Abstract
Reactivation of cytomegalovirus (CMV) from latency is a frequent complication of organ transplantation, and the molecular mechanism by which this occurs is unknown. Previous studies have shown that allogeneic stimulation induces reactivation of human CMV (HCMV) in vitro (64). We find that transplantation of vascularized allogeneic kidneys induces murine CMV (MCMV) and HCMV immediate-early (ie) gene expression. This induction is accompanied by increased expression of transcripts encoding inflammatory cytokines, including tumor necrosis factor (TNF), interleukin-2, and gamma interferon, and by activation of NF-κB. TNF alone can substitute for allogeneic transplantation in inducing HCMV and MCMV ie gene expression in some tissues. Our studies suggest that reactivation is a multistep process which is initiated by factors that induce ie gene expression, including TNF and NF-κB. Allogeneic transplantation combined with immunosuppression may be required to achieve complete reactivation in vivo.
Cytomegalovirus (CMV) is a ubiquitous herpesvirus which infects 60 to 90% of adults (reviewed in reference 50). Primary infection in immunocompetent hosts is self-limiting and is generally not associated with significant morbidity or mortality. Replicating virus is eventually cleared by the host immune response, but the virus establishes a lifelong latent or persistent infection. CD34+ hematopoietic progenitor cells and cells of the monocyte/macrophage lineage have been identified as sites of latent human CMV (HCMV) infection (27, 49, 62, 70). Reactivation of latent virus accompanied by asymptomatic viral shedding can occasionally occur in healthy, seropositive individuals. In contrast, reactivation of latent virus is frequently observed in immunocompromised individuals, such as transplant recipients and AIDS patients, and, despite the development of effective antiviral drugs, remains a significant cause of morbidity and mortality.
Due to the species specificity of HCMV, many investigators have turned to murine CMV (MCMV) as a model for the study of CMV latency and reactivation. MCMV is similar to HCMV with respect to genome organization, pathogenesis, and ability to establish latent infection and to reactivate. It has been previously demonstrated in mice latently infected with MCMV that latent viral DNA is present in bone marrow cells, in alveolar macrophages, and in endothelial cells in the kidney, liver, heart, and spleen (34). Thus, endothelial cells may represent an important site of latency for HCMV as well.
Latency has been defined operationally as the inability to detect infectious virus despite the presence of viral DNA. However, it has not been clear whether lack of virus production is due to establishment of a true latent state, in which gene products associated with lytic replication are not expressed, or whether small numbers of permissively infected cells are usually present with spread of infection blocked by the immune system. Reactivation in the former case would be due to a change in the transcriptional program of the latently infected cell, while in the latter, reactivation would be the result of failure of the immune system to remove productively infected cells (36). A number of studies have investigated expression of RNAs associated with productive infection in tissues of latently MCMV-infected mice or in latently HCMV-infected cells. Some studies have reported that MCMV immediate-early protein-1 (IE1) transcripts are not detectable in tissues of latently infected mice (34), while others have reported that they are detectable in some organs (5, 30, 37, 77, 78). It is not clear whether this discrepancy is due to differences in sensitivity of detection, to methods used for infection, or to spontaneous reactivation of the virus. The most definitive study done to date suggests that although expression of IE1 transcripts is detectable in some regions of organs from latently infected mice, it is not detectable in all regions which carry latent viral DNA and “is not a feature inherent to murine CMV latency but rather reflects foci of primordial reactivation” (37). HCMV IE1 transcripts initiated at a novel latency-specific promoter and antisense IE1 transcripts have been identified in a small percentage of granulocyte-macrophage progenitor cells infected in vitro and in bone marrow cells of healthy, seropositive individuals (27, 35, 63), but their role in the CMV life cycle is unclear (74). Others have reported that HCMV IE1 transcripts are not detectable in latently infected CD34+ hematopoietic progenitor cells (49).
Studies with animal models of CMV have shown that reactivation can be induced by immunosuppressive therapies alone, such as total body irradiation or administration of cytotoxic drugs like cyclophosphamide and azathioprine or by immunodepletion of T cells or T-cell subsets (5, 12, 47, 48, 53). One recent study concluded that, unlike the case with other herpesviruses, CMV latency is maintained primarily as a result of immune surveillance rather than by transcriptional control in latently infected cells (53). However, the immunosuppressive regimes employed in these studies result in high levels of cell death and release of inflammatory cytokines, which may themselves play a role in reactivation (7, 16, 19, 23, 25, 26, 55). In clinical settings, reactivation of CMV is frequently associated with rejection of the transplanted organ or with other conditions accompanied by high levels of inflammatory cytokines, such as graft-versus-host disease, cirrhosis, and sepsis (19, 46, 51). With less cytotoxic immunosuppressive regimens, such as the use of cyclosporine, the frequency of reactivation is much lower than that observed with earlier therapies, both in animals and in clinical settings (12, 31, 59).
Allogeneic stimulation has also been proposed as a factor in inducing reactivation of latent virus (64). The role of allogeneic stimulation in inducing CMV reactivation has been studied in animal models by using organ transplantation, tissue implantation, blood transfusion, or cell transfer. Most of these studies demonstrate that allogeneic stimulation plays an important role in the reactivation of latent CMV (11, 13, 21, 61, 76), although others have found that in the absence of immunosuppression, there is a higher frequency of reactivation with syngeneic cell transfer or tissue implantation (29, 47). More recently, reactivation of latent HCMV has been achieved by allogeneic stimulation of peripheral blood mononuclear cells in vitro (64), suggesting that allogeneic stimulation may indeed be an important factor in inducing reactivation in vivo. None of these studies has addressed the mechanism by which allostimulation might lead to reactivation of CMV. Reactivation of HCMV has also been achieved by cytokine treatment of experimentally infected granulocyte-macrophage progenitor cells in vitro (27). Tumor necrosis factor alpha (TNF-α), gamma interferon (IFN-γ), and, to a lesser extent, granulocyte-macrophage colony-stimulating factor and interleukin-4 (IL-4) were all found to induce reactivation in this system. The mechanism by which this occurred was not explored.
In view of the conflicting data on viral gene expression in latently infected cells and on the role of immune suppression and allogeneic stimulation in inducing CMV reactivation, we have further investigated the effect of allogeneic and syngeneic transplantation in inducing expression of genes required for lytic replication of MCMV. These studies were performed in the absence of immunosuppression to study the role of allostimuation alone without the potential confounding effects of cytokine release due to immunosuppression. We have compared the level of viral gene expression in transplanted donor kidneys with that in the contralateral kidneys removed at the time of transplant in order to determine the level of viral gene expression before and after transplantation. Thus, even if some ie gene expression is detectable in the latently infected animals, the specific effects of transplantation on ie gene expression can be determined. We find that allogeneic transplantation induces expression of the MCMV and HCMV ie1 genes. This induction is accompanied by increased expression of transcripts encoding inflammatory cytokines, including TNF, IL-2, and IFN-γ, suggesting that these cytokines could mediate induction of viral gene expression. In addition, we have investigated the ability of TNF to induce viral gene expression directly by injecting TNF into latently infected mice. Our studies suggest that reactivation is a multistep process which is initiated by factors induced as a result of allogeneic transplantation. TNF activates transcription factors which regulate expression of the ie1 gene, including NF-κB, and TNF alone is able to induce ie1 gene expression in some tissues. Given the fact that ie1 gene expression is very low or is not detectable in tissues from latently infected mice (34, 37) and that expression of this gene is required to initiate productive viral infection in vitro, induction of ie1 gene expression is likely to be a key step in viral reactivation. Our results suggest a mechanism by which this might occur.
MATERIALS AND METHODS
Animals.
Three-week-old female, specific-pathogen-free BALB/c mice and adult male BALB/c (H-2d), C57BL/6 (H-2b), and C3H/HeSnJ (H-2k) mice were purchased from Jackson Laboratory (Bar Harbor, Maine). Breeding pairs of MIEP-lacZ transgenic mice from the TG1JB line (4) carrying the β-galactosidase gene under the control of the HCMV ie promoter were obtained from Jay Nelson at Oregon Health Sciences University. Mice were maintained in isolation cages and were fed and watered ad libitum. This study protocol was reviewed and approved by the Northwestern University Institutional Animal Care and Use Committee.
