Abstract
Mononuclear phagocytes (MP) and T lymphocytes play a pivotal role in the host immune response to human immunodeficiency virus type 1 (HIV-1) infection. Regulation of such immune responses can be mediated, in part, through the interaction of the T-lymphocyte-expressed molecule CD40 ligand (CD40L) with its receptor on MP, CD40. Upregulation of CD40L on CD4+ peripheral blood mononuclear cells during advanced HIV-1 disease has previously been reported. Based on this observation, we studied the influence of CD40L-CD40 interactions on MP effector function and viral regulation in vitro. We monitored productive viral infection, cytokine and β-chemokine production, and β-chemokine receptor expression in monocyte-derived macrophages (MDM) after treatment with soluble CD40L. Beginning 1 day after infection and continuing at 3-day intervals, treatment with CD40L inhibited productive HIV-1 infection in MDM in a dose-dependent manner. A concomitant and marked upregulation of β-chemokines (macrophage inhibitory proteins 1α and 1β and RANTES [regulated upon activation normal T-cell expressed and secreted]) and the proinflammatory cytokine tumor necrosis factor alpha (TNF-α) was observed in HIV-1-infected and CD40L-treated MDM relative to either infected or activated MDM alone. The addition of antibodies to RANTES or TNF-α led to a partial reversal of the CD40L-mediated inhibition of HIV-1 infection. Surface expression of CD4 and the β-chemokine receptor CCR5 was reduced on MDM in response to treatment with CD40L. In addition, treatment of CCR5- and CD4-transfected 293T cells with secretory products from CD40L-stimulated MDM prior to infection with a CCR5-tropic HIV-1 reporter virus led to inhibition of viral entry. In conclusion, we demonstrate that CD40L-mediated inhibition of viral entry coincides with a broad range of MDM immune effector responses and the down-modulation of CCR5 and CD4 expression.
Mononuclear phagocytes (MP), which include circulating monocytes, tissue macrophages, and dendritic cells, are one of the first cell types to encounter and be infected by human immunodeficiency virus type 1 (HIV-1) (19, 24, 37, 47, 68, 76, 83). As innate immune cells, MP play an integral role in the host immune response against the virus. This activity occurs through a wide range of effector functions, including phagocytosis, antigen presentation, and, upon activation, secretion of proinflammatory and antiviral factors, such as interferons (13, 16, 18, 22). During the process of antigen presentation, interactions between T lymphocytes and MP can lead to the activation of both types of cells (26, 39, 43, 55, 56, 65). The interaction of the T-lymphocyte-expressed molecule CD40 ligand (CD40L) with its MP-expressed receptor, CD40, represents one mechanism through which such immune activation can be induced (5, 15, 35, 39, 40, 50, 52, 70).
Expressed primarily by activated T lymphocytes, CD40L has been shown to regulate both humoral and cellular immune responses (5, 15, 35, 39, 40, 50, 52, 70). Such regulatory effects are mediated, in part, by the ability of CD40L to stimulate the production of proinflammatory cytokines, including interleukin-1β (IL-1β), IL-6, IL-12, and tumor necrosis factor alpha (TNF-α) (35, 39). CD40L-CD40 interactions have also been shown to induce the production of chemoattractant cytokines, such as macrophage inhibitory proteins 1α and 1β (MIP-1α and MIP-1β, respectively) and RANTES (regulated upon activation normal T-cell expressed and secreted), in MP (39, 40, 52). Importantly, many of these factors have been linked to the inhibition of HIV-1 (2, 4, 8, 9, 32, 36, 39, 41, 45, 55, 59, 61, 64, 74).
Based on these observations, we hypothesized that immune activation of MP by CD40L can affect the host immune response to HIV-1 infection. Specifically, our experiments were designed to determine whether CD40L-CD40 interactions inhibit HIV-1 infection in macrophages and whether such events are directly related to the production of β-chemokines or other proinflammatory factors. For this work, monocyte-derived macrophages (MDM) were infected with HIV-1ADA and then stimulated with soluble trimeric CD40L. Virus production was measured by determining reverse transcriptase (RT) activity, and viral DNA synthesis was monitored by PCR. Cytokine and β-chemokine production was measured by an enzyme-linked immunosorbent assay (ELISA), and the expression of CD4 and the β-chemokine receptor CCR5 was determined by fluorescence-activated cell sorting (FACS).
In this report, we show that treatment with CD40L inhibited viral infection in MDM. Moreover, we demonstrate that the inhibitory effects of CD40L on HIV-1 infection were mediated, at least in part, through the production of cytokines (TNF-α) and β-chemokines (RANTES) and the downregulation of CD4 and CCR5 expression on MDM. Importantly, the treatment of CCR5- and CD4-transfected 293T cells with CD40L-stimulated MDM (CD40L MCM) inhibited the entry of an HIV-1 reporter virus pseudotyped with the CCR5 envelope protein, YU2. In contrast, the treatment of CXCR4- and CD4-transfected cells with CD40L MCM had no inhibitory effect on the entry of a virus with the CXCR4 envelope protein, HXB2. When taken together, the results of this work provide insights into how immunocompetent MP may influence viral infection and affect the tempo of disease progression in the infected human host.
MATERIALS AND METHODS
Isolation and culturing of primary monocytes.
Human monocytes were recovered from peripheral blood mononuclear cells of HIV-1-, HIV-2-, and hepatitis B virus-seronegative donors after leukapheresis and then purified by countercurrent centrifugal elutriation (25). Monocytes were cultured as adherent monolayers (3.3 × 106 cells/well in 6-well plates, 2.2 × 106 cells/well in 12-well plates, and 1.1 × 106 cells/well in 24-well plates) and differentiated for 7 days in Dulbecco modified Eagle medium (Sigma Chemical Co., St. Louis, Mo.) supplemented with 10% heat-inactivated pooled human serum, 50 μg of gentamicin (Sigma)/ml, 10 μg of ciprofloxacin (Sigma)/ml, and macrophage colony-stimulating factor (M-CSF; 1,000 U/ml; highly purified recombinant; a generous gift from Genetics Institute, Inc., Cambridge, Mass.). All tissue reagents were screened and found negative for endotoxin (<10 pg/ml) (Pyrotell Limulus amoebocyte lysate [LAL]; Associates of Cape Cod, Inc., Woods Hole, Mass.) and mycoplasma contamination (Gen-Probe II; Gen-Probe Inc., San Diego, Calif.).
Infection of MDM.
Seven days after plating, MDM were infected with HIV-1ADA, HIV-1JR-FL, or HIV-189.6 at a multiplicity of infection of 0.1 virus/target cell (25). Viral stocks were screened for mycoplasma and endotoxin using hybridization and Limulus amebocyte lysate assays, respectively. Culture media were half-exchanged every 2 to 3 days. RT activity was determined in triplicate samples of culture fluids as described below. One to seven days after infection, HIV-1ADA-infected and replicate uninfected MDM were treated with soluble trimeric CD40L (a generous gift from Immunex Corporation, Seattle, Wash.).
Measurements of RT activity.
RT activity was determined in triplicate samples of cell culture fluids. For this assay, 10 μl of supernatant was incubated in a reaction mixture of 0.05% Nonidet P-40, 10 μg of poly(A)/ml, 0.25 μg of oligo(dT)/ml, 5 mM dithiothreitol, 150 mM KCl, 15 mM MgCl2, and [3H]TTP in Tris-HCl buffer (pH 7.9) for 24 h at 37°C. Radiolabeled nucleotides were precipitated with cold 10% trichloroacetic acid on paper filters in an automatic cell harvester and washed with 95% ethanol. Radioactivity was estimated by liquid scintillation spectroscopy (37).
Detection of RT by the Lenti-RT activity assay.
Monocytes were cultured for 7 days prior to inoculation with the following HIV-1 strains: HIV-1ADA, HIV-1JR-FL, and HIV-189.6. Five days after inoculation with HIV-1, MDM were stimulated with CD40L (2 μg/ml) for 48 h. Culture fluids were collected, and the level of RT enzyme was determined using a Lenti-RT activity assay kit (Cavidi Tech, Uppsala, Sweden) in accordance with the manufacturer's instructions. Briefly, cell supernatants were incubated with bromo-dUTP, and incorporated bromo-UTP was detected by bromodeoxyuridine binding antibody conjugated to alkaline phosphatase. The level of RT in the sample was determined by colorimetric analysis of the alkaline phosphatase activity.
PCR analysis of HIV-1 DNA synthesis.
Monocytes (1.1 × 106/ml) were cultured in 24-well plates (Costar Corp.) and infected with HIV-1 as described above. Prior to infection, the HIV-1 cell-free stocks were treated with DNase I for 30 min at 37°C (57). At 4, 8, 24, 48, and 96 h following viral exposure, samples were collected for RT analysis, and the residual medium was washed off with fresh phosphate-buffered saline (PBS; Sigma). The cells were then scraped into 0.5 ml of PBS. The resultant cell pellet was used for the extraction of cellular DNA with an Iso-quick nucleic acid extraction kit (ORCA Research Inc., Bothell, Wash.). The DNA was resuspended at a concentration of 2 × 104 cell equivalents/μl. PCR was performed to identify early (primers for long terminal repeat [LTR] U3/R) and late (primers for LTR U3/gag) products of reverse transcription (79). A ratio comparing the levels of early and late viral cDNAs to the levels of mitochondrial DNA (an internal control) was then determined. Standard HIV-1 cDNAs were prepared by simultaneous amplification of serial twofold dilutions of DNA extracted from 8e5 cells harboring defective HIV-1 proviruses (14). Amplified products were run on a Southern blot, hybridized to radiolabeled oligonucleotide probes, and quantified on a PhosphorImager (Molecular Dynamics, Sunnyvale, Calif.).
Detection of MIP-1α, MIP-1β, RANTES, and TNF-α by an ELISA.
Monocytes were cultured for 7 days prior to infection with HIV-1. One to seven days after infection with HIV-1, MDM were stimulated with CD40L (2 μg/ml) for 6, 24, or 48 h. Culture fluids were collected for chemokine and cytokine determinations. Chemokine production was assayed using Quantikine ELISA kits (R & D Systems, Minneapolis, Minn.) in accordance with the manufacturer's instructions. Chemokine and cytokine levels were normalized to cell numbers by measuring cell viability (51).
MTT reduction assay.
Cell cytotoxicity was assessed by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) reduction (51). Cells were incubated with 100 μl of MTT solution for 20 min at 37°C. The extent of MTT conversion to formazan by mitochondrial dehydrogenase, indicating cell viability, was determined by measuring the optical density (OD) at 490 nm using a microplate reader. The ratio of OD from treated cells to the OD from control cells reflected the percentage of surviving cells and was used to standardize cytokine and chemokine production determined by the ELISA.
FACS analysis.
