Abstract
We investigated the ability of dengue virus to invade human primary Kupffer cells and to complete its life cycle. The virus effectively penetrated Kupffer cells, but the infection did not result in any viral progeny. Dengue virus-replicating Kupffer cells underwent apoptosis and were cleared by phagocytosis. Infected Kupffer cells produced soluble mediators that could intervene in dengue virus pathogenesis.
Dengue (DEN) viruses are arthropod-borne flaviviruses, and there are four DEN virus serotypes. DEN viruses are major human pathogens, affecting about 100 million individuals every year (8). DEN virus causes a spectrum of disease, from a harmless flu-like illness to severe illness, particularly DEN hemorrhagic fever (DHF). DHF is characterized by high fever, hemorrhagic diathesis, and DEN shock syndrome (DSS), which is often fatal in children. The pathogenesis of DEN virus is still poorly understood. In DHF and DSS, liver involvement is a characteristic sign that the disease will be fatal (1). Hepatic injury is similar to that of the early stages of yellow fever, with an increase in plasma transaminase levels, fatty changes in hepatocytes, Kupffer cell hyperplasia, and centrolobular and midzonal necrosis (11). The most characteristic sign is the presence of acidophilic or Councilman bodies, which are apoptotic bodies (7), corresponding to those seen in the liver of yellow fever patients. DEN viral antigens have been detected in both hepatocytes and Kupffer cells (1, 3, 9, 12). It has been shown that human cells of the mononuclear phagocyte lineage are targets for DEN virus replication (10), but the interactions between DEN virus and human Kupffer cells, the macrophages residing in the liver, have never been studied.
Kupffer cells may determine the outcome of the virus-driven processes (16). They may contribute to the production of viral progeny if they are permissive (22, 27) or, on the contrary, stop the virus life cycle very early (20, 28). Kupffer cells may exert antiviral properties through either autocrine or paracrine mechanisms. In this preliminary report, we addressed three issues: (i) the entry of virus particles into Kupffer cells, (ii) the outcome for such virus particles, and (iii) the production of effectors known to mediate antiviral activity and/or proinflammatory activity.
Kupffer cells were isolated from human liver specimens, obtained during partial hepatectomy for liver cancer, by collagenase perfusion and centrifugal elutriation as previously described (14). The guidelines for French institutions regarding clinical research were respected throughout our experiments. The elutriated cells were plated on eight-microchamber Lab-Tek slides (Nunc) in Dulbecco’s modified Eagle’s medium containing 20 mM HEPES, 10 mM NaHCO3, 20% heat-inactivated fetal calf serum, and 50 μg of gentamycin per ml. Slides were examined with an electron microscope, and 95 to 98% of cells had the ultrastructural features typical of Kupffer cells, with numerous vacuoles, phagolysosomes, dense bodies, and pseudopods at the cell surface. The few contaminating cells were stellate or epithelial cells.
