Abstract
The intergenic sequences (IGS) between the first nine genes of human respiratory syncytial virus (RSV) vary in length from 1 to 56 nucleotides and lack apparent conserved sequence motifs. To investigate their influence on sequential transcription and viral growth, recombinant RSV strain A2, from which the SH gene had been deleted to facilitate manipulation, was further modified to contain an M-G IGS of 16, 30, 44, 58, 65, 72, 86, 100, 120, 140, or 160 nucleotides. All of the viruses were viable. For viruses with an M-G IGS of 100 nucleotides or more, plaque size decreased with increasing IGS length. In this same length range, increasing IGS length was associated with modest attenuation during single-step, but not multistep, growth in HEp-2 cells. Surprisingly, Northern blot analysis of the accumulation of six different mRNAs indicated that there was little or no change in transcription with increasing IGS length. Thus, the RSV polymerase apparently can readily cross IGS of various lengths, including unnaturally long ones, with little or no effect on the efficiency of termination and reinitiation. This finding supports the view that the IGS do not have much effect on sequential transcription and provides evidence from infectious virus that IGS length is not an important regulatory feature. To evaluate replication in vivo, BALB/c mice were infected intranasally with RSV containing an M-G IGS of 65, 140, or 160 nucleotides. Replication of the latter two viruses was decreased up to 5- and 25-fold in the upper and lower respiratory tracts, respectively, on day 3 following infection. However, the level of replication at both sites on days 4 and 5 was very similar to that of the virus with an IGS of 65 nucleotides. Thus, the modest attenuation in vivo associated with the longer IGS was additive to that conferred by deletion of the SH gene and might be useful to incrementally increase the level of attenuation of a live-attenuated vaccine virus.
Human respiratory syncytial virus (RSV) is a member of genus Pneumovirus of family Paramyxoviridae. RSV is the most important viral agent of serious respiratory tract disease in infants and young children worldwide and is an important cause of respiratory tract disease in the general population and especially in immunocompromised individuals and the elderly (reference 8 and the references therein). A vaccine against RSV is not yet available, although significant progress toward this goal has been achieved and several live attenuated vaccine candidates have been developed (38).
The genome of RSV is a single strand of negative-sense RNA of 15,222 nucleotides that encodes 10 mRNAs. Viral RNA replication requires the polymerase (L), nucleoprotein (N), and phosphoprotein (P) (13, 39). Transcription requires in addition the M2-1 transcription antitermination factor (7, 16). Two additional proteins appear to regulate or enhance RNA synthesis, although they are not required for virus growth: the M2-2 protein appears to down-regulate transcription and up-regulate replication (2, 16), and the NS1 protein is required for efficient RNA replication (M. Teng and P. Collins, unpublished data).
The RSV genes are arranged on genomic RNA in the order 3′-NS1-NS2-N-P-M-SH-G-F-M2-L, with the M2-1 and M2-2 proteins being encoded by overlapping open reading frames in the M2 mRNA. Each of RSV genes is flanked on the upstream and downstream ends with gene-start (GS) and gene-end (GE) semiconserved sequence motifs, which direct transcriptional initiation and termination, respectively. The first nine RSV genes are separated by intergenic sequences (IGS) that are 1 to 56 nucleotides long, lack consensus elements or strong secondary structures, and vary significantly in sequence and length among RSV strains (5, 17). The last two genes, M2 and L, overlap by 68 nucleotides, and the L gene is accessed by a backsliding polymerase (10).
Among different members of order Mononegavirales, the nonsegmented negative-strand RNA viruses, the organization of the IGS exhibits two alternative patterns. A number of viruses have short conserved or semiconserved IGS. For example, the rhabdovirus vesicular stomatitis virus (VSV) has the conserved dinucleotide GA or CA as the IGS, with the sole exception that the G-L IGS of the New Jersey strain is 21 nucleotides in length (35). The paramyxoviruses Sendai virus, human parainfluenza virus type (PIV3), bovine PIV3, measles virus, and rinderpest virus have the trinucleotide GAA or GGG as the IGS (reviewed in reference 19). Besides RSV, certain other mononegaviruses have longer, more-variable IGS. For example, the rhabdovirus rabies virus has IGS of 2 to 29 nucleotides (34). Members of another genus among the paramyxoviruses, the rubulaviruses, have IGS lengths as follows: simian virus 5 (SV5), 1 to 22 nucleotides; SV41, 3 to 21 nucleotides not including the incomplete M-F gene junction; mumps virus, 1 to 7 nucleotides; Newcastle disease virus, 1 to 47 nucleotides (reviewed in references 19 and 21); and human PIV2, 4 to 45 nucleotides (18). Filoviruses also have long and diverse IGS: Marburg virus, 2 to 95 nucleotides long (EMBL accession no. Z29337; GenBank accession no. Z12132); and Ebola virus, 3 to 143 nucleotides (27; GenBank accession no. AF086833).
The role of the IGS in the regulation of RNA synthesis has been investigated for several mononegaviruses. For VSV, studies with a minireplicon showed that the IGS was essential for reinitiation at the downstream gene and that some, but not all, sequence substitutions or small length changes in the IGS strongly influenced termination at the upstream gene (1, 30–32). In contrast, substitution of the various naturally occurring RSV IGS into a dicistronic minigenome, or deletion of the IGS altogether, had no apparent effect on termination, reinitiation, or the production of a readthrough mRNA (20). In studies with an SV5 minireplicon (25), the first IGS residue immediately downstream the UUUU tract of M GE signal was necessary for efficient termination of M gene transcription, although this effect appeared to be limited to that particular GE signal. The introduction of certain nonnatural sequences into an SV5 IGS also influenced the efficiency of reinitiation at the downstream gene. Other studies compared the naturally occurring gene junctions of RSV in a minigenome system (14) or of SV5 in infectious recombinant virus (15). These results showed that the different gene junctions have different effects on sequential transcription. However, since entire gene junctions were substituted, effects due to GE, IGS, or GS signals could not be distinguished.
In this study, we manipulated the length of a single IGS in infectious recombinant RSV, varying it from 16 to 160 nucleotides, the latter being 40% of the length of the smallest RSV gene. The remainder of the gene junction and indeed the rest of the entire antigenome were held constant. The panel of recombinant RSVs was then examined for effects on sequential transcription, RNA replication, plaque formation and propagation in vitro, and replication and immunogenicity in the respiratory tracts of mice.
MATERIALS AND METHODS
Construction and structure of recombinant RSVs with a modified M-G IGS.
To facilitate cDNA manipulations, the IGS mutations were made in an antigenomic cDNA from which the SH gene had been deleted. Deletion of SH from wild-type recombinant strain A2 (wt rRSV) was described previously (37). That antigenomic backbone contained several additional mutations for vaccine development purposes that were not desirable here; we therefore imported this SH deletion into the previously described wt rRSV backbone (6) by restriction fragment replacement (not shown). As summarized in Fig. 1A and described in detail previously (37), the deletion involved replacing a ScaI-PacI restriction fragment, bearing most of the SH gene, with a small synthetic double-stranded DNA. This restored the M gene noncoding region in its entirety and recreated the GE signal (Fig. 1A). The reconstruction was designed to introduce a single nucleotide change into the M GE signal (AGTTAATAAAAAA to AGTTAATTAAAAA, with the change underlined), which created a PacI site (boldface) and made the signal identical to the original SH GE signal. Thus, in the SH deletion virus, the M-G gene junction is identical to the naturally occurring SH-G junction, consisting of the authentic SH GE signal (now attached to the M gene) followed the authentic 44-nucleotide SH-G IGS, followed by the authentic G GS. In this SH deletion virus, this gene junction will be referred to as M-G.
