Abstract
Wnt7b is a Wnt ligand that has been demonstrated to play critical roles in several developmental processes, including lung airway and vascular development and chorion-allantois fusion during placental development. Wnt signaling involves the binding of Wnt ligands to cell surface receptors of the frizzled family and coreceptors of the LRP5/6 family. However, little is known of the ligand-receptor specificity exhibited by different Wnts, Fzds, and LRPs in Wnt signaling. Expression analysis of Fzds and LRP5/6 in the developing lung and vasculature showed that Fzd1, -4, -7, and -10 and LRP5/6 are expressed in tissue-specific patterns during lung development. Fzd1, -4, and -7 are expressed primarily in the developing lung mesenchyme, and Fzd10 is expressed in airway epithelium. LRP5 and LRP6 are expressed in airway epithelium during lung development, whereas LRP5 but not LRP6 expression is observed in the muscular component of large blood vessels, including the aorta. Cell transfection studies demonstrate that Wnt7b can activate the canonical Wnt pathway but not the noncanonical Wnt pathway in a cell-specific manner. Biochemical analysis demonstrates that Wnt7b can bind to Fzd1 and -10 on the cell surface and cooperatively activate canonical Wnt signaling with these receptors in the presence of LRP5. Together, these data demonstrate that Wnt7b signals through Fzd1 and -10 and LRP5 and implicate these Wnt coreceptors in the regulation of lung airway and vascular development.
The lung develops from the anterior foregut endoderm in the region of the laryngo-tracheal groove (reviewed in reference 37). In the mouse, the early lung buds form at embryonic day 9.5 (E9.5) as an outgrowth from this region of the foregut and quickly grow through a process of branching morphogenesis. Branching morphogenesis of the lung results in the proximal-distal polarity exhibited by the airways, which is required for the proper differentiation of airway epithelium into the multiple cell lineages needed for postnatal respiration. Multiple signaling pathways have been implicated in the growth and differentiation of lung airway epithelium, including FGF, TGF-β, SHH, and BMP (reviewed in reference 37). These pathways act individually and in concert with each other to direct airway branching and to regulate epithelial and mesenchymal differentiation and proliferation.
More recently, several reports have suggested a role for Wnt signaling in lung airway differentiation (19, 26, 31). Wnts are a family of secreted cysteine rich glycoproteins which play key roles in several cellular processes, including proliferation, differentiation, migration, and programmed cell death (for a review, see reference 6). Wnts bind to cell surface receptors of the frizzled (Fzd) family of seven membrane-spanning cell surface proteins and the lipoprotein-related receptor proteins LRP5/6. Wnts can signal through at least three different pathways often referred to as (i) canonical, involving nuclear translocation of β-catenin and activation of LEF/TCF mediated gene transcription, (ii) noncanonical-planar cell polarity, involving the activation of c-Jun kinase (JNK), and (iii) noncanonical-Ca2+ pathways, involving regulation of calcium flux. The canonical pathway, which is the best understood of these pathways, involves the binding of Wnts to the cell surface and inactivation of glycogen synthase kinase 3β (GSK-3β), which phosphorylates the cell adhesion molecule β-catenin. Hypophosphorylated β-catenin translocates to the nucleus, where it binds to transcription factors of the LEF/TCF family and activates downstream target genes. Although the intracellular pathways that transmit Wnt signals have been extensively studied, little is understood about the ligand-receptor specificities that regulate the activity of different Wnts.
In the lung, several Wnt ligands are expressed, including Wnt7b, Wnt2, Wnt5a, and Wnt11 (19, 39). Wnt7b has been demonstrated to play a critical role in lung airway and vascular development. Loss of Wnt7b results in severe lung hypoplasia due to defects in branching morphogenesis and cell proliferation as well as defects in lung epithelial differentiation, including a deficiency in alveolar type 1 cell development (31). Wnt7b-deficient embryos also display loss of vascular smooth muscle integrity leading to pulmonary hemorrhage. Loss of Wnt5a leads to late airway epithelial maturation defects (19). Inactivation of β-catenin in distal airway epithelium leads to severe attenuation of epithelial differentiation (26). Although these data clearly show an important role for Wnt signaling in lung development, little is known about the molecular pathways that ligands such as Wnt7b use for signaling, in the lung or other tissues. In particular, whether Wnt7b activates canonical or noncanonical signaling is unclear as well as which Fzd/LRP receptor combination is required for signaling.
In these studies we have determined that Wnt7b activates canonical but not JNK-dependent noncanonical Wnt signaling in a cell-type-specific manner. We find that Fzd1, -4, -7, and -10 and LRP5/6 are expressed in the lung and in vascular smooth muscle at significant levels in tissue-specific patterns. Finally, we show that Wnt7b activates canonical signaling cooperatively with Fzd1, Fzd10, and LRP5. These data outline a Wnt7b pathway that regulates important aspects of lung airway and vascular development.
MATERIALS AND METHODS
Histological procedures.
Mouse embryos were fixed in 4% paraformaldehyde for 48 h and dehydrated through a series of increasing ethanol washes. Fixed embryos were embedded in paraffin, and 5 μm sections were affixed to slides, which were processed for radioactive in situ hybridization as previously described (45). Hybridized slides were coated with photographic emulsion and exposed for 7 to 10 days. Photographs were taken on a Nikon E600 microscope equipped with epifluorescence. In situ hybridization probes were generated by PCR from full-length cDNAs and contained T7 RNA polymerase binding sites in the antisense direction at the 3′ end. The oligonucleotides used to generate Fzd1-Fzd9 probes have been previously described (29). Oligonucleotides used to generate in situ probes for LRP5 and LRP6 were LRP5 sense (5′-GCGGAGTGAAGCTGGAGTCC-3′) and antisense (5′-GTAATACGACTCACTATAGGGCCTCGCATGGTGTGTGGAAGGG-3′) and LRP6 sense (5′-TACAAATGGCAAAGAGAATG-3′) and antisense (5′-GTAATACGACTCACTATAGGGCTTCCACATGGATTTGTAGCA-3′). Immunohistochemistry using the rabbit anti-Fzd10 antibody was performed as previously described (22). More details on histological procedures can be found at the University of Pennsylvania Molecular Cardiology Center web site: http://www.uphs.upenn.edu/mcrc/.
Plasmids and RT-PCR.
Plasmids containing mouse LRP5, LRP6, Fzd4, and Fzd7 cDNAs have been previously described (43). Expression vectors for Fzd1, Fzd4, Fzd7, and Fzd10 were generated in pCMVTag4A (Stratagene, Inc.) using reverse transcription-PCR (RT-PCR) from either mouse E14.5 embryonic lung RNA or the plasmids described above. Generation of the pcDNA3.1/Wnt7bHA expression vector was previously described (31). The DEP/dishevelled expression vector and the β-catenin/4145 expression plasmid in which the GSK-3β phosphorylation sites Thr 41 and Ser 45 are mutated to alanines have been previously described (31, 33).