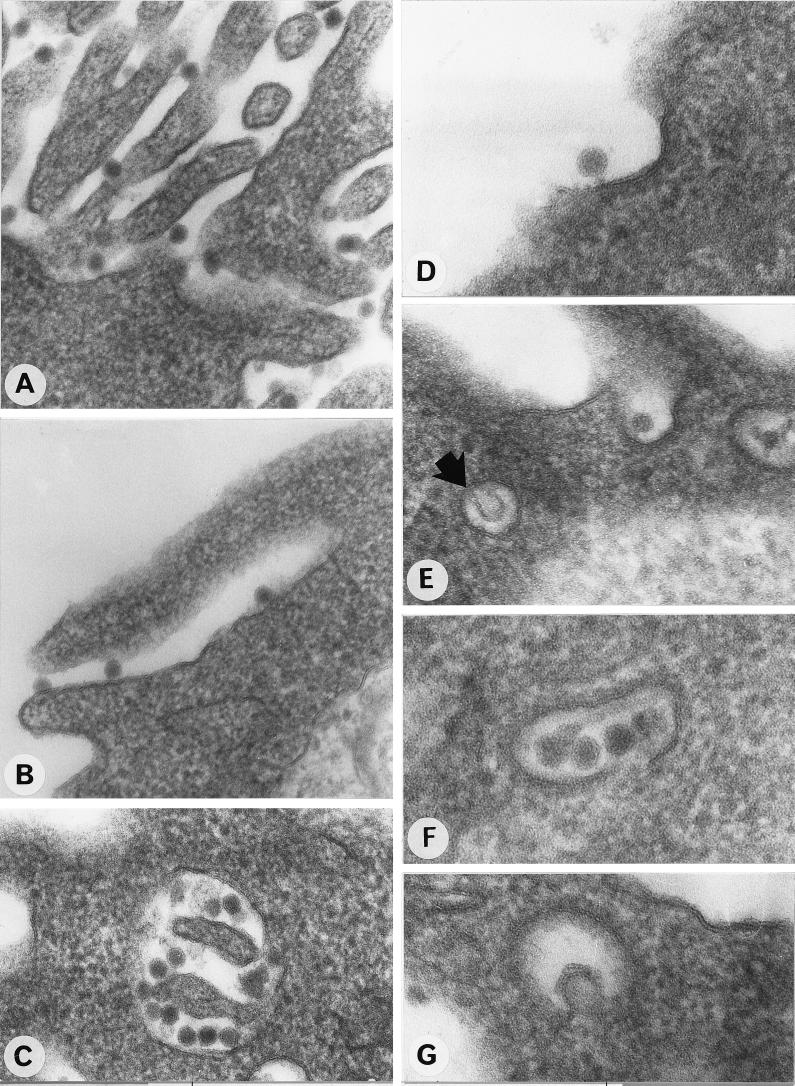
After 3 days of culture, the cells were exposed to purified DEN type 1 virus strain Oster (isolated in French Guiana from a classical case of dengue virus infection in 1989) at a multiplicity of infection (MOI) of 100 focus-forming units/cell, as previously described (19). We performed two types of experiments at various times after infection (1, 6, 24, 48, and 72 h) to determine the effects of the virus on the Kupffer cell monolayers. All of the experiments were repeated three times, each time with Kupffer cells isolated from a different liver sample. In the first set of experiments, the cells were fixed with 2.5% glutaraldehyde in 75 mM sodium cacodylate (pH 7.3) containing 4% sucrose, 1 mM MgCl2, and 1 mM CaCl2, embedded in LX 112 (Ladd Research Industries, Burlington, Vt.), and observed under a Philips electron microscope 410 (Eindhoven). In the second set of experiments, the culture supernatants were collected to determine the viral titer and the cells were fixed in 3% paraformaldehyde solution. Viral antigens were detected by an immunofluorescence assay (IFA) using either DEN type 1 virus-specific hyperimmune mouse ascitic fluid or anti-E and anti-NS1 monoclonal antibodies and fluorescein-conjugated anti-mouse immunoglobulin G (Biosys), as previously described (19). As assessed by electron microscopy studies 1 h after infection, numerous DEN virus particles were adsorbed onto the plasma membrane of most Kupffer cells (Fig. 1A). Virus particles penetrated Kupffer cells by several mechanisms. In most cases, some particles were taken up by phagocytosis, and thereafter many were engulfed by typical cellular lamellipods (Fig. 1B) and sequestered into intracytoplasmic vacuoles (Fig. 1C). DEN virus particles were also observed inside clathrin-coated pits, suggesting entry by receptor-mediated endocytosis (Fig. 1D to F). However, endocytosis appeared to occur far less frequently than phagocytosis. We performed the same experiments with a lower MOI to assess whether endocytosis was observed only at a high MOI. At an MOI of 1 or 10, the ratio of phagocytic events to receptor-mediated endocytosis was the same as that at an MOI of 100 (data not shown). Some electron micrographs strongly suggested that the envelope of the virus particle was fusing with the membrane inside the vesicle (Fig. 1E and G). After 1 h, more than 80% of the Kupffer cells had viral particles in their cytoplasm. After 6 h of infection, no viral particles could be detected in the cytoplasm of Kupffer cells. No viral proteins were detected by IFA after 1 or 6 h of infection.