MDM were cultured for 7 days at a density of 2.2 × 106 cells/ml. After 48 h in the presence or absence of CD40L (2 μg/ml), alone or in combination with CD40L antibody (M91; 20 μg/ml) or a cocktail of chemokine antibodies (anti-RANTES [5 μg/ml], anti–MIP-1β [5 μg/ml], and anti–MIP-1α [5 μg/ml]; R & D Systems), cells were washed with a 3% fetal bovine serum–PBS solution and then incubated with the following fluorescence-labeled antibodies: CCR5-PE, CD4-PE, and CD14-FITC (Pharmingen, Torrance, Calif.) (41). After 30 min of antibody incubation, cells were washed twice in a 3% fetal bovine serum–PBS solution and then fixed with 1% paraformaldehyde. Expression of cell surface antigens was assessed by immunofluorescence flow cytometry (FACSCalibur; Becton Dickinson) and analyzed with CellQuest software.
Intracellular calcium measurements.
MDM cultured on glass coverslips for 7 days were treated with human recombinant trimeric CD40L (2 μg/ml) for 24 h. Control and CD40L-treated MDM were washed and incubated with 5 μM fura II-AM (Molecular Probes, Inc., Eugene, Oreg.) for 30 min at 37°C in Ringer's solution of the following composition: 148 mM NaCl, 5 mM KCl, 1 mM MgSO4, 1.6 mM Na2HPO4, 1.5 mM CaCl2, and 5 mM d-glucose. The cells were washed twice and then incubated again for 20 min in Ringer's solution to allow for intracellular dye cleavage. The coverslips were held by an Attofluor cell chamber (Molecular Probes, Inc.), and 10 to 15 cells were chosen for imaging at room temperature. Data were recorded as the fluorescence emitted at 510 nm following excitation at 340 and 380 nm using a PTI Deltascan system as previously described (81). The concentrations of Ca2+ were calculated as follows: [Ca2+] = Kd[(R − Rmin)/(Rmax − R)] × (380min/380max), where Rmin and Rmax are the fluorescence values in the absence (with 3 mM EGTA) and presence of saturating Ca2+ (3 mM), respectively; Kd was 224 nM using PTI calcium imaging software.
Production of HIV-1 reporter viruses and the viral entry assay.
HIV-1 reporter viruses were produced as previously described (29). Briefly, envelope (Env)-defective recombinant HIV-1 luciferase reporter viruses were generated by cotransfection of 293T cells with 20 μg of pNL4–3env-LUC and 4 μg of plasmids encoding different HIV-1 Env proteins or pSVMLVenv using the calcium phosphate method (30). Pseudotyped HIVs were collected 48 h after transfection and assayed for RT enzyme activity. pNL4–3env-LUC, which encodes full-length Env-defective NL4–3 HIV-1 proviral DNA and expresses the luciferase reporter gene, was constructed as described previously (30). Complementation of Env-defective HIV-1 with HIV-1 Env expression plasmids in trans allows a single round of infection to occur and infected cells to be detected by the expression of luciferase. The Env plasmids used were YU-2env (HIV-1; CCR5 strain), HXB2env (HIV-1; CXCR4 strain), and MLVenv (amphotropic murine leukemia virus).
For the viral entry assay, 293T cells were transfected with plasmids expressing either CD4, CCR5, or CXCR4, alone or in combination. The transfected cells were plated on 24-well plates (105 cells/well) 24 h after transfection. The cells were incubated for 1 h at 37°C in macrophage-conditioned medium (MCM; diluted 1:10) from control MDM (Con MCM) or CD40L MCM prior to the addition of pseudotyped HIV-1 reporter viruses (50,000 cpm of viruses per well). The cell lysates were prepared 48 h after infection and assayed for luciferase activity (counts per second).
Statistical tests.
Data were reported as means and standard deviations (SD) of the mean. The data were normalized to cell numbers using the MTT assay for cell viability. The normalized values were used to perform statistical analyses using the analysis of variance, followed by the two-tailed Student t test for paired observations. To account for any donor-specific differences, experiments were performed with MDM derived from multiple donors. All assays were performed a minimum of three times with MDM obtained from three independent donors. Each assay was done in triplicate.
RESULTS
CD40L inhibits virus production in HIV-1-infected MDM.
To examine the effects of CD40L on ongoing HIV-1 infection and virus production in MDM, freshly elutriated human monocytes were plated and then allowed to differentiate for 7 days in medium containing M-CSF. The resulting MDM were infected with HIV-1ADA and then activated with soluble trimeric CD40L (2 μg/ml). Using this experimental system, our initial studies revealed that a single treatment with CD40L led to the inhibition of productive HIV-1 infection in the infected MDM. However, this effect was not sustainable over extended periods of time (data not shown).
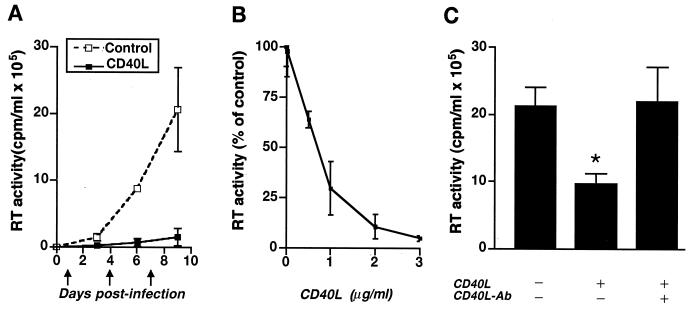
In an attempt to maintain constant levels of CD40L within our cultures, the ligand was added at 3-day intervals, beginning 1 day following virus inoculation. Compared to untreated, HIV-1-infected controls, HIV-1-infected MDM treated with CD40L (2 μg/ml) showed decreased levels of virus production, as measured by RT activity (Fig. 1A; from 20 × 105 to 2 × 105 cpm/ml). This CD40L-mediated inhibition was consistently observed at days 3, 6, and 9 following virus inoculation (Fig. 1A).
FIG. 1.
CD40L inhibits productive HIV-1 infection in MDM. (A) Beginning 1 day following virus inoculation, HIV-1ADA-infected and replicate uninfected MDM were treated at 3-day intervals (as indicated by the arrows) with CD40L at 2 μg/ml. Viral infection was monitored as RT activity in cell culture supernatants collected on days 3, 6, and 9 postinoculation. (B) Dose-dependent effects of CD40L (from 0.02 to 3 μg/ml) on HIV-1 at day 7 postinfection. (C) Neutralizing antibodies (Ab) to CD40L (M90; 8 μg/ml) were used to confirm the specificity of the effect of CD40L on virus production, as determined by measurement of RT activity. The asterisk denotes a P value of <0.01 when compared with the HIV-1-infected control. The results in panels A and C are shown as the mean and SD and are representative of three replicate assays performed with MDM from five donors. The results in panel B are shown as a percentage of the RT activity in HIV-1-infected controls (mean and SD) and are representative of three replicate assays performed with MDM from three donors.
To substantiate the inhibitory effects of CD40L on virus production, increasing doses of CD40L (ranging from 0.02 to 3.0 μg/ml) were used. CD40L-mediated inhibition of HIV-1 was shown to be dose dependent, with doses of greater than or equal to 1 μg/ml causing the most dramatic decreases in virus production, as measured by RT activity (Fig. 1B). Similar results were obtained using MDM from different donors (n ≥ 3). The specificity of the effect of CD40L on HIV-1 infection was determined by use of neutralizing antibodies to CD40L, such as M90 (Fig. 1C) and M91 (see Fig. 6A and 7A).
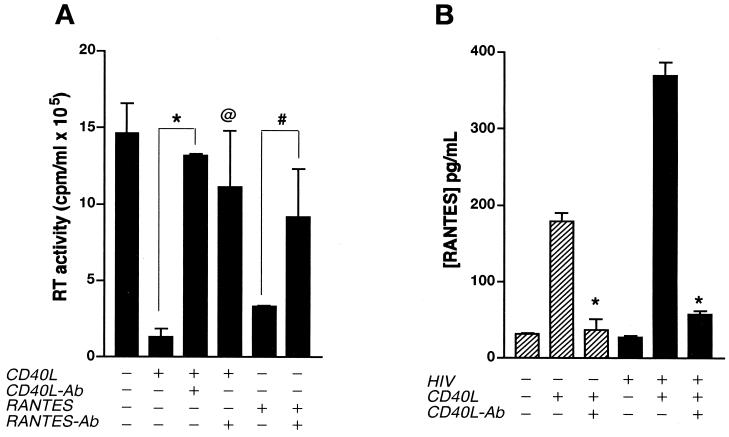
FIG. 6.
CD40L-mediated inhibition of HIV-1 infection is reversed by antibodies (Ab) to RANTES. (A) MDM cultures were treated with CD40L (2 μg/ml) 24 h after inoculation with HIV-1ADA and then retreated with CD40L every 3 days for the duration of the experiment. In replicate cultures, cells were pretreated with the positive control RANTES (0.5 μg/ml) 1 h before inoculation with HIV-1ADA and then retreated with RANTES (0.2 μg/ml) every 3 days. Virus production was measured as RT activity. (B) CD40L-induced production of RANTES in both uninfected and infected MDM. Neutralizing antibodies to CD40L (M91; 10 μg/ml) and RANTES (5 μg/ml) were used to determine the specificity of the effects mediated by CD40L (A and B) and RANTES (A), respectively. Results are expressed as the mean and SD and are representative of three independent experiments. In panel A, the asterisk denotes a P value of <0.01 and the “at” symbol denotes a P value of <0.02 when compared with HIV-1-infected, CD40L-activated MDM, and the number sign denotes a P value of <0.05 when compared with HIV-1-infected, RANTES-treated MDM. In panel B, the asterisk denotes a P value of <0.01 for comparisons with respective CD40L-treated controls.
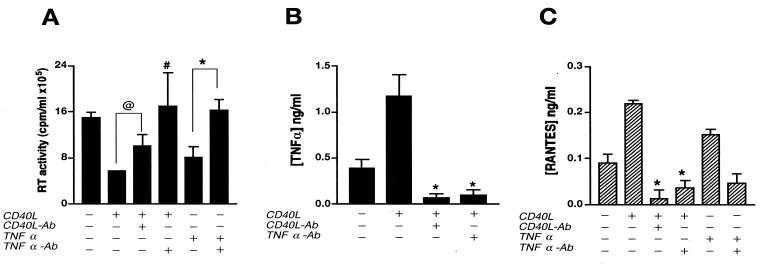
FIG. 7.
CD40L-mediated inhibition of virus production is reversed by TNF-α antibodies (Ab). (A) Seven days after infection, HIV-1ADA-infected MDM were treated with CD40L (2 μg/ml) or the positive control, TNF-α (0.02 μg/ml). Cell supernatants were collected 48 h after stimulation, and viral infection was determined as RT activity. (B and C) Levels of TNF-α (B) and RANTES (C) in supernatants collected at 6 and 24 h postactivation, respectively, were determined by an ELISA. Antibodies to CD40L (M91; 10 μg/ml) and TNF-α (2 μg/ml) were used to determine the specificity of the effects mediated by CD40L and whether such effects were mediated through the production of TNF-α (A, B, and C). Results are expressed as the mean and SD and are representative of three independent experiments. In panel A, the “at” symbol denotes a P value of <0.02 and the number sign denotes a P value of <0.05 when compared with HIV-1-infected, CD40L-treated MDM, and the asterisk denotes a P value of <0.01 when compared with HIV-1-infected, TNF-α-treated MDM. In panel B, the asterisk denotes a P value of <0.01 when compared with HIV-1-infected, CD40L-activated controls. In panel C, the asterisk denotes a P value of <0.01 when compared with CD40L- or TNF-α-treated MDM.
