Abstract
Immunoglobulin G reactive with primary isolate virions was detected in 36% of serum samples from individuals infected with human immunodeficiency virus type 1. Of these individuals, serum samples from only 7% captured significant quantities of virus. Virion-specific antibody correlated with CD4 counts and, of more significance, primary isolate neutralization. Further dissection of this response should lead to the identification of antibodies and antigenic epitopes for vaccine purposes.
The inability of the immune system to develop neutralizing antibodies to primary isolates is evident in numerous studies (14, 15, 26). The process of human immunodeficiency virus type 1 (HIV-1) infection involves a series of stepwise changes in env antigenic structures and epitope presentation during which the virus unfolds and exposes neutralizing epitopes in close proximity to the target cell surface structure. Removal of the variable loops on the surface of the virus (13) increases antibody binding to conserved regions within the core of gp120, including the CD4 binding site and CD4 binding site-related epitopes (19, 20, 24, 25). Increased exposure of these conserved epitopes within the core (11), including the chemokine receptor binding site (18), mimics that observed following CD4 binding (21, 25). Thus, it can be argued that the variable loops mask the more conserved regions of gp120 such that broadly neutralizing epitopes are not accessible to the immune system. It is known that the variable loops are immunogenic as antibodies are readily detected; however, though not exclusive, neutralizing antibodies to the variable loops tend to be strain specific. For these reasons, while a sustained antibody response to env antigens, and gp120 in particular, develops during infection, it tends to not be an effective neutralizing response. That is not to say that neutralizing antibodies to conserved regions are not produced. The isolation of anti-gp120 human monoclonal antibodies (MAbs) that broadly neutralize primary isolates such as 2G12 (23) suggests that this response can be generated but represents only a small component of the immune response in selected individuals. For that reason, serum antibodies only sporadically neutralize primary isolates.
To facilitate the identification of neutralizing epitopes on primary isolates and to study the antibody response to infection with HIV-1, we have used whole virions from primary isolates of HIV-1 to study antibody specificity of plasma from HIV-1-infected individuals. This virus capture assay was adapted from that of Orentas and Hildreth (16). Three subtype B virus isolates (92HT593, 92US660, and 92US714) which were selected based on V3 diversity were obtained from N. Halsey, Multicenter AIDS Cohort Study, and K. Nelson, respectively, through the AIDS Research and Reference Reagent Program, Division of AIDS, National Institute of Allergy and Infectious Diseases, National Institutes of Health. Viral stocks were prepared in phytohemagglutinin (PHA)-stimulated donor peripheral blood mononuclear cells (PBMCs) as described in the ACTG consensus protocol (10) and previously (6). A mix of virus isolates was prepared for use in studies by adding one vial of each isolate to PHA-stimulated donor PBMCs. When cultured alone, all isolates yielded similar quantities of virus. The p24 content of this virus stock mix was 215 ng/ml. The stock was infectious on PHA-stimulated PBMCs.
This virus stock mix was used to study the antibody response in the plasma of HIV-1-infected homosexual males involved in the Longitudinal HIV Prevention Project at the Fenway Community Health Center, Boston, Mass., and were selected at random and irrespective of clinical status. To capture human immunoglobulin G (IgG), enzyme-linked immunosorbent assay (ELISA) plates were coated overnight with goat anti-human IgG and blocked with phosphate-buffered saline (PBS) containing 1% bovine serum albumin. Serum (heat inactivated) was diluted 1:100, and human MAb was diluted to 1 μg/ml with RPMI 1640 containing 10% fetal bovine serum (FBS) and added to the ELISA plate. Human MAb F240 (7) was used as a positive control, and normal human IgG was used as a negative control. Samples were incubated for 1 h at room temperature. After washing, virus stock mix was diluted 1:2 in RPMI 1640 containing 10% FBS and incubated on the plates for 1 h at 37°C. This dilution of virus allowed 90% maximal binding with minimal background. Unbound virus was washed away, and p24 was released from the captured virus by incubation with 1% Triton X-100 in PBS for 1 h at 37°C. Released p24 was quantitated by noncommercial ELISA. For this ELISA, microtiter plates were coated with the murine anti-p24 antibody 183-H12-5C (from Bruce Chesebro and Harvy Chen through the AIDS Research and Reference Reagent Program) at 5 μg/ml. The plates were blocked with PBS containing 5% nonfat dry milk. Samples were diluted 1:2 and added to the plates for 1 h at 37°C prior to washing. Bound p24 was detected by using HIVIgG (IgG purified from HIV sera by protein G chromatography) followed by biotinylated goat anti-human IgG and streptavidin-horseradish peroxidase. The plates were developed following the addition of o-phenylene diamine substrate, and the optical density was read at 490 nm. The concentration of bound p24 was determined by a standard curve based on known concentrations of p24 (Dupont-NEN). The sensitivity of this assay is 50 pg/ml.