FIG. 1.
Ultrastructural study of the penetration of DEN virus into Kupffer cells 1 h after infection. (A) Attachment of virus particles to the cell surface; (B) engulfing of several particles by a cytoplasmic multivesicular process; (C) numerous DEN virus particles engulfed in an intracytoplasmic vacuole; (D, E, and F) various stages in the uptake of particles via clathrin-coated pits; (G and E [arrow]) electron micrographs suggesting fusion of the virion to the membrane of the vesicle. Magnifications, ×108,000 (A, B, C, and E), ×170,000 (D and G), and ×130,000 (F).
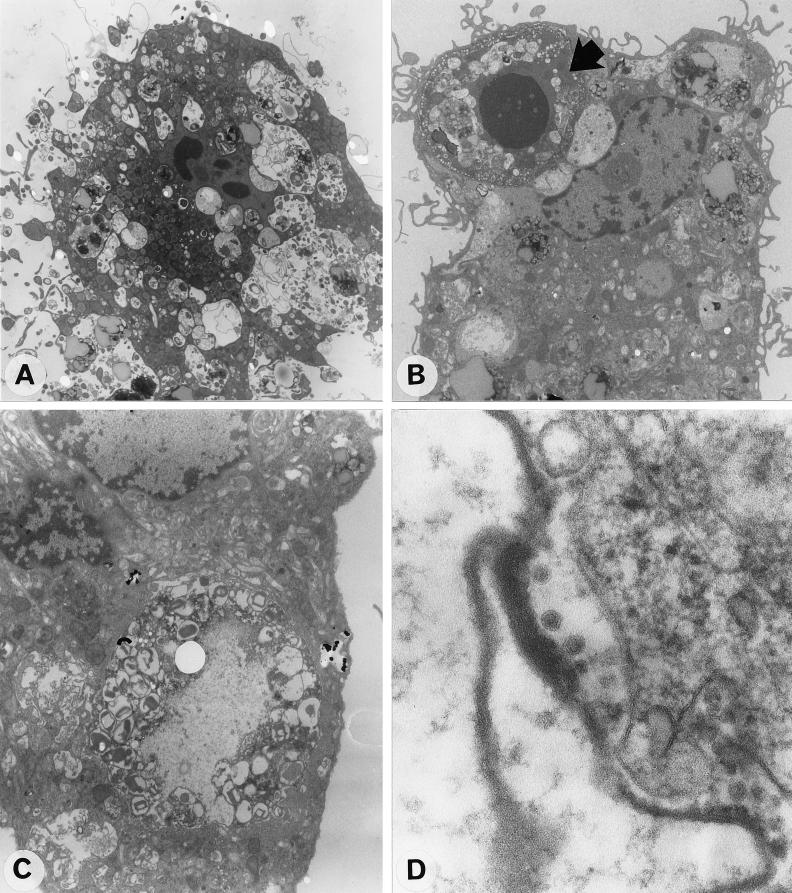
After 24 and 48 h of infection, electron microscopy revealed that about 4% of Kupffer cells showed early signs of apoptosis, with chromatin margination in nuclei, condensation, and retraction of the cytoplasm and blebbing (Fig. 2A). Several apoptotic Kupffer cells were ingested by neighboring healthy Kupffer cells (Fig. 2B). Some Kupffer cells had huge vacuolar compartments containing cells undergoing degradation (Fig. 2C). Virus-like particles were detected in some of these phagosomes (Fig. 2D). Few cells displayed ultrastructural features typical of lysis 48 h after infection (Fig. 3). DEN viral antigens were detected by IFA 24 and 48 h after infection in about 10% of the cells. The mock-infected cells and cells in cultures incubated with heat-inactivated virus (56°C, 30 min) did not show any apoptotic features and were negative in the IFA. Different patterns of fluorescence were observed 1 and 2 days after infection, speckled labeling throughout the cytoplasm of the Kupffer cells 24 h after infection and intense labeling, mostly of huge cytoplasmic inclusions at 48 h (Fig. 4). The number of cells containing viral antigens did not increase between 24 and 48 h after infection. Virus titration on AP61 mosquito cells (19) revealed that no infectious virus particles were present in the supernatants at any time after infection. In addition, viral proteins were no longer detected by IFA 72 h after infection. At this late time point, electron microscopy showed that the residual Kupffer cell monolayer was intact, with no ultrastructural cellular defects, and no virus particles could be observed.
