Abstract
The E6 and E7 oncogenes of human papillomavirus type 16 (HPV-16) are sufficient for the immortalization of human genital keratinocytes in vitro. The products of these viral genes associate with p53 and pRb tumor suppressor proteins, respectively, and interfere with their normal growth-regulatory functions. The HPV-16 E6 protein has also been shown to increase the telomerase enzyme activity in primary epithelial cells by an unknown mechanism. We report here that a study using reverse transcription-PCR and RNase protection assays in transduced primary human foreskin keratinocytes (HFKs) shows that the E6 gene (but not the E7 gene) increases telomerase hTERT gene transcription coordinately with E6-induced telomerase activity. In these same cells, the E6 gene induces a 6.5-fold increase in the activity of a 1,165-bp 5′ promoter/regulatory region of the hTERT gene, and this induction is attributable to a minimal 251-bp sequence (−211 to +40). Furthermore, there is a 35-bp region (+5 to +40) within this minimal E6-responsive promoter that is responsible for 60% of E6 activity. Although the minimal hTERT promoter contains Myc-responsive E-box elements and recent studies have suggested a role for Myc protein in hTERT transcriptional control, we found no alterations in the abundance of either c-Myc or c-Mad in E6-transduced HFKs, suggesting that there are other or additional transcription factors critical for regulating hTERT expression.
The human papillomaviruses (HPVs) designated as “high risk” types, such as HPV type 16 (HPV-16) and HPV-18, are associated with anogenital tract lesions that can progress to malignancy (44, 45). The E6 and E7 viral genes appear to be responsible for both the in vivo and in vitro transforming activity of these high-risk viruses (24, 46), and each of these genes can independently transform established rodent cell lines (3, 29, 39). Interestingly, the E6 gene can independently immortalize primary human mammary epithelial cells in culture (2).
The transforming activities of the E6 and E7 viral gene products reside in their ability to interact specifically with cellular regulatory proteins and interfere with their normal functioning. The E7 protein interacts with pRb and abrogates its tumor-suppressive activity (8, 25), while the E6 protein cooperates with E6AP, a ubiquitin E3 ligase, to target p53 tumor suppressor protein for ubiquitin-dependent degradation (16, 31, 32, 42). Other less well characterized functions for E6 oncoprotein have been proposed (9, 18, 22), including the activation of telomerase (20), which is a ribonucleoprotein enzyme important for the maintenance of telomeric structures at the ends of chromosomes (10, 27).
Telomerase activity is detected in more than 90% of immortalized and cancer cells but absent in most normal somatic cells (17, 23), suggesting that telomerase activation is an important event during the process of immortalization and malignant transformation. The absence of telomerase activity in normal cells results in progressive telomere erosion with each cell cycle due to incomplete end replication of linear DNA (13, 41), which ultimately leads to chromosomal instability and cellular senescence. Thus, telomere shortening is thought to represent the “mitotic clock” that determines normal cellular life span.
Telomerase activity is closely associated with the expression of the telomerase catalytic subunit, hTERT. The expression of hTERT RNA is detected at high levels in tumor tissues and tumor-derived cell lines but not in normal adjacent tissues or primary cells (30, 38). Ectopic expression of hTERT in telomerase-negative cells restores telomerase activity in these cells as well as extending their life span (5, 7). Introduction of a dominant-negative hTERT into cancer cells inhibits telomerase activity in these cells and limits their growth (12). These findings strongly suggest that hTERT is the rate-limiting determinant of enzymatic activity of human telomerase and that upregulation of hTERT might be a critical event in the development of human cancers. Recently, it has been shown that telomerase activity can be induced in primary human keratinocytes and mammary epithelial cells by oncogenic E6 viral protein expression (20). In this study, we investigated whether HPV-16 E6 protein could induce hTERT expression by transcriptional activation, thereby providing a mechanistic explanation for E6-mediated increases in telomerase activity.
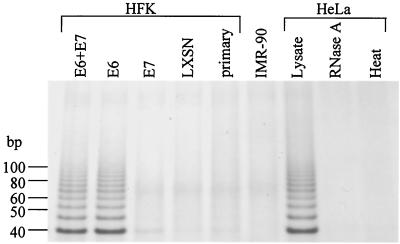
HPV-16 E6 protein increases telomerase activity in primary keratinocytes.
