Abstract
nef alleles derived from a large number of individuals infected with human immunodeficiency virus type 1 (HIV-1) were analyzed to investigate the frequency of disrupted nef genes and to elucidate whether specific amino acid substitutions in Nef are associated with different stages of disease. We confirm that deletions or gross abnormalities in nef are rarely present. However, a comparison of Nef consensus sequences derived from 41 long-term nonprogressors and from 50 individuals with progressive HIV-1 infection revealed that specific variations are associated with different stages of infection. Five amino acid variations in Nef (T15, N51, H102, L170, and E182) were more frequently observed among nonprogressors, while nine features (an additional N-terminal PxxP motif, A15, R39, T51, T157, C163, N169, Q170, and M182) were more frequently found in progressors. Strong correlations between the frequency of these variations in Nef and both the CD4+-cell count and the viral load were observed. Moreover, analysis of sequential samples obtained from two progressors revealed that several variations in Nef, which were more commonly observed in patients with low CD4+-T-cell counts, were detected only during or after progression to immunodeficiency. Our results indicate that sequence variations in Nef are associated with different stages of HIV-1 infection and suggest a link between nef gene function and the immune status of the infected individual.
A complex interplay of host genetic and immune factors and viral pathogenicity contributes to the marked differences observed among the rates of disease progression in individuals infected with human immunodeficiency virus type 1 (HIV-1) (41). One important determinant of viral pathogenicity is the nef gene of primate lentiviruses. Rhesus macaques infected with a mutant form of a pathogenic molecular clone of simian immunodeficiency virus (SIVmac239) containing a deletion in nef maintained very low viral burdens with normal CD4+-T-cell counts and remained healthy (19, 42). There is some evidence that Nef also plays an important role in disease progression in HIV-1-infected humans. Ten long-term nonprogressors (LTNPs) in whom only nef deletion forms of HIV-1 could be detected have been described (7, 20, 29, 36). All of these individuals remained asymptomatic despite 10 to 14 years of documented HIV-1 infection, and they showed clinical and virologic characteristics similar to those observed in macaques after experimental infection with nef-defective SIV.
However, further studies have shown that defects in nef are not a common explanation for nonprogressive HIV-1 infection. Huang et al. (15) found no gross defects or sequence abnormalities in nef alleles derived from 10 HIV-infected LTNPs. In a subsequent study they also showed that these nef alleles were able to increase viral infectivity and replication (16). Similarly, Michael et al. (27) found no abnormalities in nef genes derived from nine patients representing a wide range of different rates of disease progression. Furthermore, there was no apparent correlation between the phylogenetic relationship of the nef sequences and the corresponding rates of disease progression in these patients (15, 27, 33). Finally, nef alleles derived from individuals with nonprogressive or progressive infection have not been found to differ in their relative abilities to induce a decrease in CD4 surface expression (25, 27, 33).
These findings suggest that Nef is not a common mediator of the rate of HIV-1 disease progression. However, the prevalence of defective nef genes in HIV-1 infection has been investigated in only a relatively small number of infected subjects. Furthermore, it is unclear whether the in vitro activities of the primary nef alleles analyzed, such as CD4 downregulation and enhancement of infectivity, represent those Nef properties most critical for viral pathogenicity in vivo. Variations that affect functional activities of nef alleles may not necessarily alter the phylogenetic relationship of the sequences. For example, a single amino acid substitution in the SIVmac239 Nef protein, changing residue 17 in Nef from R to Y, resulted in an acutely pathogenic phenotype in infected rhesus macaques (8).
We examined nef alleles derived from LTNPs and from individuals with progressive HIV disease to investigate the frequency of defective nef genes in HIV-1 infection and to elucidate whether particular sequence variations in Nef may be associated with different stages of disease. Our study confirms that deletions in nef are rare and that the vast majority of nef genes derived from both nonprogressing and more rapidly progressing individuals are intact and predict functional Nef protein sequences. However, the analysis of a large number of deduced Nef sequences derived from 91 HIV-1-infected individuals representing a wide range of progression rates and from sequential samples obtained from two progressors revealed that some amino acid substitutions in Nef seem to be associated with different stages of disease.
MATERIALS AND METHODS
Study populations.
Most blood samples were derived from 165 HIV-1-infected individuals based at the Chelsea & Westminster Hospital, London, United Kingdom (referred to hereafter as the C&W cohort) who had been enrolled between 1994 and 1996 in a case-control study of the biological and behavioral correlates of nonprogression in HIV-1 infection. Full details of the recruitment strategy have been described recently (9). The patients were categorized into three main groups according to their clinical status and CD4+-cell count. Nonprogressors (NPs) (n = 47) were defined as individuals who had been HIV infected for at least 9 years but remained asymptomatic with an absolute CD4+-lymphocyte count of >500/mm3. Slow progressors (SPs) (n = 90) were defined as individuals who had also been infected for at least 9 years but whose CD4+-cell counts had declined to below 500/mm3. Forty-one of the SPs remained free of AIDS and Centers for Disease Control and Prevention stage IV disease; the remaining 49 SPs developed stage 4 disease or AIDS during follow-up but not within 5 years. Rapid progressors (RPs) (n = 28) were those who developed AIDS within 5 years of documented HIV-1 infection. The majority of the study participants had a first positive HIV antibody test in 1985 and 1986. Since HIV infection was introduced into the United Kingdom in the early 1980s, with a maximum incidence in 1984 (33a), it can be assumed that most patients were infected shortly before their first HIV test result. Of the 47 NPs, 90 SPs, and 28 RPs, samples from 24, 11, and 16 patients, respectively, were used for sequence analysis in this study.
Twelve patients with severe hemophilia A monitored by the New England Area Hemophilia Center at the University of Massachusetts Memorial Hospital, Worcester (named the Worcester cohort) were also investigated. Four of these patients were LTNPs, four were SPs, and four were RPs. LTNPs are those who were infected for more than 10 years and remained free of signs of HIV-1 disease in the absence of antiretroviral therapy. Further criteria were either an absolute CD4+-T-cell count maintained at >400/mm3 and a CD4 percentage of >30% or an absolute CD4+-T-cell count of >600/mm3 regardless of CD4 percentage. RPs are individuals who by 1992 progressed to death due to HIV-1 or had either an absolute CD4+-T-cell count of <200/mm3 with a CD4 percentage of <10% or an absolute CD4+-T-cell count of <100/mm3 regardless of CD4 percentage. SPs fit the definition of neither LTNPs nor RPs. The status of nef genes in one LTNP from the Worcester cohort has been described previously (12, 25). nef alleles from another LTNP in the Worcester cohort carried large deletions (20) and were not included in the present study. The 12 participants were infected with HIV-1 before 1984 through contaminated blood products.
All study participants gave informed consent, and the studies were approved by the research ethics committees of the Chelsea & Westminster Hospital and the Worcester hospital. In addition to those from these two cohorts, published nef sequences derived from patients with documented stages of disease progression and CD4+-T-cell counts (15, 27, 33, 38) were also included in the analysis.
CD4+-T-cell counts.
CD4 cell counts were determined by flow cytometry. For the C&W cohort, the same flow cytometer, monoclonal antibodies, analytical methods, and sample preparation were used throughout the 10-year period of analysis. All assays were performed with blinding as to the participant’s clinical status and CD4 measurements.
Viral load.
Proviral DNA copy numbers in patients in Worcester cohort were estimated by using a modification of the Amplicor HIV-1 test system (Roche Diagnostics Systems, Inc., Branchburg, N.J.) as described previously (12, 13, 25). Quantitative analysis of viral RNA loads was performed with the Amplicor HIV-1 Monitor assay (Roche Diagnostics Systems) according to the manufacturer’s instructions.
DNA preparation and PCR amplification.
Peripheral blood mononuclear cells (PBMCs) were isolated by centrifugation through Ficoll-Histopaque density gradients. Genomic DNA was extracted by standard methods from freshly purified PBMCs or from samples continuously frozen at −70°C since collection. Because HIV-1 proviral DNA is often present at very low copy numbers and shows substantial sequence variation, we employed nested PCR methods with degenerate sets of oligonucleotides to amplify the nef long terminal repeat (LTR) region from PBMC samples from the C&W cohort (31). The outer primers were VPRF (5′-ATGGAACAAGCMCCRGMAGACCA-3′; positions 5559 to 5581) or POLU (5′-CCCTAYAAYCCMCARAGYCARGG-3′; positions 4653 to 4675) paired with LTRR (5′-GACTACGGCCGTCTGAGGGATCTCTAGYTACCA-3′; positions 9689 to 9657); the inner primer pairs were either NOFP (5′-ATACCTASAMGAATMAGACACA RGG-3′; positions 8741 to 8763) and NORP (5′-CTGCTTATATGCAGCATCTGAGGG-3′; positions 9510 to 9487) or NEFFP (5′-TAAMATGGGKRGCAAMTGGTC-3′; positions 8783 to 8803) and NEFRP (5′-AGCAASYTCKRTGCAGCAGT-3′; positions 9420 to 9400). Standard abbreviations are used for positions of base ambiguity (18), and numbers in parentheses refer to the primer positions in the HIV-1 NL4-3 genome. For each sample 0.5 μg of template DNA was used for amplification. PCR amplification of nef genes from patients monitored by the New England Area Hemophilia Center at the University of Massachusetts Memorial Hospital was carried out as described previously (20).