Total RNA was extracted from 293 and PAC-1 cells using Trizol reagent. One microgram of DNase-treated total RNA was reverse transcribed using a commercially available kit (Invitrogen, Inc.). A total of 5% of the resulting cDNA from each cell line was subjected to PCR amplification using oligonucleotides specific for Fzd1 (sense, 5′-GGAGAGCTGTGCGTGGGCCAG-3′; antisense, 5′-AAACTTGTCGTTGCACACCAC-3′); Fzd4 (sense, 5′-CTTCACCGTGCTGACCTTCCTG-3′; antisense, 5′-TTGACCATTAGCCTTTCCAAC-3′); Fzd7 (sense, 5′-CATGCGTCGCTTCAGCTATCC-3′; antisense, 5′-CTGTCTTGGTGCCGTCGTGC-3′); and Fzd10 (sense, 5′-AGAAGCTCATGGTACGCATAG-3′; antisense, 5′-CACGCAGGCTGAAGGCTGGG-3′). The sizes of the resulting amplification products were as follows: Fzd1, 484 bp; Fzd4, 601 bp; Fzd7, 550 bp; and Fzd10, 430 bp.
Wnt reporter transfection assays.
To measure canonical Wnt signaling, the TOPFLASH and FOPFLASH reporter plasmids (0.5 μg) along with Wnt7b, Fzd, and LRP expression plasmids (0.5 μg) were transfected into 293, 3T3, PAC-1, or A7r5 cells using Fugene 6 as previously described (39). At 48 h after transfection, luciferase activity was measured using a commercially available kit (Promega) and compared to control Renilla luciferase activity.
JNK activity was measured using the PathDetect c-Jun kinase reporter system (Stratagene, Inc.). 293 and PAC-1 cells were transfected with the pFR-luc reporter plasmid, the pGAL4-c-Jun-N-term vector, and either pcDNA3/Wnt7b, pcDNA3/DEP, or pcDNA3 as a negative control. The pGAL4DBD was also used as a negative control. Luciferase activities were measured as described above.
Western blotting.
Cell extracts were made using an NE-PER extraction kit (Pierce, Inc.), and lysates were resolved in 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gels, which were transferred to Immobilon polyvinylidene difluoride membranes. For determining c-Jun phosphorylation, the membranes were probed with the phospho-c-Jun antibody KM-1 (Santa Cruz Biotechnology, Inc.) at a 1:2,000 dilution. Total c-Jun protein expression was determined using the c-Jun (N) antibody (Santa Cruz Biotechnology, Inc.) at a 1:1,000 dilution. For the β-catenin Western blots, the membranes were probed with a mouse anti-β-catenin monoclonal antibody at a 1:2,000 dilution (BD Transduction Laboratories). Western blots were further processed as previously described to detect immunospecific bands (39).
Cell surface binding experiments.
To detect cell surface binding of Wnt7b to Fzd proteins, Wnt7b conditioned medium was generated from 293 cells transfected with the pcDNA3.1/Wnt7b-HA plasmid. At 72 h after transfection, conditioned medium was concentrated 20-fold in a Centricon-20 mini concentrator. 293 cells were transfected with expression plasmids containing full-length Fzd1, Fzd4, Fzd7, and Fzd10. At 48 h after transfection of Fzd expression plasmids, the 293 cells were incubated with 200 μl of concentrated Wnt7b conditioned medium for 1 h at room temperature. Cells were then washed three times with phosphate-buffered saline, fixed for 10 min with 4% paraformaldehyde, and then incubated with mouse monoclonal to the HA epitope (Convance, Inc.) for 1 h at room temperature. Cells were washed again and incubated with goat anti-mouse immunoglobulin G-fluorescein isothiocyanate for 1 h at room temperature, after which cells were washed again three times with phosphate-buffered saline and visualized under a fluorescent microscope with a fluorescein isothiocyanate filter.
Coimmunoprecipitation assays.
293 cells were transfected using Fugene 6 and the pcDNA3.1Wnt7b-HA expression plasmid and Fzd1, Fzd4, Fzd7, and Fzd10 expression plasmids. Cell lysates were generated using a lysis buffer containing 50 mM Tris, pH 7.5, 150 mM NaCl, 0.5% NP-40, 1 mM phenylmethylsulfonyl fluoride. Mouse anti-FLAG epitope tag antibody (M2; Sigma) was used to immunoprecipitate cell lysates, which were resolved on SDS-PAGE gels, blotted to Immobilon membranes (Millipore, Inc.), and subsequently probed with mouse anti-hemagglutinin (HA) tag antibodies to detect coimmunoprecipitated proteins on Western blots.
RESULTS
Expression of frizzled and LRP5/6 receptors during lung development.
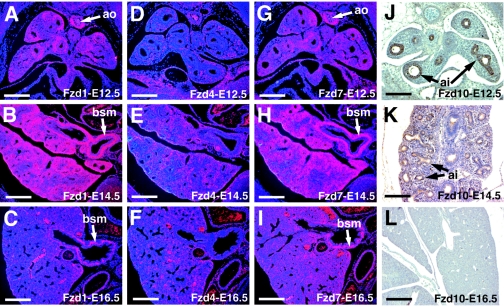
Previous studies have demonstrated an important role for Wnt7b signaling in lung airway and vascular development (19, 31). To narrow our search for receptors used by Wnt7b in the lung, in situ hybridization was performed on mouse embryonic lung sections using probes for all 10 of the known Fzd cDNAs. Of these, Fzd1, 4, 7, and 10 were found to be expressed in the lung at significant levels from E12.5 through E16.5, the time period during which the lung undergoes extensive differentiation. Fzd1 and 7 were expressed in the developing lung mesenchyme and in presumptive bronchial smooth muscle as well as vascular smooth muscle tissues such as the aorta and the large pulmonary vessels (Fig. 1A to C and G to I). Fzd4 is expressed in lung mesenchymal cells, which include precursors to vascular endothelial cells in the lung (Fig. 1D to F). This expression pattern correlates with Fzd4's previous reported expression in endothelial cells (10, 41). Immunohistochemistry shows that Fzd10 is expressed primarily in distal airway epithelium during lung development, with expression decreasing with gestational age (Fig. 1J to L).
FIG. 1.