To demonstrate and verify that E6 induced cellular telomerase activity, we infected telomerase-negative, late-passage (passage 8 [P8]) human foreskin keratinocytes (HFKs) with a control LXSN retroviral vector or one expressing HPV-16 E6, E7, or the E6 plus E7 genes. The HFKs were cultured from neonatal foreskin explants as described previously (33), maintained in keratinocyte growth medium (Gibco-BRL), and, following retroviral infection, selected in G418 (100 μg/ml) for 5 days as previously described (35). Resistant clones were pooled and passaged at a ratio of 1:5. Telomerase activity was assayed in these HFKs (as well as positive- and negative-control cell lines) using a modified telomeric repeat amplification protocol (TRAP assay) (17, 35). Telomerase activity was present in the positive-control HeLa lysates (HPV-18-positive cervical adenocarcinoma cell line) (Fig. 1) and absent in the negative-control IMR-90 cell line (normal embryonic lung fibroblasts), which does not express hTERT message (17, 23). To demonstrate telomerase-specific activity, HeLa lysates were heat treated for 10 min at 95°C or digested with 2 μg of RNase A for 30 min at 37°C prior to TRAP analysis, and both treatments resulted in the total loss of detectable telomerase activity. Telomerase activity was also detected in HFKs expressing E6 protein alone or together with E7 protein but absent in noninfected primary HFKs (P8) or HFKs containing empty vector or expressing E7 alone (Fig. 1). These results establish that our HFKs do indeed overexpress hTERT activity and confirm that the E6 protein, but not the E7 protein, mediates this increase (20, 35).
FIG. 1.
Telomerase activity in HFKs transduced with HPV-16 E6, E7, or E6 plus E7 genes. Using a modified TRAP assay (17, 35), telomerase activity was detected in HFKs expressing E6 alone or E6 plus E7 but not in HFKs expressing E7 alone. Control HFKs (either nontransduced or transduced with vector LXSN) were also negative for telomerase activity. HeLa (HPV-18-positive cervical cancer line) and IMR-90 (normal embryonic lung fibroblasts) cells were used as telomerase-positive and -negative controls, respectively. HeLa lysates were treated with either RNase A or heat prior to the telomerase reaction to demonstrate telomerase-specific activity.
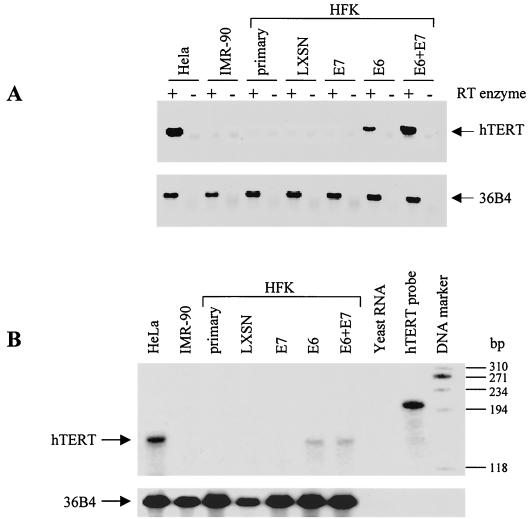
The E6 protein upregulates hTERT expression, which correlates with E6-induced activation of telomerase.
To determine if telomerase activity induced by E6 might reflect increased hTERT mRNA expression, we assayed for hTERT mRNA in the panel of keratinocytes used for Fig. 1, using both reverse transcription-PCR (RT-PCR) and RNase protection assays. Total cellular RNA was extracted from subconfluent cell cultures using TRIzol reagent (Gibco-BRL). Reverse transcription was performed on 5 μg of RNA using the Superscript preamplification system (Gibco-BRL), and the resulting cDNA was PCR amplified using hTERT- and 36B4-specific primer pairs as previously described (21, 26). hTERT mRNA was detected in HFKs expressing E6 alone or in combination with E7 (Fig. 2A). In contrast, primary HFKs and keratinocytes containing the empty vector control or E7 alone did not express hTERT message. Expression levels of the 36B4 gene were similar in all samples tested.
FIG. 2.
RT-PCR and RNase protection analyses for telomerase hTERT mRNA expression. The transduced keratinocytes described for Fig. 1 were evaluated as follows. (A) RT-PCR analysis was performed using the Superscript preamplification system (Gibco-BRL) and an hTERT-specific primer pair (26). hTERT RNA was observed only in HFKs expressing E6 alone or E6 plus E7. 36B4 primers were used to ensure cDNA integrity and to control for sample loading (21). (B) RNase protection analysis was performed on 40 μg of cellular RNA from the same cells as in panel A using radiolabeled hTERT- and 36B4-specific riboprobes and the RPA II kit (Ambion). Protected fragments are indicated by arrows. Yeast RNA was used to verify probe specificity. The 145-bp hTERT protected fragment was observed only in cells expressing E6 alone or E6 plus E7, confirming the results in panel A.