Cloning and sequencing.
PCR fragments were purified from agarose gels with a GeneClean kit (Qiagen Inc., Chatsworth, Calif.) and cloned with a TA cloning kit (Invitrogen Corp., San Diego, Calif.) as recommended by the manufacturers. To minimize the possibility of sample cross-contamination, collection of clinical samples, DNA isolation, and cloning of PCR products were performed in separate laboratory spaces and frequently in different institutions. Moreover, negative controls were included in each PCR amplification experiment. Sequencing was performed with the PRISM sequencing kit (Perkin-Elmer, Foster City, Calif.) on an Applied Biosystems 373 DNA sequencer. An overview of the number of nef sequences analyzed is given in Table 1.
TABLE 1.
Characteristics of the three progression groups and properties of the Nef sequences analyzed
| Progression group | Cohorta (n) | CD4+-cell count (no. per mm3)b | No. of nef genes analyzedc | No. (%) of defective nef genesd | NefProg scoree
|
|
|---|---|---|---|---|---|---|
| Range | Avg | |||||
| NP | C&W (24) | 875/732 (≥8.1/11.2) | 167 (3–10; 7.0) | 9 (5.4) | −4 to +4 | −0.8 |
| Worcester (4) | 1,140 (≥10.8) | 70 (6–14; 17.5) | 7 (10.0) | −4 to −2 | −3.3 | |
| ADARC (10) | 744 (≥13.3) | 90 (6–13; 9.0) | 8 (8.9) | −5 to +0 | −1.8 | |
| SFMHS (3) | 688 (≥8.0) | 27 (8–10; 9.0) | 1 (3.7) | −2 to −3 | −2.3 | |
| Total (41) | 855 (≥9.6) | 354 (3–14; 8.6) | 25 (7.0) | −5 to +4 | −1.4 | |
| SP | C&W (11) | 514/263 (≥8.5/11.1) | 49 (3–7; 4.5) | 4 (8.2) | −3 to +4 | +0.4 |
| Worcester (4) | 305 (≥11.0) | 26 (4–8; 6.5) | 0 (0) | 0 to +5 | +1.8 | |
| SFMHS (3) | 281 (≥7.7) | 32 (9–13; 10.7) | 0 (0) | +1 to +2 | +1.3 | |
| Total (18) | 429 (≥8.7) | 107 (3–13; 5.9) | 4 (3.7) | −3 to +5 | +0.9 | |
| RP | C&W (16) | 119/147 (≥6.3/7.8) | 83 (3–10; 5.1) | 10 (12.0) | −2 to +6 | +2.9 |
| Worcester (4) | 29 (≥7.7) | 29 (5–10; 7.3) | 2 (6.9) | +3 to +7 | +4.8 | |
| SFMHS (3) | 109 (≥7.7) | 29 (9–10; 9.7) | 1 (3.4) | 0 to +1 | +0.7 | |
| Chapel Hill (9) | 80 (NAf) | 30 (2–6; 3.3) | 3 (10) | 0 to +8 | +2.4 | |
| Total (32) | 96 (≥6.8) | 171 (2–10; 5.3) | 16 (9.4) | −2 to +8 | +2.8 | |
Patients analyzed by the Aaron Diamond AIDS Research Center (ADARC) (15), the San Francisco Men’s Health study (SFMHS) (27), and the AIDS Clinical Trials Group at the University of North Carolina Hospitals (Chapel Hill) (38) have been described previously. Average values for each progression group (total) are also given.
Mean values of the absolute CD4+-lymphocyte count at the time point closest to sample preparation for PCR and sequence analysis. Numbers in parentheses give the minimum number of years of documented HIV-1 infection prior to the CD4+-cell count measurement. For the C&W cohort the average number of CD4+ cells determined later in infection (indicated after the slash) is also indicated. Huang et al. (15) have given a range of CD4 values for the 10 LTNPs analyzed. The average value obtained for each patient was used for the calculation.
Only nef alleles obtained from a single time point are listed. Numbers in parentheses give the range and the average number of nef alleles analyzed per patient.
Number and percentage of nef alleles containing premature stop codons, frameshift mutations, and mutations in the initiation codon. nef genes predicting small in-frame deletions or alterations in conserved regions were considered intact.
As described in the text, the NefProg score is the number of Nef amino acid variations more frequently observed in progressors minus the number of properties typically seen in nonprogressors. The Nef sequence variations used for these calculations are shown in Fig. 2.
NA, not analyzed.
Sequence and statistical analyses.
Sequences were analyzed by using the Genetics Computer Group sequence analysis software package. Pairwise alignments of nucleotide and amino acid sequences were performed with the programs Gap and Distances. Multiple alignments were performed with the PileUp and Pretty programs and optimized manually. Statistical analysis of sequence variations in Nef protein sequences was performed with the chi-square test with the Yates correction. The relationships between Nef progression scores (NefProg scores) (see Results), viral titers, and absolute CD4+-T-cell counts were examined by using Pearson’s correlation coefficient. The significance of the correlation coefficient was assessed with Fisher’s transformation. Comparison of mean NefProg scores for the subgroups of the Worcester cohort defined by disease progression was by Student’s t test for independent samples. The software package StatView version 4.0 (Abacus Concepts, Inc., Berkely, Calif.) was used for statistical calculations.
Nucleotide sequence accession numbers.
HIV-1 nef sequences derived from the patients in the C&W and Worcester cohorts have been submitted to the GenBank sequence database and have been assigned accession numbers AF129333 to AF129395.
RESULTS
Defective nef genes are rare in HIV-1 infection.
To investigate the presence of gross deletions in nef, PCR amplification of nef LTR sequences was performed by using DNA extracted from PBMCs prepared from 165 patients (47 NPs, 90 SPs, and 28 RPs) monitored at the Chelsea & Westminster Hospital, London, United Kingdom. None of the 161 positive PCRs from these 165 HIV-1-infected individuals yielded fragments substantially shorter than that expected for a full-length nef gene (data not shown). To explore the frequency of inactivating point mutations or small deletions among the three progression groups, nef alleles derived from selected subgroups of 28 NPs, 15 SPs, and 20 RPs were cloned and sequenced. Four patients of each group were from the Worcester cohort; the remaining 24 NPs, 11 SPs, and 16 RPs were from the C&W cohort (Table 1). Consistent with previous studies (15, 27, 33), phylogenetic analysis revealed that nef sequences from patients with different rates of progression do not form distinct clusters (data not shown). Intrapatient length polymorphism of the nef gene in a previously defined variable region (38) was observed in 11 of the 28 NPs (39%) and in 14 of the 35 progressors (40%) (SPs and RPs combined). This frequency is higher than that reported by Huang et al. (15), who found no intrapatient length polymorphism in nef alleles derived from 10 LTNPs. The length of the nef open reading frames ranged from 585 to 657 bp in the NPs and from 615 to 648 bp in the progressors. The deduced amino acid sequences are shown in Fig. 1. nef open reading frames longer than 627 bp were amplified from 18 of 35 progressors (51%) and from 8 of 28 NPs (29%) (P = 0.12). This is due to a slightly higher frequency of N-terminal duplications in deduced Nef sequences derived from progressing individuals.
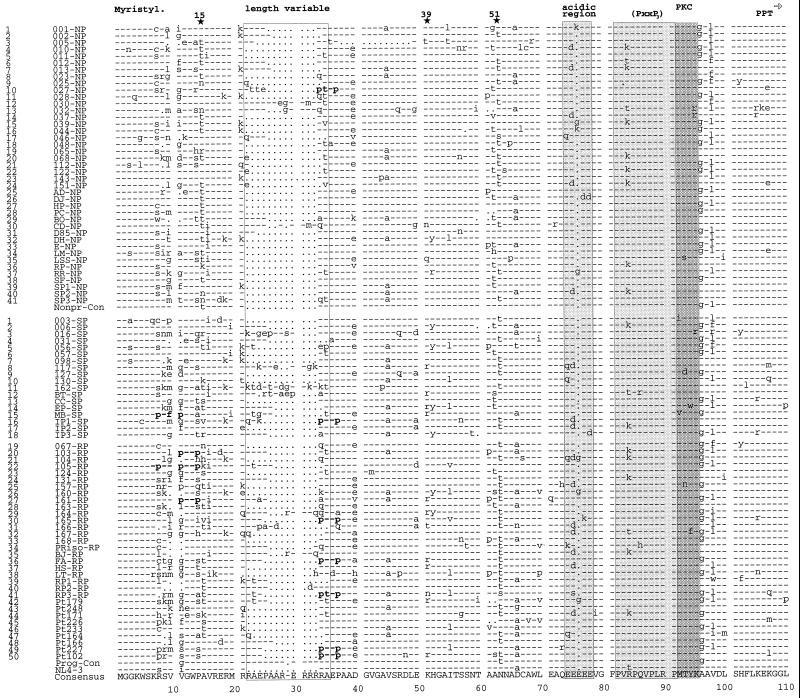
FIG. 1.