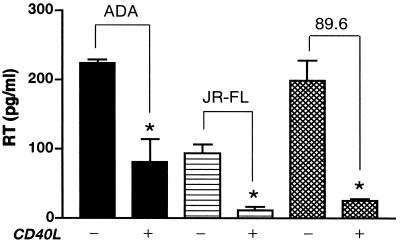
To further confirm the significance of the inhibitory effect of CD40L on HIV-1, a panel of HIV-1 strains, including the macrophage-tropic (M-tropic) strains HIV-1ADA and HIV-1JR-FL and the dual-tropic strain HIV-189.6, were used to infect MDM. Five days after inoculation, MDM were stimulated with CD40L (2 μg/ml). Supernatants were collected 48 h after activation and analyzed for RT activity using the Lenti-RT activity assay kit as described in Materials and Methods. MDM infected with HIV-1ADA and then treated with CD40L exhibited a 64% decrease (from 224 ± 4.52 to 81 ± 32.68 pg/ml) in RT levels compared to untreated HIV-1ADA-infected MDM (Fig. 2). Similarly, activation of MDM infected with HIV-1JR-FL or HIV-189.6 also led to decreased virus production, with an 88% decrease (from 94 ± 12.31 to 11 ± 5.87 pg/ml) in the JR-FL-infected cultures and an 87% decrease (from 199 ± 28.85 to 26 ± 2.22 pg/ml) in the 89.6-infected cultures (Fig. 2). Similar results were confirmed by both radioactive labeling and alkaline phosphatase RT activity assays using supernatants collected from four different MDM donors.
FIG. 2.
CD40L inhibits infection by M-tropic and dual-tropic HIV-1 strains. MDM were infected with the M-tropic strain HIV-1ADA or HIV-1JR-FL or the dual-tropic strain HIV-189.6. Beginning 5 days following virus inoculation, HIV-1-infected and replicate uninfected MDM were treated with CD40L (2 μg/ml). Viral infection was monitored by measuring the levels of RT enzyme in cell culture supernatants collected 48 h postactivation. Results are shown as the mean and SD and are representative of three replicate assays performed with MDM from four donors. The asterisk denotes a P value of <0.01 when compared with the respective HIV-1-infected controls.
Secretory products from CD40L-stimulated MDM inhibit productive HIV-1 infection.
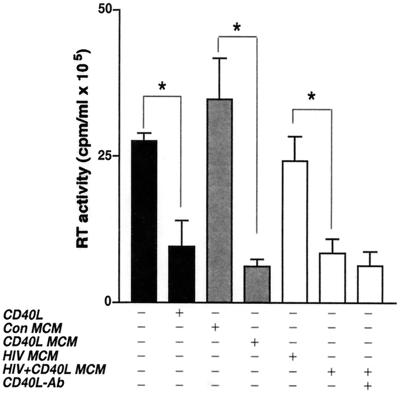
Having demonstrated that the direct addition of CD40L to cultures of infected MDM could inhibit virus production and that antibodies to CD40L could block this effect, we next examined the effects of secretory products from CD40L-activated MDM on productive HIV-1 infection. For this work, MCM collected from uninfected (Con MCM), CD40L-treated (CD40L MCM), HIV-1ADA-infected (HIV-1 MCM), or HIV-1ADA-infected and CD40L-treated (HIV-1/CD40L MCM) cells was placed on replicate cultures of infected MDM, and virus production was measured as RT activity. Figure 3 demonstrates that CD40L MCM caused an 80% reduction (from 30 × 105 to 6 × 105 cpm/ml) in RT activity when added to replicate cultures of MDM infected with HIV-1ADA. Interestingly, transfer of HIV-1/CD40L MCM also caused a 73% reduction (from 30 × 105 to 8 × 105 cpm/ml) in RT activity. The addition of neutralizing antibodies to CD40L (M90; 8 μg/ml) to MCM before addition to replicate MDM cultures did not block the inhibitory effects of either CD40L MCM (data not shown) or HIV-1/CD40L MCM (Fig. 3). However, in MDM directly treated with CD40L, neutralizing antibodies blocked the inhibitory effects of CD40L on HIV-1 (Fig. 1C). These data suggest that secretory factors produced in response to CD40L-mediated activation of MDM inhibit HIV-1 replication.
FIG. 3.
Secretory factors from CD40L-stimulated MDM inhibit virus production. Con MCM, CD40L MCM, HIV MCM, or HIV/CD40L MCM was placed on replicate cultures of infected MDM 24 h after inoculation. Viral infection was measured as RT activity. In order to determine whether residual CD40L in MCM was responsible for the effects seen, neutralizing antibodies (Ab) to CD40L (M90; 8 μg/ml) were added to one batch of HIV/CD40L MCM before transfer to the replicate cultures. Results are expressed as the mean and SD and are representative of three separate experiments performed with MDM from three donors. The asterisk denotes a P value of <0.01 when compared with the respective controls.
CD40L and CD40L MCM inhibit viral DNA synthesis in HIV-1-inoculated MDM.
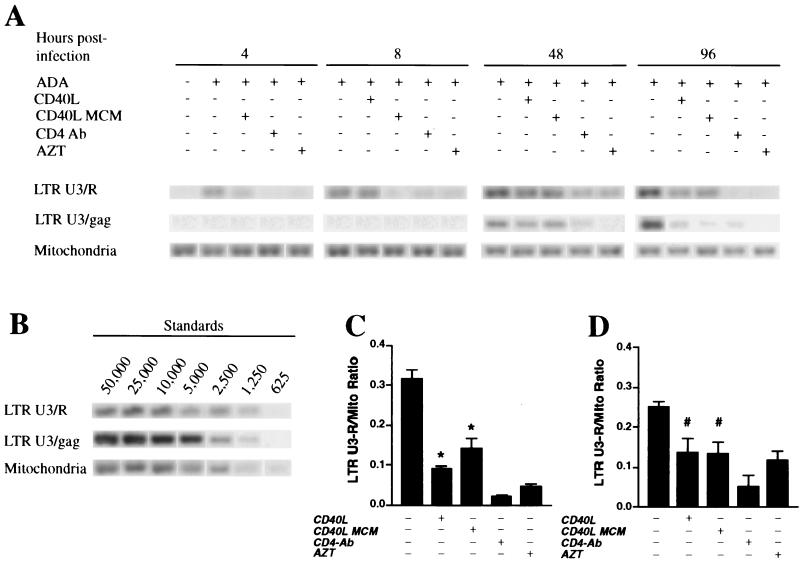
To determine at what stage CD40L or secretory products from CD40L-activated MDM affect HIV-1 infection in MDM, we examined the effects of these factors on the earliest stages of the HIV-1 life cycle. For this work, MDM were pretreated for 1 h with medium alone, CD40L MCM, or antibody to CD4 (BL4; 10 μg/ml) or for 24 h with zidovudine (AZT; 5 μM) and then inoculated with DNase-treated HIV-1ADA stocks. Four hours after inoculation, MDM were washed and then retreated with medium alone, CD40L (2 μg/ml), CD40L MCM, CD4 antibody, or AZT. At 4, 8, 24, 48, and 96 h following inoculation with virus, cells were fractured and DNA was isolated for measurement of viral nucleic acid synthesis. DNA PCR was performed to identify early (LTR U3/R) and late (LTR U3/gag) viral cDNA products of reverse transcription (79). A ratio comparing the levels of early and late viral cDNAs to the levels of mitochondrial DNA (an internal control) was then determined.
The Southern blot results from a representative experiment are shown in Fig. 4A. In HIV-1ADA-infected cells, treatment with CD40L (2 μg/ml) or with CD40L MCM led to decreased synthesis of both early and late viral cDNAs. At 8 h postinfection (Fig. 4C), cells treated with CD40L showed a 72% reduction in viral DNA synthesis, while treatment with CD40L MCM induced a 56% decrease in the synthesis of viral DNA. At 48 h following virus inoculation (Fig. 4D), CD40L- and CD40L MCM-treated cells demonstrated 44 and 48% reductions in early viral DNA synthesis, respectively. Inhibition of both early and late viral gene products was also observed at 24 h postinoculation (data not shown). In MDM pretreated with AZT or CD4 antibody (BL4), which was used as a positive control, viral DNA synthesis was suppressed (Fig. 4A, C, and D). Similar results were obtained using MDM from three different donors. The results suggest that CD40L and secretory products from CD40L-stimulated MDM inhibit early events in the HIV-1 life cycle.
FIG. 4.
CD40L affects viral DNA synthesis in HIV-1-infected MDM. (A) MDM were pretreated with CD40L MCM, antibody (Ab) to CD4, or AZT prior to infection with HIV-1ADA. Four hours postinfection, selected groups of infected MDM were treated with soluble trimeric CD40L (2 μg/ml). At 4, 8, 48, and 96 h postinfection, DNA was isolated from the fractured cells for detection of viral nucleic acid synthesis. PCR was performed to identify early (LTR U3/R) and late (LTR U3/gag) products of reverse transcription. Data from a representative experiment are shown. (B) HIV-1 cDNA extracted from 8e5 cells harboring a defective HIV-1 provirus was used as a standard (cell numbers are shown above lanes), and mitochondrial (Mito) DNA was used as an internal control. (C and D) Average ratios of early viral DNA products to mitochondrial DNA at 8 h (C) and 48 h (D) postinfection. Results are expressed as the mean and SD and are representative of three independent experiments. The asterisk denotes a P value of <0.01 and the number sign denotes a P value of <0.05 when compared with the infected controls.
CD40L induces β-chemokine and TNF-α production in MDM.
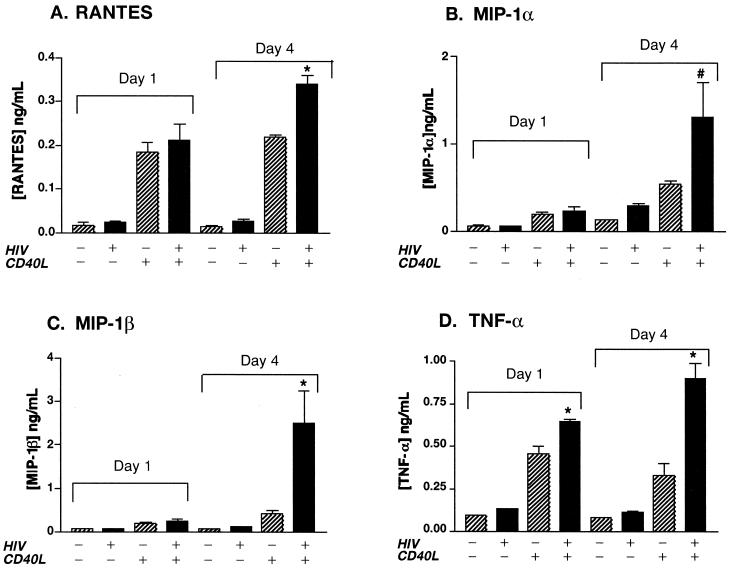
The experiments described above suggest that CD40L inhibits HIV-1 infection in MDM through a macrophage-derived soluble factor(s). In an attempt to identify the factor(s) responsible for such an effect, we next examined the effects of CD40L on MDM secretory function. Since a role for chemokines in the regulation of HIV-1 infection has been proposed by others (40, 74), we measured proinflammatory cytokine and chemokine production in CD40L-stimulated MDM. For this work, we assayed culture supernatants from uninfected, HIV-1-infected, CD40L-stimulated, and HIV-1-infected and CD40L-stimulated MDM for the presence of β-chemokines (MIP-1α, MIP-1β, and RANTES) and proinflammatory cytokines (TNF-α) with an ELISA.