Since RT-PCR analysis is not necessarily quantitative, we used RNase protection analysis to validate and better quantitate the results shown in Fig. 2A. Radiolabeled hTERT and 36B4 riboprobes were generated using linearized expression plasmids and the Maxi-script in vitro transcription kit (Ambion, Austin, Tex.) according to the manufacturer's suggested protocol. The RNase protection assay was performed using the RPA II kit (Ambion). Briefly, hTERT and 36B4 riboprobes were separately precipitated with 40 μg of total cellular RNA in ethanol, hybridized overnight, and digested with RNase A and T1. Samples were analyzed on a 6% urea polyacrylamide gel. The hTERT probe produces an expected 145-bp protected fragment as shown in Fig. 2B. Protected fragments for the 36B4 loading control are also shown. Yeast RNA was used to demonstrate probe specificity for RNA message. hTERT protected fragments were observed only in cells expressing E6 alone or together with E7, which confirms the results of the RT-PCR analysis (Fig. 2A). Unlike the RT-PCR results, however, HFKs expressing E6 or coexpressing E6 and E7 appear to have similar levels of hTERT expression, which correlates with the telomerase activity assayed in Fig. 1. Thus, upregulation of hTERT mRNA by E6 correlates with increased telomerase activity.
The E6 protein activates the hTERT promoter/regulatory region.
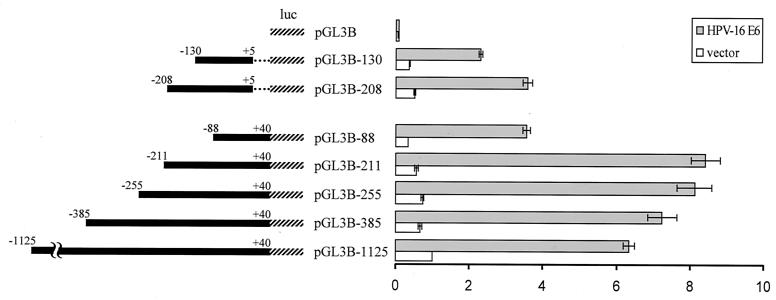
To determine if E6 protein could mediate transcriptional activation of hTERT, we performed transient-transfection assays with hTERT promoter-reporter plasmids and an E6 expression vector in telomerase-negative, late-passage HFKs (P8). A 1,165-bp fragment of the 5′ region of the hTERT gene was cloned into the pGL3-Basic vector (Promega) upstream of the firefly luciferase gene as previously described (15). Two micrograms of the resulting plasmid pGL3B-1125 (−1125 to +40) and 100 ng of an E6 expression vector or vector control were transiently cotransfected into primary HFKs, and firefly luciferase activity was measured 24 h after transfection using the dual luciferase reporter assay system (Promega). In order to control for transfection efficiency, 10 ng of the pRL-CMV plasmid, containing the Renilla reniformis luciferase gene under the control of the cytomegalovirus immediate-early enhancer/promoter, was also cotransfected, and Renilla luciferase activity was measured as described above. After normalizing for transfection efficiency, firefly luciferase activity in E6-transfected cells was compared to that in vector-transfected cells. Expression of E6 led to a 6- to 6.5-fold increase in pGL3B-1125 promoter activity relative to that of the vector control (Fig. 3). The pGL3B plasmid, containing no promoter/regulatory sequences, was used as a negative control. These results reflect the averages of at least three independent experiments. These data indicate that E6 induction of hTERT expression occurs predominantly at the level of transcriptional regulation.
FIG. 3.
E6 activation of the hTERT promoter and identification of the minimal promoter region necessary for induction. hTERT promoter fragments (solid bars) were cloned into the pGL3-Basic vector (Promega) upstream of the firefly luciferase gene (hatched lines) as described previously (15) and used in luciferase assays. Dotted lines indicate a 35-bp deleted region. Reporter plasmids are named according to the first nucleotide number at the 5′ end of each hTERT promoter fragment. Telomerase-negative keratinocytes were transiently cotransfected with an hTERT luciferase reporter plasmid, an E6 expression vector, or an empty vector and with the pRL-CMV R. reniformis reporter plasmid (to control for transfection efficiency). Relative fold activation (shown on the right) reflects the normalized luciferase activity induced by E6 or empty vector compared to the normalized activity of the largest hTERT reporter plasmid (pGL3B-1125) plus vector control. Error bars show the standard deviations for at least three independent experiments.
