Abstract
Herpes simplex virus (HSV) has several potential advantages as a vector for delivering genes to the nervous system. The virus naturally infects and remains latent in neurons and has evolved the ability of highly efficient retrograde transport from the site of infection at the periphery to the site of latency in the spinal ganglia. HSV is a large virus, potentially allowing the insertion of multiple or very large transgenes. Furthermore, HSV does not integrate into the host chromosome, removing any potential for insertional activation or inactivation of cellular genes. However, the development of HSV vectors for the central nervous system that exploit these properties has been problematical. This has mainly been due to either vector toxicity or an inability to maintain transgene expression. Here we report the development of highly disabled versions of HSV-1 deleted for ICP27, ICP4, and ICP34.5/open reading frame P and with an inactivating mutation in VP16. These viruses express only minimal levels of any of the immediate-early genes in noncomplementing cells. Transgene expression is maintained for extended periods with promoter systems containing elements from the HSV latency-associated transcript promoter (J. A. Palmer et al., J. Virol. 74:5604–5618, 2000). Unlike less-disabled viruses, these vectors allow highly effective gene delivery both to neurons in culture and to the central nervous system in vivo. Gene delivery in vivo is further enhanced by the retrograde transport capabilities of HSV. Here the vector is efficiently transported from the site of inoculation to connected sites within the nervous system. This is demonstrated by gene delivery to both the striatum and substantia nigra following striatal inoculation; to the spinal cord, spinal ganglia, and brainstem following injection into the spinal cord; and to retinal ganglion neurons following injection into the superior colliculus and thalamus.
Herpes simplex virus type 1 (HSV-1) has a number of features that could be exploited in the development of vectors for the delivery of genes to the nervous system. These include a natural tropism for neurons, a large (152-kb) genome allowing multiple gene insertions, and the ability to establish lifelong latent infections. During latency, the viral genome is maintained in an episomal state, and gene expression from the vast majority of the HSV genome is silenced. However, part of the long repeat region of the viral genome is transcribed during latency, generating a population of RNA species collectively known as the latency-associated transcripts (LATs) (58).
Considerable previous work has been reported on the development of HSV as a vector for gene delivery to neurons (reviewed in references 19 and 57). This work has focused on overcoming the two main problems with wild-type HSV-1 as a vector system: the toxicity of the virus and the transient expression of inserted genes that usually occurs.
Following infection of permissive cells, HSV-1 expresses more than 80 viral genes in a well-ordered cascade of immediate early (IE), early (E), and late (L) gene products (25). This gene cascade results in lysis of the infected cells and release of progeny virions. The IE genes are transactivated by the virion component VP16, which interacts with cellular Oct1 and host cell factor (HCF) to bind to TAATGARAT elements present in the IE gene promoters (reviewed in reference 44). The viral genome encodes five IE genes: ICP0, ICP4, ICP22, ICP27, and ICP47. Of these, ICP4 and ICP27 are absolutely required for viral replication (14, 51), and the deletion of ICP0 and ICP22 significantly impairs virus growth, especially at a low multiplicity of infection (MOI) (8, 10, 55). The ICP0, ICP4, ICP22, and ICP27 proteins regulate the expression of the later replication and structural (E and L) genes. ICP47 is not a regulatory IE protein, but inhibits the transporters of antigen processing (TAP), thereby aiding the virus in avoiding the host immune response (67). ICP47 can be deleted from the virus genome without significantly affecting virus growth (39).
ICP4 is the major viral transactivator protein, and deletion of this gene alone results in a dramatic reduction in the expression of the remaining ∼80 HSV-1 genes. Infection of nonpermissive cells with an ICP4 deletion mutant results in the expression of the four remaining IE genes, the large subunit of ribonucleotide reductase (ICP6), and open reading frame (ORF) P, but no other known HSV genes (14, 66). However, despite the fact that the majority of the virus genome is not transcribed, ICP4 deletion mutants are still highly toxic to cells (29). A number of studies have demonstrated that this toxicity is largely caused by the products of the remaining regulatory IE genes, ICP0, ICP22, and ICP27 (29, 30, 32, 52, 64). When these genes are inactivated in combination with deletion of ICP4, toxicity is eliminated (52). An optimal HSV vector should therefore not express significant amounts of any of the regulatory IE genes.
As described above, IE gene expression can be reduced or prevented by the individual deletion or inactivation of each of the IE genes. However, the efficient propagation of such viruses requires that all of the regulatory IE gene products are provided in trans by a complementing cell line. This presents a problem, because the toxicity of the IE gene products means that cell lines directing their stable expression are hard to generate. Although cell lines complementing several IE gene deletions have been described (E5 cells [ICP4], B130/2 cells [ICP27], E26 cells [ICP4 and ICP27], and FO6 cells [ICP4, ICP27, and ICP0]) (14, 26, 53, 54), the plating efficiency of disabled viruses on cells expressing multiple IE genes decreases significantly with passage. For example, the previously reported cell line expressing ICP4, ICP27, and ICP0 (FO6 cells) cannot be reliably used after passage 15 (54). No cell line complementing all of the regulatory IE genes has yet been reported, even though such a cell line would be required in order to achieve optimal growth of viruses deficient for each of the IE genes.
As an alternative approach to inactivating the IE genes individually, we have deleted the two essential IE genes ICP4 and ICP27 and prevented the transactivation of the promoters of the remaining IE genes by including a disabling mutation in the virion protein VP16. VP16 has a dual role both as a transactivator of the IE genes and as an essential structural protein (4, 46). For this reason, VP16 cannot be deleted from the virus, but a number of mutations to the C-terminal domain have been shown to reduce or abolish the transactivation ability of the protein without affecting the structural integrity of the virus (1, 56).
Disabling VP16 as a means to reduce IE gene expression has a potential advantage over deleting the genes individually, because this does not then require the complementation of all the toxic regulatory IE genes for virus growth. Mutations to VP16 can be complemented by addition of hexamethylbisacetamide (HMBA) to the growth medium (41). However, when IE genes have also been deleted, the transactivation provided by HMBA is insufficient for effective virus growth (62). The VP16 gene cannot be included in the cell line used for virus growth, because this may result in recombinational repair of the mutation in the virus or packaging of functional VP16 into the resulting virions. We therefore developed cell lines by using the equine herpesvirus 1 (EHV-1) homologue of VP16 (gene 12) together with HSV-1 ICP4 and ICP27 and found that these cell lines allow multiply disabled viruses to be grown effectively in culture (62). EHV-1 gene 12 has only minimal DNA sequence similarity to HSV-1 VP16, and the protein product of EHV-1 gene 12 is not packaged into HSV virions (62).
A second consideration in the design of HSV vectors is the choice of promoter to drive the expression of exogenous genes. Most of the HSV and non-HSV promoters that have been tested have been found to be transcriptionally silenced along with the majority of the viral genes at the onset of latency. It has been reported that the LAP2, but not the LAP1, promoter is able to drive the expression of a transgene through latency, although here the expression levels detected were very low and could only be detected by reverse transcription-PCR (22, 57). Alternatively, when an internal ribosome entry site (IRES) was inserted into the 2-kb LAT such that it directed the expression of a lacZ transgene under the control of the endogenous LAT promoter elements, strong expression that continued during latency in the peripheral nervous system and in a small number of cells in the brain was obtained from a nondisabled (essentially wild-type) virus (34). It has also been reported that when a region of DNA downstream of LAP1 was placed next to LAP1 at an exogenous site in the HSV genome, LAP1-mediated transcription was maintained during latency in the peripheral nervous system (5, 35). We have previously reported that a similar region of DNA (LAT P2, between PstI at nucleotide [nt] 118866 and BstXI at nt 120219 in HSV-1 strain 17+) can confer latent gene expression characteristics to neighboring heterologous (non-HSV) promoters or LAP1, including when such promoter cassettes are inserted at non-LAT sites in the HSV genome (45).
Previous work aimed at developing HSV vectors for persistent transgene expression has mainly used replication-competent or conditionally replication-competent viruses (5, 6, 9, 16, 34, 35, 38). Previous work has also so far been limited to testing in the peripheral nervous system, except in a few cases in which low efficiency and/or transient delivery to the central nervous system (CNS) has been reported (6, 27, 37). The aim of the current work was therefore to develop replication-incompetent vectors that express no IE genes and are therefore minimally toxic to target cells and that direct the extended expression of exogenous genes in the CNS. Extended expression of a transgene in the CNS, other than in a very few cells (6, 27, 37), has not previously been reported with HSV vectors.
MATERIALS AND METHODS
Cell culture and virus propagation.
BHK and Vero cells were maintained by standard cell culture procedures. All viruses used in this study were propagated on BHK-derived complementing cell lines that have been described previously (B130/2 cells for virus strains 17+ 27− and 1764 27− [26] and 27/12/M:4 cells for virus strain 1764 27− 4− [62]). These cell lines only complement the genes that have been deleted, and there is no sequence overlap between the viral and cellular genomes. Complementing cells were cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal calf serum and 5% tryptose phosphate broth. Continual selection was achieved by maintaining the cells in 800 μg of G418 per ml (cell line B130/2) or 800 μg of G418 per ml and 750 μg of Zeocin per ml (cell line 27/12/M:4). HMBA (3 mM) was included in the growth media of viruses containing the in1814 mutation (1).
Promoter cassettes.