Treatment of uninfected MDM with CD40L (2 μg/ml) alone induced a 16-fold increase in RANTES (from 13 ± 3 pg/ml in control cells to 219 ± 5 pg/ml in CD40L-treated MDM) (Fig. 5A), a 4-fold increase in MIP-1α (from 134 ± 2 pg/ml to 543 ± 35 pg/ml) (Fig. 5B), a 5-fold increase in MIP-1β (from 79 ± 2 pg/ml to 410 ± 81 pg/ml) (Fig. 5C), and a 4-fold increase in TNF-α (from 81 ± 1 pg/ml to 333 ± 65 pg/ml) (Fig. 5D). In our experimental system, HIV-1 infection alone induced only a modest increase in these factors, a finding that is consistent with previously published results (63). In contrast, HIV-1-infected and CD40L-activated cells consistently produced the highest levels of these factors (Fig. 5). Indeed, CD40L treatment of HIV-1-infected MDM led to enhanced β-chemokine and TNF-α production, inducing a 13-fold increase in RANTES (from 26 ± 7 pg/ml to 340 ± 18 pg/ml), a 4-fold increase in MIP-1α (from 304 ± 20 pg/ml to 1,300 ± 384 pg/ml), a 22-fold increase in MIP-1β (from 111 ± 2 pg/ml to 2,500 ± 70 pg/ml), and a nearly 8-fold increase in TNF-α (from 116 ± 4 pg/ml to 900 ± 90 pg/ml), when compared to the levels produced by HIV-1-infected MDM at day 4 postinfection. The effects of CD40L on β-chemokine and TNF-α production were demonstrated to be dose dependent (data not shown).
FIG. 5.
CD40L induces the production of β-chemokines and TNF-α. Beginning 1 day postinoculation, HIV-1ADA-infected and replicate uninfected MDM were stimulated every 3 days with CD40L (2 μg/ml). Cell culture fluids were collected 24 h after activation and assayed for RANTES, MIP-1α, MIP-1β, and TNF-α by an ELISA. Results are expressed as the mean and SD and are representative of three independent experiments. The asterisk denotes a P value of <0.01 and the number sign denotes a P value of <0.05 when compared with cells treated with CD40L alone.
These data suggested that the reduction in productive viral infection induced by CD40L (Fig. 1A) might be linked to the enhanced production of β-chemokines and TNF-α. To test this hypothesis, we examined the effects of blocking antibodies to individual cytokines and chemokines on the CD40L-mediated inhibition of HIV-1 infection. RANTES and TNF-α were selected as the primary candidates for this work, as both have been linked to the inhibition of HIV-1 infection in many cell types (4, 7, 9, 32, 41, 72).
Antibodies to RANTES and TNF-α reverse CD40L-mediated inhibition of HIV-1 infection.
To determine whether the ability of CD40L to inhibit HIV-1 was linked to its ability to enhance β-chemokine production, we examined the effect of neutralizing antibodies to RANTES (Fig. 6A) on virus production in CD40L-treated MDM. CD40L-induced inhibition of HIV-1 infection was blocked by antibodies to both CD40L (M91; 10 μg/ml) (Fig. 6A) and RANTES (5 μg/ml) (Fig. 6A). Other antibodies to CD40L (M90; 8 μg/ml) also blocked the production of RANTES in both uninfected (from 180 to 37 pg/ml) and infected (from 370 to 57 pg/ml) cells treated with CD40L (Fig. 6B). Used as a positive control for this work, RANTES (500 ng/ml) was administered to MDM 1 h prior to virus inoculation and added again (200 ng/ml) every 3 days. Treatment of MDM with RANTES caused an 80% decrease in RT activity compared to that in untreated controls (from 15 × 105 to 3 × 105 cpm/ml). Importantly, this response was also blocked by the addition of antibodies to RANTES (5 μg/ml). Moreover, treatment with MIP-1β or monocyte chemotactic protein 1 (MCP-1) (at doses ranging from 100 to 500 ng/ml) also inhibited HIV-1 infection. MIP-1β caused a 60% decrease in HIV-1 infection compared to results for untreated controls (from 15 × 105 to 6 × 105 cpm/ml), and MCP-1 induced a 72% decrease in RT activity (from 15 × 105 to 4 × 105 cpm/ml). Although the inhibitory effects of both MIP-1β and MCP-1 were blocked by the addition of their respective neutralizing antibodies (2 μg/ml), antibodies to these chemokines had no significant effect on the CD40L-mediated inhibition of HIV-1 infection in MDM (data not shown).
To further investigate the role of MDM secretory products in the CD40L-mediated inhibition of productive HIV-1 infection, we examined the relationship between decreased viral infection and cytokine production. TNF-α was selected as a representative cytokine, as it has been reported to diminish HIV-1 infection in macrophages through the production of β-chemokines (4, 32, 41). To determine whether the inhibitory effects of CD40L on HIV-1 replication were mediated by TNF-α, MDM were infected for 7 days with HIV-1ADA and then treated with CD40L (2 μg/ml) or TNF-α (20 ng/ml). Supernatants were collected 6, 24, 48, and 72 h after activation and analyzed for RT activity (Fig. 7A) or TNF-α production (Fig. 7B). As shown in Fig. 7A, the reduction in HIV-1 infection induced by CD40L was partially blocked by the addition of antibodies to CD40L (from 6 × 105 to 10 × 105 cpm/ml). Importantly, the addition of neutralizing antibodies to TNF-α (2 μg/ml), when administered in conjunction with CD40L 7 days after infection, blocked the inhibitory effects of CD40L on HIV-1 (from 6 × 105 to 17 × 105 cpm/ml) (Fig. 7A). Used as a positive control for this work, TNF-α alone caused a 45% decrease in RT activity compared to that in untreated controls (from 15 × 105 to 8 × 105 cpm/ml) (P < 0.01). This response was also blocked by the addition of neutralizing antibodies to TNF-α (2 μg/ml) (from 8 × 105 to 16 × 105 cpm/ml) (Fig. 7A).
As shown in Fig. 7B, the ability of CD40L to induce TNF-α production was blocked by preincubation with neutralizing antibodies to CD40L (from 1.2 to 0.07 ng/ml). Importantly, antibodies to both CD40L and TNF-α blocked the CD40L-induced production of RANTES in both uninfected (Fig. 7C) and infected (data not shown) MDM. Interestingly, TNF-α (20 ng/ml) induced the production of RANTES (Fig. 7C) and MIP-1α and MIP-1β (data not shown). This effect was blocked by the addition of neutralizing antibodies to TNF-α (Fig. 7C). Moreover, stimulation of MDM with CD40L led to an early (1 to 4 h postactivation) increase in TNF-α production, followed by a later (4 to 8 h poststimulation) increase in β-chemokine levels (data not shown). Together, these data suggest that increased levels of TNF-α may contribute to the inhibitory effects of CD40L through further induction of β-chemokines.
CD40L-mediated activation alters CCR5 expression on MDM.
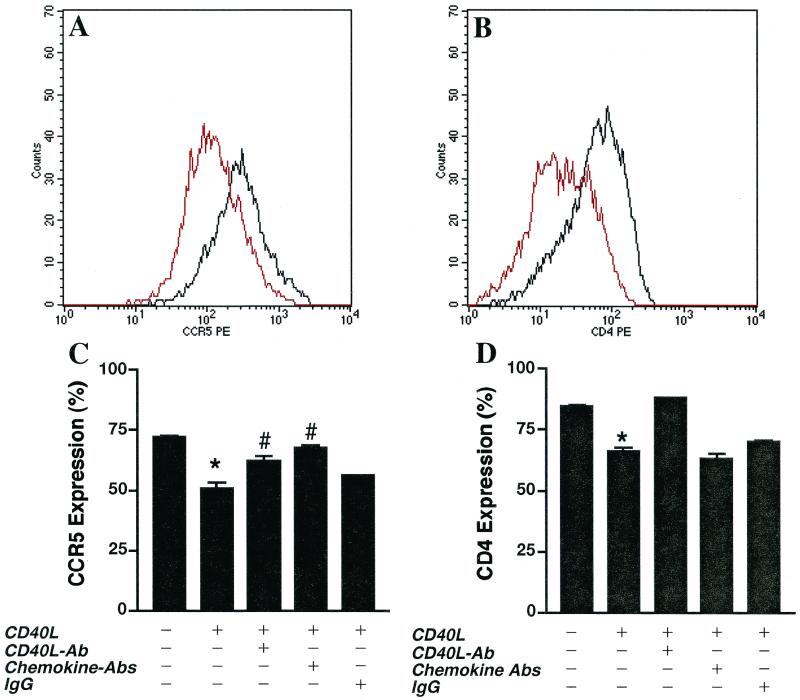
To further investigate the link between CD40L-mediated inhibition of HIV-1 infection and enhanced β-chemokine production, we examined the effects of CD40L activation on the expression of the β-chemokine receptor CCR5. MDM were treated with CD40L (2 μg/ml) for 48 h. The expression of CD14 (a monocyte marker), CCR5 (a β-chemokine receptor), and CD4 on both untreated and treated MDM was determined by FACS analysis. Macrophage populations were identified by forward- and side-scatter analysis and by CD14 immunoreactivity. The mean fluorescence intensity of CCR5 and CD4 expression on cells positive for both parameters was then determined.
Our experiments demonstrated that treatment with CD40L diminished CCR5 surface expression on MDM compared to untreated MDM. While on average CCR5 expression was decreased by 30% following CD40L treatment (Fig. 8A), the reduction in CCR5 expression ranged from 20 to 60%, depending on the donor (n = 5). This downregulation in CCR5 expression was shown to be specific, as antibodies to CD40L (M91; 20 μg/ml) were able to reverse the effect (Fig. 8C). Importantly, similar results were also observed with HIV-1-infected macrophages, where treatment with CD40L caused a 33 to 60% decrease in CCR5 expression (data not shown). In addition, MDM treated with the positive control RANTES (500 ng/ml), MIP-1β (500 ng/ml), MIP-1α (500 ng/ml), or TNF-α (100 ng/ml) also showed downregulation in CCR5 expression compared to control cells. RANTES induced a 30% reduction in CCR5 cell surface expression, MIP-1β and MIP-1α led to a 50% decrease, and TNF-α caused a 30% reduction (data not shown). Interestingly, a cocktail of chemokine antibodies (anti-RANTES [5 μg/ml], anti–MIP-1β [5 μg/ml], and anti–MIP-1α [5 μg/ml]) was also able to reverse the downregulation in CCR5 expression induced by treatment with CD40L (Fig. 8C). These results are consistent with published reports (4, 6, 41) showing that β-chemokines and TNF-α downregulate CCR5 expression. Importantly, these data provide further support for the hypothesis that soluble factors induced by CD40L-mediated activation of MDM lead to downregulation of the HIV-1 coreceptor, CCR5.