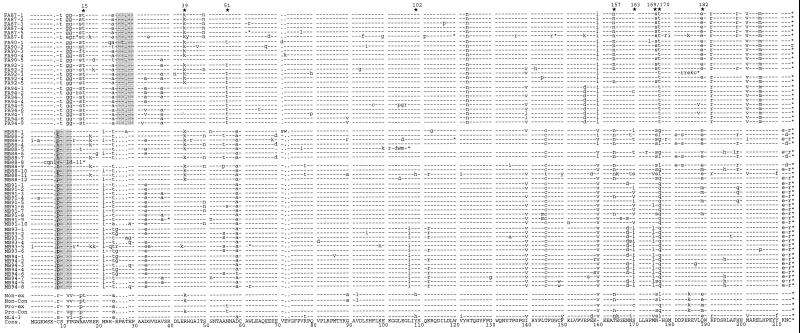
Alignment of Nef protein sequences derived from HIV-1-infected individuals with different rates of disease progression. Representative consensus Nef protein sequences from 41 NPs, 18 SPs, and 32 RPs were aligned. Nef protein sequences were derived from the present study (NPs 1 to 28, SPs 1 to 15, and RPs 19 to 38) and from studies by Huang et al. (15) (NPs 29 to 38), Michael et al. (27) (NPs 39 to 41, SPs 16 to 18, and RPs 39 to 41), and Shugars et al. (38) (patients 42 to 50). The consensus amino acid sequence is shown at the bottom. The NP and progressor consensus sequences and the HIV-1 NL4-3 sequence are also indicated. The first column indicates the index number for all NPs and progressors (SPs and RPs combined) in the study. In the second column, the first two to four numbers or letters specify the individual patient, and the last letter(s) specifies the progression grouping. Some conserved sequence elements are indicated schematically, and additional PxxP motifs close to the N terminus are in boldface. The position of the polypurine tract (PPT) and the start of the 3′ LTR (➩) are also indicated. Stars above the alignment indicate positions where amino acid variations between the different progressor groups are observed; the number gives the corresponding amino acid position in the NL4-3 Nef. Dashes indicate identity with the consensus sequence, dots indicate gaps introduced to optimize the alignment, and asterisks indicate stop codons.
The majority of analyzed nef alleles from NPs, SPs, and RPs predicted intact reading frames (Fig. 1; Table 1). All nef alleles derived from one particular NP in the C&W cohort (patient 005) contained an in-frame deletion of 36 bp downstream of the variable-length region in nef (Fig. 1 and data not shown). Surprisingly, relatively high frequencies of defective nef alleles were found in two RPs (patients 161 and 165) with low CD4+-T-lymphocyte counts (66 and 4 CD4+ cells/mm3). In both patients, 4 of 10 analyzed nef alleles from one time point contained premature in-frame stop codons and/or mutations in the initiation codon (data not shown).
Features of Nef sequences associated with different stages of HIV disease.
To investigate whether specific amino acid substitutions in Nef are associated with different stages of infection, Nef protein consensus sequences from 41 NPs and 50 patients with progressive HIV-1 infection (SPs and RPs combined) were aligned (Fig. 1). Table 1 summarizes the research cohorts that contributed to the analysis. In addition to the nef alleles analyzed in the present study, published Nef sequences from 9 AIDS patients (38), 10 LTNPs (15), and 9 patients with different rates of disease progression (27) were included (Table 1). The three SPs SP1 to SP3 (Fig. 1 and Table 1) described by Michael et al. (27) were included in the NP group in the present study, because they showed a positive CD4 slope and final CD4+-cell counts of >500/mm3 over 8 years of follow-up without antiviral therapy.
Selected laboratory characteristics of the three progression groups are summarized in Table 1. On average, the NPs had 9-fold-higher mean CD4+-cell numbers and 20-fold-lower viral RNA loads than the RPs at the time of sampling (Table 1 and data not shown). All 24 subjects from the C&W cohort who were classified as NPs had maintained CD4+-T-cell counts of >500 per mm3 for 3 years following PBMC isolation for genomic DNA preparation. The average cell number, however, declined from 875 to 732 CD4+ cells/mm3 during this time period, indicating that many of these patients are slowly progressing to immunodeficiency (Table 1). The slight increase in the RPs from the C&W cohort from an average of 119 CD4+ cells/mm3 after 6.3 years to 147 CD4+ cells/mm3 after 7.8 years of documented HIV-1 infection is due to the use of intensive combination drug therapy in these patients starting after the time of sampling for genomic DNA preparation. The average number of nef alleles analyzed per patient varied from 5.3 for the RPs to 8.6 for the NPs. For clarity, only consensus Nef protein sequences representative for each patient were aligned (Fig. 1). The amino acid variation at each position is shown in Fig. 2.
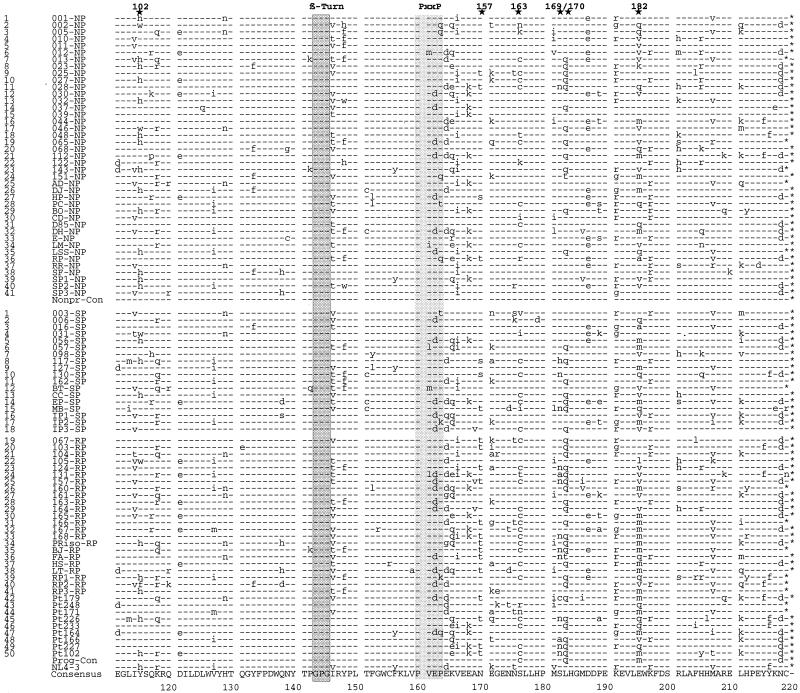
FIG. 2.
Nef sequence variation within and between NPs and progressors (P). The middle lines depict the NP (upper) and P (lower) consensus sequences with the predominant residue at each position. The sequence variation is represented above (NP) and below (P) the consensus sequences. Subscripted numbers show the number of patient-specific consensus Nef protein sequences in which that amino acid was observed. Several conserved motifs and the locations of five Nef features more frequently observed in NPs (NP-1 to NP-5) and nine features more frequently observed in progressors (P-1 to P-9) are indicated by shaded ovals. Differences between the NP and P consensus sequences are indicated by vertical bars. Amino acids that differ markedly between the two groups of patients are in white with black background. For symbols, see the legend to Fig. 1.