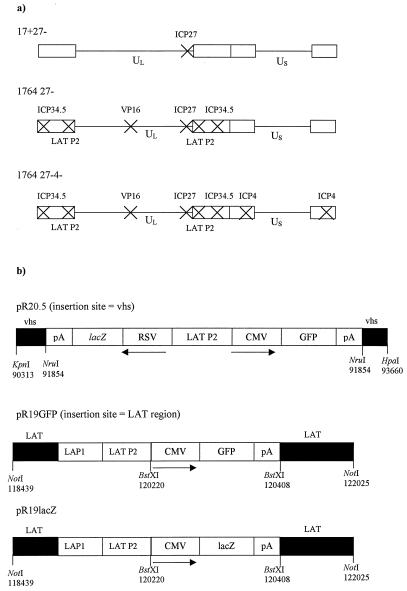
The pR20.5 and pR19 promoter cassettes have been described previously (45). The pR20.5 cassette (45) was inserted into a plasmid, allowing insertional inactivation of UL41, the gene encoding Vhs (insertion at the unique NruI site at nucleotide 91854 in UL41). The pR19lacZ and pR19GFP cassettes used here (Fig. 1) were recombined into the endogenous LAT regions between the two BstXI sites (nt 120220 and 120408). HSV-1 nucleotide numbers refer to GenBank file HE1CG. The structures of each of these shuttle plasmids are shown in Fig. 1.
FIG. 1.
Viruses used in this study. (a) Vector backbones. In each case, the indicated genes have been deleted or inactivated. Further details are given in Materials and Methods. (b) Promoter cassettes. The three promoter cassettes used in the study and their insertion sites into the viral genome are indicated. Details are given in Materials and Methods.
Virus nomenclature.
All viruses were derived from HSV-1 strain 17syn+ (7). The term “1764” describes a virus with the in1814 mutation in the gene encoding VP16 (1) and with the genes encoding ICP34.5 and ORF P completely deleted (between nt 124945 and 125723) (12). The term 27− refers to the deletion of nucleotides 113273 to 116869, which contain the genes UL54, -55, and -56 (26). UL54 is the gene encoding the essential IE protein ICP27, and UL55 and -56 are both nonessential genes. Virus strains 1764 27− and 1764 27− 4− were also deleted for the endogenous LAT P2 regions (between nt 118768 and 120470, DdeI to HpaI) in order to prevent the recombinational instability that was otherwise found to occur after insertion of LAT P2-containing expression cassettes outside of the LAT region (45).
Viruses.
The shuttle plasmids described above were recombined into either virus strain 17+ 27− (26) or virus strain 1764 27− (12) by standard calcium phosphate techniques (60). Recombinant viruses were identified by reporter gene transfer and plaque purified. Genome structures were confirmed by Southern blotting.
Western blotting.
Samples for Western blot analysis were prepared by standard techniques. Extract from approximately 105 cells (per lane) was loaded onto a sodium dodecyl sulfate (SDS)-polyacrylamide gel. Proteins were transferred to nitrocellulose and probed with appropriate antibodies by standard techniques. Antibody against HSV-1 ICP0 was purchased from ABI. Antibodies to HSV-1 ICP22, ICP6, and ICP47 were provided by Bernard Roizman (University of Chicago), Barklie Clements (MRC Institute of Virology, Glasgow, Scotland), and David Johnson (Oregon Health Sciences University, Portland), respectively. Detection was performed with the ECL enhanced chemiluminescence system (Amersham).
Primary neuronal cultures.
Organotypic hippocampal slice cultures were prepared as previously described (59) and maintained in a mixture of 50% MEM, 25 mM HEPES, 25% horse serum, and 25% Hanks' balanced salt solution supplemented with 2 mM l-glutamine and 6.4 mg of d-glucose per ml. Slices were maintained on porous rafts (Millicell; Millipore) over 1 ml of growth medium, which was changed every second day. Four organotypic slices were cultured on each raft. Slices were infected 1 week postplating by covering each raft in 2 ml of 106 PFU of virus stock per ml diluted in growth medium supplemented with 2% TCM Supplement (ICN Biomedicals) for 1 h. Dorsal root ganglion (DRG) neurons from an adult rat were prepared as described previously (13). Neurons were plated on laminin-coated coverslips and maintained in DMEM supplemented with 10% horse serum, half of which was changed every third day. Neurons were infected by incubation for 1 h with an MOI of 10 of the appropriate virus diluted in serum-free DMEM.
In vivo gene delivery.
Adult (220-g) male Lewis rats were inoculated by stereotaxic injection of vector suspensions into the striatum, superior colliculus, or spinal cord (at the C6 level). At various times postinoculation, animals were perfusion fixed in ice-cold 4% paraformaldehyde and sectioned as described in the figure legends. Sections including the injection site and connected sites within the nervous system were stained with 1× phosphate-buffered saline (PBS) containing 5 mM K3Fe(CN)6, 5 mM K4Fe(CN)6O · 6H2O, 1 mM MgCl2, and 150 μg of 4-chloro-5-bromo-3-indolyl-β-galactosidase (X-Gal) per ml in order to visualize lacZ expression. Some sections were counterstained in 0.02% neutral red.
RESULTS
Virus strain 1764 27− 4− does not express significant amounts of any IE gene.
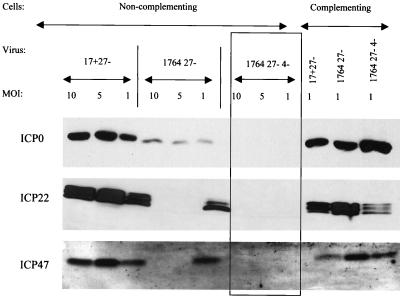
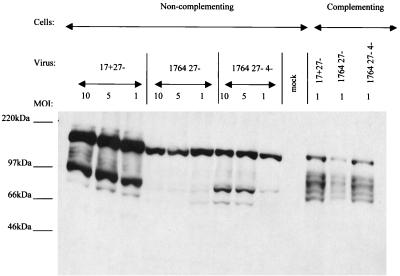
In work aimed at generating a nontoxic HSV backbone, vectors were constructed by the sequential deletion or inactivation of ICP27, VP16, ICP34.5, ICP4, and Vhs. Here we aimed to combine IE gene deletions (ICP27 and/or ICP4) with VP16 inactivation such that the remaining IE genes, which had not been specifically deleted, would not be expressed in target cells. To test the levels of residual IE gene expression, noncomplementing BHK cells were infected at MOIs of 10, 5, and 1 with 17+ 27−, 1764 27−, and 1764 27− 4− viruses. Complementing 27/12/M:4 cells (62) were infected with the same viruses at an MOI of 1 as a positive control. Cells were harvested 48 h postinfection and analyzed for the expression of ICP0, ICP22, and ICP47 by Western blotting (Fig. 2).
FIG. 2.
Virus strain 1764 27− 4− does not express significant amounts of any of the IE genes. Extracts were prepared from noncomplementing BHK cells harvested 24 h postinfection with the viruses 17+ 27− pR19GFP, 1764 27− pR20.5, and 1764 27− 4− pR20.5 over a range of MOIs. Complementing cells were infected at an MOI of 1 as a positive control. Western blots were probed for ICP0, ICP22, or ICP47.
It can be seen from Fig. 2 that the 17+ 27− virus expressed significant amounts of all three of the IE genes that have not been deleted (ICP0, ICP22, and ICP47). This is consistent with the phenotype of ICP27 deletion mutants (23, 40, 51). The subsequent inactivation of VP16 and deletion of ICP34.5 (the 1764 27− virus) leads to reduced but still significant levels of ICP0, ICP22, and ICP47 expression, although ICP22 and ICP47 are here expressed at higher levels at a low rather than high MOI, which is discussed later. Thus, the inclusion of the in1814 mutation to VP16 in the context of an ICP27-deleted virus is not sufficient to completely prevent the transactivation of the remaining IE gene promoters and leads to an unusual expression pattern for ICP22 and ICP47. However, prevention of transactivation of the other IE gene promoters can be achieved by the additional deletion of ICP4 (the 1764 27− 4− virus). The Western blots for ICP0, ICP22, and ICP47 reveal that there is no detectable expression of any of these IE genes from this virus, even at a high MOI.
The 1764 27− 4− virus does not therefore express significant amounts of any of the IE genes. The essential IE genes ICP4 and ICP27 have been completely deleted from the viral backbone, and the remaining IE genes ICP0, ICP22, and ICP47 are not expressed. This lack of IE gene expression requires the combination of VP16 inactivation and ICP4/ICP27 deletion and provides a level of disablement to the virus similar to that resulting from the individual deletion of each of the IE genes (52).
Virus strain 1764 27− 4− pR20.5 simultaneously expresses high levels of two exogenous genes.
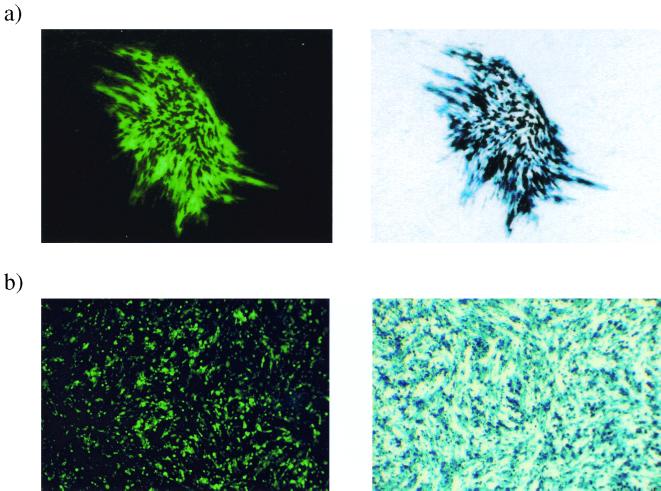
The large genome size of HSV is often cited as one of the potential benefits of HSV as a gene delivery vector. However, to date, HSV has only been used to deliver multiple genes in a highly transient fashion (31). The pR20.5 cassette has been described previously (45) and consists of a central LAT P2 element flanked by two heterologous promoters (cytomegalovirus [CMV] and Rous sarcoma virus [RSV]) arranged in a back-to-back orientation (Fig. 1), designed to allow the simultaneous expression of two exogenous genes. This is demonstrated in Fig. 3a, where the same plaque can be seen to be simultaneously expressing two transgenes (lacZ and the gene coding for green fluorescent protein [GFP]) in complementing cells.