As described in numerous studies, host cell membrane antigens are incorporated into the virion as HIV buds from the host cell (1, 2, 16, 19). Particular membrane antigens incorporated into the virion have been shown to be host cell dependent rather than isolate dependent (2). We were able to capture virus by using a number of murine MAbs reactive with cell surface antigens. For example, as has been shown for both laboratory and primary isolates, major histocompatibility complex class II antigens are incorporated into the virion as evidenced by the capture of 1,278 pg/ml (n = 3) of p24.
In preliminary experiments, a number of human MAbs were tested for primary isolate virus binding (unpublished data). Of those tested, one antibody, F240, which reacts with the immunodominant domain of gp41 (7), consistently bound significant quantities of primary isolate virions. In 10 experiments, F240 at 2 μg/ml captured 2,150 ± 514 pg (mean ± standard deviation) of virus stock per ml. To determine assay sensitivity and specificity when using plasma, the titers of plasma samples from uninfected individuals were determined in the virus capture assay. Using four different uninfected individuals and a commercial preparation of pooled human IgG, it was determined that a minimal dilution of 1:100 was needed to block nonspecific plasma effects on assay background. At this dilution, plasma from uninfected individuals captured 143 ± 71 pg of p24 per ml. Therefore, a cutoff of 300 pg/ml, which represents 2 standard deviations above the uninfected control, was selected as positive for plasma reactivity. When plasma from HIV-1-infected individuals was tested, as shown in Table 1, IgG antibodies reactive with primary isolates were not detected in the plasma samples from the majority (64%) of individuals studied. Of those with detectable antibodies, 29% captured moderate levels of virus (300 to 500 pg/ml), whereas 7% or 6 of 87 had significant antibody titers. Analysis of longitudinal samples from two individuals with high virion antibody titers demonstrated that virion-specific antibody was first detected within 18 months of infection and titers increased until a plateau was reached 5 to 7 years postinfection (data not shown). These individuals are continuing to be monitored to determine the kinetics of this antibody response. It may be argued that free gp120 in the virus stock may inhibit serum anti-gp120 binding to virus. However, two human and three murine V3 loop antibodies tested captured a similar amount of virus as gp41 (unpublished data), suggesting that there was little, if any, inhibition by free monomeric gp120 in this virus stock.
TABLE 1.
Reactivity of plasma with virions from HIV-1-infected individuals
| Concn of virus captured (pg/ml)a | No. of individuals with reactive plasma/total no. | % of individuals with reactive plasma |
|---|---|---|
| <300b | 56/87 | 64 |
| 300–500 | 25/87 | 29 |
| >500 | 6/87 | 7 |
Concentration of p24 released following addition of Triton X-100 to virus captured by human plasma (1:100) bound to goat anti-human IgG-coated ELISA plates.
The p24 assay is sensitive to 50 pg/ml. A cutoff of 300 pg/ml was selected for specificity based on results with plasma from uninfected individuals.
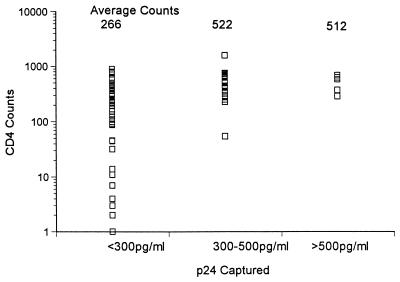
Clinical data available on the individuals in this study included antiretroviral use, clinical symptoms, and CD4 counts. For a significant number of these individuals, the length of time of infection was also known. At the time these samples were collected, plasma viral PCR data was not available. The only relationship that could be drawn was with CD4 counts, as shown in Fig. 1. Those individuals with very low or undetectable virion-specific antibody (less than 300 pg/ml) had low CD4 counts (266 on average). In contrast, those individuals with virion-specific antibody, regardless of titer, had significantly higher CD4 counts (522 for individuals whose antibody captured 300 to 500 pg/ml and 512 for individuals whose plasma captured more than 500 pg of p24 per ml). Consistent with higher CD4 counts associated with virion-specific antibody, antiretroviral drugs were used by 50% of individuals with the highest virion-specific antibody titers, 63% of those with moderate titers, and more than 81% of those with minimal titers.
FIG. 1.