With some exceptions, most previously defined conserved domains in Nef (30, 38) with putative functional relevance were conserved (Fig. 1 and 2). In Nef proteins obtained from five NPs and four SPs, changes in the N-terminal myristylation signal (MGGKWSK) were observed (Fig. 1). All deduced Nef protein sequences from one NP (patient 044) and one RP (patient 104) contained a change of a highly conserved cysteine at amino acid position 63 (044, C63→L; 104, C63→S/N). For patient 044, the C63→L substitution was present in 15 of 15 PCR clones obtained at three independent time points (Fig. 1 and data not shown). Nef sequences derived from several NPs (001, 039, 044, and RR) and a comparable number of progressors (104, 160, and S1) contained an additional G or K residue in the middle of the acidic charged region (amino acids 71 to 75). At other positions in the acidic stretch of amino acids, polar (Q [046, 104, 117, 130, and Pt164]), positively charged (K [165]) or uncharged (S [RP3]) amino acids were observed (Fig. 1 and 2). A (PxxP)3 motif, resembling an SH3 binding site and shown to be involved in Hck kinase binding (21, 22, 34), and an ExxxLL motif that may be important for Nef-mediated endocytosis of CD4 (6) were universally conserved (Fig. 1 and 2). A second PxxP motif, located close to the C terminus of Nef, was changed to PxxQ, PxxT, or PxxK in five nonprogressing and in four progressing individuals (Fig. 1 and 2). A previously identified putative protein kinase C (PKC) recognition site was almost universally conserved (Fig. 1 and 2). For most Nef proteins the sequence was RPMTYK, although some variations were observed: RPMTYR (patients 016, 032, and 037), RPMSYR (LSS1), RPISYR (003), PPMTWK (127), PPMTFK (167), PPVTWK (MB), and PPMNWK (157) (Fig. 2). All of these except the RP157 sequence still predict a PKC recognition site (R/KX0-2S/TX0-2R/K). A predicted β-turn motif (GPG) was universally conserved (Fig. 1). A previously proposed preference for a threonine (GPGT) in nonprogressors, versus isoleucine or valine (GPGV/I) in progressors (15), could not be confirmed (Fig. 1 and 2).
The high degree of conservation of these putative functional motifs suggests that Nef proteins from all progression groups are functionally active. However, the analysis of a large number of samples allowed the identification of some amino acid variations in Nef that appear to be associated either with a well-preserved immune system as among the nonprogressors or with progression to more advanced immunodeficiency. The Nef consensus sequences obtained from the 41 NPs and the 50 progressing individuals differed in only 8 of the 220 amino acid positions aligned: 11V↔G, 15T↔A, 22 · ↔R, 63N↔T, 96A↔G, 98L↔V, 183L↔Q, and 195E↔M (Fig. 2) (numbering corresponds to the alignment; · specifies a gap in the NP Nef). Some of these differences are probably coincidental and/or represent conservative substitutions that should not alter Nef function. However, other variations are highly significant (Table 2) and do affect amino acids that may be important for Nef structure and function. As mentioned above, a (PxxP)3 motif in the conserved central part of Nef and a second PxxP motif close to the C terminus showed similar degrees of conservation in nonprogressing and progressing individuals (Fig. 1 and 2). However, an additional PxxP motif close to the N terminus of Nef was present in only 1 of 41 NPs (2%) but in 8 of 32 RPs (25%) (P < 0.01) (Fig. 1; Table 2). Other variations include amino acids that may potentially be phosphorylated. At amino acid positions 51 and 157 (numbering corresponds to the NL4-3 Nef), nef alleles from RPs often predicted a threonine, whereas those from NPs usually predicted an asparagine (Fig. 2). Also, in the variable-length region, a threonine or serine was more frequently present in progressors (10 of 50; 20%) than in NPs (2 of 41; 5%) (P = 0.02) (Fig. 2). In contrast, Nef proteins from NPs more frequently contained a threonine at amino acid 15. A tyrosine at amino acid 102 in Nef was relatively conserved in progressors, whereas 34% of the NPs contained a histidine at this position. A serine at amino acid 169 was highly conserved in Nef sequences obtained from NPs (98%). In comparison, 28% of Nef sequences amplified from the RPs contained an asparagine at this position (Fig. 2; Table 2). Several of the sequence variations that seem to be associated with different stages of disease progression were located close to the carboxy terminus of the protein. Nef from progressors more frequently contained a cysteine at position 163 (Fig. 2; Table 2). At amino acid position 170, 58% of Nef proteins from RPs contained a polar amino acid (Q), whereas 68% of the NPs contained an aliphatic hydrophobic amino acid (L). This proportion was reversed at position 182, where Nef from NPs tended to have an acidic (E) or polar (Q) residue and Nef from progressors preferentially contained hydrophobic residues (M or V). These variations in the deduced amino acid sequences from NPs and rapidly progressing individuals suggest that some functional differences in Nef may be associated with different stages of disease. Some of the sequence variations close to the C terminus of Nef may be linked. For example, 79% (23 of 29) of the Nef sequences predicting a cysteine at position 163 contained a glutamine at position 170 (Fig. 1). In contrast, Nef sequences containing a serine residue at position 163 usually had a leucine at position 170 (33 of 51; 65%). These differences were highly significant (P < 0.001). Nef sequences containing the C163-Q170 combination had an asparagine at position 169 relatively frequently (8 of 23; 25%) compared to the remaining sequences (3 of 68; 4%) (P < 0.001).
TABLE 2.
Differences between Nef sequences derived from NPs, SPs, and RPs
| Nef variationa | No. (%) of occurrences in:
|
P valueb
|
||||
|---|---|---|---|---|---|---|
| NPs (n = 41) | Progressors
|
|||||
| SPs (n = 18) | RPs (n = 32) | Total (n = 50) | NPs vs all progressors | NPs vs RPs | ||
| N-terminal PxxP | 1 (2) | 2 (11) | 8 (25) | 10 (20) | <0.03 | <0.01 |
| T15 | 21 (51) | 3 (17) | 10 (31) | 13 (26) | <0.03 | 0.14 |
| A15 | 14 (34) | 10 (56) | 16 (50) | 26 (53) | 0.13 | 0.26 |
| R39 | 0 (0) | 4 (22) | 6 (19) | 10 (20) | <0.02 | <0.02 |
| T51 | 12 (29) | 9 (50) | 21 (66) | 30 (60) | <0.01 | <0.005 |
| N51 | 28 (68) | 9 (50) | 10 (31) | 19 (38) | <0.01 | <0.005 |
| H102 | 14 (34) | 3 (13) | 5 (16) | 8 (16) | 0.08 | 0.12 |
| T157 | 3 (7) | 1 (6) | 13 (41) | 14 (28) | <0.03 | <0.005 |
| C163 | 8 (20) | 5 (25) | 16 (50) | 21 (41) | <0.04 | 0.01 |
| N169 | 1 (2) | 1 (6) | 9 (28) | 10 (20) | <0.03 | <0.005 |
| L170 | 28 (68) | 11 (61) | 9 (28) | 20 (41) | <0.02 | <0.002 |
| Q170 | 12 (29) | 7 (39) | 22 (69) | 29 (58) | 0.01 | <0.002 |
| M182 | 5 (12) | 5 (28) | 14 (44) | 19 (39) | 0.01 | <0.005 |
| E182 | 16 (39) | 4 (22) | 4 (13) | 8 (16) | <0.03 | <0.03 |
Positions are those indicated by shaded ovals in Fig. 2.
Determined by the chi-square test with the Yates correction.
Relationship between the number of Nef sequence variations and CD4+-cell-count and viral load.
Next, we investigated whether individuals representing the extreme ends of the clinical spectrum of nonprogression and rapid progression were likely to harbor proviruses expressing Nef proteins showing a greater number of the features typically observed in the different progression groups. Based on the Nef protein alignment shown in Fig. 1, we identified five features that were more frequently observed in NPs (T15, N51, H102, L170, and E182) and nine features that were more typically observed in RPs at late stages (an additional N-terminal PxxP motif and the presence of amino acids A15, R39, T51, T157, C163, N169, Q170, and M182 in Nef) (Fig. 2). Most of these differences are statistically significant (Table 2). The presence or absence of these Nef features was determined for each patient analyzed, and the number of features more typical for nonprogression was subtracted from the number of features more frequently observed in rapid progression. The corresponding number was termed the Nef progression score (NefProg score). Thus, a Nef sequence that contains all eight amino acids typical for RPs and SPs and an additional PxxP would be assigned a NefProg score of +9. Conversely, a Nef sequence showing all five properties typical for NPs and none of those typical for RPs would be assigned a score of −5. For each HIV-1-infected individual the number was calculated for a representative Nef consensus sequence, obtained from PCR clones derived from a single time point. Where substantial intrapatient variation was observed, the corresponding amino acid positions were not included in the analysis.
In the 41 NPs analyzed, NefProg scores ranged from −5 to +4 with an average of −1.4 (Table 1). For the 18 SPs, scores between −3 and +5 (average of +0.9) were observed, and for the 32 RPs, scores between −2 and +8 (average of +2.8) were observed. It was noteworthy that although the NefProg score varied considerably within each group, a tendency towards more positive numbers in immunodeficient individuals, irrespective of their origins, was observed (Table 1). The greatest difference (NPs, −3.3; RPs, +4.8) was found for the patients in the Worcester cohort.