FIG. 3.
Virus strain 1764 27− 4− pR20.5 can direct the simultaneous high-level expression of two exogenous genes on complementing and noncomplementing cells. (a) Complementing cell line 27/12/M:4 (62) was infected with virus 1764 27− 4− pR20.5 at an MOI of 0.01. The plaque was photographed under fluorescence 72 h after infection, stained for lacZ expression, and photographed again. (b) Duplicate wells of noncomplementing BHK cells were infected with virus 1764 27− 4− pR20.5 at an MOI of 1. Forty-eight hours postinfection, one well was stained with X-Gal, and then the wells were photographed under bright field (for lacZ) or fluorescence (for GFP).
It has been previously reported that in the absence of IE gene products, the expression of inserted genes is undetectable in the majority of transduced cells even at short times postinfection (48, 52). It has been proposed that this absence of transgene expression is attributable to an active repression of both HSV and non-HSV promoters by a cellular factor, but that this effect is masked in less-disabled viruses by the presence of the potent viral transactivators VP16, ICP0, and ICP4 (48). In Fig. 3b, noncomplementing BHK cells were infected at an MOI of 10 and visualized by fluorescence microscopy or processed to detect lacZ expression 48 h postinfection. This shows abundant transgene expression from the 1764 27− 4− pR20.5 virus, even though IE gene expression levels are minimal (Fig. 2). This could be due to LAT P2 elements in the promoter preventing the transcriptional shutoff, which might otherwise occur (45), and/or might alternatively be due to low-level ICP0 expression, which, while undetectable in Western blots, might still aid expression from the CMV and RSV promoters used.
Exogenous gene expression from cassettes inserted into virus strain 1764 27− 4− is dose dependent in noncomplementing cells.
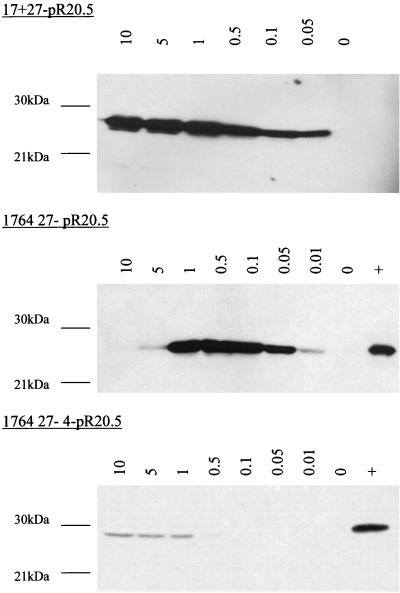
It can be seen from Fig. 2 that the expression level of ICP22 and ICP47 from the 1764 27− virus in noncomplementing cells is not dose dependent, the peak of expression occurring at an MOI of 1. This suggests that with this intermediately disabled virus, interactions between the virus genome and remaining viral factors are affecting virus gene expression. In order to examine this further, noncomplementing BHK cells were infected with a selection of disabled viruses over a wider range of MOIs, and the samples were harvested 48 h postinfection. Western blots were then probed for the expression of ICP22 and ICP47 as before. This experiment showed that in the case of the 17+ 27− virus, ICP22/47 expression is dose dependent, but with the 1764 27− virus, the peak of ICP22/47 expression is at an MOI of 0.5 (data not shown). As in Fig. 2, no expression of ICP22 was detected at any MOI from the 1764 27− 4− virus. This phenomenon was investigated further and found to also apply to exogenous genes inserted into the 1764 27− virus, but not the less-disabled 17+ 27− virus or the more-disabled 1764 27− 4− virus. As an example of an exogenous gene, a Western blot for CMV-driven GFP expression from all three viruses is shown (Fig. 4). Virus strains 17+ 27− pR19GFP and 1764 27− 4− pR20.5 demonstrate a dose-dependent pattern of GFP expression. However, with virus strain 1764 27− pR20.5, the pattern of GFP expression follows that of ICP22/47, with expression levels peaking at an MOI of 0.5. The reason for this expression pattern is unclear, but a similar dose effect has been reported previously when ICP0 and ICP4 were used in combination to activate IE gene target promoters in transient transfection assays (20, 21). Whatever the mechanism, it might be anticipated that a linearly dose-dependent pattern of transgene expression such as that directed by the most-disabled virus would be more desirable in gene delivery applications than the pattern shown by the less-disabled virus.
FIG. 4.
Expression of exogenous genes from virus 1764 27− is not dose dependent. Noncomplementing BHK cells were infected at MOIs from 0.01 to 10 with virus strains 17+ 27− pR19GFP, 1764 27− pR20.5, and 1764 27− 4− pR20.5. Complementing 27/12/M:4 cells (62) were infected at an MOI of 1 as a positive control (indicated as + in the figure). In the case of the 17+ 27− pR19GFP virus, the positive control was run on a separate gel and is not shown. All samples were harvested at 48 h postinfection.
Virus strain 1764 27− 4− expresses ICP6.
Because ICP6 (the large subunit of ribonucleotide reductase, required for virus replication in neurons) is inefficiently expressed in the absence of prior viral protein synthesis, it was originally classified as an E gene (25). However, the identification of IE-type cis-responsive elements in the promoter for ICP6 expression has led it to now be considered a hybrid IE/E gene (61). Unlike classical E genes, high levels of ICP6 are expressed in the absence of ICP4 (14). We therefore examined whether virus strain 1764 27− 4− still allowed ICP6 to be expressed.
To test levels of ICP6 expression, noncomplementing BHK cells were infected at MOIs of 10, 5, and 1 with the viruses 17+ 27− pR19GFP, 1764 27− pR20.5, and 1764 27− 4− pR20.5. Complementing 27/12/M:4 cells (62) were infected with the same viruses at an MOI of 1 as a positive control. Cells were harvested 48 h postinfection and analyzed for the expression of ICP6 by Western blotting (Fig. 5).
FIG. 5.
ICP6 expression from 17+ 27−, 1764 27−, and 1764 27− 4− viruses. Western blots of extracts from BHK cells prepared 48 h after infection with virus strains 17+ 27− pR19GFP, 1764 27− pR20.5, and 1764 27− 4− pR20.5 at MOIs of 10, 5, and 1 were probed with an anti-ICP6 antibody. 27/12/M:4 cells (62) infected with each of the viruses at an MOI of 1 are shown as a positive control.
Figure 5 shows that ICP6 was expressed from all three viruses and that the deletion of ICP4 made little difference to the levels observed, as might be expected from previous work (14, 54). However, because previous work has also shown ICP6 to be nontoxic (30), it was not anticipated that ICP6 expression would impair gene delivery when the vectors were used in the CNS.
Virus strain 1764 27− 4− directs high-efficiency gene delivery to cultured neurons.
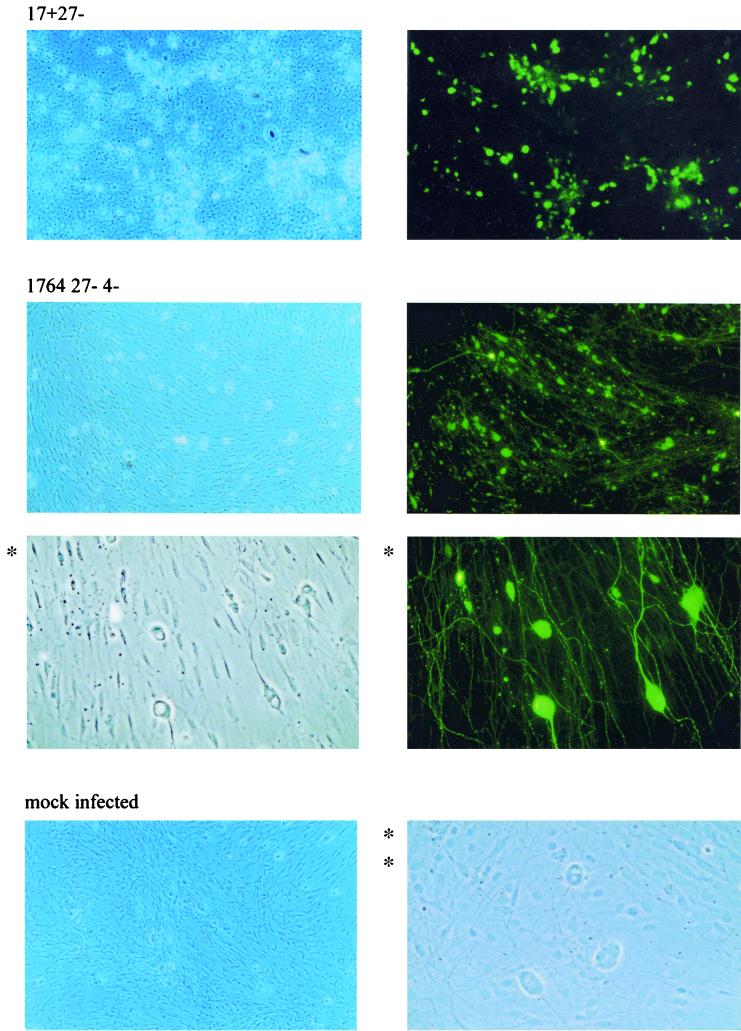
In order to test the ability of 1764 27− 4− viruses to deliver exogenous genes to cultured neurons, primary cultures of DRG neurons were infected at an MOI of 10 with 1764 27− 4− pR20.5 (Fig. 6). Mock-infected neurons or neurons infected with virus strain 17+ 27− pR19GFP were used as controls. One week postinfection, neurons were photographed under phase-contrast and fluorescence microscopy. Both viruses were able to efficiently deliver exogenous genes to neurons in culture. However, by 7 days postinfection, the 17+ 27− virus caused considerable toxic effects, with loss of supporting cells, clumping of neuronal cell bodies, and retraction of neuronal processes. In contrast, cells infected with the 1764 27− 4− virus are indistinguishable in morphology from mock-infected cells under phase-contrast microscopy. The fluorescence photomicrograph of neurons infected with the 1764 27− 4− virus shows that both neuronal cell bodies and processes show abundant levels of GFP, demonstrating efficient gene delivery.