Expression of Fzd genes during lung development. In situ hybridization was performed on E12.5, E14.5, and E16.5 mouse embryos using probes specific for Fzd1, Fzd4, and Fzd7. Immunohistochemistry was performed using a rabbit polyclonal antibody to Fzd10. Expression of Fzd1 (A to C) and Fzd7 (G to I) is observed in the aorta, bronchial smooth muscle, and pulmonary vessels. Expression of Fzd4 (D to F) is observed diffusely throughout the lung mesenchyme. Fzd10 expression (J to L) is observed primarily in distal airway epithelium from E12.5 to E14.5. Expression of all four genes decreases after E14.5 (C, F, I and L). ao, aorta; bsm, bronchial smooth muscle; pv, pulmonary vessels; ai, distal airway epithlium. Bars, 200 μm.
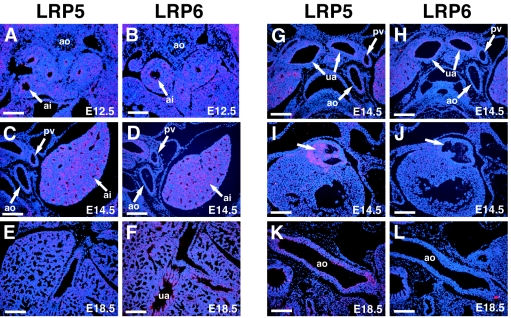
In situ hybridization for LRP5/6 expression revealed high levels of expression for both genes in the distal lung airway epithelium as early as E12.5 (Fig. 2A to F). Expression of LRP5/6 in airway epithelium decreased after E14.5, and both genes were expressed only at low levels in distal airway epithelium at E18.5 (Fig. 2E and F). LRP5 and LRP6 expression was noticed in large upper airways at E14.5 but by E18.5, only LRP6 expression was observed in upper airways (Fig. 2E to H). Expression of LRP5 was observed in developing blood vessels such as the dorsal aorta and pulmonary blood vessels (Fig. 2A to D, G, H, K, and L). Expression of LRP5 but not LRP6 was also observed at E14.5 in the outflow tract valves of the heart, where Wnt signaling has been demonstrated to play a key role in development (Fig. 2I and J) (11, 15, 21). These data suggest that LRP5 and LRP6 likely play both unique and redundant roles in Wnt signaling in pulmonary and cardiovascular development.
FIG. 2.
Expression of LRP5 and LRP6 during lung and heart development. In situ hybridization was performed on E12.5 (A and B), E14.5 (C, D, and G to J), and E18.5 (E, F, K, and L) mouse embryos using specific probes for LRP5 (A, C, E, G, I, and K) and LRP6 (B, D, F, H, J, and L). Expression of LRP5 and LRP6 is observed in distal airway epithelium at E12.5 (A and B) and E14.5 (C and D). Expression decreases significantly in distal airway epithelium by E18.5 (E and F). Expression of LRP5 and LRP6 is observed in upper airways at E14.5 (G and H), but only LRP6 expression is observed in upper airways at E18.5 (F). LRP5 but not LRP6 expression is observed in large blood vessels, including the aorta and pulmonary vessels, from E12.5 (A) through E18.5 (K). LRP5 but not LRP6 is also expressed in the developing outflow tract heart valves at E14.5 (I and J, arrow). ao, aorta; bsm, bronchial smooth muscle; pv, pulmonary vessels; ai, distal airway epithelium; ua, upper airway. Bars, 150 μm (A and B) and 200 μm (C to L).
Wnt7b activates canonical Wnt signaling in a cell-specific manner.
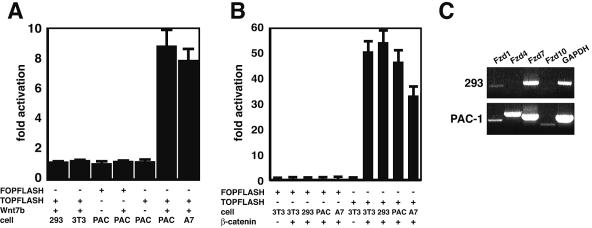
To determine whether Wnt7b could activate the canonical Wnt pathway, 293 and 3T3 cells were transfected with the TOPFLASH and FOPFLASH reporter constructs along with a Wnt7b expression vector. 293 cells are a kidney epithelial cell line whereas 3T3 cells are a mesodermally derived mesenchymal cell line. Wnt7b was unable to activate the TOPFLASH reporter in either of these cells types while a phosphorylation mutant of β-catenin, β-catenin/4145, known to activate the canonical Wnt pathway, activated the TOPFLASH reporter robustly (Fig. 3A and B) (34). Our previous work demonstrated a role for Wnt7b activity in vascular smooth muscle integrity in the lung (31). To determine whether Wnt7b activated canonical signaling in vascular smooth muscle cells (VSMCs), we performed similar transfection studies with PAC-1 and A7r5 cell lines, which are derived from rat pulmonary vascular smooth muscle and aortic smooth muscle, respectively. Surprisingly, Wnt7b was able to activate the TOPFLASH reporter approximately eightfold in both of these cell lines, indicating that Wnt7b activates canonical Wnt signaling in a cell-specific manner (Fig. 3A).
FIG. 3.
Wnt7b activates canonical signaling in vascular smooth muscle cells. The indicated cell lines were transfected with either TOPFLASH or FOPFLASH luciferase reporter plasmids along with Wnt7b expression plasmid or the β-catenin/4145 expression plasmid. All values are adjusted against TOPFLASH transfected with an empty expression plasmid (data not shown). (A) Expression of Wnt7b activates TOPFLASH approximately eightfold in PAC-1 and A7r5 cells. (B) Expression of β-catenin/4145 robustly activates TOPFLASH in all cell lines tested. Data represent the mean of three assays ± standard error of the mean. (C) RT-PCR shows that Fzd1 (low levels) and Fzd7 (high levels) are expressed in 293 cells while Fzd4 and Fzd7 are expressed at high levels and Fzd1 and Fzd10 are expressed at lower levels in PAC-1 cells. GAPDH is shown as a positive control for amplification.
Fzd expression was determined in 293 and PAC-1 cells to determine whether expression of these receptors could explain the differences in Wnt7b activation of canonical signaling (Fig. 3C). Fzd1 and Fzd7 are expressed in both 293 and PAC-1 cells. Fzd4 and Fzd10 are expressed at low levels in PAC-1 cells.
Wnt7b does not activate c-Jun kinase-dependent noncanonical Wnt signaling.