FIG. 2.
Ultrastructural features of DEN virus-infected Kupffer cells after 24 h of infection. (A) Apoptotic Kupffer cell with dense remnant nucleus and surface blebbing 6 h after infection; (B) apoptotic Kupffer cell (arrow) ingested by a neighboring Kupffer cell; (C) huge vacoular compartment containing partially digested cell material; (D) DEN virus-like particles inside phagosomes. Magnifications, ×3,900 (A), ×3,400 (B), ×7,200 (C), and ×130,000 (D).
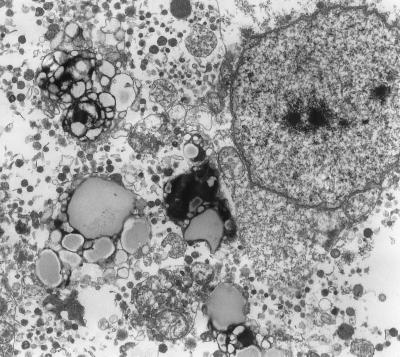
FIG. 3.
Lysed Kupffer cell observed after 48 h of infection. Magnification: ×5,400.
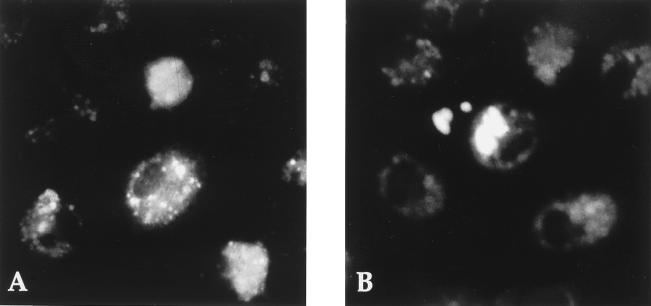
FIG. 4.
Immunofluorescence staining of DEN viral antigens. (A) Kupffer cells infected for 24 h at an MOI of 100, showing speckled staining; (B) Kupffer cells infected for 48 h, with a more diffuse and patchy pattern of fluorescence.
These results show that DEN virus can enter human Kupffer cells efficiently since more than 80% of Kupffer cells had viral particles in their cytoplasm that were visible under an electron microscope 1 h after infection. However, infection of Kupffer cells was inefficient since viral proteins were detected in only 10% of cells after 24 h of infection. This discrepancy may be due to the macrophagic nature of the cells. Most virus particles penetrated the cells by phagocytosis and were presumably degraded, since no virus particles could be detected after 6 h. Indeed, phagocytosis leads to viral degradation, and it has been shown, for example, that human immunodeficiency virus entering human macrophages by phagocytosis is noninfectious whereas that entering via the specific CD4 receptor proceeds through an infective life cycle (21). The small number of Kupffer cells in which DEN virus replication took place were probably therefore those in which the virus penetrated by receptor-mediated endocytosis and fusion.