FIG. 6.
Virus strain 1764 27− 4− is not toxic to primary neurons in culture. DRG neurons from an adult rat were either mock infected or infected at an MOI of 10 with virus strain 17+ 27− pR19GFP or virus strain 1764 27− 4− pR20.5. One week postinfection, cells were photographed under phase-contrast and fluorescence optics. Cells infected with the 1764 27− 4− virus (GFP and phase contrast) and mock-infected cells (phase contrast only) are also shown at higher magnification (* and **, respectively).
Virus strain 1764 27− 4− pR20.5 directs the expression of delivered genes for extended periods in neuronal and nonneuronal cells.
Previous work found that the genomes of viruses individually mutated for all of the IE genes are capable of persisting in Vero cells for at least 28 days postinfection (52), but that gene expression from the CMV promoter was completely repressed immediately after infection in the vast majority of cells. Gene expression could, however, be stimulated by superinfection with a less-disabled virus expressing ICP0 (52).
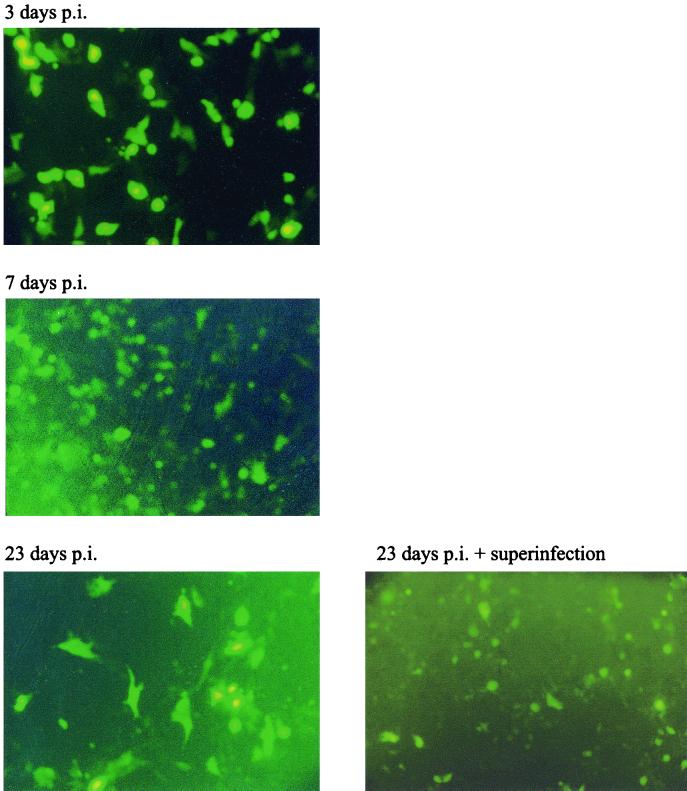
In order to test if the 1764 27− 4− virus could also establish a persistent infection, a similar experiment was performed. Vero cells at 50% confluence were infected at an MOI of 10 with virus strain 1764 27− 4− pR20.5. Following infection, cells were maintained in a low serum concentration at 34°C. At the last time point (23 days), a duplicate well was superinfected at an MOI of 5 with virus strain 17+ 27− (expressing no reporter gene). The results of this experiment are shown in Fig. 7. Here it can be seen that GFP expression was evident at all time points during the experiment and that, unlike in the previously published work (52), superinfection was not necessary for transgene expression to occur. GFP expression was present in decreasing numbers of cells over time, as in previous work (52), which is probably due to cell division in the culture and the finite life span of both transduced and untransduced cells. Superinfection of the cultures at 23 days did increase GFP expression levels slightly, but the effect was not marked. This demonstrates that the genome of virus strain 1764 27− 4− pR20.5, like that of the multiple IE gene-deleted viruses reported previously (52), is capable of persisting in the nucleus of infected cells, indicating the low toxicity of the vector. At all times up to the end of the experiment, the cells infected with virus strain 1764 27− 4− pR20.5 were indistinguishable from mock-infected cells (not shown). However, in contrast to previous work (52), virus strain 1764 27− 4− pR20.5 allowed continuous GFP expression without the need for superinfection. This previously unreported continued gene expression might be speculated to be due to the LAT P2 element in the promoter used (which has previously been shown to direct gene expression from heterologous promoters during herpesvirus latency [45]), residual ICP0 expression, or a combination of the two.
FIG. 7.
Virus strain 1764 27− 4− is persistently maintained in cultured cells. Vero cells at 50% confluency were infected at an MOI of 10 with virus strain 1764 27− 4− pR20.5. The cells were maintained in 2% serum at 34°C. At the last time point, a duplicate well was superinfected at an MOI of 5 with virus strain 17+ 27− (expressing no reporter gene). GFP expression from 1764 27− 4− pR20.5-infected cells is shown at 3, 7, and 23 days after infection without superinfection and after superinfection at 23 days.
In order to extend these findings, the expression characteristics of virus strain 1764 27− 4− pR20.5 in rat organotypic hippocampal slice cultures was also investigated. At various times after infection of the cultures with virus strain 1764 27− 4− pR20.5, a single slice was photographed under a fluorescence microscope (Fig. 8).
FIG. 8.
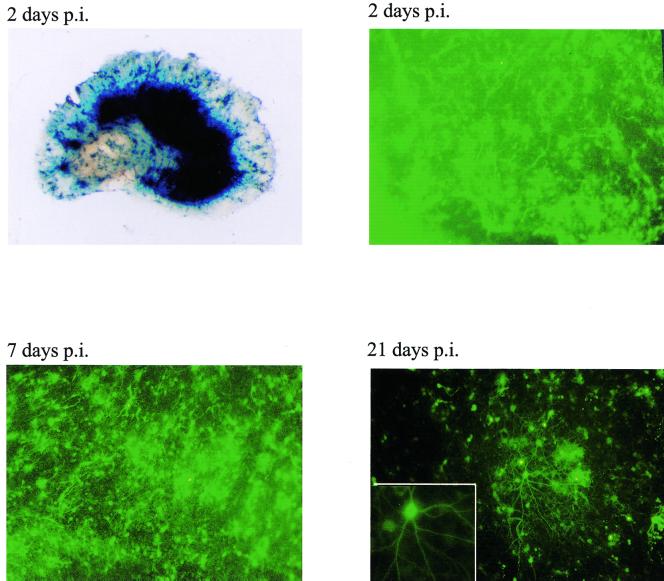
Transgene expression from virus strain 1764 27− 4− pR20.5 is maintained for at least 3 weeks in organotypic hippocampal slice cultures. Organotypic hippocampal slice cultures were prepared and infected 7 days later with 106 PFU of 1764 27− 4− pR20.5. An overview of an infected slice stained for lacZ and expression of GFP at 2, 7, and 21 days after infection are shown. The insert shows a higher-magnification image of an infected neuron at 21 days after infection.
Figure 8 shows that the high level of transgene expression observed at 2 days postinfection was still clearly detectable in the cultures 3 weeks after infection, albeit in a reduced number of cells. The processes of transduced neurons, even at the 3-week time point, were not swollen or beaded, suggesting that degeneration of transduced neurons had not occurred. Infected cultures maintained normal morphology and did not differ from mock-infected control cultures at any time. These results again suggest that the toxic effects of 1764 27− 4− pR20.5 on transduced cells are minimal, at least by morphological criteria, even over extended periods in culture.
Virus strain 1764 27− 4− gives widespread gene expression in the CNS following retrograde transport from the site of injection.
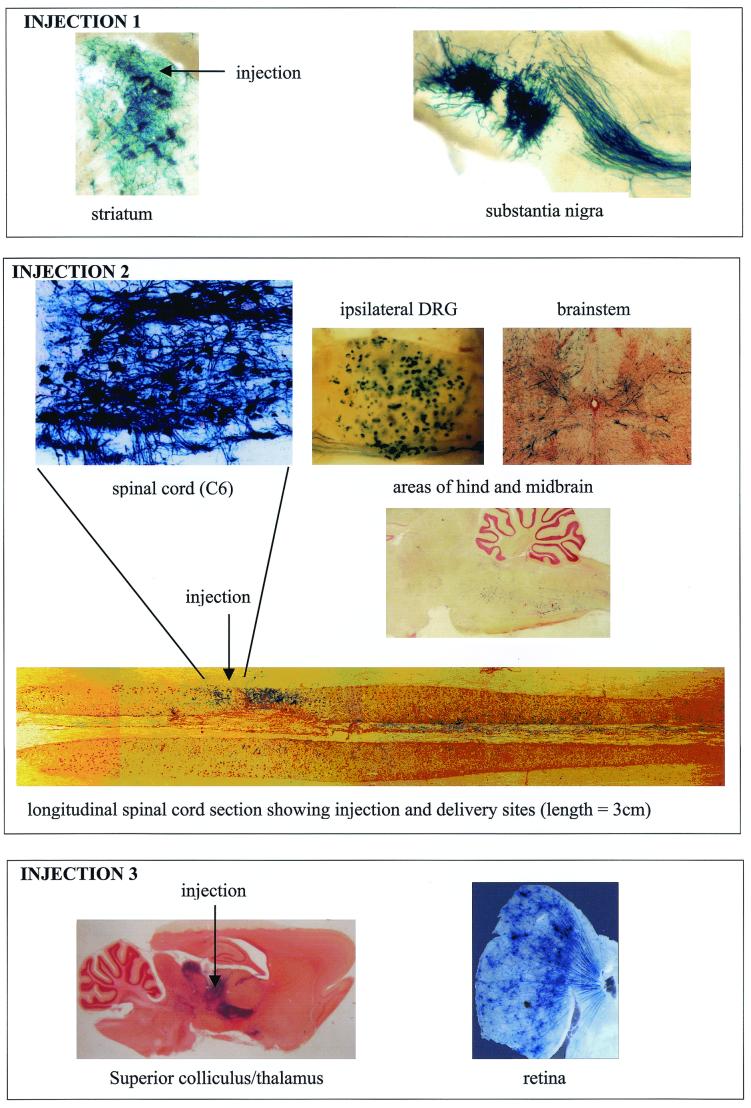
In order to examine transgene expression in the CNS in vivo with the viruses developed in this study, injections into the rat striatum, spinal cord, or superior colliculus were carried out with the 1764 27− 4− pR19lacZ virus. Transgene expression (lacZ) at both the injection site and connected sites within the nervous system was analyzed. The results of these experiments are shown in Fig. 9. In each case, the virus gave high-level gene expression at the site of injection and was also efficiently transported from the site of injection to the cell bodies at connected sites. This was clearly demonstrated by transport of the disabled virus from the striatum to the substantia nigra, from the superior colliculus via the optic nerve to the retinal ganglion cells, and from the C6 level of the spinal cord to DRG, the brainstem, and areas of the hind- and midbrain.