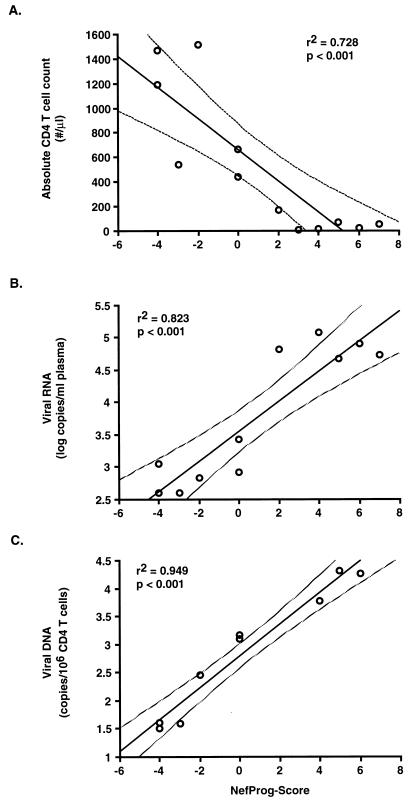
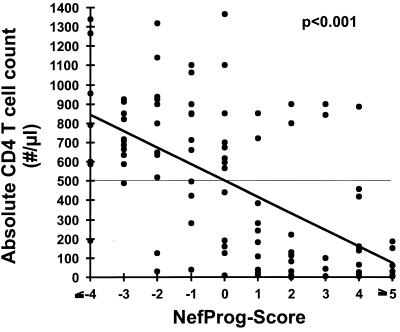
Each of the three progression groups analyzed in this study is, to some extent, heterogeneous with regard to duration of infection, CD4+-T-cell count, and viral load. In order to further investigate how the stage of HIV disease relates to the Nef features identified, we compared the CD4+-cell counts and the viral load data with the NefProg scores. First, the 12 individuals in the Worcester cohort were analyzed, because very stringent criteria were used for nonprogressive infection and concurrent or nearly concurrent (within half a year of sampling for PCR analysis) data on CD4+-T-cell counts and plasma viral RNA load were available for most of these patients. Average viral DNA copy frequencies were determined with samples from 1994, the same calendar year as the NefProg score determination for most individuals. There was a strong correlation between the NefProg score and the absolute CD4+-cell counts (correlation coefficient, −0.854; P value, <0.001) (Fig. 3A), the plasma viral RNA load (correlation coefficient, +0.907; P value, <0.001) (Fig. 3B), and the proviral DNA copy number (correlation coefficient, +0.974; P value, <0.001) (Fig. 3C). The CD4+-T-cell count decreased and the viral load increased for patients harboring Nef protein sequences with high NefProg scores (Fig. 3). Next, we expanded the analysis to Nef consensus sequences derived from all 91 HIV-1-infected individuals listed in Table 1 and from 9 additional patients described by Ratner et al. (33). Concurrent data on viral load were not available for some of these patients and/or were obtained by using different methods. Therefore, only the relation between the CD4+-cell count and the NefProg score was investigated (Fig. 4). Although there were some exceptions, there was a strong correlation between the absolute CD4+-cell count and the NefProg score (correlation coefficient, −0.6; P value, <0.001). The majority of Nef proteins with a score below 0 were derived from individuals with >500 CD4+ cells/mm3 (34 of 42; 81%). In contrast, Nef proteins with a score greater than 0 were usually obtained from patients with <500 CD4+ cells/mm3 (38 of 45; 84%). These differences are even more significant for the extreme ends of the spectrum of the Nef sequence variations identified. Only 2 of 18 individuals (11%) (patients 490 and 191) from whom deduced Nef protein sequences with a score of ≤−3 were obtained had <500 CD4+ cells/mm3 at the time of sampling. In comparison, 1 of 18 patients (6%) from whom Nef proteins with a score ≥+4 were derived had >500 CD4+ cells/mm3. Our data suggest that certain sequence variations in Nef predominate in NPs with relatively normal CD4+-cell counts, while others are found in severely immunodeficient RPs with low CD4+-cell counts.
FIG. 3.
Correlation between the NefProg score and the CD4+-cell count and the viral load in patients in the Worcester cohort. The circles represent the absolute CD4+-cell counts (A), the plasma viral RNA levels (B), and the proviral copy numbers (C) in relation to the NefProg score given on the x axis. Pearson correlation coefficients obtained with the Fisher transformation test were used to assess the statistical significance.
FIG. 4.
CD4+-cell counts in relation to NefProg score. The dots represent the CD4+-cell count and NefProg score for each of the 100 patients analyzed. In addition to those from the patients described in the legend to Fig. 1, data from nine additional HIV-1-infected persons were included (33). The linear regression line is indicated.
Sequence variations in Nef during progression from the asymptomatic stage to AIDS.
To investigate whether the sequence variations in Nef that appear to be associated with progressive disease are already present early during the asymptomatic stage or emerge during or after AIDS progression, sequential samples derived from two individuals from the Worcester cohort with progressive HIV-1 disease, FA and MB, were analyzed. Both patients have severe type A hemophilia and are seropositive for both hepatitis B and C viruses.
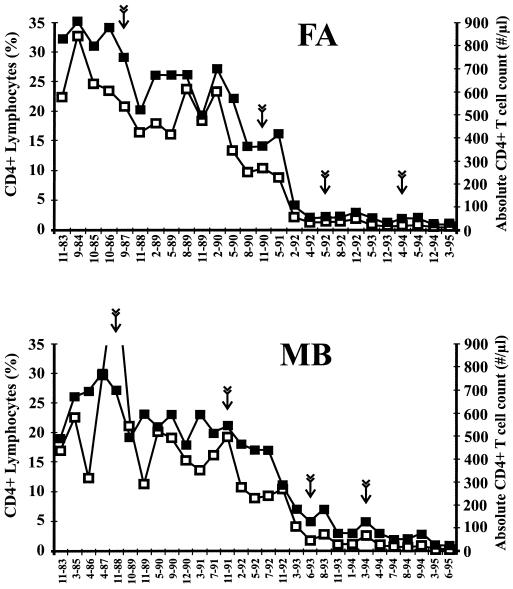
From the RP FA, blood samples were obtained between 1987 and 1994. In 1987, the CD4+-lymphocyte counts were just >500/mm3; by 1990, they had declined to 280/mm3; and in 1992 and 1994, they were <50 cells/mm3 (Fig. 5). FA initiated therapy in 1990 with zidovudine (AZT), changing to dideoxyinosine (ddI) in 1992, to AZT with Nevirapine in 1993, and to AZT alternating with ddI in 1994 until 1996. He has been treated for cytomegalovirus disease since 1995, for thrush (1994), and for presumed Pneumocystis carinii pneumonia (1993). Amino acid dominance in Nef changed at 12 positions between 1987 and 1994: 31D→A/V, 36V→A, 42K→R, 48N→S, 54N→T, 70 · →E, 138I→V/L, 154E→D, 162N→T, 174S→N, 187E→Q, and 197R→H (numbering corresponds to the alignment shown in Fig. 6; · specifies a gap). Three of these changes (48N→S, 70 · →E, and 197R→H) were already apparent in 1990. Seven additional alterations (31D→A/V, 36V · →A, 42K→R, 54N→T, 138I→V, 162N→T, and 174S→N) were observed in a subset of Nef protein sequences obtained from the 1992 blood sample, while the remaining two substitutions (154E→D and 187E→Q) were observed in 1994 (Fig. 6). Several of these changes in Nef, which coincided with progression to AIDS in FA, correspond to those that were more frequently observed in RPs. In addition, Nef proteins detected in FA in 1987 contained several features that were typically associated with a nonprogressive status (T15, K39, N51, N157, S169, and E182). In 1994, when the patient was severely immunodeficient, four of these positions (39K→R, 51N→T, 157N→T, and 169S→N) had changed to amino acids that were more frequently found in progressing individuals (Fig. 6; Table 3). Also, two other substitutions (154E→D and 187E→Q) observed during the late stages of infection in FA were more frequently observed in progressing infection (Fig. 2). Based on our criteria described above, the NefProg score changed from −2 in 1987 to +4 in 1994 (Table 3).
FIG. 5.
Profile of CD4+-T-cell counts in patients FA and MB. The month and year of blood sampling are given on the x axis. The vertical arrows indicate the time points at which PBMCs were collected for genomic DNA extraction and PCR analysis. ■, percentage of CD4+ T lymphocytes; □, absolute number of CD4+ T lymphocytes.
FIG. 6.
Alignment of the predicted Nef amino acid sequences derived from patients FA and MB. The two-digit number in the left column gives the year of PBMC collection, and the last number specifies the individual clone. The Nef consensus sequences derived from NPs (Non-Con) and progressors (Pro-Con) are shown at the bottom for comparison. The Non-ex and Pro-ex sequences also contain those variations that were more frequently observed in NPs or RPs but did not alter the NP and RP consensus Nef sequences. The nine amino acid positions corresponding to those at which differences between Nef sequences derived from NPs and RPs were observed are indicated by stars above the alignment. The positions of additional N-terminal PxxP motifs in deduced FA and MB Nef sequences are shaded. Abbreviations and symbols are described in the legend to Fig. 1.