FIG. 1.
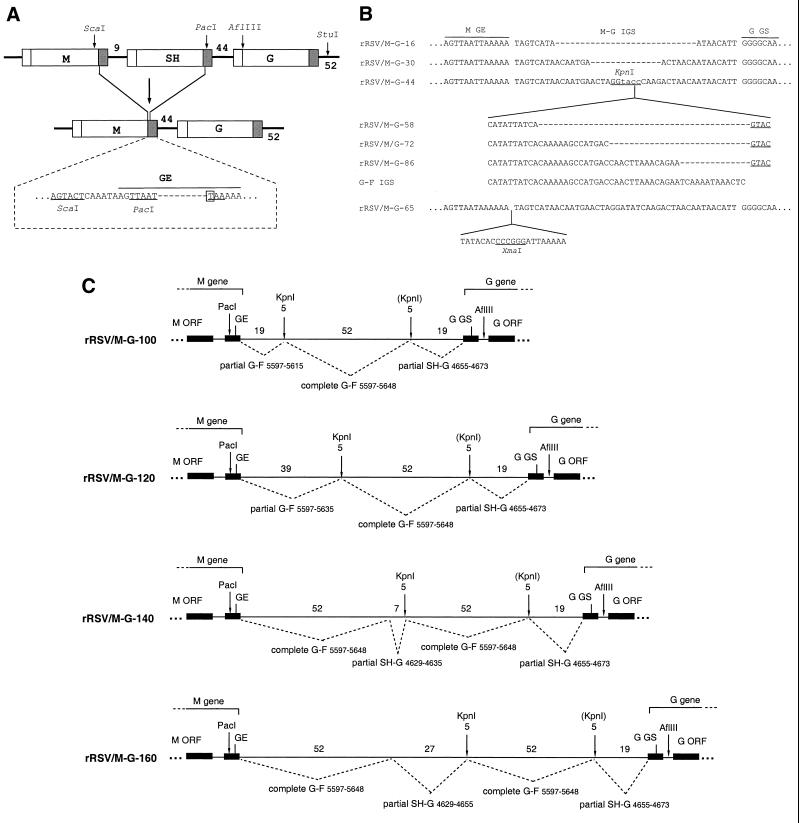
Structures of artificial IGS inserted between the M and G genes of recombinant RSVs lacking the SH gene. (A) Deletion of the SH gene. The ScaI-PacI restriction fragment bearing most of the SH gene was replaced by a synthetic double-stranded DNA that restored the downstream noncoding region of the M gene and restored a GE signal. The sequence below the diagram shows the restored ScaI and PacI sites (underlined) and GE signal (overlined), and the dashes in the sequence indicate the boundary between sequence originally derived from the M (left) and SH (right) genes. The restored signal contains a single nucleotide substitution (boxed) that creates a PacI site and makes the GE signal identical to the naturally occurring SH GE signal. GS and GE signals are shown as white and gray boxes, respectively, and the number of nucleotides in the IGS is indicated. (B) Structures of IGS between the M and G genes of rRSV/M-G-16, -30, -44, -58, -72, -86, and -65. rRSV/M-G-44 contains the parental 44-nucleotide IGS modified by nucleotide substitution at four positions (lowercase letters) to create a KpnI site (underlined). To create rRSV/M-G-16 and -30, the indicated deletions (dashed line) were made in the 44-nucleotide IGS between the M and G genes. The KpnI site introduced into this IGS was used to accept addition sequence segments, all of which were derived from increasing increments of the naturally occurring 52-nucleotide G-F IGS, which is shown. The inserts had an additional GTAC (underlined) at the downstream end that represents an incomplete KpnI site. Thus, the introduction of incrementally longer segments of the G-F IGS resulted in the creation of rRSV/M-G-58, -72, and -86. rRSV/M-G-65, constructed in previous work (4), contains a 21-nucleotide insertion with an XmaI site (underlined) and was placed immediately downstream of the M GE signal. (C) Structures of IGS between the M and G genes of rRSV/M-G-100, -120, -140, and -160. The diagram shows the downstream end of the M gene on the left, the upstream end of the G gene on the right, and the intervening M-G IGS. The open reading frames (ORF) and GS and GE transcription signals are shown as filled rectangles; nontranslated gene regions and the M-G IGS are shown as thin lines. The set of synthetic M-G IGS was assembled from sequence segments copied from the naturally occurring G-F and SH-G IGS. These are identified according to their original sequence position in the complete 15,223-nucleotide recombinant wt rRSV antigenome, and the nucleotide length of each segment is indicated above the lines. The left-hand KpnI site is indicated as 5 nucleotides because it overlaps with the last nucleotide of the upstream G-F intergenic region; the second KpnI site, also indicated as 5 nucleotides, is in parentheses because the complete site was not regenerated by the design of the construction.
To manipulate the M-G IGS, we subcloned this region as a PacI-StuI fragment (Fig. 1A) and used PacI and AflIII restriction sites to make additions, deletions, or substitutions by restriction fragment replacement using small double-stranded cDNAs made either by annealing synthetic oligonucleotides or by PCR using mutagenic primers, with sticky ends made by restriction enzyme digestion of the PCR product. All of these manipulations employed conventional methods and will not be described in detail. All structures were confirmed by dideoxynucleotide sequencing. The resulting mutant IGS (Fig. 1B and C) had lengths of 16, 30, 44, 58, 72, 86, 100, 120, 140, and 160 nucleotides. As indicated in Fig. 1B and C, assembly of the various mutant IGS involved sequence derived from the naturally occurring SH-G and G-F IGS of strain A2.
The one exception to the construction scheme described above is rRSV/M-G-65, which was constructed in previous work (4). This virus does not contain the point mutation and resulting PacI site in the M GE signal mentioned above. The structure of its IGS is shown in Fig. 1B.
Recovery and propagation of recombinant RSV.
Recombinant RSV was recovered by cotransfection of each antigenomic cDNA with support plasmids encoding the N, P, M2-1, and L proteins into HEp-2 cells infected with vaccinia virus recombinant MVA encoding the bacteriophage T7 RNA polymerase (6). The MVA recombinant was kindly supplied by Linda Wyatt and Bernie Moss. Each recombinant virus was designated according to the length of its M-G IGS, i.e., rRSV/M-G-16, -30, -44, and so forth. The modified viruses were amplified by four to five sequential passages in HEp-2 cells. Opti-MEM (Life Technologies) containing 2% fetal bovine serum (Summit Biotechnologies) was used in all infections. After the last passage, the cells and overlying medium were harvested and centrifuged at 500 × g, the supernatants were taken and adjusted to contain 100 mM MgSO4 and 50 mM HEPES sodium salt, and the viruses were aliquoted and frozen. wt rRSV was used as one of the controls; however, this was for descriptive purposes rather than direct comparison because it contains the SH gene.
Reverse transcription-PCR (RT-PCR) analysis of the modified viral IGS.