Relationship of serum antibody reactivity with virions and CD4 counts. A scatter plot of the correlation of virus captured by human plasma as described in Table 1 with CD4 counts is shown. For a concentration of <300 pg/ml, n = 56; for 300 to 500 pg/ml, n = 25; for >500 pg/ml, n = 6.
To measure neutralization of primary isolates of a large number of serum samples, an assay that measures the amount of p24 produced by a constant inoculum of virus was used. In this assay, serum was diluted 1:50 in growth medium (RPMI 1640 with 20% FBS and 5% interleukin-2), and 25 μl was added to 25 μl of viral stock (from same date as that used in binding assay; 100 50% tissue culture infective doses) and incubated for 1 h at 37°C. After 1 h, donor PHA-stimulated PBMCs (2 × 105 cells) were added and the plates were incubated overnight at 37°C in 5% CO2. The plates were washed twice, and the medium was replaced with fresh growth medium. Seven days after the infection was initiated, the supernatant was removed and tested for p24 by ELISA. A dilution of 1:50 was determined in preliminary experiments to be required for sensitive differential neutralization to be detected.
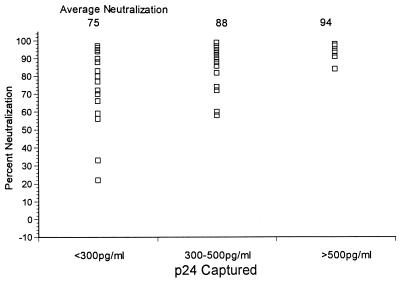
As shown in Fig. 2, 33% (6 of 18) of individuals with <300 pg of virion-specific antibody per ml neutralized more than 90% of virus production (range, 0 to 97%). Of 24 individuals with 300 to 500 pg of virion-specific antibody per ml, 18 or 75% neutralized more than 90% of virus production (range, 60 to 99%). Of significance, all six of the individuals with >500 pg of virion-specific antibody per ml neutralized more than 90% of virus production. Thus, while high titers of antibody reactive with primary isolate virions was not absolutely essential for viral neutralization, it was clearly associated with neutralization. As described above, F240 reacts significantly with primary isolate virions; however, this anti-gp41 antibody fails to neutralize laboratory or primary isolates, including the three isolates used in these studies (reference 7 and unpublished data).
FIG. 2.
Association of virion-specific antibodies with virus neutralization. The correlation of virus captured by human plasma as described in Table 1 with primary isolate neutralization is shown. For a concentration of <300 pg/ml, n = 18; for 300 to 500 pg/ml, n = 24; for >500 pg/ml, n = 6.
Although the epitope specificity of the serum virion binding antibody response remains to be determined, this data does conclusively demonstrate that antibodies reactive with primary isolate virions are not prevalent in the majority of HIV-1-infected individuals. Consistent with these findings, it has been reported that antibodies from phage display libraries tend to react predominantly with nonnative forms of envelope rather than native envelope on the surface of infected cells or virions (17). We propose that it is difficult for the immune system to respond in a higher-order integration in which a two-step antibody response can (i) first modify the envelope to expose the neutralizing epitopes and then (ii) secondarily recognize the exposed neutralizing epitopes. This higher-order integration must be imposed on the immune system through vaccine design. Therefore, to generate effective neutralizing antibody responses, immunogens must be designed to induce multiple classes of antibodies which together mediate viral neutralization through an integration of these two steps, exposure and neutralization. Binding of antibodies to laboratory or primary isolates may be necessary for neutralization but not sufficient (4, 5, 9), which is consistent with the hypothesis that neutralizing antibodies would not be expected to neutralize in the absence of exposure of the neutralizing site. Studies using human MAbs directed to different epitopes of gp120 have demonstrated that the combination of selected antibodies results in enhanced binding and neutralization of virus (3, 12, 22). It has also been shown that immunization of goats with CD4-gp120 complexes results in the generation of a polyclonal antibody response that neutralizes primary isolates (8). These results suggest that if the immune system is primed appropriately such that neutralizing epitopes are exposed, a neutralizing antibody response may be induced.
Acknowledgments
We thank the HIV-1-infected individuals in this study for their selfless commitment to participation in these studies. We also appreciate the support of the research staff at the Fenway Community Health Center, especially Judy Erdman and Brian Glaser.
This work was supported by Public Health Service grant AI-26926 from the National Institute for Allergy and Infectious Diseases, National Institutes of Health.