FIG. 9.
1764 27− 4− pR19lacZ is transported from the injection site in the CNS, giving widespread gene delivery. Adult Lewis rats were stereotaxically injected with 2.5 × 105 PFU of 1764 27− 4− pR19lacZ either in the striatum (injection 1), at the C6 level of the spinal cord (injection 2), or in the superior colliculus and thalamus (injection 3). One week (injection 1) or 3 weeks (injections 2 and 3) after injection, the animals were perfusion fixed, and relevant areas of the nervous system were sectioned and stained for lacZ expression. The sections from injection 1 are 200 μm thick, and the sections from injections 2 and 3 are 80 μm thick.
Both the promoter cassette and the vector backbone affect the levels of transgene expression in the CNS at inoculated and distant sites.
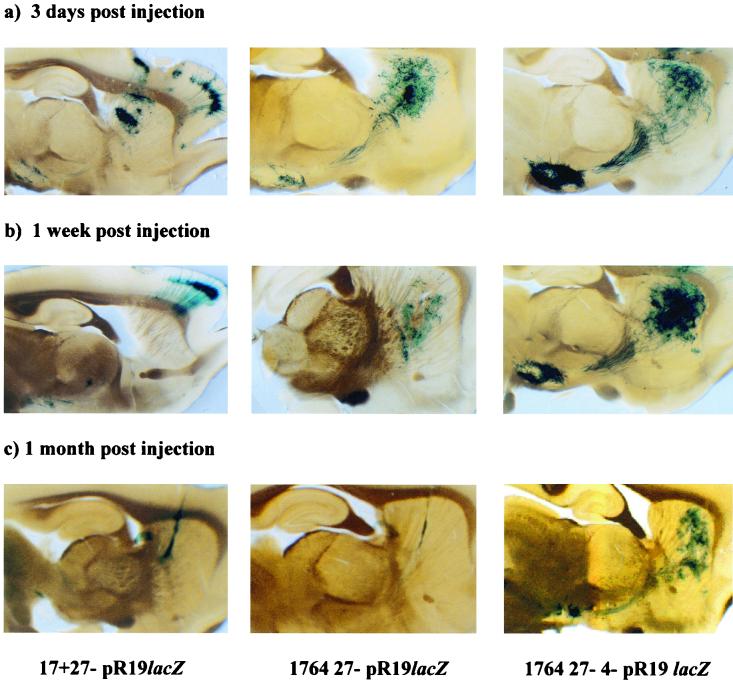
In order to test whether a promoter cassette containing the LAT P2 region was sufficient to drive the expression of a transgene and allow gene delivery to connected sites in the CNS regardless of the virus backbone, the following experiment was performed. Virus strain 17+ 27−, 1764 27−, or 1764 27− 4−, each containing the pR19lacZ cassette (Fig. 1), was stereotaxically injected (2 × 105 PFU) into the striatum of adult rats. The results of injection of this set of viruses are shown in Fig. 10. All three viruses gave lacZ expression at the early time points, but expression levels from the 17+ 27− and 1764 27− viruses were dramatically reduced between 3 days and 1 week postinjection, and expression was only detectable in a few cells by 1 month. However, with the 1764 27− 4− virus, expression was strong at 1 week and was still clearly evident at 1 month postinjection, in both the striatum and the substantia nigra. This suggests that the lack of expression with the 17+ 27− and 1764 27− viruses at later time points was due to the residual toxicity of these less-disabled viruses, rather than transcriptional shutoff of the LAT P2/CMV promoter, because this was able to remain active in the 1764 27− 4− virus.
FIG. 10.
Transgene expression from differently disabled viruses containing the pR19lacZ expression cassette in the rat CNS in vivo. Either 1764 27− 4− pR19lacZ, 1764 27− pR19lacZ, or 17+ 27− pR19lacZ (2.5 × 105 PFU) was stereotaxically injected into the rat striatum. Animals were perfusion fixed, and 200-μm sections were cut in the parasagittal plane to visualize the striatum and substantia nigra in the same section. (a) lacZ expression at 3 days after injection. (b) lacZ expression at 1 week after injection. (c) lacZ expression at 1 month after injection. The left panel shows transgene expression following injection of the 17+ 27− pR19lacZ virus, the middle panel shows the 1764 27− pR19lacZ virus, and the right panel shows the 1764 27− 4− pR19lacZ virus, as indicated at the bottom of the figure.
It is also noteworthy that at early times postinjection, the expression pattern differed according to the level of disablement of the virus. In the case of the 17+ 27− virus, the striatum, substantia nigra, and cortex were all stained at 3 days postinjection. However, by 1 week postinjection, the striatum was not labeled, and gene expression was only apparent at connected sites—the majority of gene expression was in the cortex, although some expression in the substantia nigra could still be seen. By 1 month postinjection, labeling of only a few cells was seen with the 17+ 27− virus. However, with the intermediately disabled 1764 27− virus, a different expression pattern was observed. Here, intense X-Gal staining was seen in the striatum, with some staining of the substantia nigra at 3 days postinjection. This pattern was maintained, but significantly reduced, by 1 week postinjection, and again only low-level gene expression was seen at 1 month postinjection. Unlike with the 17+ 27− virus, no gene delivery to cortical neurons other than those adjacent to the needle track occurred with this virus at any time point. With the 1764 27− 4− virus, lacZ expression was of considerably greater intensity in both the striatum and the substantia nigra at all time points during the experiment, but no delivery to cortical neurons was seen.
DISCUSSION
A number of studies have concluded that expression of ICP4, ICP27, ICP0, and ICP22 must be prevented to generate a nontoxic HSV vector (29, 30, 32, 52, 64). ICP4 is the major transcriptional regulatory protein expressed by HSV-1. ICP4 is required for the expression of the E and L genes (14) and is known to be highly toxic (29). ICP4 is able to transactivate E and L genes and downregulate the expression of some IE genes, especially ICP0 and ICP4 itself (50). ICP4 can also repress the activity of the latency promoters, either on its own or in combination with ICP0 (3, 22). ICP27 is the other essential regulatory IE gene (51), encoding a nuclear phosphoprotein which performs a number of diverse functions. These include repression of IE genes, control of the switch between E and L gene expression, and selection of transcriptional termination sites (40, 42). ICP27 normally mediates the shutoff of host protein synthesis by sequestering snRNPs (23, 24). ICP0 is not essential for a productive infection, but its deletion has been shown to significantly impair virus replication, especially at a low MOI (8, 10). Transient transfection studies have shown that ICP0 is a promiscuous transactivator of almost any target promoter, and this is enhanced by ICP4 (17, 18). ICP22 is nonessential for virus growth, but its deletion delays and reduces synthesis of Ε and L proteins, respectively (55). These effects are possibly mediated through an alteration in the phosphorylation of the large subunit of cellular RNA polymerase II (49). ICP22 is cytotoxic (30). A virus with deletions of ICP27, ICP22, and ICP4 is less toxic than a virus with deletions of only ICP27 and ICP4 (32, 64).
Bearing in mind these diverse functions, it is unsurprising that expression of HSV IE genes results in numerous toxic effects and that prevention of IE gene expression is necessary for the construction of a nontoxic HSV vector.
Prevention of HSV IE gene expression has only been achieved previously by the deletion or inactivation of each individual IE gene (52). In order to produce such disabled viruses at high titers, each of the deleted functions should be provided in the complementing cell line used for virus growth. This has proven problematic due to the toxicity of the IE genes, and no cell line expressing all of the IE genes has been reported. Here, we have taken the alternative approach of deleting the two essential IE genes (ICP4 and ICP27) and preventing the transactivation of the promoters of the remaining IE genes by including a disabling mutation in the virion protein VP16 (the in1814 mutation) (1). This approach was based on the ability of the in1814 mutation to reduce the transactivation capability of VP16 (1). Previous work has combined the in1814 mutation with IE gene mutations (ICP0 deficiency with a temperature-sensitive mutation in ICP4), but these viruses are capable of expressing IE genes and are replication competent below the completely nonpermissive temperature of 38.5°C (38, 47).
Relevant to the choice of VP16 mutation in the vectors described here, a recent report has demonstrated that a virus with the in1814 mutation in VP16 and with ICP0 inactivated was more toxic than a virus with the entire transactivation domain of VP16 deleted (the V422 mutation) and ICP0 inactivated (43). Viruses with the in1814 mutation probably therefore retain a residual transactivation activity (43). However, Fig. 2 demonstrates no detectable expression of ICP0, ICP22, or ICP47 from virus strain 1764 27− 4− pR20.5 in noncomplementing cells. It has previously been shown that the in1814 mutation does not reduce the level of ICP4 at all (1), whereas the V422 mutation decreases ICP4 levels significantly (56). The previously observed differences in toxicity between the ICP0/in1814 and ICP0/V422 viruses (43) are therefore probably primarily due to differences in ICP4 expression levels mediated by the different mutations to VP16. These results therefore suggest that there would be little difference between the toxicity of a virus containing the in1814 mutation and that of a virus containing the V422 mutation when ICP4 is deleted, as in the 1764 27− 4− viruses described here, although a 1764 27− 4− virus containing the V422 mutation rather than the in1814 mutation is currently under construction (and with ICP6 deleted [see below]) to test the gene delivery properties of such viruses.