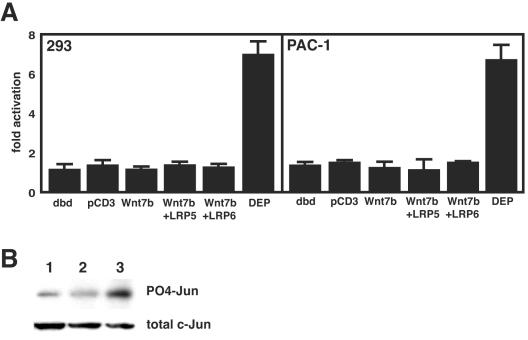
Wnt ligands can activate either canonical or noncanonical pathways, often in a cell context-dependent manner. Since Wnt7b could not activate canonical signaling in 293 or 3T3 cells, both of which are receptive to Wnt signaling (1, 14, 17, 25), we wanted to determine whether Wnt7b could activate the noncanonical planar cell polarity pathway. This pathway has been shown to activate c-Jun kinase (JNK), resulting in the phosphorylation of its downstream targets such as c-Jun (4, 20, 40, 44). To determine whether Wnt7b activated the JNK pathway, we used a transcriptional reporter system to measure activation of JNK by Wnt7b in 293 and PAC-1 cells. This assay measures the change in gene transcription through phosphorylation of the activation domain of c-Jun when fused to the GAL4 DNA binding domain (20). Wnt7b was unable to activate JNK while the Dishevelled-EGL10-Pleckstrin (DEP) domain of dishevelled, which has been shown to be critical for JNK mediated Wnt signaling (12, 32), significantly activated JNK in both 293 and PAC-1 cells (Fig. 4A). Cotransfection of LRP5 or LRP6 did not affect the ability of Wnt7b to activate JNK (Fig. 4A). Western blots demonstrated that expression of Wnt7b in 293 cells did not alter the level of c-Jun phosphorylation while expression of the DEP domain significantly increased c-Jun phosphorylation (Fig. 4B). These data indicate that Wnt7b is unable to activate JNK-dependent noncanonical Wnt signaling.
FIG. 4.
Wnt7b does not activate JNK dependent Wnt signaling. The PathDetect reporter system was used to measure JNK activity in both 293 and PAC-1 cells. (A) The GAL4 DNA binding domain (dbd) alone or in combination with pcDNA3 (pCD3) did not activate JNK. Expression of Wnt7b, in the presence or absence of LRP5 and LRP6, did not activate JNK, while expression of the DEP domain of dishevelled did. Data represent the means of three assays ± standard errors of the means. (B) Western blots showing Jun phosphorylation in response to pcDNA3 expression (lane 1), pcDNA3.1/Wnt7b expression (lane 2), or pcDNA3.1/DEP expression (lane 3). The total amount of c-Jun protein was used to control for loading.
Wnt7b binds to cells expressing Fzd1 and Fzd10.
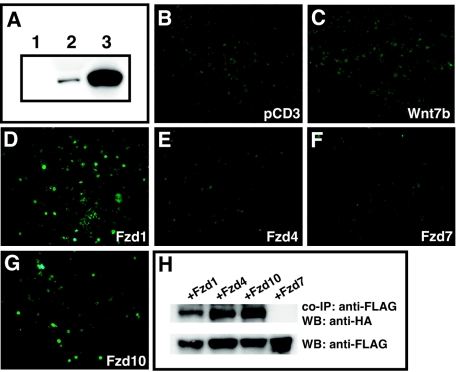
The ability of Wnt7b to activate canonical Wnt signaling in a cell-dependent manner, along with the cell-restricted pattern of Fzd expression, suggested that Wnt7b activity is likely regulated by the expression pattern and ability to interact with specific members of the Fzd family. To determine whether Wnt7b can bind to any of the four Fzds expressed in the lung during development, conditioned medium containing hemagglutinin (HA)-tagged Wnt7b (Fig. 5A) was incubated with 293 cells transfected with expression constructs encoding Fzd1, 4, 7, and 10. Equivalent expression of each of these four Fzd proteins in transfected 293 cells was confirmed by Western blotting (data not shown). After incubation and staining with an anti-HA epitope antibody, cells expressing Fzd1 and Fzd10 were found to bind Wnt7b at significant levels (Fig. 5D and G). However, cells expressing Fzd4 and Fzd7 did not bind Wnt7b at detectable levels (Fig. 5E and F). These data suggest that Wnt7b binds avidly to Fzd1 and Fzd10.
FIG. 5.
Fzd1 and Fzd10 bind Wnt7b at the cell surface. 293 cells were transfected with an expression plasmid encoding a HA-tagged Wnt7b protein. Conditioned medium from these cells was used to determine binding of Wnt7b to different Fzd receptors. (A) Western blots of control transfected 293 cells (lane 1), culture supernatant from Wnt7b-HA-expressing 293 cells (lane 2), and cell lysates from Wnt7b-HA-expressing 293 cells (lane 3). Concentrated Wnt7b-HA containing supernatant was incubated with untransfected 293 cells (B) or cells transfected with either pcDNA3 (C) or expression plasmids encoding Fzd1 (D), Fzd4 (E), Fzd7 (F), and Fzd10 (G). Only cells expressing Fzd1 and Fzd10 bound the HA-tagged Wnt7b protein at detectable levels. (H) 293 cells were transfected with a HA-tagged Wnt7b expression plasmid and FLAG-tagged Fzd1, Fzd4, Fzd7, and Fzd10 expression plasmids. Cell lysates were immunoprecipitated (co-IP) with a monoclonal antibody to FLAG, resolved on SDS-PAGE gels, Western blotted (WB), and probed with anti-HA epitope monoclonal antibody.
Wnt7b coimmunoprecipitates with Fzd1, Fzd4, and Fzd10.
Coimmunoprecipitation assays were performed to further assess the ability of Wnt7b to bind to Fzd1 and Fzd10. 293 cells were transfected with an HA-tagged Wnt7b expression vector along with a FLAG-tagged Fzd1, Fzd4, Fzd7, and Fzd10. Cell lysates were immunoprecipitated with anti-FLAG antibody, and coprecipitated proteins were detected on Western blots with the anti-HA epitope antibody. Interestingly, Wnt7b precipitates with Fzd1, Fzd4, and Fzd10 but not Fzd7 (Fig. 5H). These data show that Wnt7b binds efficiently to Fzd1 and Fzd10, supporting the findings from the above-described cell binding experiments. The ability of Wnt7b to interact with Fzd4 in a coimmunoprecipitation assay but not in a cell surface binding assay suggests either that there is additional specificity for ligand-receptor interactions that is conferred at the cell surface or that the cell surface binding assay is less sensitive than the coimmunoprecipitation assay.
Wnt7b activates canonical Wnt signaling in cooperation with Fzd1, Fzd10, and LRP5 in 293 cells.