REFERENCES
- 1.Arthur L O, Bess J W J, Sowder II R C, Benveniste R E, Mann D L, Cherman J-C, Henderson L E. Cellular proteins bound to immunodeficiency viruses: implication for pathogenesis and vaccines. Science. 1992;258:1935–1938. doi: 10.1126/science.1470916. [DOI] [PubMed] [Google Scholar]
- 2.Bastiani L, Laal S, Kim M, Zolla-Pazner S. Host cell-dependent alterations in envelope components of human immunodeficiency virus type 1 virions. J Virol. 1997;71:3444–3450. doi: 10.1128/jvi.71.5.3444-3450.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cavacini L A, Emes C, Power J, Gorny M, Zolla-Pazner S, Posner M R. Human monoclonal antibodies to the V3 loop of gp120 mediate variable and distinct effects on binding and viral neutralization by a human monoclonal antibody to the CD4 binding site. J Acquir Immun Defic Syndr. 1993;6:353–358. [PubMed] [Google Scholar]
- 4.Cavacini L A, Emes C L, Power J, Duval M, Posner M R. Effect of antibody valency on interaction with cell-surface expressed HIV-1 and viral neutralization. J Immunol. 1994;152:2538–2545. [PubMed] [Google Scholar]
- 5.Cavacini L A, Emes C L, Power J, Desharnais F, Duval M, Montefiori D, Posner M R. Influence of heavy chain constant regions on antigen binding and HIV-1 neutralization by a human monoclonal antibody. J Immunol. 1995;155:3638–3644. [PubMed] [Google Scholar]
- 6.Cavacini L A, Samore M H, Gambertoglio J, Jackson B, Duval M, Wisnewski A, Hammer S, Koziel C, Trapnell C, Posner M R. Phase I study of a human monoclonal antibody directed against the CD4 binding site of HIV-1/gp120. AIDS Res Hum Retroviruses. 1998;14:545–550. doi: 10.1089/aid.1998.14.545. [DOI] [PubMed] [Google Scholar]
- 7.Cavacini L A, Emes C L, Wisnewski A V, Power J, Lewis G, Montefiori D, Posner M R. Functional and molecular characterization of a human monoclonal antibody reactive with the immunodominant region of HIV-1/gp41. AIDS Res Hum Retroviruses. 1998;14:1271–1280. doi: 10.1089/aid.1998.14.1271. [DOI] [PubMed] [Google Scholar]
- 8.Devico A, Silver A, Thornton A M, Sarngadharan M G, Pal R. Covalently crosslinked complexes of human immunodeficiency virus type 1 (HIV-1) gp120 and CD4 receptor elicit a neutralizing immune response that includes antibodies selective for primary virus isolates. Virology. 1996;218:258–263. doi: 10.1006/viro.1996.0188. [DOI] [PubMed] [Google Scholar]
- 9.Fouts T R, Trkola A, Fung M S, Moore J P. Interactions of polyclonal and monoclonal anti-glycoprotein 120-glycoprotein 41 complexes of a primary HIV type 1 isolate: relationship to neutralization. AIDS Res Hum Retroviruses. 1998;14:591–597. doi: 10.1089/aid.1998.14.591. [DOI] [PubMed] [Google Scholar]
- 10.Hollinger F B, Bremer J W, Myers L E, Gold J W M, McQuay L the NIH/NIAID/DAIDS/ACTG Virology Laboratories. Standardization of sensitive human immunodeficiency virus coculture procedures and establishment of a multicenter quality assurance program for the AIDS clinical trials group. J Clin Microbiol. 1992;30:1787–1794. doi: 10.1128/jcm.30.7.1787-1794.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kwong P D, Wyatt R, Robinson J, Sweet R W, Sodroski J, Hendrickson W A. Structure of an HIV gp120 envelope glycoprotein in complex with the CD4 receptor and a neutralizing human antibody. Nature. 1998;393:648–659. doi: 10.1038/31405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laal S, Burda S, Gorny M K, Karwowska S, Buchbinder A, Zolla-Pazner S. Synergistic neutralization of human immunodeficiency virus type 1 by combinations of human monoclonal antibodies. J Virol. 1994;68:4001–4008. doi: 10.1128/jvi.68.6.4001-4008.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moore J P, Sattentau Q J, Wyatt R, Sodroski J. Probing the structure of the surface glycoprotein gp120 of human immunodeficiency virus type 1 with a panel of human monoclonal antibodies. J Virol. 1994;68:469–484. doi: 10.1128/jvi.68.1.469-484.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moore J P, Cao Y, Qing L, Sattentau Q J, Pyati J, Koduri R, Robinson J, Barbas III C F, Burton D R, Ho D D. Primary isolates of human immunodeficiency virus type 1 are relatively resistant to neutralization by monoclonal antibodies to gp120, and their neutralization is not predicted by studies with monomeric gp120. J Virol. 