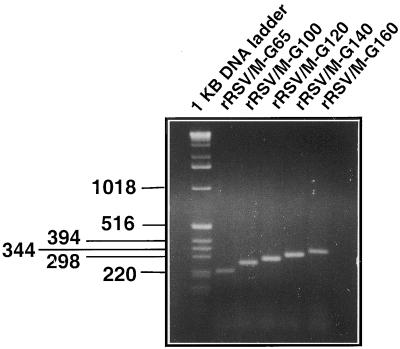
Total RNA was isolated 48 h postinfection from virus-infected HEp-2 cells, using Trizol reagent (Life Technologies) according manufacturer's recommendations with addition of two phenol-chloroform extractions followed by ethanol precipitation. Reverse transcription was performed using SuperScript II reverse transcriptase (Life Technologies) and the positive-sense oligonucleotide primer 5′-TCATCCCAAGTCATTGTT-3′ (nucleotides 4158 to 4175 of wt rRSV antigenome) upstream of the M-G gene junction. An aliquot of the cDNA product was used as the template in PCR using the above-mentioned primer together with the negative-sense primer 5′-TATATAAGCACGATGATATG-3′, corresponding to nucleotides 4763 to 4782 of the antigenome, downstream of the M-G gene junction. An initial 2-min denaturation step was performed during which the Taq DNA polymerase was added, and then 25 cycles were performed (denaturation, 1 min at 94°C; annealing, 1 min at 40°C; elongation, 2 min at 72°C). The products were analyzed on a 2.5% agarose gel.
Immunostaining of viral plaques.
Monolayers of HEp-2 cells in six-well plates were infected with virus, covered with Opti-MEM (Life Technologies) containing 2% fetal bovine serum and 0.9% methylcellulose, and incubated at 32°C in a CO2 incubator. Six days later, the monolayers were fixed with cold 80% methanol and stained with a mixture of three murine monoclonal antibodies against RSV F protein (23). The plaques were visualized using horseradish peroxidase-labeled goat anti-mouse immunoglobulin G (IgG; Kirkegaard & Perry Laboratories). The average plaque diameter was calculated by measurement of 30 plaques for each virus.
Kinetics of viral replication in HEp-2 cells.
Duplicate wells of six-well plates were infected with virus at a multiplicity of infection (MOI) of either 5 or 0.01 PFU in a total volume of 1 ml. After 3 h of adsorption at 37°C, the cells were washed three times and incubated at 37°C. At the indicated time points, 120-μl aliquots of medium were taken and replaced with an equal volume of fresh medium. Magnesium sulfate and HEPES were added to the aliquots as indicated above, and the samples were flash-frozen. Each of the duplicate samples was titrated in duplicates, and the mean concentration of the virus at each time point was determined.
Northern blot hybridization.
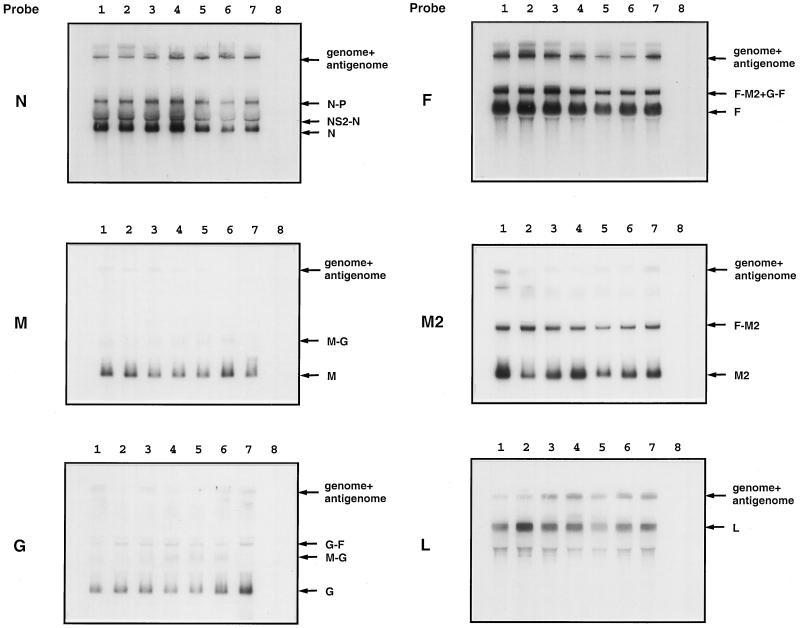
Total intracellular RNA was isolated from virus-infected HEp-2 cells using Trizol reagent as described above, electrophoresed in denaturing agarose gel containing formaldehyde (13), transferred onto nitrocellulose membranes, and hybridized with a 32P-labeled double-stranded cDNA probe or strand-specific RNA probe specific to the RSV N, M, G, F, M2, or L gene, as indicated in the legends to Fig. 5 and 6. The bands corresponding to the individual RSV mRNAs or genomic RNA were quantitated using a Molecular Dynamics PhosphorImager. For each band, the background amount of radioactivity was subtracted.
FIG. 5.
Northern blot analysis of RNAs synthesized by recombinant RSVs bearing M-G IGS of increasing length. HEp-2 cells were infected with the indicated virus (MOI of 5 PFU), incubated for 48 h, and harvested; then total intracellular RNA was extracted. The RNA was subjected to electrophoresis in a formaldehyde-containing agarose gel, transferred onto nitrocellulose membranes, and hybridized with a double-stranded cDNA probe specific to the N, M, G, F, M2 or L mRNA, as indicated to the left. Lanes: 1, rRSV/M-G-44; 2, rRSV/M-G-65; 3, rRSV/M-G-100; 4, rRSV/M-G-120; 5, rRSV/M-G-140; 6, rRSV/M-G-160; 7, wt rRSV; 8, noninfected. Positions of the various RSV and readthrough mRNAs, as well as genomic and antigenomic RNAs, are indicated to the right.
FIG. 6.
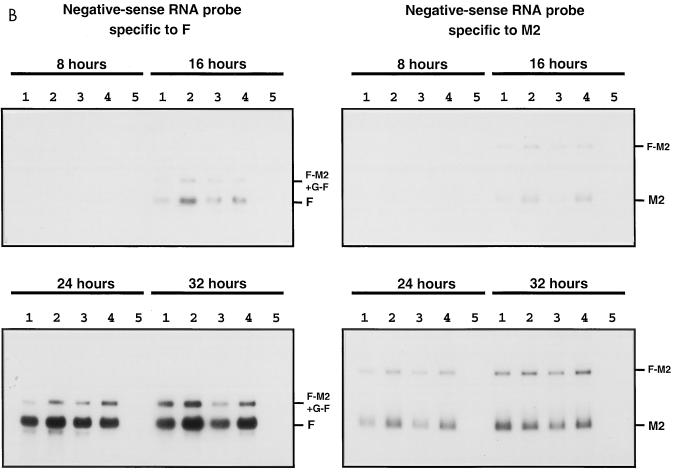
Kinetics of genome transcription of recombinant RSVs bearing M-G IGS of 44, 65, 140, or 160 nucleotides in length. The accumulation viral RNAs in infected HEp-2 cells (MOI of 5 PFU) was evaluated by Northern blot hybridization of total intracellular RNA harvested 8, 16, 24, and 32 h postinfection. The blots were hybridized with a negative-sense RNA probe specific to the N, F, or M2 mRNA, a double-stranded cDNA probe specific to the G mRNA, or a positive-sense RNA probe specific to RSV genomic RNA. Lanes: 1, rRSV/M-G-44; 2, rRSV/M-G-65; 3, rRSV/M-G-140; 4, rRSV/M-G-160; 5, uninfected. Positions of the individual mRNAs and readthrough mRNAs, and of genomic and antigenomic RNAs, are indicated to the right.
Virus replication and immunogenicity in mice.