Virus infection and establishment of MCMV latency.
Three- to 4-week-old mice were injected intraperitoneally with 105 PFU of MCMV (Smith strain) and maintained for 6 months to establish latent infection as previously described (34).
Transplants and organ processing.
Mouse kidney transplants were performed as previously described (80). The right donor kidney from latently infected mice was removed for use as a control, and the left donor kidney was transplanted into syngeneic BALB/c (H-2d) or allogeneic C57BL/6 (H-2b) adult males and removed at 1, 2, 5, 8, or 15 days after transplant. Kidneys from MIEP-lacZ transgenic mice were transplanted into allogeneic C3H/HeSnJ (H-2k) mice and were removed 2 days after transplant. All organs were frozen in liquid nitrogen immediately after removal.
Cytokine injections.
For RNA analysis, mice were injected intraperitoneally with 10 μg of TNF (R & D Systems, Minneapolis, Minn.) or in the tail vein with 2.5 μg of TNF and were sacrificed at various times after injection. No difference in MCMV RNA expression was observed with intraperitoneal or intravenous injections. For gel shifts or analysis of β-galactosidase expression, mice were injected intravenously with 2.5 μg of TNF and were sacrificed after 2 or 24 h, respectively.
RT-PCR analysis.
Mice were anesthetized with Metofane (Schering-Plough) and were sacrificed by cervical dislocation. For RNA extraction, frozen tissues were sonicated in TriReagent (Molecular Systems, Cinncinati, Ohio) to disrupt the tissue and the RNA was purified according to the directions of the manufacturer. Reverse transcriptase PCRs (RT-PCRs) were performed with an RNA PCR kit (Perkin-Elmer Cetus) according to the directions of the manufacturer. RNA (1.5 μg) was reverse transcribed using random hexamer primers, and the cDNA was amplified in 40 cycles of 94°C, 30 s; 58°C, 30 s; and 72°C, 30 s, followed by a 7-min incubation at 72°C. PCR products were electrophoretically separated on 1.5% agarose gels, transferred to nitrocellulose, and hybridized to oligonucleotide probes at 42°C for 2 h. Probes were labeled with fluorescein-dUTP and terminal transferase using an enhanced chemiluminescence 3′ oligonucleotide labeling kit (Amersham) and were detected according to the directions of the manufacturer. The following primers and probes were used to amplify and detect RT-PCR products: for ie1, primer 1, primer 2 (1), 179 bp, probe, CH15 (30); for ie3, CH17 (30), IE3-RT/R (38), 229 bp, probe, CH15 (30); for E-1, forward, reverse (5), 266 bp, probe, GGCAGCGGCAGCGGAGGCAGCAGCGGCCTCAGTACAAAGC; for gB, forward, reverse (5), 400 bp, probe, AACAGAAACCATGTTCTCCGTCTCGTTCACGAAGGGAAC; for TNF, sens, antisens (33), 678 bp, probe, CAGTAGACAGAAGAGCGTGGTGGCCCCTGCCACAAGCAGG; for IL-2, 5′, 3′ (57), 247 bp, probe, GAGCTCCTGAGCAGGATGGAGAATTACAGGAACCTGAAAC; for IFN-γ, sens, antisens (33), 401 bp, probe, GAGCCAGATTATCTCTTTCTACCTCAGACTCTTTGAAGTC; for IL-1β, 5′ AAGCTCTCCACCTCAATGGACAG and 3′ CTCAAACTCCACTTTGCTCTTGA, 260 bp; for β-actin, 5′ TGAGAGGGAAATCGTGCGTG and 3′ ATCTGCTGGAAGGTGGACAGTGAG, 453 bp.
Analysis of lacZ transgene expression.
Histochemical staining of tissue sections from MIEP-lacZ transgenic mice for β-galactosidase activity was performed as previously described (4). β-Galactosidase activity in tissues from MIEP-lacZ transgenic mice was assayed with a Galacto-Star chemiluminescent reporter gene assay system (Tropix PE Biosystems, Bedford, Mass.) in triplicate as described by the manufacturer and was quantitated with a Monolight 2010 luminometer. Protein concentration in the extract was determined by Bio-Rad protein assay, and values were expressed as average light units of β-galactosidase per nanogram of protein.
Electrophoretic mobility shift assay.
Nuclear extracts were prepared from renal and lung tissue as previously described (18), assayed for protein concentration (9), and stored at −80°C. NF-κB and AP-1 consensus oligonucleotides (Promega Co., Madison, Wis.) were labeled with [γ-32]ATP (3,000 Ci/mmol, 10 mCi/ml; Amersham, Arlington Heights, Ill.) using T4 polynucleotide kinase. Extract (3.5 μg) in 10 μl was preincubated with 4 μl of gel shift binding buffer (18) at 25°C for 15 min and was then incubated with 1 μl of probe for 20 min at 25°C and analyzed as previously described (17, 18). Competition experiments were performed by incubation of extracts with a 100-fold excess of unlabeled oligonucleotide containing consensus or mutant NF-κB binding sites (Santa Cruz Biotechnology, Santa Cruz, Calif.) for 20 min prior to addition of the probe. Supershift experiments were performed by incubating extracts, oligonucleotides, and 1 μg of p50, p65, p52, RelB, or c-Rel antibody (Santa Cruz Biotechnology) for 20 min prior to electrophoresis.
RESULTS
Allogeneic transplantation induces ie1 gene expression.
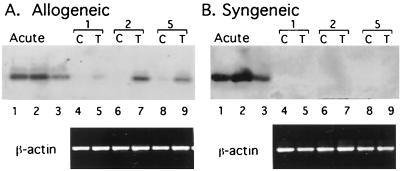
In order to investigate the role of allogeneic stimulation in inducing MCMV lytic cycle gene expression, kidneys from latently infected BALB/c mice were transplanted into uninfected syngeneic BALB/c (H-2d) or allogeneic C57BL/6 (H-2b) recipients and were analyzed for expression of MCMV RNAs at various times after transplant. The contralateral donor kidneys were removed at the time of transplant and were analyzed as controls. Representative results of allogeneic and syngeneic transplants removed at days 1, 2, and 5 are shown in Fig. 1. IE1 transcripts were not detectable in most of the control kidneys removed from latently infected donors at the time of transplant, although some expression was observed in some of the controls (e.g., Fig. 1A, lane 8). Expression of IE1 RNA was induced by allogeneic transplantation, with a peak of induction at 2 days posttransplant. On the basis of these results, additional transplants were analyzed at 2 days posttransplant. Expression of IE1 RNA was induced in five of five kidneys transplanted into allogeneic recipients and in zero of four syngeneic transplants harvested 48 h after transplant. Thus, expression of MCMV IE1 RNA was induced by allogeneic transplantation, and this induction was due to the allogeneic response rather than ischemia/reperfusion injury.
FIG. 1.
Effect of transplantation on expression of MCMV IE1 RNA. Kidneys from latently infected mice were transplanted into allogeneic or syngeneic recipients and were removed 1, 2, or 5 days after transplant (lanes 5, 7, and 9, respectively). RNAs from transplanted kidneys (T) or contralateral control (C) kidneys or from productively infected murine embryo fibroblasts (lanes 1 and 2) were analyzed for MCMV IE1 RNA expression by RT-PCR. Transplanted and contralateral control kidneys are indicated by brackets. Lane 3, RNA from lung tissue of a latently infected mouse injected with TNF. β-Actin RNA was analyzed as a positive control.
RNAs isolated from control and transplanted kidneys were also examined for expression of IE3, an alternatively spliced transcript initiated at the ie promoter; E-1, an early gene product; and gB, a late gene product. No expression of these transcripts was observed, indicating that although the initial phase of viral replication was induced by allogeneic stimulation, full reactivation with lytic replication was not induced (data not shown). The lack of expression of IE1 at later times after transplant and the lack of expression of other genes associated with productive infection are likely to be due to destruction of cells expressing foreign antigens by immune surveillance in these immunocompetent recipients.
NF-κB is activated by allogeneic transplantation.