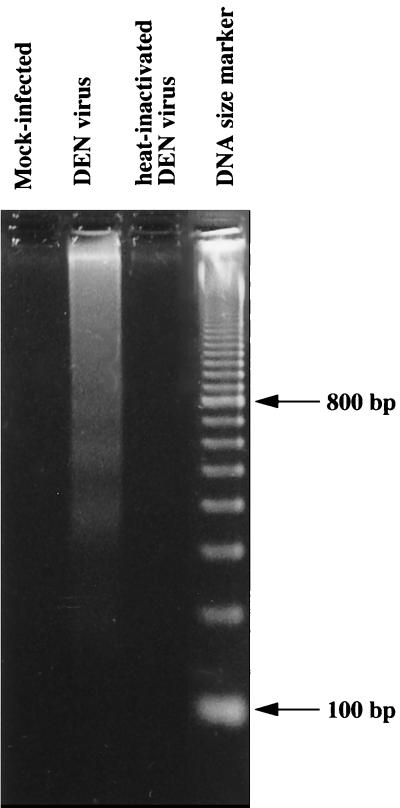
No viral progeny was detected in the supernatants, and thus DEN virus replication was aborted in human Kupffer cells. It should be noted that infection of human Kupffer cells, isolated from and cultured under conditions identical to those used here, with human immunodeficiency virus leads to productive infection (27). Furthermore, it appeared that DEN virus-infected Kupffer cells underwent early and rapid cell death by apoptosis, as previously shown in other DEN virus-infected mammalian cell lines (5, 18). The apoptotic process was detected in about 4% of cells by the terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling technique and confirmed by DNA analysis, which revealed internucleosomal DNA fragmentation typical of apoptotic cells 48 h after infection (Fig. 5). No apoptotic cells were detected by either technique in mock-infected cells or in cells exposed to heat-inactivated virus, suggesting that one or several steps in the replication process are required to trigger cell death. As assessed by electron microscopy, healthy Kupffer cells were able to ingest apoptotic Kupffer cells (Fig. 2B). Furthermore the presence of virus-like particles in the huge vacuolar compartment (Fig. 2D) suggests that the ingested cells may have been sites of viral replication. Thus, these apoptotic bodies may correspond to the intracytoplasmic inclusions containing DEN viral antigens observed by IFA 48 h after infection. Seventy-two hours after infection, apoptotic and lysed Kupffer cells were no longer visible and no fluorescent DEN virus antigen-containing cells were detected. We suggest that the few cells in which DEN virus replication and maturation were initiated died by apoptosis. This event could prevent the release of infectious viral progeny. Furthermore, apoptotic bodies may be cleared by neighboring Kupffer cells: Kupffer cells have been shown to have a scavenger function for apoptotic peripheral blood lymphocytes (6) and for simian immunodeficiency virus-infected lymphocytes in monkeys (23). The mechanism triggering apoptosis of DEN virus-infected Kupffer cells remains unknown.
FIG. 5.
Apoptotic DNA degradation in DEN virus-infected Kupffer cells. Soluble DNA was extracted from Kupffer cell lysates 48 h after mock infection, infection with DEN virus, or exposure to heat-inactivated DEN virus. Sizes of DNA markers are indicated.
Like other macrophages, Kupffer cells may be reactive to the entry of virus particles and transiently release various mediators (4). The mediators released include interleukins (ILs), interferon (IFN), tumor necrosis factor (TNF), and nitric oxide (NO) and may interfere with the virus life cycle and/or initiate an inflammatory process, thereby contributing to the pathological changes seen in DEN virus pathogenesis (13, 15). We investigated whether Kupffer cells react to DEN virus infection by incubating them with DEN type 1 virus at an MOI of 100 for 1, 6, and 30 h. IL-1α, IL-1β, IL-6, TNF-α, and IFN-α were assayed in the supernatants with enzyme-linked immunosorbent assay kits (the Quantikine kit from R&D Systems was used for all cytokines except IFN-α, which was tested with a kit from Endogen). Similar amounts of IL-1α and IL-1β were released from infected and mock-infected cells (Table 1). The synthesis of TNF-α, IFN-α, and IL-6 increased with time after infection (Table 1). IFN-α release increased as early as 1 h after infection. The levels of IL-6 and TNF-α synthesis were higher than those for mock-infected cells after 6 and 30 h, respectively.