1995;69:101–109. doi: 10.1128/jvi.69.1.101-109.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moore J P, Cao Y, Leu J, Qin L, Korber B, Ho D D. Inter- and intraclade neutralization of human immunodeficiency virus type 1: genetic clades do not correspond to neutralization serotypes but partially correspond to gp120 antigenic serotypes. J Virol. 1996;70:427–444. doi: 10.1128/jvi.70.1.427-444.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Orentas R J, Hildreth J E K. Association of host cell surface adhesion receptors and other membrane proteins with HIV and SIV. AIDS Res Hum Retroviruses. 1993;9:1157–1165. doi: 10.1089/aid.1993.9.1157. [DOI] [PubMed] [Google Scholar]
- 17.Parren P W H I, Gauduin M-C, Koup R A, Poignard P, Sattentau Q J, Fisicaro P, Burton D R. Relevance of the antibody response against human immunodeficiency virus type 1 envelope to vaccine design. Immunol Lett. 1997;57:105–112. doi: 10.1016/s0165-2478(97)00043-6. [DOI] [PubMed] [Google Scholar]
- 18.Rizzuto C D, Wyatt R, Hernandez-Ramos N, Sun Y, Kwong P D, Hendrickson W A, Sodroski J. A conserved HIV gp120 glycoprotein structure involved in chemokine receptor binding. Science. 1998;280:1949–1953. doi: 10.1126/science.280.5371.1949. [DOI] [PubMed] [Google Scholar]
- 19.Saifuddin M, Parker C J, Peeples M E, Gorny M K, Zolla-Pazner S, Ghassemi M, Rooney I A, Atkinson J P, Spear G T. Role of virion-associated glycosyl-phosphatidylinositol-linked proteins CD55 and CD59 in complement resistance of cell line-derived and primary isolates of HIV-1. J Exp Med. 1995;182:501–509. doi: 10.1084/jem.182.2.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sullivan N, Sun Y, Sattentau Q, Thali M, Wu D, Denisova G, Gershoni J, Robinson J, Moore J, Sodroski J. CD4-induced conformational changes in the human immunodeficiency virus type 1 gp120 glycoprotein: consequences for virus entry and neutralization. J Virol. 1998;72:4694–4703. doi: 10.1128/jvi.72.6.4694-4703.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thali M, Moore J P, Furman C, Charles M, Ho D D, Robinson J, Sodroski J. Characterization of conserved human immunodeficiency virus type 1 gp120 neutralization epitopes exposed upon gp120-CD4 binding. J Virol. 1993;67:3978–3988. doi: 10.1128/jvi.67.7.3978-3988.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tilley S A, Honnen W J, Racho M E, Chou T-C, Pinter A. Synergistic neutralization of HIV-1 by human monoclonal antibodies against the V3 loop and the CD4-binding site of gp120. AIDS Res Hum Retroviruses. 1992;4:461–467. doi: 10.1089/aid.1992.8.461. [DOI] [PubMed] [Google Scholar]
- 23.Trkola A, Purtscher M, Muster T, Ballaun C, Buchacher A, Sullivan N, Srinivasan K, Sodroski J, Moore J P, Katinger H. Human monoclonal antibody 2G12 defines a distinctive neutralization epitope on the gp120 glycoprotein of human immunodeficiency virus type 1. J Virol. 1996;70:1100–1108. doi: 10.1128/jvi.70.2.1100-1108.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wyatt R, Sullivan N, Thali M, Repke H, Ho D, Robinson J, Posner M, Sodroski J. Functional and immunologic characterization of human immunodeficiency virus type 1 envelope glycoproteins containing deletions of the major variable regions. J Virol. 1993;67:4557–4565. doi: 10.1128/jvi.67.8.4557-4565.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wyatt R, Moore J, Accola M, Desjardin E, Robinson J, Sodroski J. Involvement of the V1/V2 variable loop structure in the exposure of human immunodeficiency virus type 1 gp120 epitopes induced by receptor binding. J Virol. 1995;69:5723–5733. doi: 10.1128/jvi.69.9.5723-5733.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou J Y, Montefiori D C. Antibody-mediated neutralization of primary isolates of human immunodeficiency virus type 1 in peripheral blood mononuclear cells is not affected by the initial activation state of the cells. J Virol. 1997;71:2512–2517. doi: 10.1128/jvi.71.3.2512-2517.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]