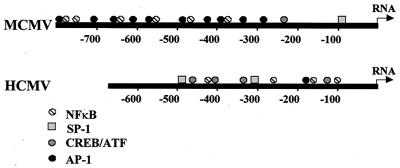
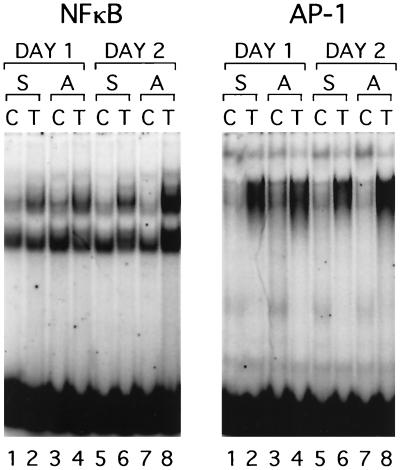
Regulation of HCMV and MCMV ie gene expression is controlled by the CMV ie promoter/enhancer (8, 20, 68, 72). Both the MCMV and HCMV ie promoter/enhancer regions contain multiple binding sites for NF-κB, as well as other transcription factors (Fig. 2). The NF-κB sites and the ATF(CREB) sites have been shown to be important in regulation of the HCMV promoter (32, 41, 60, 68). The factors important in regulation of the MCMV promoter have not been studied. In order to investigate the effect of transplantation on activation of transcription factors that are likely to be important in controlling MCMV ie gene expression, we performed gel shift analysis on nuclear extracts of kidneys of latently infected mice transplanted into syngeneic or allogeneic recipients (Fig. 3). The contralateral kidney from each donor was removed at the time of transplant and was analyzed as a control. Two NF-κB complexes were observed in extracts of control and transplanted kidneys. Both complexes bound specifically to the consensus NF-κB binding site, since an unlabeled NF-κB oligonucleotide competitively abolished binding, but an oligonucleotide with a mutated NF-κB binding site did not (data not shown). The more slowly migrating complex is a classical NF-κB complex, since it was supershifted by antibodies specific for the p65 and p50 subunits of NF-κB (data not shown). The faster-migrating complex was not supershifted with antibodies to any of the known NF-κB subunits, including p65, p50, p52, c-Rel, and RelB, and its composition is therefore unknown. A slight activation of NF-κB was observed in syngeneic transplants (Fig. 3, left panel, compare lanes 1 and 2 and lanes 5 and 6). This is likely to be due to ischemia/reperfusion injury, which has been previously reported to activate NF-κB. However, much greater induction was observed at day 2 in the allogeneic transplants than in syngeneic transplants (Fig. 3, left panel, compare lanes 7 and 8 with lanes 5 and 6). This coincides with the peak of IE1 RNA expression previously observed in latently infected kidneys transplanted into allogeneic recipients (Fig. 1). Activation of AP-1 was observed in both allogeneic and syngeneic transplants and was therefore due to ischemia/reperfusion injury. Previous reports have demonstrated activation of AP-1 and NF-κB due to ischemia/reperfusion injury and activation of NF-κB due to allogeneic transplantation (10, 14, 81).
FIG. 2.
Putative transcription factor binding sites in the MCMV and HCMV ie1 promoter/enhancer regions. Binding sites in the MCMV promoter/enhancer are based on analysis of the nucleotide sequence (20). Binding sites in the HCMV promoter/enhancer are based on previous analyses (41).
FIG. 3.
Effect of transplantation on activation of NF-κB and AP-1. Nuclear extracts from transplanted (T) and contralateral control kidneys (C) were analyzed for activation of NF-κB and AP-1 by electrophoretic mobility shift assay. Kidneys were transplanted into allogeneic (lanes 4 and 8) or syngeneic (lanes 2 and 6) recipients and were removed 1 day (lanes 2 and 4) or 2 days (lanes 6 and 8) after transplant. Results shown are representative of two sets of experiments. S, syngeneic transplant; A, allogeneic transplant.
Expression of inflammatory cytokines is induced by allogeneic transplantation.
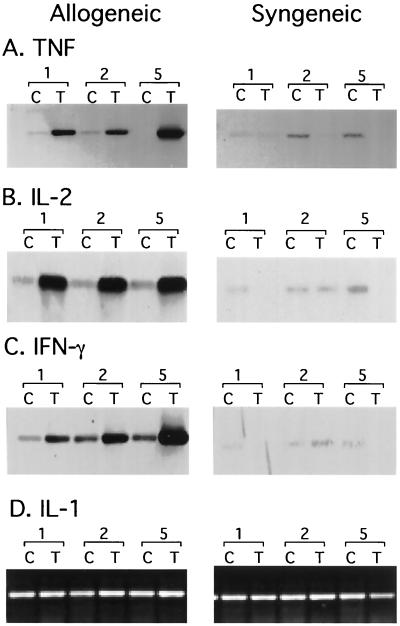
NF-κB is activated by several different mediators of the immune and inflammatory response, including the proinflammatory cytokines IL-1 and TNF. Allogeneic stimulation results in activation of TH1 cells and release of inflammatory cytokines. We therefore analyzed RNAs from transplanted kidneys for expression of TNF, IL-2, IFN-γ, and IL-1 (Fig. 4). Expression of IL-1 was present in control kidneys and was not significantly induced in allogeneic or syngeneic transplants. In contrast, little or no expression of TNF, IL-2, and IFN-γ was observed in control kidneys. Expression of these cytokines was specifically induced after 24 h in allogeneic but not in syngeneic transplants. Induction of ie1 gene expression was observed 24 to 48 h after allogeneic transplantation. Thus, expression of these cytokines was induced prior to or coincident with induction of ie1 gene expression. These data are consistent with the hypothesis that inflammatory cytokines activate transcription factors which induce expression of the ie1 gene. TNF is known to induce activation of both NF-κB and AP-1 (42, 52, 65, 73), and TNF has been shown to induce expression of HCMV ie promoter-driven reporter constructs in vitro via activation of NF-κB (54, 67). These observations suggest that TNF could mediate induction of MCMV ie1 gene expression in vivo in response to allogeneic transplantation.
FIG. 4.
Effect of transplantation on expression of inflammatory cytokines. Kidneys were transplanted (lanes T) into allogeneic or syngeneic mice and were removed 1, 2, and 5 days after transplant. The contralateral kidney was removed at the time of transplant and analyzed as a control (lanes C). RNAs were analyzed for expression of TNF, IL-2, IFN-γ, or IL-1 by RT-PCR. PCR products were detected by agarose gel electrophoresis (D) or by Southern blot hybridization (A to C). Induction of TNF expression was observed in six of six allogeneic and zero of six syngeneic transplants.
TNF induces MCMV ie1 gene expression in the lung.
In order to test this hypothesis directly, latently infected mice were injected with recombinant murine TNF and analyzed for ie1 gene expression at various times after injection. Induction of IE1 RNA was consistently observed in lung tissue from latently infected mice 8 to 24 h after injection of TNF (Fig. 5), although the kinetics of induction varied somewhat. No expression of IE1 RNA was detected in the lung at 48 h after injection, and no induction of E-1 or gB transcripts was observed at any time point (data not shown). This is likely to be due to immune destruction of cells expressing IE1 protein in these immunocompetent mice with immunological memory of MCMV. Surprisingly, no induction of IE1 transcripts was observed in kidney, heart, liver, or spleen from latently infected mice injected with TNF.
FIG. 5.