FIG. 8.
CD40L downregulates CD4 and CCR5 cell surface expression on MDM. (A and B) After 7 days in culture, elutriated and M-CSF-differentiated MDM were stimulated in the presence or absence of CD40L (2 μg/ml) for 48 h. Cells were dually immunostained for the monocyte antigen CD14 (CD14-FITC), the β-chemokine receptor CCR5 (CCR5-PE), or CD4 (CD4-PE). MDM populations, identified by forward- and side-scatter analyses and CD14 immunoreactivity, were examined for changes in the cell surface expression of CCR5 and CD4. The mean fluorescence intensity of CCR5 (A) and CD4 (B) expression is shown. Effects of treatment with CD40L are shown in red, and the expression of CCR5 and CD4 on untreated controls is shown in black. Profiles are representative of triplicate determinations with five donors. (C and D) Antibodies (Ab) to CD40L (M91; 20 μg/ml) or a cocktail of β-chemokine antibodies (anti-RANTES [5 μg/ml], anti–MIP-1β [5 μg/ml], and anti–MIP-1α [5 μg/ml]) was used to determine the specificity of the effects of CD40L on CCR5 (C) and CD4 (D) expression. In panel C, the asterisk denotes a P value of <0.01 when compared with the untreated control and the number sign indicates a P value of <0.01 when compared with CD40L-treated MDM. In panel D, the asterisk denotes a P value of <0.01 when compared with the untreated control.
Importantly, the addition of CD40L (2 μg/ml) to cultures of MDM led to a 40% reduction in CD4 cell surface expression. This downregulation in CD4 was specific, as antibodies to CD40L (M91; 20 μg/ml) were able to reverse the effect (Fig. 8D). In contrast, a cocktail of chemokine antibodies (anti-RANTES [5 μg/ml], anti–MIP-1β [5 μg/ml], and anti–MIP-1α [5 μg/ml]) had no significant effect on the CD40L-induced downregulation of CD4 expression (Fig. 8D). The profiles shown in Fig. 8 are representative of the trends observed using MDM from five donors. These data suggest that the inhibitory effects of CD40L on productive HIV-1 infection in MDM may be mediated, at least in part, through the production of soluble factors, which in turn decrease CCR5 cell surface expression.
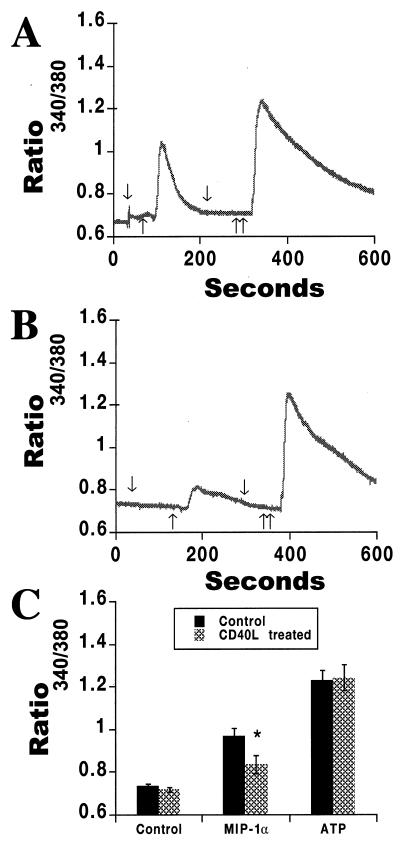
To further confirm the role of CD40L in regulation of CCR5, we determined the level of CCR5 activity on both untreated and CD40L-treated MDM using calcium imaging analysis. MIP-1α (1 μg/ml), a natural ligand for CCR5, was used to assay for chemokine receptor-mediated increases in intracellular calcium levels (Fig. 9). ATP (100 μM) was used as a control, as it affects intracellular calcium levels through CCR5-independent pathways (Fig. 9). In MDM pretreated with CD40L (2 μg/ml for 24 h), the calcium response induced by application of the CCR5 ligand MIP-1α was reduced (Fig. 9B) in comparison to the response evoked in untreated MDM (Fig. 9A). In contrast, ATP-induced calcium responses remained unchanged (Fig. 9A and B). This observation was confirmed in three separate experiments performed with three sets of MDM donors. The average of these data is shown in Fig. 9C, where the intracellular calcium response induced by MIP-1α was significantly higher (P < 0.01) in untreated MDM (0.965 ± 0.037; n = 3) than in CD40L-treated MDM (0.834 ± 0.043). These data are expressed as ratios of the absorbances at 340 and 380 nm. This finding, in conjunction with the results of the FACS analysis, demonstrates that the levels and activity of CCR5 on MDM are decreased after treatment with CD40L.
FIG. 9.
CD40L alters levels of functional CCR5 on MDM. MDM were cultured for 7 days and then treated overnight with CD40L (2 μg/ml). Replicate controls were left untreated. MIP-1α (1 μg/ml), a natural ligand for CCR5, was used to assay for chemokine receptor-mediated increases in intracellular calcium levels. ATP (100 μM) was used as a control for this assay, as it affects intracellular calcium levels through CCR5-independent pathways. The expression of functional CCR5, as determined by changes in intracellular calcium, was then measured with fura II. In panels A and B, arrows pointing down denote the addition of buffer, a single arrow pointing up denotes the addition of MIP-1α (1 μg/ml), and double arrows pointing up denote the addition of ATP (100 μM). In MDM pretreated for 24 h with CD40L (2 μg/ml), the MIP-1α-mediated calcium response was reduced (B) in comparison to the response evoked in untreated MDM (A), while ATP-induced calcium responses remained unchanged (A and B). The data shown in panels A and B are representative of three replicate experiments performed with MDM from three donors. The average of these data is shown in panel C and is expressed as the mean and SD. In panel C, the asterisk denotes a P value of <0.01 when compared with MDM treated with medium alone.
Secretory factors from CD40L-stimulated MDM inhibit M-tropic HIV-1 entry.
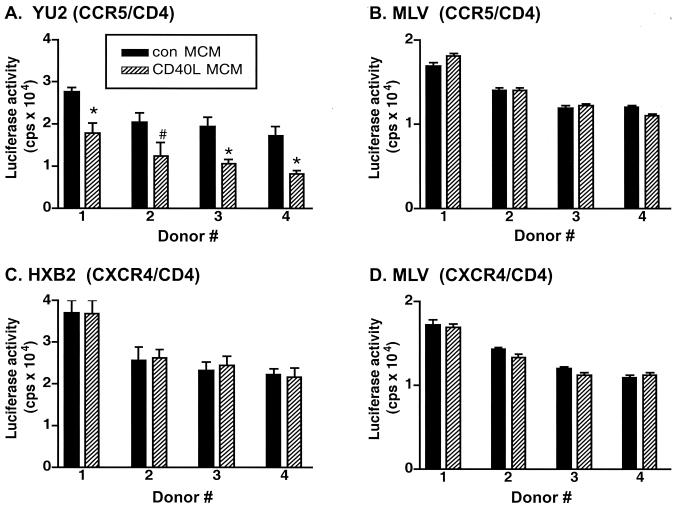
To determine whether factors produced by CD40L-treated MDM could block the entry of HIV-1, Con MCM or CD40L MCM (CD40L was used at 2 μg/ml) was placed on CCR5- and CD4-, CXCR4- and CD4-, CCR5-, CXCR4-, or CD4-transfected 293T cells. Viral entry was detected by measuring HIV-1 luciferase reporter virus activity. For this work, transfected 293T cells were incubated for 1 h at 37°C with either Con MCM or CD40L MCM and then infected with Env-defective recombinant HIV-1 luciferase reporter viruses pseudotyped with either YU2, a representative M-tropic CCR5 Env protein, or HXB2, a T-lymphocyte-tropic CXCR4 Env protein (29). The amphotropic murine leukemia virus (A-MLV) envelope was used as a control for specificity. The efficiency of viral entry was determined by measuring luciferase activity (counts per second) 48 h after infection. Results were obtained from triplicate determinations using MCM collected from four MDM donors.
As shown in Fig. 10A, CD40L MCM inhibited infection with HIV-1 luciferase reporter viruses containing YU2 Env by 40%, compared to the results for cells treated with Con MCM. In contrast, entry of a reporter virus containing HXB2 Env was not inhibited by the addition of CD40L MCM (Fig. 10C). Neither Con MCM nor CD40L MCM inhibited infection by virus pseudotyped with nonspecific A-MLV Env (Fig. 10B and 10D) or Env-defective HIV-1 reporter virus (data not shown). In addition, the direct addition of fresh medium or soluble CD40L alone had no inhibitory effects in any of the cell systems described above. These data suggest that the observed inhibition of viral entry is due to soluble factors produced by CD40L-activated MDM.
FIG. 10.
Secretory factors from CD40L-stimulated MDM inhibit M-tropic HIV-1 entry. Con MCM or CD40L MCM was placed on 293T cells transfected with CCR5- and CD4- or CXCR4- and CD4-expressing plasmids for 1 h prior to infection with HIV-1 luciferase reporter viruses pseudotyped with either YU2 (CCR5 Env) (A), HXB2 (CXCR4 Env) (C), or MLV (nonspecific control Env acquired from A-MLV) (B and D). Forty-eight hours after infection, viral entry into CCR5- and CD4- or CXCR4- and CD4-transfected 293T cells was determined by measurement of luciferase activity (counts per second). Results are expressed as the mean and SD (n = 3) for 293T cells treated with MCM from four human donors. In panel A, the asterisk denotes a P value of <0.01 and the number sign denotes a P value of <0.05 when compared with cells treated with Con MCM.
DISCUSSION
In this report, we demonstrated that CD40L-mediated activation of HIV-1-infected MDM led to inhibition of HIV-1 infection, enhanced TNF-α and β-chemokine secretion, and decreased expression of CD4 and the β-chemokine receptor CCR5. Interestingly, secretory products from CD40L-activated MDM, when used to treat replicate cultures of infected MDM, were also shown to inhibit virus production. We propose that the mechanism for these effects could be linked to the increased levels of β-chemokines and cytokines produced in response to stimulation with CD40L. While it is generally accepted that β-chemokines play an important role in the regulation of HIV-1 infection (6), the role of cytokines, such as TNF-α, in such events is still widely debated. Nonetheless, previous reports (4, 41), as well as our own work, have shown that TNF-α can induce the secretion of β-chemokines and retard HIV-1 infection. Moreover, cytokines such as alpha interferon have been shown to inhibit HIV-1 infection in MDM (22, 26). While the inhibitory effects of CD40L on HIV-1 infection may be regulated by both chemokines and cytokines acting in tandem, the individual contributions of these factors to the CD40L-mediated inhibition of HIV-1 infection require further investigation.