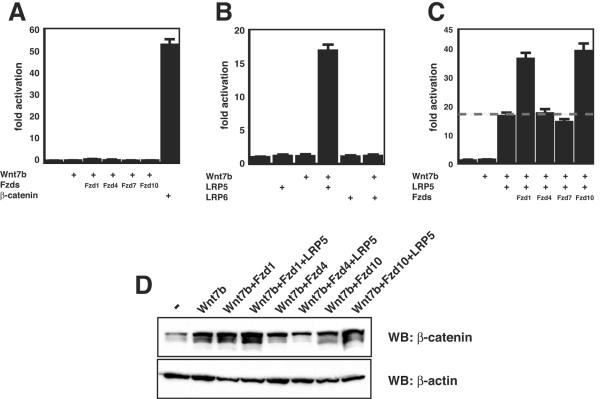
Since the above-described data suggest that Fzd1 and Fzd10 were capable of binding Wnt7b at the cell surface, we sought to determine whether they could activate canonical Wnt signaling in cooperation with Wnt7b in a cell line not responsive to Wnt7b alone, such as the kidney epithelial cell line 293. 293 cells were transfected with expression vectors for Wnt7b and Fzd1, 4, 7, or 10. None of these combinations resulted in active signaling (Fig. 6A). LRP5/6 coreceptors, along with Fzd proteins, are required for canonical Wnt signaling (24, 33, 38). To determine whether LRP5 or LRP6 could cooperatively activate canonical Wnt signaling with Wnt7b, 293 cells were transfected with expression vectors for Wnt7b and LRP5 or LRP6. Data from these experiments demonstrate that LRP5 but not LRP6 can cooperatively activate canonical Wnt signaling with Wnt7b (Fig. 6B). These data suggest that 293 cells express an Fzd receptor at low levels or that an Fzd receptor binds to Wnt7b only weakly and that the receptor's activity is augmented by addition of LRP5. Expression of low levels of Fzd1 in 293 cells may help explain this finding (Fig. 3C).
FIG. 6.
Wnt7b activates canonical Wnt signaling cooperatively through Fzd1, Fzd10, and LRP5. (A) Coexpression of Wnt7b and Fzd1, -4, -7, and -10 does not activate the TOPFLASH reporter, while β-catenin/4145 does. (B) Coexpression of Wnt7b and LRP5 but not LRP6 activates TOPFLASH reporter. (C) Coexpression of Wnt7b/Fzd1/LRP5 and Wnt7b/Fzd10/LRP5 activates the TOPFLASH reporter. Data represent the mean of three assays ± standard error of the mean. (D) Western blots (WB) show that coexpression of Wnt7b/Fzd1/LRP5 and Wnt7b/Fzd10/LRP5 results in the highest levels of β-catenin stabilization. Note that expression of Wnt7b alone or with only Fzd1, Fzd4, and Fzd10 did result in a detectable but less-dramatic increase in β-catenin stabilization.
To determine whether the addition of Fzds and LRP5 would further cooperatively activate Wnt signaling with Wnt7b, 293 cells were cotransfected with a combination of these expression vectors. As shown in Fig. 6C, addition of Fzd1 and Fzd10 resulted in an additional increase in Wnt signaling as measured by the TOPFLASH reporter. Western blot analyses were performed to determine whether this increase in TOPFLASH activity corresponded with an increase in steady-state levels of β-catenin, which occurs in canonical Wnt signaling. The Wnt7b/Fzd1/LRP5 and Wnt7b/Fzd10/LRP5 combinations significantly increased stabilized levels of β-catenin in transfected 293 cells while Wnt7b/Fzd4/LRP5 increased stabilized levels of β-catenin protein modestly. Interestingly, Wnt7b/Fzd1 and Wnt7b/Fzd10 caused an increase in stabilized levels of β-catenin even though Wnt activity as measured by TOPFLASH activation did not significantly change (Fig. 6A and D). This suggests that a threshold of β-catenin stabilization must be meet before Wnt7b activation of gene transcription is measurable by the TOPFLASH reporter plasmid. Together, these data demonstrate that Wnt7b cooperatively activates canonical Wnt signaling with LRP5 and Fzd1 and Fzd10.
Wnt7b activates canonical Wnt signaling in cooperation with Fzd1, Fzd10, and LRP5 in PAC-1 cells.
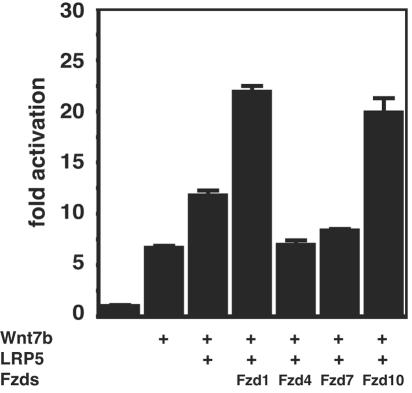
Wnt7blacZ −/− embryos exhibit defects in pulmonary vascular smooth muscle integrity (31). Since Wnt7b is expressed in the airway epithelium in the lung and not VSMCs or their mesenchymal precursors (31), it must act in a paracrine manner to regulate VSMC development. Previous reports have suggested that VSMCs are responsive to β-catenin/Wnt signaling (36). To determine whether Wnt7b signals through LRP5 and Fzd1 and Fzd10 in VSMCs, PAC-1 cells were transfected with expression plasmids encoding combinations of Wnt7b, LRP5, and Fzd1, 4, 7, and 10. As expected based on data in Fig. 2, Wnt7b activated the TOPFLASH reporter in PAC-1 cells (Fig. 7). However, Wnt7b plus LRP5 generated a significant increase in Wnt activity as measured by the TOPFLASH reporter (Fig. 7). Addition of Fzd1 and Fzd10 further increased Wnt activity over LRP5 alone (Fig. 7). These data demonstrate that Wnt7b activates canonical Wnt signaling in VSMCs cooperatively through LRP5 and Fzd1 and Fzd10.
FIG. 7.
Wnt7b/Fzd1/LRP5 and Wnt7b/Fzd10/LRP5 cooperatively activate canonical Wnt activity in PAC-1 cells. PAC-1 cells were transfected with expression plasmids encoding the indicated proteins. Wnt7b alone activated the TOPFLASH reporter approximately 7-fold, and coexpression of LRP5 increased this activation to 12-fold. However, coexpression of Wnt7b/Fzd1/LRP5 and Wnt7b/Fzd10/LRP5 resulted in a significant increase in TOPFLASH activity to more than 20-fold. Data represent the mean of three assays ± standard error of the mean.
DISCUSSION
Previous studies have demonstrated that Wnt signaling is a critical signaling pathway during lung airway development (19, 26, 31). Although Wnt7b is one of the key Wnt ligands expressed in the lung, little is known about how it transmits signals in this pathway, including which Fzd-LRP coreceptor complex is required for signaling. In this report, we show that Wnt7b activates canonical Wnt signaling in a cell-specific manner through Fzd1 and 10 and the Wnt coreceptor LRP5. We also show that this pathway is active in VSMC cells, which suggests, along with a loss of large blood vessel integrity in Wnt7blacZ−/− embryos, that Wnt signaling plays an important role in VSMC development and homeostasis. Together, these data provide key evidence on the receptor complex Wnt7b signals through and begins to provide a mechanism of how this signaling pathway regulates lung vascular and airway development.