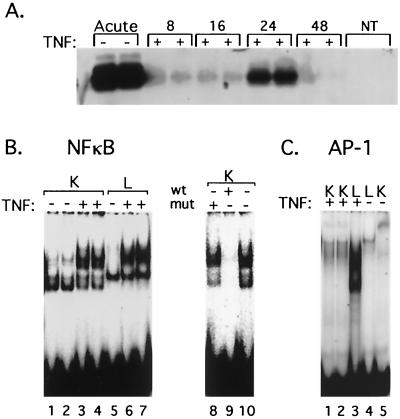
Effect of TNF on MCMV ie1 gene expression and transcription factor activation in lung and kidney tissue of latently infected mice. (A) RT-PCR analysis of IE1 RNA expression in lungs of latently infected mice at 8, 16, 24, and 48 h after injection with TNF. RNA from productively infected murine embryo fibroblasts (Acute) was analyzed as a positive control. NT, no template. (B and C) Electrophoretic mobility shift assay analysis of activation of NF-κB and AP-1 in lung (L) and kidney (K) extracts 2 h after injection with TNF (+) or with phosphate-buffered saline (−). Results shown are representative of two sets of experiments. Competition assays (lanes 8 to 10) were performed by incubating extracts with excess unlabeled oligonucleotides containing mutant (lane 8) or wild-type NF-κB binding sites (lane 9) or no competitor (lane 10) with kidney extracts from mice injected with TNF. wt, wild type; mut, mutant.
Gel shift analysis of nuclear extracts of lung and kidney tissue from mice injected with TNF was performed to identify the transcription factors activated by TNF (Fig. 5). As was the case with transplanted kidneys (Fig. 3), two complexes with NF-κB-binding activity were observed. Both complexes bound specifically to the consensus NF-κB binding site, since binding was abolished by excess unlabeled NF-κB oligonucleotide (Fig. 5B, lane 9) but not by a mutant NF-κB oligonucleotide (Fig. 5B, lane 8). Supershift analysis demonstrated that the more slowly migrating complex contained p65 and p50 (data not shown) subunits of NF-κB, while the more rapidly migrating complex was not recognized by antibodies specific for any NF-κB subunit, including p65, p50, p52, c-Rel, and RelB. Injection with TNF induced significant activation of NF-κB in both the lung and the kidney (Fig. 5B). The mobility of the NF-κB complex appeared to be slightly different in kidney and lung extracts, suggesting that the composition of the complex could be different. However, supershift analysis indicated no obvious differences in the compositions of the lung and kidney complexes (data not shown). In contrast, examination of AP-1 activation demonstrated that TNF activated AP-1 in the lung but not in the kidney (Fig. 5C). The mobility of this complex was significantly greater than that present in transplanted kidneys, suggesting that the composition of the AP-1 complex could differ between lung and kidney. Thus, our gel shift analysis indicates that induction of ie1 gene expression correlates with activation of both NF-κB and AP-1, suggesting that NF-κB and AP-1 may act cooperatively to induce MCMV ie1 gene expression. TNF can substitute for allogeneic transplantation in inducing MCMV ie1 gene expression in tissues where both AP-1 and NF-κB are activated.
TNF and allogeneic transplantation induce HCMV ie gene expression.
Both the HCMV and MCMV ie promoter/enhancer regions contain multiple NF-κB consensus binding sites (20, 32, 60, 68, 72). Previous studies have demonstrated that HCMV ie promoter-driven reporter constructs are induced by TNF in vitro by activation of NF-κB (54, 67). To investigate the response of the HCMV ie promoter/enhancer region to TNF and allogeneic transplantation, we used MIEP-lacZ transgenic mice carrying a β-galactosidase reporter gene under the control of the HCMV ie promoter/enhancer (4). As reported previously, expression of the transgene was observed in the kidneys of untreated transgenic mice (4). Injection with TNF induced a higher level of expression of the transgene (Fig. 6A). The extent of induction was determined using a quantitative chemiluminescence assay for β-galactosidase activity. Injection with TNF resulted in a statistically significant induction in β-galactosidase activity which is more than twofold higher than that found in controls (P = 0.01) (Fig. 6B). Preliminary studies with injection of IL-1, which also induces activation of NF-κB, and with IFN-γ, which activates JAK/STAT signaling, did not show any induction of the transgene and were not pursued further.
FIG. 6.
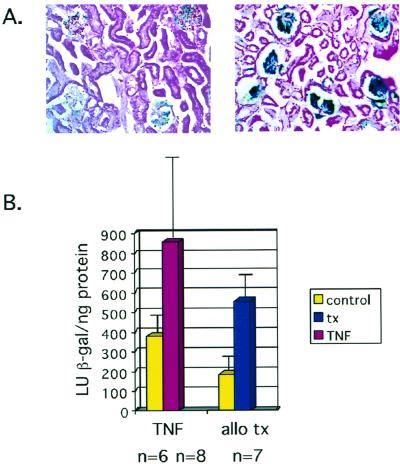
Effect of TNF and transplantation on HCMV ie1 gene expression in vivo. (A) Histochemical analysis of expression of the β-galactosidase transgene under the control of the HCMV major ie promoter in the kidneys of control (left) and TNF-injected (right) MIEP-lacZ mice. (B) Quantitative chemiluminescence analysis of β-galactosidase activity in kidneys of control mice and mice injected with TNF, in kidneys transplanted into allogeneic recipients, and in contralateral control kidneys. Values shown are average light units per nanogram of protein plus standard deviation. TNF (P = 0.01, two-tailed t test) and allogeneic transplantation (P = 0.001, paired t test) induce a statistically significant increase in β-galactosidase activity over that of controls. allo tx, allogeneic transplant.
In order to investigate the response of the HCMV ie promoter/enhancer to allogeneic stimulation, kidneys from transgenic mice were transplanted into allogeneic C3H/HeSnJ (H-2k) recipients, and the level of β-galactosidase activity was compared with that found in the control contralateral kidney. Because the transgenic mice were derived by injecting the transgene into the ova of mice heterozygous at the H2 locus, it was not possible to perform syngeneic transplants with these mice. However, it is clear from a comparison of the transplanted and contralateral control kidneys that allogeneic transplantation resulted in a statistically significant induction of ie promoter/enhancer activity of approximately threefold at 2 days posttransplant (P = 0.001). While differences in the context of a gene may influence its expression, the results with the MIEP-lacZ transgenic mice indicate that both TNF and allogeneic transplantation induce HCMV ie gene expression. These results are consistent with our studies of latently MCMV-infected mice, in which the ie promoter/enhancer is in its natural context in the viral genome, and with previous studies of regulation of the HCMV ie promoter/enhancer (54, 67).
DISCUSSION
Reactivation of CMV from latency is a frequent complication of organ transplantation, resulting in significant morbidity and mortality. The molecular mechanism by which this occurs is largely unknown. Because the ie1 gene either is not expressed or is expressed at very low levels in latently infected cells (5, 34, 37, 49, 63, 71) and ie1 expression is required to initiate viral replication, induction of ie1 gene expression may be a crucial first step in the reactivation process. In this report we have demonstrated that allogeneic transplantation of kidneys induces expression of the MCMV ie1 gene. This induction occurs only in allogeneic and not syngeneic transplants, indicating that the induction is due to the allogeneic response rather than to ischemia/reperfusion injury. Some IE1 RNA expression was observed in some of the latently infected controls. CMV is known to reactivate periodically, and the observed expression of IE1 RNA may reflect the foci of nascent reactivation observed by others (37). Regardless of whether IE1 RNA was expressed in latently infected mice, it is clear from comparison of RNA expression in the transplanted and contralateral control kidneys that allogeneic transplantation induces MCMV ie1 gene expression. Using transgenic mice harboring the β-galactosidase gene under the control of the HCMV ie promoter/enhancer, we find that allogeneic transplantation also induces HCMV ie gene expression.
Expression of the MCMV and HCMV ie genes is controlled by the ie promoter/enhancer region (8, 20, 68, 72). The HCMV ie promoter/enhancer consists of repeats of 18-, 19-, 16-, and 21-bp motifs. The 18-, 19-, and 21-bp elements contain binding sites for NF-κB, ATF(CREB), and Sp1, respectively, and the NF-κB and ATF(CREB) sites have been demonstrated to play an important role in regulation of ie gene expression (32, 41, 60, 68). The transcription factors binding to sites in the MCMV ie promoter/enhancer and the sites important in regulating MCMV ie gene expression have not been studied. The MCMV promoter/enhancer (20) has five sites matching the AP-1 consensus site TGANT(C/A)A (39, 40), four AP-1 sites juxtaposed to classic NF-κB sites (GGGRNNYYCC) (2), and an AP-1 site paired with an inverted NF-κB site. While the HCMV promoter/enhancer (8, 72) has only one AP-1 site, it has paired NF-κB/ATF(CREB) sites and single ATF(CREB) sites matching the consensus sequence TGACGTCA (41). AP-1 and ATF(CREB) are a family of related transcription factors which share subunits and bind to similar sequences (28, 39, 43). These observations suggest that although the configuration of the transcription factor binding sites is somewhat different, regulation of the HCMV and MCMV ie genes may be similar.