TABLE 3.
Nef amino acid variations observed during AIDS progression
| Patient | PBMC sample (mo-yr) | No. of clones | No. (%) of CD4+ cells | No. defec-tivea | PxxP | Amino acid at position:b
|
Properties (RP/NP)c | NefProg score | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 15 | 39 | 51 | 102 | 157 | 163 | 169 | 170 | 182 | ||||||||
| FA | 9-87 | 6 | 537 (29) | 2 | + | T | K | N | Y | N | S | S | T | E | 1/3 | −2 |
| 11-90 | 5 | 266 (14) | 0 | + | T | K4R1 | N4/T1 | Y | N | S | S | T | E | 1/3 | −2 | |
| 5-92 | 5 | 32 (2) | 1 | + | T | K | T3/A1 | Y | N3/T1 | S | S3/N1 | T | E3/Q1 | 2/2 | +0 | |
| 4-94 | 9 | 18 (2) | 0 | + | T | R | T | Y | T | S8/C1 | N | T | Q | 5/1 | +4 | |
| MB | 11-88 | 12 | >1,000 (27) | 3 | +4/−5 | A | K8R1 | N | Y | N | I/T/S | S | L5/Q4 | E7/V/M | 1/2 | −1 |
| 11-91 | 7 | 496 (21) | 0 | + | A | R | N6/S1 | Y | T6/N1 | I/V/S | N | Q | E/M/Q | 6/1 | +5 | |
| 6-93 | 6 | 46 (5) | 1 | + | A | R | N | Y | T | I | N | Q | Q | 6/1 | +5 | |
| 3-94 | 8 | 64 (5) | 1 | + | A | R | N | Y | T | I | N6/S1 | Q | Q | 6/1 | +5 | |
Number of clones containing premature stop codons or frameshift mutations (Fig. 6).
Amino acid positions refer to the NL4-3 Nef sequence. Amino acids typically observed in RPs are underlined; amino acids that were more often observed in NPs are in boldface. Subscript numbers are the numbers of individual clones for positions at which sequence variations were observed. Only Nef protein sequences predicted from intact nef open reading frames were included in the analysis.
Number of properties observed more often in RPs and number of properties typically observed in NPs. Numbers were calculated for the representative consensus sequence.
Four sequential samples were also analyzed from the individual MB (Fig. 5). The first sample was drawn in 1988, when the patient was asymptomatic with a CD4+-cell count of >1,000/mm3. This declined subsequently to <500/mm3 in 1991 and <50/mm3 after 1993 (Fig. 5). MB initiated therapy in 1991 with AZT, changing to ddI in 1993 and then to AZT alternating with ddI in 1993. In 1996, he changed therapy again to stavudine and Indinavir, with a good clinical response. Three of the 12 nef alleles derived from the 1988 PBMC sample predicted premature stop codons or frameshift mutations (Fig. 6). The nine intact Nef protein sequences showed a relatively high degree of divergence (up to 10%). Five of these Nef sequences showed features corresponding to an NefProg score of −1. The remaining four Nef sequences predicted an additional N-terminal PxxP and Q170, changing the score to +1. At the remaining three time points, all deduced Nef sequences predicted a PxxP close to the N terminus. Furthermore, in comparison with the majority of Nef sequences obtained in 1988, amino acid dominance changed at four positions after 1991: K39→R, N157→T, S169→N, L170→Q, and E182→Q. Similar to the results obtained with sequential samples from patient FA, these alterations represented changes to amino acids that were more typical for RPs (Fig. 6; Table 3).
Thus, several Nef features that were more frequently observed in RPs were not detected in early samples when the CD4+-T-cell numbers were high but came to predominate during (MB) or after (FA) progression to AIDS (Table 3). At four positions, the same changes of K39→R, N157→T, S169→N, and E182→Q were observed in both patients. In FA these changes occurred very late in infection when the CD4+-cell numbers were already below 50/mm3. In comparison, in patient MB the predominance at these amino acid positions changed when the CD4+-T-cell counts declined from the normal range in 1988 to just below 500/mm3 in 1991 (Table 3).
Localization of amino acid variations on the three-dimensional structure of Nef.
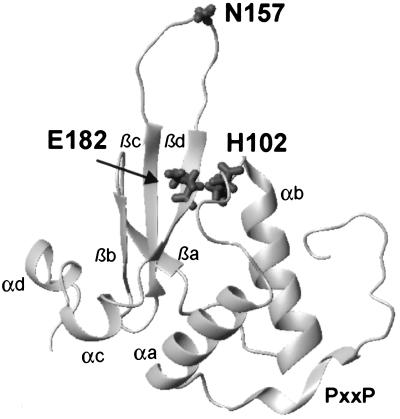
Finally, we investigated the localization of the amino acid variations observed between NPs and RPs on the published three-dimensional nuclear magnetic resonance solution structure of HIV-1 Nef (14). Six of the nine amino acid substitutions that may be associated with different stages of infection (Fig. 2) are located within the N-terminal domain (residues 15, 39, and 51) or in the unstructured loop between β-sheets c and d (residues 163, 169, and 170). These regions were deleted in the structural analysis of Nef to circumvent problems associated with aggregation (14). The localization of the three remaining positions (102, 157, 182) is shown in Fig. 7. Interestingly, amino acids 102 and 182, although well separated on the linear sequence, are in close proximity on the tertiary Nef structure. The presence of a His or Tyr at position 102, however, was not significantly associated with the presence of a certain amino acid at position 182. The remaining amino acid position, 157, is located at the tip of a loop structure and well exposed on the Nef surface (Fig. 7).
FIG. 7.
Localization of amino acid positions at which variations between NPs and RPs were observed on the tertiary structure of Nef. Residues H102, N157, and E182 were frequently found at these positions in NPs. The four α-helices (αa to αd), the four β-strands (βa to βd), and the approximate position of the (PxxP)3 motif are also indicated. Regions encompassing the remaining six amino acid positions used to define the NefProg score (residues 15, 39, 51, 63, 169, and 170) were deleted from the molecule used to determine the nuclear magnetic resonance solution structure of Nef (14).
DISCUSSION
Consistent with previous studies (15, 25, 27, 33), we found that large deletions in nef are rarely present in HIV-1 infection. Overall, we have investigated the presence of gross deletions in nef in about 200 HIV-1-infected individuals. Large deletions in nef were detected in only one subject (20). This frequency of 0.5% is lower than the 6% found in HIV-2 infection (40). Overall, the prevalence of inactivating point mutations was less than 10% (Table 1). One LTNP with a high frequency of defective nef alleles has been described (25). In a second LTNP, a 36-bp deletion was universally detected (4). We also found a high frequency (40%) of disrupted nef alleles in two AIDS patients, which is consistent with the results of McNearney et al. (26), who reported that defects in nef are more common during the late stages of disease. On average, the prevalence of inactivating point mutations in RPs was 9.4%, which is 2.5-fold higher than the 3.7% observed for the SPs (Table 1). It remains to be elucidated whether the selective pressure for open functional nef genes is reduced during the final stage of infection, when the host immune system is destroyed and most T cells are already activated.
Our study also confirms that several previously defined motifs in Nef with putative functional relevance (38) are usually conserved and that this is independent of the stage of disease progression. This suggests that the majority of nef alleles derived from all three progression groups are functionally active. However, in contrast to previous studies (15, 27, 33), we observed that certain sequence variations in Nef are found more frequently in NPs with normal and stable CD4+-T-cell counts, while others are more frequently found in patients whose HIV disease has progressed. A reanalysis of previously published Nef sequences derived from LTNPs or patients with AIDS in other studies shows that our results are complementary (Table 1). Although a high NefProg score was more often found in subjects with low CD4+-T-cell counts and low scores were more often found in LTNPs, these Nef features varied considerably within each of the three progression groups. This is not unexpected, since the clinical status of infection depends on the delicate balance of a multitude of virus-host interactions and each of these groups is heterogenous with regard to CD4+-cell count, viral RNA load, and duration of infection. Furthermore, Nef protein sequences are highly variable, and some variations were observed only in a subset of individuals within the same progression group. Therefore, the analysis of large sample numbers was critical for the identification of differences in Nef that might be associated with different stages of disease progression.