Each of 12-week-old, respiratory-pathogen-free BALB/c mice (Charles River, Wilmington, Mass.) in groups of 20 to 21 were inoculated intranasally under light methoxyflurane anesthesia with 106 PFU of rRSV/M-G/65, rRSV/M-G-140, rRSV/M-G-160, or medium alone. On days 3, 4, and 5 following inoculation, five mice per group were sacrificed (except on day 5 for rRSV/M-G-160, when six mice were sacrificed) by CO2 asphyxiation, and nasal turbinates and lungs were harvested for quantification of virus titer by plaque assay (23, 24). Serum samples were taken from the remaining mice on day 53 postinfection. Serum IgG antibodies specific to the RSV F protein were quantitated in by enzyme-linked immunosorbent assay (ELISA) using immunoaffinity-purified F protein from cells infected with RSV Long strain as the solid-phase antigen (23).
RESULTS
Construction of rRSVs with M-G IGS of increasing length.
Initially, we sought to manipulate the 44-nucleotide IGS between the SH and G genes in a complete RSV strain A2 antigenomic cDNA. However, the RSV antigenome has a tendency to acquire the insertion of a discrete fragment of 1.4 kb of heterologous sequence into the noncoding region of the SH gene during propagation in Escherichia coli. Therefore, we chose instead to work with a version of the antigenomic cDNA from which the SH gene had been deleted (Materials and Methods; Fig. 1A). As described previously, recombinant RSV lacking the SH gene grows as well as or better than wt rRSV in cell culture (forming larger plaques in HEp-2 cells, as shown in Fig. 2 below) and is slightly attenuated in the upper respiratory tracts of mice and in the upper and lower respiratory tracts of chimpanzees (4, 37). Thus, while the function of the SH protein remains unknown, its gene can be deleted with little apparent effect on RSV gene expression and replication in vitro and only a slight effect in vivo. In this SH deletion virus, the SH GE signal was fused to the M gene, replacing the M GE signal, and was followed by the 44-nucleotide SH-G IGS and the G gene (Fig. 1A). Thus, although we designate this gene junction in the SH deletion virus as M-G, it actually is identical to the naturally occurring SH-G gene junction, and hence all of the subsequent manipulations involved an authentic gene junction structure.
FIG. 2.
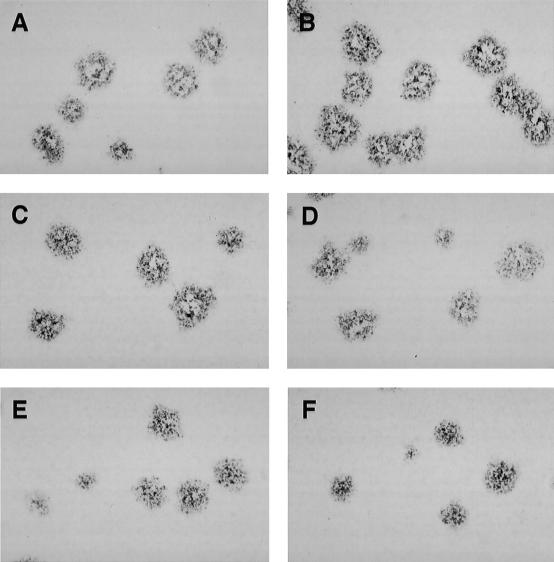
Plaque morphology in HEp-2 cells of recombinant RSVs bearing M-G IGS of increasing length. HEp-2 cell monolayers were infected with wt rRSV (A), rRSV/M-G-65 (B), rRSV/M-G-100 (C), rRSV/M-G-120 (D), rRSV/M-G-140 (E), or rRSV/M-G-160 (F). Note that rRSV/M-G-65 (B), rather than wt rRSV, should be used as the parent for comparison because the latter virus differs from the others in containing the SH gene. The cells were incubated for 6 days at 32°C and fixed with methanol; plaques were visualized by immunostaining with F-specific monoclonal antibodies.
Using the SH deletion virus as the parent, we prepared and recovered the following panel of recombinant viruses, which are designated according the length of the M-G IGS: rRSV/M-G-16, rRSV/M-G-30, rRSV/M-G-44, rRSV/M-G-58, rRSV/M-G-72, rRSV/M-G-86, rRSV/M-G-100, rRSV/M-G-120, rRSV/M-G-140, and rRSV/M-G-160. The IGS structures of the first six viruses are shown in Fig. 1B, and those of the last four are outlined in Fig. 1C. We also had available the original SH deletion virus prepared previously (4), which has a 65-nucleotide M-G IGS and for the purposes of this report was renamed rRSV/M-G-65 (Fig. 1B). In addition, we made a second version of rRSV/M-G-100 that differed only in the sequence composition of the 100-nucleotide IGS; however, the two different versions were indistinguishable in cell culture, and only the results for the one shown in Fig. 1C will be presented. As indicated in Fig. 1, the artificially long IGS were constructed from segments derived from the naturally occurring SH-G and G-F IGS and did not contain any non-RSV sequence except for the indicated KpnI and XmaI restriction sites. The viruses were indistinguishable with regard to efficiency of recovery.
Increased length of M-G IGS resulted in reduced plaque size.
The plaques formed by the various recombinant viruses in HEp-2 cell monolayers were visualized by staining with monoclonal antibodies against the F protein (Fig. 2); plaque size was measured in several independent experiments. Viruses that had an M-G IGS of 16 to 86 nucleotides were indistinguishable on the basis of plaque size (represented in Fig. 2 by rRSV/M-G-65); wt rRSV also was included, but it contains the SH gene and hence rRSV/M-G-65 rather than wt rRSV should be considered the parental control for the IGS mutants. The viruses with an M-G IGS of 100 nucleotides or more had progressively smaller plaques and also exhibited greater heterogeneity in plaque size compared to rRSV/M-G-65 (Fig. 2). Taking one typical experiment as an example, with the average plaque size of rRSV/M-G-65 normalized to 1.0 (standard deviation [SD] 0.13), the relative plaque sizes were as follows: rRSV/M-G-100, 0.90 (SD 0.13); rRSV/M-G-120, 0.83 (SD 0.16); rRSV/M-G-140, 0.80 (SD 0.26); rRSV/M-G-160, 0.68 (SD 0.28); and wt rRSV, 0.88 (SD 0.17).
The modified IGS are stable.
Preparations of the various viruses were made by amplification by four or five passages in HEp-2 cells. Intracellular RNA from each final passage was isolated and analyzed by RT-PCR using primers that flanked the M-G IGS. The length of the PCR product representing the M-G IGS of each recombinant RSV corresponded to the calculated value, and shorter PCR products that might represent deletions were not detected (Fig. 3). Thus, the modified M-G IGS appeared to be stable.
FIG. 3.
RT-PCR analysis of the M-G gene junction from recovered recombinant RSV. Total intracellular RNA isolated from HEp-2 cells after the fourth or fifth passage of the indicated recombinant RSVs was subjected to RT-PCR using primers spanning positions 4158 to 4782 of antigenomic RNA, which includes the M-G IGS. The products were analyzed on a 2.5% agarose gel and visualized with ethidium bromide. The lengths of DNA fragments of a commercial DNA molecular size marker are indicated in nucleotides at the left. The length of the RT-PCR product produced from rRSV/M-G-65 is predicted to be 226 nucleotides.
Effect of increasing IGS length on single-step and multistep replication in vitro.