In addition to the remaining IE genes, a virus with ICP4 deleted continues to express the large subunit of ribonucleotide reductase (ICP6) and ORF P, a protein of unknown function (14, 66). Although ORF P has been deleted from the 1764 27− 4− viruses (along with the deletion of ICP34.5), ICP6 has not, and so ICP6 expression levels were assessed. Figure 5 shows that ICP6 is expressed from a 1764 27− 4− virus in noncomplementing cells. The ICP6 promoter is not greatly transactivated by VP16, but is sensitive to transactivation by low levels of ICP0 (15, 54, 61). ICP6 expressed by the 1764 27− 4− virus may not therefore be due to residual transactivation by the mutated VP16, but due to low levels of ICP0, although if so, these are undetectable by Western blotting (Fig. 2). In agreement with previous work showing that ICP6 is nontoxic, we found that despite ICP6 expression, 1764 27− 4− pR20.5 was nontoxic to cultured neurons and Vero cells at a high MOI (Fig. 6, 7, and 8), at least to the extent of not obviously affecting cellular morphology over extended periods.
Previous work with HSV vectors in primary neuronal cultures found that a virus deficient for ICP4, ICP22, and ICP27 was nontoxic to DRG neurons, but caused almost complete elimination of supporting cells by 4 days postinfection (32). This is in contrast to Fig. 6, where the number of supporting cells in cultures infected with virus strain 1764 27− 4− pR20.5 is similar to that in mock-infected cultures. This suggests that the elimination of supporting cells seen previously (32) is due to ICP0 expression and that if low levels of ICP0 are expressed by 1764 27− 4− viruses, this is not sufficient to have such a toxic effect.
Interestingly, the expression of ICP22/47 and exogenous genes in noncomplementing cells was found not to be linearly dependent on virus dose with the intermediately disabled 1764 27− virus, the peak of expression occurring at an MOI of 0.5. A similar phenomenon has been observed previously in transient transfection assays (20, 21). Here, when plasmids encoding both ICP0 and ICP4 were transfected with any ΙΕ gene promoter linked to the chloramphenicol acetyltransferase (CAT) reporter gene, the ratio of the ICP0 plus ICP4 plasmid to the target plasmid determined if CAT activity was activated or repressed. When the ratio of ICP0 plus ICP4 plasmid to target plasmid was low, activation occurred, but when the ratio was high, repression occurred. This was described as a “spike response” (20, 21).
Figures 2 and 3 show a similar spike response with the 1764 27− virus. At a high MOI, the levels of ICP4 and ICP0 may here result in the repression of the ICP22/47 and CMV promoters. At a lower MOI, ICP4 and ICP0 may activate the promoters, as in the previous work (20, 21). Activation of IE promoters when ICP0 and ICP4 levels are low (e.g., directly after infection of a cell with a wild-type virus) and repression of IE promoters when ICP0 and ICP4 levels are high (e.g., at the end of ΙΕ gene expression) probably normally provide a negative feedback mechanism that regulates expression of the IE proteins. With 17+ 27−, functional VP16 presumably masks these effects, and with 1764 27− 4−, the deletion of ICP4 and lack of expression of ICP0 probably render any transactivation dependent on cellular factors, and thus expression levels of the delivered gene are dose dependent. Interestingly, the previous work (20, 21) observed that the spike response affects all of the ΙΕ gene promoters. This is in contrast to Fig. 2, where unlike ICP22 and ICP47, ICP0 is expressed in a linear, dose-dependent manner from the 1764 27− virus.
As well as vector cytotoxicity, HSV vector development has been hampered by a lack of promoters capable of driving exogenous gene expression at latent times after infection. Recent work has shown that DNA elements from the LAT region (LAP1 linked to LAP2), an IRES placed within the 2-kb LAT transcript, and the Moloney murine leukemia virus (MMLV) promoter in combination with the LAP1 promoter all allow expression of a reporter gene during latency (5, 16, 34–36). We have reported that the LAT P2 region is capable of conferring latent gene expression characteristics on neighboring promoters from either within the LAT region or at sites away from the LAT region (45). In the current work, we aimed to combine these promoter cassettes (45) with a nontoxic vector backbone and test for gene expression characteristics in primary neuronal cultures in vitro and the CNS in vivo.
Figure 6 shows highly efficient gene delivery to DRG neurons, and Fig. 8 shows that in organotypic hippocampal cultures, transgene expression is maintained for at least 23 days, albeit in a reduced number of cells compared to those at earlier time points. Previous work with highly disabled HSV, while showing the vectors to be nontoxic, has also shown that transgene expression is repressed in cultured cells immediately after infection. The 1764 27− 4− vectors described here are nontoxic by morphological criteria, while also allowing gene expression in cultured cells (Fig. 3, 6, 7, and 8). This could be due to either the LAT P2 elements in the promoters used, which have previously been shown to aid the maintenance of gene expression (45), residual but nontoxic levels of ICP0, or a combination of the two.
The vectors were also tested in the rat CNS in vivo. Previous work with disabled HSV to deliver genes to the CNS in vivo has been severely limited by the toxicity of the vector systems used, which results in high levels of neuronal damage shortly after injection and gene expression in only a very few cells in the longer term (11, 27, 37). Gene delivery to the CNS with low-toxicity, multiple IE gene-deficient vectors has not been reported.
Figure 9 shows the results of a set of injections with virus strain 1764 27− 4− pR19LacZ (Fig. 1). The virus was inoculated at various sites in the rat CNS, and transgene expression at both the injection site and at connected sites within the nervous system was examined. The axonal transport capability of wild-type HSV1 has long been known (2), and this property has been exploited to trace neuroanatomical pathways (reviewed in reference 33). This capability is unique among viruses currently used as gene delivery vectors and is thought to be mediated by the interaction of the virion protein UL34 with cytoplasmic dynein (65). However, in the CNS, HSV vectors that take advantage of these capabilities have not been described, due to either toxicity of the vectors or a lack of transgene expression due to the promoters used (see below). Figure 9 shows that unlike these previously reported HSV vectors, the 1764 27− 4− pR19lacZ virus is efficiently retrogradely transported, allowing high-level transgene expression not only at the injection site, but also at connected sites in the nervous system. Indeed, the widespread gene delivery observed in the striatum and substantia nigra following striatal injection; in the thalamus, superior colliculus, and retina following injection into the thalamus or superior colliculus; and in the spinal cord, DRGs, and mid- and hindbrain following injection into the spinal cord has not been observed previously with any vector, HSV or otherwise. These unique capabilities might have important implications for gene therapeutic or functional genomic applications where target neurons are in regions of the nervous system that are inaccessible by surgical techniques. This might include, for example, the dopaminergic neurons of the substantia nigra, which are affected in Parkinson's disease; the ganglion cells of the retina, which are affected in macular degeneration; or the delivery of genes to the spinal cord and DRG in the study and treatment of pain or in spinal cord or nerve repair.
Both a highly disabled vector backbone and an appropriate promoter cassette are required for widespread gene delivery to the CNS, allowing gene expression over extended periods. This is demonstrated in Fig. 10, where vectors with various levels of disablement but containing the same promoter cassette have been tested for gene delivery following injection into the rat striatum. It can be seen that less-disabled viruses containing the same promoter cassette do not drive widespread gene expression as effectively as the fully disabled 1764 27− 4− virus and that gene expression is more transient. We have previously shown that similar promoter cassettes not containing LAT P2 do not give gene expression for more than a few days in the peripheral nervous system (45), and thus these were not tested here.
Variable patterns of lacZ gene expression have been previously reported following injection of HSV vectors into the rat striatum (11, 27, 28, 37, 63). For example, an ICP0-deficient virus gave expression in the striatum and cortex but not the substantia nigra (11), and an ICP4-deficient virus gave expression in the striatum and a small number of cells in the substantia nigra (11, 37).
The results in Fig. 10 for the 17+ 27− virus (striatal and cortical expression) are more consistent with the expression pattern that had previously been attributed to a replication-attenuated (ICP0 deficient) rather than replication-incompetent virus (11). This suggests that cortical staining is associated more with high toxicity than replication competence per se, as had previously been suggested (11). The 1764 27− 4− virus would be expected to be considerably less toxic than any of the viruses discussed above, intense lacZ staining is observed in both the striatum and the substantia nigra and not in the cortex, and this expression is maintained for more extended periods than have previously been reported with HSV.
Overall, the current work has combined a promoter system previously shown to give transgene expression during HSV latency (45) with a nontoxic, replication-incompetent HSV vector backbone and achieved efficient gene transfer to cultured neurons and widespread gene expression in the CNS in vivo. The vectors described here have the advantage of not requiring the individual complementation of each of the IE genes by a complementing cell line, yet are still of low toxicity to target cells. The vectors contain promoters that are able to drive the expression of exogenous genes for extended periods and fully utilize the retrograde transport capabilities of HSV. Therefore, unlike with other vector systems, widespread gene delivery can result from a single injection and expression can be detected at sites far removed from the site of inoculation. This retrograde transport capability is unique among the viruses commonly used as gene delivery vectors and potentially has important applications in therapeutic gene delivery to surgically inaccessible areas of the brain and spinal cord.
ACKNOWLEDGMENTS
We are grateful to Barklie Clements (MRC Institute of Virology, Glasgow, Scotland), David Johnson (Oregon Health Sciences University, Portland), and Bernard Roizman (University of Chicago) for providing antibodies to HSV-1 ICP6, ICP47, and ICP22, respectively. We are also grateful to Greg Campbell (University College London) for performing some of the animal surgery.