The observation that some discernible differences in Nef are associated with different stages of disease progression does not allow us to conclude that different Nef activities determine the rate of disease progression in HIV-1 infection. However, our preliminary results on Nef sequence analysis of sequential samples obtained from two progressors suggest that some of these Nef sequence variations are selected for during or after progression to immunodeficiency. The in vitro functions of Nef that are critical for viral pathogenicity and the selective forces that may drive these Nef sequence variations with progressive disease remain to be elucidated. Nonetheless, it seems that Nef is a multifunctional protein which may enhance viral replication in vivo by various independent mechanisms. Some well-established Nef activities, like the downregulation of CD4 (2, 10, 24) or major histocompatibility complex class I molecules (23, 37) and alteration of T-cell signaling (3, 17), may allow the virus to escape the immune system. Other Nef activities, such as the enhancement of viral infectivity (5, 11) or the activation of T cells (1, 28, 39), may enhance viral spread in a more direct manner. Since primate immunodeficiency viruses adapt very rapidly to their host environment, it is tempting to speculate that slightly different properties of Nef may mediate optimal viral spread and replication at different stages of infection.
Some of the amino acid positions that appear to vary between different progression groups have been investigated in previous studies. For example, it was shown that a cysteine at position 163 in Nef (170 in Eli Nef), which we detected more frequently detected in RPs, was important for efficient replication of HIV-1 Eli in Jurkat T cells and in primary PBMCs (43). A cysteine at position 151 that was implicated in nonprogressive HIV-1 infection (32) was present at the corresponding position in 2 of 41 NPs and 3 of 50 progressors, indicating that it does not play a role in disease progression. Two other cysteines, at positions 55 and 143 in Nef, that may form a disulfide bond (43) were conserved in all but 3 of the 91 subjects analyzed. Similarly, deduced Nef protein sequences from a single progressor did not contain the putative PKC recognition site. In comparison, mutations in the acidic stretch of amino acids and the C-terminal PxxP motif, which may contribute to the higher in vitro replicative potential of Nef+ viruses (34), were more frequently found (Fig. 2). Interestingly, we observed a higher frequency of N-terminal PxxP motifs, which resemble minimal SH3 binding domains, in Nef derived from RPs (Table 2). SIV variants that contain an additional SH2 binding domain close to the N terminus of Nef show an acutely pathogenic phenotype in rhesus macaques (8). However, in addition to the PxxP motif, an arginine and an aromatic, hydrophobic residue (Ø = A, I, L, or V) are critical for SH3 binding (consensus, RxØPxxP or PxØPxR) (35). These requirements were fulfilled in only 2 of the 11 subjects in whom Nef sequences with an additional N-terminal PxxP motif could be detected (RPs 103 [RxLPxxP] and 161 [RxVPxxP]). Therefore, it seems unlikely that the majority of these N-terminal PxxP motifs would interact with SH3 domains of Src family tyrosine kinases.
In summary, our results show that deduced HIV-1 Nef sequences derived from individuals who have maintained a high stable CD4+-T-cell count and from patients with progressive disease show notable amino acid differences. These variations involve amino acids that may potentially be phosphorylated (T/A15, T/N51, Y/H102, T/N157, and S/N169), charged residues (K/R39 and E/M182), and a cysteine (S/C163), all of which may affect Nef structure and function. The selective forces that drive these sequence variations and the phenotypic consequences of these variations in in vitro assay systems will need to be investigated in future studies.
ACKNOWLEDGMENTS
We thank Marion Hamacher and Mandy Krumbiegel for excellent technical assistance. We are grateful to Bernhard Fleckenstein and B. G. Gazzard for support and encouragement. We also thank Ronald C. Desrosiers and Klaus Überla for critical reading of the manuscript, Doreen Brettler for coordination of the clinical evaluation and management of the Worcester cohort, and Natalie Ives for help with statistical analysis. We are also indebted to all of the patients who participated in the various research cohorts.
N.D. was funded by an MRC Ph.D. studentship. This work was supported by the Deutsche Forschungsgesellschaft (DFG), the Wilhelm-Sander-Stiftung, the UK Medical Research Council, the Arthur Ashe Foundation for the defeat of AIDS, the Wellcome Trust, and National Institutes of Health grants HL-42257, AI-39400, AI-01382, and AI-26507.
REFERENCES
- 1.Alexander L, Du Z, Rosenzweig M, Jung J J, Desrosiers R C. A role for natural simian immunodeficiency virus and human immunodeficiency virus type 1 nef alleles in lymphocyte activation. J Virol. 1997;71:6094–6099. doi: 10.1128/jvi.71.8.6094-6099.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson S, Shugars D C, Swanstrom R, Garcia J V. Nef from primary isolates of human immunodeficiency virus type 1 suppresses surface CD4 expression in human and mouse T cells. J Virol. 1993;67:4923–4931. doi: 10.1128/jvi.67.8.4923-4931.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baur A S, Sawai E T, Dazin P, Fantl W J, Cheng-Mayer C, Peterlin B M. HIV-1 Nef leads to inhibition or activation of T cells depending on its intracellular localization. Immunity. 1994;1:373–384. doi: 10.1016/1074-7613(94)90068-x. [DOI] [PubMed] [Google Scholar]
- 4.Carl, S., R. Daniels, A. J. Iafrate, M. Troop, P. Easterbrook, J. Skowronski, and F. Kirchhoff. Partial “repair” of defective nef genes in a long-term nonprogressor of HIV-1 infection selectively restores the ability of Nef to enhance infectivity and to down-modulate MHC class I cell surface expression. Submitted for publication.
- 5.Chowers M Y, Spina C A, Kwoh T J, Fitch N J, Richman D D, Guatelli J C. Optimal infectivity in vitro of human immunodeficiency virus type 1 requires an intact nef gene. J Virol. 1994;68:2906–2914. doi: 10.1128/jvi.68.5.2906-2914.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Craig H M, Pandori M W, Guatelli J C. Interaction of HIV-1 Nef with the cellular dileucine-based sorting pathway is required for CD4 down-regulation and optimal viral infectivity. Proc Natl Acad Sci USA. 1998;95:11229–11234. doi: 10.1073/pnas.95.19.11229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deacon N J, Tsykin A, Solomon A, Smith K, Ludford-Menting M, Hooker D J, McPhee D A, Greenway A L, Ellett A, Chatfield C. Genomic structure of an attenuated quasi species of HIV-1 from a blood transfusion donor and recipients. Science. 1995;270:988–991. doi: 10.1126/science.270.5238.988. [DOI] [PubMed] [Google Scholar]
- 8.Du Z, Lang S M, Sasseville V G, Lackner A A, Ilyinskii P O, Daniel M D, Jung J U, Desrosiers R C. Identification of a nef allele that causes lymphocyte activation and acute disease in macaque monkeys. Cell. 1995;82:665–674. doi: 10.1016/0092-8674(95)90038-1. [DOI] [PubMed] [Google Scholar]
- 9.Easterbrook P J. Research potentials and pitfalls in the use of an HIV clinical database: Chelsea and Westminster Hospital. J Acquired Immune Defic Syndr Hum Retrovirol. 1998;17:28–33. doi: 10.1097/00042560-199801001-00009. [DOI] [PubMed] [Google Scholar]
- 10.Garcia J V, Miller A D. Serine phosphorylation-independent downregulation of cell-surface CD4 by nef. Nature. 1991;350:508–511. doi: 10.1038/350508a0. [DOI] [PubMed] [Google Scholar]
- 11.Goldsmith M A, Warmerdam M T, Atchison R E, Miller M D, Greene W C. Dissociation of the CD4 downregulation and viral infectivity enhancement functions of human immunodeficiency virus type 1 Nef. J Virol. 1995;69:4112–4121. doi: 10.1128/jvi.69.7.4112-4121.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Greenough T C, Somansundaran M, Brettler D B, Hesselton R M, Alimenti A, Kirchhoff F, Panicali D, Sullivan J L. Normal immune function and inability to isolate virus in culture in an individual with long-term human immunodeficiency type-1 infection. AIDS Res Hum Retroviruses. 1994;10:395–403. doi: 10.1089/aid.1994.10.395. [DOI] [PubMed] [Google Scholar]
- 13.Greenough, T. C., D. B. Brettler, F. Kirchhoff, L. Alexander, R. C. Desrosiers, S. J. O’Brien, M. Somasundaran, K. Luzuriaga, and J. L. Sullivan. Immunological and virological characterization of individuals with long-term non-progressive HIV-1 infection in a hemophilia cohort. Submitted for publication.