NF-κB is a family of dimeric transcription factor complexes consisting of p50, p52, p65 (RelA), c-Rel, and RelB subunits. In normal tissues, NF-κB is inactive (6, 22, 44, 69, 81) because it is sequestered in the cytoplasm by the inhibitory subunit I-κB (reviewed in references 2 and 3). NF-κB is activated by exposure to lipopolysaccharide or inflammatory cytokines such as TNF or IL-1, viral infection, B- or T-cell activation, and oxidative stress. These diverse stimuli activate different signaling pathways which result in phosphorylation and degradation of I-κB, allowing free NF-κB to translocate to the nucleus and activate transcription of NF-κB-responsive genes. These include many of the genes involved in mediating immune and inflammatory responses, including proinflammatory cytokines, chemokines, adhesion molecules, inflammatory enzymes, and receptors (reviewed in reference 3).
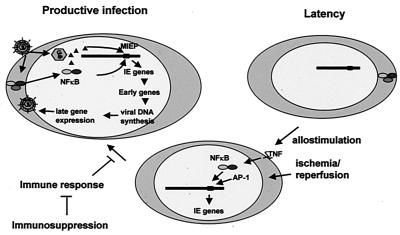
During productive HCMV infection, contact between the viral glycoproteins gB and gH and receptors on the cell surface induces activation of NF-κB (79). Activated NF-κB and pp71, the viral transactivator protein released from the virion, translocate to the nucleus and activate the major ie promoter (reviewed in reference 50). Viral components which activate NF-κB during productive infection are not present in latently infected cells. Our model (Fig. 7) postulates that in a transplant recipient, cellular factors activated as a result of allogeneic stimulation and ischemia/reperfusion injury substitute for these viral components in activating the transcription factors required for ie gene expression.
FIG. 7.
Model for reactivation of CMV from latency. See text for explanation.
Allogeneic transplantation induces expression of a complex array of genes involved in an inflammatory immune response, including cytokines which activate transcription factors which in turn induce expression of additional genes (10, 14, 15, 24, 56, 69, 81). Previous investigators have noted an association between TNF levels and reactivation of CMV in transplant recipients and suggested that TNF may play an important role in reactivation by inducing ie gene expression through activation of NF-κB (19, 23, 54, 55, 67). We have demonstrated that allogeneic transplantation induces expression of TNF and ie1 and activation of NF-κB in the kidney and that the kinetics of induction are consistent with the hypothesis that TNF mediates induction of ie gene expression through activation of NF-κB in vivo. In contrast, other cytokines which are induced by allogeneic transplantation, such as IFN-γ and IL-2, activate other signaling pathways and are unlikely to play a role in inducing CMV ie gene expression. IL-1, which can induce activation of NF-κB, is not significantly induced by allogeneic transplantation. Preliminary investigation of the effects of IL-1 and IFN-γ on MIEP-lacZ transgene expression showed no induction (data not shown). Thus, our model postulates that TNF plays an important role in mediating induction of ie gene expression in an allogeneic transplant.
We have tested the ability of TNF to induce ie gene expression in vivo by injecting latently MCMV-infected mice and MIEP-lacZ transgenic mice with TNF. We find that TNF alone is able to induce expression of the MCMV ie1 gene in the lungs of latently infected mice, although it appears to be insufficient to induce expression of IE1 RNA in other tissues. Gel shift analysis of the transcription factors activated by allogeneic transplantation and by TNF revealed that allogeneic kidney transplantation activated AP-1 as well as NF-κB. Both of these transcription factors were also activated by TNF in the lung, but only NFκB was activated by TNF in the kidney. The MCMV ie promoter/enhancer has multiple repeats of AP-1 and NF-κB binding sites separated by 3 nucleotides. This suggests that AP-1 and NF-κB could act cooperatively to control ie gene expression. Synergistic interactions between NF-κB and AP-1 have been previously demonstrated (45, 66). Taken together, our results suggest that activation of both AP-1 and NF-κB is required for induction of the MCMV ie promoter/enhancer and that activation of AP-1 in the kidney occurs at least in part as a result of ischemia/reperfusion injury and is not mediated by TNF. In contrast, TNF alone was sufficient to induce expression of the HCMV ie promoter/enhancer in the kidneys of MIEP-lacZ transgenic mice. These results are consistent with previous reports that TNF induces expression of the HCMV ie gene expression in vitro (54, 67). Gel shift analysis of extracts from MIEP-lacZ transgenic mice injected with TNF demonstrated that as is the case with BALB/c mice latently infected with MCMV, TNF induces activation of both NF-κB and AP-1 in the lung but of NF-κB alone in the kidney (data not shown). The differences in the response of the transgene and the MCMV ie1 gene to TNF in the kidney are therefore likely to reflect differences in the promoters of these genes rather than differences in the strains of mice in TNF-mediated signal transduction.
Thus, our results are consistent with a model for reactivation from latency in which TNF released as a result of allostimulation activates the transcription factors which drive ie gene expression, which in turn activates lytic replication of the virus (Fig. 7). These studies will provide the framework for future studies using genetically deficient strains of mice to further define the components required for this induction.
Although we observed induction of ie1 gene expression in transplants of mice latently infected with MCMV, expression of IE3, an alternatively spliced transcript initiated at the ie1 promoter, was not observed. This may be due to differences in sensitivity of detection. Alternatively, some investigators have suggested that posttranscriptional regulation of ie3 (or ie2 in the case of HCMV) may play an important role in inducing reactivation (38, 71). Expression of genes representative of later phases of MCMV replication, E-1 and gB, was also not observed. This is likely due to immune destruction of cells expressing foreign antigens. Complete reactivation of HCMV with production of infectious virus can be achieved in vitro by allogeneic stimulation of latently infected peripheral blood mononuclear cells (64). Reactivation of infectious virus has also been demonstrated in animal models by allogeneic transplantation or cell transfer combined with immunosuppression (11, 12, 47, 61, 76). Thus, it is likely that reactivation is a multistep process which is initiated by induction of ie gene expression and that immunosuppression is required to achieve full lytic replication in vivo. Further studies will be required to determine the roles of the immune response and additional factors in achieving complete reactivation in vivo.
The ability to establish a lifelong latent infection and to reactivate periodically is a characteristic common to all herpesviruses. Presumably, this ability has evolved because it confers a selective advantage to the virus (58). TNF is released by activated T cells and macrophages as part of the inflammatory response to infection. Reactivation of viral replication in response to inflammation would allow the virus to escape from a host which might succumb to other infections and thus would be expected to have an important survival advantage. Furthermore, a host suffering from acute infection is likely to be immunocompromised, and reactivation under these circumstances would favor virus escape over immune destruction. The observation that reactivation of CMV is associated with sepsis (19) as well as with allogeneic transplantation supports the hypothesis that the natural stimulus for reactivation is an inflammatory immune response and that allogeneic transplantation mimics this process. The observations that reactivation of Epstein-Barr virus occurs as a result of B-cell activation (which would occur during infection) and that infection or tissue damage (75) induces reactivation of herpes simplex virus (hence the terms “cold sore” and “fever blister”) suggest that reactivation in response to inflammatory mediators may be a more general strategy which has contributed to the success of herpesviruses as pathogens.
ACKNOWLEDGMENTS
This work is supported by NIH grant R01AI42898–02 to M.I.A.
We thank the members of the Transplant Division for advice and support, Jay Nelson for MIEP-lacZ transgenic mice, Dave Ivancic for help with histology, Laimonis Laimins for luminometer use, Sarah Martin for early analysis of transgenic mice, Patricia Spear and Richard Longnecker for advice and critical review of the manuscript, and Wei Hsueh for support and use of laboratory space.