As it is currently understood, Wnt proteins bind to and signal through Fzd cell surface receptors. Binding to Fzd proteins is essential but not always sufficient for activation of canonical Wnt signaling, and genetic evidence obtained in both Drosophila and mouse species suggests that additional cell surface proteins are necessary for efficient Wnt signaling (3, 8, 13, 43). These additional factors include the LRP5/6 coreceptors, which are required for most aspects of canonical Wnt signaling (24, 33, 38). Loss of function experiments have clearly demonstrated a requirement for LRP5/6 in Wnt signaling (24, 33, 38), although exactly how they work within the Wnt pathway is controversial. Some studies have demonstrated a direct binding of LRP5/6 to Wnt proteins but this has not been confirmed in other studies (23, 33, 42). LRP5/6 are also targets of Wnt signaling regulation through direct interactions with the secreted Wnt inhibitor dikkopf-1 (2, 5, 23, 30). Together, Fzds and LRP5/6 provide a specific coreceptor complex required for Wnt signaling.
Our findings demonstrate that Wnt7b can activate canonical Wnt signaling in the VSMC lines PAC-1 and A7r5 but not 3T3 or 293 cells by itself. Our data also show that Wnt7b cannot activate the noncanonical JNK-dependent Wnt pathway in 293 or PAC-1 cells, indicating cell-specific activation of the canonical Wnt pathway by Wnt7b. These findings allowed us to reconstitute an active ligand-receptor signaling complex in 293 cells for canonical Wnt signaling via Wnt7b, which demonstrated that Fzd1 and Fzd10 are able to bind and cooperatively activate canonical Wnt signaling in the presence of LRP5. Such cell-specific activation of the canonical pathway has been previously demonstrated and suggests a requirement for expression of specific cell surface receptors (7, 42). Only a few studies have examined Wnt ligand-receptor specificities. Wu and Nusse examined Drosophila Wnt ligand-receptor specificities and showed that with the three DWnts examined, wg, DWnt2, DWnt4, and DWnt8 all bind with different specificities to fzd, Dfzd2, Dfzd3, and Dfzd4 (42). This was the first thorough study of ligand receptor interactions for Wnts and clearly demonstrated specificity between Wnt ligands and Fzd receptors, at least in Drosophila. Evidence for specific cooperative activation of canonical Wnt signaling by Wnt7a, Fzd5, and LRP6 has also been reported (7).
The finding that Wnt7b binds and activates Wnt signaling through Fzd1 and Fzd10 has several implications. Due to its expression pattern, these data suggest that Fzd1 is the primary Fzd expressed in the lung mesenchyme that cooperatively activates canonical Wnt signaling with Wnt7b. Fzd1 expression in both lung mesenchyme and in the mature vasculature such as the aorta and large pulmonary vessels also suggests that it plays an important role in blood vessel development. Little is known about the role of Wnt signaling in vascular development. Loss of Wnt7b results in a loss of VSMC integrity in the lung while loss of Fzd5 leads to defects in yolk sac and placental angiogenesis (16, 31). Both β-catenin and LRP6 are important for VSMC proliferation induced by arterial injury (35, 36). In the case of Wnt7b, our previous data clearly demonstrate that it is expressed in the airway epithelium of the lung and not in VSMCs or other mesenchymal derivatives (31). This suggests that Wnt7b acts in a paracrine manner to regulate VSMC development and differentiation in the lung. The finding that Fzd1 is highly expressed in vascular smooth muscle tissues such as the aorta and pulmonary vessels supports the hypothesis that Wnt signaling is active in these cells, possibly playing a critical role in their development and response to environmental stimuli. Interestingly, other studies have shown an important role for Wnt7b in chorion-allantois fusion in the placenta where it appears to regulate expression of alpha 4 integrin (28). Since alpha 4 integrin is expressed at high levels in VSMCs (9) and is known to be important for cardiovascular development (46), it will be of interest in the future to determine whether Wnt signaling, in particular through Wnt7b, regulates its expression in VSMCs through paracrine mechanisms in the lung and placenta and in other tissues such as the brain.
Along with data demonstrating cooperative signaling with Wnt7b and LRP5, our data suggests that Fzd10 may be the major Fzd receptor that transmits Wnt7b signals in lung airway epithelium. Interestingly, Wnt7a has been implicated in cooperative signaling with Fzd10 in limb development (18). The expression patterns of Wnt7a, Wnt7b, and Fzd10 are similar in many tissues such as the neural tube, limb bud, and developing brain (18, 27, 31). However, Wnt7a is not expressed in the airway epithelium during embryonic development (W. Shu and E. Morrisey, unpublished observations). Given its ability to cooperatively activate canonical Wnt signaling and its expression pattern during embryogenesis, Fzd10 likely is one of the main Fzd receptors for Wnt7a and Wnt7b. Several studies have implied an important role for Wnt signaling in airway epithelial development. Loss of Wnt7b results in reduced branching morphogenesis as well as defective epithelial differentiation (31). Loss of Wnt5a also leads to defects in airway epithelial differentiation (19). Distal lung airway-specific loss of β-catenin results in defective distal airway development (26). However, due to the dual roles that β-catenin plays in cell-cell adhesion and Wnt signaling, the role of canonical Wnt signaling in distal lung epithelial development is still unclear. A better understanding of the requirement of Fzd10 for lung airway epithelial development should provide key insights into the Wnt pathway in lung morphogenesis.
These studies point to an important interaction between Wnt7b, LRP5, and Fzd1 and Fzd10. The paucity of information regarding Wnt-Fzd-LRP specificities in mammals has limited progress into understanding the tissue specific functions of Wnt ligands during development. Thus, the identification of these receptors for Wnt7b should lead to a better understanding of how Wnt7b signaling regulates epithelial-mesenchymal interactions in the lung and in other tissues during development.
Acknowledgments
We thank Sarah Miller for the frizzled in situ probe oligonucleotides, Jen Chih Hsieh, Jeremy Nathans, Jim Smith, and Arnold Levine for Wnt component expression plasmids, and the members of the University of Pennsylvania Molecular Cardiology Histology Core for their excellent technical assistance.
This work was supported by funding from the National Institutes of Health to E.E.M. (P01 HL075215).