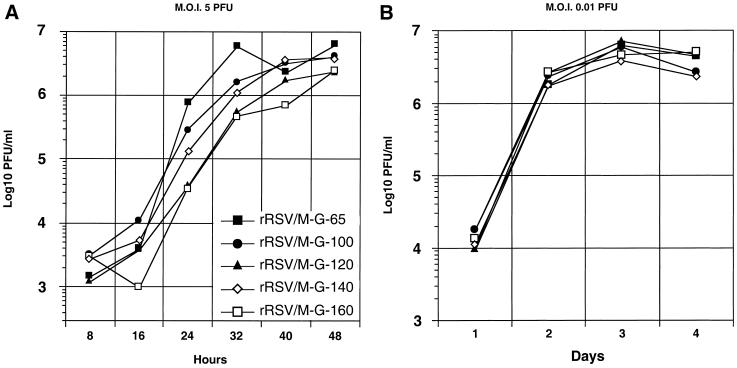
Analysis of in vitro replication of the viruses with M-G IGS of 86 nucleotides or less revealed no differences between the viruses (results not shown). We therefore focused on the viruses with an M-G IGS of 100 nucleotides or more. To study the kinetics of virus replication in a single-step growth cycle, HEp-2 cell monolayers were infected at an MOI of 5 PFU per cell with various recombinant viruses containing M-G IGS of increasing length. Aliquots from the overlying medium were taken at 8-h intervals and analyzed later in parallel by plaque assay (Fig. 4A). In general, virus growth was delayed and somewhat reduced with increasing IGS length, although in this particular experiment rRSV/M-G-140 grew somewhat better than rRSV/M-G-120. The largest differences between titers of the fastest-growing and most attenuated viruses, rRSV/M-G-65 and rRSV/M-G-160, respectively, were 21-fold at 24 h and 12-fold at 32 h. However, by 48 h postinfection the slower-growing viruses had largely caught up with the rRSV/M-G-65 virus, and the difference in titer was small.
FIG. 4.
Kinetics of replication in vitro of recombinant RSVs bearing M-G IGS of increasing length. Confluent HEp-2 cell monolayers were infected in duplicate with the indicated viruses at an input MOI of 5 (A) or 0.01 (B) PFU per cell. The cells were washed three times and incubated at 37°C. Aliquots of the medium overlying the cells were taken at 8 (A)- or 24 (B)-h intervals, replaced with an equal volume of fresh medium, flash-frozen, and analyzed later in a single assay by plaque titration.
To analyze multistep growth kinetics, HEp-2 monolayers were infected at an MOI of 0.01 PFU per cell, and samples were taken for analysis at 24-h intervals (Fig. 4B). It had been expected that the kinetics of multistep growth with liquid overlay would reflect the plaque size analysis described above. Unexpectedly, this was not the case, and there were no significant differences between the various viruses.
Sequential transcription is not affected by the increased length of the M-G IGS.
Transcription of individual genes of wt rRSV and of the viruses with M-G IGS of increasing length was analyzed by Northern blot hybridization. HEp-2 cells were infected at an MOI of 5 PFU per cell and harvested 48 h later, and total intracellular RNA was isolated and analyzed by Northern blot hybridization with double-stranded cDNA probes. We tested transcription of the genes located upstream of the modified IGS (N and M), as well as ones located downstream (G, F, M2, and L). Typical results are shown in Fig. 5, and the results of quantitation of the bands corresponding to G and F mRNA using phosphorimagery are presented in Table 1. Surprisingly, there was little or no consistent difference between the different viruses in the level of expression of the various mRNAs. In particular, the efficiency of transcription of genes downstream of the M-G IGS, and the frequency of readthrough transcription across the M-G IGS, appeared to be unaffected by its increasing length. The accumulation of genomic and antigenomic RNAs, which migrate as a single band, also did not appear to vary in a consistent manner among the different viruses.
TABLE 1.
Accumulation of G and F mRNAs in virus-infected HEp-2 cellsa
| Virus | Relative radioactivity
|
|||
|---|---|---|---|---|
| G mRNA
|
F mRNA
|
|||
| Expt 1 | Expt 2 | Expt 1 | Expt 2 | |
| rRSV/M-G-65 | 1.05 | 1.26 | 0.74 | 1.21 |
| rRSV/M-G-100 | 2.61 | 1.34 | 0.86 | 1.09 |
| rRSV/M-G-120 | 1.94 | 1.09 | 0.76 | 0.93 |
| rRSV/M-G-140 | 1.44 | 1.63 | 0.61 | 0.80 |
| rRSV/M-G-160 | 1.10 | 2.71 | 0.37 | 1.27 |
HEp-2 cells were infected with the indicated virus, and total intracellular RNA was isolated 48 h later and analyzed by Northern blot hybridization as described in Materials and Methods. The amount of radioactivity in the bands corresponding to individual mRNAs was quantitated by phosphorimagery and normalized relative to rRSV/M-G-44, which was assigned a value of 1.00.
It was possible that differences in transcription or replication occurred that were not apparent by analyzing total RNA harvested late in infection. Therefore, we also investigated the kinetics of accumulation of several mRNAs in HEp-2 cells infected as described above. Total intracellular RNA was isolated 8, 16, 24, and 32 h postinfection and analyzed by Northern blot hybridization. Representative results for rRSV/M-G-44, rRSV/M-G-65, rRSV/M-G-140, and rRSV/M-G-160 are shown in Fig. 6.
The accumulation of mRNA was analyzed by hybridization with a double-stranded cDNA probe specific to the G gene, or with single-stranded RNA probes of negative polarity specific to N, G, F, or M2 mRNA. No significant differences were found between viruses with regard to the overall mRNA patterns or the rates of accumulation of the various mRNAs. Small differences were seen in some experiments, but these were not consistent between experiments. In these particular experiments, antigenomic RNA was not readily detectable.
The accumulation of genomic RNA was analyzed by hybridization with a positive-sense M2-specific (not shown) or F-specific (Fig. 6) RNA probe and also was visualized with the double-stranded G cDNA probe mentioned above. Genomic RNA was undetectable at 8 h postinfection and was barely detectable at 16 h. Surprisingly, at 24 and 32 h, the amounts of detected genomic RNA or genomic/antigenomic RNA appeared to be somewhat increased for the viruses with longer IGS compared to rRSV/M-G-44. Since the transfer and detection of very large RNAs are subject to considerable experimental variability, further work will be necessary to determine whether this increase is a consistent effect. However, it seems clear that the increasing length of the M-G IGS was not associated with a diminution of RNA replication; this is a further sign that sequential transcription was not reduced significantly.
Replication in vivo and immunogenicity of viruses containing M-G IGS of increasing length.
It was of interest to determine whether the decreased plaque size and reduction in single-cycle growth in vitro was reflected by decreased replication of virus in vivo. We compared the abilities of rRSV/M-G-65, rRSV/M-G-140, and rRSV/M-G-160 to replicate in the respiratory tracts of mice. Tissues from BALB/c mice inoculated intranasally were harvested on days 3, 4, and 5 postinfection, and virus titers in the nasal turbinates and lungs were measured (Table 2). In the nasal turbinates, day 3 titers of rRSV/M-G-140 and rRSV/M-G-160 were 2.4-fold (P < 0.02) and 5.0-fold (P < 0.001), respectively, lower than that of rRSV/M-G-65. On the same day, titers of rRSV/M-G-140 and rRSV/M-G160 in the lungs were 25.3-fold (P < 0.05) and 17.6-fold (P < 0.02) respectively, lower than that of rRSV/M-G-65. On days 4 and 5, no significant difference was found among the three viruses.
TABLE 2.