REFERENCES
- 1.Abecassis M M, Jiang X, O'Neil M E, Bale J F., Jr Detection of murine cytomegalovirus (MCMV) DNA in skin using the polymerase chain reaction (PCR) Microb Pathog. 1993;15:17–22. doi: 10.1006/mpat.1993.1053. [DOI] [PubMed] [Google Scholar]
- 2.Baldwin A S. The NF-kB and IkB proteins: new discoveries and insights. Annu Rev Immunol. 1996;14:649–681. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- 3.Barnes P J, Karin M. Nuclear factor-kappaB: a pivotal transcription factor in chronic inflammatory diseases. N Engl J Med. 1997;336:1066–1071. doi: 10.1056/NEJM199704103361506. [DOI] [PubMed] [Google Scholar]
- 4.Baskar J F, Smith P P, Nilaver G, Jupp R A, Hoffmann S, Peffer N J, Tenney D J, Colberg-Poley A M, Ghazal P, Nelson J A. The enhancer domain of the human cytomegalovirus major immediate-early promoter determines cell type-specific expression in transgenic mice. J Virol. 1996;70:3207–3214. doi: 10.1128/jvi.70.5.3207-3214.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bevan I S, Sammons C C, Sweet C. Investigation of murine cytomegalovirus latency and reactivation in mice using viral mutants and the polymerase chain reaction. J Med Virol. 1996;48:308–320. doi: 10.1002/(SICI)1096-9071(199604)48:4<308::AID-JMV3>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 6.Blackwell T S, Blackwell T R, Holden E P, Christman B W, Christman J W. In vivo antioxidant treatment suppresses nuclear factor-kappa B activation and neutrophilic lung inflammation. J Immunol. 1996;157:1630–1637. [PubMed] [Google Scholar]
- 7.Book B, Pescovitz M, Leapman S, Filo R. Polyclonal antilymphocyte sera induce immune activation in human renal allograft recipients. Transplant Proc. 1998;30:1348–1350. doi: 10.1016/s0041-1345(98)00271-1. [DOI] [PubMed] [Google Scholar]
- 8.Boshart M, Weber F, Jahn G, Dorsch-Hasler K, Fleckenstein B, Schaffner W. A very strong enhancer is located upstream of an immediate early gene of human cytomegalovirus. Cell. 1985;41:521–530. doi: 10.1016/s0092-8674(85)80025-8. [DOI] [PubMed] [Google Scholar]
- 9.Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 10.Bradham C A, Stachlewitz R F, Gao W, Qian T, Jayadev S, Jenkins G, Hannun Y, Lemasters J J, Thurman R G, Brenner D A. Reperfusion after liver transplantation in rats differentially activates the mitogen-activated protein kinases. Hepatology. 1997;25:1128–1135. doi: 10.1002/hep.510250514. [DOI] [PubMed] [Google Scholar]
- 11.Bruning J H, Bruggeman C A, van Boven C P, van Breda Vriesman P J. Passive transfer of cytomegalovirus by cardiac and renal organ transplants in a rat model. Transplantation. 1986;41:695–698. doi: 10.1097/00007890-198606000-00005. [DOI] [PubMed] [Google Scholar]
- 12.Bruning J H, Bruggeman C A, van Breda Vriesman P J. The transfer of cytomegalovirus infection in rats by latently infected renal allografts, and the role of various immunosuppressive regimens in virus reactivation. Transplantation. 1988;46:623–624. doi: 10.1097/00007890-198810000-00041. [DOI] [PubMed] [Google Scholar]
- 13.Cheung K S, Lang D J. Transmission and activation of cytomegalovirus with blood transfusion: a mouse model. J Infect Dis. 1977;135:841–845. doi: 10.1093/infdis/135.5.841. [DOI] [PubMed] [Google Scholar]
- 14.Cooper M, Lindholm P, Pieper G, Seibel R, Moore G, Nakanishi A, Dembny K, Komorowski R, Johnson C, Adams M, Roza A. Myocardial nuclear factor-kappaB activity and nitric oxide production in rejecting cardiac allografts. Transplantation. 1998;66:838–844. doi: 10.1097/00007890-199810150-00005. [DOI] [PubMed] [Google Scholar]
- 15.Dallman J M, Larsen C P, Morris P J. Cytokine gene transcription in vascularised organ grafts: analysis using semiquantitative polymerase chain reaction. J Exp Med. 1991;174:493–496. doi: 10.1084/jem.174.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Debets J M, Leunissen K M, Hooff H J van C, van der Linden J, Buurman W A. Evidence of involvement of tumor necrosis factor in adverse reactions during treatment of kidney allograft rejection with antithymocyte globulin. Transplantation. 1989;47:487–492. doi: 10.1097/00007890-198903000-00018. [DOI] [PubMed] [Google Scholar]
- 17.De Plaen I G, Tan X D, Chang H, Qu X W, Liu Q P, Hsueh W. Intestinal NF-kappaB is activated, mainly as p50 homodimers, by platelet-activating factor. Biochim Biophys Acta. 1998;1392:185–192. doi: 10.1016/s0005-2760(98)00024-1. [DOI] [PubMed] [Google Scholar]
- 18.Deryckere F, Gannon F. A one-hour minipreparation technique for extraction of DNA-binding proteins from animal tissues. BioTechniques. 1994;16:405. [PubMed] [Google Scholar]
- 19.Docke W D, Prosch S, Fietze E, Kimel V, Zuckermann H, Klug C, Syrbe U, Kruger D H, von Baehr R, Volk H D. Cytomegalovirus reactivation and tumour necrosis factor. Lancet. 1994;343:268–269. doi: 10.1016/s0140-6736(94)91116-9. [DOI] [PubMed] [Google Scholar]
- 20.Dorsch-Hasler K, Keil G M, Weber F, Jasin M, Schaffner W, Koszinowski U H. A long and complex enhancer activates transcription of the gene coding for the highly abundant immediate early mRNA in murine cytomegalovirus. Proc Natl Acad Sci USA. 1985;82:8325–8329. doi: 10.1073/pnas.82.24.8325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dowling J N, Wu B C, Armstrong J A, Ho M. Enhancement of murine cytomegalovirus infection during graft-vs.-host reaction. J Infect Dis. 1977;135:990–994. doi: 10.1093/infdis/135.6.990. [DOI] [PubMed] [Google Scholar]
- 22.Essani N A, McGuire G M, Manning A M, Jaeschke H. Endotoxin-induced activation of the nuclear transcription factor kappa B and expression of E-selectin messenger RNA in hepatocytes, Kupffer cells, and endothelial cells in vivo. J Immunol. 1996;156:2956–2963. [PubMed] [Google Scholar]
- 23.Fietze E, Prosch S, Reinke P, Stein J, Docke W D, Staffa G, Loning S, Devaux S, Emmrich F, von Baehr R. Cytomegalovirus infection in transplant recipients. The role of tumor necrosis factor. Transplantation. 1994;58:675–680. [PubMed] [Google Scholar]
- 24.Gao W, Topham P S, King J A, Smiley S T, Csizmadia V, Lu B, Gerard C J, Hancock W W. Targeting of the chemokine receptor CCR1 suppresses development of acute and chronic cardiac allograft rejection. J Clin Investig. 2000;105:35–44. doi: 10.1172/JCI8126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grant S C, Lamb W R, Brooks N H, Brenchley P E, Hutchinson I V. Serum cytokines in human heart transplant recipients. Is there a relationship to rejection? Transplantation. 1996;62:480–491. doi: 10.1097/00007890-199608270-00010. [DOI] [PubMed] [Google Scholar]
- 26.Guttmann R D, Caudrelier P, Alberici G, Touraine J L. Pharmacokinetics, foreign protein immune response, cytokine release, and lymphocyte subsets in patients receiving thymoglobuline and immunosuppression. Transplant Proc. 1997;29:24S–26S. [PubMed] [Google Scholar]
- 27.Hahn G, Jores R, Mocarski E S. Cytomegalovirus remains latent in a common precursor of dendritic and myeloid cells. Proc Natl Acad Sci USA. 1998;95:3937–3942. doi: 10.1073/pnas.95.7.3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hai T W, Liu F, Allegretto E A, Karin M, Green M R. A family of immunologically related transcription factors that includes multiple forms of ATF and AP-1. Genes Dev. 1988;2:1216–1226. doi: 10.1101/gad.2.10.1216. [DOI] [PubMed] [Google Scholar]