Providing further support for the hypothesis that CD40L inhibits HIV-1 infection through the production of soluble factors, neutralizing antibodies to both RANTES and TNF-α were shown to block the inhibitory effects of CD40L on productive HIV-1 infection in MDM. Interestingly, antibodies to MIP-1β and MCP-1 had no effect on the CD40L-mediated inhibition of HIV-1 infection. Importantly, secretory products from CD40L-stimulated MDM inhibited the entry of an HIV-1 luciferase reporter virus pseudotyped with the M-tropic CCR5 envelope protein, YU2, into CCR5- and CD4-transfected 293T cells. In contrast, CD40L MCM had no inhibitory effect on the entry of virus with the CXCR4 envelope protein, HXB2, into CXCR4- and CD4-transfected cells. Together, these data suggest that multiple factors induced by CD40L stimulation of MDM may operate in tandem to regulate HIV-1 infection. Such inhibition appears to be mediated, at least in part, through the regulation of viral entry.
One potential mechanism through which CD40L may inhibit productive HIV-1 infection in MDM is the regulation of CD4 and the HIV-1 coreceptor, CCR5. In support of this notion, we observed decreased levels of CD4 and CCR5 on MDM in response to treatment with CD40L. Based on reports demonstrating that chemokines can induce chemokine receptor endocytosis (3, 46, 54, 77), we hypothesize that CD40L or the secretory products induced by it may decrease the cell surface expression of CCR5 through mechanisms of internalization. Support for this hypothesis was provided by data (Fig. 8C) demonstrating that a cocktail of chemokine antibodies (anti-RANTES, anti–MIP-1β, and anti–MIP-1α) could reverse the CD40L-mediated downregulation of CCR5. Moreover, unpublished data from our group has shown that CCR5 mRNA expression in MDM is not altered after treatment with CD40L. Although these data suggest that receptor internalization, as opposed to altered transcription, is responsible for the reduction in CCR5 cell surface expression, this hypothesis requires further investigation. Moreover, the mechanism by which CD40L activation causes a downregulation in CD4 expression remains to be determined.
The ability of HIV-1-infected and CD40L-activated MDM to produce substantially higher levels of β-chemokines and TNF-α than MDM stimulated with CD40L alone suggests that HIV-1 infection “primes” macrophages for subsequent immune stimulation. Such priming events may enhance the ability of MP to produce factors that regulate HIV-1 infection. This may explain why virus is so tightly regulated in tissue, such as the brain and lungs, during the early stages of infection, when CD40L-expressing T lymphocytes are still plentiful (23, 37, 53, 76). While sustained increases in cytokines and chemokines can regulate the spread of HIV-1 infection among MP, such factors can also alter the protective functions of the MP and cause adverse effects, depending upon the environment in which they are expressed (21, 48, 69, 82). Indeed, the pathogenic potential of such events is clearly seen in the brain during HIV-1-associated dementia (HAD), where the number of immune-activated MP correlates with the severity of cognitive impairment (10, 17, 20, 34, 57, 58, 60, 80). While the mechanism by which MP become immune activated during HAD is still not completely understood, increasing evidence suggests that factors, such as CD40L, may contribute to this process. Indeed, several lines of evidence suggest a role for CD40L-mediated activation in HAD. First, the expression of CD40L on peripheral blood mononuclear cells has been reported to be increased in HAD patients (62). Second, findings from our laboratory and findings of others suggest that CD40L-CD40 interactions can stimulate the production of factors, such as chemokines, cytokines, and proteinases (1, 11, 27, 28, 35, 38–40, 42, 49, 67, 71, 75, 78), which compromise the integrity of the blood-brain barrier and promote infiltration of monocytes into the brain. Third, factors that are capable of inducing neuronal injury (12, 17, 31, 33, 44, 66, 73, 81) are substantially upregulated when MDM are infected with HIV-1 and then activated with CD40L. While we assume that the principal biological function of CD40L is to regulate host immune responses, the evidence presented above suggests that CD40L-mediated activation of MP could adversely affect the outcome of HIV-1 infection in many tissues, including the brain. However, this hypothesis certainly requires further investigation.
When taken together, the data presented in this paper implicate a role for CD40L-mediated activation in the regulation of viral infection in MP and a possible mechanism for such events. The finding that CD40L stimulation inhibits viral entry and retards the HIV-1 life cycle in MP implicates a role for CD40L-mediated activation in host antiviral defenses. Moreover, these findings reinforce the importance of innate immune responses in regulating the tempo of disease onset and progression in the HIV-1-infected human host.
ACKNOWLEDGMENTS
We kindly thank Immunex Corporation for providing soluble, trimeric CD40L; Anuja Ghorpade, Charles Kuszynski, Linda Wilkie, Lisa Ryan, and Walter Zink for scientific discussion and technical suggestions; Alicia Lopez, Clancy Williams, Lori Todd, Michael Bauer, and David Erichsen for technical support; and Julie Ditter and Robin Taylor for outstanding administrative and secretarial support.
This work was supported in part by research grants from the National Institutes of Health: P01 NS31492–01, R01 NS34239–01, R01 NS34239–02, and R01 NS36126–01 (to Howard E. Gendelman), P20 RR15635–01 (to Jialin Zheng), and R01 NS39804 (to Johnny He).
REFERENCES
- 1.Alderson R M, Armitage R J, Tough T W, Strockbine L, Fanslow W C, Spriggs M K. CD40 expression by human monocytes: regulation by cytokines and activation of monocytes by the ligand for CD40. J Exp Med. 1993;178:669–674. doi: 10.1084/jem.178.2.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alkhatib G, Combadiere C, Broder C C, Feng Y, Kennedy P E, Murphy P M, Berger E A. CC CKR5: a RANTES, MIP-1α, MIP-1β receptor as a fusion cofactor for macrophage-tropic HIV-1. Science. 1996;272:1955–1958. doi: 10.1126/science.272.5270.1955. [DOI] [PubMed] [Google Scholar]
- 3.Alkhatib G, Locati M, Kennedy P E, Murphy P M, Berger E A. HIV-1 coreceptor activity of CCR5 and its inhibition by chemokines: independence from G protein signaling and importance of coreceptor downmodulation. Virology. 1997;234:340–348. doi: 10.1006/viro.1997.8673. [DOI] [PubMed] [Google Scholar]
- 4.Bailer R T, Lee B, Montaner L J. IL-13 and TNF-alpha inhibit dual-tropic HIV-1 in primary macrophages by reduction of surface expression of CD4, chemokine receptors CCR5, CXCR4 and post-entry viral gene expression. Eur J Immunol. 2000;30:1340–1349. doi: 10.1002/(SICI)1521-4141(200005)30:5<1340::AID-IMMU1340>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 5.Caux C, Massacrier C, Vanbervliet B, Dubois B, Kooten C V, Durand I, Banchereau J. Activation of human dendritic cells through CD40 cross-linking. J Exp Med. 1994;180:1263–1272. doi: 10.1084/jem.180.4.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choe H, Farzan M, Sun Y, Sullivan N, Rollins B, Ponath P D, Wu L, Mackay C R, LaRosa G, Newman W, Gerard N, Gerard C, Sodroski J. The β-chemokine receptors CCR3 and CCR5 facilitate infection by primary HIV-1 isolates. Cell. 1996;85:1135–1148. doi: 10.1016/s0092-8674(00)81313-6. [DOI] [PubMed] [Google Scholar]
- 7.Cocchi F, DeVico A L, Garzino-Demo A, Arya S K, Gallo R C, Lusso P. Identification of RANTES, MIP-1α, and MIP-1β as the major HIV-suppressive factors produced by CD8+ T cells. Science. 1995;270:1811–1815. doi: 10.1126/science.270.5243.1811. [DOI] [PubMed] [Google Scholar]
- 8.Cocchi F, DeVico A L, Garzino-Demo A, Cara A, Gallo R C, Lusso P. The v3 domain of the HIV-1 gp120 envelope glycoprotein is critical for chemokine-mediated blockade of infection. Nat Med. 1996;2:1244–1247. doi: 10.1038/nm1196-1244. [DOI] [PubMed] [Google Scholar]
- 9.Coffey J M, Woffendin C, Phare S M, Strieter R M, Markovitz D M. RANTES inhibits HIV-1 replication in human peripheral blood monocytes and alveolar macrophages. Am J Physiol. 1997;272:L1025–L1029. doi: 10.1152/ajplung.1997.272.5.L1025. [DOI] [PubMed] [Google Scholar]
- 10.Conant K, Garzino-Demo A, Nath A, McArthur J C, Halliday W, Power C, Gallo R C, Major E O. Induction of monocyte chemoattractant protein-1 in HIV-1 Tat-stimulated astrocytes and elevation in AIDS dementia. Proc Natl Acad Sci USA. 1998;95:3117–3121. doi: 10.1073/pnas.95.6.3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cotter R, Zheng J, Niemann D, Thomas E, Gendelman H. Proceedings of the XIth International Congress of Virology. Sydney, New South Wales, Australia: International Union of Microbiological Societies; 1999. CD40L activation of mononuclear phagocytes: regulation of HIV-1 replication and beta-chemokine production; p. 421. [Google Scholar]
- 12.Endres J M, Clapham P R, Marsh M, Ahuja M, Turner J D, McKnight A, Thomas J F, Stoebenau-Haggarty B, Choe S, Vance P J, Wells T N C, Powers C A, Sutterwala S S, Doms R W, Landau N R, Hoxie J A. CD4-independent infection by HIV-2 is mediated by fusin/CXCR4. Cell. 1996;87:745–756. doi: 10.1016/s0092-8674(00)81393-8. [DOI] [PubMed] [Google Scholar]
- 13.Fan S X, Turpin J A, Aronovitz J R, Meltzer M S. Interferon-gamma protects primary monocytes against infection with human immunodeficiency virus type 1. J Leukoc Biol. 1994;56:362–368. doi: 10.1002/jlb.56.3.362. [DOI] [PubMed] [Google Scholar]