A minimal 251-bp promoter/regulatory region of hTERT is essential for maximal promoter activity induced by E6.
To identify the minimal promoter/regulatory region of the hTERT gene necessary for full transcriptional activation by E6, a series of luciferase plasmids containing 5′-truncated hTERT promoter fragments were constructed as previously described (15) and used in luciferase assays. As shown in Fig. 3, E6 expression induced promoter activity that increased with serial deletions of the pGL3B-1125 plasmid, with peak promoter activity exhibited by a 251-bp promoter fragment (pGL3B-211). The activity of the pGL3B-211 plasmid induced by E6 was 8- to 8.5-fold higher than the activity of the pGL3B-1125 plasmid plus vector control. In addition, E6 induced about a 25% increase in activity of the pGL3B-211 plasmid above the E6-induced activity of the pGL3B-1125 plasmid, which suggests the presence of repressor sequences in the deleted region (−1125 to −211). However, a 123-bp truncation of pGL3B-211, represented by the pGL3B-88 plasmid, resulted in a 60% reduction of full promoter activity. To demonstrate E6-specific induction of each truncated hTERT promoter-reporter plasmid, basal promoter activity was measured in transient-transfection assays with an E6-negative vector. Basal activity levels for each promoter plasmid are similar and are low compared to E6-induced activity levels (Fig. 3). Taken together, these results indicate that the proximal 251-bp (−211 to +40) promoter/regulatory region of the hTERT gene functions as the core regulatory region essential for full transcriptional activation of hTERT by E6. Although the precise mechanism for E6-mediated transactivation of hTERT remains uncertain, the identification of SP1, AP2, and Myc regulatory elements within the 251-bp core regulatory region (15, 37) suggests potential targets.
Identification of a 35-bp sequence that accounts for 60% of E6-induced transcriptional activation of hTERT.
To examine whether sequences downstream of the transcription start site might participate in hTERT transcriptional regulation, we deleted a 35-bp region (+5 to +40) of the hTERT core promoter and performed luciferase assays. Transient cotransfections of late passage keratinocytes with the resulting plasmid, pGL3B-208, and an E6 expression vector resulted in a 60% reduction of full luciferase activity (Fig. 3), suggesting the presence of a positive-acting element(s) within this 35-bp region. While there is a theoretical possibility that region −211 to −208 might contribute to this activity, preliminary analysis of point mutants indicates that the 35-bp region constitutes the major E6-responsive element (data not shown). Previous reports have identified two Myc-responsive sites, known as E-box elements (CACGTG), located in the 5′ regulatory region of hTERT (6, 15, 37). Interestingly, one E-box element is located in the 35-bp region, while the other E box is situated approximately 200 bp upstream (15). The potential role for Myc as a mediator of transcriptional activation of hTERT by E6 is underscored by the observation that a deletion of either E box (see pGL3B-208 and pGL3B-88 in Fig. 3) results in a similar reduction of promoter activity, 60% of maximal activity. However, the simultaneous deletion of both E boxes, represented by the pGL3B-130 plasmid (−130 to +5), did not appear to have a marked additive effect compared to deletion of either E box alone. In fact, pGL3B-130 plus E6 showed significant promoter activity above that of the vector control. Taken together, our results define a minimal 35-bp sequence of the hTERT promoter/regulatory region that contains a site for Myc/Max binding and that appears to account for at least 60% of the full transcriptional activity induced by E6.
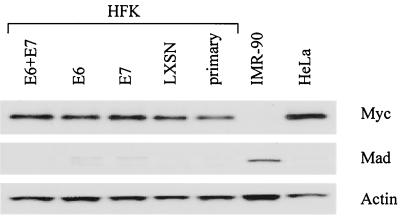
E6 expression does not alter Myc or Mad protein expression.