- 29.Hamilton J D, Seaworth B J. Transmission of latent cytomegalovirus in a murine kidney tissue transplantation model. Transplantation. 1985;39:290–296. doi: 10.1097/00007890-198503000-00017. [DOI] [PubMed] [Google Scholar]
- 30.Henry S C, Hamilton J D. Detection of murine cytomegalovirus immediate early 1 transcripts in the spleens of latently infected mice. J Infect Dis. 1993;167:950–954. doi: 10.1093/infdis/167.4.950. [DOI] [PubMed] [Google Scholar]
- 31.Hibberd P L, Tolkoff-Rubin N E, Cosimi A B, Schooley R T, Isaacson D, Doran M, Delvecchio A, Delmonico F L, Auchincloss H, Jr, Rubin R H. Symptomatic cytomegalovirus disease in the cytomegalovirus antibody seropositive renal transplant recipient treated with OKT3. Transplantation. 1992;53:68–72. doi: 10.1097/00007890-199201000-00013. [DOI] [PubMed] [Google Scholar]
- 32.Hunninghake G W, Monick M M, Liu B, Stinski M F. The promoter-regulatory region of the major immediate-early gene of human cytomegalovirus responds to T-lymphocyte stimulation and contains functional cyclic AMP-response elements. J Virol. 1989;63:3026–3033. doi: 10.1128/jvi.63.7.3026-3033.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kita M, Tanaka K, Shinmura K, Tanaka Y, Liu Y, Imanishi J. Expression des genes des cytokines et des genes associés a l'interferon chez l'embryon de la souris. C R Séances Soc Biol Fil. 1994;188:593–600. [PubMed] [Google Scholar]
- 34.Koffron A J, Hummel M, Patterson B K, Yan S, Kaufman D B, Fryer J P, Stuart F P, Abecassis M I. Cellular localization of latent murine cytomegalovirus. J Virol. 1998;72:95–103. doi: 10.1128/jvi.72.1.95-103.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kondo K, Xu J, Mocarski E S. Human cytomegalovirus latent gene expression in granulocyte-macrophage progenitors in culture and in seropositive individuals. Proc Natl Acad Sci USA. 1996;93:11137–11142. doi: 10.1073/pnas.93.20.11137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kurz S, Steffens H P, Mayer A, Harris J R, Reddehase M J. Latency versus persistence or intermittent recurrences: evidence for a latent state of murine cytomegalovirus in the lungs. J Virol. 1997;71:2980–2987. doi: 10.1128/jvi.71.4.2980-2987.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kurz S K, Rapp M, Steffens H P, Grzimek N K, Schmalz S, Reddehase M J. Focal transcriptional activity of murine cytomegalovirus during latency in the lungs. J Virol. 1999;73:482–494. doi: 10.1128/jvi.73.1.482-494.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kurz S K, Reddehase M J. Patchwork pattern of transcriptional reactivation in the lungs indicates sequential checkpoints in the transition from murine cytomegalovirus latency to recurrence. J Virol. 1999;73:8612–8622. doi: 10.1128/jvi.73.10.8612-8622.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee W, Haslinger A, Karin M, Tjian R. Activation of transcription by two factors that bind promoter and enhancer sequences of the human metallothionein gene and SV40. Nature. 1987;325:368–372. doi: 10.1038/325368a0. [DOI] [PubMed] [Google Scholar]
- 40.Lee W, Mitchell P, Tjian R. Purified transcription factor AP-1 interacts with TPA-inducible enhancer elements. Cell. 1987;49:741–752. doi: 10.1016/0092-8674(87)90612-x. [DOI] [PubMed] [Google Scholar]
- 41.Liu B, Stinski M F. Human cytomegalovirus contains a tegument protein that enhances transcription from promoters with upstream ATF and AP-1 cis-acting elements. J Virol. 1992;66:4434–4444. doi: 10.1128/jvi.66.7.4434-4444.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu Z G, Hsu H, Goeddel D V, Karin M. Dissection of TNF receptor 1 effector functions: JNK activation is not linked to apoptosis while NF-kappaB activation prevents cell death. Cell. 1996;87:565–576. doi: 10.1016/s0092-8674(00)81375-6. [DOI] [PubMed] [Google Scholar]
- 43.Macgregor P F, Abate C, Curran T. Direct cloning of leucine zipper proteins: Jun binds cooperatively to the CRE with CRE-BP1. Oncogene. 1990;5:451–458. [PubMed] [Google Scholar]
- 44.Manning A M, Bell F P, Rosenbloom C L, Chosay J G, Simmons C A, Northrup J L, Shebuski R J, Dunn C J, Anderson D C. NF-kappa B is activated during acute inflammation in vivo in association with elevated endothelial cell adhesion molecule gene expression and leukocyte recruitment. J Inflamm. 1995;45:283–296. [PubMed] [Google Scholar]
- 45.Martin T, Cardarelli P M, Parry G C, Felts K A, Cobb R R. Cytokine induction of monocyte chemoattractant protein-1 gene expression in human endothelial cells depends on the cooperative action of NF-kappa B and AP-1. Eur J Immunol. 1997;27:1091–1097. doi: 10.1002/eji.1830270508. [DOI] [PubMed] [Google Scholar]
- 46.Matthes-Martin S, Aberle S W, Peters C, Holter W, Popow-Kraupp T, Potschger U, Fritsch G, Ladenstein R, Rosenmayer A, Dieckmann K, Gadner H. CMV-viraemia during allogenic bone marrow transplantation in paediatric patients: association with survival and graft-versus-host disease. Bone Marrow Transplant. 1998;21(Suppl. 2):S53–S56. [PubMed] [Google Scholar]
- 47.Mayo D, Armstrong J A, Ho M. Activation of latent murine cytomegalovirus infection: cocultivation, cell transfer, and the effect of immunosuppression. J Infect Dis. 1978;138:890–896. doi: 10.1093/infdis/138.6.890. [DOI] [PubMed] [Google Scholar]
- 48.Mayo D R, Armstrong J A, Ho M. Reactivation of murine cytomegalovirus by cyclophosphamide. Nature. 1977;267:721–723. doi: 10.1038/267721a0. [DOI] [PubMed] [Google Scholar]
- 49.Mendelson M, Monard S, Sissons P, Sinclair J. Detection of endogenous human cytomegalovirus in CD34+ bone marrow progenitors. J Gen Virol. 1996;77:3099–3102. doi: 10.1099/0022-1317-77-12-3099. [DOI] [PubMed] [Google Scholar]
- 50.Mocarski E S. Cytomegaloviruses and their replication. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. 3rd ed. Vol. 2. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 2447–2492. [Google Scholar]
- 51.Mutimer D, Mirza D, Shaw J, O'Donnell K, Elias E. Enhanced (cytomegalovirus) viral replication associated with septic bacterial complications in liver transplant recipients. Transplantation. 1997;63:1411–1415. doi: 10.1097/00007890-199705270-00007. [DOI] [PubMed] [Google Scholar]
- 52.Natoli G, Costanzo A, Ianni A, Templeton D J, Woodgett J R, Balsano C, Levrero M. Activation of SAPK/JNK by TNF receptor 1 through a noncytotoxic TRAF2-dependent pathway. Science. 1997;275:200–203. doi: 10.1126/science.275.5297.200. [DOI] [PubMed] [Google Scholar]
- 53.Polic B, Hengel H, Krmpotic A, Trgovcich J, Pavic I, Luccaronin P, Jonjic S, Koszinowski U H. Hierarchical and redundant lymphocyte subset control precludes cytomegalovirus replication during latent infection. J Exp Med. 1998;188:1047–1054. doi: 10.1084/jem.188.6.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Prosch S, Staak K, Stein J, Liebenthal C, Stamminger T, Volk H-D, Kruger D. Stimulation of the human cytomegalovirus IE enhancer/promoter in HL-60 cells by TNF alpha is mediated via induction of NF-kB. Virology. 1995;208:197–206. doi: 10.1006/viro.1995.1143. [DOI] [PubMed] [Google Scholar]