REFERENCES
- 1.Ace C I, McKee T A, Ryan J M, Cameron J M, Preston C M. Construction and characterization of a herpes simplex virus type 1 mutant unable to transinduce immediate-early gene expression. J Virol. 1989;63:2260–2269. doi: 10.1128/jvi.63.5.2260-2269.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bak I J, Markham C H, Cook M L, Stevens J G. Intraaxonal transport of herpes simplex virus in the rat central nervous system. Brain Res. 1977;136:415–429. doi: 10.1016/0006-8993(77)90067-1. [DOI] [PubMed] [Google Scholar]
- 3.Batchelor A H, O'Hare P. Regulation and cell-type-specific activity of a promoter located upstream of the latency-associated transcript of herpes simplex virus type 1. J Virol. 1990;64:3269–3279. doi: 10.1128/jvi.64.7.3269-3279.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Batterson W, Roizman B. Characterization of the herpes simplex virion-associated factor responsible for the induction of α genes. J Virol. 1983;46:371–377. doi: 10.1128/jvi.46.2.371-377.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berthomme H, Lokensgard J, Yang L, Margolis T, Feldman L T. Evidence for a bidirectional element located downstream from the herpes simplex virus type 1 latency-associated promoter that increases its activity during latency. J Virol. 2000;74:3613–3622. doi: 10.1128/jvi.74.8.3613-3622.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bloom D C, Maidment N T, Tan A, Dissette V B, Feldman L T, Stevens J G. Long-term expression of a reporter gene from latent herpes simplex virus in the rat hippocampus. Brain Res Mol Brain Res. 1995;31:48–60. doi: 10.1016/0169-328x(95)00031-m. [DOI] [PubMed] [Google Scholar]
- 7.Brown S M, Ritchie D A, Subak-Sharpe J H. Genetic studies with herpes simplex virus type 1. The isolation of temperature-sensitive mutants, their arrangement into complementation groups and recombination analysis leading to a linkage map. J Gen Virol. 1973;18:329–346. doi: 10.1099/0022-1317-18-3-329. [DOI] [PubMed] [Google Scholar]
- 8.Cai W, Schaffer P A. Herpes simplex virus type 1 ICP0 regulates expression of immediate-early, early, and late genes in productively infected cells. J Virol. 1992;66:2904–2915. doi: 10.1128/jvi.66.5.2904-2915.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carpenter D E, Stevens J G. Long-term expression of a foreign gene from a unique position in the latent herpes simplex virus genome. Hum Gene Ther. 1996;7:1447–1454. doi: 10.1089/hum.1996.7.12-1447. [DOI] [PubMed] [Google Scholar]
- 10.Chen J, Silverstein S. Herpes simplex viruses with mutations in the gene encoding ICP0 are defective in gene expression. J Virol. 1992;66:2916–2927. doi: 10.1128/jvi.66.5.2916-2927.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chiocca E A, Choi B B, Cai W Z, DeLuca N A, Schaffer P A, DiFiglia M, Breakefield X O, Martuza R L. Transfer and expression of the lacZ gene in rat brain neurons mediated by herpes simplex virus mutants. New Biol. 1990;2:739–746. [PubMed] [Google Scholar]
- 12.Coffin R S, MacLean A R, Latchman D S, Brown S M. Gene delivery to the central and peripheral nervous systems of mice using HSV1 ICP34.5 deletion mutant vectors. Gene Ther. 1996;3:886–891. [PubMed] [Google Scholar]
- 13.De Felipe C, Hunt S P. The differential control of c-jun expression in regenerating sensory neurons and their associated glial cells. J Neurosci. 1994;14:2911–2923. doi: 10.1523/JNEUROSCI.14-05-02911.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DeLuca N A, McCarthy A M, Schaffer P A. Isolation and characterization of deletion mutants of herpes simplex virus type 1 in the gene encoding immediate-early regulatory protein ICP4. J Virol. 1985;56:558–570. doi: 10.1128/jvi.56.2.558-570.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Desai P, Ramakrishnan R, Lin Z W, Osak B, Glorioso J C, Levine M. The RR1 gene of herpes simplex virus type 1 is uniquely trans activated by ICP0 during infection. J Virol. 1993;67:6125–6135. doi: 10.1128/jvi.67.10.6125-6135.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dobson A T, Margolis T P, Sedarati F, Stevens J G, Feldman L T. A latent, nonpathogenic HSV-1-derived vector stably expresses beta-galactosidase in mouse neurons. Neuron. 1990;5:353–360. doi: 10.1016/0896-6273(90)90171-b. [DOI] [PubMed] [Google Scholar]
- 17.Everett R D. Trans activation of transcription by herpes virus products: requirement for two HSV-1 immediate-early polypeptides for maximum activity. EMBO J. 1984;3:3135–3141. doi: 10.1002/j.1460-2075.1984.tb02270.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Everett R D. A detailed mutational analysis of Vmw110, a trans-acting transcriptional activator encoded by herpes simplex virus type 1. EMBO J. 1987;6:2069–2076. doi: 10.1002/j.1460-2075.1987.tb02472.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fink D J, DeLuca N A, Goins W F, Glorioso J C. Gene transfer to neurons using herpes simplex virus-based vectors. Annu Rev Neurosci. 1996;19:265–287. doi: 10.1146/annurev.ne.19.030196.001405. [DOI] [PubMed] [Google Scholar]
- 20.Gelman I H, Silverstein S. Dissection of immediate-early gene promoters from herpes simplex virus: sequences that respond to the virus transcriptional activators. J Virol. 1987;61:3167–3172. doi: 10.1128/jvi.61.10.3167-3172.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gelman I H, Silverstein S. Herpes simplex virus immediate-early promoters are responsive to virus and cell trans-acting factors. J Virol. 1987;61:2286–2296. doi: 10.1128/jvi.61.7.2286-2296.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goins W F, Sternberg L R, Croen K D, Krause P R, Hendricks R L, Fink D J, Straus S E, Levine M, Glorioso J C. A novel latency-active promoter is contained within the herpes simplex virus type 1 UL flanking repeats. J Virol. 1994;68:2239–2252. doi: 10.1128/jvi.68.4.2239-2252.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hardwicke M A, Sandri-Goldin R M. The herpes simplex virus regulatory protein ICP27 contributes to the decrease in cellular mRNA levels during infection. J Virol. 1994;68:4797–4810. doi: 10.1128/jvi.68.8.4797-4810.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hardy W R, Sandri-Goldin R M. Herpes simplex virus inhibits host cell splicing, and regulatory protein ICP27 is required for this effect. J Virol. 1994;68:7790–7799. doi: 10.1128/jvi.68.12.7790-7799.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Honess R W, Roizman B. Regulation of herpesvirus macromolecular synthesis. I. Cascade regulation of the synthesis of three groups of viral proteins. J Virol. 1974;14:8–19. doi: 10.1128/jvi.14.1.8-19.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Howard M K, Kershaw T, Gibb B, Storey N, MacLean A R, Zeng B Y, Tel B C, Jenner P, Brown S M, Woolf C J, Anderson P N, Coffin R S, Latchman D S. High efficiency gene transfer to the central nervous system of rodents and primates using herpes virus vectors lacking functional ICP27 and ICP34.5. Gene Ther. 1998;5:1137–1147. doi: 10.1038/sj.gt.3300700. [DOI] [PubMed] [Google Scholar]
- 27.Huang Q, Vonsattel J P, Schaffer P A, Martuza R L, Breakefield X O, DiFiglia M. Introduction of a foreign gene (Escherichia coli lacZ) into rat neostriatal neurons using herpes simplex virus mutants: a light and electron microscopic study. Exp Neurol. 1992;115:303–316. doi: 10.1016/0014-4886(92)90196-w. [DOI] [PubMed] [Google Scholar]
- 28.Jin B K, Belloni M, Conti B, Federoff H J, Starr R, Son J H, Baker H, Joh T H. Prolonged in vivo gene expression driven by a tyrosine hydroxylase promoter in a defective herpes simplex virus amplicon vector. Hum Gene Ther. 1996;7:2015–2024. doi: 10.1089/hum.1996.7.16-2015. [DOI] [PubMed] [Google Scholar]
- 29.Johnson P A, Miyanohara A, Levine F, Cahill T, Friedmann T. Cytotoxicity of a replication-defective mutant of herpes simplex virus type 1. J Virol. 1992;66:2952–2965. doi: 10.1128/jvi.66.5.2952-2965.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johnson P A, Wang M J, Friedmann T. Improved cell survival by the reduction of immediate-early gene expression in replication-defective mutants of herpes simplex virus type 1 but not by mutation of the virion host shutoff function. J Virol. 1994;68:6347–6362. doi: 10.1128/jvi.68.10.6347-6362.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krisky D M, Marconi P C, Oligino T J, Rouse R J, Fink D J, Cohen J B, Watkins S C, Glorioso J C. Development of herpes simplex virus replication-defective multigene vectors for combination gene therapy applications. Gene Ther. 1998;5:1517–1530. doi: 10.1038/sj.gt.3300755. [DOI] [PubMed] [Google Scholar]
- 32.Krisky D M, Wolfe D, Goins W F, Marconi P C, Ramakrishnan R, Mata M, Rouse R J, Fink D J, Glorioso J C. Deletion of multiple immediate-early genes from herpes simplex virus reduces cytotoxicity and permits long-term gene expression in neurons. Gene Ther. 1998;5:1593–1603. doi: 10.1038/sj.gt.3300766. [DOI] [PubMed] [Google Scholar]
- 33.Kuypers H G, Ugolini G. Viruses as transneuronal tracers. Trends Neurosci. 1990;13:71–75. doi: 10.1016/0166-2236(90)90071-h. [DOI] [PubMed] [Google Scholar]