To examine whether E6-mediated activation of hTERT might involve changes in the abundance of endogenous Myc or Mad proteins, we performed Western blot analyses as described previously (36) on 50 μg of total cell lysates from keratinocytes transduced with E6. Protein blots were reacted with either the c-Myc 9E10 monoclonal antibody (Pharmingen; 2 μg/ml) or a polyclonal Mad antibody (Pharmingen; 1:1,000 dilution). Immunoblots were stripped and reprobed using a monoclonal actin antibody (Amersham; 5 to 10 μg/ml) to demonstrate equal loading of protein. Results show no obvious differences in either cellular Myc or Mad protein levels in keratinocytes expressing and not expressing E6 (Fig. 4). Therefore, we conclude that protein levels of Myc and Mad do not appear to be important for E6-mediated transcriptional control of telomerase hTERT. However, we cannot exclude the possibility that our Western blot assay is insensitive to subtle E6-mediated changes in Myc or Mad protein expression that are sufficient to induce hTERT gene activity. Alternatively, it is possible that E6 is altering the state of Myc activity by modifying other regulators of Myc, such as Max or Mxi.
FIG. 4.
Immunoblot analyses of cellular Myc and Mad proteins in transduced HFKs. Protein extracts of the transduced keratinocytes used for Fig. 1 were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, blotted onto nylon filters, and incubated with either Myc 9E10 monoclonal antibody (Pharmingen; 2 μg/ml) or Mad polyclonal antibody (Pharmingen; 1:1,000 dilution). The specified proteins were detected using alkaline phosphatase-conjugated goat anti-mouse or -rabbit immunoglobulin G antibodies (Tropix; 1:5,000 dilution) and visualized using CDP-Star chemiluminescent substrate (Tropix). To ensure equal loading of protein, immunoblots were stripped and reprobed using an actin monoclonal antibody (Amersham; 5 to 10 μg/ml).
Discussion.
The list of p53-independent activities of HPV-16 E6 protein is growing and includes, among others, the inhibition of keratinocyte differentiation in response to calcium and serum (34), the direct transactivation or repression of viral promoters (9, 18, 22), and the activation of telomerase enzyme in primary epithelial cells (20). However, in regard to the latter function of E6, the mechanism of telomerase activation remains unknown. In this report, we show that HPV-16 E6 induces hTERT expression in primary human keratinocytes and that this upregulation correlates strongly with telomerase activity. In addition, we demonstrate that E6 is able to transactivate the hTERT promoter, indicating that the mechanism for E6-mediated increases in hTERT expression occurs predominantly at the level of transcriptional regulation, although it is possible that other mechanisms such as RNA stability or processing may have a contributing role. However, the biological relevance of telomerase activation in primary keratinocytes by E6 is unclear. E6 and E7 proteins independently can extend the life spans of keratinocytes (19, 35), but both are required for the efficient immortalization of these cells (24). Interestingly, ectopic expression of hTERT in telomerase-negative, E7-expressing keratinocytes not only restores telomerase activity in these cells but also causes them to become immortalized (19). Therefore, E6 might contribute to the immortalization of keratinocytes by mediating upregulation of hTERT and concomitant activation of telomerase.
The precise mechanism for transcriptional activation of the hTERT gene by E6 remains to be identified. Recent reports showing that E6 can increase Myc protein expression in human mammary epithelial cells (40) and that c-Myc protein can directly activate hTERT transcription (43) led us to examine Myc as a potential mediator of hTERT transcription by E6. Results from our Western blot analyses showed no detectable differences in c-Myc expression in HFKs expressing E6 and those lacking E6 (Fig. 4). The results of our luciferase assays, however, suggest that c-Myc may still have a role in E6-mediated control of hTERT transcription. Promoter analysis of truncated hTERT promoter-reporter constructs led us to identify two regions (−211 to −88 and +5 to +40) whose individual deletions resulted in a reduction of promoter activity up to 60% of maximal activity (Fig. 3). Interestingly, each region contains a c-Myc/Max binding element known as an E box (15, 37). The activity of Myc is regulated by switches through its dimerization partners. Although Myc can form homodimers, Myc preferentially heterodimerizes with Max to induce Myc-responsive genes (4). The family of Mad proteins oppose Myc activity by competing with Myc for Max binding (1). Based on recent reports showing direct repression of hTERT transcription by Mad proteins (11, 28), we examined relative levels of Mad protein in E6-transduced keratinocytes but found no differences (Fig. 4). However, we cannot exclude the possibility that alterations in other regulators of Myc activity may contribute to hTERT transcriptional activation.
Acknowledgments
We thank Dan Hartmann for assistance with the telomerase assay and Hiroshi Nakai for reviewing the manuscript. We also thank Stefanie Kühn for preparing the primary keratinocytes.
This work was supported by a grant from the National Cancer Institute, R01 CA 53371. T.V. was supported by an NRSA predoctoral grant from the National Cancer Institute, 1F31 CA90203-01.