- 55.Prosch S, Volk H-D, Reinke P, Pioch K, Docke W-D, Kruger D H. Human cytomegalovirus infection in transplant recipients: role of TNF-alpha for reactivation and replication of human cytomegalovirus. In: Scholz M, Rabenau H F, Doerr H W, Cinatl J, editors. CMV-related immunopathology. Vol. 21. Basel, Switzerland: Karger; 1998. pp. 29–41. [Google Scholar]
- 56.Quan D, Grant D R, Zhong R Z, Zhang Z, Garcia B M, Jevnikar A M. Altered gene expression of cytokine, ICAM-1, and class II molecules precedes mouse intestinal allograft rejection. Transplantation. 1994;58:808–816. [PubMed] [Google Scholar]
- 57.Reiner S L, Zheng S, Corry D B, Locksley R M. Constructing polycompetitor cDNAs for quantitative PCR. J Immunol Methods. 1993;165:37–46. doi: 10.1016/0022-1759(93)90104-f. . (Errata, 173:133 and 175:275, 1994.) [DOI] [PubMed] [Google Scholar]
- 58.Roizman B, Sears A. Herpes simplex viruses and their replication. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. 3rd ed. Vol. 2. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 2231–2295. [Google Scholar]
- 59.Rubin R H. Impact of cytomegalovirus infection on organ transplant recipients. Rev Infect Dis. 1990;12(Suppl. 7):S754–S766. doi: 10.1093/clinids/12.supplement_7.s754. [DOI] [PubMed] [Google Scholar]
- 60.Sambucetti L C, Cherrington J M, Wilkinson G W, Mocarski E S. NF-kappa B activation of the cytomegalovirus enhancer is mediated by a viral transactivator and by T cell stimulation. EMBO J. 1989;8:4251–4258. doi: 10.1002/j.1460-2075.1989.tb08610.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schmader K E, Rahija R, Porter K R, Daley G, Hamilton J D. Aging and reactivation of latent murine cytomegalovirus. J Infect Dis. 1992;166:1403–1407. doi: 10.1093/infdis/166.6.1403. [DOI] [PubMed] [Google Scholar]
- 62.Sindre H, Tjoonnfjord G E, Rollag H, Ranneberg-Nilsen T, Veiby O P, Beck S, Degre M, Hestdal K. Human cytomegalovirus suppression of and latency in early hematopoietic progenitor cells. Blood. 1996;88:4526–4533. [PubMed] [Google Scholar]
- 63.Slobedman B, Mocarski E S. Quantitative analysis of latent human cytomegalovirus. J Virol. 1999;73:4806–4812. doi: 10.1128/jvi.73.6.4806-4812.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Soderberg-Naucler C, Fish K N, Nelson J A. Reactivation of latent human cytomegalovirus by allogeneic stimulation of blood cells from healthy donors. Cell. 1997;91:119–126. doi: 10.1016/s0092-8674(01)80014-3. [DOI] [PubMed] [Google Scholar]
- 65.Song H Y, Regnier C H, Kirschning C J, Goeddel D V, Rothe M. Tumor necrosis factor (TNF)-mediated kinase cascades: bifurcation of nuclear factor-kappaB and c-jun N-terminal kinase (JNK/SAPK) pathways at TNF receptor-associated factor 2. Proc Natl Acad Sci USA. 1997;94:9792–9796. doi: 10.1073/pnas.94.18.9792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stein B, Baldwin A S, Jr, Ballard D W, Greene W C, Angel P, Herrlich P. Cross-coupling of the NF-kappa B p65 and Fos/Jun transcription factors produces potentiated biological function. EMBO J. 1993;12:3879–3891. doi: 10.1002/j.1460-2075.1993.tb06066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Stein J, Volk H D, Liebenthal C, Kruger D H, Prosch S. Tumour necrosis factor alpha stimulates the activity of the human cytomegalovirus major immediate early enhancer/promoter in immature monocytic cells. J Gen Virol. 1993;74:2333–2338. doi: 10.1099/0022-1317-74-11-2333. [DOI] [PubMed] [Google Scholar]
- 68.Stinski F M, Roehr T J. Activation of the major immediate early gene of human cytomegalovirus by cis-acting elements in the promoter-regulatory sequence and by virus-specific trans-acting components. J Virol. 1985;55:431–441. doi: 10.1128/jvi.55.2.431-441.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tacchini L, Radice L, Pogliaghi G, Bernelli-Zazzera A. Differential activation of heat shock and nuclear factor kappaB transcription factors in postischemic reperfused rat liver. Hepatology. 1997;26:186–191. doi: 10.1053/jhep.1997.v26.pm0009214468. [DOI] [PubMed] [Google Scholar]
- 70.Taylor-Wiedeman J, Sissons J G, Borysiewicz L K, Sinclair J H. Monocytes are a major site of persistence of human cytomegalovirus in peripheral blood mononuclear cells. J Gen Virol. 1991;72:2059–2064. doi: 10.1099/0022-1317-72-9-2059. [DOI] [PubMed] [Google Scholar]
- 71.Taylor-Wiedeman J, Sissons P, Sinclair J. Induction of endogenous human cytomegalovirus gene expression after differentiation of monocytes from healthy carriers. J Virol. 1994;68:1597–1604. doi: 10.1128/jvi.68.3.1597-1604.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Thomsen D R, Stenberg R M, Goins W F, Stinski M F. Promoter-regulatory region of the major immediate early gene of human cytomegalovirus. Proc Natl Acad Sci USA. 1984;81:659–663. doi: 10.1073/pnas.81.3.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Westwick J K, Weitzel C, Minden A, Karin M, Brenner D A. Tumor necrosis factor alpha stimulates AP-1 activity through prolonged activation of the c-Jun kinase. J Biol Chem. 1994;269:26396–26401. [PubMed] [Google Scholar]
- 74.White K L, Slobedman B, Mocarski E S. Human cytomegalovirus latency-associated protein pORF94 is dispensable for productive and latent infection. J Virol. 2000;74:9333–9337. doi: 10.1128/jvi.74.19.9333-9337.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Whitley R J. Herpes simplex viruses. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. 3rd ed. Vol. 2. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 2297–2342. [Google Scholar]
- 76.Yagyu K, Takeshita M, Otsuka T, Kubota H, Furuse A, van Breda Vriesman P J. Reactivation of latent cytomegalovirus in allografts: comparison of lung graft and kidney graft in rats. Transplant Proc. 1994;26:2343–2344. [PubMed] [Google Scholar]
- 77.Yu Y, Henry S C, Xu F, Hamilton J D. Expression of a murine cytomegalovirus early-late protein in “latently” infected mice. J Infect Dis. 1995;172:371–379. doi: 10.1093/infdis/172.2.371. [DOI] [PubMed] [Google Scholar]
- 78.Yuhasz S A, Dissette V B, Cook M L, Stevens J G. Murine cytomegalovirus is present in both chronic active and latent states in persistently infected mice. Virology. 1994;202:272–280. doi: 10.1006/viro.1994.1343. [DOI] [PubMed] [Google Scholar]
- 79.Yurochko A D, Hwang E-S, Rasmussen L, Keay S, Pereira L, Huang E-S. The human cytomegalovirus UL55 (gB) and UL75 (gH) glycoprotein ligands initiate the rapid activation of Sp1 and NF-κB during infection. J Virol. 1997;71:5051–5059. doi: 10.1128/jvi.71.7.5051-5059.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhang Z, Schlachta C, Duff J, Stiller C, Grant D, Zhong R. Improved techniques for kidney transplantation in mice. Microsurgery. 1995;16:103–109. doi: 10.1002/micr.1920160212. [DOI] [PubMed] [Google Scholar]
- 81.Zwacka R M, Zhang Y, Zhou W, Halldorson J, Engelhardt J F. Ischemia/reperfusion injury in the liver of BALB/c mice activates AP-1 and nuclear factor kappaB independently of IkappaB degradation. Hepatology. 1998;28:1022–1030. doi: 10.1002/hep.510280417. [DOI] [PubMed] [Google Scholar]