- 14.Folks T M, Powell D, Lightfoote M, Koenig S, Fauci A S, Benn S, Rabson A, Daugherty D, Gendelman H E, Hoggan M D, et al. Biological and biochemical characterization of a cloned Leu-3− cell surviving infection with the acquired immune deficiency syndrome retrovirus. J Exp Med. 1986;164:280–290. doi: 10.1084/jem.164.1.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Foy T M, Aruffo A, Bajorath J, Buhlmann J E, Noelle R J. Immune regulation by CD40 and its ligand GP39. Annu Rev Immunol. 1996;14:591–617. doi: 10.1146/annurev.immunol.14.1.591. [DOI] [PubMed] [Google Scholar]
- 16.Francis L M, Meltzer M S, Gendelman H E. Interferons in the persistence, pathogenesis, and treatment of HIV infection. AIDS Res Hum Retrovir. 1992;8:199–207. doi: 10.1089/aid.1992.8.199. [DOI] [PubMed] [Google Scholar]
- 17.Gabuzda D, He J, Ohagen A, Vallat A. Chemokine receptors in HIV-1 infection of the central nervous system. Immunology. 1998;10:203–213. doi: 10.1006/smim.1998.0133. [DOI] [PubMed] [Google Scholar]
- 18.Gabuzda D H, Hirsch M S. Neurologic manifestations of infection with human immunodeficiency virus. Clinical features and pathogenesis. Ann Intern Med. 1987;107:383–391. doi: 10.7326/0003-4819-107-2-383. [DOI] [PubMed] [Google Scholar]
- 19.Gabuzda D H, Ho D D, de la Monte S M, Hirsch M S, Rota T R, Sobel R A. Immunohistochemical identification of HTLV-III antigen in brains of patients with AIDS. Ann Neurol. 1986;20:289–295. doi: 10.1002/ana.410200304. [DOI] [PubMed] [Google Scholar]
- 20.Gartner S. HIV infection and dementia. Science. 2000;287:602–604. doi: 10.1126/science.287.5453.602. [DOI] [PubMed] [Google Scholar]
- 21.Gendelman H E. The neuropathogenesis of HIV-1-dementia: a panel discussion. In: Gendelman H E, Lipton S A, Epstein L G, Swindells S, editors. The neurology of AIDS. 1st ed. New York, N.Y: Chapman & Hall; 1997. pp. 1–10. [Google Scholar]
- 22.Gendelman H E, Baca L, Turpin J A, Kalter D C, Hansen B D, Orenstein J M, Friedman R M, Meltzer M S. Restriction of HIV replication in infected T cells and monocytes by interferon-alpha. AIDS Res Hum Retrovir. 1990;6:1045–1049. doi: 10.1089/aid.1990.6.1045. [DOI] [PubMed] [Google Scholar]
- 23.Gendelman H E, Leonard J M, Dutko F, Koenig S, Khillan J, Meltzer M S. Immunopathogenesis of human immunodeficiency virus infection in the central nervous system. Ann Neurol. 1988;23:S78–S81. doi: 10.1002/ana.410230721. [DOI] [PubMed] [Google Scholar]
- 24.Gendelman H E, Morahan P. The macrophage in viral infections. In: Lewis C, McGee J, editors. The macrophage. New York, N.Y: IRL Press; 1992. pp. 157–195. [Google Scholar]
- 25.Gendelman H E, Orenstein J M, Martin M A, Ferrua C, Mitra R, Phipps T, Wahl L A, Lane H C, Fauci A S, Burke D S. Efficient isolation and propagation of human immunodeficiency virus on recombinant colony-stimulating factor 1-treated monocytes. J Exp Med. 1988;167:1428–1441. doi: 10.1084/jem.167.4.1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gendelman H E, Skillman D R, Meltzer M S. Interferon alpha (IFN)-macrophage interactions in human immunodeficiency virus (HIV) infection: role of IFN in the tempo and progression of HIV disease. Int Rev Immunol. 1992;8:43–54. doi: 10.3109/08830189209056640. [DOI] [PubMed] [Google Scholar]
- 27.Graf D, Muller S, Korthauer U, van Kooten C, Weise C, Kroczek R A. A soluble form of TRAP (CD40 ligand) is rapidly released after T cell activation. Eur J Immunol. 1995;25:1749–1754. doi: 10.1002/eji.1830250639. [DOI] [PubMed] [Google Scholar]
- 28.Grewal I S, Borrow P, Pamer E G, Oldstone M B, Flavell R A. The CD40-CD154 system in anti-infective host defense. Curr Opin Immunol. 1997;9:491–497. doi: 10.1016/s0952-7915(97)80100-8. [DOI] [PubMed] [Google Scholar]
- 29.He J, Chen Y, Farzan M, Choe H, Ohagen A, Gartner S, Busciglio J, Yang X, Hofmann W, Newman W, Mackay C R, Sodroski J, Gabuzda D. CCR3 and CCR5 are co-receptors for HIV-1 infection of microglia. Nature. 1997;385:645–649. doi: 10.1038/385645a0. [DOI] [PubMed] [Google Scholar]
- 30.He J, Landau N R. Use of a novel human immunodeficiency virus type 1 reporter virus expressing human placental alkaline phosphatase to detect an alternative viral receptor. J Virol. 1995;69:4587–4592. doi: 10.1128/jvi.69.7.4587-4592.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hesselgesser J, Taub D, Baskar P, Greenberg M, Hoxie J, Kolson D L, Horuk R. Neuronal apoptosis induced by HIV-1 gp120 and the chemokine SDF-1α mediated by the chemokine receptor CXCR4. Curr Biol. 1998;8:595–598. doi: 10.1016/s0960-9822(98)70230-1. [DOI] [PubMed] [Google Scholar]
- 32.Hornung F, Scala G, Lenardo M. TNF-α induced secretion of C-C chemokines modulates C-C chemokine receptor 5 expression on peripheral blood lymphocytes. J Immunol. 2000;164:6180–6187. doi: 10.4049/jimmunol.164.12.6180. [DOI] [PubMed] [Google Scholar]
- 33.Horuk R, Martin A W, Wang Z, Schweitzer L, Gerassimides A, Guo H, Lu Z, Hesselgesser J, Perez H D, Kim J, Parker J, Hadley T J, Peiper S C. Expression of chemokine receptors by subsets of neurons in the central nervous system. J Immunol. 1997;158:2882–2890. [PubMed] [Google Scholar]
- 34.Jones M, Olafson K, Del Bigio M R, Peeling J, Nath A. Intraventricular injection of human immunodeficiency virus type 1 (HIV-1) tat protein causes inflammation, gliosis, apoptosis, and ventricular enlargement. J Neuropathol Exp Neurol. 1998;57:563–570. doi: 10.1097/00005072-199806000-00004. [DOI] [PubMed] [Google Scholar]
- 35.Kiener P A, Moran-Davis P, Rankin B M, Wahl A F, Aruffo A, Hollenbaugh D. Stimulation of CD40 with purified soluble gp39 induces proinflammatory responses in human monocytes. J Immunol. 1995;1995:4917–4925. [PubMed] [Google Scholar]
- 36.Kinter A L, Ostrowski M, Goletti D, Oliva A, Weissman D, Gantt K, Hardy E, Jackson R, Ehler L, Fauci A S. HIV replication in CD4+ T cells of HIV-infected individuals is regulated by a balance between the viral suppressive effects of endogenous beta-chemokines and the viral inductive effects of other endogenous cytokines. Proc Natl Acad Sci USA. 1996;93:14076–14081. doi: 10.1073/pnas.93.24.14076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Koenig S, Gendelman H E, Orenstein J M, Canto M C D, Pezeshkpour G H, Yungbluth M, Janotta F, Aksamit A, Martin M A, Fauci A S. Detection of AIDS virus in macrophages in brain tissue from AIDS patients with encephalopathy. Science. 1986;233:1089–1093. doi: 10.1126/science.3016903. [DOI] [PubMed] [Google Scholar]
- 38.Kooten C V, Banchereau J. CD40-CD40 ligand: a multifunctional receptor-ligand pair. Adv Immunol. 1996;61:1–77. doi: 10.1016/s0065-2776(08)60865-2. [DOI] [PubMed] [Google Scholar]
- 39.Kornbluth R S. The emerging role of CD40 ligand in HIV infection. J Leukoc Biol. 2000;68:373–382. [PubMed] [Google Scholar]
- 40.Kornbluth R S, Kee K, Richman D D. CD40 ligand (CD154) stimulation of macrophages to produce HIV-1-suppressive beta-chemokines. Proc Natl Acad Sci USA. 1998;95:5205–5210. doi: 10.1073/pnas.95.9.5205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lane B R, Markovitz D M, Woodford N L, Rochford R, Strieter R M, Coffey M J. TNF-alpha inhibits HIV-1 replication in peripheral blood monocytes and alveolar macrophages by inducing the production of RANTES and decreasing C-C chemokine receptor 5 (CCR5) expression. J Immunol. 1999;163:3653–3661. [PubMed] [Google Scholar]
- 42.Lane P, Brocker T, Hubele S, Padovan E, Lanzavecchia A, McConnell F. Soluble CD40 ligand can replace the normal T cell-derived CD40 ligand signal to B cells in T cell-dependent activation. J Exp Med. 1993;177:1209–1213. doi: 10.1084/jem.177.4.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Laurence J. Reservoirs of HIV infection or carriage: monocytic, dendritic, follicular dendritic, and B cells. Ann N Y Acad Sci. 1993;693:52–64. doi: 10.1111/j.1749-6632.1993.tb26256.x. [DOI] [PubMed] [Google Scholar]
- 44.Lavi E, Strizki J M, Ulrich A M, Zhang W, Fu L, Wang Q, O'Connor M, Hoxie J A, Gonzalez-Scarano F. CXCR-4 (fusin), a co-receptor for the type 1 human immunodeficiency virus (HIV-1), is expressed in the human brain in a variety of cell types, including microglia and neurons. Am J Pathol. 1997;151:1035–1042. [PMC free article] [PubMed] [Google Scholar]
- 45.Lee B, Rucker J, Doms R W, Tsang M, Hu X, Dietz M, Bailer R, Montaner L J, Gerard C, Sullivan N, Sodroski J, Stantchev T S, Broder C C, Arenzana-Seisdedos F, Amara A, Thomas D, Virelizier J L, Baleux F, Clark-Lewis I, Legler D F, Moser B, Baggiolini M, DeVico A L, Pal R, Markham P D, Garzino-Demo A, Gallo R C. β-Chemokine MDC and HIV-1 infection. Science. 1998;281:487. [Google Scholar]
- 46.Lee S, Lapham C K, Chen H, King L, Manischewitz J, Romantseva T, Mostowski H, Stantchev T S, Broder C C, Golding H. Coreceptor competition for association with CD4 may change the susceptibility of human cells to infection with T-tropic and macrophage-tropic isolates of human immunodeficiency virus type 1. J Virol. 2000;74:5016–5023. doi: 10.1128/jvi.74.11.5016-5023.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li L, Smith P. Immunobiology of mucosal HIV-1 infection. Curr Opin Gastroenterol. 1996;12:560–563. [Google Scholar]
- 48.Lipton S A, Gendelman H E. Dementia associated with the acquired immunodeficiency syndrome. N Engl J Med. 1995;16:934–940. doi: 10.1056/NEJM199504063321407. [DOI] [PubMed] [Google Scholar]
- 49.Ludewig B, Henn V, Schroder J M, Graf D, Kroczek R A. Induction, regulation, and function of soluble TRAP (CD40 ligand) during interaction of primary CD4+ CD45RA+ T cells with dendritic cells. Eur J Immunol. 1996;26:3137–3143. doi: 10.1002/eji.1830261246. [DOI] [PubMed] [Google Scholar]