REFERENCES
- 1.Ayer D E, Kretzner L, Eisenman R N. Mad: a heterodimeric partner for Max that antagonizes Myc transcriptional activity. Cell. 1993;72:211–222. doi: 10.1016/0092-8674(93)90661-9. [DOI] [PubMed] [Google Scholar]
- 2.Band V, Zajchowski D, Kulesa V, Sager R. Human papilloma virus DNAs immortalize normal human mammary epithelial cells and reduce their growth factor requirements. Proc Natl Acad Sci USA. 1990;87:463–467. doi: 10.1073/pnas.87.1.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bedell A M, Jones K H, Grossman S R, Laimins L A. Identification of human papillomavirus type 18 transforming genes in immortalized and primary cells. J Virol. 1989;63:1247–1255. doi: 10.1128/jvi.63.3.1247-1255.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blackwood E M, Eisenman R N. Max: a helix-loop-helix zipper protein that forms a sequence-specific DNA-binding complex with Myc. Science. 1991;251:1211–1217. doi: 10.1126/science.2006410. [DOI] [PubMed] [Google Scholar]
- 5.Bodnar A G, Ouellette M, Frolkis M, Holt S E, Chiu C P, Morin G B, Harley C B, Shay J W, Lichtsteiner S, Wright W E. Extension of life span by introduction of telomerase into normal human cells. Science. 1998;279:349–352. doi: 10.1126/science.279.5349.349. [DOI] [PubMed] [Google Scholar]
- 6.Cong Y S, Wen J, Bacchetti S. The human telomerase catalytic subunit hTERT: organization of the gene and characterization of the promoter. Hum Mol Genet. 1999;8:137–142. doi: 10.1093/hmg/8.1.137. [DOI] [PubMed] [Google Scholar]
- 7.Counter C M, Meyerson M, Eaton E N, Ellisen L W, Caddle S D, Haber D A, Weinberg R A. Telomerase activity is restored in human cells by ectopic expression of hTERT (hEST2), the catalytic subunit of telomerase. Oncogene. 1998;16:1217–1222. doi: 10.1038/sj.onc.1201882. [DOI] [PubMed] [Google Scholar]
- 8.Dyson N, Howley P M, Munger K, Harlow E. The human papilloma virus-16 E7 oncoprotein is able to bind to the retinoblastoma gene product. Science. 1989;243:934–937. doi: 10.1126/science.2537532. [DOI] [PubMed] [Google Scholar]
- 9.Etscheid B G, Foster S A, Galloway D A. The E6 protein of human papillomavirus type 16 functions as a transcriptional repressor in a mechanism independent of the tumor suppressor protein, p53. Virology. 1994;205:583–585. doi: 10.1006/viro.1994.1684. [DOI] [PubMed] [Google Scholar]
- 10.Greider C W. Telomere length regulation. Annu Rev Biochem. 1996;65:337–365. doi: 10.1146/annurev.bi.65.070196.002005. [DOI] [PubMed] [Google Scholar]
- 11.Gunes C, Lichtsteiner S, Vasserot A P, Englert C. Expression of the hTERT gene is regulated at the level of transcriptional initiation and repressed by Mad1. Cancer Res. 2000;60:2116–2121. [PubMed] [Google Scholar]
- 12.Hahn W C, Stewart S A, Brooks M W, York S G, Eaton E, Kurachi A, Beijersbergen R L, Knoll J H, Meyerson M, Weinberg R A. Inhibition of telomerase limits the growth of human cancer cells. Nat Med. 1999;5:1164–1170. doi: 10.1038/13495. [DOI] [PubMed] [Google Scholar]
- 13.Harley C B, Futcher A B, Greider C W. Telomeres shorten during ageing of human fibroblasts. Nature. 1990;345:458–460. doi: 10.1038/345458a0. [DOI] [PubMed] [Google Scholar]
- 14.Hawley-Nelson P, Vousden K H, Hubbert N L, Lowy D R, Schiller J T. HPV16 E6 and E7 proteins cooperate to immortalize human foreskin keratinocytes. EMBO J. 1989;8:3905–3910. doi: 10.1002/j.1460-2075.1989.tb08570.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Horikawa I, Cable P L, Afshari C, Barrett J C. Cloning and characterization of the promoter region of human telomerase reverse transcriptase gene. Cancer Res. 1999;59:826–830. [PubMed] [Google Scholar]