Replication and immunogenicity in mice of recombinant RSVs bearing an M-G intergenic region of increasing length
| Animal infected with indicated virus | Virus titer ± SE, log10 PFU/mla
|
Mean serum IgG titer, log2 on day 53b | |||||
|---|---|---|---|---|---|---|---|
| Day 3
|
Day 4
|
Day 5
|
|||||
| Nasal turbinates | Lungs | Nasal turbinates | Lungs | Nasal turbinates | Lungs | ||
| rRSV/M-G-65 | 2.9 ± 0.08 | 4.3 ± 0.30 | 2.7 ± 0.08 | 4.1 ± 0.03 | 2.4 ± 0.07 | 4.4 ± 0.05 | 14.1 ± 0.49 |
| rRSV/M-G-140 | 2.5 ± 0.11c | 3.2 ± 0.13d | 2.6 ± 0.06 | 3.9 ± 0.08 | 2.5 ± 0.06 | 4.7 ± 0.05 | 14.5 ± 0.49 |
| rRSV/M-G-160 | 2.2 ± 0.11e | 3.4 ± 0.06f | 2.5 ± 0.04 | 3.9 ± 0.07 | 2.5 ± 0.13 | 4.2 ± 0.04 | 16.1 ± 0.49 |
| Mock | NAg | NA | NA | NA | NA | NA | 6.1 ± 0.49 |
BALB/c mice in groups of 20 (or 21 for rRSV/M-G-160) were infected with 106 PFU per mouse of the indicated virus or with medium alone on day 0. On days 3, 4, and 5, five mice per group (six mice for rRSV/M-G-160 on day 5) were sacrificed, nasal turbinates and lungs were harvested, and virus titers were determined by plaque assay. Limits of RSV detection: in the nasal turbinates, 100 PFU/ml; in the lungs, 50 PFU/ml. The mock group had five animals.
Serum IgG antibody response was quantitated in an ELISA, using purified RSV F protein. Five animals per group were used.
Statistical significance calculated by Student's t test compared to rRSV/M-G-65 control, P < 0.02
Statistical significance calculated by Student's t test compared to rRSV/M-G-65 control, P < 0.05;
Statistical significance calculated by Student's t test compared to rRSV/M-G-65 control, P < 0.001;
Statistical significance calculated by Student's t test compared to rRSV/M-G-65 control, P < 0.02.
NA, not applicable.
The immunogenicity of the viruses was compared by taking serum samples on days 53 (Table 2) and 66 (not shown) postinfection and determining the titers of IgG specific to the RSV F protein by ELISA. The viruses were equally and highly immunogenic.
DISCUSSION
Mononegaviruses exhibit two alternative patterns with respect to IGS. Viruses such as VSV (a vesiculovirus), Sendai virus (a respirovirus), and measles virus (a morbillivirus) contain highly conserved di- or trinucleotide IGS. Studies with minireplicons indicate that these are important in termination and reinitiation during sequential transcription (see the introduction). In contrast, other mononegaviruses such as RSV, SV5 (rubulavirus), and the filoviruses have IGS that vary in length and do not appear to contain a conserved sequence motif. In an RSV minigenome, the variable IGS did not appear to play an important role in transcription, although in an SV5 minigenome at least one IGSinfluenced transcription (25). Previous studies with these two types of IGS, conserved and variable, focused on comparing the naturally occurring IGS or involved nucleotide substitutions or small length changes (1, 14, 15, 20, 31, 32). Particularly in the case of RSV, we felt that the possibility that the length of the IGS might be an important regulatory feature remained to be further explored, and that it would be useful to do so in the context of an infectious virus rather than a minireplicon. This was done in the present study by creating a panel of recombinant RSVs with M-G IGS of increasing length, such that the longest version was nearly three times the length of the longest naturally occurring RSV IGS. These experiments were performed using an SH-minus viral backbone in order to facilitate assembly of the full-length cDNAs. Effects on gene expression and growth in vitro and in vivo were examined.
Increasing the M-G IGS from 16 to 86 nucleotides had no discernible effect on the growth of recombinant RSV in cell culture. An IGS of 100 nucleotides or longer resulted in a modest decrease in viral growth as assayed by plaque size or single-step growth yield, but attenuation was not observed in multistep growth. The basis for this modest attenuation remains unclear. In other work, we have found that, other factors being equal, the replication of RSV in cell culture is very sensitive to the length of the genome, a characteristic that is in sharp contrast with other mononegaviruses (26, 29). For example, deletion of the SH gene (4) or of a noncoding gene segment (A. Bukreyev and P. Collins, unpublished data) resulted in increased plaque size and in some cases an increased yield of virus in liquid overlay. Conversely, the addition of a foreign gene whose encoded protein would not be expected to affect RSV growth, such as chloramphenicol acetyltransferase or luciferase, resulted in decreased plaque size and decreased virus yield, and the magnitude of these effects was much greater with longer genes (3; Bukreyev and Collins, unpublished).
In the present study, the level of attenuation of in vitro growth increased as the length of the IGS increased. For example, taking the plaque size of rRSV/M-G-65 (genome of 14,825 nucleotides) as 1.0, rRSV/M-G-120 (14,880 nucleotides) had a plaque size of 0.83 and rRSV/M-G-160 (14,920 nucleotides) had a plaque size of 0.68. Thus, plaque size seemed to be remarkably sensitive to small changes in length. It is not clear whether this was simply a function of increasing genome length, or whether there was some augmented effect because the length change was in an IGS.
We included wt rRSV in the comparison for descriptive purposes. However, it should not be used as a comparison to evaluate the effect of length on plaque size because it expresses an additional gene, the SH gene, whose effect on growth remains unclear. The average plaque size of wt rRSV was intermediate (0.88, normalized to a value of 1.0 for rRSV/M-G-65) among the IGS mutants even though its genome was substantially longer (15,223 nucleotides). We speculate that the inhibitory effect of the greater length might have been compensated for by the expression of the SH protein, which certainly enhances growth in vivo (4, 37) and likely does the same in vitro.
We had anticipated that the diminution in plaque size observed with viruses containing an M-G IGS of 100 to 160 nucleotides would be associated with a change in the efficiency of sequential transcription. In particular, we had anticipated that increased IGS length would be associated with increased polymerase falloff and consequential decreased transcription of downstream genes. Surprisingly, Northern blot analysis did not detect any difference in the amount or kinetics of accumulation for several mRNAs and readthrough mRNAs from genes upstream and downstream of the M-G IGS. We cannot rule out the possibility that there was an effect on transcription that was too small to be detected by Northern blots but was sufficient to alter growth, but such an effect would have to be small indeed to have been missed here.
A simple model of RSV transcription is as follows. The transcriptase switches into a transcribing mode upon encountering a GS signal. The transcribing complex is then converted to a stable elongation complex by association with the P protein and likely is further stabilized by the M2-1 antitermination protein (7, 9). Upon encountering a GE signal, the transcriptase then switches into a stuttering-termination mode, which adds a poly(A) tail and releases the mRNA. Thereafter, the transcriptase can either dissociate or remain template bound. In the latter case, it scans for the next GS signal. At least for the M2-L gene junction, the transcriptase apparently can scan in either direction (10). The present study indicates that the transcriptase can scan for at least 160 nucleotides in the downstream direction with no discernible increase in fall off or reduction in the efficiency of reinitiation. Given that the longest artificial IGS was 160 nucleotides—nearly 40% the length of the smallest RSV gene—it is difficult to imagine that this scanning can occur while the transcriptase remains at the GE signal. The same point of view is supported by noting that the incremental increases in length for the IGS of 100, 120, 140, and 160 nucleotides are much smaller than would correspond to turns in the nucleocapsid helix. Thus, higher-order structure likely does not provide access from the upstream GE signal to the downstream GS signal. We therefore imagine that the transcriptase slides along the IGS without synthesis; prokaryotic and eukaryotic DNA-dependent RNA polymerases also are thought to slide along the template prior to initiation at a transcription start site (12). If the RSV transcriptase indeed slides on the IGS, this apparently involves a stable interaction, since increasing IGS length was not associated with a detectable increase in falloff within the site range examined here. We are presently extending this study using longer IGS in a minigenome system, which will allow greater experimental control and in particular will allow us to examine whether the M2-1 transcription antitermination factor is involved in sliding along the IGS.