REFERENCES
- 1.Bafico, A., A. Gazit, S. S. Wu-Morgan, A. Yaniv, and S. A. Aaronson. 1998. Characterization of Wnt-1 and Wnt-2 induced growth alterations and signaling pathways in NIH3T3 fibroblasts. Oncogene 16:2819-2825. [DOI] [PubMed] [Google Scholar]
- 2.Bafico, A., G. Liu, A. Yaniv, A. Gazit, and S. A. Aaronson. 2001. Novel mechanism of Wnt signalling inhibition mediated by Dickkopf-1 interaction with LRP6/Arrow. Nat. Cell Biol. 3:683-686. [DOI] [PubMed] [Google Scholar]
- 3.Bhanot, P., M. Brink, C. H. Samos, J. C. Hsieh, Y. Wang, J. P. Macke, D. Andrew, J. Nathans, and R. Nusse. 1996. A new member of the frizzled family from Drosophila functions as a Wingless receptor. Nature 382:225-230. [DOI] [PubMed] [Google Scholar]
- 4.Boutros, M., N. Paricio, D. I. Strutt, and M. Mlodzik. 1998. Dishevelled activates JNK and discriminates between JNK pathways in planar polarity and wingless signaling. Cell 94:109-118. [DOI] [PubMed] [Google Scholar]
- 5.Brott, B. K., and S. Y. Sokol. 2002. Regulation of Wnt/LRP signaling by distinct domains of Dickkopf proteins. Mol. Cell. Biol. 22:6100-6110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cadigan, K. M., and R. Nusse. 1997. Wnt signaling: a common theme in animal development. Genes Dev. 11:3286-3305. [DOI] [PubMed] [Google Scholar]
- 7.Caricasole, A., T. Ferraro, L. Iacovelli, E. Barletta, A. Caruso, D. Melchiorri, G. C. Terstappen, and F. Nicoletti. 2003. Functional characterization of WNT7A signaling in PC12 cells: interaction with A FZD5 x LRP6 receptor complex and modulation by Dickkopf proteins. J. Biol. Chem. 278:37024-37031. [DOI] [PubMed] [Google Scholar]
- 8.Culi, J., and R. S. Mann. 2003. Boca, an endoplasmic reticulum protein required for wingless signaling and trafficking of LDL receptor family members in Drosophila. Cell 112:343-354. [DOI] [PubMed] [Google Scholar]
- 9.Duplaa, C., T. Couffinhal, P. Dufourcq, B. Llanas, C. Moreau, and J. Bonnet. 1997. The integrin very late antigen-4 is expressed in human smooth muscle cell. Involvement of alpha 4 and vascular cell adhesion molecule-1 during smooth muscle cell differentiation. Circ. Res. 80:159-169. [DOI] [PubMed] [Google Scholar]
- 10.Favre, C. J., M. Mancuso, K. Maas, J. W. McLean, P. Baluk, and D. M. McDonald. 2003. Expression of genes involved in vascular development and angiogenesis in endothelial cells of adult lung. Am. J. Physiol. Heart Circ. Physiol. 285:H1917-H1938. [DOI] [PubMed] [Google Scholar]
- 11.Gitler, A. D., M. M. Lu, Y. Q. Jiang, J. A. Epstein, and P. J. Gruber. 2003. Molecular markers of cardiac endocardial cushion development. Dev. Dyn. 228:643-650. [DOI] [PubMed] [Google Scholar]
- 12.Heisenberg, C. P., M. Tada, G. J. Rauch, L. Saude, M. L. Concha, R. Geisler, D. L. Stemple, J. C. Smith, and S. W. Wilson. 2000. Silberblick/Wnt11 mediates convergent extension movements during zebrafish gastrulation. Nature 405:76-81. [DOI] [PubMed] [Google Scholar]
- 13.Hsieh, J. C., L. Lee, L. Zhang, S. Wefer, K. Brown, C. DeRossi, M. E. Wines, T. Rosenquist, and B. C. Holdener. 2003. Mesd encodes an LRP5/6 chaperone essential for specification of mouse embryonic polarity. Cell 112:355-367. [DOI] [PubMed] [Google Scholar]
- 14.Hsieh, J. C., A. Rattner, P. M. Smallwood, and J. Nathans. 1999. Biochemical characterization of Wnt-frizzled interactions using a soluble, biologically active vertebrate Wnt protein. Proc. Natl. Acad. Sci. USA 96:3546-3551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hurlstone, A. F., A. P. Haramis, E. Wienholds, H. Begthel, J. Korving, F. Van Eeden, E. Cuppen, D. Zivkovic, R. H. Plasterk, and H. Clevers. 2003. The Wnt/beta-catenin pathway regulates cardiac valve formation. Nature 425:633-637. [DOI] [PubMed] [Google Scholar]
- 16.Ishikawa, T., Y. Tamai, A. M. Zorn, H. Yoshida, M. F. Seldin, S. Nishikawa, and M. M. Taketo. 2001. Mouse Wnt receptor gene Fzd5 is essential for yolk sac and placental angiogenesis. Development 128:25-33. [DOI] [PubMed] [Google Scholar]
- 17.Julius, M. A., S. D. Rai, and J. Kitajewski. 1999. Chimeric Wnt proteins define the amino-terminus of Wnt-1 as a transformation-specific determinant. Oncogene 18:149-156. [DOI] [PubMed] [Google Scholar]
- 18.Kawakami, Y., N. Wada, S. Nishimatsu, and T. Nohno. 2000. Involvement of frizzled-10 in Wnt-7a signaling during chick limb development. Dev. Growth Differ. 42:561-569. [DOI] [PubMed] [Google Scholar]
- 19.Li, C., J. Xiao, K. Hormi, Z. Borok, and P. Minoo. 2002. Wnt5a participates in distal lung morphogenesis. Dev. Biol. 248:68-81. [DOI] [PubMed] [Google Scholar]
- 20.Li, L., H. Yuan, W. Xie, J. Mao, A. M. Caruso, A. McMahon, D. J. Sussman, and D. Wu. 1999. Dishevelled proteins lead to two signaling pathways. Regulation of LEF-1 and c-Jun N-terminal kinase in mammalian cells. J. Biol. Chem. 274:129-134. [DOI] [PubMed] [Google Scholar]
- 21.Liebner, S., A. Cattelino, R. Gallini, N. Rudini, M. Iurlaro, S. Piccolo, and E. Dejana. 2004. Beta-catenin is required for endothelial-mesenchymal transformation during heart cushion development in the mouse. J. Cell Biol. 166:359-367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lu, M. M., S. Li, H. Yang, and E. E. Morrisey. 2002. Foxp4: a novel member of the Foxp subfamily of winged-helix genes co-expressed with Foxp1 and Foxp2 in pulmonary and gut tissues. Mech. Dev. 119(Suppl. 1):S197-S202. [DOI] [PubMed] [Google Scholar]