TABLE 1.
Nitrite or nitrate, IL-1α, IL-1β, IFN-α, IL-6, and TNF-α concentrations in the supernatants of infected or mock-infected Kupffer cellsa
| Time after infection (h) | Concn ofb:
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nitrite or nitrate (μM)
|
IL-1α (pg/ml)
|
IL-1β (pg/ml)
|
IFN-α (pg/ml)
|
IL-6 (pg/ml)
|
TNF-α (pg/ml)
|
|||||||
| Mock | Inf | Mock | Inf | Mock | Inf | Mock | Inf | Mock | Inf | Mock | Inf | |
| 1 | 12.5 | 26 | 5 | 5 | 5 | 3 | 3 | 10 | 2 | 2.5 | 5 | 7 |
| 6 | 14 | 24.5 | 5 | 6 | 5 | 6 | 0.5 | 25 | 5.1 | 8.5 | 9 | 32 |
| 30 | 13.5 | 23.5 | 6 | 6 | 7 | 5 | 0.5 | 85 | 8.9 | 42 | 10 | 150 |
Kupffer cells were either exposed to infectious DEN virus at an MOI of 100 (Inf) or mock infected (Mock) at various times after infection.
Each value is the mean of two determinations in duplicate.
Because NO is rapidly scavenged from cells, its production is generally detected indirectly. NO is generated in macrophages if the inducible NO synthase (iNOS) is present. A monoclonal anti-iNOS antibody (Transduction Laboratories, Lexington, Ky.) failed to label mock-infected cells, whereas about 80% of the Kupffer cells were labeled as early as 1 h after exposure to DEN virus. Furthermore, the number of iNOS-containing cells was constant after 1, 6, and 30 h, indicating that NO production was sustained. The production of NO was confirmed by demonstrating an increase in the concentration of its end products, nitrite and nitrate, in the supernatants of DEN virus-infected Kupffer cells by a fluorometric assay (Cayman Chemical, Ann Arbor, Mich.) (Table 1). NO may have several functions in viral infection (25), and it has been shown to have both direct and indirect antiviral effects in infected cells and animal models (2, 17, 24, 26, 29).
DEN virus infection seemed to induce a biphasic activation of Kupffer cells: there was one peak of activation shortly after infection which involved the production of NO and IFN-α, and a second peak occurred a few hours later and involved IL-6 and TNF-α synthesis. The timing of the synthesis of these soluble mediators suggests the involvement of a very early step of infection in their activation. Lower levels or the absence of soluble mediators in the supernatants of Kupffer cells incubated with heat-inactivated virus suggests that cell activation was induced by virus infection. However, further investigation is required to determine the mechanisms by which DEN virus infection activates Kupffer cells and the roles of the various mediators in DEN virus-driven processes.
This preliminary in vitro study suggests that Kupffer cells may protect against DEN virus infection by (i) eliminating the few infected cells driven through apoptosis and (ii) exerting intrinsic and extrinsic antiviral effects through autocrine and/or paracrine mechanisms, resulting in the rapid clearance of the initial virus input.
Acknowledgments
We thank Geneviève Milon for helpful comments and expert critical reading of the manuscript.