- 14.Grzesiek S, Bax A, Clore G M, Gronenborn A M, Hu J S, Kaufman J, Palmer I, Stahl S J, Wingfield P T. The solution structure of HIV-1 Nef reveals an unexpected fold and permits delineation of the binding surface for the SH3 domain of Hck tyrosine protein kinase. Nat Struct Biol. 1996;3:340–345. doi: 10.1038/nsb0496-340. [DOI] [PubMed] [Google Scholar]
- 15.Huang Y, Zhang L, Ho D D. Characterization of nef sequences in long-term survivors of human immunodeficiency virus type 1 infection. J Virol. 1995;69:93–100. doi: 10.1128/jvi.69.1.93-100.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang Y, Zhang L, Ho D D. Biological characterization of Nef in long-term survivors of human immunodeficiency virus type 1 infection. J Virol. 1995;69:8142–8146. doi: 10.1128/jvi.69.12.8142-8146.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iafrate A J, Bronson S, Skowronski J. Separable functions of Nef disrupt two aspects of T cell receptor machinery: CD4 expression and CD3 signaling. EMBO J. 1997;16:673–684. doi: 10.1093/emboj/16.4.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.International Union of Biochemistry Nomenclature Committee. Nomenclature for incompletely specified bases in nucleic acid sequences. Eur J Biochem. 1985;150:1–5. doi: 10.1111/j.1432-1033.1985.tb08977.x. [DOI] [PubMed] [Google Scholar]
- 19.Kestler H W, Ringler D J, Mori K, Panicali D L, Sehgal P K, Daniel M D, Desrosiers R C. Importance of the nef gene for maintenance of high virus loads and for development of AIDS. Cell. 1991;65:651–662. doi: 10.1016/0092-8674(91)90097-i. [DOI] [PubMed] [Google Scholar]
- 20.Kirchhoff F, Greenough T C, Brettler D B, Sullivan J L, Desrosiers R C. Absence of intact nef sequences in a long-term, non-progressing survivor of HIV-1 infection. N Engl J Med. 1995;332:228–232. doi: 10.1056/NEJM199501263320405. [DOI] [PubMed] [Google Scholar]
- 21.Lee C H, Leung B, Lemmon M A, Zheng J, Cowburn D, Kuriyan J, Saksela K. A single amino acid in the SH3 domain of Hck determines its high affinity and specificity in binding to HIV-1 Nef protein. EMBO J. 1995;14:5006–5015. doi: 10.1002/j.1460-2075.1995.tb00183.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee C H, Saksela K, Mirza U A, Chait B T, Kuriyan J. Crystal structure of the conserved core of HIV-1 Nef complexed with a Src family SH3 domain. Cell. 1996;85:931–942. doi: 10.1016/s0092-8674(00)81276-3. [DOI] [PubMed] [Google Scholar]
- 23.Le Gall S, Prevost M C, Heard J M, Schwartz O. Human immunodeficiency virus type I Nef independently affects virion incorporation of major histocompatibility complex class I molecules and virus infectivity. Virology. 1997;229:295–301. doi: 10.1006/viro.1996.8417. [DOI] [PubMed] [Google Scholar]
- 24.Mariani R, Skowronski J. CD4 down-regulation by nef alleles isolated from human immunodeficiency virus type 1-infected individuals. Proc Natl Acad Sci USA. 1993;90:5549–5553. doi: 10.1073/pnas.90.12.5549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mariani R, Kirchhoff F, Greenough T C, Sullivan J L, Desrosiers R C, Skowronski J. High frequency of defective nef alleles in a long-term survivor with nonprogressive human immunodeficiency virus type 1 infection. J Virol. 1996;70:7752–7764. doi: 10.1128/jvi.70.11.7752-7764.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McNearney T, Hornickova Z, Templeton A, Birdwell A, Arens M, Markham R, Saah A, Ratner L. Nef and LTR sequence variation from sequentially derived human immunodeficiency virus type 1 isolates. Virology. 1995;208:388–398. doi: 10.1006/viro.1995.1166. [DOI] [PubMed] [Google Scholar]
- 27.Michael N L, Chang G, d’Arcy L A, Tseng C J, Birx D L, Sheppard H W. Functional characterization of human immunodeficiency virus type 1 nef genes in patients with divergent rates of disease progression. J Virol. 1995;69:6758–6769. doi: 10.1128/jvi.69.11.6758-6769.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miller M D, Warmerdam M T, Gaston I, Greene W C, Feinberg M B. The human immunodeficiency virus-1 nef gene product: a positive factor for viral infection and replication in primary lymphocytes and macrophages. J Exp Med. 1994;179:101–114. doi: 10.1084/jem.179.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mills J. Effects of 13–17 years of infection with a nef-deleted strain of HIV: the Sydney Blood Bank Cohort. 1997. pp. 293–299. . Report of the Onzieme Colloque Cent Gardes meeting. [Google Scholar]
- 30.Myers G, Korber B T, Foley B, Jeang K-T, Mellors J W, Wain-Hobson S. Human retroviruses and AIDS 1996: a compilation and analysis of nucleic acid and amino acid sequences. Los Alamos, N.Mex: Los Alamos National Laboratory; 1996. [Google Scholar]
- 31.Penny M A, Thomas S J, Douglas N W, Ranjbar S, Holmes H, Daniels R S. Env-gene sequences of primary HIV-1 isolates of subtypes B, C, D, E and F obtained from the WHO-Network for HIV: isolation and characterisation. AIDS Res Hum Retroviruses. 1996;12:741–747. doi: 10.1089/aid.1996.12.741. [DOI] [PubMed] [Google Scholar]
- 32.Premkumar D R, Ma X Z, Maitra P K, Chakrabarti B K, Salkowitz J, Yen-Lieberman B, Hirsch M S, Kestler H W. The nef gene from a long-term HIV type 1 non-progressor. AIDS Res Hum Retroviruses. 1996;12:337–345. doi: 10.1089/aid.1996.12.337. [DOI] [PubMed] [Google Scholar]
- 33.Ratner L, Joseph T, Bandres J, Ghosh S, Vander Heyden N, Templeton A, Hahn B, Powderly W, Arens M. Sequence heterogenicity of Nef transcripts in HIV-1-infected subjects at different stages of disease. Virology. 1996;223:245–250. doi: 10.1006/viro.1996.0474. [DOI] [PubMed] [Google Scholar]
- 33a.Report of an expert group (chairman, N. E. Ray) The incidence and prevalence of AIDS and prevalence of other severe HIV disease in England and Wales for 1995 to 1999: projection using data to the end of 1994. Commun Dis Rep Rev. 1996;6:R1–R21. [PubMed] [Google Scholar]
- 34.Saksela K, Cheng G, Baltimore D. Proline-rich (PxxP) motifs in HIV-1 Nef bind to SH3 domains of a subset of Src kinases and are required for the enhanced growth of Nef+ viruses but not for down-regulation of CD4. EMBO J. 1995;14:484–491. doi: 10.1002/j.1460-2075.1995.tb07024.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saksela K. HIV-1 Nef and host protein kinases. Frontiers Biosci. 1997;2:606–618. doi: 10.2741/a217. [DOI] [PubMed] [Google Scholar]
- 36.Salvi R, Garbuglia A R, Di Caro A, Pulciani S, Montella F, Benedetto A. Grossly defective nef gene sequences in a a human immunodeficiency virus type 1-seropositive long-term nonprogressor. J Virol. 1998;72:3646–3657. doi: 10.1128/jvi.72.5.3646-3657.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schwartz O, Marechal V, Le Gall S, Lemonnier F, Heard J M. Endocytosis of major histocompatibility complex class I molecules is induced by the HIV-1 Nef protein. Nat Med. 1996;2:338–342. doi: 10.1038/nm0396-338. [DOI] [PubMed] [Google Scholar]
- 38.Shugars D C, Smith M S, Glueck D H, Nantermet P V, Seillier-Moiseiwitsch F, Swanstrom R. Analysis of human immunodeficiency virus type 1 nef gene sequences present in vivo. J Virol. 1993;67:4639–4650. doi: 10.1128/jvi.67.8.4639-4650.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Spina C A, Kwoh T J, Chowers M Y, Guatelli J C, Richman D D. The importance of nef in the induction of human immunodeficiency virus type 1 replication from primary quiescent CD4 lymphocytes. J Exp Med. 1994;179:115–123. doi: 10.1084/jem.179.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Switzer W M, Wiktor S, Soriano V, Silva-Graca A, Mansinho K, Coulibaly I M, Ekpini E, Greenberg A E, Folks T M, Heneine W. Evidence of Nef truncation in human immunodeficiency virus type 2 infection. J Infect Dis. 1998;177:65–71. doi: 10.1086/513819. [DOI] [PubMed] [Google Scholar]
- 41.Weiss R A. How does HIV cause AIDS? Science. 1993;260:1273–1279. doi: 10.1126/science.8493571. [DOI] [PubMed] [Google Scholar]
- 42.Wyand M S, Manson K H, Garcia-Moll M, Monfiori D, Desrosiers R C. Vaccine protection by a triple deletion mutant of simian immunodeficiency virus. J Virol. 1996;70:3724–3733. doi: 10.1128/jvi.70.6.3724-3733.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zazopoulos E, Haseltine W A. Effect of nef alleles on replication of human immunodeficiency virus type 1. Virology. 1993;194:20–27. doi: 10.1006/viro.1993.1230. [DOI] [PubMed] [Google Scholar]