- 23.Mao, B., W. Wu, Y. Li, D. Hoppe, P. Stannek, A. Glinka, and C. Niehrs. 2001. LDL-receptor-related protein 6 is a receptor for Dickkopf proteins. Nature 411:321-325. [DOI] [PubMed] [Google Scholar]
- 24.Mao, J., J. Wang, B. Liu, W. Pan, G. H. Farr III, C. Flynn, H. Yuan, S. Takada, D. Kimelman, L. Li, and D. Wu. 2001. Low-density lipoprotein receptor-related protein-5 binds to Axin and regulates the canonical Wnt signaling pathway. Mol. Cell 7:801-809. [DOI] [PubMed] [Google Scholar]
- 25.Maye, P., J. Zheng, L. Li, and D. Wu. 2004. Multiple mechanisms for Wnt11-mediated repression of the canonical Wnt signaling pathway. J. Biol. Chem. 279:24659-24665. [DOI] [PubMed] [Google Scholar]
- 26.Mucenski, M. L., S. E. Wert, J. M. Nation, D. E. Loudy, J. Huelsken, W. Birchmeier, E. E. Morrisey, and J. A. Whitsett. 2003. β-Catenin is required for specification of proximal/distal cell fate during lung morphogenesis. J. Biol. Chem. 278:40231-40238. [DOI] [PubMed] [Google Scholar]
- 27.Nunnally, A. P., and B. A. Parr. 2004. Analysis of Fz10 expression in mouse embryos. Dev. Genes Evol. 214:144-148. [DOI] [PubMed] [Google Scholar]
- 28.Parr, B. A., V. A. Cornish, M. I. Cybulsky, and A. P. McMahon. 2001. Wnt7b regulates placental development in mice. Dev. Biol. 237:324-332. [DOI] [PubMed] [Google Scholar]
- 29.Reddy, S. T., T. Andl, M. M. Lu, E. E. Morrisey, and S. E. Millar. 2004. Expression of Frizzled genes in developing and postnatal hair follicles. J. Investig. Dermatol. 123:275-282. [DOI] [PubMed] [Google Scholar]
- 30.Semenov, M. V., K. Tamai, B. K. Brott, M. Kuhl, S. Sokol, and X. He. 2001. Head inducer Dickkopf-1 is a ligand for Wnt coreceptor LRP6. Curr. Biol. 11:951-961. [DOI] [PubMed] [Google Scholar]
- 31.Shu, W., Y. Q. Jiang, M. M. Lu, and E. E. Morrisey. 2002. Wnt7b regulates mesenchymal proliferation and vascular development in the lung. Development 129:4831-4842. [DOI] [PubMed] [Google Scholar]
- 32.Tada, M., and J. C. Smith. 2000. Xwnt11 is a target of Xenopus Brachyury: regulation of gastrulation movements via Dishevelled, but not through the canonical Wnt pathway. Development 127:2227-2238. [DOI] [PubMed] [Google Scholar]
- 33.Tamai, K., M. Semenov, Y. Kato, R. Spokony, C. Liu, Y. Katsuyama, F. Hess, J. P. Saint-Jeannet, and X. He. 2000. LDL-receptor-related proteins in Wnt signal transduction. Nature 407:530-535. [DOI] [PubMed] [Google Scholar]
- 34.Tao, W., D. Pennica, L. Xu, R. F. Kalejta, and A. J. Levine. 2001. Wrch-1, a novel member of the Rho gene family that is regulated by Wnt-1. Genes Dev. 15:1796-1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang, X., N. Adhikari, Q. Li, and J. L. Hall. 2004. The LDL receptor related protein LRP6 regulates proliferation and survival through the Wnt cascade in vascular smooth muscle cells. Am. J. Physiol. Heart Circ. Physiol. 287:2376-2383. [DOI] [PubMed] [Google Scholar]
- 36.Wang, X., Y. Xiao, Y. Mou, Y. Zhao, W. M. Blankesteijn, and J. L. Hall. 2002. A role for the beta-catenin/T-cell factor signaling cascade in vascular remodeling. Circ. Res. 90:340-347. [DOI] [PubMed] [Google Scholar]
- 37.Warburton, D., M. Schwarz, D. Tefft, G. Flores-Delgado, K. D. Anderson, and W. V. Cardoso. 2000. The molecular basis of lung morphogenesis. Mech. Dev. 92:55-81. [DOI] [PubMed] [Google Scholar]
- 38.Wehrli, M., S. T. Dougan, K. Caldwell, L. O'Keefe, S. Schwartz, D. Vaizel-Ohayon, E. Schejter, A. Tomlinson, and S. DiNardo. 2000. arrow encodes an LDL-receptor-related protein essential for Wingless signalling. Nature 407:527-530. [DOI] [PubMed] [Google Scholar]
- 39.Weidenfeld, J., W. Shu, L. Zhang, S. E. Millar, and E. E. Morrisey. 2002. The WNT7b promoter is regulated by TTF-1, GATA6, and Foxa2 in lung epithelium. J. Biol. Chem. 277:21061-21070. [DOI] [PubMed] [Google Scholar]
- 40.Weston, C. R., and R. J. Davis. 2002. The JNK signal transduction pathway. Curr. Opin. Genet. Dev. 12:14-21. [DOI] [PubMed] [Google Scholar]
- 41.Wright, M., M. Aikawa, W. Szeto, and J. Papkoff. 1999. Identification of a Wnt-responsive signal transduction pathway in primary endothelial cells. Biochem. Biophys. Res. Commun. 263:384-388. [DOI] [PubMed] [Google Scholar]
- 42.Wu, C. H., and R. Nusse. 2002. Ligand receptor interactions in the Wnt signaling pathway in Drosophila. J. Biol. Chem. 277:41762-41769. [DOI] [PubMed] [Google Scholar]
- 43.Xu, Q., Y. Wang, A. Dabdoub, P. M. Smallwood, J. Williams, C. Woods, M. W. Kelley, L. Jiang, W. Tasman, K. Zhang, and J. Nathans. 2004. Vascular development in the retina and inner ear: control by Norrin and Frizzled-4, a high-affinity ligand-receptor pair. Cell 116:883-895. [DOI] [PubMed] [Google Scholar]
- 44.Yamanaka, H., T. Moriguchi, N. Masuyama, M. Kusakabe, H. Hanafusa, R. Takada, S. Takada, and E. Nishida. 2002. JNK functions in the non-canonical Wnt pathway to regulate convergent extension movements in vertebrates. EMBO Rep. 3:69-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yang, H., M. M. Lu, L. Zhang, J. A. Whitsett, and E. E. Morrisey. 2002. GATA6 regulates differentiation of distal lung epithelium. Development 129:2233-2246. [DOI] [PubMed] [Google Scholar]
- 46.Yang, J. T., H. Rayburn, and R. O. Hynes. 1995. Cell adhesion events mediated by alpha 4 integrins are essential in placental and cardiac development. Development 121:549-560. [DOI] [PubMed] [Google Scholar]