- 50.Malik N, Greenfield B W, Wahl A F, Kiener P A. Activation of human monocytes through CD40 induces matrix metalloproteinases. J Immunol. 1996;156:3952–3960. [PubMed] [Google Scholar]
- 51.Manthrope M, Fagnani R, Skaper S D, Varon S. An automated colorimetric microassay for neurotrophic factors. Dev Brain Res. 1986;25:191–198. doi: 10.1016/s0006-8993(86)80227-x. [DOI] [PubMed] [Google Scholar]
- 52.McDyer J F, Dybul M, Goletz T J, Kinter A L, Thomas E K, Berzofsky J A, Fauci A S, Seder R A. Differential effects of CD40 ligand/trimer stimulation on the ability of dendritic cells to replicate and transmit HIV infection: evidence for CC-chemokine-dependent and -independent mechanisms. J Immunol. 1999;162:3711–3717. [PubMed] [Google Scholar]
- 53.Meltzer M S, Nakamura M, Hansen B D, Turpin J A, Kalter D C, Gendelman H E. Macrophages as susceptible targets for HIV infection, persistent viral reservoirs in tissue, and key immunoregulatory cells that control levels of virus replication and extent of disease. AIDS Res Hum Retrovir. 1990;6:967–971. doi: 10.1089/aid.1990.6.967. [DOI] [PubMed] [Google Scholar]
- 54.Moore J P. Coreceptors: implications for HIV pathogenesis and therapy. Science. 1997;276:51–52. doi: 10.1126/science.276.5309.51. [DOI] [PubMed] [Google Scholar]
- 55.Moriuchi H, Moriuchi M, Fauci A S. Factors secreted by human T lymphotropic virus type I (HTLV-I)-infected cells can enhance or inhibit replication of HIV-1 in HTLV-I-uninfected cells: implications for in vivo coinfection with HTLV-I and HIV-1. J Exp Med. 1998;187:1689–1697. doi: 10.1084/jem.187.10.1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Muller F, Aukrust P, Nordoy I, Froland S S. Possible role of interleukin-10 (IL-10) and CD40 ligand expression in the pathogenesis of hypergammaglobulinemia in human immunodeficiency virus infection: modulation of IL-10 and Ig production after intravenous Ig infusion. Blood. 1998;92:3721–3729. [PubMed] [Google Scholar]
- 57.Nath A, Conant K, Chen P, Scott C, Major E O. Transient exposure to HIV-1 Tat protein results in cytokine production in macrophages and astrocytes. A hit and run phenomenon. J Biol Chem. 1999;274:17098–17102. doi: 10.1074/jbc.274.24.17098. [DOI] [PubMed] [Google Scholar]
- 58.New D R, Maggirwar S B, Epstein L G, Dewhurst S, Gelbard H A. HIV-1 Tat induces neuronal death via tumor necrosis factor alpha and activation of non-NMDA receptors by a NFκB-independent mechanism. J Biol Chem. 1998;273:17852–17858. doi: 10.1074/jbc.273.28.17852. [DOI] [PubMed] [Google Scholar]
- 59.Oravecz T, Pall M, Norcross M A. Beta-chemokine inhibition of monocytotropic HIV-1 infection. Interference with a postbinding fusion step. J Immunol. 1996;157:1329–1332. [PubMed] [Google Scholar]
- 60.Perry S W, Hamilton J A, Tjoelker L W, Dbaibo G, Dzenko K A, Epstein L G, Hannun Y, Whittaker J S, Dewhurst S, Gelbard H A. Platelet-activating factor receptor activation. An initiator step in HIV-1 neuropathogenesis. J Biol Chem. 1998;273:17660–17664. doi: 10.1074/jbc.273.28.17660. [DOI] [PubMed] [Google Scholar]
- 61.Rucker J, Samson M, Doranz B J, Libert F, Berson J F, Yi Y, Smyth R J, Collman R G, Broder C C, Vassart G, Doms R W, Parmentier M. Regions in beta-chemokine receptors CCR5 and CCR2b that determine HIV-1 cofactor specificity. Cell. 1996;87:437–446. doi: 10.1016/s0092-8674(00)81364-1. [DOI] [PubMed] [Google Scholar]
- 62.Ryan L, Swindells S, Zheng J, Brester M, Ratansuwan W, Cotter R, Bohac D, Anderson J, Gendelman H. Proceedings of the 7th Conference on Retroviruses and Opportunistic Infections. San Francisco, Calif: Foundation for Retrovirology and Human Health; 2000. Monocyte immunity as a predictor for HIV-1-associated dementia; p. 132. .. [Google Scholar]
- 63.Schmidtmayerova H, Nottet H S, Nuovo G, Raabe T, Flanagan C R, Dubrovsky L, Gendelman H E, Cerami A, Bukrinsky M, Sherry B. HIV-1 infection alters chemokine β peptide expression in human monocytes: implications for recruitment of leukocytes into brain and lymph nodes. Proc Natl Acad Sci USA. 1996;93:700–704. doi: 10.1073/pnas.93.2.700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schmidtmayerova H, Sherry B, Bukrinsky M. Chemokines and HIV replication. Nature. 1996;382:767. doi: 10.1038/382767a0. [DOI] [PubMed] [Google Scholar]
- 65.Severino E M, Sipsas N V, Nguyen P T, Kalams S A, Walker B D, Johnson R P, Yang O O. Inhibition of human immunodeficiency virus type 1 replication in primary CD4+ T lymphocytes, monocytes, and dendritic cells by cytotoxic T lymphocytes. J Virol. 2000;74:6695–6699. doi: 10.1128/jvi.74.14.6695-6699.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shieh J T C, Albright A V, Sharron M, Gartner S, Strizki J, Doms R W, Gonzalez-Scarano F. Chemokine receptor utilization by human immundeficiency virus type 1 isolates that replicate in microglia. J Virol. 1998;72:4243–4249. doi: 10.1128/jvi.72.5.4243-4249.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shu U, Kiniwa M, Wu C, Maliszewski C, Vezzio N, Hakimi J, Gately M, Delespesse G. Activated T cells induce interleukin-12 production by monocytes via CD40-CD40 ligand interaction. Eur J Immunol. 1995;25:1125–1128. doi: 10.1002/eji.1830250442. [DOI] [PubMed] [Google Scholar]
- 68.Smith P, Wahl S. Immunobiology of mucosal HIV-1 infection. In: Ogra P, Lamm M, Strober W, McGhee J, Bienenstock J, editors. Mucosal immunology. San Diego, Calif: Academic Press, Inc.; 1999. pp. 977–989. [Google Scholar]
- 69.Soontornniyomkij V, Wang G, Pittman C A, Wiley C A, Achim C L. Expression of brain-derived neurotrophic factor protein in activated microglia of human immunodeficiency virus type 1 encephalitis. Neuropathol Appl Neurobiol. 1998;24:453–460. doi: 10.1046/j.1365-2990.1998.00134.x. [DOI] [PubMed] [Google Scholar]
- 70.Stout R D, Suttles J, Xu J, Grewal I S, Flavell R A. Impaired T cell-mediated macrophage activation in CD40 ligand-deficient mice. J Immunol. 1996;156:8–11. [PubMed] [Google Scholar]
- 71.Tian L, Noelle R, Lawrence D. Activated T-cells enhance nitric oxide production by murine splenic macrophages through gp39 and LFA-1. Eur J Immunol. 1995;25:306–309. doi: 10.1002/eji.1830250152. [DOI] [PubMed] [Google Scholar]
- 72.Torre V S, Marozsan A J, Albright J L, Collins K R, Hartley O, Offord R E, Quinones-Mateu M E, Arts E J. Variable sensitivity of CCR5-tropic human immunodeficiency virus type 1 isolates to inhibition by RANTES analogs. J Virol. 2000;74:4868–4876. doi: 10.1128/jvi.74.10.4868-4876.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vallat A-V, Girolami U D, He J, Mhashikar A, Marasco W, Shi B, Gray F, Bell J, Keohane C, Smith T W, Gabuzda D. Localization of HIV-1 co-receptors CCR5 and CXCR4 in the brain of children with AIDS. Am J Pathol. 1998;152:167–178. [PMC free article] [PubMed] [Google Scholar]
- 74.Verani S, Scarlatti G, Comar M, Tresoldi E, Polo S, Giacca M, Lusso P, Siccardi A G, Vercelli D. C-C chemokines released by lipopolysaccharide (LPS)-stimulated human macrophages suppress HIV-1 infection in both macrophages and T cells. J Exp Med. 1997;185:805–816. doi: 10.1084/jem.185.5.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wagner D, Stout R, Suttles J. Role of the CD40-CD40 ligand interaction in CD4+ T cell contact-dependent activation of monocyte interleukin-1 synthesis. Eur J Immunol. 1994;24:3148–3154. doi: 10.1002/eji.1830241235. [DOI] [PubMed] [Google Scholar]
- 76.Wiley C A, Schrier R D, Nelson J A, Lampert P W, Oldstone M B A. Cellular localization of human immunodeficiency virus infection within the brains of acquired immune deficiency syndrome patients. Proc Natl Acad Sci USA. 1986;83:7089–7093. doi: 10.1073/pnas.83.18.7089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wu L, Paxton W A, Kassam N, Ruffing N, Rottman J B, Sullivan N, Choe H, Sodroski J, Newman W, Koup R A, Mackay C R. CCR5 levels and expression pattern correlate with infectability by macrophage-tropic HIV-1, in vitro. J Exp Med. 1997;185:1681–1691. doi: 10.1084/jem.185.9.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yellin J M, Sippel K, Inghirami G, Covey L R, Lee J J, Sinning J, Clark E A, Chess L, Lederman S. CD40 molecules induce down-modulation and endocytosis of T cell surface T cell-B cell activating molecule/CD40-L. Potential role in regulating helper effector function. J Immunol. 1994;152:598–608. [PubMed] [Google Scholar]
- 79.Zack J, Arrigo S, Weitsman S, Go A, Haislip A, Chen I. HIV-1 entry into quiescent primary lymphocytes: molecular analysis reveals a labile, latent viral structure. Cell. 1990;61:213–222. doi: 10.1016/0092-8674(90)90802-l. [DOI] [PubMed] [Google Scholar]
- 80.Zheng J, Gendelman H E. The HIV-1 associated dementia complex: a metabolic encephalopathy fueled by viral replication in mononuclear phagocytes. Curr Opin Neurol. 1997;10:319–325. [PubMed] [Google Scholar]
- 81.Zheng J, Thylin M, Ghorpade A, Xiong H, Persidsky Y, Cotter R, Niemann D, Che M, Zeng Y, Gelbard H, Shepard R, Swartz J, Gendelman H. Intracellular CXCR4 signaling, neuronal apoptosis and neuropathogenic mechanisms of HIV-1-associated dementia. J Neuroimmunol. 1999;98:185–200. doi: 10.1016/s0165-5728(99)00049-1. [DOI] [PubMed] [Google Scholar]
- 82.Zheng, J., M. R. Thylin, Y. Persidsky, C. E. Williams, R. L. Cotter, W. Zink, L. A. Ryan, A. Ghorpade, K. Lewis, and H. E. Gendelman. HIV-1 infected immune competent mononuclear phagocytes influence the pathways to neuronal demise. Neurotoxicity Res., in press. [DOI] [PubMed]
- 83.Zhu T, Mo H, Wang N, Nam D S, Cao Y, Koup R A, Ho D D. Genotypic and phenotypic characterization of HIV-1 in patients with primary infection. Science. 1993;261:1179–1181. doi: 10.1126/science.8356453. [DOI] [PubMed] [Google Scholar]