Changes in the length of an IGS would not be expected to have any direct effect on RNA replication. However, the Northern blot analysis indicated that the amount of intracellular genomic RNA was slightly increased for the longer genomes (rRSV/M-G-140 and rRSV/M-G-160) at 24 and 32 h postinfection (Fig. 6A and C). Further work will be necessary to determine whether this apparent increase is a consistent effect of increased IGS length or, more likely, of increased genome length in general. These time points corresponded with diminished release of infectious particles for these viruses (Fig. 4A). One possibility is that the two effects are linked, and that greater genome length decreases the efficiency of packaging, thereby increasing the intracellular accumulation of viral genome.
The replication of rRSV/M-G-140 and rRSV/M-G-160 in mice was modestly attenuated compared to rRSV/M-G-65 on day 3 in both the upper and lower respiratory tracts. However, there was not a significant difference on days 4 and 5 postinfection. These results appear to parallel the kinetics of virus replication observed in vitro, where a reduction of virus replication associated with increased IGS length was observed during single-cycle growth but not during multicycle growth. We previously showed that recombinant RSV with the SH gene deleted (rRSV/M-G-65) displayed a 10-fold reduction in replication in the nasal turbinates of BALB/c mice, whereas replication in the lungs was unaffected (4). Therefore, the attenuation in the upper respiratory tract observed here in response to an increase in IGS length presumably was additive to that due to the deletion of the SH gene. Attenuation due to increasing IGS length was not accompanied by a decrease in immunogenicity. This was not unexpected, since reduced immunogenicity typically is observed only with strongly attenuated viruses. The ability to introduce a small increase in attenuation is of potential value for designing a live-attenuated RSV vaccine. In this context, it will be important to compare the effects of artificially long IGS in complete wt rRSV containing the SH gene versus highly attenuated vaccine candidate viruses.
One of the themes that has emerged from studies of recombinant mononegaviruses is the surprising flexibility and stability of the genome in accommodating mutations. These can include the deletion of genes (2, 4, 33), the insertion of foreign genes (3, 22, 28), rearrangement of gene order (36), and substitution of cis-acting signals (11). In the present study, this list is expanded by the observation that an unnaturally long IGS could be introduced into the RSV genome with no apparent effect on sequential transcription and little effect on virus growth.
ACKNOWLEDGMENTS
We thank Myron Hill, Kim Tran, Ena Camargo, and Chris J. Cho for technical assistance.
REFERENCES
- 1.Barr J N, Whelan S P, Wertz G W. Role of the intergenic dinucleotide in vesicular stomatitis virus RNA transcription. J Virol. 1997;71:1794–1801. doi: 10.1128/jvi.71.3.1794-1801.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bermingham A, Collins P L. The M2–2 protein of human respiratory syncytial virus is a regulatory factor involved in the balance between RNA replication and transcription. Proc Natl Acad Sci USA. 1999;96:11259–11264. doi: 10.1073/pnas.96.20.11259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bukreyev A, Camargo E, Collins P L. Recovery of infectious respiratory syncytial virus expressing an additional, foreign gene. J Virol. 1996;70:6634–6641. doi: 10.1128/jvi.70.10.6634-6641.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bukreyev A, Whitehead S S, Murphy B R, Collins P L. Recombinant respiratory syncytial virus from which the entire SH gene has been deleted grows efficiently in cell culture and exhibits site-specific attenuation in the respiratory tract of the mouse. J Virol. 1997;71:8973–8982. doi: 10.1128/jvi.71.12.8973-8982.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Collins P L, Dickens L E, Buckler-White A, Olmsted R A, Spriggs M K, Camargo E, Coelingh K V. Nucleotide sequences for the gene junctions of human respiratory syncytial virus reveal distinctive features of intergenic structure and gene order. Proc Natl Acad Sci USA. 1986;83:4594–4598. doi: 10.1073/pnas.83.13.4594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Collins P L, Hill M G, Camargo E, Grosfeld H, Chanock R M, Murphy B R. Production of infectious human respiratory syncytial virus from cloned cDNA confirms an essential role for the transcription elongation factor from the 5′ proximal open reading frame of the M2 mRNA in gene expression and provides a capability for vaccine development. Proc Natl Acad Sci USA. 1995;92:11563–11567. doi: 10.1073/pnas.92.25.11563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Collins P L, Hill M G, Cristina J, Grosfeld H. Transcription elongation factor of respiratory syncytial virus, a nonsegmented negative-strand RNA virus. Proc Natl Acad Sci USA. 1996;93:81–85. doi: 10.1073/pnas.93.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Collins P L, McIntosh K, Chanock R M. Respiratory syncytial virus. In: Fields B N, Knipe D M, Howley P M, Chanock R M, Melnick J L, Monath T P, Roizman B, Straus S E, editors. Fields virology. 3rd ed. Vol. 2. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 1313–1352. [Google Scholar]
- 9.Dupuy L C, Dobson S, Bitko V, Barik S. Casein kinase 2-mediated phosphorylation of respiratory syncytial virus phosphoprotein P is essential for the transcription elongation activity of the viral polymerase; phosphorylation by casein kinase 1 occurs mainly at Ser215 and is without effect. J Virol. 1999;73:8384–8392. doi: 10.1128/jvi.73.10.8384-8392.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fearns R, Collins P L. Model for polymerase access to the overlapped L gene of respiratory syncytial virus. J Virol. 1999;73:388–397. doi: 10.1128/jvi.73.1.388-397.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garcin D, Pelet T, Calain P, Roux L, Curran J, Kolakofsky D. A highly recombinogenic system for the recovery of infectious Sendai paramyxovirus from cDNA: generation of a novel copy-back nondefective interfering virus. EMBO J. 1995;14:6087–6094. doi: 10.1002/j.1460-2075.1995.tb00299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gelles J, Landick R. RNA polymerase as a molecular motor. Cell. 1998;93:13–16. doi: 10.1016/s0092-8674(00)81140-x. [DOI] [PubMed] [Google Scholar]
- 13.Grosfeld H, Hill M G, Collins P L. RNA replication by respiratory syncytial virus (RSV) is directed by the N, P, and L proteins; transcription also occurs under these conditions but requires RSV superinfection for efficient synthesis of full-length mRNA. J Virol. 1995;69:5677–5686. doi: 10.1128/jvi.69.9.5677-5686.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hardy R W, Harmon S B, Wertz G W. Diverse gene junctions of respiratory syncytial virus modulate the efficiency of transcription termination and respond differently to M2-mediated antitermination. J Virol. 1999;73:170–176. doi: 10.1128/jvi.73.1.170-176.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.He B, Lamb R. Effect of inserting paramyxovirus simian virus 5 gene junctions at the HN/L gene junction: analysis of accumulation of mRNAs transcribed from rescued viable viruses. J Virol. 1999;73:6228–6234. doi: 10.1128/jvi.73.8.6228-6234.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jin H, Cheng X, Zhou H, Li S, Seddiqui A. Respiratory syncytial virus that lacks open reading frame 2 of the M2 gene (M2–2) has altered growth characteristics and is attenuated in rodents. J Virol. 2000;74:74–82. doi: 10.1128/jvi.74.1.74-82.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson P R, Collins P L. The A and B subgroups of human respiratory syncytial virus: comparison of intergenic and gene-overlap sequences. J Gen Virol. 1988;69:2901–2906. doi: 10.1099/0022-1317-69-11-2901. [DOI] [PubMed] [Google Scholar]