- 34.Lachmann R H, Efstathiou S. Utilization of the herpes simplex virus type 1 latency-associated regulatory region to drive stable reporter gene expression in the nervous system. J Virol. 1997;71:3197–3207. doi: 10.1128/jvi.71.4.3197-3207.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lokensgard J R, Berthomme H, Feldman L T. The latency-associated promoter of herpes simplex virus type 1 requires a region downstream of the transcription start site for long-term expression during latency. J Virol. 1997;71:6714–6719. doi: 10.1128/jvi.71.9.6714-6719.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lokensgard J R, Bloom D C, Dobson A T, Feldman L T. Long-term promoter activity during herpes simplex virus latency. J Virol. 1994;68:7148–7158. doi: 10.1128/jvi.68.11.7148-7158.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maidment N T, Tan A M, Bloom D C, Anton B, Feldman L T, Stevens J G. Expression of the lacZ reporter gene in the rat basal forebrain, hippocampus, and nigrostriatal pathway using a nonreplicating herpes simplex vector. Exp Neurol. 1996;139:107–114. doi: 10.1006/exnr.1996.0085. [DOI] [PubMed] [Google Scholar]
- 38.Marshall K R, Lachmann R H, Efstathiou S, Rinaldi A, Preston C M. Long-term transgene expression in mice infected with a herpes simplex virus type 1 mutant severely impaired for immediate-early gene expression. J Virol. 2000;74:956–964. doi: 10.1128/jvi.74.2.956-964.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mavromara-Nazos P, Ackermann M, Roizman B. Construction and properties of a viable herpes simplex virus 1 recombinant lacking coding sequences of the α47 gene. J Virol. 1986;60:807–812. doi: 10.1128/jvi.60.2.807-812.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McCarthy A M, McMahan L, Schaffer P A. Herpes simplex virus type 1 ICP27 deletion mutants exhibit altered patterns of transcription and are DNA deficient. J Virol. 1989;63:18–27. doi: 10.1128/jvi.63.1.18-27.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McFarlane M, Daksis J I, Preston C M. Hexamethylene bisacetamide stimulates herpes simplex virus immediate early gene expression in the absence of trans-induction by Vmw65. J Gen Virol. 1992;73:285–292. doi: 10.1099/0022-1317-73-2-285. [DOI] [PubMed] [Google Scholar]
- 42.McLauchlan J, Simpson S, Clements J B. Herpes simplex virus induces a processing factor that stimulates poly(A) site usage. Cell. 1989;59:1093–1105. doi: 10.1016/0092-8674(89)90765-4. [DOI] [PubMed] [Google Scholar]
- 43.Mossman K L, Smiley J R. Truncation of the C-terminal acidic transcriptional activation domain of herpes simplex virus VP16 renders expression of the immediate-early genes almost entirely dependent on ICP0. J Virol. 1999;73:9726–9733. doi: 10.1128/jvi.73.12.9726-9733.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.O'Hare P. The virion transactivator of herpes simplex virus. Semin Virol. 1993;4:145–155. [Google Scholar]
- 45.Palmer J A, Branston R H, Lilley C E, Robinson M J, Groutsi F, Smith J, Latchman D S, Coffin R S. Development and optimization of herpes simplex virus vectors for multiple long-term gene delivery to the peripheral nervous system. J Virol. 2000;74:5604–5618. doi: 10.1128/jvi.74.12.5604-5618.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pellett P E, McKnight J L, Jenkins F J, Roizman B. Nucleotide sequence and predicted amino acid sequence of a protein encoded in a small herpes simplex virus DNA fragment capable of trans-inducing alpha genes. Proc Natl Acad Sci USA. 1985;82:5870–5874. doi: 10.1073/pnas.82.17.5870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Preston C M, Mabbs R, Nicholl M J. Construction and characterization of herpes simplex virus type 1 mutants with conditional defects in immediate early gene expression. Virology. 1997;229:228–239. doi: 10.1006/viro.1996.8424. [DOI] [PubMed] [Google Scholar]
- 48.Preston C M, Nicholl M J. Repression of gene expression upon infection of cells with herpes simplex virus type 1 mutants impaired for immediate-early protein synthesis. J Virol. 1997;71:7807–7813. doi: 10.1128/jvi.71.10.7807-7813.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rice S A, Long M C, Lam V, Schaffer P A, Spencer C A. Herpes simplex virus immediate-early protein ICP22 is required for viral modification of host RNA polymerase II and establishment of the normal viral transcription program. J Virol. 1995;69:5550–5559. doi: 10.1128/jvi.69.9.5550-5559.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Roberts M S, Boundy A, O'Hare P, Pizzorno M C, Ciufo D M, Hayward G S. Direct correlation between a negative autoregulatory response element at the cap site of the herpes simplex virus type 1 IE175 (α4) promoter and a specific binding site for the IE175 (ICP4) protein. J Virol. 1988;62:4307–4320. doi: 10.1128/jvi.62.11.4307-4320.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sacks W R, Greene C C, Aschman D P, Schaffer P A. Herpes simplex virus type 1 ICP27 is an essential regulatory protein. J Virol. 1985;55:796–805. doi: 10.1128/jvi.55.3.796-805.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Samaniego L A, Neiderhiser L, DeLuca N A. Persistence and expression of the herpes simplex virus genome in the absence of immediate-early proteins. J Virol. 1998;72:3307–3320. doi: 10.1128/jvi.72.4.3307-3320.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Samaniego L A, Webb A L, DeLuca N A. Functional interactions between herpes simplex virus immediate-early proteins during infection: gene expression as a consequence of ICP27 and different domains of ICP4. J Virol. 1995;69:5705–5715. doi: 10.1128/jvi.69.9.5705-5715.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Samaniego L A, Wu N, DeLuca N A. The herpes simplex virus immediate-early protein ICP0 affects transcription from the viral genome and infected-cell survival in the absence of ICP4 and ICP27. J Virol. 1997;71:4614–4625. doi: 10.1128/jvi.71.6.4614-4625.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sears A E, Halliburton I W, Meignier B, Silver S, Roizman B. Herpes simplex virus 1 mutant deleted in the α22 gene: growth and gene expression in permissive and restrictive cells and establishment of latency in mice. J Virol. 1985;55:338–346. doi: 10.1128/jvi.55.2.338-346.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Smiley J R, Duncan J. 1814 linker insertion mutation. J. Virol. 71:6191–6193. 1997. Truncation of the C-terminal acidic transcriptional activation domain of herpes simplex virus VP16 produces a phenotype similar to that of the. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Soares K, Goins W F, Glorioso J C, Fink D. Advances in engineering HSV vectors for gene transfer to the nervous system. In: Meager A, editor. Gene therapy technologies, applications and regulations. New York, N.Y: John Wiley & Sons, Inc.; 1999. pp. 127–163. [Google Scholar]
- 58.Stevens J G, Wagner E K, Devi-Rao G B, Cook M L, Feldman L T. RNA complementary to a herpesvirus alpha gene mRNA is prominent in latently infected neurons. Science. 1987;235:1056–1059. doi: 10.1126/science.2434993. [DOI] [PubMed] [Google Scholar]
- 59.Stoppini L, Buchs P A, Muller D. A simple method for organotypic cultures of nervous tissue. J Neurosci Methods. 1991;37:173–182. doi: 10.1016/0165-0270(91)90128-m. [DOI] [PubMed] [Google Scholar]
- 60.Stow N D, Wilkie N M. An improved technique for obtaining enhanced infectivity with herpes simplex virus type 1 DNA. J Gen Virol. 1976;33:447–458. doi: 10.1099/0022-1317-33-3-447. [DOI] [PubMed] [Google Scholar]
- 61.Sze P, Herman R C. The herpes simplex virus type 1 ICP6 gene is regulated by a ‘leaky’ early promoter. Virus Res. 1992;26:141–152. doi: 10.1016/0168-1702(92)90153-z. [DOI] [PubMed] [Google Scholar]
- 62.Thomas S K, Lilley C E, Latchman D S, Coffin R S. Equine herpesvirus 1 gene 12 can substitute for vmw65 in the growth of herpes simplex virus (HSV) type 1, allowing the generation of optimized cell lines for the propagation of HSV vectors with multiple immediate-early gene defects. J Virol. 1999;73:7399–7409. doi: 10.1128/jvi.73.9.7399-7409.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wood M J, Byrnes A P, Kaplitt M G, Pfaff D W, Rabkin S D, Charlton H M. Specific patterns of defective HSV-1 gene transfer in the adult central nervous system: implications for gene targeting. Exp Neurol. 1994;130:127–140. doi: 10.1006/exnr.1994.1192. [DOI] [PubMed] [Google Scholar]
- 64.Wu N, Watkins S C, Schaffer P A, DeLuca N A. Prolonged gene expression and cell survival after infection by a herpes simplex virus mutant defective in the immediate-early genes encoding ICP4, ICP27, and ICP22. J Virol. 1996;70:6358–6369. doi: 10.1128/jvi.70.9.6358-6369.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ye G-J, Vaughan K T, Vallee R B, Roizman B. The herpes simplex virus 1 UL34 protein interacts with a cytoplasmic dynein intermediate chain and targets nuclear membrane. J Virol. 2000;74:1355–1363. doi: 10.1128/jvi.74.3.1355-1363.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yeh L, Schaffer P A. A novel class of transcripts expressed with late kinetics in the absence of ICP4 spans the junction between the long and short segments of the herpes simplex virus type 1 genome. J Virol. 1993;67:7373–7382. doi: 10.1128/jvi.67.12.7373-7382.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.York I A, Roop C, Andrews D W, Riddell S R, Graham F L, Johnson D C. A cytosolic herpes simplex virus protein inhibits antigen presentation to CD8+ T lymphocytes. Cell. 1994;77:525–535. doi: 10.1016/0092-8674(94)90215-1. [DOI] [PubMed] [Google Scholar]