- 16.Huibregtse J M, Scheffner M, Howley P M. A cellular protein mediates association of p53 with the E6 oncoprotein of human papillomavirus types 16 or 18. EMBO J. 1991;10:4129–4135. doi: 10.1002/j.1460-2075.1991.tb04990.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim N W, Piatyszek M A, Prowse K R, Harley C B, West M D, Ho P L, Coviello G M, Wright W E, Weinrich S L, Shay J W. Specific association of human telomerase activity with immortal cells and cancer. Science. 1994;266:2011–2015. doi: 10.1126/science.7605428. [DOI] [PubMed] [Google Scholar]
- 18.Kinoshita T, Shirasawa H, Shino Y, Moriya H, Desbarats L, Eilers M, Simizu B. Transactivation of prothymosin alpha and c-myc promoters by human papillomavirus type 16 E6 protein. Virology. 1997;232:53–61. doi: 10.1006/viro.1997.8536. [DOI] [PubMed] [Google Scholar]
- 19.Kiyono T, Foster S A, Koop J I, McDougall J K, Galloway D A, Klingelhutz A J. Both Rb/p16INK4a inactivation and telomerase activity are required to immortalize human epithelial cells. Nature. 1998;396:84–88. doi: 10.1038/23962. [DOI] [PubMed] [Google Scholar]
- 20.Klingelhutz A J, Foster S A, McDougall J K. Telomerase activation by the E6 gene product of human papillomavirus type 16. Nature. 1996;380:79–82. doi: 10.1038/380079a0. [DOI] [PubMed] [Google Scholar]
- 21.Laborda J. 36B4 cDNA used as an estradiol-independent mRNA control is the cDNA for human acidic ribosomal phosphoprotein PO. Nucleic Acids Res. 1991;19:3998. doi: 10.1093/nar/19.14.3998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lamberti C, Morrissey L C, Grossman S R, Androphy E J. Transcriptional activation by the papillomavirus E6 zinc finger oncoprotein. EMBO J. 1990;9:1907–1913. doi: 10.1002/j.1460-2075.1990.tb08317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meyerson M, Counter C M, Eaton E N, Ellisen L W, Steiner P, Caddle S D, Ziaugra L, Beijersbergen R L, Davidoff M J, Liu Q, Bacchetti S, Haber D A, Weinberg R A. hEST2, the putative human telomerase catalytic subunit gene, is up-regulated in tumor cells and during immortalization. Cell. 1997;90:785–795. doi: 10.1016/s0092-8674(00)80538-3. [DOI] [PubMed] [Google Scholar]
- 24.Munger K, Phelps W C, Bubb V, Howley P M, Schlegel R. The E6 and E7 genes of the human papillomavirus type 16 together are necessary and sufficient for transformation of primary human keratinocytes. J Virol. 1989;63:4417–4421. doi: 10.1128/jvi.63.10.4417-4421.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Munger K, Werness B A, Dyson N, Phelps W C, Harlow E, Howley P M. Complex formation of human papillomavirus E7 proteins with the retinoblastoma tumor suppressor gene product. EMBO J. 1989;8:4099–4105. doi: 10.1002/j.1460-2075.1989.tb08594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakamura T M, Morin G B, Chapman K B, Weinrich S L, Andrews W H, Lingner J, Harley C B, Cech T R. Telomerase catalytic subunit homologs from fission yeast and human. Science. 1997;277:955–959. doi: 10.1126/science.277.5328.955. [DOI] [PubMed] [Google Scholar]
- 27.Nugent C I, Lundblad V. The telomerase reverse transcriptase: components and regulation. Genes Dev. 1998;12:1073–1085. doi: 10.1101/gad.12.8.1073. [DOI] [PubMed] [Google Scholar]
- 28.Oh S, Song Y H, Yim J, Kim T K. Identification of Mad as a repressor of the human telomerase (hTERT) gene. Oncogene. 2000;19:1485–1490. doi: 10.1038/sj.onc.1203439. [DOI] [PubMed] [Google Scholar]
- 29.Phelps W C, Yee C L, Munger K, Howley P M. The human papillomavirus type 16 E7 gene encodes transactivation and transformation functions similar to those of adenovirus E1A. Cell. 1988;53:539–547. doi: 10.1016/0092-8674(88)90570-3. [DOI] [PubMed] [Google Scholar]
- 30.Ramakrishnan S, Eppenberger U, Mueller H, Shinkai Y, Narayanan R. Expression profile of the putative catalytic subunit of the telomerase gene. Cancer Res. 1998;58:622–625. [PubMed] [Google Scholar]