- 18.Kawano M, Okamoto K, Bando H, Kondo K, Tsurudome M, Komada H, Nishio M, Ito Y. Characterizations of the human parainfluenza type 2 virus gene encoding the L protein and the intergenic sequences. Nucleic Acids Res. 1991;19:2739–2746. doi: 10.1093/nar/19.10.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kolakofsky D, Pelet T, Garcin D, Hausmann S, Curran J, Roux L. Paramyxovirus RNA synthesis and the requirement for hexamer genome length: the rule of six revisited. J Virol. 1998;72:891–899. doi: 10.1128/jvi.72.2.891-899.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuo L, Fearns R, Collins P L. The structurally diverse intergenic regions of respiratory syncytial virus do not modulate sequential transcription by a dicistronic minigenome. J Virol. 1996;70:6143–6150. doi: 10.1128/jvi.70.9.6143-6150.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lamb R A, Kolakofsky D. Paramyxoviridae: the viruses and their replication. In: Fields B N, Knipe D M, Howley P M, Chanock R M, Melnick J L, Monath T P, Roizman B, Straus S E, editors. Fields virology. 3rd ed. Vol. 1. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 1177–1204. [Google Scholar]
- 22.Mebatsion T, Schnell M J, Cox J H, Finke S, Conzelmann K K. Highly stable expression of a foreign gene from rabies virus vectors. Proc Natl Acad Sci USA. 1996;93:7310–7314. doi: 10.1073/pnas.93.14.7310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murphy B R, Sotnikov A V, Lawrence L A, Banks S M, Prince G A. Enhanced pulmonary histopathology is observed in cotton rats immunized with formalin-inactivated respiratory syncytial virus (RSV) or purified F glycoprotein and challenged with RSV 3-6 months after immunization. Vaccine. 1990;8:497–502. doi: 10.1016/0264-410x(90)90253-i. [DOI] [PubMed] [Google Scholar]
- 24.Prince G A, Jenson A B, Horswood R L, Camargo E, Chanock R M. The pathogenesis of respiratory syncytial virus infection in cotton rats. Am J Pathol. 1978;93:771–791. [PMC free article] [PubMed] [Google Scholar]
- 25.Rassa J C, Parks G D. Highly diverse intergenic regions of the paramyxovirus simian virus 5 cooperate with the gene end U tract in viral transcription termination and can influence reinitiation at a downstream gene. J Virol. 1999;73:3904–3912. doi: 10.1128/jvi.73.5.3904-3912.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sakai Y, Kiyotani K, Fukumura M, Asakawa M, Kato A, Shioda T, Yoshida T, Tanaka A, Hasegawa M, Nagai Y. Accommodation of foreign genes into the Sendai virus genome: sizes of inserted genes and viral replication. FEBS Lett. 1999;456:221–226. doi: 10.1016/s0014-5793(99)00960-6. [DOI] [PubMed] [Google Scholar]
- 27.Sanchez A, Kiley M, Holloway B, Auperin D. Sequence analysis of the Ebola virus genome: organization, genetic elements, and comparison with the genome of Marburg virus. Virus Res. 1993;29:215–240. doi: 10.1016/0168-1702(93)90063-s. [DOI] [PubMed] [Google Scholar]
- 28.Schnell M J, Buonocore L, Whitt M A, Rose J K. The minimal conserved transcription stop-start signal promotes stable expression of a foreign gene in vesicular stomatitis virus. J Virol. 1996;70:2318–2323. doi: 10.1128/jvi.70.4.2318-2323.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Skiadopoulos M H, Surman S R, Durbin A P, Collins P L, R. M B. Long nucleotide insertions between the HN and the L protein coding regions of human parainfluenza virus type 3 yield viruses with temperature sensitive and attenuation phenotypes. Virology. 2000;272:225–234. doi: 10.1006/viro.2000.0372. [DOI] [PubMed] [Google Scholar]
- 30.Stillman E, Whitt M. Transcript initiation and 5′-end modifications are separable events during vesicular stomatitis virus transcription. J Virol. 1999;73:7199–7209. doi: 10.1128/jvi.73.9.7199-7209.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stillman E A, Whitt M A. The length and sequence composition of vesicular stomatitis virus intergenic regions affect mRNA levels and the site of transcript initiation. J Virol. 1998;72:55565–55572. doi: 10.1128/jvi.72.7.5565-5572.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stillman E A, Whitt M A. Mutational analyses of the intergenic dinucleotide and the transcriptional start sequence of vesicular stomatitis virus (VSV) define sequences required for efficient termination and initiation of VSV transcripts. J Virol. 1997;71:2127–2137. doi: 10.1128/jvi.71.3.2127-2137.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Teng M N, Collins P L. Altered growth characteristics of recombinant respiratory syncytial viruses which do not produce NS2 protein. J Virol. 1999;73:466–473. doi: 10.1128/jvi.73.1.466-473.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tordo N P O, Ermine A, Keith G, Rougeon F. Walking along the rabies genome: is the large G-L intergenic region a remnant gene? Proc Natl Acad Sci USA. 1986;83:3914–3918. doi: 10.1073/pnas.83.11.3914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wagner R R, Rose J K. Rhabdoviridae: the viruses and their replication. In: Fields B N, Knipe D M, Howley P M, Chanock R M, Melnick J L, Monath T P, Roizman B, Straus S E, editors. Fields virology. 3rd ed. Vol. 1. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 1121–1135. [Google Scholar]
- 36.Wertz G, Perepelitsa V, Ball L. Gene rearrangement attenuates expression and lethality of a nonsegmented negative strand RNA virus. Proc Natl Acad Sci USA. 1998;95:3501–3506. doi: 10.1073/pnas.95.7.3501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Whitehead S S, Bukreyev A, Teng M N, Firestone C Y, St. Claire M, Elkins W R, Collins P L, Murphy B R. Recombinant respiratory syncytial virus bearing a deletion of either the NS2 or SH gene is attenuated in chimpanzees. J Virol. 1999;73:3438–3442. doi: 10.1128/jvi.73.4.3438-3442.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Whitehead S S, Firestone C Y, Karron R A, Crowe J E, Jr, Elkins W R, Collins P L, Murphy B R. Addition of a missense mutation present in the L gene of respiratory syncytial virus (RSV) cpts530/1030 to RSV vaccine candidate cpts248/404 increases its attenuation and temperature sensitivity. J Virol. 1999;73:871–877. doi: 10.1128/jvi.73.2.871-877.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yu Q, Hardy R W, Wertz G W. Functional cDNA clones of the human respiratory syncytial (RS) virus N, P, and L proteins support replication of RS virus genomic RNA analogs and define minimal trans-acting requirements for RNA replication. J Virol. 1995;69:2412–2419. doi: 10.1128/jvi.69.4.2412-2419.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]