REFERENCES
- 1.Bhamarapravati N, Tuchinda P, Boonyapaknavik V. Pathology of Thailand haemorrhagic fever: a study of 100 autopsy cases. Ann Trop Med Parasitol. 1967;61:500–510. doi: 10.1080/00034983.1967.11686519. [DOI] [PubMed] [Google Scholar]
- 2.Bi Z, Reiss C S. Inhibition of vesicular stomatitis virus infection by nitric oxide. J Virol. 1995;69:2208–2213. doi: 10.1128/jvi.69.4.2208-2213.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Couvelard, A., P. Marianneau, C. Bedel, M. T. Drouet, F. Vachon, D. Hénin, and V. Deubel. Report of a fatal case of dengue infection with hepatitis: demonstration of dengue antigens in hepatocytes and liver apoptosis. Human Pathol., in press. [DOI] [PubMed]
- 4.Decker K. Biologically active products of stimulated liver macrophages (Kupffer cells) Eur J Biochem. 1990;192:245–261. doi: 10.1111/j.1432-1033.1990.tb19222.x. [DOI] [PubMed] [Google Scholar]
- 5.Desprès P, Flamand M, Ceccaldi P-E, Deubel V. Human isolates of dengue type 1 virus induce apoptosis in mouse neuroblastoma cells. J Virol. 1996;70:4090–4096. doi: 10.1128/jvi.70.6.4090-4096.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Falasca L, Bergamini A, Serafino A, Balabaud C, Dini L. Human Kupffer cell recognition and phagocytosis of apoptotic peripheral blood lymphocytes. Exp Cell Res. 1996;224:152–162. doi: 10.1006/excr.1996.0123. [DOI] [PubMed] [Google Scholar]
- 7.Feldmann G. Liver apoptosis. J Hepatol. 1997;26:1–11. doi: 10.1016/s0168-8278(97)80491-6. [DOI] [PubMed] [Google Scholar]
- 8.Gubler D J. Dengue and dengue hemorrhagic fever. Clin Microbiol Rev. 1998;11:480–496. doi: 10.1128/cmr.11.3.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hall W C, Crowell T P, Watts D M, Barros V L, Kruger H, Pinheiro F, Peters C J. Demonstration of yellow fever and dengue antigens in formalin-fixed paraffin-embedded human liver by immunohistochemical analysis. Am J Trop Med Hyg. 1991;45:408–417. doi: 10.4269/ajtmh.1991.45.408. [DOI] [PubMed] [Google Scholar]
- 10.Halstead S B. Antibody, macrophages, dengue virus infection, shock, and hemorrhage: a pathogenetic cascade. Rev Infect Dis. 1989;11:S830–S839. doi: 10.1093/clinids/11.supplement_4.s830. [DOI] [PubMed] [Google Scholar]
- 11.Innis B. Dengue and dengue hemorrhagic fever. In: Portefield J S, editor. Exotic viral infections. London, United Kingdom: Chapman & Hall Medical; 1995. pp. 103–146. [Google Scholar]
- 12.Kangwanpong D, Bhamarapravati N, Lucia H L. Diagnosing dengue virus infection in archived autopsy tissues by means of the in situ PCR method: a case report. Clin Diagn Virol. 1995;3:165–172. doi: 10.1016/0928-0197(94)00032-p. [DOI] [PubMed] [Google Scholar]
- 13.Khare M, Chaturvedi U C. Role of nitric oxide in transmission of dengue virus specific suppressor signal. Indian J Exp Biol. 1997;35:855–860. [PubMed] [Google Scholar]
- 14.Kirn A, Steffan A M, Bingen A, Cinqualbre J, Gendrault J L. Isolement et culture de cellules de Kupffer humaines. C R Acad Sci Paris. 1980;291:249–251. [PubMed] [Google Scholar]
- 15.Kurane I, Ennis F A. Cytokines in dengue virus infections: role of cytokines in the pathogenesis of dengue hemorrhagic fever. Semin Virol. 1994;5:443–448. [Google Scholar]
- 16.Latham P S. The role of hepatocytes and sinusoidal cells in the pathogenesis of viral hepatitis. Int Rev Cytol. 1988;112:185–223. doi: 10.1016/s0074-7696(08)62009-4. [DOI] [PubMed] [Google Scholar]