- 31.Scheffner M, Huibregtse J M, Vierstra R D, Howley P M. The HPV-16 E6 and E6-AP complex functions as a ubiquitin-protein ligase in the ubiquitination of p53. Cell. 1993;75:495–505. doi: 10.1016/0092-8674(93)90384-3. [DOI] [PubMed] [Google Scholar]
- 32.Scheffner M, Werness B A, Huibregtse J M, Levine A J, Howley P M. The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell. 1990;63:1129–1136. doi: 10.1016/0092-8674(90)90409-8. [DOI] [PubMed] [Google Scholar]
- 33.Schlegel R, Phelps W C, Zhang Y L, Barbosa M. Quantitative keratinocyte assay detects two biological activities of human papillomavirus DNA and identifies viral types associated with cervical carcinoma. EMBO J. 1988;7:3181–3187. doi: 10.1002/j.1460-2075.1988.tb03185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sherman L, Jackman A, Itzhaki H, Stoppler M C, Koval D, Schlegel R. Inhibition of serum- and calcium-induced differentiation of human keratinocytes by HPV16 E6 oncoprotein: role of p53 inactivation. Virology. 1997;237:296–306. doi: 10.1006/viro.1997.8778. [DOI] [PubMed] [Google Scholar]
- 35.Stoppler H, Hartmann D P, Sherman L, Schlegel R. The human papillomavirus type 16 E6 and E7 oncoproteins dissociate cellular telomerase activity from the maintenance of telomere length. J Biol Chem. 1997;272:13332–13337. doi: 10.1074/jbc.272.20.13332. [DOI] [PubMed] [Google Scholar]
- 36.Suprynowicz F A, Sparkowski J, Baege A, Schlegel R. E5 oncoprotein mutants activate phosphoinositide 3-kinase independently of platelet-derived growth factor receptor activation. J Biol Chem. 2000;275:5111–5119. doi: 10.1074/jbc.275.7.5111. [DOI] [PubMed] [Google Scholar]
- 37.Takakura M, Kyo S, Kanaya T, Hirano H, Takeda J, Yutsudo M, Inoue M. Cloning of human telomerase catalytic subunit (hTERT) gene promoter and identification of proximal core promoter sequences essential for transcriptional activation in immortalized and cancer cells. Cancer Res. 1999;59:551–557. [PubMed] [Google Scholar]
- 38.Takakura M, Kyo S, Kanaya T, Tanaka M, Inoue M. Expression of human telomerase subunits and correlation with telomerase activity in cervical cancer. Cancer Res. 1998;58:1558–1561. [PubMed] [Google Scholar]
- 39.Vousden K H, Doniger J, DiPaolo J A, Lowy D R. The E7 open reading frame of human papillomavirus type 16 encodes a transforming gene. Oncogene Res. 1988;3:167–175. [PubMed] [Google Scholar]
- 40.Wang J, Xie L Y, Allan S, Beach D, Hannon G J. Myc activates telomerase. Genes Dev. 1998;12:1769–1774. doi: 10.1101/gad.12.12.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Watson J D. Origin of concatemeric T7 DNA. Nat New Biol. 1972;239:197–201. doi: 10.1038/newbio239197a0. [DOI] [PubMed] [Google Scholar]
- 42.Werness B A, Levine A J, Howley P M. Association of human papillomavirus types 16 and 18 E6 proteins with p53. Science. 1990;248:76–79. doi: 10.1126/science.2157286. [DOI] [PubMed] [Google Scholar]
- 43.Wu K J, Grandori C, Amacker M, Simon-Vermot N, Polack A, Lingner J, Dalla-Favera R. Direct activation of TERT transcription by c-MYC. Nat Genet. 1999;21:220–224. doi: 10.1038/6010. [DOI] [PubMed] [Google Scholar]
- 44.zur Hausen H. Human papillomaviruses in the pathogenesis of anogenital cancer. Virology. 1991;184:9–13. doi: 10.1016/0042-6822(91)90816-t. [DOI] [PubMed] [Google Scholar]
- 45.zur Hausen H. Viruses in human cancers. Science. 1991;254:1167–1173. doi: 10.1126/science.1659743. [DOI] [PubMed] [Google Scholar]
- 46.zur Hausen H. Papillomaviruses causing cancer: Evasion from host-cell control in early events in carcinogenesis. J Natl Cancer Inst. 2000;92:690–698. doi: 10.1093/jnci/92.9.690. [DOI] [PubMed] [Google Scholar]