.73.6.4640-4650.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.VanCott T C, Polonis V R, Loomis L D, Michael N L, Nara P L, Birx D L. Differential role of V3-specific antibodies in neutralization assays involving primary and laboratory-adapted isolates of HIV type 1. AIDS Res Hum Retrovir. 1995;11:1379–1390. doi: 10.1089/aid.1995.11.1379. [DOI] [PubMed] [Google Scholar]
- 87.van't Wout A B, Kootstra N A, Mulder-Kampinga G A, Albrecht-van Lent N, Scherpbier H J, Veenstra J, Boer K, Coutinho R A, Miedema F, Schuitemaker H. Macrophage-tropic variants initiate human immunodeficiency virus type 1 infection after sexual, parenteral, and vertical transmission. J Clin Investig. 1994;94:2060–2067. doi: 10.1172/JCI117560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Walker C M, Moody D J, Stites D P, Levy J A. CD8 lymphocytes can control HIV infection in vitro by suppressing virus replication. Science. 1986;234:1563–1566. doi: 10.1126/science.2431484. [DOI] [PubMed] [Google Scholar]
- 89.Wrin T, Crawford L, Sawyer L, Weber P, Sheppard H W, Hanson C V. Neutralizing antibody responses to autologous and heterologous isolates of human immunodeficiency virus. J Acquir Immune Defic Syndr. 1994;7:211–219. [PubMed] [Google Scholar]
- 90.Wyatt R, Kwon P D, Desjardins E, Sweet R W, Robinson J, Hendrickson W A, Sodroski J G. The antigenic structure of the HIV gp120 envelope glycoprotein. Nature. 1998;393:705–711. doi: 10.1038/31514. [DOI] [PubMed] [Google Scholar]
- 91.Wyatt R, Sodroski J. The HIV-1 envelope glycoproteins: fusogens, antigens, and immunogens. Science. 1998;280:1884–1888. doi: 10.1126/science.280.5371.1884. [DOI] [PubMed] [Google Scholar]
- 92.Yasutomi Y, Reimann K A, Lord C I, Miller M D, Letvin N L. Simian immunodeficiency virus-specific CD8+ lymphocyte responses in acutely infected rhesus monkeys. J Virol. 1993;67:1707–1711. doi: 10.1128/jvi.67.3.1707-1711.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhou J Y, Montefiori D C. Antibody-mediated neutralization of primary isolates of human immunodeficiency virus type 1 in peripheral blood mononuclear cells is not affected by the initial activation state of the cells. J Virol. 1997;71:2512–2517. doi: 10.1128/jvi.71.3.2512-2517.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]