- 17.Lin Y L, Huang Y L, Ma S H, Yeh C T, Chiou S Y, Chen L K, Liao C L. Inhibition of Japanese encephalitis virus infection by nitric oxide: antiviral effect of nitric oxide on RNA virus replication. J Virol. 1997;71:5227–5235. doi: 10.1128/jvi.71.7.5227-5235.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marianneau P, Cardona A, Edelman L, Deubel V, Desprès V. Dengue virus replication in human hepatoma cells activates NF-κB which in turn induces apoptotic cell death. J Virol. 1997;71:3244–3249. doi: 10.1128/jvi.71.4.3244-3249.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marianneau P, Mégret F, Olivier R, Morens D M, Deubel V. Dengue 1 virus binding to human hepatoma HepG2 and simian Vero cell surfaces differs. J Gen Virol. 1996;77:2547–2554. doi: 10.1099/0022-1317-77-10-2547. [DOI] [PubMed] [Google Scholar]
- 20.Mogensen S C. Macrophages and age-dependent resistance to hepatitis induced by herpes simplex virus type 2 in mice. Infect Immun. 1978;19:46–50. doi: 10.1128/iai.19.1.46-50.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pauza C D, Price T M. Human immunodeficiency virus infection of T cells and monocytes proceeds via receptor-mediated endocytosis. J Cell Biol. 1988;107:959–968. doi: 10.1083/jcb.107.3.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pereira C A, Steffan A M, Kirn A. Kupffer and endothelial liver cell damage renders A/J mice susceptible to mouse hepatitis virus type 3. Virus Res. 1984;1:557–563. doi: 10.1016/0168-1702(84)90013-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Persidsky Y, Steffan A M, Gendrault J L, Hurtrel B, Berger S, Royer C, Stutte H J, Muchmore E, Aubertin A M, Kirn A. Permissiveness of Kupffer cells for simian immunodeficiency virus (SIV) and morphological changes in the liver of rhesus monkeys at different periods of SIV infection. Hepatology. 1995;21:1215–1225. [PubMed] [Google Scholar]
- 24.Pertile T L, Karaca K, Sharma J M, Walser M M. An antiviral effect of nitric oxide: inhibition of reovirus replication. Avian Dis. 1996;40:342–348. [PubMed] [Google Scholar]
- 25.Peterhans E. Reactive oxygen species and nitric oxide in viral diseases. Biol Trace Elem Res. 1997;56:107–116. doi: 10.1007/BF02778986. [DOI] [PubMed] [Google Scholar]
- 26.Sanders S P, Siekierski E S, Porter J D, Richards S M, Proud D. Nitric oxide inhibits rhinovirus-induced cytokine production and viral replication in a human respiratory epithelial cell line. J Virol. 1998;72:934–942. doi: 10.1128/jvi.72.2.934-942.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schmitt M P, Gendrault J L, Schweitzer C, Steffan A M, Beyer C, Royer C, Jaeck D, Pasquali J L, Kirn A, Aubertin A M. Permissivity of primary cultures of human Kupffer cells for HIV-1. AIDS Res Hum Retroviruses. 1990;6:987–991. doi: 10.1089/aid.1990.6.987. [DOI] [PubMed] [Google Scholar]
- 28.Selgrade M K, Osborn J E. Role of macrophages in resistance to murine cytomegalovirus. Infect Immun. 1974;10:1383–1390. doi: 10.1128/iai.10.6.1383-1390.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tucker P C, Griffin D E, Choi S, Bui N, Wesselingh S. Inhibition of nitric oxide synthesis increases mortality in Sindbis virus encephalitis. J Virol. 1996;70:3972–3977. doi: 10.1128/jvi.70.6.3972-3977.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]